1. Introduction
The bone has a remarkable ability to provide structural support, protect vital organs, and facilitate locomotion, so it is an extraordinary tissue essential for the integrity and functionality of the human body [
1]. However, when it is subjected to traumatic injuries, diseases such as osteoporosis, or congenital defects, the regenerative capacity of the bone tissue may be compromised, which leads to impaired function and quality of life for the affected individuals. Therefore, addressing these challenges necessitates innovative approaches in tissue engineering and regenerative medicine [
2].
Over the years, researchers and clinicians alike have sought effective strategies to promote bone regeneration and restore skeletal function. Bone tissue regeneration remains a significant challenge in the field of biomedical engineering. Traditional treatment approaches often fall short in achieving optimal functional and aesthetic outcomes, necessitating the exploration of innovative biomaterials and techniques, such as autologous bone grafts and metallics implants, which, while valuable, are not without limitations [
3]. Developing biomaterial-based scaffolds capable of mimicking the intricate microenvironment of the native bone emerges as a promising avenue for therapeutic intervention. Chitosan-based hydrogels have emerged as promising candidates due to their biocompatibility, tunable properties [
4], and the ability to mimic the extracellular matrix (ECM) environment conducive to bone regeneration [
5].
It also offers several advantages, including high water content, porous structure, ability to encapsulate bioactive agents, biodegradability, antimicrobial properties, and nontoxicity, facilitating controlled release kinetics crucial for tissue regeneration processes [
6,
7,
8]. Moreover, specific composite chitosan formulations efficiently support electrical conduction, which is crucial for regeneration and wound healing owing to its hemostatic and antimicrobial properties. Studies have demonstrated that chitosan (CS) biomaterials could facilitate cell proliferation at the wound site and mediate complete wound generation and epithelial reconstruction [
9].
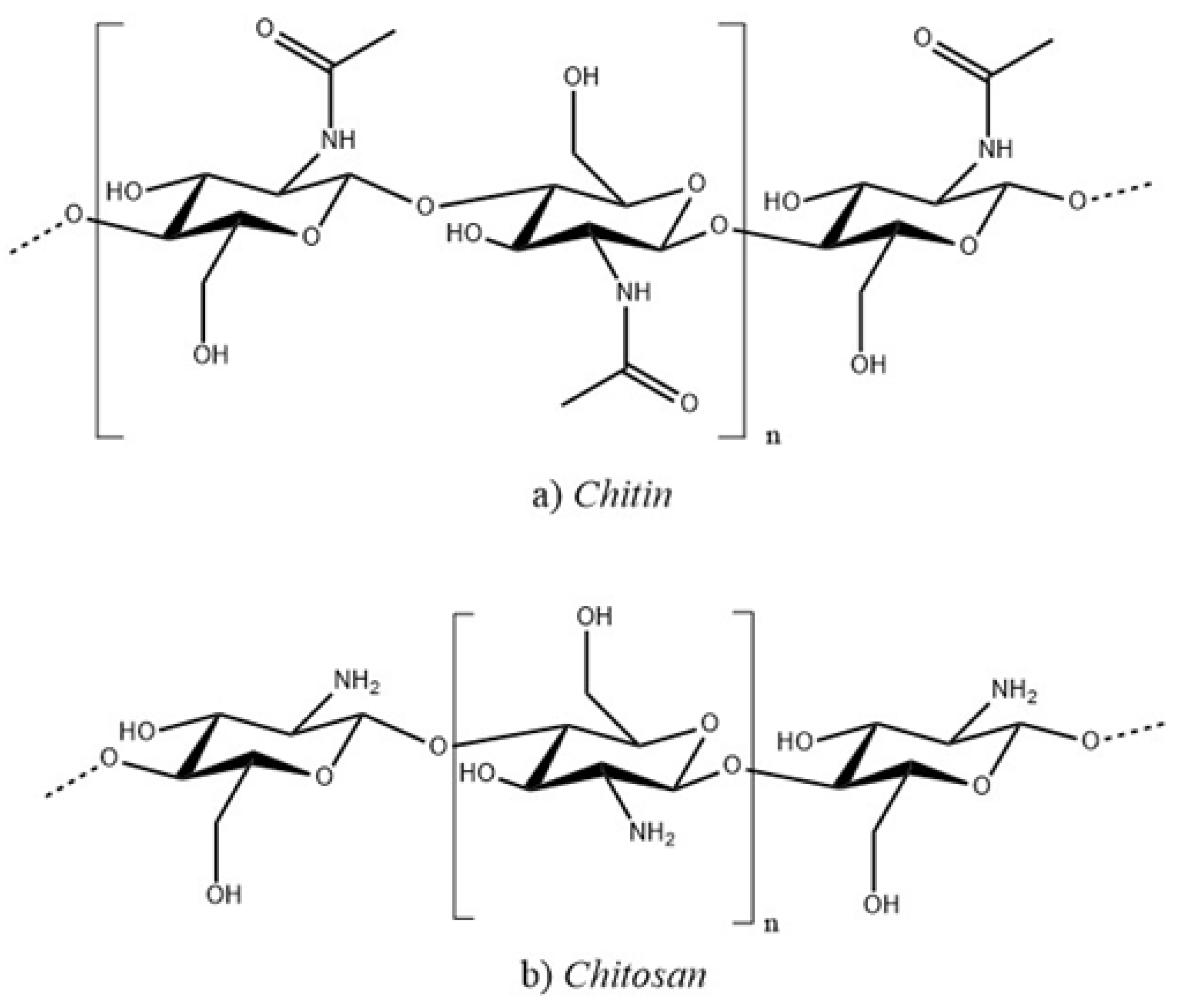
Chitin and its deacetylated derived, chitosan, are a family of linear polysaccharides composed of varying amounts of (
) linked residues of N-acetyl-2 amino-2-deoxy-D-glucose (glucosamine, GlcN) and 2-amino-2-deoxy-D-glucose (N-acetyl-glucosamine, GlcNAc) residues [
10], as shows in
Figure 1. Therefore, chitosan is soluble in aqueous acidic media via primary amine protonation (pKa = 6.3) [
11]. It is a polysaccharide that is widely used in various biomedical applications. It is obtained through partial deacetylation of chitin, the second most abundant polysaccharide, and is found in the exoskeletons of crustaceans and insects and in the cell walls of fungi. If chitin or poly(N-acetyl-1,4-glucosamine) undergoes chemical deacetylation it generates a random distribution of acetylated (GlcNAc;
-(1,4)-2-acetamido-2-deoxy-D-4-glucopyranose) and deacetylated (GlcN;
-(1,4)-2-amino-2-deoxy-D-glucopyranose) units in the main chain structure, depending on the degree of deacetylation (DD), which usually ranges between 0.50 and 0.95 resulting in chitosan, when the degree of deacetylation reaches 100%, the polymer is known as chitan. This implies a structural relationship between chitin, chitosan, and chitan [
12].
Chitosan, a cationic polymer made from (1-4)-2-amino-2-deoxy-
-D-glucan, has gained popularity due to its pH sensitivity, biocompatibility, and bioactive properties, surpassing the base polymer chitin [
13]. There are two methods for chitosan synthesis, which are chemical and biological. Chemical methods rely exclusively on chemicals, whereas biological techniques utilize enzymes or bacteria to produce chitosan.
This study explores the latest advancements in chitosan-based hydrogels for bone tissue regeneration; By synthesizing and analyzing existing literature, we seek to cohesively evaluate the efficacy, mechanisms, and challenges associated with utilizing these biomaterials in clinical settings.
2. Methodology
The methodology used in the development of this research was qualitative-documentary-exploratory, based on:
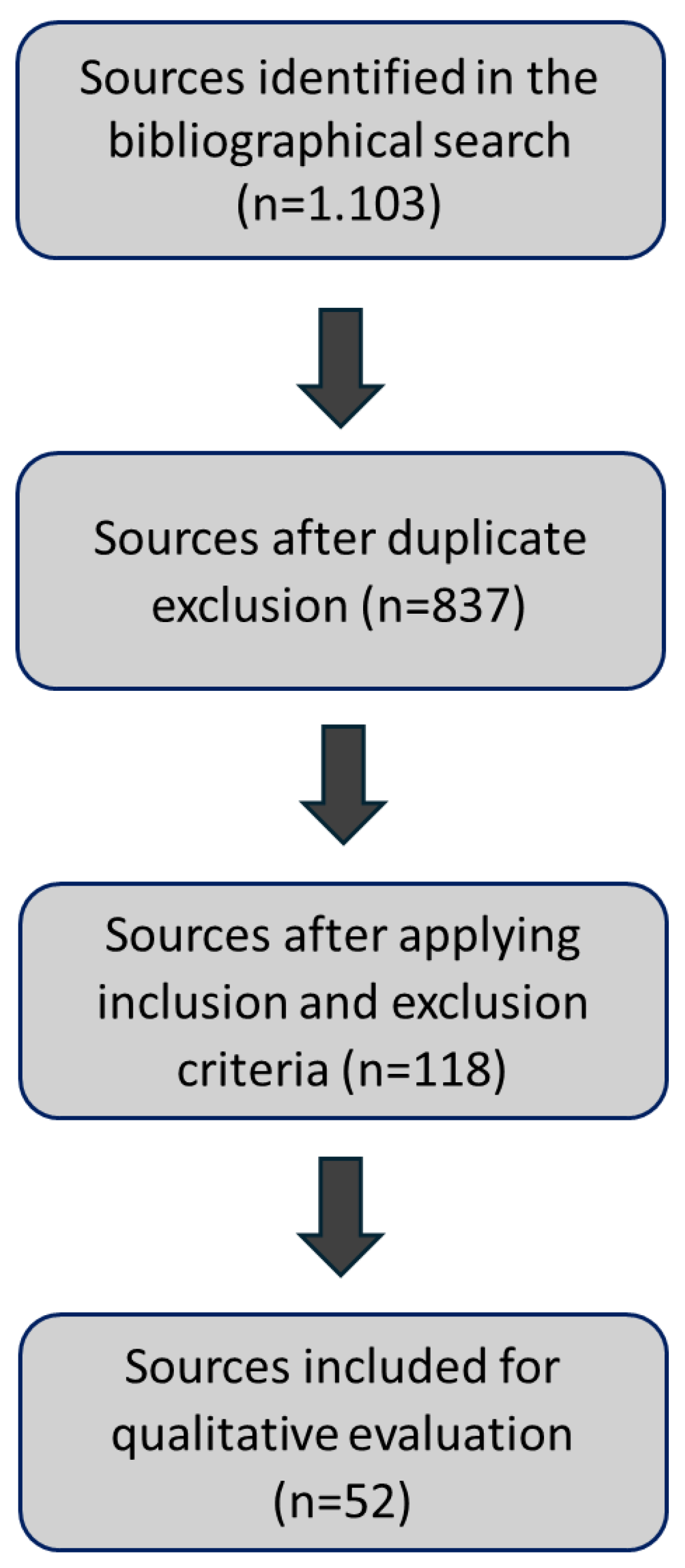
Search and date compilations: the research involved an exhaustive search across a wide range of databases, including PubMed, Frontiers, ScienceDirect, SciELO, and Google Scholar. Key search terms included “Chitosan-based hydrogels” and “Bone regeneration,” with a search period from 1994 to 2023.
Information selection and refinement of information: a thorough exploration of the collected data was conducted using Mendeley [
14] as a crucial bibliographic management tool to organize and manage references. The information was systematically refined and categorized based on five main criteria that form the foundation of this study: bone tissue regeneration, properties, synthesis, types of chitosan hydrogels, and hydrogels for bone tissue regeneration. These bases were prioritized to ensure that the most relevant and impactful information guided the research direction.
Selection of subtopics: the refined information facilitated the organization of the research structure, clarifying the selection of subtopics directly related to the study’s objectives. This step allowed for a focused examination of each aspect of chitosan-based hydrogels concerning bone regeneration.
Data Analysis: an indepth analysis of the selected information was performed, interpreting the findings within the context of the defined subtopics. The data analysis aimed to synthesize the insights from the literature, discuss key findings and draw conclusions that contribute to a broader understanding of the role of chitosan-based hydrogels in bone tissue regeneration.
Figure 2.
3. Discussion and Results
3.1. Bone
The bone represents major supporting structures, the binding place for muscles, ligaments, and tendons, mechanical support, and the shield of most essential tissues. It is also a bioceramic composite. One needs to understand that there are two stages of bone formation, referred to as primary and secondary osteogenesis, and that the mechanism of bone formation differs substantially in these two stages [
15]. Epiphysial cartilage, which serves as the locus for primary bone formation, combines ground substance and very loose, small fibril bundles of collagen. Secondary bone development occurs when the original woven bone is modified to have a more optimum structure, such as parallel-fibered or lamellar bone, which in the case of humans is organized into concentric lamella that make up the osteons of the Haversian canal system [
2]. The osteoblasts secrete collagen fibrils in secondary bone, which is more significant than in primary bone. During secondary bone development, the crystal organization is guided by the collagen fibrils that form within them. This produces exceedingly small intrafibrillar crystals that would not be thermodynamically stable if not embedded in the organic matrix. Crystals, or interfibrillar crystals, can also form on the surface and between collagen fibers.
3.2. Bone Tissue Regeneration
The repair and/or regeneration of injured bone is a significant problem in orthopedic surgery. Bone grafts are the second most transplanted tissue after blood. These transplants are used to treat bone defects caused by severe trauma or developmental deformities, replacement surgeries to relieve pain or repair joint damage, revision surgeries to replace or compensate for a failed implant, and tumor resection, which is the surgical removal of tumor-affected bone tissue [
16]. An ideal bone tissue-engineered scaffold must possess several important characteristics, including biocompatibility, osteoinductivity, bioactivity, good mechanical integrity through the bone healing process, a degradation rate such that the strength of the scaffold is maintained until the regeneration tissue can provide the necessary mechanical support [
17,
18].
3.3. Structure and Properties of Chitosan
Chitosan is a polysaccharide that is widely used for various biomedical applications. It is very abundant, and its production is a meager cost. It is obtained through partial deacetylation of chitin, found in the exoskeletons of crustaceans and insects and the cell walls of fungi. Chitosan is the only polycation in nature, and its charge density depends on the degree of acetylation and pH of the media [
19]. Its acetylation degree and molecular weight determine the polymer’s solubility. Chitosan oligomers are soluble at various pH levels, from acidic to primary (physiological pH 7.4). Depending on the agents employed to remove chitin from organisms and convert it to chitosan [
20].
One of the most valuable properties of chitosan is chelation. Chitosan can selectively bind desired materials such as cholesterol, fats, metal ions, proteins, and tumor cells. Chitosan has shown an affinity for proteins, such as wheat germ agglutinin and trypsin; other properties that make this very useful include inhibition of tumor cells, antifungal effects, acceleration of wound healing, and stimulation of the immune system. It is also an appropriate cationic polymer for membrane production. Early studies demonstrated that membranes formed from polymers might be used for water purification, filtration, surgical dressing, and controlled release [
21,
22].
3.4. Solubility
As mentioned, chitosan is a biocompatible and biodegradable polymer that has gained much attention in tissue engineering, including tissue bone regeneration. So, the solubility properties play a significant role in the development of hydrogels. Therefore, since chitosan is produced by deacetylation of chitin, some N-acetylglucosamine moieties are converted into glucosamine units and the presence of large amounts of protonated
groups on the chitosan structure accounts for its solubility in acid aqueous media since its pKa value approximately 6.5 which means that when it is around 50% of all amino groups are protonated, chitosan becomes soluble [
11]. Solubility depends on factors such as polymer molecular weight, degree of acetylation, pH, temperature, and polymer crystallinity [
23]. Homogeneous deacetylation of chitin permits the production of polymers soluble in aqueous acetic solutions with DD as low as 28%.
In the studied role of crystallinity and inter- or intramolecular forces on chitosan solubility, in this work by Sogias et al. [
24], a parent chitosan sample was half reacetylated with anhydride acetic or fully N-deacetylated under homogeneous conditions. After reacetylation, the polymer’s solubility was expanded to pH 7.4, while the reduction in the solubility range of the fully deacetylated chitosan was determined. The lower solubility was attributed to increased polymer crystallinity following deacetylation, which counteracted the effects of the increased glucosamine moieties. In contrast, the half-acetylated sample showed a drop in crystallinity. This means that using hydrogen bond disruptors like urea or guanidine hydrochloride affects chitosan’s solubility window. Broad solubility can be increased by combining chemical and physical disruption of hydrogen bonds using a variety of approaches, including acidic solutions, derivatization, ionic liquids, and cosolvents. These factors must be optimized in order for the hydrogel to form successfully [
24].
3.5. Viscosity
Polymer viscosity is an essential technological property because extremely viscous solutions are challenging to manage. Viscometry is a potent instrument for estimating the molecular weight of chitosan since it is a simple and rapid method, even though it is not an absolute method, and so requires the measurement of solvent-specific constants [
25]. The viscosity of chitosan is regulated by the polymer’s molecular weight and the degree of deacetylation, and it decreases as the polymer’s molecular weight is reduced. The viscosity of a polymer in a solution can be used to evaluate its durability, as it decreases with polymer storage due to degradation. In this, the deacetylation advances of the chitosan produce a kind of viscosity named shear viscosity.
The shear viscosity at the same rate was studied by Wang and Xu [
26] in two samples with different deacetylation degrees (91% vs. 75%) and represented versus intrinsic viscosity; it was stated that shear viscosity was more significant for those samples with highest deacetylation degree, therefore when the curves were evaluated, straight lines were observed in both samples in which this is explained due to the nature of chitosan, as this polymer is a cationic polyelectrolyte because if the amine protonation in acidic media. Which means that the higher the DD, the larger chain expansion is expected [
10,
26].
3.6. Synthesis of Chitosan
Chitosan has several advantageous properties, such as biocompatibility, biodegradability, and antimicrobial activity [
27]. Depending on the agents that are used to extract chitin from living organisms and transform it into chitosan, there are two methods of chitosan synthesis, namely chemical or biological. The chemical methods rely exclusively on chemicals, whereas biological techniques utilize enzymes or bacteria for chitosan production [
28]. These techniques have three typical steps: demineralization, deproteinization, and deacetylation. Demineralization entails the removal of minerals, so this is the first step aimed at removing
, which, in a chemical approach, is performed using hydrochloric acid. The second step eliminates proteins and other organic compounds with an alkaline solution generating chitin. Chitosan is known to be degraded in vertebrates mainly by lysozyme and certain bacterial enzymes in the colon. The lysozyme, present in several bodily fluids such as serum, tears, saliva, and others like those surrounding cartilage, hydrolyzes
(
) linkages between N-acetyl-D-glucosamine residues of chitosan [
29]. The rate and extent of chitosan degradation in living organisms depends on the degree of deacetylation. Therefore, giving an adequate time interval and appropriate conditions will mean that chitosan will degrade sufficiently to be eliminated by normal metabolic processes [
20].
Lastly, the third synthesis step involves the deacetylation of chitin, commonly using a sodium hydroxide solution. In the biological approach to chitosan preparation, these three steps are performed by bacteria and/ or enzymes. Overall, chemical methods of chitosan synthesis tend to be cheaper, more rapid, less complicated, and produce a higher yield with greater degrees of deacetylation compared to biological alternatives, even though the biological methods could generate chitosan with superior mechanical properties [
30]. These qualities include improved tensile strength, which provides excellent durability and resistance to deformation, and an increased elastic modulus, which indicates greater stiffness and appropriateness for structural applications. Chitosan’s increased durability allows it to absorb greater force without breaking, making it ideal for packaging and biomedical devices. Its increased flexibility makes it optimal for bending and flexing applications like films and coatings. Higher elongation at break implies that chitosan can stretch further before failing, which is useful in wound dressings and flexible electronics. Chitosan’s high compressive strength makes it ideal for load-bearing applications such as tissue engineering scaffolds; not only that, but its superior viscoelastic qualities allow for more efficient energy absorption and dissipation, making it excellent for dynamic settings [
31]. Furthermore, better barrier characteristics against gases and moisture increase its efficacy in packaging applications. Enhanced biocompatibility and bioactivity, while not strictly mechanical qualities, help to improve its overall performance in biomedical applications by assuring positive interactions with biological tissue [
32,
33].
Chitosan can be chemically and physically modified to address its limitations, and formulations can be designed to deliver a desirable outcome. It possesses hydroxy- and amino-functional groups, which can be subjected to carboxyalkylation, sulphonation, acetylation with acids and esters, and with the addition of sugars, producing derivatives with improved solubility in water, enhanced regenerative properties, and ameliorated drug-delivery capabilities [
34]. Adding other chemical groups enriches the bioactive properties of chitosan, enabling it to interact with a greater range of active molecules and cells. Another approach to address the inadequacies of pure chitosan and enhance its properties is to combine it with different natural and synthetic polymers. Chitosan is frequently used with various natural biomaterials, including but not limited to gelatin, hydraulic acid, alginate, collagen, silk fibroin, and other compounds [
35]. Other composites of chitosan and various synthetic polymers such as polyvinyl alcohol, polypyrrole, poly(-caprolactone), polyvinylpyrrolidone, among others, are widely utilized. These synthetic compounds were shown to ameliorate significantly the native properties of chitosan, making it a more efficient drug delivery agent and improving its regenerative potential [
30,
36].
3.7. Types of Chitosan Hydrogels
Two chitosan hydrogels can be prepared: without and with a chemical cross-linker, respectively known as physically cross-linked hydrogel and physical/chemical co-cross-linked hydrogel. Depending on crosslinking density, chemical hydrogels can be less sensitive to degradation and often have mechanical properties than physical hydrogels. Hydrogels, particularly in situ forming hydrogels, have acquired popularity in various biomedical applications, serving as carriers and scaffolds for tissue engineering [
37]. These injectable fluids undergo
in situ sol-gel transitions in response to physical or chemical stimuli. These have several advantages over conventional hydrogels formed outside the body, including high tissue-like water content, the ability to homogeneously incorporate therapeutic agents prior to administration, minimally invasive implantation, easily manipulable physical properties, and fluid properties that allow for the even filling of any size or shape of the defect [
38,
39].
3.8. Bone Regeneration Using Hydrogel
Injectable hydrogels containing chitosan have been demonstrated to enhance bone tissue regeneration. Glycol chitosan and oxidized hyaluronic acid injectable hydrogel to optimize the delivery of graphene oxide, a known osteoinductive material. It was expected that the hydrogel might be employed to transport the necessary amounts of graphene oxide to stimulate osteogenesis in cell cultures. The hydrogel containing graphene oxide induced osteogenic differentiation of human adipose-derived mesenchymal stem cells, as evidenced by positive staining for alkaline phosphatase activity and calcium deposition, as well as increased expression of osteogenic markers COL1, OCN, BSP, and RUNX2 [
39].
Chitosan-based injectable hydrogels have been used to administer drugs [
40]. For example, Tao et al. [
41], created a thermosensitive chitosan-glycerol phosphate hydrogel/nanoparticle system to transport vancomycin to the diseased bone and improve healing. The researchers implanted the hydrogel, and after eight weeks, bone inflammation was dramatically reduced while bone regeneration was increased, as evidenced by a reduction in inflammation markers and radiographic and histological inspection. A study was conducted using chitosan-based injectable hydrogels that may induce bone regeneration on their own; hence a highly osteoinductive injectable in situ forming hydrogel composed of methacrylate glycol chitosan and montmorillonite was created [
41]. This hydrogel alone could recruit native cells and mediate osseous tissue regeneration in a calvarial defect. Another technique for chitosan production is using nanofiber membranes, which can mediate, control, and sustain the release of diverse bioactive chemicals.
3.9. Injectable Hydrogels
Injectable hydrogels containing chitosan have been shown to promote bone tissue regeneration. Glycol chitosan and oxidized hyaluronic acid injectables can be used to optimize the distribution of graphene oxide, an osteoinductive substance. It was demonstrated that hydrogel may transfer the necessary amounts of graphene oxide to stimulate osteogenesis in cell cultures. The hydrogel containing graphene oxide enhanced osteogenic development of human adipose-derived mesenchymal stem cells, as evidenced by positive staining for alkaline phosphatase activity and calcium distribution [
42,
43].
3.10. pH-responsive Chitosan
Protonation generates electrostatic repulsion at lower pH levels, increasing water solubility, whereas higher pH levels cause deprotonation and structural collapse, decreasing solubility. Therefore, the solubility and swelling capabilities of CS-based hydrogels are regulated by their pKa value and external pH conditions, making them suitable for bone regeneration applications. Incorporating other polymers can improve CS’s pH responsiveness, although its poor solubility in alkaline and neutral solutions and insufficient mechanical performance are limitations. Various physical and chemical modifications have been developed to enhance CS solubility without compromising its properties. For example, Rogina et al. [
44] , created a pH-responsive CS-hydroxyapatite hydrogel using
as a gelling agent, achieving quick gelation within four minutes and supporting cell proliferation and differentiation. This hydrogel showed good biocompatibility, cell adhesion, and proliferation, and it enhanced bone marker expression and new bone regeneration [
44].
3.11. Thermo-Responsive Chitosan
Chitosan lacks thermosensitive properties, but thermo-responsive CS-based hydrogels can be created by incorporating thermo-responsive polymers [
45]. Temperature is a standard stimulus for hydrogel systems due to its simple regulation and applicability for
in vitro and
in vivo testing. Thermo-responsive CS injectable hydrogels with
sol-gel transitions at physiological temperatures have recently been developed for tissue engineering. These hydrogels offer temperature responsiveness, moldability, tailorable rheological properties, excellent biocompatibility, and biodegradability, making them suitable for enhancing cellular activity in bone and dental regenerations. The CS/
-glycerophosphate (GP) hydrogel is widely used as a scaffold for delivering growth factors, cells, small drugs, and nucleic acids. The cationic CS chains interact with negatively charged GP molecules via electrostatic attraction and hydrogen bonds, resulting in injectable CS/GP hydrogels with an optimized structural system and a
sol-gel transition temperature of 37°C, ideal for bone regeneration [
46,
47].
3.12. Polymers Introducing Chitosan
Incorporating various natural (e.g., alginate, hyaluronic acid, collagen, starch, and silk fibroin) and synthetic polymers (e.g., poly(vinyl alcohol), polyethylene glycol, and polycaprolactone) into chitosan hydrogels enhance their multifunctionality and thermo-responsive properties for effective bone repair. For example, Bagheri et al. [
48], developed an electroactive hydrogel combining chitosan, aniline oligomer, and agarose. The aniline oligomer modulated the hydrogel’s swelling, degradation rate, thermal characteristics, and conductivity, enabling regulated electrical stimulation to increase cell activity, growth, and proliferation [
48]. An example is that Tang et al. [
42], created a biocompatible, injectable, thermo-responsive hydrogel (HA-CPN/BCP) based on hyaluronic acid-g-chitosan-g-poly(N-isopropylacrylamide) with biphasic calcium phosphate (BCP) ceramic microparticles. This hydrogel supported human fetal osteoblast cell attachment, proliferation, and differentiation, and its mechanical strength and elasticity promoted calcium deposition, extracellular matrix mineralization, and ectopic bone tissue formation [
42].
3.13. Nanoparticles Encapsulated CS
Incorporation of functional nanoparticles (NPs) into hydrogels can increase the mechanical, biological, and chemical properties and therefore expand their applications [
49]. Especially metal/metal-oxide NPs (e.g., Au, Ag, and
), inorganic/ceramic NPs (e.g., hydroxyapatite, calcium phosphate, silica, and silicates), and polymeric NPs (natural/synthetic polymers, dendrimers, and hyperbranched polyesters) can promote the osteogenic differentiation and mineralization for bone regeneration [
50,
51] .
3.14. Dental Regeneration
Studies in dentistry have shown that chitosan can be used to generate biocompatible dental materi-als with antibacterial properties. Chitosan has an anti-inflammatory action that regulates the release of inflammatory factors, which is a crucial element of its immunomodulatory property. In dentistry, a primary issue is the health of the oral cavity, which frequently struggles with diseases and edema. There was a study conducted in which they compared a standard pulp capping material, mineral trioxide aggregate (MTA), with injectable bioactive silver-doped glass nanoparticles (Ag-BG) blended with chitosan // Beta B-sodium glycerophosphate gel (CS). There were four treatments generated (MTA, Ag-BG, CS, Ag-BG/ CS), and the analysis of their cytokine profiles showed that the rats treated with an Ag-BG/CS had a significant decrease in the levels of inflammatory cytokines; however, the effect was more substantial in combination [
42,
52]. This, in turn, suggests that both Ag-BG contribute to the anti-inflammatory activity of the hydrogel, providing additional evidence of the modulatory role of chitosan.
4. Conclusions and Future Perpestives
There are numerous notable applications for chitosan in biomedical engineering, with great potential in bone tissue regeneration due to its biocompatible, biodegradable properties and its ability to mimic the extracellular matrix. Additionally, chitosan-based scaffolds have a three-dimensional structure that promotes cell adhesion, proliferation, and differentiation. Chitosan-based hydrogels, particularly those that respond to stimuli such as pH and temperature, offer advanced solutions for bone tissue engineering applications. Chitosan’s ability to retain water, form porous structures, and encapsulate bioactive agents allows for the controlled release of factors that promote bone regeneration.
Incorporating functional nanoparticles, such as metals and metal oxides, further enhances the properties of chitosan hydrogels, boosting osteogenic differentiation and mineralization, which are key for bone repair. Studies also show that these hydrogels improve cell proliferation and differentiation and optimize bone marker expression, favoring tissue regeneration. Chitosan hydrogels, especially when combined with nanoparticles and other chemical modifications, represent an innovative and effective tool for bone tissue regeneration. Their ability to adapt to physiological conditions and improve cellular activity positions them as a fundamental material in the advancement of regenerative bone therapies.
Author Contributions
Conceptualization, J.R.; Y.G.; J.P.S.V and E.A.; formal analysis, J.R.; Y.G.; J.P.S.V and E.A.; writing—original draft preparation, J.R. and Y.G; writing—review and editing, J.R.; Y.G.; J.P.S.V and E.A.; supervision, J.R.; funding acquisition, J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Biomedical Engineering Department, Polytechnic University of Puerto Rico, PR 00918 USA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Florencio-Silva, R.; Sasso, G.R.d.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: structure, function, and factors that influence bone cells. BioMed research international 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M. Bone tissue regeneration: biology, strategies and interface studies. Progress in biomaterials 2019, 8, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: recent advances and challenges. Critical Reviews™ in Biomedical Engineering 2012, 40. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.A.; Otoni, C.G.; Espitia, P.J. Fundamentals of chitosan-based hydrogels: elaboration and characterization techniques. Materials for Biomedical Engineering.
- Jin, R.; Teixeira, L.M.; Dijkstra, P.J.; Karperien, M.; Van Blitterswijk, C.; Zhong, Z.; Feijen, J. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2544–2551. [Google Scholar] [CrossRef]
- Do, N.H.; Truong, Q.T.; Le, P.K.; Ha, A.C. Recent developments in chitosan hydrogels carrying natural bioactive compounds. Carbohydrate Polymers 2022, 294, 119726. [Google Scholar] [CrossRef]
- Rondón, J.; Vázquez, J.; Lugo, C. Biomateriales utilizados en ingeniería de tejidos para la fabricación de andamios Biomaterials used in tissue engineering for the manufacture of scaffolds. Ciencia e Ingeniería 2023, 44, 297–308. [Google Scholar]
- Lv, S.; Zhang, S.; Zuo, J.; Liang, S.; Yang, J.; Wang, J.; Wei, D. Progress in preparation and properties of chitosan-based hydrogels. International Journal of Biological Macromolecules 2023, 242, 124915. [Google Scholar] [CrossRef]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and chitosan as polymers of the future—obtaining, modification, life cycle assessment and main directions of application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Chatterjee, R.; Maity, M.; Hasnain, M.S.; Nayak, A.K. Chitosan: source, chemistry, and properties. In Chitosan in Drug Delivery; Elsevier, 2022; pp. 1–22.
- Velásquez, C.L. Chitosan-based nanomaterials on controlled bioactive agents delivery: a review. Journal of Analytical & Pharmaceutical Research 2018, 7, 484–489. [Google Scholar]
- Pellá, M.C.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydrate polymers 2018, 196, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Elsevier. Mendeley (Version v1.19.8)[Computer software]. Available from www.mendeley.com.
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Materials Science and Engineering: R: Reports 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Basha, R.Y.; TS, S.K.; Doble, M. Design of biocomposite materials for bone tissue regeneration. Materials Science and Engineering: C 2015, 57, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering. Advanced Functional Materials 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Xue, X.; Zhang, H.; Liu, H.; Wang, S.; Li, J.; Zhou, Q.; Chen, X.; Ren, X.; Jing, Y.; Deng, Y.; others. Rational design of multifunctional CuS nanoparticle-PEG composite soft hydrogel-coated 3D hard polycaprolactone scaffolds for efficient bone regeneration. Advanced Functional Materials 2022, 32, 2202470. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: a potential biopolymer in drug delivery and biomedical applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan: derivatives, composites and applications; John Wiley & Sons, 2017.
- Rinaudo, M. Characterization and properties of some polysaccharides used as biomaterials. Macromolecular Symposia. Wiley Online Library, 2006, Vol. 245, pp. 549–557.
- Spoială, A.; Ilie, C.I.; Ficai, D.; Ficai, A.; Andronescu, E. Chitosan-based nanocomposite polymeric membranes for water purification—A review. Materials 2021, 14, 2091. [Google Scholar] [CrossRef]
- Pillai, C.K.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Progress in polymer science 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Sogias, I.A.; Khutoryanskiy, V.V.; Williams, A.C. Exploring the factors affecting the solubility of chitosan in water. Macromolecular Chemistry and Physics 2010, 211, 426–433. [Google Scholar] [CrossRef]
- Norzita, Y.; Norhashidah, T.; Maznah, M. Determination of viscosity-average molecular weight of chitosan using intrinsic viscosity measurement 2013.
- Wang, W.; Xu, D. Viscosity and flow properties of concentrated solutions of chitosan with different degrees of deacetylation. International journal of biological macromolecules 1994, 16, 149–152. [Google Scholar] [CrossRef]
- Pokhrel, S.; Yadav, P.N.; Adhikari, R. Applications of chitin and chitosan in industry and medical science: a review. Nepal Journal of Science and Technology 2015, 16, 99–104. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and chitosan: production and application of versatile biomedical nanomaterials. International journal of advanced research 2016, 4, 411. [Google Scholar] [PubMed]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. International Journal of Biological Macromolecules 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-based biomaterials for tissue regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y. Chitosan-based hydrogels: functions and applications 2016.
- Rinaudo, M. Chitin and chitosan: Properties and applications. Progress in polymer science 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Stevens, C.V. Chitin and chitosan: properties and applications 2019.
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. Journal of Controlled Release 2020, 326, 150–163. [Google Scholar] [CrossRef]
- Luna-Bárcenas, G.; Prokhorov, E.; Elizalde-Peña, E.; Nuno-Licona, A.; Sanchez, I. Chitosan-Based Hydrogels for Tissue Engineering Applications, Biotechnology in Agriculture, Industry and Medicine Series, 2011.
- Doan, L.; Tran, K. Relationship between the Polymer Blend Using Chitosan, Polyethylene Glycol, Polyvinyl Alcohol, Polyvinylpyrrolidone, and Antimicrobial Activities against Staphylococcus aureus. Pharmaceutics 2023, 15, 2453. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of scaffolds for bone-tissue regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: characteristics and pharmaceutical applications. Res Pharm Sci 2015, 10, 1–16. [Google Scholar]
- Taokaew, S.; Kaewkong, W.; Kriangkrai, W. Recent development of functional chitosan-based hydrogels for pharmaceutical and biomedical applications. Gels 2023, 9, 277. [Google Scholar] [CrossRef]
- Garshasbi, H.; Salehi, S.; Naghib, S.M.; Ghorbanzadeh, S.; Zhang, W. Stimuli-responsive injectable chitosan-based hydrogels for controlled drug delivery systems. Frontiers in Bioengineering and Biotechnology 2023, 10, 1126774. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhang, Y.; Shen, A.; Yang, Y.; Diao, L.; Wang, L.; Cai, D.; Hu, Y. Injectable chitosan-based thermosensitive hydrogel/nanoparticle-loaded system for local delivery of vancomycin in the treatment of osteomyelitis. International Journal of Nanomedicine, 5855. [Google Scholar]
- Tang, G.; Tan, Z.; Zeng, W.; Wang, X.; Shi, C.; Liu, Y.; He, H.; Chen, R.; Ye, X. Recent advances of chitosan-based injectable hydrogels for bone and dental tissue regeneration. Frontiers in Bioengineering and Biotechnology 2020, 8, 587658. [Google Scholar] [CrossRef] [PubMed]
- A. Alamir, H.T.; Ismaeel, G.L.; Jalil, A.T.; Hadi, W.H.; Jasim, I.K.; Almulla, A.F.; Radhea, Z.A. Advanced injectable hydrogels for bone tissue regeneration. Biophysical Reviews 2023, 15, 223–237. [Google Scholar]
- Rogina, A.; Ressler, A.; Matić, I.; Ferrer, G.G.; Marijanović, I.; Ivanković, M.; Ivanković, H. Cellular hydrogels based on pH-responsive chitosan-hydroxyapatite system. Carbohydrate polymers 2017, 166, 173–182. [Google Scholar] [CrossRef]
- Argüelles-Monal, W.M.; Lizardi-Mendoza, J.; Fernández-Quiroz, D.; Recillas-Mota, M.T.; Montiel-Herrera, M. Chitosan derivatives: introducing new functionalities with a controlled molecular architecture for innovative materials. Polymers 2018, 10, 342. [Google Scholar] [CrossRef]
- Dhivya, S.; Saravanan, S.; Sastry, T.; Selvamurugan, N. Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. Journal of nanobiotechnology 2015, 13, 1–13. [Google Scholar] [CrossRef]
- Saravanan, S.; Vimalraj, S.; Thanikaivelan, P.; Banudevi, S.; Manivasagam, G. A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. International journal of biological macromolecules 2019, 121, 38–54. [Google Scholar] [CrossRef]
- Bagheri, B.; Zarrintaj, P.; Surwase, S.S.; Baheiraei, N.; Saeb, M.R.; Mozafari, M.; Kim, Y.C.; Park, O.O. Self-gelling electroactive hydrogels based on chitosan–aniline oligomers/agarose for neural tissue engineering with on-demand drug release. Colloids and Surfaces B: Biointerfaces 2019, 184, 110549. [Google Scholar] [CrossRef]
- Verma, D.K.; Srivastava, R.; Kumar, S. Synthesis of chitosan-based nanomaterials. In Role of Chitosan and Chitosan-Based Nanomaterials in Plant Sciences; Elsevier, 2022; pp. 33–57.
- Wang, Q.; Zhang, Y.; Ma, Y.; Wang, M.; Pan, G. Nano-crosslinked dynamic hydrogels for biomedical applications. Materials Today Bio 2023, 20, 100640. [Google Scholar] [CrossRef]
- Karchoubi, F.; Ghotli, R.A.; Pahlevani, H.; Salehi, M.B. New insights into nanocomposite hydrogels; a review on recent advances in characteristics and applications. Advanced Industrial and Engineering Polymer Research 2024, 7, 54–78. [Google Scholar] [CrossRef]
- Arora, S.; Das, G.; Alqarni, M.; Grover, V.; Manzoor Baba, S.; Saluja, P.; Hassan, S.A.B.; Abdulla, A.M.; Bavabeedu, S.S.; Abullais, S.S.; others. Role of chitosan hydrogels in clinical dentistry. Gels 2023, 9, 698. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).