Submitted:
14 October 2024
Posted:
14 October 2024
You are already at the latest version
Abstract
Newts, a type of urodele amphibian, offer remarkable insights into regenerative medicine due to their extraordinary tissue regeneration capabilities—a challenging feat in humans. During limb regeneration of adult newts, fascinating cellular and molecular processes are revealed, including scarless healing, de-differentiation of mature cells, and regeneration of limbs and digits. Sonic hedgehog (Shh), crucial for vertebrate limb development, is regulated by the zone of polarizing activity regulatory sequence (ZRS) in the limb bud zone of polarizing activity (ZPA). The metamorphosed (terrestrial) newt can reactivate Shh during regeneration, facilitating proper limb patterning. Cell types capable of regulating the ZRS in metamorphosed newts remain unknown. The identification of such cell types provides invaluable insight to novel regenerative mechanisms. Therefore, in this study, we developed the first newt ZRS reporter. First, we isolated and characterized the newt ZRS enhancer (nZRS), identifying conserved DNA binding sites. Several binding sites with medical relevance were conserved in the newt ZRS. In a functional analysis, we developed a system composed of a transgenic nZRS reporter newt and a new newt anti-Shh antibody, together allowing Shh monitoring during limb regeneration. This system provides a valuable in vivo approach for future genetic studies of patterning during limb regeneration.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Anesthesia and Surgical Dissections
2.3. Molecular Cloning of the Newt nZRS Enhancer Region
2.4. Molecular Cloning of C. pyrrhogaster Shh and Antibody
2.5. Bioinformatic Analysis
2.6. Construction of the nZRS Reporter
2.7. Newt Transgenesis
2.8. Tissue Fixation
2.9. Immunolabeling
2.10. Image Acquisition and Data Analysis
2.11. Digital Software
3. Results
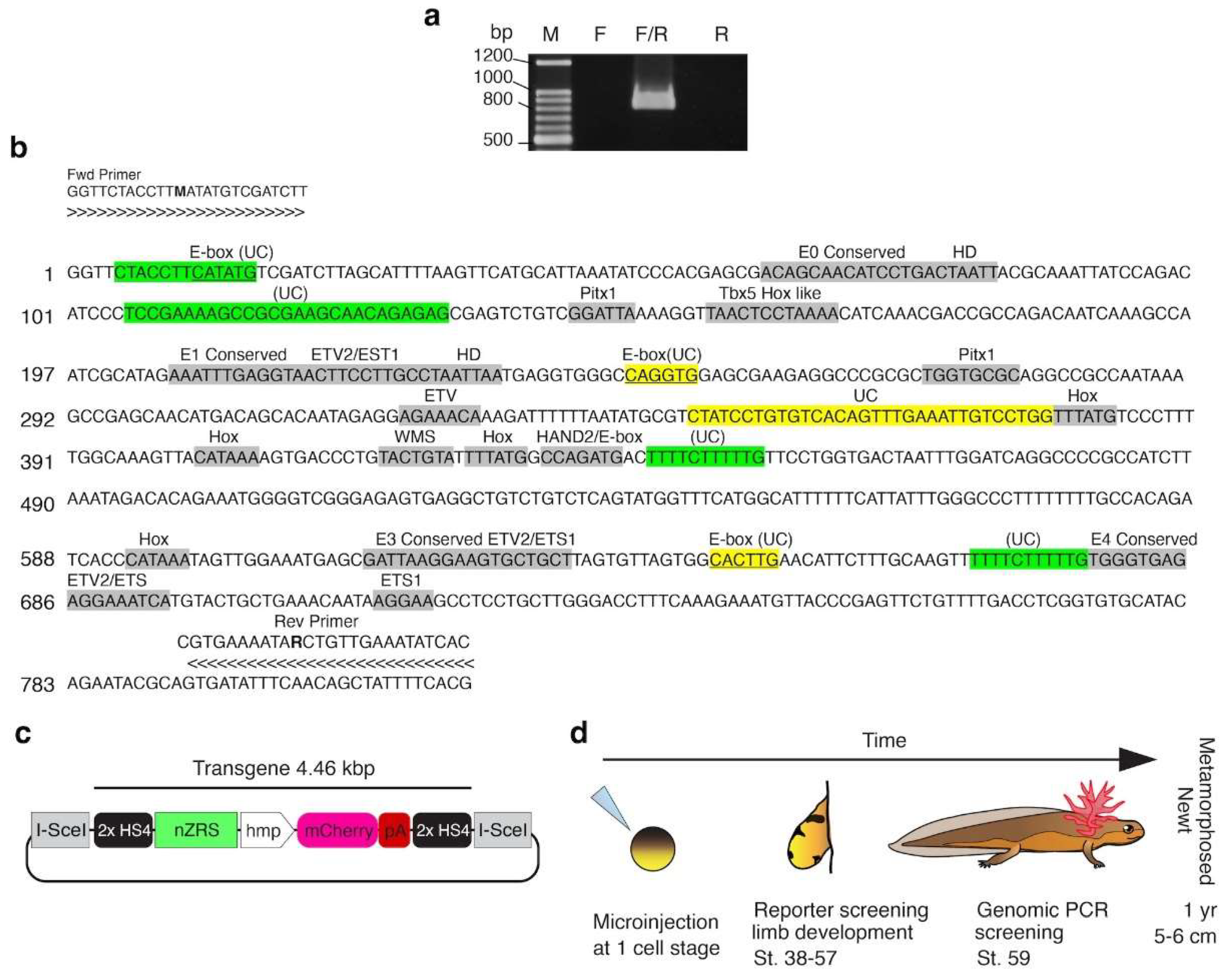
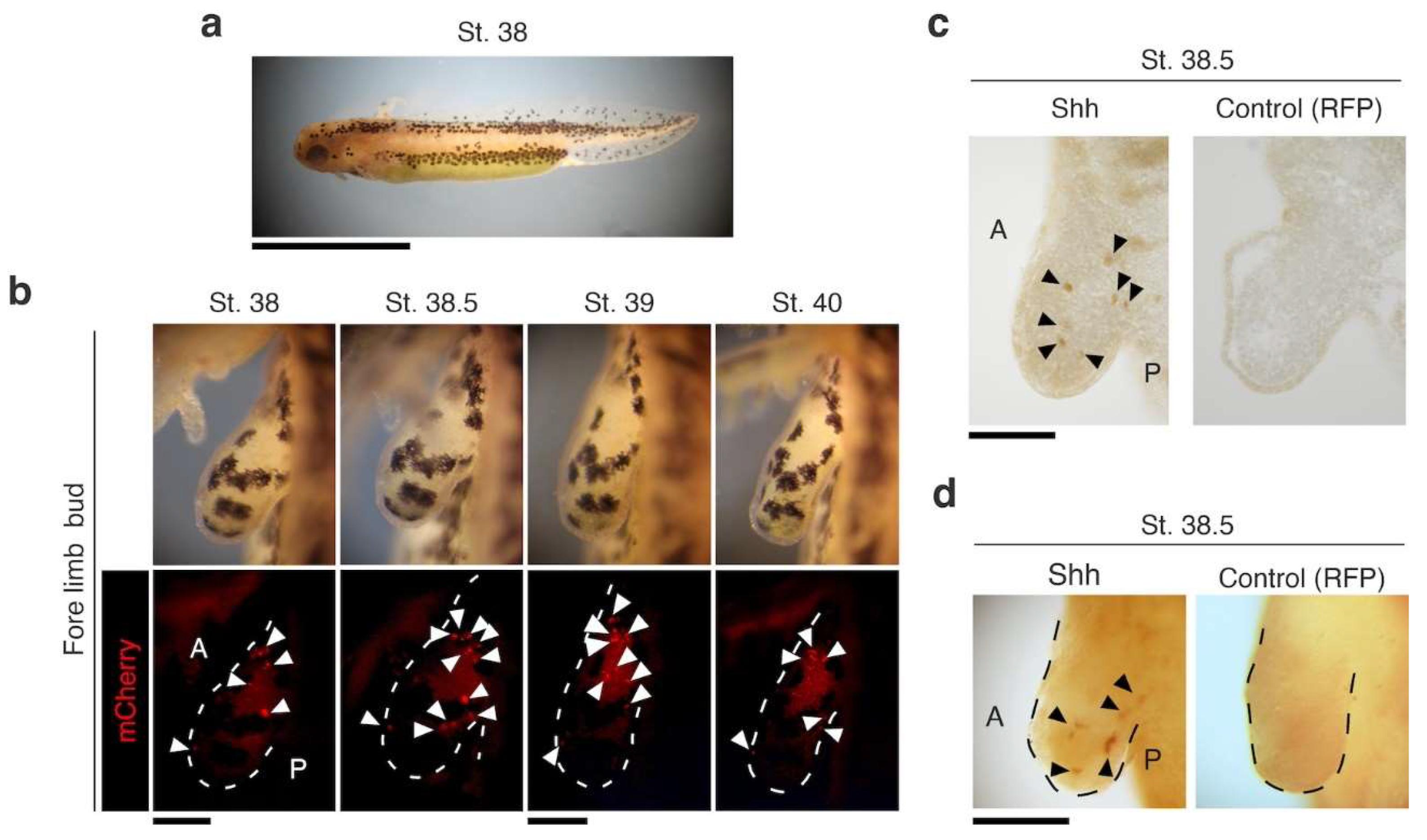
3.1. Characterization of the Newt nZRS Enhancer
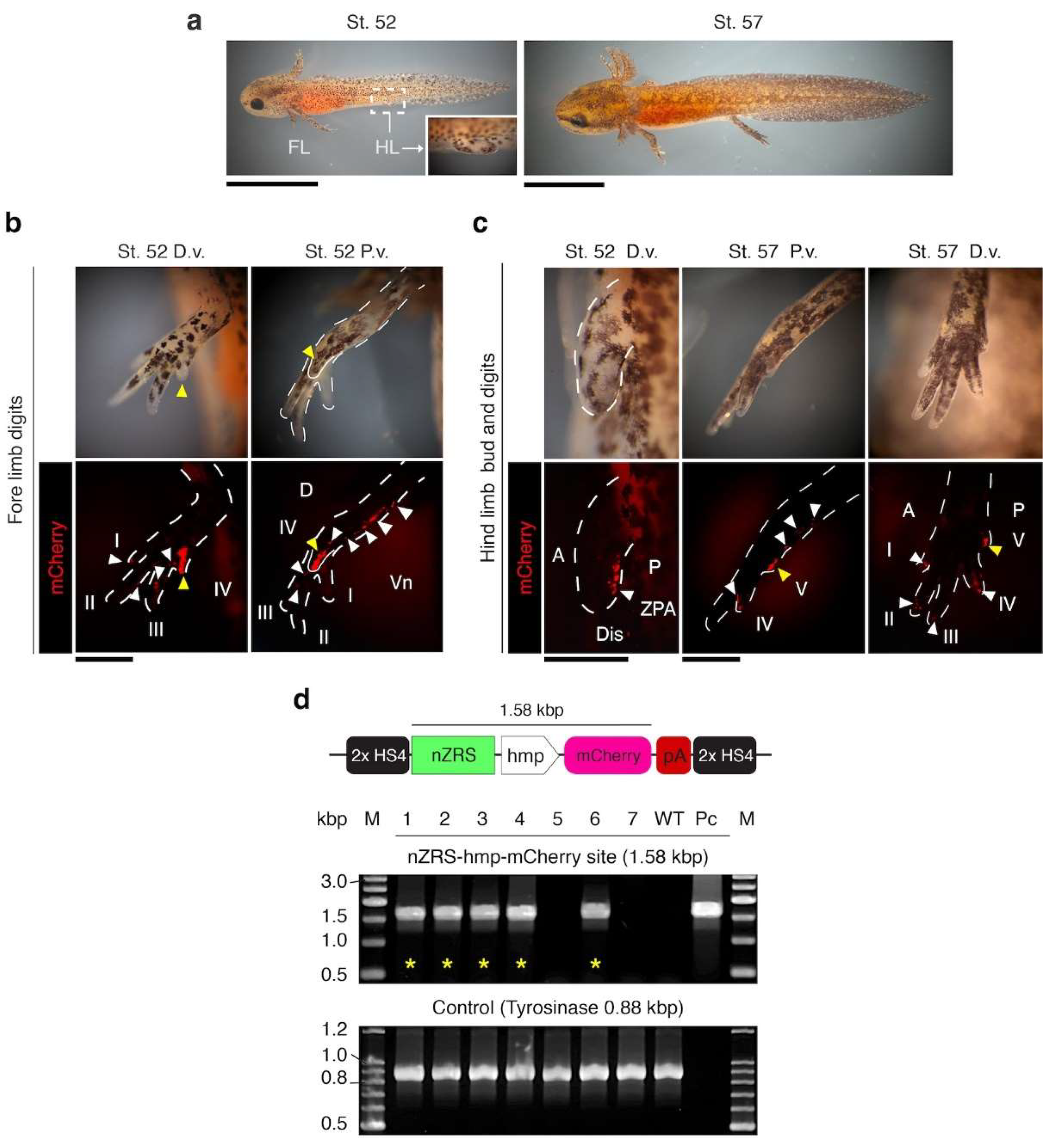
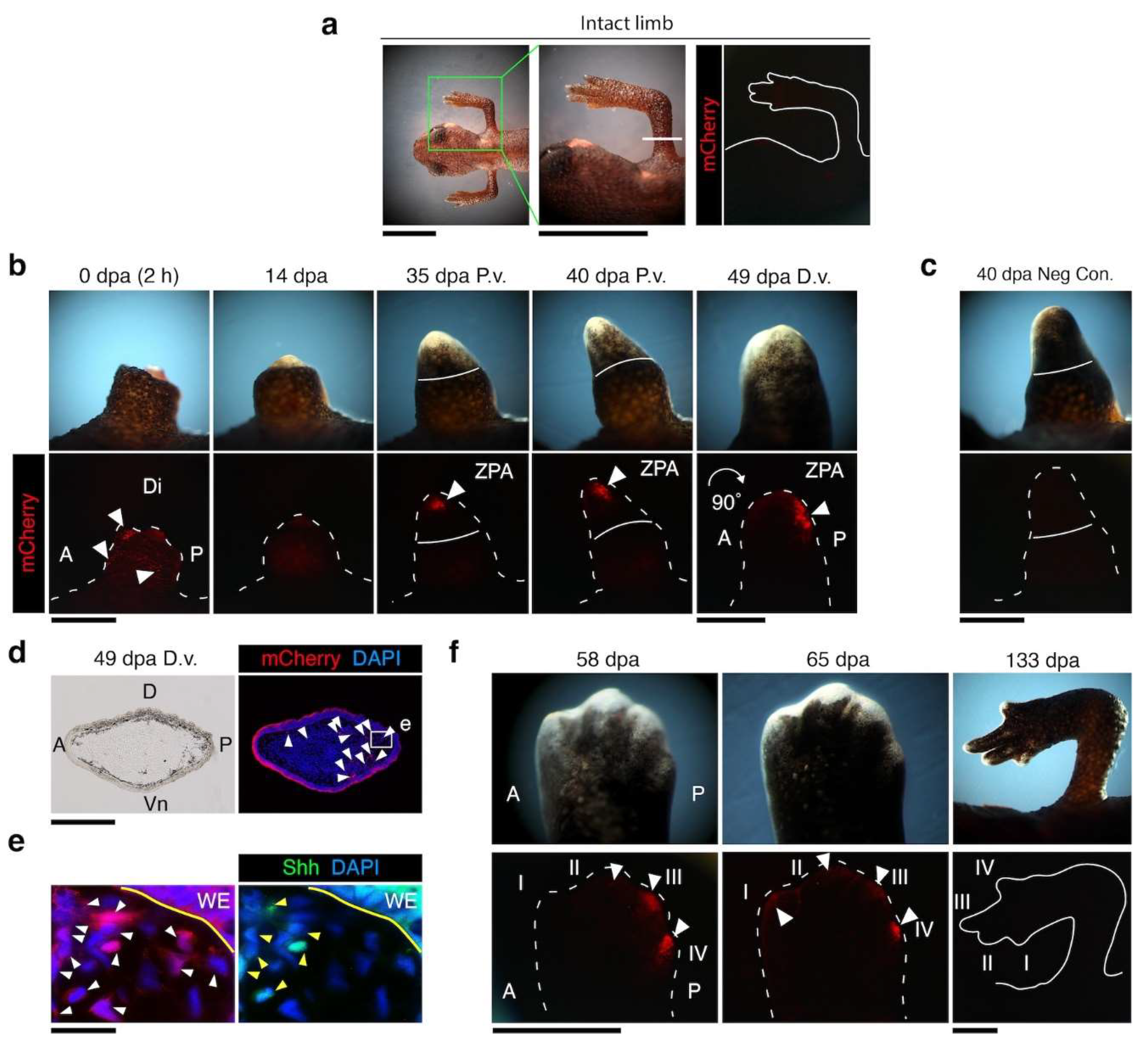
3.2. Functional Analysis of the nZRS Enhancer
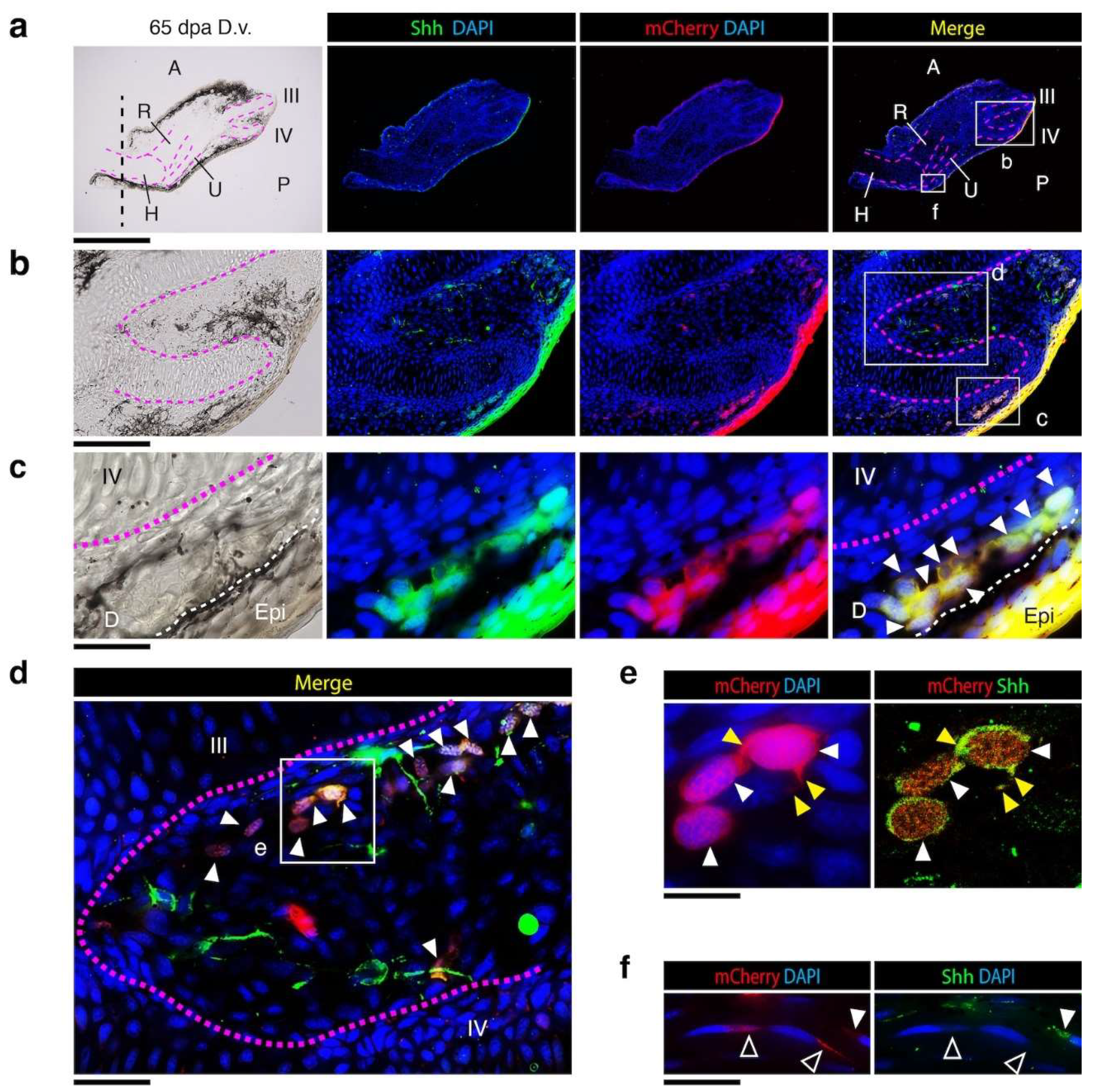
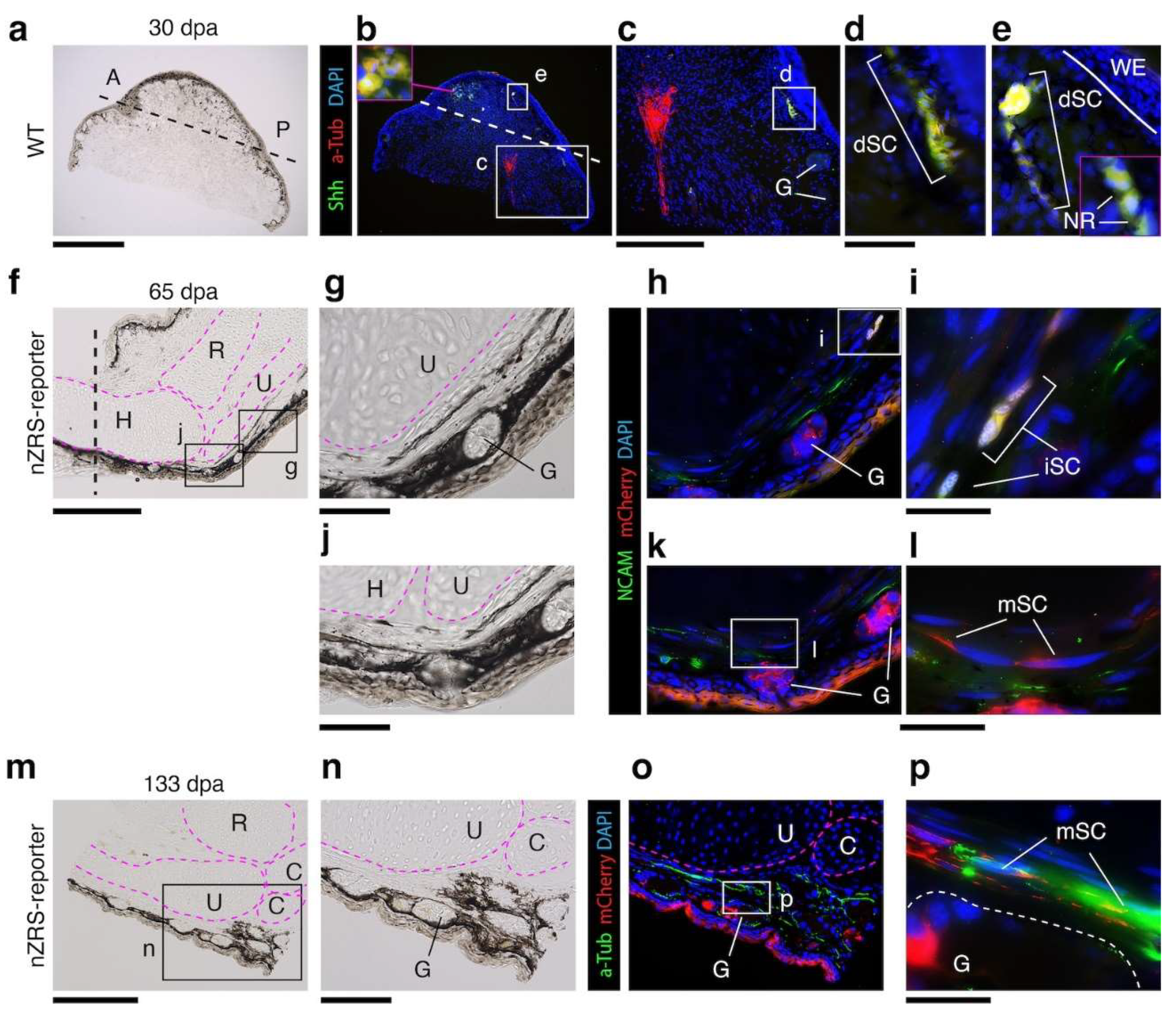
3.5. Dermal Regenerating Schwann Cells and Glands Express Shh
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casco-Robles, M.M.; Islam, M.R.; Inami, W.; Tanaka, H.V.; Kunahong, A.; Yasumuro, H.; Hanzawa, S.; Casco-Robles, R.M.; Toyama, F.; Maruo, F.; et al. Turning the Fate of Reprogramming Cells from Retinal Disorder to Regeneration by Pax6 in Newts. Sci. Rep. 2016, 6, 33761. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Shiga, S.; Casco-Robles, M.M.; Takeshima, K.; Maruo, F.; Chiba, C. The Latent Dedifferentiation Capacity of Newt Limb Muscles Is Unleashed by a Combination of Metamorphosis and Body Growth. Sci. Rep. 2022, 12, 11653. [Google Scholar] [CrossRef]
- Banda, C.H.; Shiraishi, M.; Mitsui, K.; Okada, Y.; Danno, K.; Ishiura, R.; Maemura, K.; Chiba, C.; Mizoguchi, A.; Imanaka-Yoshida, K.; et al. Structural and Functional Analysis of the Newt Lymphatic System. Sci. Rep. 2023, 13, 6902. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, M.; Hameed, L.S.; Berg, D.A.; Wang, H.; Simon, A. Progenitor Cell Dynamics in the Newt Telencephalon during Homeostasis and Neuronal Regeneration. Stem Cell Rep. 2014, 2, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tsissios, G.; Sallese, A.; Smucker, B.; Nguyen, A.-T.; Chen, J.; Wang, H.; Del Rio-Tsonis, K. In Vivo Imaging of Newt Lens Regeneration: Novel Insights Into the Regeneration Process. Trans. Vis. Sci. Tech. 2021, 10, 4. [Google Scholar] [CrossRef]
- Eroglu, E.; Yen, C.Y.T.; Tsoi, Y.-L.; Witman, N.; Elewa, A.; Joven Araus, A.; Wang, H.; Szattler, T.; Umeano, C.H.; Sohlmér, J.; et al. Epicardium-Derived Cells Organize through Tight Junctions to Replenish Cardiac Muscle in Salamanders. Nat. Cell Biol. 2022, 24, 645–658. [Google Scholar] [CrossRef]
- Kurosaka, H.; Takano-Yamamoto, T.; Yamashiro, T.; Agata, K. Comparison of Molecular and Cellular Events during Lower Jaw Regeneration of Newt (Cynops pyrrhogaster) and West African Clawed Frog (Xenopus tropicalis). Dev. Dyn. 2008, 237, 354–365. [Google Scholar] [CrossRef]
- Tanaka, H.V.; Ng, N.C.Y.; Yang Yu, Z.; Casco-Robles, M.M.; Maruo, F.; Tsonis, P.A.; Chiba, C. A Developmentally Regulated Switch from Stem Cells to Dedifferentiation for Limb Muscle Regeneration in Newts. Nat. Commun. 2016, 7, 11069. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Zhou, Y.; Gaggar, A.; Duncan, S.R. Fibrosis: Ultimate and Proximate Causes. J. Clin. Invest. 2014, 124, 4673–4677. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, L.; Wang, M.; Zhou, S.; Lu, Y.; Cui, H.; Racanelli, A.C.; Zhang, L.; Ye, T.; Ding, B.; et al. Targeting Fibrosis: Mechanisms and Clinical Trials. Sig. Transduct. Target Ther. 2022, 7, 206. [Google Scholar] [CrossRef]
- Stocum, D.L. Mechanisms of Urodele Limb Regeneration. Regeneration 2017, 4, 159–200. [Google Scholar] [CrossRef] [PubMed]
- Joven, A.; Elewa, A.; Simon, A. Model Systems for Regeneration: Salamanders. Development 2019, 146, dev167700. [Google Scholar] [CrossRef]
- Casco-Robles, R.M.; Watanabe, A.; Eto, K.; Takeshima, K.; Obata, S.; Kinoshita, T.; Ariizumi, T.; Nakatani, K.; Nakada, T.; Tsonis, P.A.; et al. Novel Erythrocyte Clumps Revealed by an Orphan Gene Newtic1 in Circulating Blood and Regenerating Limbs of the Adult Newt. Sci. Rep. 2018, 8, 7455. [Google Scholar] [CrossRef]
- Kumar, A.; Godwin, J.W.; Gates, P.B.; Garza-Garcia, A.A.; Brockes, J.P. Molecular Basis for the Nerve Dependence of Limb Regeneration in an Adult Vertebrate. Science 2007, 318, 772–777. [Google Scholar] [CrossRef]
- Kumar, A.; Gates, P.B.; Brockes, J.P. Positional Identity of Adult Stem Cells in Salamander Limb Regeneration. Comptes Rendus. Biologies 2007, 330, 485–490. [Google Scholar] [CrossRef]
- Da Silva, S.M.; Gates, P.B.; Brockes, J.P. The Newt Ortholog of CD59 Is Implicated in Proximodistal Identity during Amphibian Limb Regeneration. Developmental Cell 2002, 3, 547–555. [Google Scholar] [CrossRef]
- McQueen, C.; Towers, M. Establishing the Pattern of the Vertebrate Limb. Development 2020, 147, dev177956. [Google Scholar] [CrossRef] [PubMed]
- Tickle, C. An Historical Perspective on the Pioneering Experiments of John Saunders. Dev. Biol 2017, 429, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, Y.; Yoshizato, K. Expression of Sonic Hedgehog Gene in Regenerating Newt Limb Blastemas Recapitulates That in Developing Limb Buds. Proc. Natl. Acad. Sci. U.S.A. 1997, 94, 9159–9164. [Google Scholar] [CrossRef]
- Kvon, E.Z.; Kamneva, O.K.; Melo, U.S.; Barozzi, I.; Osterwalder, M.; Mannion, B.J.; Tissières, V.; Pickle, C.S.; Plajzer-Frick, I.; Lee, E.A.; et al. Progressive Loss of Function in a Limb Enhancer during Snake Evolution. Cell 2016, 167, 633–642.e1. [Google Scholar] [CrossRef]
- Lettice, L.A. A Long-Range Shh Enhancer Regulates Expression in the Developing Limb and Fin and Is Associated with Preaxial Polydactyly. Human Molecular Genetics 2003, 12, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gates, P.B.; Czarkwiani, A.; Brockes, J.P. An Orphan Gene Is Necessary for Preaxial Digit Formation during Salamander Limb Development. Nat. Commun. 2015, 6, 8684. [Google Scholar] [CrossRef] [PubMed]
- Casco-Robles, M.M.; Yasuda, K.; Yahata, K.; Maruo, F.; Chiba, C. Reviewing the Effects of Skin Manipulations on Adult Newt Limb Regeneration: Implications for the Subcutaneous Origin of Axial Pattern Formation. Biomedicines 2021, 9, 1426. [Google Scholar] [CrossRef] [PubMed]
- Percie Du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. BMJ Open Science 2020, 44. [Google Scholar] [CrossRef]
- Casco-Robles, M.M.; Yamada, S.; Miura, T.; Nakamura, K.; Haynes, T.; Maki, N.; Del Rio-Tsonis, K.; Tsonis, P.A.; Chiba, C. Expressing Exogenous Genes in Newts by Transgenesis. Nat. Protoc. 2011, 6, 600–608. [Google Scholar] [CrossRef]
- Casco-Robles, M.M.; Yamada, S.; Miura, T.; Chiba, C. Simple and Efficient Transgenesis with I-SceI Meganuclease in the Newt, Cynops pyrrhogaster. Dev. Dyn. 2010, 239, 3275–3284. [Google Scholar] [CrossRef]
- Shkumatava, A.; Fischer, S.; Müller, F.; Strahle, U.; Neumann, C.J. Sonic Hedgehog, Secreted by Amacrine Cells, Acts as a Short-Range Signal to Direct Differentiation and Lamination in the Zebrafish Retina. Development 2004, 131, 3849–3858. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Schloissnig, S.; Kawaguchi, A.; Nowoshilow, S.; Falcon, F.; Otsuki, L.; Tardivo, P.; Timoshevskaya, N.; Keinath, M.C.; Smith, J.J.; Voss, S.R.; et al. The Giant Axolotl Genome Uncovers the Evolution, Scaling, and Transcriptional Control of Complex Gene Loci. Proc. Natl. Acad. Sci. U.S.A. 2021, 118, e2017176118. [Google Scholar] [CrossRef]
- Smith, J.J.; Timoshevskaya, N.; Timoshevskiy, V.A.; Keinath, M.C.; Hardy, D.; Voss, S.R. A Chromosome-Scale Assembly of the Axolotl Genome. Genome Res. 2019, 29, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Sandelin, A. JASPAR: An Open-Access Database for Eukaryotic Transcription Factor Binding Profiles. Nucleic Acids Res. 2004, 32, 91D–94. [Google Scholar] [CrossRef] [PubMed]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-Coffee: A Novel Method for Fast and Accurate Multiple Sequence Alignment Edited by J. Thornton. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Casco-Robles, M.M.; Miura, T.; Chiba, C. The Newt (Cynops pyrrhogaster) RPE65 Promoter: Molecular Cloning, Characterization and Functional Analysis. Transgenic Res. 2015, 24, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Yee, S.P.; Rigby, P.W. The Regulation of Myogenin Gene Expression during the Embryonic Development of the Mouse. Genes & Development 1993, 7, 1277–1289. [Google Scholar] [CrossRef]
- Richter, K.N.; Revelo, N.H.; Seitz, K.J.; Helm, M.S.; Sarkar, D.; Saleeb, R.S.; D’Este, E.; Eberle, J.; Wagner, E.; Vogl, C.; et al. Glyoxal as an Alternative Fixative to Formaldehyde in Immunostaining and Super-resolution Microscopy. The EMBO Journal 2018, 37, 139–159. [Google Scholar] [CrossRef]
- Konno, K.; Yamasaki, M.; Miyazaki, T.; Watanabe, M. Glyoxal Fixation: An Approach to Solve Immunohistochemical Problem in Neuroscience Research. Sci. Adv. 2023, 9, eadf7084. [Google Scholar] [CrossRef]
- Lettice, L.A.; Williamson, I.; Wiltshire, J.H.; Peluso, S.; Devenney, P.S.; Hill, A.E.; Essafi, A.; Hagman, J.; Mort, R.; Grimes, G.; et al. Opposing Functions of the ETS Factor Family Define Shh Spatial Expression in Limb Buds and Underlie Polydactyly. Developmental Cell 2012, 22, 459–467. [Google Scholar] [CrossRef]
- Koyano-Nakagawa, N.; Gong, W.; Das, S.; Theisen, J.W.M.; Swanholm, T.B.; Van Ly, D.; Dsouza, N.; Singh, B.N.; Kawakami, H.; Young, S.; et al. Etv2 Regulates Enhancer Chromatin Status to Initiate Shh Expression in the Limb Bud. Nat. Commun. 2022, 13, 4221. [Google Scholar] [CrossRef]
- Osterwalder, M.; Speziale, D.; Shoukry, M.; Mohan, R.; Ivanek, R.; Kohler, M.; Beisel, C.; Wen, X.; Scales, S.J.; Christoffels, V.M.; et al. HAND2 Targets Define a Network of Transcriptional Regulators That Compartmentalize the Early Limb Bud Mesenchyme. Developmental Cell 2014, 31, 345–357. [Google Scholar] [CrossRef]
- Lettice, L.A.; Devenney, P.; De Angelis, C.; Hill, R.E. The Conserved Sonic Hedgehog Limb Enhancer Consists of Discrete Functional Elements That Regulate Precise Spatial Expression. Cell Reports 2017, 20, 1396–1408. [Google Scholar] [CrossRef]
- Nemec, S.; Luxey, M.; Jain, D.; Huang Sung, A.; Pastinen, T.; Drouin, J. Pitx1 Directly Modulates the Core Limb Development Program to Implement Hindlimb Identity. Development 2017, 144, 3325–3335. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jin, Y.-Q.; Chen, L.; Wang, Y.; Yang, X.; Cheng, J.; Wu, W.; Qi, Z.; Shen, Z. Specific Marker Expression and Cell State of Schwann Cells during Culture In Vitro. PLoS ONE 2015, 10, e0123278. [Google Scholar] [CrossRef] [PubMed]
- Bolívar, S.; Navarro, X.; Udina, E. Schwann Cell Role in Selectivity of Nerve Regeneration. Cells 2020, 9, 2131. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell. Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef]
- Maier, C.E.; Watanabe, M.; Singer, M.; McQuarrie, I.G.; Sunshine, J.; Rutishauser, U. Expression and Function of Neural Cell Adhesion Molecule during Limb Regeneration. Proc. Natl. Acad. Sci. U.S.A. 1986, 83, 8395–8399. [Google Scholar] [CrossRef]
- Armati, P.J.; Mathey, E.K. An Update on Schwann Cell Biology — Immunomodulation, Neural Regulation and Other Surprises. J. Neurol. Sci. 2013, 333, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.W.; Thompson, W.J. Biology and Pathology of Nonmyelinating Schwann Cells. Glia 2008, 56, 1518–1531. [Google Scholar] [CrossRef]
- Ramesh, R.; Manurung, Y.; Ma, K.H.; Blakely, T.; Won, S.; Moreno-Ramos, O.A.; Wyatt, E.; Awatramani, R.; Svaren, J. JUN Regulation of Injury-Induced Enhancers in Schwann Cells. J. Neurosci. 2022, 42, 6506–6517. [Google Scholar] [CrossRef]
- Angius, D.; Wang, H.; Spinner, R.J.; Gutierrez-Cotto, Y.; Yaszemski, M.J.; Windebank, A.J. A Systematic Review of Animal Models Used to Study Nerve Regeneration in Tissue-Engineered Scaffolds. Biomaterials 2012, 33, 8034–8039. [Google Scholar] [CrossRef]
- Arthur-Farraj, P.; Coleman, M.P. Lessons from Injury: How Nerve Injury Studies Reveal Basic Biological Mechanisms and Therapeutic Opportunities for Peripheral Nerve Diseases. Neurotherapeutics 2021, 18, 2200–2221. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J.A.; Pilch, K.S.; Van Der Lans, M.; Fazal, S.V.; Benito, C.; Wagstaff, L.J.; Mirsky, R.; Jessen, K.R. After Nerve Injury, Lineage Tracing Shows That Myelin and Remak Schwann Cells Elongate Extensively and Branch to Form Repair Schwann Cells, Which Shorten Radically on Remyelination. J. Neurosci. 2017, 37, 9086–9099. [Google Scholar] [CrossRef]
- Lin, H.-P.; Oksuz, I.; Hurley, E.; Wrabetz, L.; Awatramani, R. Microprocessor Complex Subunit DiGeorge Syndrome Critical Region Gene 8 (Dgcr8) Is Required for Schwann Cell Myelination and Myelin Maintenance. J. Biol. Chem. 2015, 290, 24294–24307. [Google Scholar] [CrossRef] [PubMed]
- Arthur-Farraj, P.J.; Latouche, M.; Wilton, D.K.; Quintes, S.; Chabrol, E.; Banerjee, A.; Woodhoo, A.; Jenkins, B.; Rahman, M.; Turmaine, M.; et al. C-Jun Reprograms Schwann Cells of Injured Nerves to Generate a Repair Cell Essential for Regeneration. Neuron 2012, 75, 633–647. [Google Scholar] [CrossRef]
- Widera, D.; Heimann, P.; Zander, C.; Imielski, Y.; Heidbreder, M.; Heilemann, M.; Kaltschmidt, C.; Kaltschmidt, B. Schwann Cells Can Be Reprogrammed to Multipotency by Culture. Stem Cells and Development 2011, 20, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Kintner, C.R.; Brockes, J.P. Monoclonal Antibodies to the Cells of a Regenerating Limb. Development 1985, 89, 37–55. [Google Scholar] [CrossRef]
- Maki, N.; Suetsugu-Maki, R.; Tarui, H.; Agata, K.; Del Rio-Tsonis, K.; Tsonis, P.A. Expression of Stem Cell Pluripotency Factors during Regeneration in Newts. Dev. Dyn. 2009, 238, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, S.; Kitazawa, R.; Tamada, H.; Maeda, S. Promoter Structure of Human Sonic Hedgehog Gene. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression 1998, 1443, 358–363. [Google Scholar] [CrossRef]
- Patterning Activities of Hedgehog Proteins in the Developing Eye and Brain. Trends in Genetics 1995, 11, 434. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




