1. Introduction
Janus kinase inhibitors (JAKis) are a novel class of disease-modifying antirheumatic drugs (DMARDs) (i.e., targeted synthetic DMARDs [tsDMARDs]) that have revolutionized the landscape of autoimmune and inflammatory disease management *1+. Acting as intracellular signaling modulators, these molecules intervene in the Janus kinase - Signal Transducer and Activator of Transcription (JAK-STAT) pathway, a crucial cascade involved in the transmission of signals from type I and type II cell surface receptors to the nucleus *2+. This pathway is central to the regulation of immune responses, making JAKi a targeted and effective therapeutic strategy for diseases characterized by dysregulated immunity such as rheumatoid arthritis (RA), psoriasis/psoriatic arthritis (Pso/PsA), and ulcerative colitis (UC)*3+. Compared with biologic DMARDs (bDMARDs), the versatility of JAKi lies in their ability to selectively block specific JAK isoforms, including JAK1, JAK2, JAK3, and TYK2, thereby disrupting the signaling pathways mediated by different cytokines*4+, with the overarching goal to modulate immune responses without causing global immunosuppression.
Filgotinib – the newest member of the JAKi class – exerts its therapeutic effects by selectively targeting and inhibiting the kinase activity of JAK1*5+, disrupting the intracellular signaling cascade initiated by key cytokines (e.g., interleukin-6 *IL-6+ and type-I interferons *IFN+), that are integral to the inflammatory processes involved in the pathophysiology of RA. Compared to non-selective JAKis, the targeted approach of filgotinib may theoretically allow for a more precise interference with inflammatory pathways while minimizing the impact on other JAK isoforms; this has been translated into a favorable efficacy and safety profile, as already demonstrated in randomized clinical trials (RCTs) and long-term extension (LTE) studies *6–10+. However, it is now recognized that real-world evidence (RWE) plays a critical role in complementing findings from RCTs by providing insights into the performance of pharmaceutical interventions in everyday clinical practice. This concept is even more emphasized when applied to JAKis, particularly in the aftermath of the ORAL Surveillance trial *11+, a post-marketing safety study that failed to demonstrate non-inferiority of tofacitinib compared to TNF inhibitors (TBFis) with regard to the risk of major cardiovascular events (MACEs) and cancer. While this trial initially cast shadows on the safety of JAKis, massive RWE data provided robust reassuring results *12–14+. Unfortunately, a similar amount of data is not yet available for other members of the JAKi class, including filgotinib.
On this basis, the aim of our study was to provide additional evidence on the effectiveness and safety of filgotinib in real-world RA patients.
2. Methods
Study Design
Multicenter, retrospective cohort study to evaluate the real-life effectiveness and safety of filgotinib in adult patients with RA.
Participants
Consecutive patients, aged 18 years and older, diagnosed with RA and starting filgotinib during the study period (January 2021 – December 2021) were retrospectively recruited from 11 rheumatology clinics distributed across the Calabria Region (Southern Italy). According to a previous report *15+, these centers account for > 90% of total bDMARDs (and tsDMARDs) prescriptions for the rheumatology area in Calabria Region.
Of note, we deliberately included only patients who initiated filgotinib therapy before January 2022, prior to the publication of the results of the ORAL Surveillance study *11+. This decision was prompted by the recognition that the emergence of new clinical data and updated recommendations from the regulatory authorities had a significant impact on physicians’ prescribing behaviors. By incorporating patients treated before this milestone, we aimed at capturing a broader picture of filgotinib use in clinical practice, reflecting the temporal context in which prescriptions were made and mitigating the risk of an immediate influence of new evidence on patient management.
Data Collection
Baseline data, including demographic information, disease characteristics, prior treatment history, and comorbid conditions (with special focus on MACE risk factors - smoking, past history of atherosclerotic cardiovascular disease *ASCVD+, obesity, high blood pressure, dyslipidemia, diabetes) were collected. Similarly, clinical assessment and laboratory results were retrieved from the clinical records of individual follow-up visits at baseline (T0, before starting filgotinib), at 3 ± 1 months (T3), and at 6 ± 1 months (T6). According to local practice, a core set of outcome measures is required to complete the prescription form for bDMARDs and tsDMARDs; therefore, these measures were available for all patients. The disease activity score including 28 joints and C-reactive protein (DAS28-CRP)*16+ and the simplified disease activity index (SDAI)*17+ were used to define disease states as follows: a) remission (DAS28-CRP < 2.6 or SDAI ≤ 3.3), b) low disease activity (DAS28-CRP ≥ 2.6 and ≤ 3.1 or SDAI > 3.3 or ≤ 11), c) moderate disease activity (DAS28-CRP ≥ 3.1 and ≤ 5.1 or SDAI > 11 and ≤ 26), or d) high disease activity (DAS28-CRP > 5.1 or SDAI > 26). A 10-cm visual analogue scale (VAS) was used to prospectively assess pain. Adverse events (AEs) were systematically recorded at each follow-up visit.
Ethical Approval
The study protocol was approved by the local Ethics Committee (Comitato Etico Territoriale – Regione Calabria, Italy), protocol number 70/2023. Informed consent was obtained from all patients at the time of enrollment. All procedures were performed in accordance with the 1964 Declaration of Helsinki and its later amendments.
Data Analysis
Data are expressed as mean ± standard deviation (SD), median *range+ or number (percentage), as appropriate. Student’s t-test and Mann–Whitney U test were used to compare differences between normally and non-normally distributed continuous variables, respectively. Fisher’s exact test was used to compare categorical variables. A p-value < 0.05 was considered statistically significant. All analyses were performed using the Statistical Package for Social Sciences (SPSS) software version 26.0 (IBM, Armonk, NY, USA).
3. Results
Baseline Characteristics
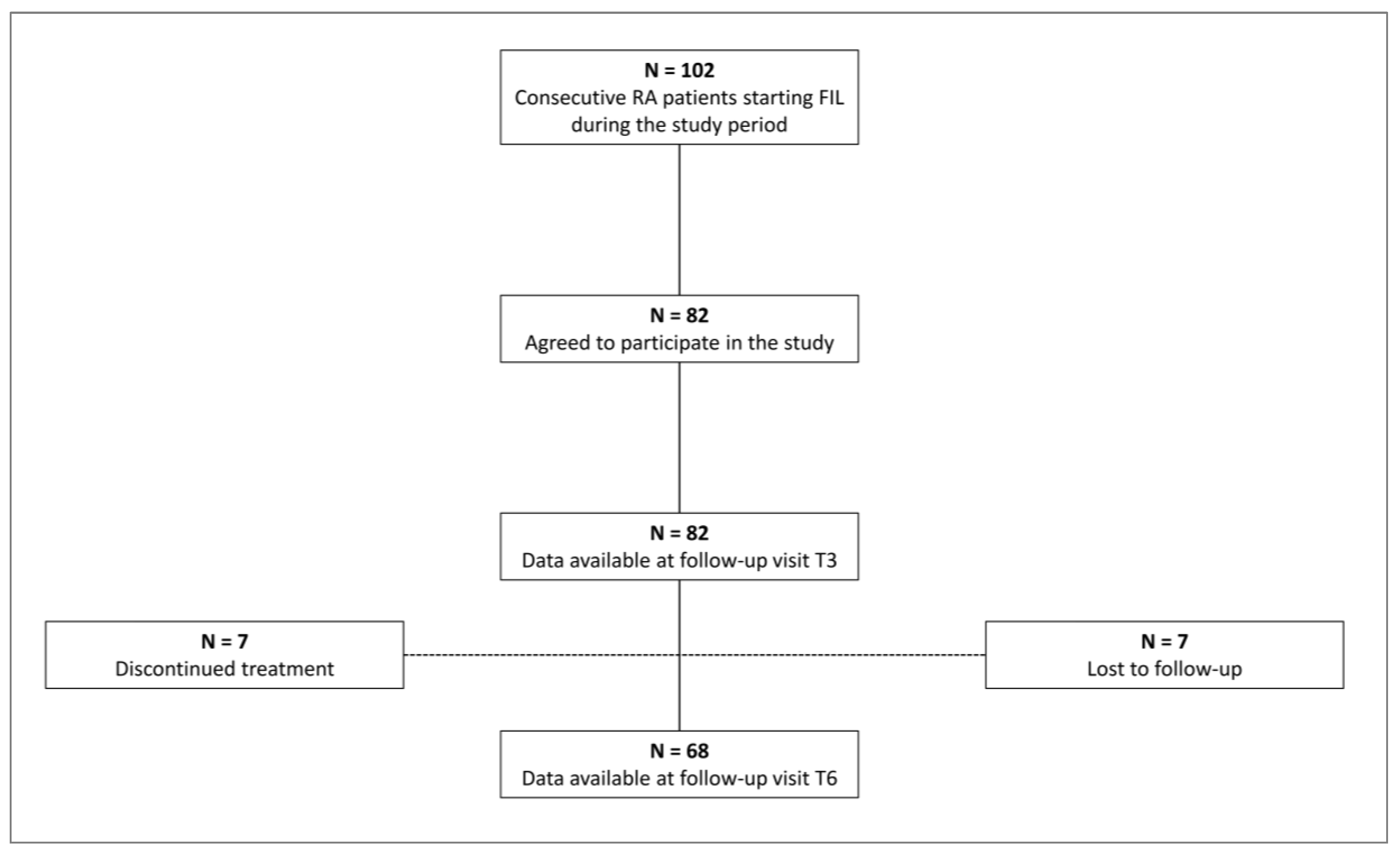
During the recruitment period, a total of 101 patients started treatment with FIL for inadequate control of disease activity or steroid dependence. Of these, 82 (63 females) agreed to participate in the study and signed the informed consent form (
Figure 1). General characteristics of the study population are detailed in
Table 1. Mean age was 62 ± 13 years; notably 39 patients (47.6%) were older than 65 years. Average disease duration was 13 ± 9 years. Regarding other cardiovascular risk factors, 19 patients (23.1%) were current or former smokers, 4 patients had a past history of ASCVD, 12 patients (14.6) had diabetes, 43 patients (52.4%) had high blood pressure and 28 patients (34.1%) had dyslipidemia. Most patients (61%) received at least one bDMARD [range 1 – 6] and 18 (22%) received three or more bDMARDs; furthermore, 11 patients (13.4%) have been already exposed to another JAKi. Filgotinib was administered as monotherapy in 57 patients (69.5%).
Effectiveness of Filgotinib in Real Life
The number of patients available for analysis at each follow-up visit are reported in
Figure 1. Seven patients did not attend T6 visit and thus were lost to follow-up; 7 patients discontinued treatment after the T3 follow-up visit because of primary failure (n = 3) or adverse events (nausea, n = 1; diplopia, n = 1; malaise, n = 1; recent diagnosis of metastatic cancer, n = 1). Accordingly, a total of 82 patients were available for analysis at T3 and 68 at T6.
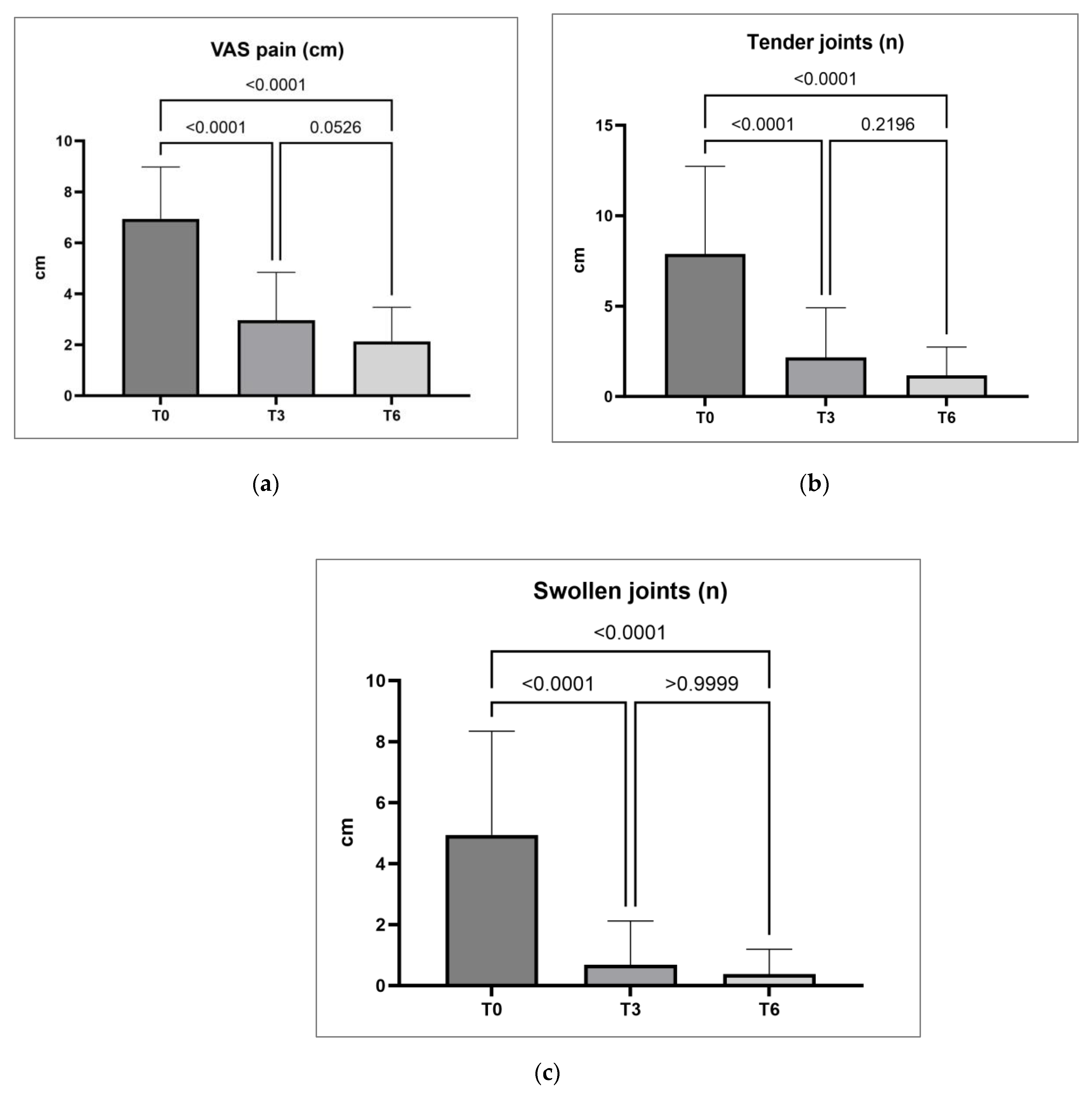
A significant reduction in VAS pain, TJC and SJC was observed at month three (T3) and six (T6) compared to baseline values (T0) as reported in
Table 2 and
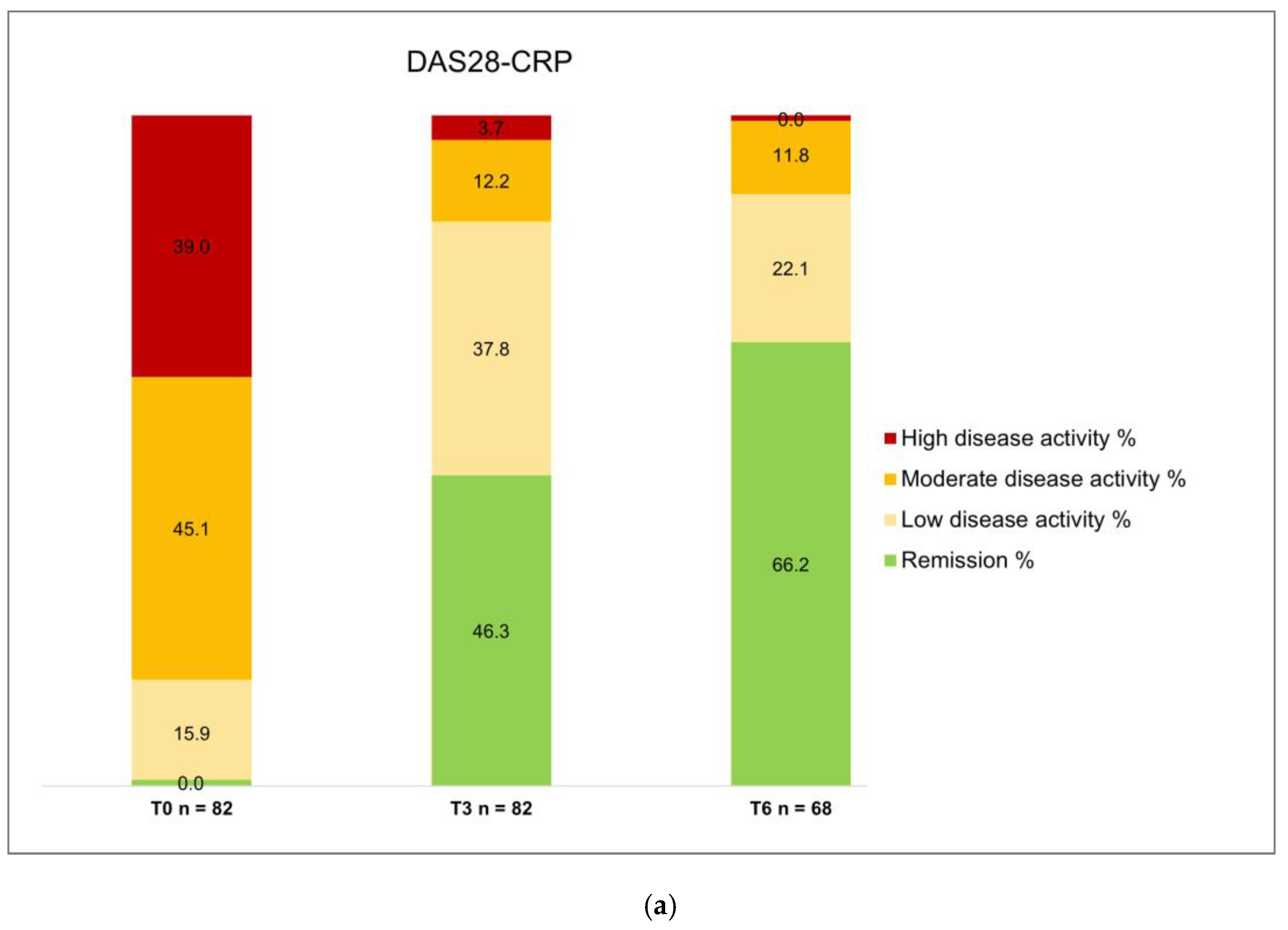
Figure 2. A relevant proportion of patients obtained DAS28-CRP remission at T3 and T6 (46.3% and 66.2%, respectively) or DAS28-CRP low disease activity (37.8% and 22.1%, respectively) (
Table 2,
Figure 3). Consequently, only 13 (15.9%) patients at T3 and 8 (11.8%) patients at T6 were classified has moderate or high disease activity on the basis of DAS28-CRP values.
4. Discussion
Filgotinib is the newest molecule of the JAKi class, and what distinguishes this product from other members of the same family is its stronger preferential binding with JAK1 *33741556+. Indeed, while all JAKi have demonstrated a favorable safety profile in RCTs, preclinical data suggest that a higher selectivity for JAK1 may confer additional benefits, especially in preserving hematopoietic and thrombotic homeostasis *18– 20+.
The clinical development program for filgotinib included two phase II RCTs (DARWIN 1, 2, 3) and three phase III RCTs, followed by LTE studies (FINCH 1, 2, 3, 4) *6–10+. These studies consistently demonstrated the efficacy of filgotinib 200 mg in improving signs and symptoms of RA, enhancing physical function, and inhibiting radiographic progression, both in combination therapy and in monotherapy.
In addition, a favorable safety profile was demonstrated, with filgotinib exhibiting the lowest incidence of Herpes Zoster infection in its class *21,22+, and an incidence of cardiovascular, thromboembolic, and neoplastic events lower than that expected in the RA population *23–27+.
While RCTs are vital for evaluating the safety and efficacy of novel medications in a highly controlled setting, overly rigid inclusion and exclusion criteria may not adequately address the heterogeneity of vulnerable characteristics that are found in real-world populations *28+. Validating RCTs results requires an analysis of the performance of these molecules in a real population under real life conditions. To date, real-world reports on filgotinib are limited, primarily because most studies have focused on earlier-generation agents. Tofacitinib, in particular, came under increased scrutiny following the publication of the ORAL Surveillance post-marketing safety trial *11+, which failed to demonstrate its non-inferiority compared to TNFi with respect to the risks of MACE and cancers.
In this context, we conducted a retrospective review of clinical data encompassing all patients treated with filgotinib before January 2022, prior to the publication of the results from the ORAL Surveillance trial *11+. Indeed, as a result of these findings, the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) issued a set of measures aimed to mitigate the risk of serious side effects that was extended to all members of the JAKi family across all approved indications *29+, leading to restrictions in the use of this drug class. Specifically, these restrictions apply to individuals aged 65 years or above, those at an elevated risk of MACE, individuals who smoke or have a prolonged history of smoking, and those at an increased risk of cancer.
Based on data obtained before the release of the EMA recommendations, our cohort provides an authentic snapshot of filgotinib use in real-world clinical practice by a collaborative group of Italian rheumatologists. This cohort, which reflects a pragmatic prescription of filgotinib, included 82 individuals with a high prevalence of the risk factors identified by EMA: 47.6% of individuals were aged 65 years or older, 23.1% were current or former smokers and 4.9% had a history of ASCVD. Moreover, other traditional cardiovascular risk factors were present in a significant proportion of patients: obesity/overweight in 64.6%, hypertension in 52.4%, dyslipidemia in 34.1%, and type 2 diabetes in 14.6%.
Although these characteristics collectively describes a population at increased risk of MACE, only three patients in our cohort reported adverse events requiring drug discontinuation, none of which was related to the cardiovascular system. One patient with metastatic cancer diagnosed one month after treatment initiation, and thus likely preexisting, discontinued treatment. These findings align seamlessly with the results presented in a recent study named RELIFIRA*30+, wherein the authors retrospectively analyzed 120 patients treated with filgotinib. In this study, 54.6% of the population was aged 65 years or older, 7.21% had a history of ASCVD, 15.5% had diabetes, and 47.4% had hypertension.
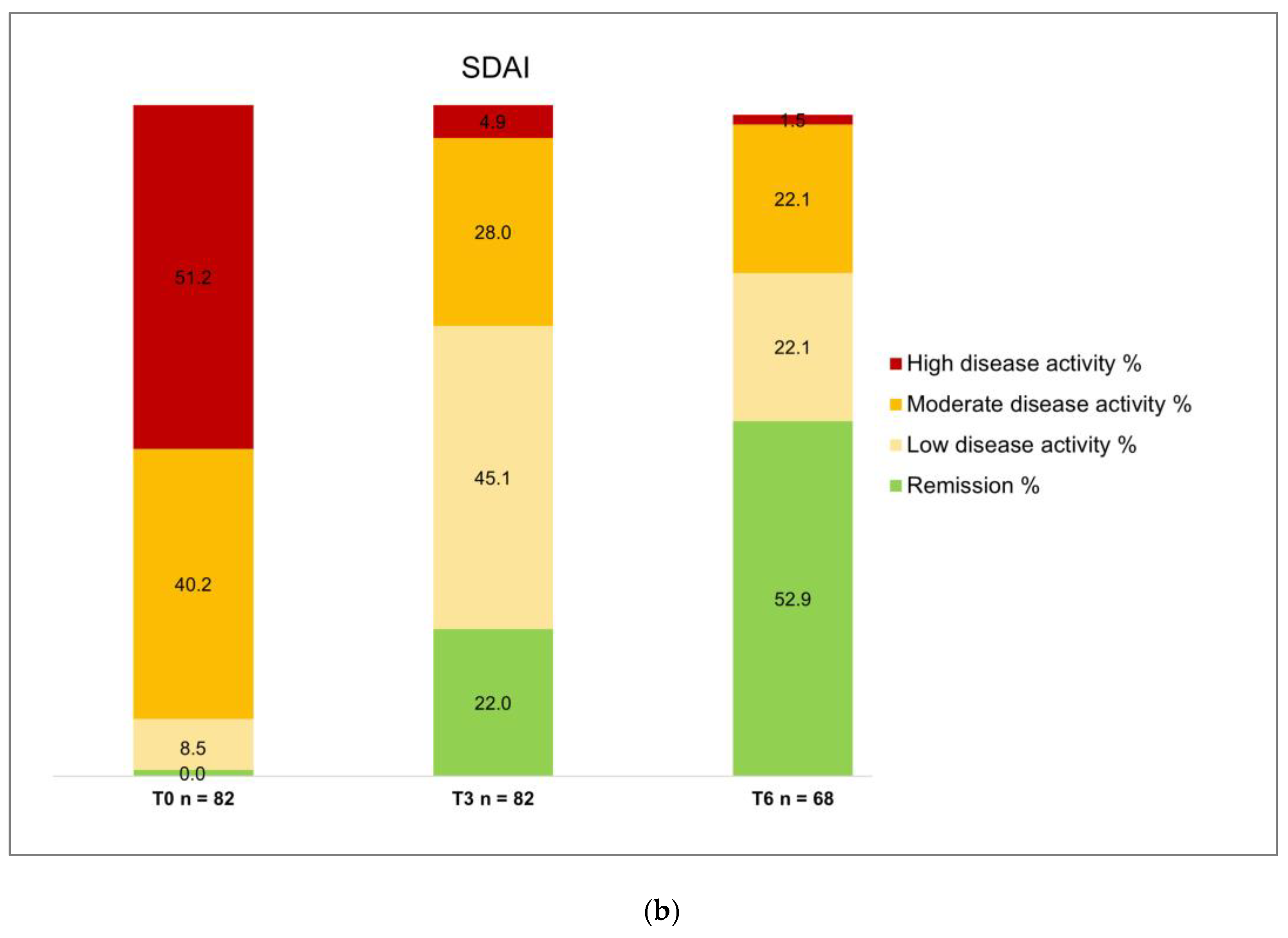
Concerning effectiveness, it is crucial to note that all patients had previously failed conventional DMARDs, and 61% received at least one bDMARD (22% three or more – up to six – previous bDMARDs); in addition, 11 patients (13.4%) had been already exposed to another JAKi. These characteristics delineate a specific subset of "difficult-to-treat" RA patients*31+. Despite the challenging clinical scenario, filgotinib demonstrated remarkable efficacy, with only three primary failures. At month 3, 46.3% achieved DAS28-CRP remission, and 37.8% attained low disease activity. At month 6, 66.2% achieved remission, with 21.1% in DAS28-CRP low disease activity. Regarding the SDAI index, at three months, 45.1% exhibited moderate disease activity, with 22% achieving remission. At six months, 22.1% had moderate disease activity, and 52.9% were in remission. In addition, a significant effect was observed on VAS pain, which decreased at 3 months and remained consistently low at 6 months, and on the number of tender and swollen joints. Again, these results are consistent with the RELIFIRA study, confirming filgotinib effectiveness in managing difficult-to-treat real-world RA patients *30+. A final notable finding relates to the number of patients treated with filgotinib monotherapy, representing 69.5% of the observed cohort. This percentage underscores that the positive outcomes in terms of effectiveness and safety can be mainly attributed to the effects of filgotinib, despite the challenging clinical context in which both conventional and biologic therapies have previously failed.
In conclusion, despite the inherent limitations of a retrospective study, our data provide additional evidence of the effectiveness and safety of filgotinib in a real-world setting, even among patients with difficult-to-treat RA, and a high prevalence of risk factors currently limiting the prescription of this class of molecules.
Author Contributions
All authors contributed equally.
Funding
Manuscript writing support and Open Access fees were funded by an independent grant from Alfasigma S.p.A. Authors of the manuscript were solely responsible for the manuscript development and content.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Editorial Assistance: Momento Medico srl, Italy.
Conflicts of Interest
Nothing to declare.
References
- Tanaka Y, Luo Y, O’Shea JJ, et al. Janus kinase-targeting therapies in rheumatology: a mechanisms-based approach. Nat Rev Rheumatol. 2022;18:133–45. [CrossRef]
- Hu X, Li J, Fu M, et al. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6:402. [CrossRef]
- Malemud CJ. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2018;10:117–27.
- Leonard WJ. Role of Jak kinases and STATs in cytokine signal transduction. Int J Hematol. 2001;73:271–7. [CrossRef]
- Namour F, Anderson K, Nelson C, et al. Filgotinib: A Clinical Pharmacology Review. Clin Pharmacokinet. 2022;61:819–32. [CrossRef]
- Genovese MC, Kalunian K, Gottenberg J-E, et al. Effect of Filgotinib vs Placebo on Clinical Response in Patients With Moderate to Severe Rheumatoid Arthritis Refractory to Disease-Modifying Antirheumatic Drug Therapy: The FINCH 2 Randomized Clinical Trial. JAMA. 2019;322:315–25.
- Westhovens R, Taylor PC, Alten R, et al. Filgotinib (GLPG0634/GS-6034), an oral JAK1 selective inhibitor, is effective in combination with methotrexate (MTX) in patients with active rheumatoid arthritis and insufficient response to MTX: results from a randomised, dose-finding study (DARWIN 1). Ann Rheum Dis. 2017;76:998–1008.
- Kavanaugh A, Kremer J, Ponce L, et al. Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: results from a randomised, dose- finding study (DARWIN 2). Ann Rheum Dis. 2017;76:1009–19.
- Westhovens R, Rigby WFC, van der Heijde D, et al. Filgotinib in combination with methotrexate or as monotherapy versus methotrexate monotherapy in patients with active rheumatoid arthritis and limited or no prior exposure to methotrexate: the phase 3, randomised controlled FINCH 3 trial. Ann Rheum Dis. 2021;80:727–38.
- Combe B, Kivitz A, Tanaka Y, et al. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: a phase III randomised clinical trial. Ann Rheum Dis. 2021;80:848–58.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med. 2022;386:316–26.
- Kremer JM, Bingham CO, Cappelli LC, et al. Postapproval Comparative Safety Study of Tofacitinib and Biological Disease-Modifying Antirheumatic Drugs: 5-Year Results from a United States-Based Rheumatoid Arthritis Registry. ACR Open Rheumatol. 2021;3:173–84. [CrossRef]
- Khosrow-Khavar F, Kim SC, Lee H, et al. Tofacitinib and risk of cardiovascular outcomes: results from the Safety of TofAcitinib in Routine care patients with Rheumatoid Arthritis (STAR-RA) study. Ann Rheum Dis. 2022;81:798–804. [CrossRef]
- Khosrow-Khavar F, Desai RJ, Lee H, et al. Tofacitinib and Risk of Malignancy: Results From the Safety of Tofacitinib in Routine Care Patients With Rheumatoid Arthritis (STAR-RA) Study. Arthritis Rheumatol. 2022;74:1648–59. [CrossRef]
- Palleria C, Iannone L, Leporini C, et al. Implementing a simple pharmacovigilance program to improve reporting of adverse events associated with biologic therapy in rheumatology: Preliminary results from the Calabria Biologics Pharmacovigilance Program (CBPP). PLoS One. 2018;13:e0205134.
- Fuchs HA, Brooks RH, Callahan LF, et al. A simplified twenty-eight-joint quantitative articular index in rheumatoid arthritis. Arthritis Rheum. 1989;32:531–7.
- Smolen JS, Breedveld FC, Schiff MH, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford). 2003;42:244–57.
- Besancenot R, Roos-Weil D, Tonetti C, et al. JAK2 and MPL protein levels determine TPO-induced megakaryocyte proliferation vs differentiation. Blood. 2014;124:2104–15. [CrossRef]
- Sopjani M, Morina R, Uka V, et al. JAK2-mediated Intracellular Signaling. Curr Mol Med. 2021;21:417– 25. [CrossRef]
- Park SO, Wamsley HL, Bae K, et al. Conditional deletion of Jak2 reveals an essential role in hematopoiesis throughout mouse ontogeny: implications for Jak2 inhibition in humans. PLoS One. 2013;8:e59675. [CrossRef]
- Winthrop KL, Curtis JR, Lindsey S, et al. Herpes Zoster and Tofacitinib: Clinical Outcomes and the Risk of Concomitant Therapy. Arthritis Rheumatol. 2017;69:1960–8.
- Smolen JS, Genovese MC, Takeuchi T, et al. Safety Profile of Baricitinib in Patients with Active Rheumatoid Arthritis with over 2 Years Median Time in Treatment. J Rheumatol. 2019;46:7–18.
- Molander V, Bower H, Frisell T, et al. Risk of venous thromboembolism in rheumatoid arthritis, and its association with disease activity: a nationwide cohort study from Sweden. Ann Rheum Dis. 2021;80:169–75. [CrossRef]
- Chen C-P, Kung P-T, Chou W-Y, et al. Effect of introducing biologics to patients with rheumatoid arthritis on the risk of venous thromboembolism: a nationwide cohort study. Sci Rep. 2021;11:17009. [CrossRef]
- Sivaraman P, Cohen SB. Malignancy and Janus Kinase Inhibition. Rheum Dis Clin North Am. 2017;43:79– 93.
- Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524–9.
- De Cock D, Hyrich K. Malignancy and rheumatoid arthritis: Epidemiology, risk factors and management. Best Pract Res Clin Rheumatol. 2018;32:869–86. [CrossRef]
- Tan YY, Papez V, Chang WH, et al. Comparing clinical trial population representativeness to real-world populations: an external validity analysis encompassing 43 895 trials and 5 685 738 individuals across 989 unique drugs and 286 conditions in England. Lancet Healthy Longev. 2022;3:e674–89. [CrossRef]
- EMA. Measures to minimise risk of serious side effects with Janus kinase inhibitors for chronic inflammatory disorders. https://www.ema.europa.eu/en/medicines/human/referrals/janus-kinase- inhibitors-jaki.
- Benucci M, Bardelli M, Cazzato M, et al. ReLiFiRa (Real Life Filgotinib in Rheumatoid Arthritis): Retrospective Study of Efficacy and Safety in Common Clinical Practice. J Pers Med. 2023;13:1303. [CrossRef]
- Nagy G, Roodenrijs NM, Welsing PM, et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2021;80:31–5.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).