1. Introduction
One of the great and popular fruit produces in tropical and subtropical areas of the world is the mango (
Mangifera indica Linn.), stated as the “King of Fruits,”. This is somewhat clarified by its exceptional flavor, color, scent, juicy pulp, and nourishing worth [
20]. Mango comprises iron, calcium, phosphorus and vitamins A and C [
15]. Mango manufacture touched 58.3 million metric tons international in 2021; by 2026, it is likely to have bigger by 1.9% to 65 million metric tons [
1]. Concluded 90 nations international harvest mangoes, with India presence the world’s highest creator [
7]. More than 70% of the world’s mangoes are produced annually in Asia, making it the region with the greatest production capacity [
6]. In 2019-2020 total production of mangoes in Pakistan was 10340 kg per hectare with Punjab being largest producer [
21]. Pakistan’s mango industry is concerned that the country’s output of high-quality mangos is not rising at the rate required to compete in the global market. In addition to pests and diseases, one of the main causes is the lack of genetic diversity. Plant material can be improved by chromosome doubling, hybridization, random seedling selection, genetic mutation, and other processes that occur within species or varieties [
22]. It is important to understand that every available germplasm has some sort of use [
13]. If it has little commercial value, it might be useful for other things like home gardening, disease resistance, and climate adaptation [
4].
Varietal description is a vital constituent of mango enhancement and breeding. Breeding plans that usage germplasm with exclusive traits necessity precise information in instruction to make innovative cultivars [
27]. Numerous mango cultivars everywhere the world have a diversity of drawbacks, counting substitute behavior, low yields, thin maturing spaces, and poor fruit quality. Therefore, in order to expand the genetic resource repository, it is crucial to identify, utilize, and protect the essential germplasm of promising and endangered mango types and cultivars [
11]. To increase the productivity and fruit quality of the already available mango germplasm, there is currently a huge demand on the amount of time needed to locate, define, evaluate, and protect the scarce genetic resources [
22]. A number of approaches and techniques for the documentation and detailed report of mango genotypes founded on structural, biochemical, agronomic, and genetic structures have been issued [
11].
The learning’s goals were to find, describe, and suggest mango germplasm in South Punjab; additionally, to expand the varietal spectrum by choosing good fruit characteristics to meet the changing demands of the market. Choosing important morphological and biochemical indicators for mangos to serve as future reference points for varietal identification and breeding efforts was another objective.
2. Materials and Methods
The study was showed in the Horticulture Research Station Bahawalpur. Fruits of five verities were used in this study. These varieties include Anwar Ratol, Azeem Chaunsa, Alishan, Dusehri, Haden, Mango Research Centre Shujabad’s experimental mango orchard provided fresh fruit samples (5 kg) of each type that were uniformly sized and of high quality. To start the natural ripening process, the fruit trials were kept at room temperature.
2.1. Physical Attributes
Using a vernier caliper for dimensions of length and width were recorded after a week, when the fruit’s color had fully matured. The pulp, skin, and stone are then separated from this fruit. The mass of the peel, pulp, and stone are among the factors that are then ascertained using a weight balance.
2.2. Biochemical Attributes for Fruit Quality
2.2.1. Total Soluble Solids and Titrateable Acidity
A digital refrigerator was applied to test the total soluble solids in a mango fruit juice trial. For the estimation of titratable acidity method, a conical flask containing 10 ml of pulp solution was filled. The conical flask was shaken briskly after adding two to three drops of phenolphthalein indicator. Following that, 0.1 N NaOH solutions were added to a burette and quickly filtered until a persistent pink hue was achieved. The amount of NaOH solution needed for the titration was measured and recorded. The following formula was used to get the percent titratable acidity.
where V1 is the volume made up, E is the equivalent weight of acid, T is the titre, N is the normality of NaOH, V2 is the volume of extract, and W is the weight of the sample.
2.2.2. Ascorbic Acid Content
Using the procedure of
Sogi et al., [
26] the quantity of ascorbic acid in the pulp was estimated. Whatman® filter paper was used to filter the extracted juice from each sample. In a 100 mL round-bottom flask, 10 mL of filtered aliquot was collected, and 0.4% oxalic acid was additional to bring the volume up to the required level. From a 100 mL aliquot, 5 mL was placed in a beaker and titrated against newly made dye 2, 6-dichlorophenol indophenol until a bright pink endpoint was reached, which lasted for ten to fifteen seconds
. In order to prepare the dye, 200 milliliters of Ascorbic acid (mg 100 mL-1) and 52 milliliters of 2, 6-dichlorophenol indophenol were added to a volumetric flask. The volume was then accustomed by addition distilled water to get the anticipated level.
2.2.3. Total Phenolic and Total Protein Contents
Following a 5-minute incubation period at 22°C, 100 mL of the vegetable extract and 0.75 mL of the folin-Ciocalteu reagent were mixed. After that, the mixture was kept at 22°C for 90 minutes and 0.75 mL of Na2CO3 solution was added. Using a spectrophotometer, the absorbance of the sample was determined at 725 nm [
26].
The total protein content was quantified by means of the technique mention by
Hanif et al., [
9] wherein ordinary bovine serum albumin was utilized as average. The antioxidative enzyme activity was estimated using the protein content.
2.2.4. Carotenoid and Anthocyanin Content
For the estimation of carotenoid and anthocyanin content method of
Haider et al., [
8] was followed. One gram of the material was extracted using a 15:85 HCl-methanol result in a trembling water soak at 25 °C for six hours. After that, the sample was spun in a temperature-controlled centrifuge for 20 minutes at 4 °C at 4000 × g. The absorbance of anthocyanin was restrained at 650, 620, and 530 nm by means of a UV–Vi’s spectrophotometer (2326 K, Hermle Labortechnik GmbH, Wehingen, Germany). Whole anthocyanins (mg Kg−1 FW) were computed using the following formula:
The photosynthetic pigments were measured using spectrophotometry. A little pinch of acid-washed sand was added to 5 ml of acetone to homogenize new pulp samples (0.1 g) (BDH Chemicals, England). After the combination was clean using Whatman No. 1 filter paper, it was centrifuged at 9,000 rpm for three minutes at room temperature. The resultant filtrate was restrained at 470, 645, and 662 nm by the absorption. The pigment content, reported as μg per g of fresh weight (FW), was measured using the following formulae.
A470 shows the absorbance at 470 nm (the wavelength or absorbance range for carotenoids), Ca indicates chlorophyll a, and Cb represents chlorophyll b. Cx + c is the concentration of total carotenoids founded on xanthophyll’s and carotenes.
2.2.5. Antioxidative Enzyme’s Activity
For the quantification of Peroxidase activity. The reaction mixture (2 mL) comprised 1 mL of water, 200 μl enzyme extract, and 200 μl H2O2 (27.5 mM). Following seven minutes of exposure to fluorescent light for both the reaction mixture and the blank, the absorbance was restrained at 470 nm by means of a spectrophotometer [
25].
The procedure indicated by
Lo’Ay et al., [
17] was used to assess the CAT activity: 1000 μL of newly manufactured H2O2 (5.9 Mm) was mixed with 1000 μL of fruit extract, and absorbance was measured at 240 nm.
3. Results
3.1. Physical Attributes
3.1.1. Fruit Size (Length and Width)
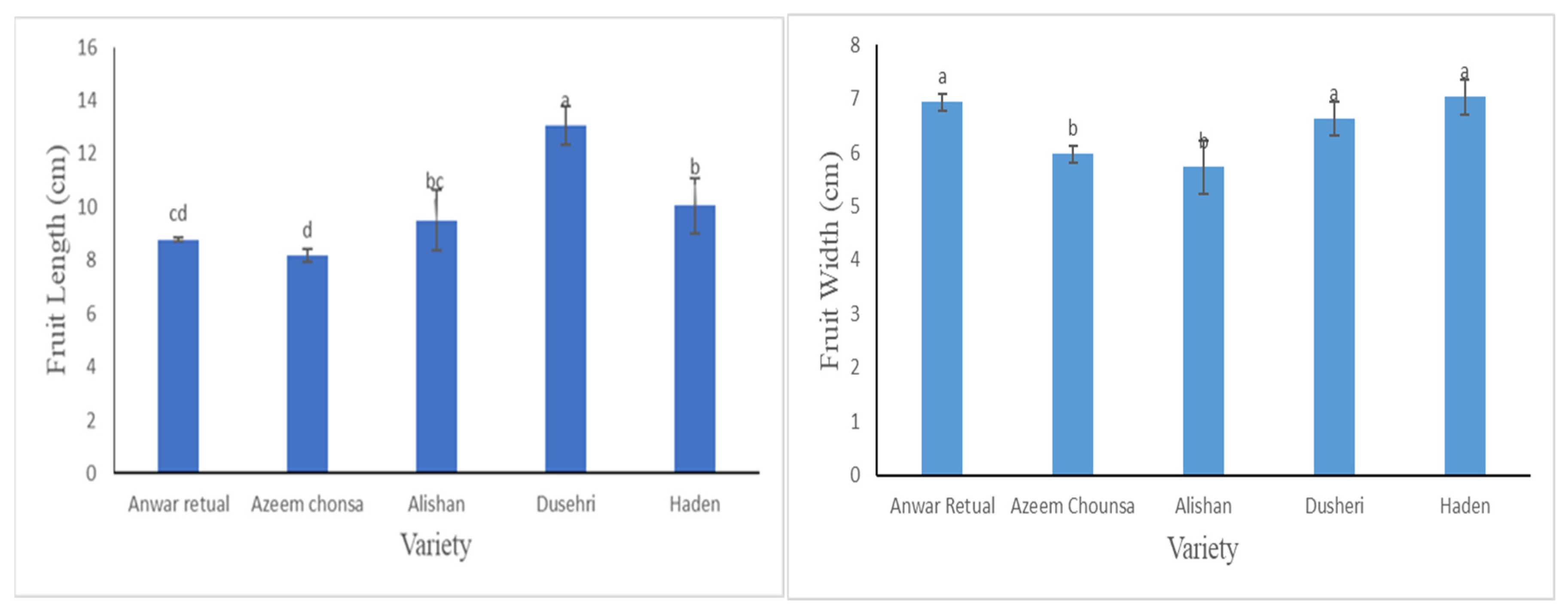
Facts associated to fruit length accessible in
Figure 1a exposed that Dusehri has significantly more length as compared to other 4 varaieties like Anwar retual, Azeem chonsa, Alishan and Haden. Anwar retual and Alishan have little difference as compare to other varaieties.
Numerical study depicted that there was no important difference in fruit Width of Haden, Dusehri and Anwar retual (
Figure 1b).
3.1.2. Fruit Weight, Pulp Weight, Peel Weight and Stone Weight (g)
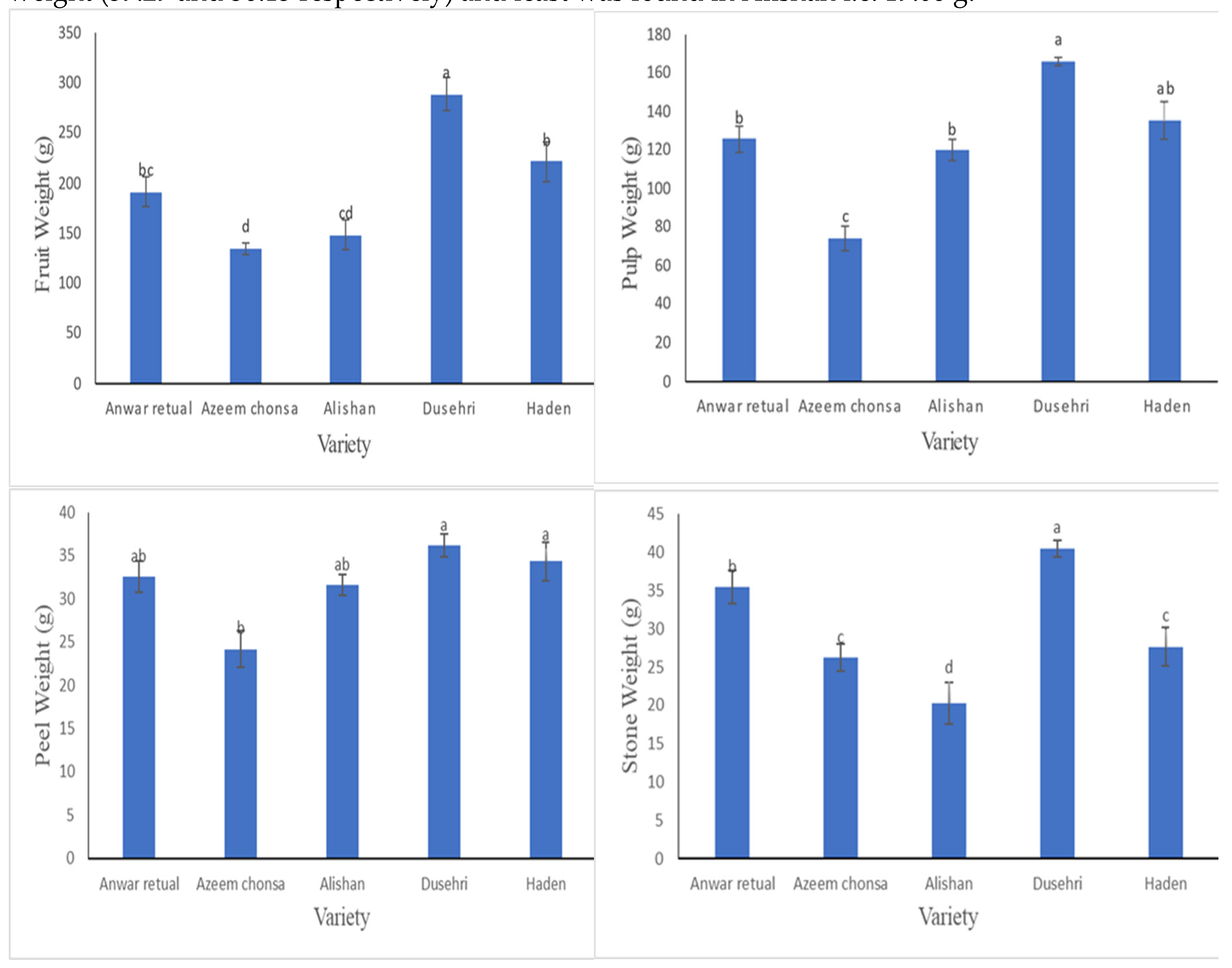
The mean value of fruit weight showed a range from 315g to 131g. Maximum fruit weight was recorded in Dusehri as compared to Anwar retual, Azeem chonsa, Alishan and Haden. While least weight was found in Alishan as shown in
Figure 2a.
Pulp weight varied significantly amongst the dissimilar varieties below learning. The lowest pulp weight was observed in Azeem chounsa i.e. 72.16g while highest was observed in Dusehri i.e. 171g (
Figure 2b).
Taking into consideration
Figure 2c, no important changes were originated amongst the diversities. While in case of stone weight Dusehri and Anwar retual were found to have highest stone weight (39.29 and 36.13 respectively) and least was found in Alishan i.e. 19.06 g.
3.2. Biochemical Attributes
3.2.1. TSS (Brix) and TA (%)
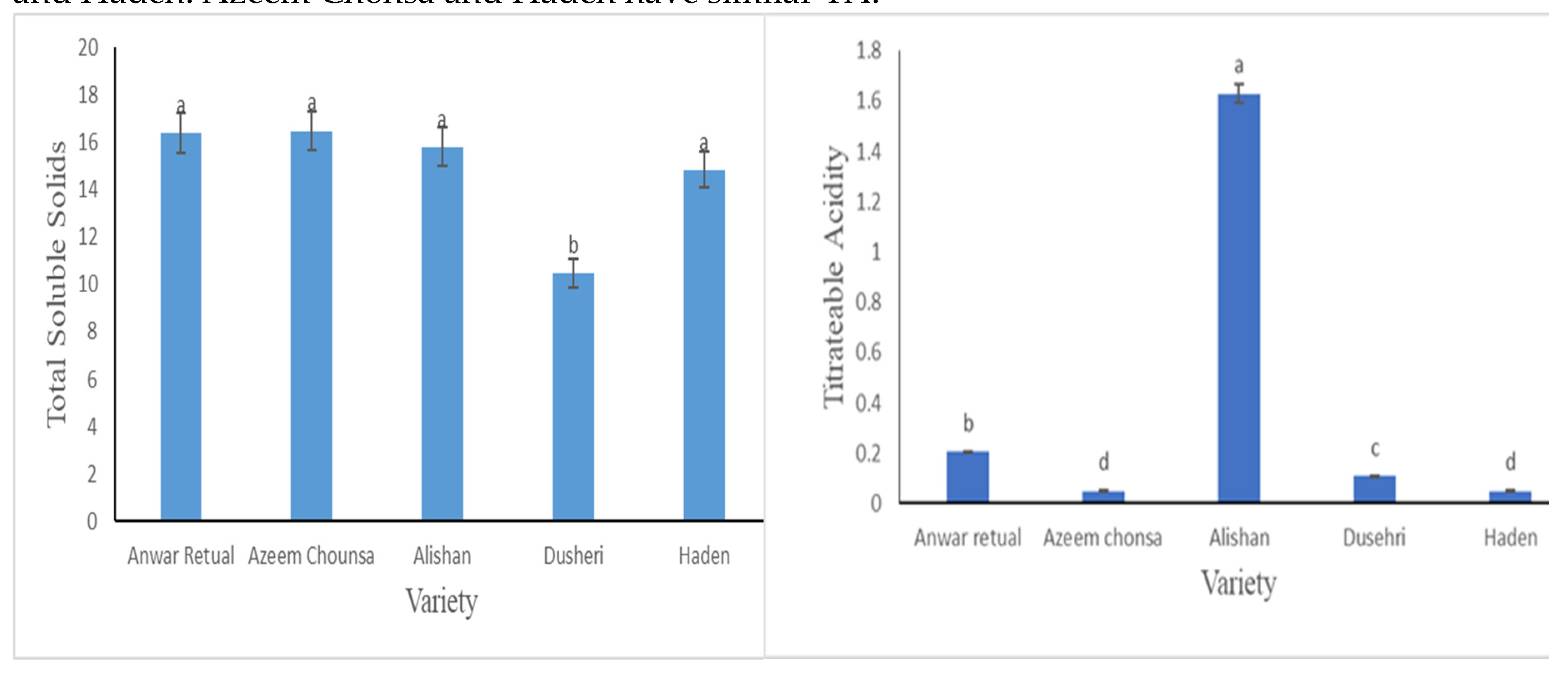
TSS content ranged from 16.36 to 14.83, Anwar retuaal and Azeem chonsa depicted high Soluble Solids content as compare to other varieties but not varied significantly as compare to Alishan and Haden. Alishan has maximum TSS value than Dusehri and Haden but minimum value as compare to Anwar retuaal and Azeem chonsa (
Figure 3a). Taking into consideration
Figure 3b, Alishan has more titratable acidity (1.63%) as compare to Anwar retuaal, Azeem Chonsa, Dusehri and Haden. Anwar retual has minimum TA as compare to Alishan but maximum from Azeem Chonsa, Dusehri and Haden. Azeem Chonsa and Haden have similar TA.
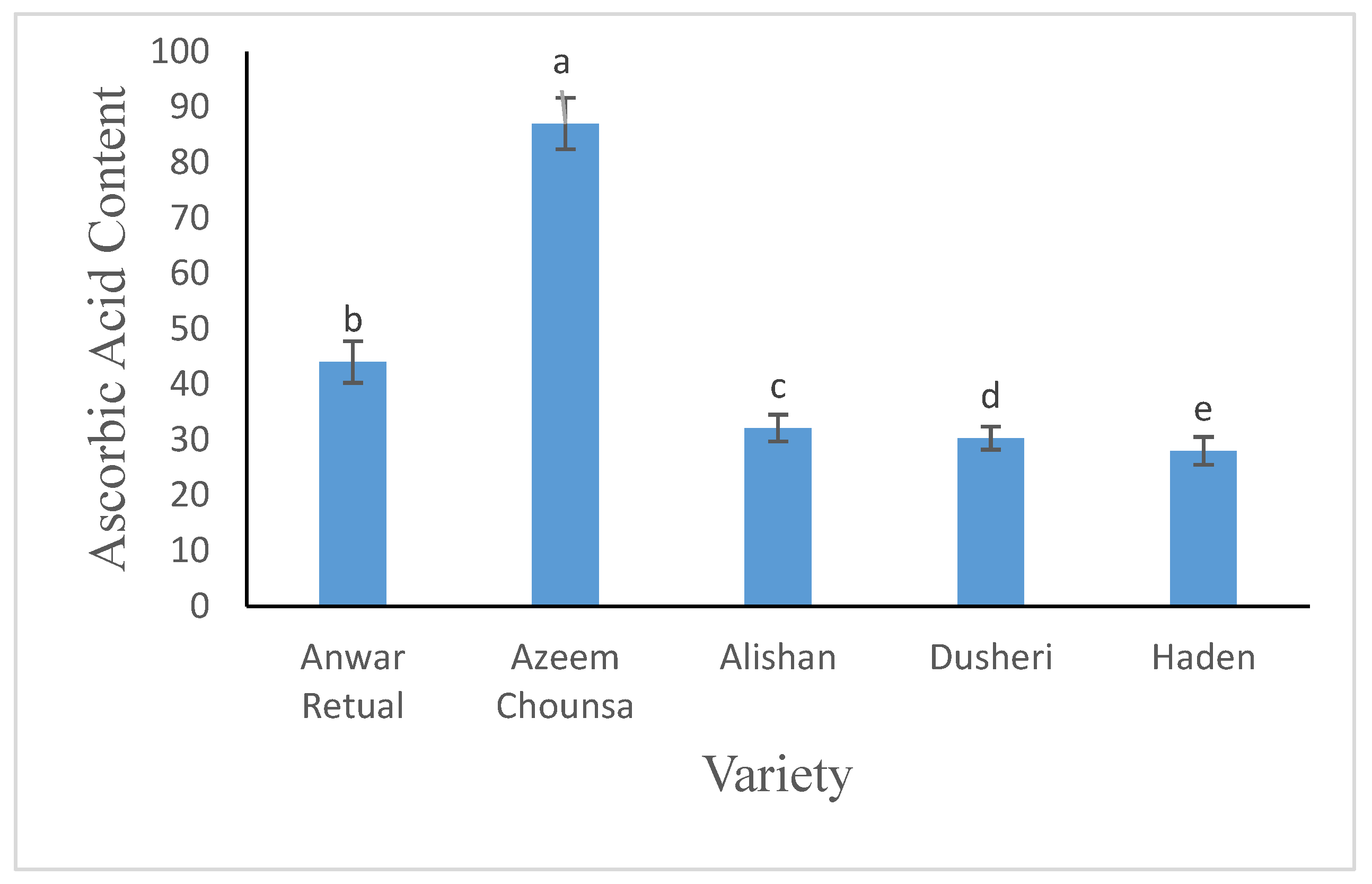
3.2.2. Ascorbic Acid (mg/100g)
Ascorbic acid content was found maximum in Alishan (87 mg/100g) as compare to Anwar retual, Azeem Chounsa, Dusehri and Haden. Statistical analysis depicted significant variations among all the varieties (
Figure 4).
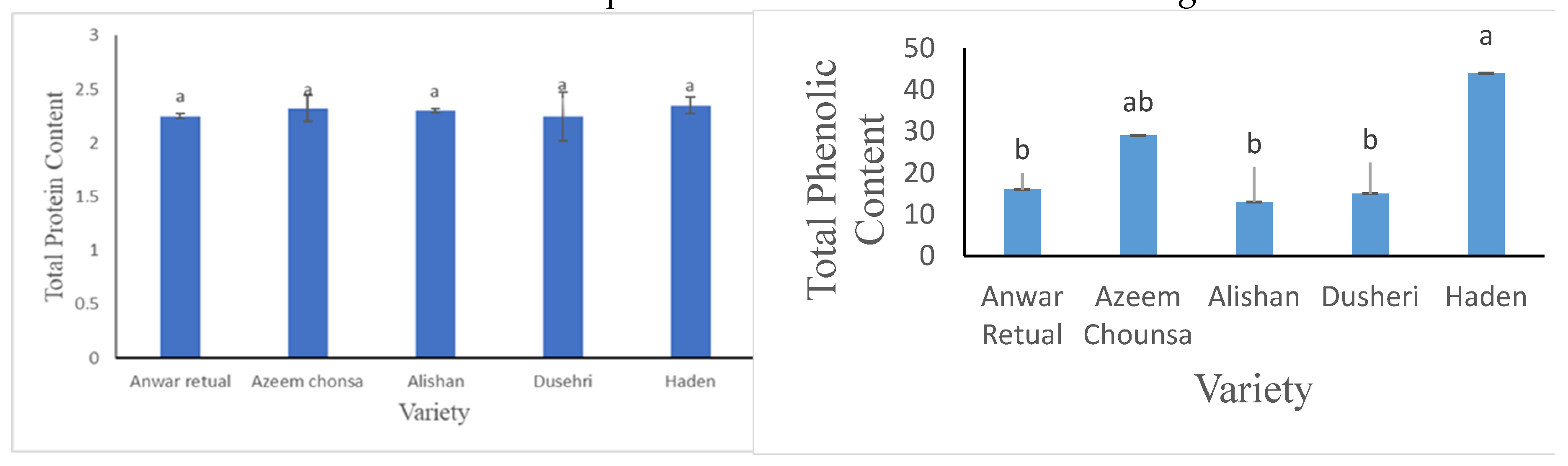
3.2.3. Total Protein Content (g) and Total Phenolic Content (mg GAE/g DW)
Taking into consideration
Figure 5a statistical analysis depicted that there were no important dissimilarities among the diversities in case of total protein content ranging from 2.32g- 2.25g.
Haiden has maximum value (44 mg GAE/g DW) of total phenolic content as compare to other 4 varaieties mentioned in this research. Haden has more phenolic content as compare to Anwar retual, Alishan and Dusehri but lower as compare to Azeem chonsa as shown in
Figure 5b.
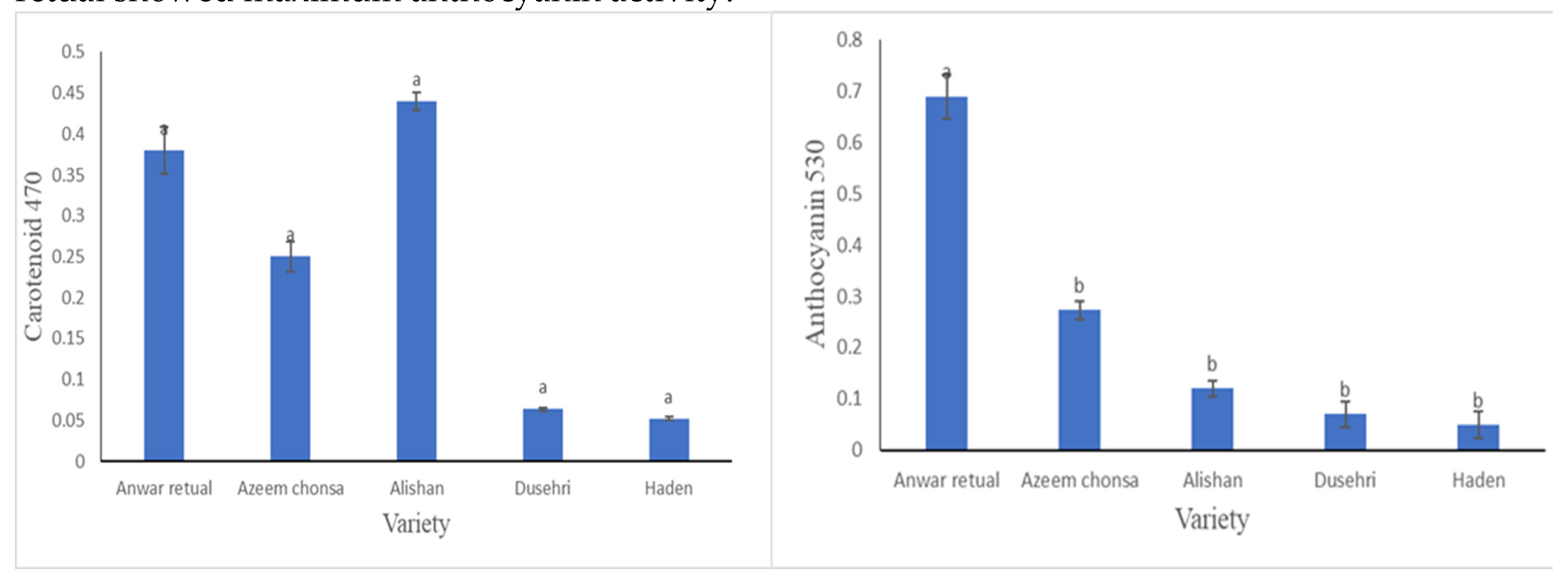
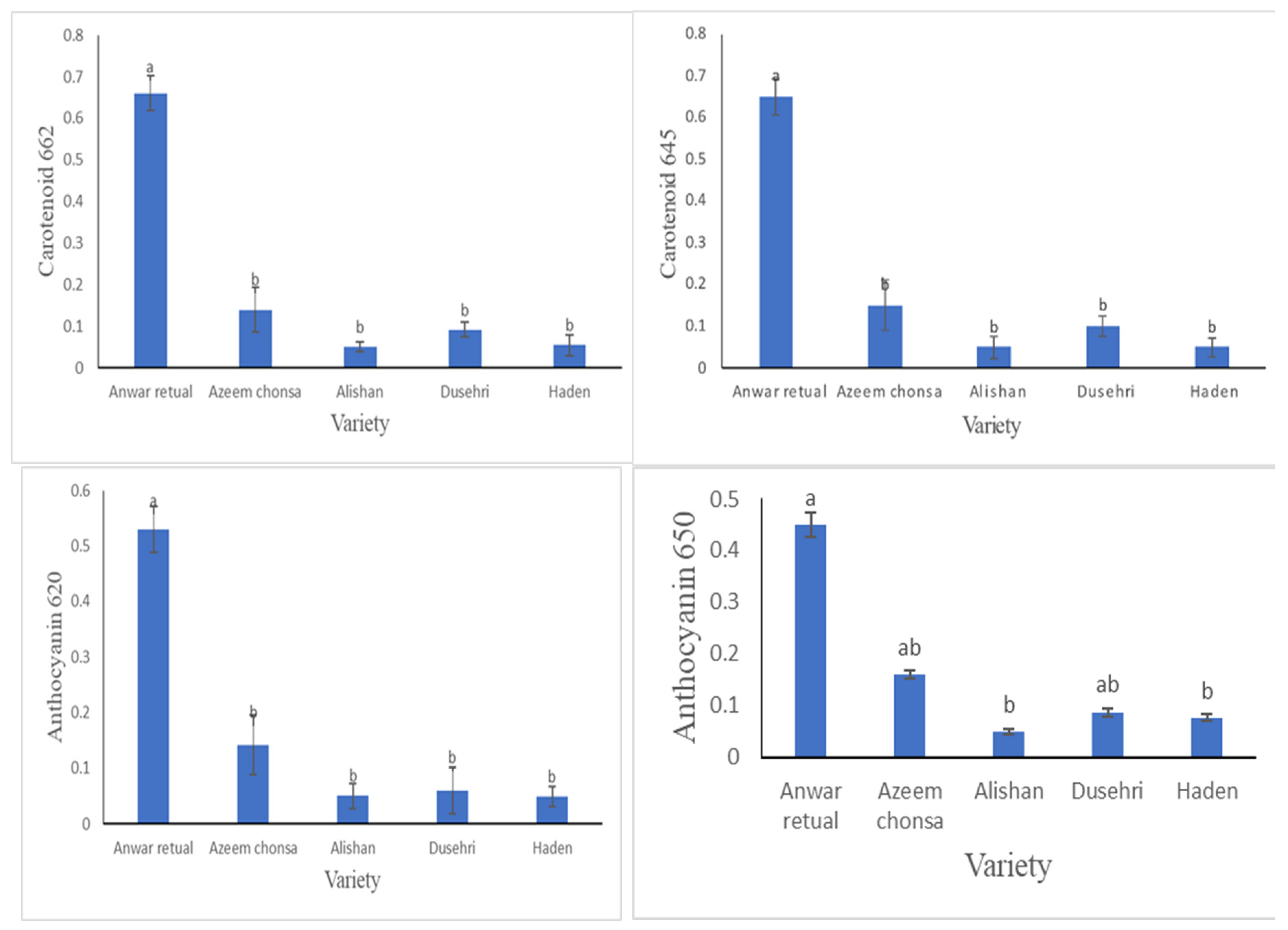
3.2.4. Carotenoid Content (mg/ 100 g FW) and Anthocyanin Content
Carotenoid and Anthocyanin content were calculated through 3 Wavelengths i.e. 662, 645, 470 and 530, 620 and 650 respectively. For the estimation of carotenoid content, when subjected to wavelengths of 662 and 645, Anwar retual has maximum value as compare to other four varieties. While Alishan was found found to have maximum value in case of 470 Wavelength. For the quantification of anthocyanin content when subjected to wavelengths of 530, 620 and 650 Anwar retual showed maximum anthocyanin activity.
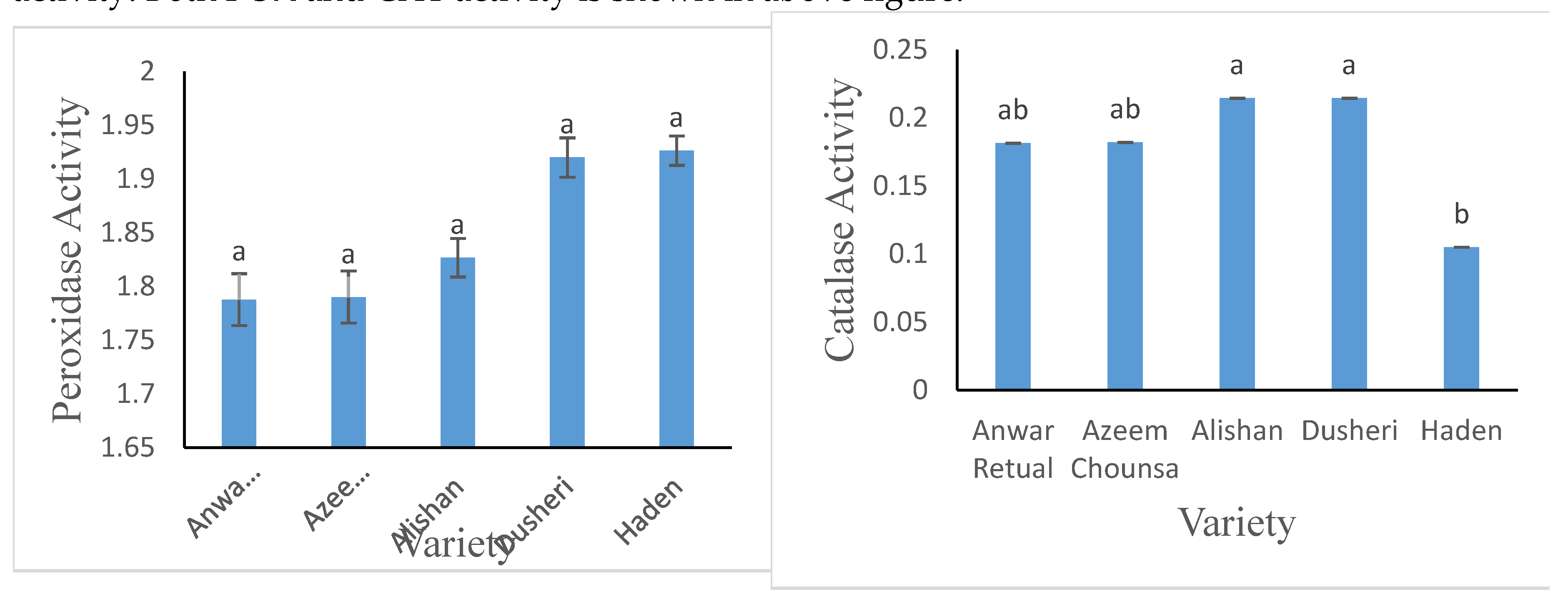
3.2.5. Antioxidative Enzyme’s Activity (U/mg protein)
Qunatification of POX activity showed similar values of Duesehri and Haden (1.92) while 1.78 least was obeserved in Anwar Retual with no significant variation among them. In case of CAT activity, a range of value from 0.21 to 0.10 was quantified with Alishan shown maximum CAT activity. Both POX and CAT activity is shown in above figure.
Figure 7.
Peroxidase and Catalase activity of 5 Mango Cultivars. Information composed from the mean of three duplicates, and vertical bars specify the standard deviation of the means. Mean values tracked by dissimilar superscript literatures differs significantly (P < 0.05).
Figure 7.
Peroxidase and Catalase activity of 5 Mango Cultivars. Information composed from the mean of three duplicates, and vertical bars specify the standard deviation of the means. Mean values tracked by dissimilar superscript literatures differs significantly (P < 0.05).
4. Discussion
The purpose of this study was to identify South Punjab’s native and historically cultivated mango varieties. This learning providing a foundation for the explanation of the main mango diversities in south Punjab region of Pakistan. Pomological markers presented less degree of Significance amongst different varieties. Based on Factorial Analysis, all of the characteristics examined were effective for characterizing mango cultivars. Consequences found were highly valuable for germplasm description and to design local Mango varietial catalog. Alike trainings were done previously on local varieties on Mango [
12], Citrus [
5], fig [
23], that demonstrated that the pomological traits under consideration exhibited a continual diversity while current study didn’t indicate high degree of variation which may be due to genetic composition of each variety which determines different characteristics, which are also greatly impacted by environmental variables.
Mean value of fruit measurement showed meaningful variation extended from 10.33 to 8.83 our outcomes are in confirmation with [
12]. Fruit width didn’t depict significant variation in current study however in previous studies Bora et al., [
3] and Kher and Sharma, [
12] significant variations were observed which may be because of different genetic makeup of different diversities and impact of environmental factors.
The mean value of fruit weight showed a range from 315g to 131g which showed high degree of variation which may be due to difference in respiration rates of different varieties. Peel Weight was observed between 33.6 to 32.1g didn’t indicated significance among varieties. It is evident from
Figure 1b that pulp weight of different mango varieties doesn’t significantly differed our results which is not in confirmation previous study conducted by
Bora et al., [
3] which may be due to environmental variables and difference in growing conditions. Stone weight depicted significant variations ranging from 39.29 to 19.09g which is confirmed by the study of
Bora et al., [
3].
Fruit biochemical features for instance, soluble solids, acidity, sugar: acid proportion, ascorbic acid, sugars, antioxidants, phenolics and flavonoids are also very imperious for respectable cultivar/diversity. Taste, texture, aroma volatiles, and biochemical characteristics are some of the essential features that altogether subsidize for the superiority of mango [
19]. TSS content ranged from 16.36 to 14.83, result showed that there were no significant variations in all varieties except Duesehri which showed least TSS content, TSS range is slightly lesser than as reported in previous existing literature which may be due to environmental factors or varietial characters
Bora et al., [
3]. Maximum titratable acidity was found in Alishan (1.63%), overall, it ranged from 1.63% to 0.05% showed high degree of variation. Wide degree of variation in different mango varieties was also supported with
Bakhshi and Bajwa [
2]. Ascorbic acid content was found maximum in Alishan (87 mg/100g) as compare to Anwar retual, Azeem Chounsa, Dusehri and Haden. Statistical analysis depicted significant variations among all the varieties. Wide range in ascorbic acid content is also supported by
Mitra et al., [
18] experiential the ascorbic acid content in the choice of 21.66 mg/100 g -125.40 mg/100 g. Such difference in ascorbic acid content could be accredited to the landscape and scope of genetic inconsistency current in the investigational material.
No significant variations in total protein content was observed while in terms of total phenolic contents showed a high degree of variation 15-44 (mg GAE/g DW) is somehow in agreement with the
Liu et al., [
16] may be due to genetic composition of each variety.
A fruit product’s natural appearance is expressed by total carotenoids, and fruits with higher levels of these compounds have certain benefits, especially in international trade where chemical color addition is prohibited. Carotenoid and Anthocyanin content were calculated through 3 Wavelengths i.e. 662, 645, 470 and 530, 620 and 650 respectively. For the estimation of carotenoid content, when subjected to wavelengths of 662 and 645, Anwar retual has maximum value as compare to other four varieties. While Alishan was found found to have maximum value in case of 470 Wavelength. For the quantification of anthocyanin content when subjected to wavelengths of 530, 620 and 650 Anwar retual showed maximum anthocyanin activity. Variation in Carotenoids content was also detected by
Hoda et al., [
10].
The poor flavors, texture loss, and color changes are all caused by POD. Qunatification of POX activity showed similar values of Duesehri and Haden (1.92) while 1.78 least was obeserved in Anwar Retual with no significant variation among them. Our study is somehow in agreement with
Liu et al., [
16] difference may be due to environmental factors. In case of CAT activity, a range of value from 0.21 to 0.10 was quantified with Alishan shown maximum CAT activity.
Conclusion
The results of this investigation support the hypothesis that there is substantial variance among genotypes depending on physical and biochemical attributes. South Punjab has a varied germplasm that can be castoff for crossbreeding, and effective exploration will be carried out to fully realize the probable. Description is therefore a crucial prerequisite for initiating a breeding effort.
Author Contributions
Methodology, A.N.; Formal analysis, M.A.B.; Writing—original draft preparation, M.A.A.; Writing—review and editing, A.L, A.S, F.R.; Supervision, M.I. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgement
The authors would like to thank Dr. Waqar Jaleel (Entomology Section, Horticultural Research Station, Bahawalpur) for manuscript formatting.
Conflicts of Interest
The authors have declared that no competing interests exist. The funders have no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Antwi-Boasiako, A.; Amponsah, P.; Opoku, J.A.; Coulibaly, D.; Mintah, P. Increasing Mango Production Efficiency under the Fast-Changing Climate. Abiotic Stress in Crop Plants - Ecophysiological Responses and Molecular Approaches. IntechOpen. 2024.
- Bakhshi, J.C.; Bajwa, B.S. Studies on varietal differences in fruit quality of the mango varieties grown in the Punjab. INDIAN J HORTIC. 1959, 16, 216–220. [Google Scholar]
- Bora, L.; Singh, A.K.; Singh, C.P. Characterization of mango (Mangifera indica L.) genotypes based on physio-chemical quality attributes. J. appl. nat. sci. 2017, 9, 2199–2204. [Google Scholar] [CrossRef]
- Campbell, C.D.B.; Bernard, R. Mangos in the United States: A year-long supply. In Florida State Horticultural Society. Meeting (USA). 1995.
- Debbabi, O.S.; Bouhlal, R.; Abdelaali, N.; Mnasri, S.; Mars, M. Pomological study of sweet orange (Citrus sinensis L. Osbeck) cultivars from Tunisia. Int. J. Fruit Sci. 2013, 13, 274–284. [Google Scholar] [CrossRef]
- FAO. FAOSTAT crop production [Online]. 2021. Available from: http://www.fao.org/faostat/en/#home.
- FAO. FAOSTAT crop production [Online]. 2023. Available from: http://www.fao.org/faostat/en/#home.
- Haider, M.W.; Nafees, M.; Iqbal, R.; Ali, S.; Asad, H.U.; Azeem, F.; Arslan, M.; Rahman, M.H.U.; Gaafar, A.R.Z.; Elshikh, M.S. Combined application of hot water treatment and eucalyptus leaf extract postpones seneṣcence in harvested green chilies by conserving their antioxidants: a sustainable approach. BMC Plant Biol. 2023, 23, 576. [Google Scholar] [CrossRef] [PubMed]
- Hanif, A.; Ahmad, S.; Jaskani, M.J.; Ahmad, R. Papaya treatment with putrescine maintained the overall quality and promoted the antioxidative enzyme activities of the stored fruit. Sci. Hortic. 2020, 268, 109367. [Google Scholar] [CrossRef]
- Hoda, M.N.; Sanjay Singh, S.S.; Jayant Singh, J.S. Evaluation of ecological groups of Mango (Magnifera indica) cultivars for flowering and fruiting under Bihar conditions Indian J. Agric. Sci. 2003, 73, 101–105. [Google Scholar]
- Khan, A.S.; Ali, S.; Khan, I.A. Morphological and molecular characterization and evaluation of mango germplasm: An overview. Sci. Hortic. 2015, 194, 353–366. [Google Scholar] [CrossRef]
- Kher, R.; Sharma, R.M. Performance of some mango cultivars under sub-tropical rainfed region of Jammu. Haryana J. Hortic. Sci. 2002, 31, 8–9. [Google Scholar]
- Knight, R.J. Evaluating important fruit characters in mango germplasm. Fruit Var. J. 1993, 47, 25–31. [Google Scholar]
- Krishna, H.; Singh, S.K. Biotechnological advances in mango (Mangifera indica L.) and their future implication in crop improvement—a review. Biotech. Adv. 2007, 25, 223-243.
- Lebaka, V.R.; Wee, Y.J.; Ye, W.; Korivi, M. Nutritional composition and bioactive compounds in three different parts of mango fruit. Int. J. Environ. Res. Public Health. 2021, 18, 741. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.X.; Fu, S.F.; Bi, X.F.; Chen, F.; Liao, X.J.; Hu, X.S.; Wu, J.H. Physico-chemical and antioxidant properties of four mango (Mangifera indica L.) cultivars in China. Food chem. 2013, 138, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Lo’Ay, A.A.; Mostafa, N.A.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Hassan, S.; Abdein, M.A. Influence of the position of mango fruit on the tree (Mangifera indica L. CV.‘Zibda’) on chilling sensitivity and antioxidant enzyme activity. Horticulturae. 2021, 7, 515. [Google Scholar] [CrossRef]
- Mitra, S.; Kundu, S.; Mitra, S.K. Evaluation of local strains of mango (Mangifera indica) grown in West Bengal. Indian J. Agric. Sci. 2001, 71. [Google Scholar]
- Nayak, D. , Singh, A.K.; Srivastav, M. Estimation of genetic parameters of fruit quality traits in mango hybrid population. INDIAN J HORTIC. 2013, 70, 13–17. [Google Scholar]
- Ntsoane, M.L.; Zude-Sasse, M.; Mahajan, P.; Sivakumar, D. Quality assesment and postharvest technology of mango: A review of its current status and future perspectives. Sci. Hortic. 2019, 249, 77–85. [Google Scholar] [CrossRef]
- Pakistan Bureau of Statistics. 2022.
- Rajwana, I.A.; Khan, I.A.; Malik, A.U.; Saleem, B.A.; Khan, A.S.; Ziaf, K.; Anwar, R.; Amin, M. Morphological and biochemical markers for varietal characterization and quality assessment of potential indigenous mango (Mangifera indica) germplasm. Int. J. Agric. Biol. 2011, 13, 151–158. [Google Scholar]
- Saddoud, O.; Baraket, G.; Chatti, K.; Trifi, M.; Marrakchi, M.; Salhi-Hannachi, A.; Mars, M. Morphological variability of fig (Ficus carica L.) cultivars. Int. J. Fruit Sci. 2008, 8, 35–51. [Google Scholar] [CrossRef]
- Sanjay S,; S.S. Evaluation of mango cultivars for their flowering, fruiting and fruit quality attributes. Progressive Hortic. 2002, 34, 240–243.
- Shahbaz, M.; Akram, A.; Raja, N.I.; Mukhtar, T.; Mehak, A.; Fatima, N.; Ajmal, M.; Ali, K.; Mustafa, N.; Abasi, F. Antifungal activity of green synthesized selenium nanoparticles and their effect on physiological, biochemical, and antioxidant defense system of mango under mango malformation disease. PLoS One. 2023, 18, 0274679. [Google Scholar] [CrossRef] [PubMed]
- Sogi, D.S.; Siddiq, M.; Roidoung, S.; Dolan, K.D. Total phenolics, carotenoids, ascorbic acid, and antioxidant properties of fresh-cut mango (Mangifera indica L., cv. Tommy Atkin) as affected by infrared heat treatment. J. Food Sci. 2012, 77, 1197–C1202. [Google Scholar] [CrossRef] [PubMed]
- Vasugi, C.; Dinesh, M.R.; Sekar, K.; Shivashankara, K.S.; Padmakar, B.; Ravishankar, K.V. Genetic diversity in unique indigenous mango accessions (Appemidi) of the Western Ghats for certain fruit characteristics. Curr. Sci. 2012, 199–207. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).