1. Introduction
Highly pathogenic avian influenza viruses (HPAIVs) of the H5N1 subtype (clade 2.3.4.4b) have spread widely among dairy cattle in the United States [
1,
2]. Unexpectedly, high levels of viable H5N1 HPAIVs have been detected in raw milk from those infected cows [
3,
4]. It has raised serious concerns regarding the safety of the cow’s milk supply [
5,
6]. Pasteurization, a heat-treatment process to eliminate pathogens, ensures the safety of commercial milk. However, recent studies have shown inconsistent results when simulating pasteurization conditions to inactivate the virus in contaminated milk.
One study found that the heat treatment of H5N1 HPAIV-positive milk samples at 63℃ for 5-30 minutes or at 72℃ for 15 or 20 seconds reduced the virus titers below the detection limit that was conducted in Madin–Darby canine kidney (MDCK) cells with the traditional 50% tissue-culture infectious dose (TCID
50) as the readout [
3]. Nevertheless, this study also reported that the heat treatment of H5N1 HPAIV-positive milk samples at 72℃ for 15 or 20 seconds did not completely disarm the virus as inoculation of the treated samples into embryonated chicken eggs still resulted in detectable infectious virus particles [
3]. Despite a similar conclusion being reached, another independent study noticed that if initial titers for H5N1 HPAIVs were substantially higher, relatively small amounts of the virus remained infectious in milk even following 15 seconds of treatment at 72°C, which were determined by TCID
50 assay in MDCK cells [
7]. The latest study examining the effects of pasteurization-like temperatures on influenza viruses in retail and unpasteurized milk showed that heat treatment at 72°C for 20 seconds in a 20 µL sample volume significantly reduced influenza virus titer from ~10
8 TCID
50/mL to ~10
4 TCID
50/mL [
8], which implied incomplete killing of influenza viruses under such conditions. However, this study revealed that heat treatment at 63°C for 30 minutes could effectively reduce influenza viruses viability below the limit of detection in MDCK cells [
8]. In addition, a separate study showed the complete inactivation of an H5 virus in raw milk after treatment at 72°C for 15 seconds in a polymerase-chain-reaction (PCR) thermocycler [
9]. Moreover, another latest study reported that no viable virus could be detected when HPAIV-artificially-contaminated raw milk was treated under closely approximate commercial milk-pasteurization conditions [
10].
These divergent results highlight the need for further investigation towards elucidating the impact of heat treatment on the inactivation of influenza viruses. In the present study, we investigated the thermal stability of influenza viruses in artificially contaminated milk and Dulbecco's Modified Eagle Medium (DMEM) control media. In addition to HPAIV H5N1 and influenza A/WSN/1933 viruses, we also include an influenza D virus strain with bovine as primary reservoir.
2. Materials and Methods
Cells and viruses. MDCK cells were maintained at 37 ℃ with 5% CO
2. High glucose DMEM (Cytiva HyClone, USA) supplemented with 10% (v/v) fetal bovine serum (FBS, Procell) and 100 U/mL penicillin-streptomycin (Life Technologies, USA) was used to culture MDCK cells. The human A/WSN/1933 (A/H1) virus was rescued by the reverse genetics system [
11], A/chicken/CHN/Cangzhou/2023 (A/H5) virus (GenBank: PQ278123-PQ278130) was isolated from chicken by inoculation of a clinical sample into embryonated chicken eggs, and D/bovine/CHN/JY3002/2022 virus was isolated from cattle and stored in the laboratory [
12]. These viruses were propagated in MDCK cells or embryonated chicken eggs. The experiments involving the influenza A/H5 virus were carried out in the Biosafety Level 3 laboratory.
Heat treatment. Before heat treatment, a volume of influenza A/H1, A/H5 or D/Yama2019 virus was mixed with three volumes of milk to make the artificially contaminated milk, or to three volumes of DMEM to serve as a control. The mixture was aliquoted into 12.5µL or 50µL per tube, and then incubated under different temperatures (4°C, 37°C, 49°C, 53°C, and 57°C) or pasteurized with different procedures (63°C for 30 minutes, 72°C for 15 seconds, or 80°C for 15 seconds) in a PCR thermocycler (Bio-Rad, T100 Thermal Cycler). After incubation for indicated times at 4°C, each virus solution in milk or DMEM was titered by TCID50 assays. The influenza A/H1 or D/Yama2019 virus in milk or DMEM was treated under 37°C, 49°C, 53°C, and 57°C for one hour and incubated for another half hour in 4°C prior to the TCID50 titration. The influenza A/H1, A/H5 or D/Yama2019 virus in milk or DMEM was pasteurized at 63°C for 30 minutes, 72°C for 15 seconds, or 80°C for 15 seconds in the PCR thermocycler. Briefly, the aliquoted sample (12.5µL or 50µL per PCR tube) was incubated at room temperature for 10 minutes. At the same time, the thermocycler lid was preheated to the temperature for treatment, and then set procedures as follows: (1) 25°C for 45 seconds, (2) 63°C for 30 minutes, 72°C for 15 seconds, or 80°C for 15 seconds, and (3) 4°C for 5 minutes. Samples in PCR vials were placed into the thermocycler and treated by running the indicated procedures. The treated samples were took out from the machine and immediately placed on ice. Infectious viruses in heat-treated and untreated samples were measured by TCID50 assays or tested by inoculation into embryonated chicken eggs. For testing in embryonated chicken eggs, a mixture of two tubes of pasteurized samples with a reaction volume of 50µL or a mixture of eight tubes of pasteurized samples containing a reaction volume of 12.5µL was prepared prior to inoculation. and then were inoculated into embryonated chicken eggs to detect survival virus. Upon death, eggs were tested for hemagglutination titers (HA). Remaining live eggs were also tested 4 days after inoculation.
Statistical analysis. The significant differences between different groups were determined by the Student’s t test or the one-way ANOVA followed by Tukey’s multiple comparison test in GraphPad Prism 8.0. Values of p< 0.05 were considered significant (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001).
3. Results
One volume of a low titer (~4x10
3 TCID
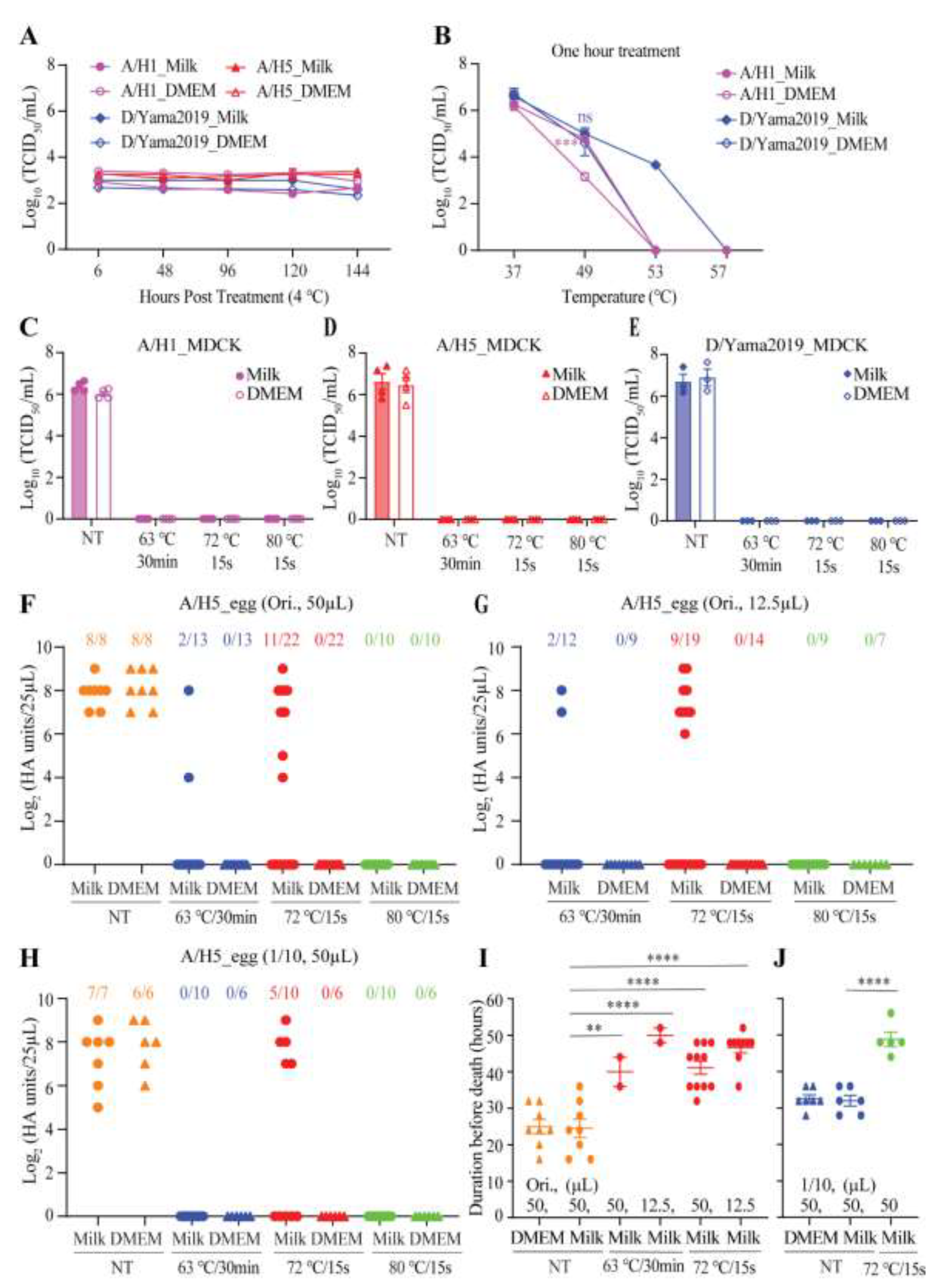
50/mL) of influenza A/WSN/1933 (A/H1), A/chicken/CHN/Cangzhou03/2023 (A/H5), or D/bovine/CHN/JY3002/2022 (D/Yama2019) was respectively mixed with three volumes of commercial whole-fat milk or DMEM. There was no significant decline of virus titers over one week storage at 4°C (
Figure 1A). These results demonstrated the stability of influenza viruses in milk or in DMEM held at refrigerated temperatures. To determine if milk could protect the virus against the heat treatment, the A/H1 (1.66x10
6 TCID
50/mL) or D/Yama2019 (4.57x10
6 TCID
50/mL) in milk or DMEM were treated at 37°C, 49°C, 53°C, and 57°C for one hour, respectively, followed by another half hour incubation on ice. The residual titer of A/H1 in milk was ~1.6 log
10TCID
50/mL higher than that in DMEM when treated at 49°C, while the A/H1 in milk or DMEM was completely inactivated when treated at 53°C (
Figure 1B). The D/Yama2019 in milk retained a high residual infectivity (4.68x10
3 log10TCID
50/mL) after treatment at 53°C but the D/Yama2019 in DMEM completely lost its infectivity after treatment under the same condition (
Figure 1B). Based on these results, milk compositions, especially fat, probably enhanced influenza virus heat resistance.
To evaluate the effect of thermal pasteurization on the inactivation of influenza viruses in milk, one volume of the virus was added to three volumes of milk or DMEM and the mixture was aliquoted into 12.5µL or 50µL per tube, which was then pasteurized with three different procedures in a PCR thermocycler. Regardless of the pasteurization procedures, virus strains, and mixing with milk or DMEM, all treatments resulted in no detectable viruses when assayed using the MDCK-based TCID
50 experiments (
Figure 1C-E). Since the A/H5 virus was isolated from chicken, and its titer in embryonated chicken eggs (1.91x10
8 EID
50/mL) (50% egg infectious dose per milliliter) was higher than that measured in MDCK cells (3.55x10
6 TCID
50/mL), pasteurized samples were also inoculated into embryonated chicken eggs for detecting survival of viruses. A mixture of two tubes of pasteurized samples with a reaction volume of 50µL or a mixture of eight tubes of pasteurized samples containing a reaction volume of 12.5µL was prepared prior to inoculation. Upon death, eggs were tested for hemagglutination titers (HA). Remaining live eggs were also tested for HA titers 4 days after inoculation.
After heating, each virus-contaminated milk sample at 63°C for 30 minutes, 2 out of 13 samples with a reaction volume of 50µL (
Figure 1F) and 2 out of 12 samples with a reaction volume of 12.5µL (
Figure 1G) retained viable viruses. Notably, 11 out of 22 samples with a reaction volume of 50µL (
Figure 1F) and 9 out of 19 samples with a reaction volume of 12.5µL (
Figure 1G) retained infectious virus particles when virus-contaminated milk samples were treated at 72°C for 15 seconds. Under treatment at 72°C for 15 seconds (reaction volume: 50µL), virus survival was also found in 5 out of 10 milk samples containing 1/10 diluted A/H5 (
Figure 1H). In milk, only 80°C for 15 seconds destroyed the A/H5 virus completely, while in DMEM, all three pasteurization procedures inactivated the virus completely (
Figure 1F-H). In dead eggs, recovered HA titers of pasteurized virus samples were relatively lower or comparable to HA titers of non-treated virus samples (
Figure 1F-H), while it took treated virus samples significantly longer to cause egg death (
Figure 1I-J). These results suggested that milk could protect influenza viruses against heat inactivation, and pasteurization at 80°C for 15 seconds was superior to pasteurization at 72°C for 15 seconds or at 63°C for 30 minutes in fully killing the virus in milk.
4. Discussion
There is widespread recognition that pasteurization of raw milk inactivates potential pathogens. Due to the recent discovery of HPAIV H5N1 in cattle and the observation of virus tropism for the mammary gland [
1,
2,
13], a greater focus is being given to determining which parameters are necessary to specifically inactivate HPAIV H5N1 in milk. The data from this study provide convincing evidence that milk increases influenza viruses thermal resistance. Influenza A/H1, A/H5, and D/Yama2019 viruses used in the study all displayed greater survivability against heat treatment in milk than in DMEM (
Figure 1B, and 1F-H). Milk fat and protein probably protect viruses from inactivation by pasteurization [
14]. This might make milk products with a higher fat and protein content particularly resistant to virus inactivation. A recent study found that the bovine influenza H5N1 virus stayed active in concentrated lactose solution for up to 14 days under refrigerated conditions [
15]. Fortunately, heat treatment at 66 °C for a minimum of 5 minutes could efficiently inactivate the virus in lactose [
15].
This work also emphasizes that various variables including different equipment or procedures, virus strains, cell-free or cell-associated status of the virus, and survival virus detection methods should be considered when using a heat inactivation experiment to evaluate the impact of temperature on the stability of influenza viruses including HPAIV H5N1 circulated in dairy cows. Both this (
Figure 1D and 1F-H) and a recent publication [
3] showed that residual viruses post-pasteurization could not be detected using MDCK cells-based TCID
50 tests, but can be recovered by inoculating embryonated chicken eggs. A sensitive and efficient measuring method might be necessary to determine whether the remaining viable virus was present.
This study observed that pasteurization at 80°C for 15 seconds could completely inactivate HPAIV H5N1 in milk (
Figure 1F-H). Neither pasteurization at 72°C for 15 seconds nor at 63°C for 30 minutes could fully kill the virus in milk (
Figure 1F-H). The latest published study showed that pasteurization at 63°C for 30 minutes could effectively reduce viral viability below the limit of detection, but viable viruses could still be detected after pasteurization at 72°C for 20 seconds [
8]. Typically, milk safety is enhanced by higher pasteurization temperatures and/or longer treatment times. However, milk quality and taste are also crucial in the dairy industry and may be compromised by excessive pasteurization. Given that influenza viruses in milk are more resistant to inactivation by pasteurization than those in DMEM, this study highlights the need to improve current pasteurization standards.
Author Contributions
Conceptualization, J.Y., W-K.W. and Y.S.; methodology, W.H., Z.W., Y.C., T.L. and S.W.; software, W.H. and J.Y.; validation, J.Y., W-K.W., Z.W. and W.H.; formal analysis, Z.W., W.H. and J.Y.; investigation, Z.W. and W.H.; resources, J.Y., W-K.W. and Y.S.; data curation, J.Y. and W-K.W.; writing—original draft preparation, J.Y.; writing—review and editing, J.Y., W-K.W., Y.S. W.H., Z.W., Y.C., T.L., X.J. and S-L.Z.; visualization, W.H., Z.W. and J.Y.; supervision, J.Y. and W-K.W.; project administration, J.Y.; funding acquisition, J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the sub project of "Discipline Construction of Swine and Poultry Breeding Industry" of Special Project on Science and Technology Innovation Strategy (ZX202401-04), the National Natural Science Foundation of China (NSFC) grant (No. 32202795), the Natural Science Foundation of Guangdong Province (No. 2024A1515011049), and the special funds (Nos. R2021YJ-QG008, R2023PY-JX025 and R2021QD-034) for Talent Introduction Program from Guangdong Academy of Agricultural Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Burrough ER, Magstadt DR, Petersen B, Timmermans SJ, Gauger PC, Zhang J, Siepker C, Mainenti M, Li G, Thompson AC, Gorden PJ, Plummer PJ, Main R. 2024. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024. Emerg Infect Dis 30:1335-1343.
- Caserta LC, Frye EA, Butt SL, Laverack M, Nooruzzaman M, Covaleda LM, Thompson AC, Koscielny MP, Cronk B, Johnson A, Kleinhenz K, Edwards EE, Gomez G, Hitchener G, Martins M, Kapczynski DR, Suarez DL, Alexander Morris ER, Hensley T, Beeby JS, Lejeune M, Swinford AK, Elvinger F, Dimitrov KM, Diel DG. 2024. Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle. Nature. [CrossRef]
- Guan L, Eisfeld AJ, Pattinson D, Gu C, Biswas A, Maemura T, Trifkovic S, Babujee L, Presler R, Jr., Dahn R, Halfmann PJ, Barnhardt T, Neumann G, Thompson A, Swinford AK, Dimitrov KM, Poulsen K, Kawaoka Y. 2024. Cow's Milk Containing Avian Influenza A(H5N1) Virus - Heat Inactivation and Infectivity in Mice. N Engl J Med 391:87-90.
- Neumann G, Kawaoka Y. 2024. Highly pathogenic H5N1 avian influenza virus outbreak in cattle: the knowns and unknowns. Nat Rev Microbiol 22:525-526.
- Nowogrodzki J. 2024. Bird flu in US cows: is the milk supply safe? Nature. [CrossRef]
- Harris E. 2024. Does Infectious Bird Flu Virus Persist After Pasteurization? JAMA 332:362.
- Kaiser F, Morris DH, Wickenhagen A, Mukesh R, Gallogly S, Yinda KC, de Wit E, Lloyd-Smith JO, Munster VJ. 2024. Inactivation of Avian Influenza A(H5N1) Virus in Raw Milk at 63 degrees C and 72 degrees C. N Engl J Med 391:90-92.
- Caceres CJ, Gay LC, Faccin FC, Regmi D, Palomares R, Perez DR. 2024. Influenza A(H5N1) Virus Resilience in Milk after Thermal Inactivation. Emerg Infect Dis 30.
- Cui P, Zhuang Y, Zhang Y, Chen L, Chen P, Li J, Feng L, Chen Q, Meng F, Yang H, Jiang Y, Deng G, Shi J, Chen H, Kong H. 2024. Does pasteurization inactivate bird flu virus in milk? Emerg Microbes Infect 13:2364732.
- Spackman E, Anderson N, Walker S, Suarez DL, Jones DR, McCoig A, Colonius T, Roddy T, Chaplinski NJ. 2024. Inactivation of highly pathogenic avian influenza virus with high temperature short time continuous flow pasteurization and virus detection in bulk milk tanks. [CrossRef]
- Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. 2000. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A 97:6108-13.
- Yu J, Wen Z, Hu W, Chen M, Zhang Y, Liu S, Wang G, Wang Z, Wang D, Zhai SL, Wei WK, Li T, Liao M. 2024. Influenza D virus infection in China, 2022-2023. Emerg Microbes Infect 13:2343907.
- Nelli RK, Harm TA, Siepker C, Groeltz-Thrush JM, Jones B, Twu NC, Nenninger AS, Magstadt DR, Burrough ER, Pineyro PE, Mainenti M, Carnaccini S, Plummer PJ, Bell TM. 2024. Sialic Acid Receptor Specificity in Mammary Gland of Dairy Cattle Infected with Highly Pathogenic Avian Influenza A(H5N1) Virus. Emerg Infect Dis 30:1361-1373.
- Tomasula PM, Kozempel MF, Konstance RP, Gregg D, Boettcher S, Baxt B, Rodriguez LL. 2007. Thermal inactivation of foot-and-mouth disease virus in milk using high-temperature, short-time pasteurization. J Dairy Sci 90:3202-11.
- Kwon T, Gebhardt JT, Lyoo EL, Nooruzzaman M, Gaudreault NN, Morozov I, Diel DG, Richt JA. 2024. Bovine Highly Pathogenic Avian Influenza Virus Stability and Inactivation in the Milk Byproduct Lactose. Viruses 16.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).