1. Introduction
In Bangladesh, indigenous poultry farming plays a vital role in providing nutrition to many people, with almost 89 % of rural households engaged in this sector [
1]. Commercial strains and indigenous chicken breeds contribute almost equal numbers of eggs (50:50) and meat (60:40) to satisfy the national demand in Bangladesh [
2,
3]. Among the Indigenous chicken breeds, the Hilly breed has exhibited superior disease resistance, early sexual maturity, and higher egg production when compared with nondescriptive Deshi chickens in Bangladesh’s hot weather. Additionally, this breed has shown a lower mortality percentage in rearing in rural areas, further highlighting its potential as a promising indigenous chicken breed in Bangladesh [
4].
However, because of global climate change, indigenous poultry farming in Bangladesh also faces the economically harmful challenge of high ambient temperatures [
5]. During heat stress, birds generally thermoregulate through reduced feed intake, hormonal regulation, and panting; thus, subsequently negatively influencing reproduction [
6,
7]. Chicken thermoregulation also involves expressing heat shock-related genes such as heat shock proteins (HSPs) and heat shock transcription factors (HSF) [
8,
9].
The heat shock protein 70 (HSP70) gene is a member of the HSP family and is expressed in almost all types of cells [
10]. This gene plays a protective role in various stress responses, including heat stress, and maintains homeostasis by balancing the synthesis and degradation of cellular proteins [
11,
12]. Previous studies have shown that genetic variations (i.e., single nucleotide polymorphisms (SNPs) in the 5ʹ-flanking region of HSP70 affect the functions of this gene, leading to changes in cellular processes and influencing phenotypic performance [
13,
14]. Similarly, certain SNPs (e.g., A258G, C276G, and C1431A) in the coding region of chicken HSP70 have been linked to thermotolerance, production, and reproductive performance [
15,
16]. Moreover, polymorphisms in the 5´-flanking region of HSP70 have also been associated with thermotolerance and reproductive traits in mammals, but the correlation between these SNPs and egg production traits in chickens remains unclear [
17,
18,
19].
Heat shock proteins, including HSP70, are transcriptionally regulated by HSF3, a member of the HSF protein family in chickens [
20,
21]. HSF3 mainly regulates the expression of HSP70 and acts as a primary defense against heat stress [
21,
22]. A previous study found that the genetic polymorphism A-1388G alters the activity of the CdxA transcription binding site, resulting in changes to the promoter activity of the HSF3 gene in chickens [
23,
24]. This alteration has been associated with heat-resistance parameters in Lingshan chickens, but associations between chicken reproductive traits and SNPs of HSF3 remain unexplored [
23].
The poultry industry in Bangladesh, particularly the indigenous chicken breeds, plays a critical role in ensuring food security and nutrition for rural households [
25]. However, the increasing impacts of global climate change, specifically rising ambient temperatures, pose significant challenges to the productivity and reproductive performance of chickens [
26]. Heat stress negatively affects poultry through reduced feed intake and altered reproductive processes, significantly hindering production [
27,
28]. Although indigenous breeds like the Hilly chicken exhibit resilience to harsh conditions, the genetic mechanisms underlying their thermotolerance, particularly in relation to HSPs and heat HSFs, remain inadequately explored [
29]. Specifically, understanding how genetic variations in key genes such as HSP70 and HSF3 affect reproductive traits under heat stress is crucial to optimizing poultry breeding programs for improved productivity in tropical climates [
30,
31].
Building on previous studies and the challenges identified by earlier research, the following key research questions arise: What genetic variations (SNPs) are present in the HSP70 and HSF3 genes of the Hilly chicken breed in Bangladesh? How do these SNPs relate to egg production and reproductive traits in chickens exposed to heat stress? Furthermore, can these specific SNPs in the HSP70 and HSF3 genes serve as genetic markers to enhance thermotolerance and improve reproductive performance in chickens?
This research hypothesizes that genetic variations (SNPs) in the HSP70 and HSF3 genes are associated with significant differences in reproductive traits and egg production in Hilly chickens. These variations may serve as potential genetic markers for enhancing thermotolerance and productivity in chickens raised under high ambient temperatures.
The main objective of the study was to identify and characterize genetic variations (SNPs) in the HSP70 and HSF3 genes in the Hilly chicken breed of Bangladesh. The study further aims to evaluate the associations between these SNPs and reproductive traits, with a particular focus on identifying potential genetic markers that could be used for selecting chickens with enhanced thermotolerance and improved egg production traits in hot environments. The findings of the study could contribute to the development of genetic selection strategies aimed at improving thermotolerance and reproductive performance in poultry, enhancing the sustainability of chicken farming in hot climates.
2. Materials and Methods
2.1. Experimental Birds and Trait Records
A total of 150 female Hilly chickens maintained at Bangladesh Livestock Research Institute (9th generation of the breeding flock) were used in the present study (
Figure 1). These hens were reared under the standard management protocol of the BLRI from hatching. At 16 weeks of age, the birds were transferred to separate cages in a naturally ventilated poultry house with a 16-h photoperiod that included 12 h of daylight and 4 h of artificial light. During the laying age period (17–72 weeks), the birds were fed twice daily (morning and evening) with a diet containing 16.33 % crude protein and 2845 Kcal ME/kg DM. They were provided free access to water. From that point until reaching 310 days of age, the following parameters were recorded: the age at sexual maturity (ASM), body weight (BW) at ASM, egg weight (EW) at ASM (g), monthly egg production (number/bird), and EW at 40 weeks of age (g).
2.2. Blood Collection and Genomic DNA Extraction
Blood samples from mature Hilly hens were collected at 310 days of age and stored on FTA cards (QIAGEN GmbH, Hilden, Germany). Genomic DNA was extracted from the cards according to the manufacturer’s instructions. After genomic DNA was extracted, the DNA concentrations were measured using a Bio Spec-Nano (Shimadzu Corp., Kyoto, Japan) and stored at -20 °C for further analysis.
2.3. Primer Design, PCR Amplification, and Sequencing of the HSP70 Fragment
A pair of primers listed in
Table 1 were designed using Primer3 software from NCBI utilizing the complete DNA sequence of HSP70 (NC_052536.1). PCR was performed with a 20 μL final reaction volume containing 100 ng of genomic DNA, 10 μL of 2 × GoTaq Green Master Mix (Promega Corp., WI, USA), and 10 pmol/μL of each primer (HSP70_F_Common and HSP70_R) to amplify the 5ʹ-flanking region of the HSP70 gene. Amplification was conducted for 30 cycles, beginning with an initial denaturation at 94°C for 5 min, then denaturation at 94 °C for 30 s, followed by 30 s of annealing at 64 °C, 30 s of extension at 72 °C, and finally 5 min of final extension at 72 °C. The PCR products were electrophoresed on a 1 % agarose gel and stained with ethidium bromide to visualize the amplicons. The amplified PCR products were purified from the agarose gel using a NucleoSpin Gel and PCR Clean-up Kit (Macherey–Nagel GmbH & Co. KG, Germany). The purified DNA provided the template for direct sequencing utilizing the Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Inc., Foster City, CA, USA) for each strand primer (HSP70_F/Seq and HSP70_R/Seq) (
Table 1). An AB1 3130 sequencer (Applied Biosystems) was used to sequence the products according to the manufacturer’s protocol. The sequence data for the 5ʹ-flanking region of HSP70 were edited, assembled, and aligned; and polymorphism detection was conducted using GENETYX ver. 15 (GENETYX Corp., Tokyo, Japan).
2.4. Genotyping of SNPs and Reconstruction of Haplotypes in the HSP70 Gene
SNPs were genotyped separately using allele-specific PCR (AS-PCR). The AS-PCR was performed using a 20 μL reaction volume containing 10 pmol/μL of each primer (
Table 1) using combinations of P1, P5 or P2, P5 for G-399A; P3, P5 or P4, P5 for A-68G; P9, P13 or P10, P13 for A258G; P11, P13 or P12, P13 for C276G; and P14, P16 or P15, P16 for C1431A SNPs. In the PCR reaction, 100 ng of DNA, 10 μL of 2 × GoTaq Green Master Mix (Promega), and the remainder of the reaction volume were made up with nuclease-free water. The PCR protocol consisted of the following steps: initial denaturation at 94 °C for 5 min, denaturing at 94 °C for 30 s, annealing temperatures, and cycle numbers as in
Table 1, extension at 72 °C for 30 s, and final extension at 72 °C for 5 min. The PCR product was separated by 1.5 % (>200bp) or 2 % (<200bp) agarose gel depending on the amplicon size and was stained with ethidium bromide. Haplotypes were constructed using population genotyping data (
Table 2).
2.5. Genotyping of SNPs and Reconstruction of Haplotypes within HSF3 Gene
Genotyping of SNPs within HSF3 was performed using AS-PCR. The primer (
Table 3) combinations of P17, P19 or P18, P19 for the SNP A-1388G; and P20,P22 or P21,P22, for the A-1703G SNP, were utilized. The PCR conditions and mixtures are detailed above. Haplotypes were constructed using genotyping data as mentioned above.
2.6. Statistical Analysis
To assess the association between egg production traits and SNPs or haplotypes, a general linear model procedure (GLM) in IBM SPSS Statistics for Windows, ver. 20.0 (IBM Corp., Armonk, NY, USA), and the following equation was employed for the analysis [
32].
Where Yij is the phenotypic value of the specific traits (e.g., egg production, EW, ASM, and BW), μ is the population mean of the target trait, Gi is the genotype effect (where I = 3 genotypes), and eij is the random residual error associated with the Yij observation. The population in the Hardy–Weinberg Equilibrium (HWE) was fixed using a χ2 test. The parameter values are presented as the least square means ± standard error, and statistical significance (least significant difference) was evaluated at P<0.05. Haplotype frequencies with a minimum threshold of >3 % were considered for the association study.
3. Results
3.1. Identification of Novel SNPs in the 5ʹ-Flanking Region of HSP70
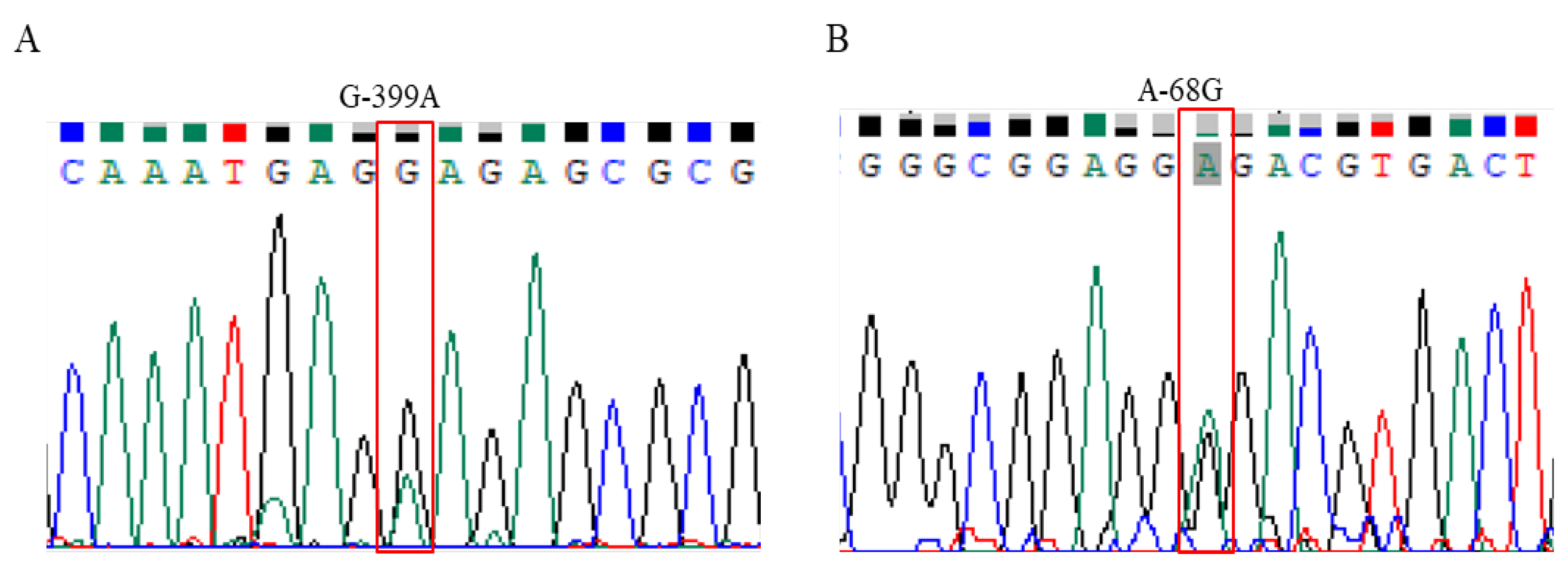
Based on a reference sequence of HSP70 from the NCBI database (
https://www.ncbi.nlm.nih.gov/; NC_052536.1), two novel SNPs, G-399A (
Table 4 and
Figure 2A) and A-68G (
Table 4 and
Figure 2B) were identified in the 729-bp length of the 5ʹ-flanking region of the HSP70 gene in Bangladeshi Hilly chickens.
3.2. Genotypic and Allelic Frequencies and Haplotype Combinations in HSP70
A total of five SNPs (
Table 4) were genotyped, including two novel SNPs, G-399A (
Figure 3A) and A-68G (
Figure 3B) as well as three previously reported SNPs, A258G (
Figure 3C), C276G (
Figure 3D), and C1431A (
Figure 3E) in the
HSP70 of Hilly chickens.
Table 4 shows the genotypic and allelic frequency for the analyzed SNPs in
HSP70. For the G-399A SNP, the wild-type GG genotype frequency (0.92) was significantly (
P<0.05) greater than those of AG (0.08) genotypes, and the frequency of the G allele (0.95) was notably greater (
P<0.05) than its A allele (0.05) counterpart. Regarding the A-68G SNP, the ratio of the AA genotype (0.67) was significantly greater (
P<0.05) compared with the AG (0.22) and GG (0.10) genotypes, and the frequency of the A allele (0.78) was greater than that of the G allele (0.22).
The genotypic frequency of AG (0.77) in the previously known SNP A258G was greater (
P<0.05) relative to the AA (0.20) and GG (0.03) genotypes. The frequency of the A allele (0.58) was comparatively greater (
P<0.05) than the G allele (0.42) for this SNP. Regarding, the C276G SNP, the CC genotype had a greater (
P<0.05) frequency (0.58) than the CG (0.39) and GG (0.03) genotypes. Additionally, an increased frequency of the C allele (0.77) was noted compared with the G allele (0.23). In the C1431A SNP of
HSP70, only two genotypes, CC (0.74) and CA (0.26), were observed with greater (
P<0.05) frequencies for the C allele (0.87) compared with the A allele (0.13). All SNPs, except C276G and C1431A, were outside the Hardy–Weinberg Equilibrium (
P<0.05). Based on the genotyping data from the five SNPs, haplotypes were constructed and 15 distinct haplotypes (H1 to H15) were identified within the study population as shown in
Table 5. The most common haplotype was H1, with a frequency of 34 %, while the frequencies of the other haplotypes ranged from 0.019 to 11.70 %.
3.3. Association between Genotypes and Egg Production Traits for HSP70
Table 6 shows the association of specific polymorphisms in the HSP70 gene with egg production traits in chickens, where significant effects were observed for certain SNPs on traits like egg number and egg weight. Notably, G-399A and A258G were linked to egg production during specific intervals, while A-68G is associated with egg weight at 40 weeks (
Table 6).
The G-399A was significantly (
P<0.05) correlated with egg number (EN) at 161–190 days of age. Birds with the GA genotype of this SNP had significantly (
P<0.05) greater EN than birds with the GG genotype. The A-68G SNP was significantly associated with EW at 40 weeks of age, and a significantly (
P<0.05) higher value was observed in birds with the mutant GG genotype compared with the AA and AG genotypes (
Table 7).
In the case of the A258G SNP, it was related (
P<0.05) to the EN at 221–250 days of age. Additionally, the AA genotype hens produced more eggs than the AG and GG genotype hens. The SNP C276G showed a significant (
P<0.05) association with the EN at 281–310 days of age, and a notably higher EN (
P<0.05) was observed in hens with the GG genotype compared with those with the CC and CG genotypes. For the SNP C1431A, it was found to be associated (
P<0.05) with EN at 191–220 days of age, and the birds with the heterozygous CA genotype produced significantly higher EN than the CC genotype birds (
Table 7).
3.4. Association of the Haplotypes for HSP70 with Egg Production Traits in the Hilly Chicken
The study considered a total of eight haplotypes (H1 to H3, and H7 to H11) with frequencies of >3 % that were used in the association analysis. These haplotypes were significantly (
P<0.05) associated with ASM, EW at ASM, EW at 40 weeks, BW at ASM, and EN. Among all these haplotypes, H11 had significantly earlier ASM (days) and greater EN at 130–160 and 161–190 days of age compared with the other haplotypes. Haplotype 2 (H2) significantly correlated with lower BW at ASM and higher EN at 191–220 days of age compared with the other haplotypes. Furthermore, H7 showed significantly higher EW values at ASM and 40 weeks compared with the other haplotypes (
Table 8).
3.5. Genotypic and Allelic Frequencies and Haplotype Combinations in HSF3
Two previously known SNPs, A-1388G (
Figure 4A) and A-1703G (
Figure 4B), in the 5ʹ-untranslated region (UTR) of the HSF3 gene, were genotyped in the studied flock.
Table 9 presents the genotype and allele frequencies and reveals that the A-1388G SNP showed only two categories of genotypes, with the dominant AA genotype (0.94) remarkably greater than the AG genotype (0.06). The percentage of allele A (0.97) was much higher compared with the G allele (0.03), and the allocation of genotypes in the studied flock did not conflict with the HWE (
P>0.05).
Regarding the A-1703G SNP, the frequency of the AA genotype was notably greater (0.91) than the AG genotype (0.09), with a significantly greater frequency of the A allele (0.96) compared with the G allele (0.04). Additionally, according to the χ2 test, this SNP deviated from the HWE (P<0.05). The genotype data was used to perform haplotype reconstruction, revealing the existence of only two haplotype categories: H1(AA) and H2(AG), among the 150 individual birds that were examined. Among these haplotypes, H1 was the most frequently observed with a frequency of 85 %, while H2 was observed in only 15 % of the individuals.
3.6. Association between Genotypes and Egg Production Traits and HSF3
The significant association analyses between the SNPs and egg production traits are shown in
Table 10. For the SNP A-1388G, a significant (
P<0.05) association was found in the EN at 130–160 days of age, with a greater value observed in the AG genotype compared with the AA genotype.
However, a significantly reduced EN was also found at 251–280 days of age for this SNP compared with the AA genotypes. Lastly, the A-1703G SNP did not show any significant (
P<0.05) correlation with egg production traits in the studied flock (
Table 11).
3.7. Association of HSF3 Haplotypes with Hilly Chicken Egg Production Traits
A significant association (
P<0.05) was observed between haplotypes and ASM and EN at different ages. Compared with Haplotype H1, Haplotype H2 exhibited significantly earlier ASM (days) and higher EN during the 130–160 days of age period. In contrast, Haplotype H1 showed a significant correlation with higher EN during the 251–280 days of age period compared with Haplotype H2 (
Table 12).
3.8. Evaluation of Combined Genotypic Effects of SNPs G-399A and A-68G in HSP70 with A-1388G SNP in HSF3 on Egg Production Traits
We analyzed the effects of combined genotypes of G-399A with A-1388G SNP and A-68G with A-1388G SNP on the phenotypic performance of the studied population. Birds with wild/heterozygote combinations for G-399A and A-1388G SNPs showed significantly (
P<0.05) earlier ASM and higher EN at 130 – 160 days of age compared to wild/wild. BW at 40 weeks, EN at 161 – 190, and 251 – 280 days of age were also significantly (
P<0.05) influenced by different combinations of these two SNPs (
Table 13).
Regarding the combined effects of A-68G and A-1388G SNPs, birds with any mutant combinations within these SNPs significantly (
P<0.05) influenced the EW compared to the combinations with wild/wild (
Table 14).
4. Discussion
High ambient temperatures negatively affect livestock reproduction, and this impact may be exacerbated by ongoing global warming, particularly for backyard poultry farms. Using heat-resistant markers in animal breeding programs proves beneficial to efforts aimed at improving productivity in hot climates [
34,
35]. Heat shock protein 70 (HSP70) is one of the most common biological response markers of thermal stress and participates in numerous physiological processes including protein folding, transportation, and assembly within the cells [
36,
37].
It also protects the cells by preventing the apoptotic pathway which might positively impact animal health and productivity [
38]. The present study investigated SNP identification in the HSP70 and HSFF3 genes which regulate the transcription of HSP70 and their association with the reproductive traits of Bangladeshi Hilly chickens.
In this study, analysis of the HSP70 gene in Hilly chickens exhibited heterogeneity in the 5ʹ-flanking region. Genetic variations observed in the 5ʹ-flanking region of the HSP70 gene are caused by the transitions of the nucleotides at positions -399 and -68 bp 5ʹ-upstream from the start codon. The 5ʹ-flanking region of HSP70 is polymorphic and several SNPs have also been reported in the broiler, Naked Neck, and Indonesian local chickens [
18,
39,
40]. Furthermore, three previously reported synonymous SNPs in the coding region of this gene (A258G, C276G, and C1431A) were also found in the studied flock. All the SNPs in HSP70, except C276G and C1431A, were outside Hardy–Weinberg equilibrium (
Table 3). This might be due to the selection and breeding strategy used to improve the studied flock.
Nevertheless, the present study revealed a significant association between the tested HSP70 SNPs and specific egg production traits among Hilly chickens (
Table 6). The novel G-399A SNP was found to be significantly correlated with greater EN (
P<0.05), and A-68G showed a significant association with increased EW (
P<0.05) (
Table 7). These associations indicate that genetic variations in HSP70 may influence egg production efficiency in chickens in hot environments. Similar effects associated with HSP70 SNPs in the 5ʹ-flanking region were previously reported in other vertebrates. This includes the finding that several SNPs in the 5ʹ-flanking region of the HSP70 gene were associated with mRNA stability, stress response, milk production, and calf weaning weight in cattle [
14,
17]. Variations in the 5ʹ-flanking region of HSP70 may alter the specific binding site of the transcription factors and could modulate the binding efficiency that affects gene functions. This could lead to changes in cellular processes and ultimately alter phenotype performance [
14,
41]. The precise mechanism of how HSP70 affects egg production in chickens remains unknown. However, a possible explanation might be that during heat stress, HSP70 regulates thermotolerance that inhibits apoptosis in ovarian cells, which may lead to protecting granulosa cells and ultimately improving folliculogenesis and egg production [
42,
43,
44]. Therefore, further physiological research is required.
Regarding the three examined synonymous SNPs of HSP70, the AA genotype of A258G had higher EN (
P<0.05) at 221–250 days compared with other genotypes. The AA genotype of the same SNP was significantly associated with improved thermotolerance and BW at an early stage, but not EW or EN until 280 days of age in Taiwanese chickens [
16]. This discrepancy might be due to differences in chicken breeds or environmental factors such as temperature and duration of heat stress exposure. Furthermore, the GG genotype of C276G SNP was significantly associated with greater EN (Table
7), and in a previous study the C276G polymorphism was found to produce a novel haplotype in combination with the A258G SNP reported as a heat stress marker in Indonesian Walik chickens [
15]. These silent mutations in the coding region of the HSP70 gene have been previously reported as heat-resistant markers in chickens [
45]. However, this earlier study was not an association study considering reproductive performance in chickens. In the present study, the C1431A SNP was significantly correlated with increased EN as shown in
Table 7. A significant association between this SNP and EW, fertility, and hatchability percentage was previously observed in Iranian Mazandaran native breeder chicken but the authors did not report the association of this SNP with EN [
33].
Although important egg production traits such as ASM, EW at ASM, and BW at ASM were not correlated with any individual HSP70 SNPs (
Table 6), the haplotype combinations resulting from five SNPs significantly affected these traits (
Table 8). This suggests that the individual effects of these SNPs are relatively small compared with their combined effects. In chickens, combined genotypes have a greater impact on BW than individual genotypes [
46].
Regarding the SNPs in the HSF3 gene, although the A-1703G SNP did not significantly affect egg production traits in the studied flock, the A-1388G SNP was found to be significantly associated with EN (
Table 11). Notably, A-1388G polymorphism has been found to change the CdxA transcription factor binding site associated with thermotolerance in Chinese Lingshan chickens [
23]. Haplotype analysis of HSF3 also revealed significant associations with several egg production traits, including EN and ASM for H2 (
Table 12). Laying hens with the H2 haplotype exhibited earlier ASM and significantly greater EN compared with hens with the H1 haplotype. However, it is noteworthy that EN was reduced at a later stage (251–280 days) for H2, indicating that this haplotype may be less advantageous for long-term egg production.
Our findings revealed significant associations between specific SNPs within the HSP70 and HSF3 genes and egg production traits in the studied population. Moreover, analyzing the combined effects of two novel SNPs (G-399A and A-68G) in HSP70 and the A-1388G SNP in HSF3 genes on phenotypic performance, we found a significant interaction that suggests that these genes may play a synergistic or compensatory role in egg production in the hot environments.
5. Conclusions
In conclusion, two novel SNPs (G-399A and A-68G) were identified in the 5ʹ-flanking region of HSP70 in Hilly chickens, and three previously known SNPs (A258G, C276G, and C1431A) of HSP70 were also existed in HSP70 in the same breed. Moreover, HSF3 also possessed two reported SNPs (A-1388G and A-1703G) in this studied breed. All the SNPs in both genes, except A-1703G in HSF3, and their corresponding haplotypes, were associated with egg production traits in Hilly chickens. These significant SNPs and haplotypes might be available in the future for molecular marker-based selection programs to enhance Hilly chickens’ egg productivity in the high ambient temperature experiences in Bangladesh. Further research is needed to elucidate the precise mechanisms enabling HSPs to improve chickens' reproductive performance.
Author Contributions
Conception and design of the study, T.O. and M.Y.A; curation of data, S.F.; formal analysis, M.Y.A; methodology, T.O.; investigation, S.A.; original draft preparation, M.Y.A; supervision, review and editing, T.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted following the rules and standards established by the Institutional Animal Ethics Executive Committee of the Bangladesh Livestock Research Institute (BLRI), Bangladesh (Approval number: AEEC/BLRI 0012/22).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors confirmed and declared no conflict of interest.
References
- Bhuiyan, A.; Bhuiyan, M.; Deb, G. Indigenous chicken genetic resources in Bangladesh: current status and future outlook. Anim. Genet. Resour. Inf. 2005, 36, 73–84. [Google Scholar] [CrossRef]
- Rashid, M.A.; Manjula, P.; Faruque, S.; Bhuiyan, A.K.F.H.; Seo, D.; Alam, J.; Lee, J.H.; Alam Bhuiyan, M.S. Genetic diversity and population structure of indigenous chicken of Bangladesh using microsatellite markers. Asian-Australasian J. Anim. Sci. 2020, 33, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Afrin, A.; Ahmed, T.; Lahiry, A.; Rahman, S.; Dey, B.; Hashem, A.; Das, S.C. Profitability and meat quality of fast-, medium- and slow-growing meat-type chicken genotypes as affected by growth and length of rearing. Saudi J. Biol. Sci. 2024, 31, 104025. [Google Scholar] [CrossRef] [PubMed]
- I Khan, M.K.; Siddiki, A.Z.; Ali, M.R.; A Akter, M. Identification of best performer hilly chickens of Bangladesh in consideration of climate change factors: light and heat. Indian J. Anim. Sci. 2017, 87, 991–995–991–995. [Google Scholar] [CrossRef]
- Chowdhury, Q.M.M.K.; Hossain, M.; Ahmed, J.; Shykat, C.A.; Islam, S.; Hasan, M. Impact of Climate Change on Livestock in Bangladesh: A Review of What We Know and What We Need to Know. Am. J. Agric. Sci. Eng. Technol. 2019, 3, 89–96. [Google Scholar] [CrossRef]
- He, S.; Arowolo, M.; Medrano, R.; Li, S.; Yu, Q.; Chen, J.; He, J. Impact of heat stress and nutritional interventions on poultry production. World's Poult. Sci. J. 2018, 74, 647–664. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, Y.-K.; Lee, S.-D.; Kim, S.-H.; Lee, S.-R.; Lee, H.-G.; Lee, K.-W. Changes in Production Parameters, Egg Qualities, Fecal Volatile Fatty Acids, Nutrient Digestibility, and Plasma Parameters in Laying Hens Exposed to Ambient Temperature. Front. Veter- Sci. 2020, 7, 412. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. HEAT-SHOCK PROTEINS, MOLECULAR CHAPERONES, AND THE STRESS RESPONSE: Evolutionary and Ecological Physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- Sawka, M.N.; Wenger, C.B.; Pandolf, K.B. Thermoregulatory responses to acute exercise-heat stress and heat acclimation. In Comprehensive Physiology; Wiley, 2011; pp. 157–185.
- Lindquist, S.; Craig, E.A. THE HEAT-SHOCK PROTEINS. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Huang, L.-Z.; Zhou, M.; Ding, Y.-F.; Zhu, C. Gene Networks Involved in Plant Heat Stress Response and Tolerance. Int. J. Mol. Sci. 2022, 23, 11970. [Google Scholar] [CrossRef]
- Kan, Y.; Mu, X.-R.; Gao, J.; Lin, H.-X.; Lin, Y. The molecular basis of heat stress responses in plants. Mol. Plant 2023, 16, 1612–1634. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman; Ahmed, I. ; Huq, S.; Mahmud, S.A.; Begum, S.; Amin, U.M.; Rahman, H.; Sarker, P.K.; Hossain, M.U.; Das, K.C.; et al. Association of polymorphism in heat shock protein 70 genes with type 2 diabetes in Bangladeshi population. Mol. Genet. Genom. Med. 2020, 8, e1073. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; Nawaz, A.; Rehman, M.S.; Ali, M.A.; Dilshad, S.M.; Yang, C. Prospects of HSP70 as a genetic marker for thermo-tolerance and immuno-modulation in animals under climate change scenario. Anim. Nutr. 2019, 5, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Aryani, A.; Solihin, D.D.; Sumantri, C.; Afnan, R.; Sartika, T. Genetic Diversity of the Structure of HSP70 Gene in Kampung Unggul Balitbangtan (KUB), Walik, and Kate Walik Chickens. Trop. Anim. Sci. J. 2019, 42, 180–188. [Google Scholar] [CrossRef]
- Liang, H.-M.; Lin, D.-Y.; Hsuuw, Y.-D.; Huang, T.-P.; Chang, H.-L.; Lin, C.-Y.; Wu, H.-H.; Hung, K.-H. Association of heat shock protein 70 gene polymorphisms with acute thermal tolerance, growth, and egg production traits of native chickens in Taiwan. Arch. Anim. Breed. 2016, 59, 173–181. [Google Scholar] [CrossRef]
- Abbas, Z.; Hu, L.; Fang, H.; Sammad, A.; Kang, L.; Brito, L.F.; Xu, Q.; Wang, Y. Association Analysis of Polymorphisms in the 5′ Flanking Region of the HSP70 Gene with Blood Biochemical Parameters of Lactating Holstein Cows under Heat and Cold Stress. Animals 2020, 10, 2016. [Google Scholar] [CrossRef] [PubMed]
- Prihandini, P.W.; Primasari, A.; Aryogi, A.; Luthfi, M.; Hariyono, D.N.H. Genetic polymorphisms of the 5' untranslated regions of the HSP70 gene in Indonesian cattle populations. Veter- World 2022, 15, 168–172. [Google Scholar] [CrossRef]
- Suhendro, I.; Noor, R.R.; Jakaria, J.; Priyanto, R.; Manalu, W.; Andersson, G. Association of heat-shock protein 70.1 gene with physiological and physical performance of Bali cattle. Veter- World 2024, 17, 17–25. [Google Scholar] [CrossRef]
- Takii, R.; Fujimoto, M.; Matsuura, Y.; Wu, F.; Oshibe, N.; Takaki, E.; Katiyar, A.; Akashi, H.; Makino, T.; Kawata, M.; et al. HSF1 and HSF3 cooperatively regulate the heat shock response in lizards. PLOS ONE 2017, 12, e0180776–e0180776. [Google Scholar] [CrossRef]
- Gouda, A.; Tolba, S.; Mahrose, K.; Felemban, S.G.; Khafaga, A.F.; Khalifa, N.E.; Jaremko, M.; Moustafa, M.; Alshaharni, M.O.; Algopish, U.; et al. Heat shock proteins as a key defense mechanism in poultry production under heat stress conditions. Poult. Sci. 2024, 103, 103537. [Google Scholar] [CrossRef]
- Shehata, A.M.; Saadeldin, I.M.; Tukur, H.A.; Habashy, W.S. Modulation of Heat-Shock Proteins Mediates Chicken Cell Survival against Thermal Stress. Animals 2020, 10, 2407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-W.; Kong, L.-N.; Zhang, D.-X.; Ji, C.-L.; Zhang, X.-Q.; Luo, Q.-B. Effect of the C.–1 388 A>G polymorphism in chicken heat shock transcription factor 3 gene on heat tolerance. J. Integr. Agric. 2015, 14, 1808–1815. [Google Scholar] [CrossRef]
- Wang, X.-H.; Yu, H.-L.; Zou, W.-B.; Mi, C.-H.; Dai, G.-J.; Zhang, T.; Zhang, G.-X.; Xie, K.-Z.; Wang, J.-Y. Study of the Relationship between Polymorphisms in the IL-8 Gene Promoter Region and Coccidiosis Resistance Index in Jinghai Yellow Chickens. Genes 2020, 11, 476. [Google Scholar] [CrossRef] [PubMed]
- Padhi, M.K. Importance of Indigenous Breeds of Chicken for Rural Economy and Their Improvements for Higher Production Performance. Scientifica 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oke, O.E.; Akosile, O.A.; Uyanga, V.A.; Oke, F.O.; Oni, A.I.; Tona, K.; Onagbesan, O.M. Climate change and broiler production. Veter- Med. Sci. 2024, 10, e1416. [Google Scholar] [CrossRef] [PubMed]
- Apalowo, O.O.; Ekunseitan, D.A.; Fasina, Y.O. Impact of Heat Stress on Broiler Chicken Production. Poultry 2024, 3, 107–128. [Google Scholar] [CrossRef]
- Oluwagbenga, E.; Fraley, G. Heat stress and poultry production: a comprehensive review. Poult. Sci. 2023, 102, 103141. [Google Scholar] [CrossRef] [PubMed]
- Perini, F.; Cendron, F.; Rovelli, G.; Castellini, C.; Cassandro, M.; Lasagna, E. Emerging Genetic Tools to Investigate Molecular Pathways Related to Heat Stress in Chickens: A Review. Animals 2020, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Juiputta, J.; Chankitisakul, V.; Boonkum, W. Appropriate Genetic Approaches for Heat Tolerance and Maintaining Good Productivity in Tropical Poultry Production: A Review. Veter- Sci. 2023, 10, 591. [Google Scholar] [CrossRef]
- Onagbesan, O.M.; Uyanga, V.A.; Oso, O.; Tona, K.; Oke, O.E. Alleviating heat stress effects in poultry: updates on methods and mechanisms of actions. Front. Veter- Sci. 2023, 10, 1255520. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, L.; Zhao, X.; Ran, J.; Wang, Y.; Yin, H.; Li, D.; Zhu, Q. Analysis of Expression and Single Nucleotide Polymorphisms of INHA Gene Associated with Reproductive Traits in Chickens. BioMed Res. Int. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Rouhi, M.; Mokhtari, R.; Kazemi, H. Genetic analysis of a novel polymorphism in coding region of HSP70 gene and its association with some productive and reproductive traits in Mazandaran native breeder hens. J. Genet. Disord. Genet. Med. 2019, 2, 1–5. [Google Scholar]
- Deb, R.; Sajjanar, B.; Singh, U.; Kumar, S.; Brahmane, M.; Singh, R.; Sengar, G.; Sharma, A. Promoter variants at AP2 box region of Hsp70.1 affect thermal stress response and milk production traits in Frieswal cross bred cattle. Gene 2013, 532, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Ratwan, P.; Dahiya, S.; Nehra, A.K. Climate change and heat stress: Impact on production, reproduction and growth performance of poultry and its mitigation using genetic strategies. J. Therm. Biol. 2021, 97, 102867. [Google Scholar] [CrossRef]
- Singh, M.K.; Shin, Y.; Ju, S.; Han, S.; Choe, W.; Yoon, K.-S.; Kim, S.S.; Kang, I. Heat Shock Response and Heat Shock Proteins: Current Understanding and Future Opportunities in Human Diseases. Int. J. Mol. Sci. 2024, 25, 4209. [Google Scholar] [CrossRef] [PubMed]
- Archana, P.R.; Aleena, J.; Pragna, P.; Vidya, M.K.; Abdul Niyas, P.A.; Bagath, M.; Krishnan, G.; Manimaran, A.; Beena, V.; Kurien, E.K.; et al. Role of Heat Shock Proteins in Livestock Adaptation to Heat Stress. J. Dairy Veter- Anim. Res. 2017, 5, 00127. [Google Scholar] [CrossRef]
- Grepper, D.; Tabasso, C.; Zanou, N.; Aguettaz, A.K.; Castro-Sepulveda, M.; Ziegler, D.V.; Lagarrigue, S.; Arribat, Y.; Martinotti, A.; Ebrahimi, A.; et al. BCL2L13 at endoplasmic reticulum-mitochondria contact sites regulates calcium homeostasis to maintain skeletal muscle function. iScience 2024, 27, 110510. [Google Scholar] [CrossRef]
- Galal, A.; Radwan, L.M. Identification of single nucleotide polymorphism in heat shock protein HSP70 and HSP90 after four selection generations in two lines of chickens. Ann. Agric. Sci. 2020, 65, 124–128. [Google Scholar] [CrossRef]
- Galal, A.; Radwan, L.M.; Rezik, H.H.; Ayoub, H. Expression levels of HSP70 and CPT-1 in three local breeds of chickens reared under normal or heat stress conditions after the introduction of the naked neck gene. J. Therm. Biol. 2018, 80, 113–118. [Google Scholar] [CrossRef]
- Basiricò, L.; Morera, P.; Primi, V.; Lacetera, N.; Nardone, A.; Bernabucci, U. Cellular thermotolerance is associated with heat shock protein 70.1 genetic polymorphisms in Holstein lactating cows. Cell Stress Chaperon- 2011, 16, 441–448. [Google Scholar] [CrossRef]
- Sirotkin, A.V. Effect of two types of stress (heat shock/high temperature and malnutrition/serum deprivation) on porcine ovarian cell functions and their response to hormones. J. Exp. Biol. 2010, 213, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, J.; Luo, M.; Sun, Y.; Wang, G. The effect of heat stress on gene expression, synthesis of steroids, and apoptosis in bovine granulosa cells. Cell Stress Chaperon- 2016, 21, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Hu, M.; Gu, L.; Lei, M.; Chen, Z.; Zhu, H.; Chen, R. Effect of Heat Stress on Egg Production, Steroid Hormone Synthesis, and Related Gene Expression in Chicken Preovulatory Follicular Granulosa Cells. Animals 2022, 12, 1467. [Google Scholar] [CrossRef] [PubMed]
- Budi, T.; Singchat, W.; Tanglertpaibul, N.; Thong, T.; Panthum, T.; Noito, K.; Wattanadilokchatkun, P.; Jehangir, M.; Chaiyes, A.; Wongloet, W.; et al. Research Note: Possible influence of thermal selection on patterns of HSP70 and HSP90 gene polymorphisms in Thai indigenous and local chicken breeds and red junglefowls. Poult. Sci. 2024, 103, 103503. [Google Scholar] [CrossRef]
- Cao, Z.P.; Wang, S.Z.; Wang, Q.G.; Wang, Y.X.; Li, H. Association of Spot14α Gene Polymorphisms with Body Weight in the Chicken. Poult. Sci. 2007, 86, 1873–1880. [Google Scholar] [CrossRef]
Figure 1.
The study is conducted at the Bangladesh Livestock Research Institute (BLRI).
Figure 1.
The study is conducted at the Bangladesh Livestock Research Institute (BLRI).
Figure 2.
Determination of SNPs in the 5´-flanking region of the HSP70 gene by sequencing. (A) The unique SNP G-399A was detected as multiple peaks at the same positions of 399 bp upstream from the start codon. (B) The unique SNP A-68G was detected as multiple peaks at the same positions of 68 bp upstream from the start codon.
Figure 2.
Determination of SNPs in the 5´-flanking region of the HSP70 gene by sequencing. (A) The unique SNP G-399A was detected as multiple peaks at the same positions of 399 bp upstream from the start codon. (B) The unique SNP A-68G was detected as multiple peaks at the same positions of 68 bp upstream from the start codon.
Figure 3.
The electrophoresis image of AS-PCR for 5 SNPs including two novel SNPs (G-399A and A-68G) and three known in the coding region (CDs) of the HSP70 gene. (A) Image of AS-PCR for G-399A SNP generated 300bp. Samples 1,2,5 represent wild GG while 3,4 for AG genotypes respectively. M shows a 1kb DNA ladder marker. (B) Photograph of AS-PCR for the A-68G SNP, which produced 630 bp. AA (Sample 1, 2), and AG (Sample 3, 4 & 6) while GG (Sample 5) represent mutated homozygous genotypes. M shows a 1kb DNA ladder marker. (C) Photograph of AS-PCR for the A258G SNP produced 600bp. AA (Sample 1) represented wild type, while AG (Sample 2) indicates heterozygous, and mutated homozygous GG represented in Sample 3 respectively. M shows a 1kb DNA ladder marker. (D) The image of AS-PCR for the C276G SNP yielded 616 bp. Mutated GG (Sample 4) genotypes while, CC (Sample 1&3), and CG (Sample 2) indicate wild and heterozygous genotypes respectively. M shows a 1kb DNA ladder marker. (E) Picture of AS-PCR for the SNP of C1431A created 198bp. Heterozygous CA (Sample 1-3) genotype while CC (Sample 4) represents a wild genotype. M shows a 100bp DNA ladder marker.
Figure 3.
The electrophoresis image of AS-PCR for 5 SNPs including two novel SNPs (G-399A and A-68G) and three known in the coding region (CDs) of the HSP70 gene. (A) Image of AS-PCR for G-399A SNP generated 300bp. Samples 1,2,5 represent wild GG while 3,4 for AG genotypes respectively. M shows a 1kb DNA ladder marker. (B) Photograph of AS-PCR for the A-68G SNP, which produced 630 bp. AA (Sample 1, 2), and AG (Sample 3, 4 & 6) while GG (Sample 5) represent mutated homozygous genotypes. M shows a 1kb DNA ladder marker. (C) Photograph of AS-PCR for the A258G SNP produced 600bp. AA (Sample 1) represented wild type, while AG (Sample 2) indicates heterozygous, and mutated homozygous GG represented in Sample 3 respectively. M shows a 1kb DNA ladder marker. (D) The image of AS-PCR for the C276G SNP yielded 616 bp. Mutated GG (Sample 4) genotypes while, CC (Sample 1&3), and CG (Sample 2) indicate wild and heterozygous genotypes respectively. M shows a 1kb DNA ladder marker. (E) Picture of AS-PCR for the SNP of C1431A created 198bp. Heterozygous CA (Sample 1-3) genotype while CC (Sample 4) represents a wild genotype. M shows a 100bp DNA ladder marker.
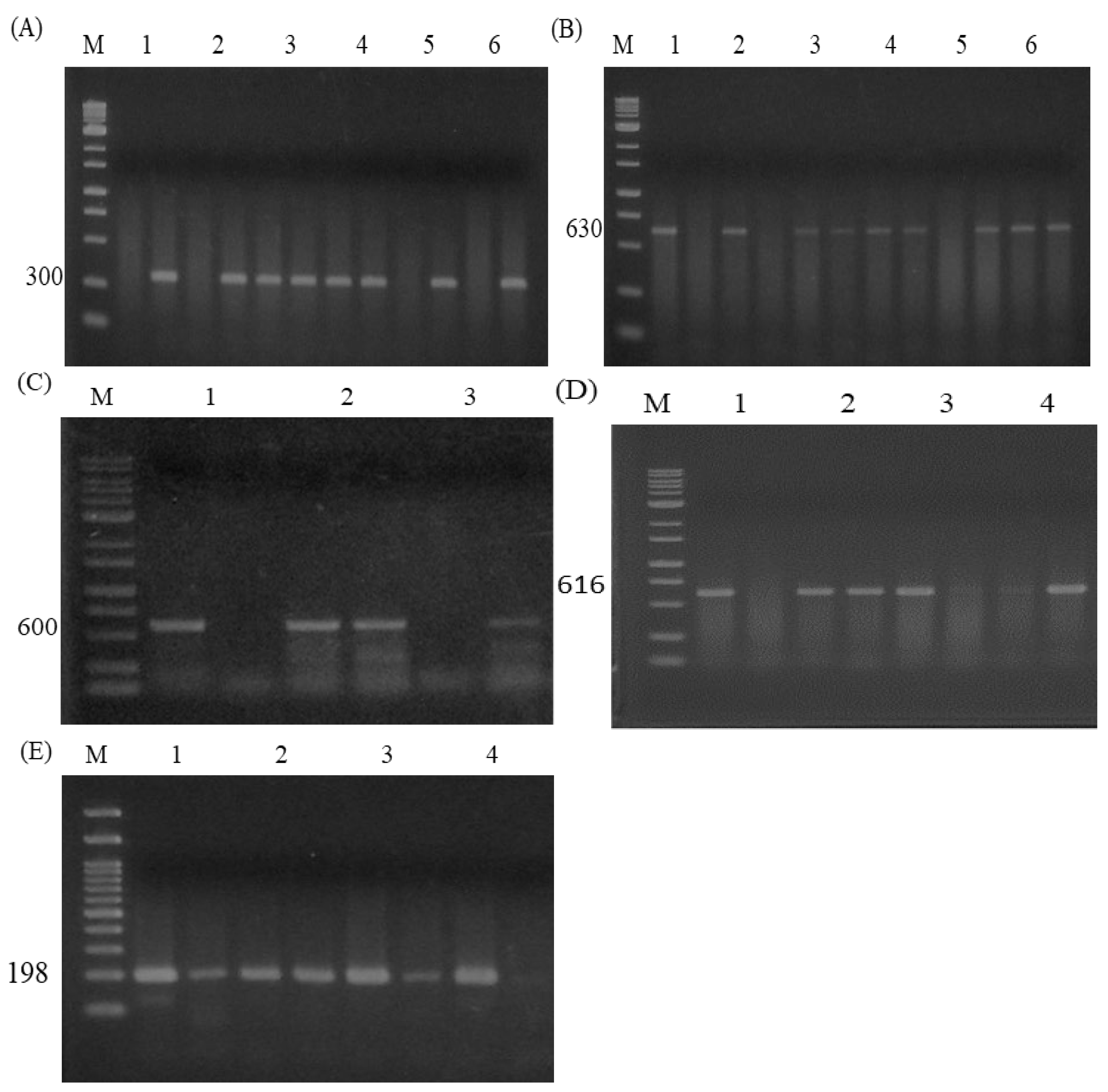
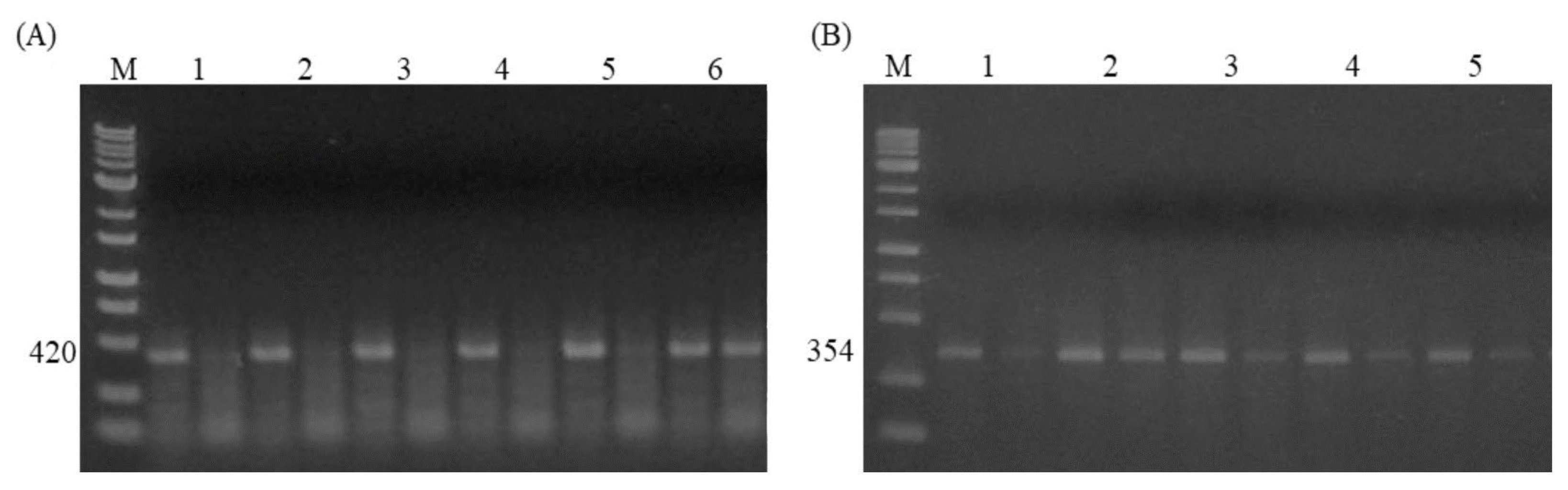
Figure 4.
The electrophoresis image of AS-PCR for two SNPs in the 5´-UTR region of HSF3 gene (A) Photograph of AS-PCR for the A-1388G SNP produced 420bp. Only two categorized genotypes, AA (Samples 1-5) and AG (Sample 6) were found. M shows a 1kb DNA ladder marker. (B) Image of AS-PCR for the SNP A-1703G produced 354bp. Samples 1,3,4,5, represent AA while sample 2 indicates AG genotype. M shows a 1kb DNA ladder marker.
Figure 4.
The electrophoresis image of AS-PCR for two SNPs in the 5´-UTR region of HSF3 gene (A) Photograph of AS-PCR for the A-1388G SNP produced 420bp. Only two categorized genotypes, AA (Samples 1-5) and AG (Sample 6) were found. M shows a 1kb DNA ladder marker. (B) Image of AS-PCR for the SNP A-1703G produced 354bp. Samples 1,3,4,5, represent AA while sample 2 indicates AG genotype. M shows a 1kb DNA ladder marker.
Table 1.
Primer sequences for sequencing and genotyping of HSP70 gene.
Table 1.
Primer sequences for sequencing and genotyping of HSP70 gene.
| No |
Primer name |
Sequence (5´ to 3´) |
Anneal. Temp. |
PCR cycle |
Product length |
P1
P2
P3
P4
P5
P6
P7
P8 |
HSP70_R_S1_G
HSP70_R_S1_A
HSP70_R_S2_A
HSP70_R_S2_G
HSP70_F_Com
HSP70_R
HSP70_F_Seq
HSP70_R_Seq |
CCAATCACAACGCGCTCTC
CCAATCACAACGCGCTCTT
TCGCTCGCAGTCACGTCT
TCGCTCGCAGTCACGTCC
AGAAGTTGTGTGAGTCGCGA
AATACGTGGTGCCCAGATCG
GTCGCGACCAAATAAGGGTA
GTGCCCAGATCGATGCCGATG |
64 |
30
30
30 |
300
630
729
|
P9
P10
P11
P12
P13 |
HSP70_R_S1_A
HSP70_R_S1_G
HSP70_R_S2_C
HSP70_R_S2_G
HSP70_F_Com |
GAAGGGCCAGTGCTTCATGTCT
GAAGGGCCAGTGCTTCATGTCC
CCTCGTTCACCACACGGAAG CCTCGTTCACCACACGGAAC
CGATCTGGCTGCAATCTACG |
60
58 |
30
30 |
600
616
|
P14
P15
P16 |
HSP70_R_S3_C
HSP70_R_S3_A
HSP70_S3_F_Com |
CTATGTCAAAAGTGACCTCG CTATGTCAAAAGTGACCTCT AGCGTAACACCACCATTC |
55 |
30 |
198 |
Table 2.
Haplotype construction using 5 SNPs in the HSP70 gene and their frequencies.
Table 2.
Haplotype construction using 5 SNPs in the HSP70 gene and their frequencies.
| Haplotype |
Position of SNP |
Frequency |
| |
G-399A |
A-68G |
A258G |
C276G |
C1431A |
|
| H1 |
G |
A |
A |
C |
C |
0.340 |
| H2 |
G |
A |
A |
C |
A |
0.039 |
| H3 |
G |
A |
G |
G |
C |
0.078 |
| H4 |
G |
A |
G |
C |
A |
0.029 |
| H5 |
A |
A |
A |
C |
C |
0.029 |
| H6 |
G |
A |
A |
G |
C |
0.029 |
| H7 |
G |
G |
A |
C |
C |
0.087 |
| H8 |
G |
A |
G |
G |
A |
0.068 |
| H9 |
G |
A |
G |
C |
C |
0.117 |
| H10 |
G |
G |
G |
C |
C |
0.039 |
| H11 |
G |
G |
G |
C |
A |
0.039 |
| H12 |
G |
G |
G |
G |
A |
0.019 |
| H13 |
G |
G |
A |
G |
A |
0.029 |
| H14 |
G |
G |
A |
C |
A |
0.029 |
| H15 |
G |
G |
G |
G |
C |
0.029 |
Table 3.
Primer sequences for genotyping of HSF3 gene.
Table 3.
Primer sequences for genotyping of HSF3 gene.
| No |
Primer name |
Sequence (5´ to 3´) |
Anneal. Temp. |
PCR cycle |
Product length |
P17
P18
P19 |
HSF3_F_S1_A
HSF3_F_S1_G HSF3_S1_R_Com |
GTCCCCATAATACCTCCCCA
GTCCCCATAATACCTCCCCG
TTTTAGCTGCCAGTTCCTTT |
60 |
25 |
354 |
P20
P21
P22 |
HSF3_R_S2_ A
HSF3_R_S2_G HSF3_S2_F_Com |
TTTTAGCTGCCAGTTCCTTT
TTTTAGCTGCCAGTTCCTTC
AAGAATGGCTCCTTGCCACC |
59 |
30 |
420 |
Table 4.
Genotypic and allelic frequencies with Hardy-Weinberg equilibrium test at the SNPs locus of the HSP70 gene.
Table 4.
Genotypic and allelic frequencies with Hardy-Weinberg equilibrium test at the SNPs locus of the HSP70 gene.
| SNPs |
Genotype frequency |
Allele frequency |
χ2 (HWE)
|
P-value |
| G-399A |
GG |
AG |
AA |
G |
A |
|
|
| |
0.92(137) |
0.08(12) |
– |
0.95 |
0.05 |
7.44 |
P<0.01 |
| A-68G |
AA |
AG |
GG |
A |
G |
|
|
| |
0.67(72) |
0.22(24) |
0.10(11) |
0.78 |
0.22 |
11.68 |
P<0.01 |
| A258G |
AA |
AG |
GG |
A |
G |
|
|
| |
0.20(28) |
0.77(113) |
0.03(5) |
0.58 |
0.42 |
50.44 |
P<0.00 |
| C276G |
CC |
CG |
GG |
C |
G |
|
|
| |
0.58(86) |
0.39(58) |
0.03(5) |
0.77 |
0.23 |
1.63 |
P>0.05 |
| C1431A |
CC |
CA |
AA |
C |
A |
|
|
| |
0.74(110) |
0.26(39) |
– |
0.87 |
0.13 |
3.42 |
P>0.05 |
Table 5.
Analyzed Single-Nucleotide Polymorphisms( SNPs) in the Chicken HSP70 gene.
Table 5.
Analyzed Single-Nucleotide Polymorphisms( SNPs) in the Chicken HSP70 gene.
| SNP name |
Mutation |
Location |
Genomic position |
| G-399A |
G>A* |
5'-flanking |
52383334 |
| A-68G |
A>G* |
5'-flanking |
52383665 |
| A258G |
A>G |
Coding |
[15] |
| C276G |
C>G |
Coding |
[15] |
| C1431A |
C>A |
Coding |
[33] |
Table 6.
Association of Polymorphisms in HSP70 Gene with egg production traits.
Table 6.
Association of Polymorphisms in HSP70 Gene with egg production traits.
| SNPs |
Traits (P value of significant test) |
ASM
(days) |
BW at ASM |
EW at ASM |
EW at
40 WK |
EN
130-160d |
EN
161-190d |
EN
191-220d |
EN
221-250d |
EN
251-280d |
EN
281-310d |
| G-399A |
0.890 |
0.148 |
0.129 |
0.660 |
0.578 |
0.020 |
0.421 |
0.218 |
0.484 |
0.225 |
| A-68G |
0.365 |
0.537 |
0.707 |
0.009 |
0.547 |
0.520 |
0.478 |
0.444 |
0.413 |
0.186 |
| A258G |
0.543 |
0.593 |
0.426 |
0.960 |
0.561 |
0.563 |
0.590 |
0.016 |
0.377 |
0.805 |
| C276G |
0.382 |
0.246 |
0.275 |
0.795 |
0.568 |
0.304 |
0.759 |
0.262 |
0.222 |
0.050 |
| C1431A |
0.121 |
0.464 |
0.651 |
0.855 |
0.969 |
0.681 |
0.012 |
0.058 |
0.341 |
0.363 |
Table 7.
Genotypic effects of SNPs in HSP70 gene on egg production traits.
Table 7.
Genotypic effects of SNPs in HSP70 gene on egg production traits.
| SNPs |
Traits |
Genotypes (Mean ± SE) |
P value |
| G-399A |
EN at 161–190d |
GG
16.27±0.26b
|
AG
18.83±0.86a
|
AA
– |
0.020 |
| A-68G |
EW at 40 wk |
AA
46.56±0.11b
|
AG
47.03±0.18a
|
GG
47.30±0.27a
|
0.009 |
| A258G |
EN at 221–250d |
AA
16.00±0.19a
|
AG
14.82±0.63b
|
GG
14.60±1.53ab
|
0.016 |
| C276G |
EN at 281–310d |
CC
13.01±0.24ab
|
CG
12.67±0.29b
|
GG
13.60±0.93a
|
0.050 |
| C1431A |
EN at 191–220d |
CC
16.49±0.21b
|
CA
17.61±0.39a
|
AA
– |
0.012 |
Table 8.
Association of haplotypes of the HSP70 polymorphisms with egg production traits in Hilly chicken.
Table 8.
Association of haplotypes of the HSP70 polymorphisms with egg production traits in Hilly chicken.
| Haplotypes |
Traits (Mean ± SE) |
| |
ASM |
EW at ASM |
EW at 40 wk |
BW at ASM |
EN
130-160d |
EN
161-190d |
EN
191-220d |
| H1(GAACC) |
160.71±1.61ab
|
25.85±0.52b
|
46.812±0.16ab
|
1728.52±24.49b
|
2.00±0.74b
|
15.91±0.54b
|
16.03±0.39b
|
| H2(GAACA) |
156.75±4.68 ab
|
26.00±0.74 ab
|
45.915±0.46b
|
1543.75±77.24a
|
4.50±2.15ab
|
17.25±1.58 ab
|
18.75±1.13a
|
| H3(GAGGC) |
157.00±5.41 ab
|
26.62±0.52 ab
|
46.356±0.33b
|
1648.25±54.62ab
|
4.25±1.53 ab
|
16.25±1.12 ab
|
16.75±0.80 ab
|
| H7(GGACC) |
161.44±3.12 ab
|
27.00±0.49 a
|
47.342±0.31a
|
1839.55±51.49c
|
4.33±1.44 ab
|
14.66±1.05 ab
|
16.44±0.75 ab
|
| H8(GAGGA) |
165.28±3.54b
|
25.85±0.55 ab
|
46.526±0.35 ab
|
1688.85±58.39abc
|
1.71±1.63 ab
|
15.57±1.19 ab
|
17.57±0.86 ab
|
| H9(GAGCC) |
158.88±3.12 ab
|
26.33±0.49 ab
|
46.399±0.31b
|
1778.88±51.49c
|
2.66±1.43 ab
|
17.22±1.05 ab
|
17.11±0.75 ab
|
| H10(GGGCC) |
159.25±4.68 ab
|
26.75±0.74 ab
|
47.125±0.46 ab
|
1711.25±77.24 abc
|
2.75±2.15 ab
|
16.75±1.58ab
|
16.75±1.13 ab
|
| H11(GGGCA) |
153.25±4.68a
|
27.00±0.73 ab
|
46.853±0.46ab
|
1685.25±77.24 abc
|
7.00±2.16a
|
18.25±1.58a
|
18.25±1.13ab
|
Table 9.
Genotypic and allelic frequencies with Hardy-Weinberg equilibrium (HWE) test at the SNPs locus of HSF3 gene.
Table 9.
Genotypic and allelic frequencies with Hardy-Weinberg equilibrium (HWE) test at the SNPs locus of HSF3 gene.
| SNPs |
Genotype frequency |
Allele frequency |
χ2 (HWE)
|
P-value |
| A-1388G |
AA |
AG |
GG |
A |
G |
|
|
| |
0.94(141) |
0.06(9) |
– |
0.97 |
0.03 |
0.143 |
P>0.05 |
| A-1703G |
AA |
AG |
GG |
A |
G |
|
|
| |
0.91(136) |
0.09(14) |
– |
0.96 |
0.04 |
6.88 |
P<0.01 |
Table 10.
Effects of SNPs in HSF3 gene on egg production.
Table 10.
Effects of SNPs in HSF3 gene on egg production.
| SNPs |
Traits |
Genotypes (mean ± SE) |
P value |
| A-1388G |
|
AA |
AG |
– |
|
| |
EN at 130–160d
EN at 251–280d |
2.85±0.36a
11.04±0.25a
|
5.55±1.22b
8.82±0.84b
|
–
– |
0.037
0.013 |
Table 11.
Association of polymorphisms in HSF3 gene with egg production traits.
Table 11.
Association of polymorphisms in HSF3 gene with egg production traits.
| SNPs |
Traits (P value of significant test) |
ASM
(days) |
BW at ASM(g) |
EW at ASM(g) |
EW at 40 wk(g) |
EN
130-160d |
EN
161-190d |
EN
191-220d |
EN
221-250d |
EN
251-280d |
EN
281-310d |
| A-1388G |
0.071 |
0.948 |
0.679 |
0.450 |
0.037 |
0.803 |
0.083 |
0.913 |
0.013 |
0.200 |
| A-1703G |
0.363 |
0.484 |
0.406 |
0.510 |
0.731 |
0.572 |
0.342 |
0.569 |
0.598 |
0.818 |
Table 12.
Association of haplotypes in HSF3 gene with egg production traits in Hilly chicken.
Table 12.
Association of haplotypes in HSF3 gene with egg production traits in Hilly chicken.
| Haplotypes |
Traits (Mean ± SE) |
| |
ASM |
EW at ASM |
EW at 40 wk |
BW at ASM |
EN
130-160d |
EN
161-190d |
EN
251-280d |
| H1(AA) |
159.95±0.76b
|
26.14±0.14 |
46.77±0.08 |
1746.38±14.54 |
2.76±0.35b
|
16.48±0.27 |
11.03±.25a
|
| H2(AG) |
155.44±1.78a
|
25.96±0.33 |
46.85±0.18 |
1704.08±34.18 |
4.60±0.82a
|
16.61±0.64 |
9.52±.59b
|
Table 13.
Combined genotypic effects of two SNP(G-399A) in HSP70 and SNP(A-1388G) in HSF3 genes on productive and reproductive performances.
Table 13.
Combined genotypic effects of two SNP(G-399A) in HSP70 and SNP(A-1388G) in HSF3 genes on productive and reproductive performances.
| Parameter |
Genotype (Mean±SE) |
P value |
WildxWild(GGxAA)
0.84(120) |
WildxHet(GGxAG)
0.06(9) |
HetxWild(AGxAA)
0.08(11) |
MutxWild(AAxAA)
0.01(2) |
| ASM(d) |
160.08±0.79b
|
154.11±2.90 a
|
156.0±2.63 ab
|
157.0±6.16 ab
|
0.05 |
| EW at ASM(g) |
26.16±0.15 |
25.56±0.53 |
25.91±0.48 |
27.0±1.13 |
0.28 |
| BW at ASM(g) |
1748.71±14.11 |
1730.89±51.51 |
1672.91±46.60 |
1715.0±109.27 |
0.74 |
| BW at 40 Wks |
2047.77±24.65 a
|
1947.78±90.0 ab
|
1838.73±81.41 b
|
2260.5±190.92 a
|
0.02 |
| EW at 40 Wks |
46.78±0.08 |
46.39±0.30 |
46.95±0.28 |
46.72±0.65 |
0.23 |
| EN at 130-160d |
2.71±0.36 b
|
6.11±1.33 a
|
3.64±1.20 ab
|
3.49±2.82 ab
|
0.02 |
| EN at 161-190d |
16.26±0.28 b
|
16.89±1.02 ab
|
18.91±0.92 a
|
16.50±2.16 ab
|
0.01 |
| EN at 191-220d |
16.85±0.20 |
15.44±0.73 |
16.45±0.66 |
18.5±1.55 |
0.07 |
| EN at 221-250d |
15.13±0.19 |
15.0±0.71 |
14.91±0.64 |
12.50±1.51 |
0.86 |
| EN at 251-280d |
11.14±0.25 a
|
8.56±0.92 b
|
10.18±0.84 ab
|
12.5±1.96 ab
|
0.01 |
| EN at 281-310d |
13.0±0.19 |
13.67±0.69 |
11.91±0.63 |
13.0±1.48 |
0.36 |
Table 14.
Combined genotypic effects of A-68G SNP in HSP70 and A-1388G SNP of HSF3 genes on productive and reproductive performance.
Table 14.
Combined genotypic effects of A-68G SNP in HSP70 and A-1388G SNP of HSF3 genes on productive and reproductive performance.
| Parameter |
Combined genotypes(Mean±SE) |
P value |
WildxWild(AAxAA)
0.62(65) |
WildxHet(AAxAG)
0.07(7) |
HetxWild(AGxAA)
0.22(23) |
MutxWild(GGxAA)
0.09(10) |
| ASM(d) |
160.56±1.11 |
156.42±3.29 |
160.18±1.86 |
164.0±2.76 |
0.37 |
| EW at ASM(g) |
26.27±0.18b
|
25.85±0.56 b
|
26.09±0.31 b
|
27.4±0.47 a
|
0.03 |
| BW at ASM(g) |
1731.06±20.81 |
1757.85±61.92 |
1763.36±34.93 |
1834.1±51.81 |
0.31 |
| BW at 40 Wks(g) |
2008.54±33.73 |
2075.0±100.38 |
2027.14±56.62 |
2093.5±83.98 |
0.77 |
| EW at 40 Wks(g) |
46.56±0.12 b
|
46.49±0.34 ab
|
47.07±0.19 a
|
47.33±0.29 a
|
0.03 |
| EN at 130 to 160 d |
2.17±0.48 |
4.43±1.42 |
3.0±0.80 |
2.1±1.19 |
0.43 |
| EN at 161 to 190 d |
16.45±0.42 |
16.86±1.26 |
16.27±0.71 |
14.9±1.05 |
0.55 |
| EN at 191 to 220 d |
16.66±0.28 |
16.43±0.84 |
16.95±0.48 |
16.5±0.70 |
0.92 |
| EN at 221 to 250 d |
15.05±0.28 |
15.0±0.84 |
15.36±0.47 |
14.7±0.70 |
0.88 |
| EN at 251 to 280 d |
11.56±0.37 a
|
8.57±1.09 b
|
10.32±0.62 ab
|
10.5±0.92 ab
|
0.01 |
| EN at 281 to 310 d |
13.08±0.23 b
|
14.57±0.69 a
|
12.45±0.39 b
|
12.9±0.58 ab
|
0.04 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).