Submitted:
11 October 2024
Posted:
14 October 2024
You are already at the latest version
Abstract
Keywords:
Introduction
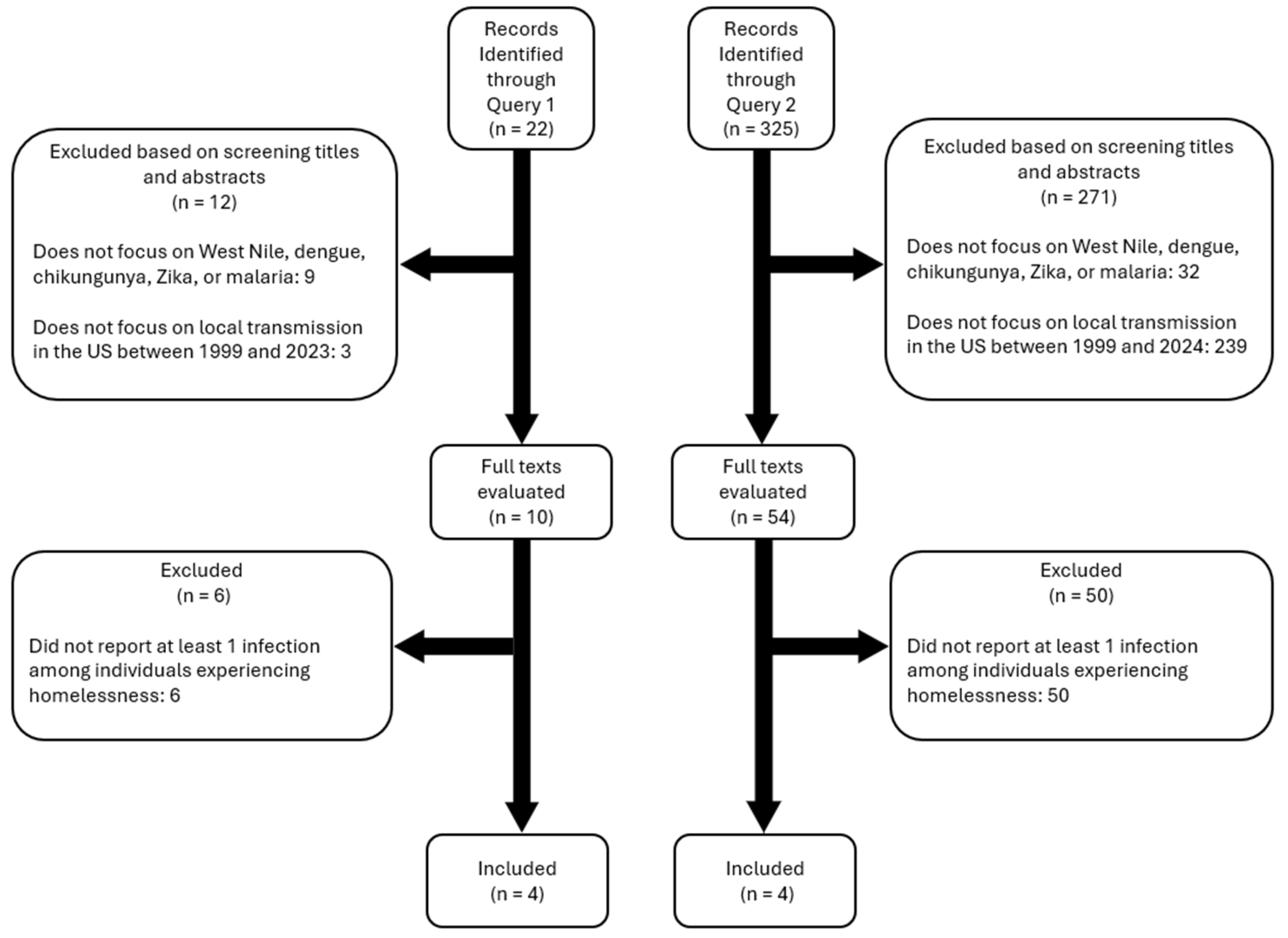
Methods
Diseases of Interest
Search Queries
- Query 1: (homeless* OR "transient population" OR "transient communit*") AND (mosquito* OR arbovir* OR dengue OR malaria OR Zika OR chikungunya OR "West Nile" OR DENV OR CHIKV OR ZIKV OR WNV) AND ("United States" OR "USA")
- Query 2: (Outbreak OR Epidemic) AND (locally acquired OR autochthonous) AND (Mosquito-borne OR Mosquito-Transmitted OR arbovir* OR dengue OR malaria OR Zika OR chikungunya OR "West Nile" OR DENV OR CHIKV OR ZIKV OR WNV) AND ("United States" OR "USA")
Inclusion and Exclusion Criteria
Scope of Review and Analysis
Data Availability
Results
Discussion
Funding
Conflict of Interest
References
- Mutebi, J.-P.; Wilke, A.B.B.; Ostrum, E.; Vasquez, C.; Cardenas, G.; Carvajal, A. , et al. Diel activity patterns of two distinct populations of Aedes aegypti in Miami, FL and Brownsville, TX. Sci Rep. 2022;12: 5315. [CrossRef]
- Smith, M.; Dixon, D.; Bibbs, C.; Autry, D.; Xue, R. De. Diel patterns of Aedes aegypti (Diptera: Culicidae) after resurgence in St. Augustine, Florida as collected by a mechanical rotator trap. Journal of Vector Ecology. 2018;43: 201–204.
- Martin, E.; Medeiros, M.C.I.; Carbajal, E.; Valdez, E.; Juarez, J.G.; Garcia-Luna, S. , et al. Surveillance of Aedes aegypti indoors and outdoors using Autocidal Gravid Ovitraps in South Texas during local transmission of Zika virus, 2016 to 2018. Acta Trop. 2019;192: 129–137. [CrossRef]
- Koyoc-Cardeña, E.; Medina-Barreiro, A.; Cohuo-Rodríguez, A.; Pavía-Ruz, N.; Lenhart, A.; Ayora-Talavera, G. , et al. Estimating absolute indoor density of Aedes aegypti using removal sampling. Parasit Vectors. 2019;12: 250. [CrossRef]
- Mutebi, J.P.; Hughes, H.R.; Burkhalter, K.L.; Kothera, L.; Vasquez, C.; Kenney, J.L. Zika virus MB16-23 in mosquitoes, Miami-Dade County, Florida, USA, 2016. Emerg Infect Dis. 2018;24: 808–810. [CrossRef]
- Ventura, P.C.; Wilke, A.B.B.; Chitturi, J.; Kummer, A.G.; Agrawal, S.; Vasquez, C. , et al. Unveiling the role of mosquito and human diel activity patterns in the risk of mosquito-borne disease infection. medRxiv. 2024. [Google Scholar] [CrossRef]
- Meyer, T.E.; Bull, L.M.; Holmes, K.C.; Pascua, R.F.; Travassos da Rosa, A.; Gutierrez, C.R. , et al. West Nile Virus Infection among the Homeless, Houston, Texas. Emerg Infect Dis. 2007;13: 1500–1503. [CrossRef]
- Molaei, G.; Andreadis, T.G.; Armstrong, P.M.; Bueno, R.; Dennett, J.A.; Real, S.V. , et al. Host feeding pattern of Culex quinquefasciatus (Diptera: Culicidae) and its role in transmission of West Nile virus in Harris County, Texas. American Journal of Tropical Medicine and Hygiene. 2007;77: 73–81. [CrossRef]
- LaDeau, S.; Leisnham, P.; Biehler, D.; Bodner, D. Higher mosquito production in low-income neighborhoods of Baltimore and Washington, DC: Understanding ecological drivers and mosquito-borne disease risk in temperate cities. Int J Environ Res Public Health. 2013;10: 1505–1526. [CrossRef]
- Rothman, S.E.; Jones, J.A.; Ladeau, S.L.; Leisnham, P.T. Higher West Nile virus infection in Aedes albopictus (Diptera: Culicidae) and Culex (Diptera: Culicidae) mosquitoes from lower income neighborhoods in urban Baltimore, MD. J Med Entomol. 2021;58: 1424–1428. [CrossRef]
- Ajelli, M.; Moise, I.K.; Hutchings, T.C.S.G.; Brown, S.C.; Kumar, N.; Johnson, N.F. , et al. Host outdoor exposure variability affects the transmission and spread of Zika virus: Insights for epidemic control. PLoS Negl Trop Dis. 2017;11: e0005851. [CrossRef]
- US Department of Housing and Urban Development. The 2020 Annual Homeless Assessment Report to Congress, Part 1: Point-In-Time Estimates of Homelessness. Washington, DC; 2021.
- Petrovich, J.C.; Cronley, C.C. Deep in the heart of Texas: A phenomenological exploration of unsheltered homelessness. American Journal of Orthopsychiatry. 2015;85: 315–323. [CrossRef]
- Hopper, K.; Shinn, M.; Laska, E.; Meisner, M.; Wanderling, J. Estimating numbers of unsheltered homeless people through plant-capture and postcount survey methods. Am J Public Health. 2008;98: 1438–1442. [CrossRef]
- US Department of Housing and Urban Development. The 2023 Annual Homeless Assessment Report to Congress, Part 1: Point-in-Time Estimates of Homelessness. Washington, DC: U.S. Department of Housing and Urban Development.
- Olivet, J.; Wilkey, C.; Richard, M.; Dones, M.; Tripp, J.; Beit-Arie, M. , et al. Racial inequity and homelessness: Findings from the SPARC Study. Ann Am Acad Pol Soc Sci. 2021;693: 82–100. [CrossRef]
- Geller, A.; Curtis, M.A. A sort of homecoming: Incarceration and the housing security of urban men. Soc Sci Res. 2011;40: 1196–1213. [CrossRef]
- Fargo, J.; Metraux, S.; Byrne, T.; Munley, E.; Montgomery, A.E.; Jones, H. , et al. Prevalence and risk of homelessness among US veterans. Prev Chronic Dis. 2012;9: E45. [CrossRef]
- Culhane, D.P.; Metraux, S.; Byrne, T.; Stino, M.; Bainbridge, J. The age structure of contemporary homelessness: Evidence and implications for public policy. Analyses of Social Issues and Public Policy. 2013;13: 228–244. [CrossRef]
- Roncarati, J.S.; Baggett, T.P.; O’Connell, J.J.; Hwang, S.W.; Cook, E.F.; Krieger, N. , et al. Mortality among unsheltered homeless adults in Boston, Massachusetts, 2000-2009. JAMA Intern Med. 2018;178: 1242. [CrossRef]
- Levitt, A.J.; Culhane, D.P.; DeGenova, J.; O’Quinn, P.; Bainbridge, J. Health and social characteristics of homeless adults in Manhattan who were chronically or not chronically unsheltered. Psychiatric Services. 2009;60: 978–981.
- Montgomery, A.E.; Szymkowiak, D.; Marcus, J.; Howard, P.; Culhane, D.P. Homelessness, unsheltered status, and risk factors for mortality. Public Health Reports. 2016;131: 765–772.
- Nyamathi, A.M.; Leake, B.; Gelberg, L. Sheltered versus nonsheltered homeless women. J Gen Intern Med. 2000;15: 565–572. [CrossRef]
- Byrne, T.; Montgomery, A.E.; Fargo, J.D. Unsheltered homelessness among veterans: Correlates and profiles. Community Ment Health J. 2016;52: 148–157. [CrossRef]
- Fazel, S.; Geddes, J.R.; Kushel, M. The health of homeless people in high-income countries: Descriptive epidemiology, health consequences, and clinical and policy recommendations. The Lancet. 2014;384: 1529–1540. [CrossRef]
- Anderson, M.-C.; Hazel, A.; Perkins, J.; Almquist, Z. The ecology of unsheltered homelessness: Environmental and social-network predictors of well-being among an unsheltered homeless population. Int J Environ Res Public Health. 2021;18: 7328. [CrossRef]
- Centers for Disease Control and Prevention (CDC). Arbovirus Catalog. Available at: https://wwwn.cdc.gov/arbocat/.
- Zardini, A.; Menegale, F.; Gobbi, A.; Manica, M.; Guzzetta, G.; D’Andrea, V. , et al. Estimating the potential risk of transmission of arboviruses in the Americas and Europe: A modelling study. Lancet Planet Health. 2024;8: e30–e40. [CrossRef]
- Monaghan, A.J.; Eisen, R.J.; Eisen, L.; McAllister, J.; Savage, H.M.; Mutebi, J.P. , et al. Consensus and uncertainty in the geographic range of Aedes aegypti and Aedes albopictus in the contiguous United States: Multi-model assessment and synthesis. PLoS Comput Biol. 2019;15: 1–19. [CrossRef]
- Wilke, A.B.B.; Vasquez, C.; Beier, J.C. Presence and abundance of malaria vector species in Miami-Dade County, Florida. Malar J. 2023; 1–8. [CrossRef]
- Levine, R.S.; Peterson, A.T.; Benedict, M.Q. Distribution of members of Anopheles quadrimaculatus say s.l. (Diptera: Culicidae) and implications for their roles in malaria transmission in the United States. J Med Entomol. 2004;41: 607-13. [CrossRef]
- Murray, K.O.; Baraniuk, S.; Resnick, M.; Arafat, R.; Kilborn, C.; Cain, K. , et al. Risk factors for encephalitis and death from West Nile virus infection. Epidemiol Infect. 2006;134: 1325–1332. [CrossRef]
- Blackburn, D.; Drennon, M.; Broussard, K.; Morrison, A.M.; Stanek, D.; Sarney, E. , et al. Outbreak of locally acquired mosquito-transmitted (autochthonous) malaria - Florida and Texas, May-July 2023. MMWR Morb Mortal Wkly Rep. 2023;72: 973-978.
- Conway, K.L.; Jasuja, R.M.; Hauser, N.E.; Foley, J.E. Benefits, companion animal zoonotic disease prevalence and public perceptions of pet ownership among people experiencing homelessness in northern California. Zoonoses Public Health. 2022;69: 806–815. [CrossRef]
- Centers for Disease Control and Prevention, Filler SJ, MacArthur JR, Parise M, Wirtz R, Eliades MJ, et al. Locally acquired mosquito-transmitted malaria: a guide for investigations in the United States. MMWR Recomm Rep. 2006;55: 1-9.
- Eliades, M.J.; Shah, S.; Nguyen-Dinh, P.; Newman, R.D.; Barber, A.M.; Nguyen-Dinh, P. , et al. Malaria surveillance--United States, 2003. MMWR Surveill Summ. 2005;54: 25–40.
- Centers for Disease Control and Prevention (CDC). Local transmission of Plasmodium vivax malaria--Palm Beach County, Florida, 2003. MMWR Morb Mortal Wkly Rep. 2003;52: 908-11.
- Leibler, J.H.; Zakhour, C.M.; Gadhoke, P.; Gaeta, J.M. Zoonotic and vector-borne infections among urban homeless and marginalized people in the United States and Europe, 1990–2014. Vector-Borne and Zoonotic Diseases. 2016;16: 435–444. [CrossRef]
- Perez, M.T.; Morand, J.; Bush, L.M.; Crankshaw, K.; Sudduth, N.C. Hematological laboratory findings in patients of an autochthonous Plasmodium vivax Malaria Outbreak. Lab Med. 2004;35: 420–426.
- Centers for Disease Control and Prevention (CDC). West Nile virus disease cases reported to CDC by state of residence. Available at: https://www.cdc.gov/west-nile-virus/data-maps/index.html.
- Ramin, B.; Svoboda, T. Health of the homeless and climate change. Journal of Urban Health. 2009;86: 654–664. [CrossRef]
- Centers for Disease Control and Prevention (CDC). Chikungunya disease cases reported to CDC by state of residence. Available at: https://www.cdc.gov/chikungunya/data-maps/chikungunya-us.html.
- Centers for Disease Control and Prevention (CDC). Zika disease cases reported to CDC by state of residence. Available at: https://www.cdc.gov/zika/zika-cases-us/index.html.
- Centers for Disease Control and Prevention (CDC). Dengue disease cases reported to CDC by state of residence. Available at: https://www.cdc.gov/dengue/areaswithrisk/in-the-us.html.
- Shankar, M.B.; Rodríguez-Acosta, R.L.; Sharp, T.M.; Tomashek, K.M.; Margolis, H.S.; Meltzer, M.I. Estimating dengue under-reporting in Puerto Rico using a multiplier model. PLoS Negl Trop Dis. 2018;12: e0006650. [CrossRef]
- Walter Reed Biosystematics Unit. Systematic Catalog of Culicidae. Available at: https://wrbu.si.edu.
- Centers for Disease Control and Prevention. Arbovirus Catalog. Available at: https://wwwn.cdc.gov/Arbocat/Default.aspx.
- World Health Organization. Handbook for integrated vector management. World Health Organization; 2012.
- Lizzi, K.M.; Qualls, W.A.; Brown, S.C.; Beier, J.C. Expanding Integrated Vector Management to promote healthy environments. Trends Parasitol. 2014;30: 394–400. [CrossRef]
- Lindsay, S.W.; Jawara, M.; Paine, K.; Pinder, M.; Walraven, G.E.L.; Emerson, P.M. Changes in house design reduce exposure to malaria mosquitoes. Tropical Medicine and International Health. 2003;8: 512–517. [CrossRef]
- Centers for Disease Control and Prevention (CDC). West Nile Virus in the United States: Guidelines for Surveillance, Prevention. Available at: https://www.cdc.gov/westnile/resources/pdfs/wnvGuidelines.pdf.
- Byrne, T.; Huang, M.; Nelson, R.E.; Tsai, J. Rapid rehousing for persons experiencing homelessness: A systematic review of the evidence. Hous Stud. 2021; 1–27. [CrossRef]
- Rog, D.J.; Marshall, T.; Dougherty, R.H.; George, P.; Daniels, A.S.; Ghose, S.S. , et al. Permanent supportive housing: Assessing the evidence. Psychiatric Services. 2014;65: 287–294. [CrossRef]
- Baxter, A.J.; Tweed, E.J.; Katikireddi, S.V.; Thomson, H. Effects of Housing First approaches on health and well-being of adults who are homeless or at risk of homelessness: Systematic review and meta-analysis of randomised controlled trials. J Epidemiol Community Health. 2019;73: 379–387. [CrossRef]
- Padgett, D.K.; Herman, D. From Shelters to hotels: An enduring solution to ending homelessness for thousands of Americans. Psychiatric Services. 2021;72: 986–987. [CrossRef]
- Colburn, G. .; Fyall, R.., Ed.; Thompson, S., et al. Impact of hotels as non-congregate emergency shelters: An analysis of investments in hotels as emergency shelter in King County, WA during the COVID-19 pandemic. University of Washington. November 2020. Available at: https://kcrha.org/wp-content/uploads/2020/11/Impact-of-Hotels-as-ES-Study_Full-Report_Final-11302020.pdf. [Google Scholar]
- Rosenberg, R.; Lindsey, N.P.; Fischer, M.; Gregory, C.J.; Hinckley, A.F.; Mead, P.S. , et al. Vital Signs: Trends in reported vectorborne disease cases - United States and Territories, 2004-2016. MMWR Morb Mortal Wkly Rep. 2018;67: 496-501.
- Reiner, R.C.; Perkins, T.A.; Barker, C.M.; Niu, T.; Chaves, L.F.; Ellis, A.M. , et al. A systematic review of mathematical models of mosquito-borne pathogen transmission: 1970–2010. J R Soc Interface. 2013;10: 20120921. [CrossRef]
- Smith, D.L.; Perkins, T.A.; Reiner, R.C.; Barker, C.M.; Niu, T.; Chaves, L.F. , et al. Recasting the theory of mosquito-borne pathogen transmission dynamics and control. Trans R Soc Trop Med Hyg. 2014;108: 185–197. [CrossRef]
- Lega, J.; Brown, H.E.; Barrera, R. A 70% Reduction in mosquito populations does not require removal of 70% of mosquitoes. J Med Entomol. 2020;57: 1668–1670. [CrossRef]
- Morin, C.W.; Monaghan, A.J.; Hayden, M.H.; Barrera, R.; Ernst, K. Meteorologically driven simulations of dengue epidemics in San Juan, PR. PLoS Negl Trop Dis. 2015;9: e0004002. [CrossRef]
- Puggioni, G.; Couret, J.; Serman, E.; Akanda, A.S.; Ginsberg, H.S. Spatiotemporal modeling of dengue fever risk in Puerto Rico. Spat Spatiotemporal Epidemiol. 2020;35: 100375. [CrossRef]
- Tjaden, N.B.; Caminade, C.; Beierkuhnlein, C.; Thomas, S.M. Mosquito-borne Diseases: advances in modelling climate-change impacts. Trends Parasitol. 2018;34: 227–245. [CrossRef]
- Gibb, R.; Colón-González, F.J.; Lan, P.T.; Huong, P.T.; Nam, V.S.; Duoc, V.T. , et al. Interactions between climate change, urban infrastructure and mobility are driving dengue emergence in Vietnam. Nat Commun. 2023;14: 8179. [CrossRef]
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl Trop Dis. 2019;13: e0007213. [CrossRef]
- Caminade, C.; Kovats, S.; Rocklov, J.; Tompkins, A.M.; Morse, A.P.; Colón-González, F.J. , et al. Impact of climate change on global malaria distribution. Proceedings of the National Academy of Sciences. 2014;111: 3286–3291. [CrossRef]
- Wilke, A.B.B.; Chang, N.B.; Townsend, J.; Benelli, G.; Ajelli, M. Unintended effects of urban policies on the risk of arbovirus transmission. Trends Parasitol. 2023;39: 1001-1003. [CrossRef]
| Reference | Title | Relevant findings for homelessness and MBDs |
|---|---|---|
| Murray et al. [32] | Risk factors for encephalitis and death from West Nile virus infection. | Individuals experiencing homelessness showed a higher likelihood of hospitalization due to West Nile compared to the general population. |
| Meyer et al. [7] | West Nile virus infection among the homeless, Houston, Texas. | Factors associated with an increased risk of West Nile infection were found to be chronic homelessness and >6 hours outdoor exposure to mosquitoes. |
| Blackburn et al. [33] | Outbreak of locally acquired mosquito-transmitted (autochthonous) malaria - Florida and Texas, May-July 2023. | Eight cases of locally acquired Plasmodium vivax malaria were reported in Florida and Texas. Three of the eight patients were reported experiencing homelessness. |
| Conway et al. [34] | Benefits, companion animal zoonotic disease prevalence and public perceptions of pet ownership among people experiencing homelessness in northern California. | Five out of 42 samples taken from dogs of unhoused owners tested positive for West Nile virus. |
| Eliades et al. [36] | Malaria surveillance--United States, 2003 | In 2003, eight cases of locally transmitted malaria were reported in Palm Beach County, Florida. The 8 affected individuals were found to spend extended periods outdoors, including one homeless individual. |
| CDC et al. [37] | Local transmission of Plasmodium vivax malaria--Palm Beach County, Florida, 2003 | Examines seven cases of Plasmodium vivax malaria that occurred in Palm Beach County, Florida, during July and August 2003. One case involved a man who had been sleeping in an outdoor homeless camp near a canal. |
| Leibler et al. [38] | Zoonotic and vector-borne infections among urban homeless and marginalized people in the United States and Europe, 1990-2014. | Serological evidence of exposure to pathogens, including Rickettsia typhi, West Nile virus, and Seoul hantavirus in individuals experiencing homelessness. HIV infection, injection drug use, and heavy drinking were common risk factors for these infections. |
| Perez et al. [39] | Hematological laboratory findings in patients of an autochthonous Plasmodium vivax malaria outbreak | Hematological findings of the 2003 malaria outbreak in Palm Beach County, Florida, showed that all 8 reported cases, one of which is an individual experiencing homelessness, carried the same strain of Plasmodium vivax. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





