1. Introduction
Intestinal parasitic infections are caused by intestinal helminths and protozoa [
1]. They are mostly prevalent in developing countries and are diseases of poor people belonging to Neglected Tropical Diseases (NTDs) affecting around 1.7 billion people worldwide [
2,
3,
4]. They may cause different health conditions such as epigastric pain, diarrhea, abdominal pain, vomiting, discomfort, and burping even though a person can leave with intestinal parasites without knowing that they have them [
5].
Ascaris lumbricoides, Hookworm (
Ancylostoma duodenale), whipworm (
Trichuris trichiura), Taenia spp., and
Schistosoma mansoni were the focus of this study. Intestinal nematodes (
A. lumbricoides,
T. trichiura, and Hookworms) are extremely widespread in sub-Saharan Africa, China, and East Asian countries, causing 1.97 million DALYs globally and are most prevalent in children, as reported by the global burden of disease [
6].
Schistosomiasis is a debilitating infection due to parasites of the genus Schistosoma. These are blood-dwelling helminths with five different species that affect humans:
S. mansoni,
S haematobium,
S. japonicum,
S. mekongi, and
S. intercalatum.(ref)
S. mansoni was reported to be responsible for 1.64 million DALYs in 2019 [
7].
Intestinal parasitosis and schistosomiasis are responsible for high morbidity and mortality worldwide, especially in developing countries, owing to inadequate sanitation, unsafe water sources, and poverty (limited resources), which results in difficulties in the implementation of control measures [
8,
9]. They cause iron deficiency anemia; approximations show that 740 million people worldwide are infected with Hookworms, which is a clinical cause of iron deficiency anemia due to the consumption of iron in the intestine [
10]. Intestinal parasites also cause growth retardation in children, physical and mental problems that affect their studies [
11].
Intestinal parasites affect the social well-being and finance of the population, as well as the economy of the country [
11]. In 2021, the World Health Organization (WHO)reported that, 1.15 billion people globally required preventive chemotherapy for intestinal parasites and Schistosoma including 914.3 and 251.3 million people for soil-transmitted helminths and schistosoma, respectively. It has been reported that 461.3 million (40%) people who required preventive chemotherapy are from Africa [
12]. Worldwide chemotherapy coverage is 55.96% and 29.94% for soil-transmitted helminths and schistosoma, respectively. This coverage is relatively low in Africa, at 33.62% and 31.20%, respectively [
12].
Studies conducted in different countries among different groups of people have shown that intestinal helminthiasis is a problem faced by the population. For instance, a review conducted in Africa demonstrated that the prevalence of intestinal parasites was 25.8% among school children [
13]. In Madagascar, in a survey of stunted children aged two and five years, intestinal parasites were prevalent at 96.3% [
14]. In a District in Ethiopia, 48.7% of children under five years of age were infested with intestinal parasites [
15].
The Rwanda National Survey of 2008 presented the distribution of soil-transmitted helminths among schoolchildren in the Huye district and reported a prevalence of 61%, 31%, and 13% for
A. lumbricoides, hookworms, and
T. trichiura, respectively [
16]. Another study conducted in the Bugesera district reported a prevalence of 47.5% of intestinal parasites, with 86.7%, 8.9%, and 4.4% of
A. lumbricoides,
T. trichiura, and hookworms, respectively [
17]. A cross-sectional survey conducted in Rutsiro District, Rwanda, in a population aged two years and below revealed that the prevalence of soil-transmitted helminths was 44.8% [
18].
Intestinal helminthiases and schistosomiasis have been associated with different factors such as inadequate preparation of vegetables and fruits [
15,
19,
20], not practicing hand washing [
19,
20,
21,
22], inadequate knowledge of the mode of transmission of intestinal parasites, [
19,
22], open defecation [
15], not wearing shoes [
15], having many family members [
15], drinking unsafe water [
14,
18,
21], malnutrition [
23], residence [
22], and the age of the child [
14,
24,
25,
26,
27] can influence intestinal helminthiases [
28].
Schistosomiasis have been associated with different factors such as living in proximity to freshwater body, swimming in open waterbodies, age, poverty, access to potable water and safe human excreta disposal [
29,
30,
31].This study aimed to assess the prevalence of intestinal helminthiases, schistosomiasis, and associated factors among preschool-aged children (PSAC) in Rwanda.
Intestinal helminthiases and schistosomiasis continue to become public health issues in developing countries. The United Nations Sustainable Development Goal 3, which emphasizes good health and well-being, highlights the prevention, control, and elimination and/or eradication of NTDs, including intestinal helminths and schistosomiasis, by 2030 [
32]. The WHO has established a roadmap that will help to eliminate NTDs by 2030, and there is hope to achieve this if there is a multilateral partnership and country ownership. Kigali Declaration on NTDs committed to reducing the number of people requiring intervention for NTDs, including intestinal helminthiases and schistosomiasis, by 90% [
4].
There are few publications on the status of intestinal helminthiases and schistosomiasis in Rwanda, particularly among preschool-aged children. Additionally, information on factors that may be associated with disease trends at the national level in this age group is scarce. The current study aimed to determine the prevalence of helminthiases and schistosomiasis, investigate their spatial distribution, and identify factors potentially associated with helminthiases and schistosomiasis among preschool-aged children in Rwanda.
2. Materials and Methods
This was an analytical cross-sectional study and a secondary analysis of the data collected in a study entitled “Prevalence mapping of Schistosoma mansoni among preschool-aged children in Rwanda.”
Seventeen [
17] Districts located in five provinces of Rwanda were included in this study. According to the mother study, the districts were chosen as endemic areas of Schistosoma mansoni. These are Bugesera, Gatsibo, Kayonza, Ngoma, Nyagatare, and Rwamagana in the Eastern province; Gasabo in the City of Kigali; Gicumbi, and Musanze in the Northern province; Karongi, Nyamasheke, Rubavu, Rusizi and Rutsiro in the Western province; and Gisagara, Kamonyi, and Nyanza in the Southern province. Being endemic to schistosomiasis in this study meant that the district was assessed for schistosomiasis in schoolchildren, and the prevalence was found to be 10% or greater.
This study focused on preschool-aged children (PSAC) in a schistosomiasis-endemic area. The sample size was comprised of 4675 children aged 7-68 months old that were enrolled in the mother study from the selected villages. All participants in the maternal study were included in the secondary data analysis.
Villages were purposively selected according to their proximity to water bodies or wetlands. From the mother study, the districts that are endemic to schistosomiasis were identified, as well as sectors that have water bodies and wetlands so that the population is exposed were identified too. In each targeted sector, two villages were selected purposively depending on their proximity to the water body or wetland. The children who participated in this study were randomly selected. All the children who participated in the mother study were also part of this study.
Intestinal helminths (Ascaris, Trichuris, ankylostoma, and tenia) and schistosoma were dichotomous dependent variables. Dependent variables were added to form a new categorical variable that justified the outcome. To confirm that the child had an intestinal helminth or schistosoma (positive outcome), there might have been at least one intestinal helminth or schistosoma per child. The absence of any intestinal helminths or schistosoma was considered a negative outcome. This variable was recorded as a new variable (parasite), which was a binary variable, where 0 was used for the absence of parasites and 1 for the presence of parasites.
Independent variables or factors included sociodemographic factors (age, gender (sex), residence, parent occupation, child caregiver during the day, and stunting), WASH Factors (fetching water into the lake or water body, bathing in the lake or water body), and environmental factors such as rainfall, temperature, land cover, soil pH, and soil type.
This secondary data analysis used the existing data. The data collected for the primary purpose were as follows: First, a structured questionnaire was developed to guide interviews on the risk factors that may be associated with intestinal helminthiases and schistosomiasis. This questionnaire was administered to the children’s guardians after they agreed to participate in the study. Second, laboratory tests were performed on the collected stool samples of each participating child. Parasites in stools were detected using Kato Katz’s laboratory techniques [
33]. Third, a document review was performed to determine other possible environmental factors that could potentially influence the prevalence and distribution of these parasites. Environmental factors values were extracted from GIS software for the study area, where other data were collected. These variables are essential for understanding the ecological factors contributing to the distribution of intestinal helminths and schistosoma. Environmental variables (precipitation, temperature, soil pH, land cover, and soil type) were extracted at specific sampling points using the "Extract Multiple Values to Points" tool in ArcGIS. These points were based on samples collected at the village level (village centroids were used), enabling a localized understanding of the spatial distribution patterns of the variables. The values generated for each environmental variable were organized into a tabular format. This table, which contains the environmental variable values, forms the basis for further analysis. By exporting this table, we created a structured dataset that was ready for subsequent statistical and correlation analyses. A prevalence distribution map was produced from the data aggregated at the village level (village prevalence was mapped).
Descriptive analysis was performed on the data. Prevalence was assessed as a measure of frequency. The results are presented as frequencies and percentages. Odds ratios were assessed as a measure of the association between independent variables and the outcome of interest (parasites). The chi-square test was used to test the statistical relationship between parasites and factors (independent variables). logistic regression analysis was conducted to identify significant variables linked to the outcome; multivariable logistic regression analysis was conducted. The decision was made based on the p-value (alpha). The confidence level was set at 95%. For a p-value less than 0.05, the two variables were considered to be associated. Stata 15 software was used to perform statistical analysis, and the output tables were transformed into tables using Excel. ArcGIS was used to produce the prevalence distribution map.
Ethical approval was provided by the Institutional Review Board (IRB) of the University of Rwanda at the College of Medicine and Health Sciences. This study was conducted to determine the benefits to the population. Data were kept confidential.
3. Results
3.1. Description of Socio-Demographic, WASH, and Environmental Characteristics
Table 1 illustrates the survey participants according to sociodemographic factors, WASH, and environmental characteristics. In total, 4675 preschool-aged children (PSAC) were enrolled in this study. Among them, 49.56% (n = 2317) were females and 50.44% (n = 2358) were males. More than 81% of the children were older than two years of age. A large proportion of the PSAC (90.31%, n = 4062) had parents who were farmers. 74.62% (n = 3479) were cared for by their mothers during the day. Some PSAC (34.16%, n = 1597) were stunted. The highest proportions of 83.32% (n = 3895) and 63.76% (n = 2937) of PSAC-fetched water were in the lake and the water body and bathed in the lake or water body, respectively. A small proportion (9.03%, n = 422) of the PSAC lived in regions of high rainfall. The proportion of PSAC who lived in regions with normal LST was 41.41% (n = 1936). Most of the PSAC (81.13%; n = 3793) lived in regions with clay soil type. All children lived in acidic regions (pH less than 6.5), and 65.09% (n=3043) of the PSAC lived in regions reserved for agricultural activities.
3.2. Prevalence of Intestinal Parasites and Schistosomiasis among Preschool-Aged Children
Table 2 illustrates the prevalence of schistosomiasis, Ascaris, ankylostoma,
Trichirus trichura, and tenia and the overall prevalence of parasites (either an intestinal parasite or schistosoma). The overall prevalence of intestinal parasites and schistosoma infections was 20.3%. Ascaris had the highest prevalence: 15%, Trichuris trichiura was prevalent at 7.7%, Ankylostoma was prevalent at 1.5, Schistosoma at 0.8%, and taenia and other parasites were prevalent at 0.2% each (
Table 2).
3.3. Distribution of Intestinal Parasites and Schistosomiasis among Preschool-Aged Children in Rwanda
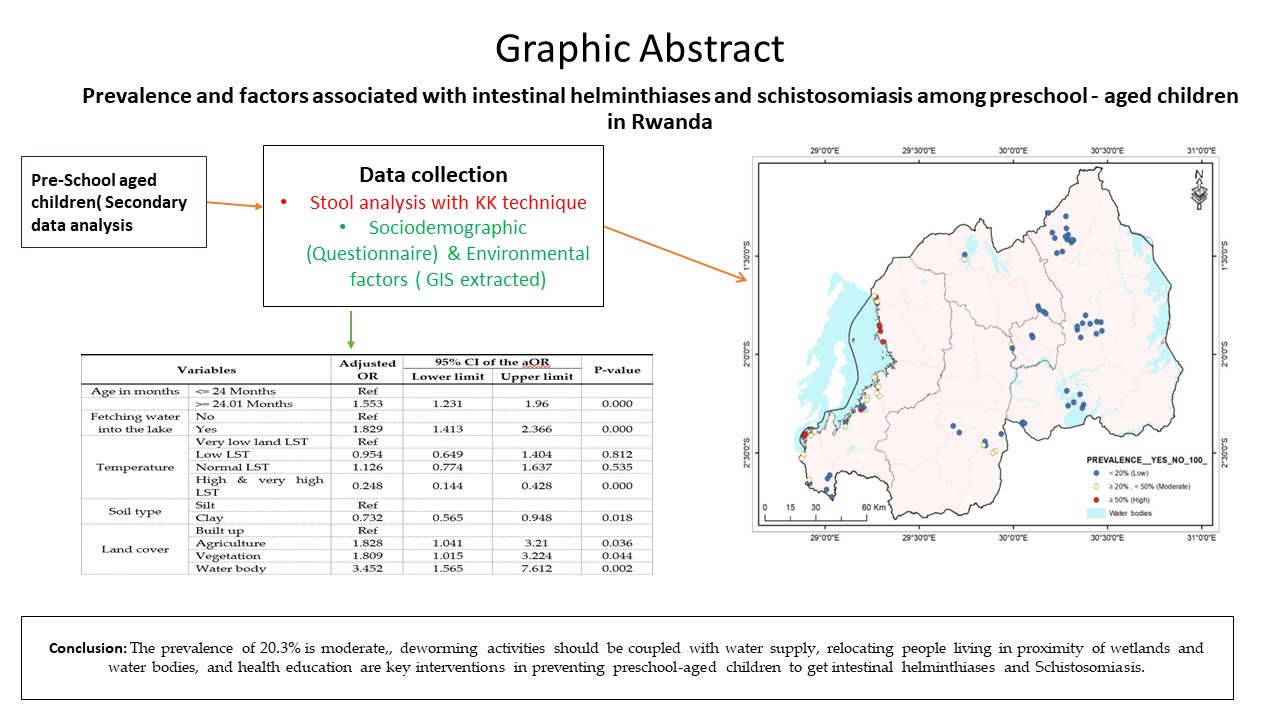
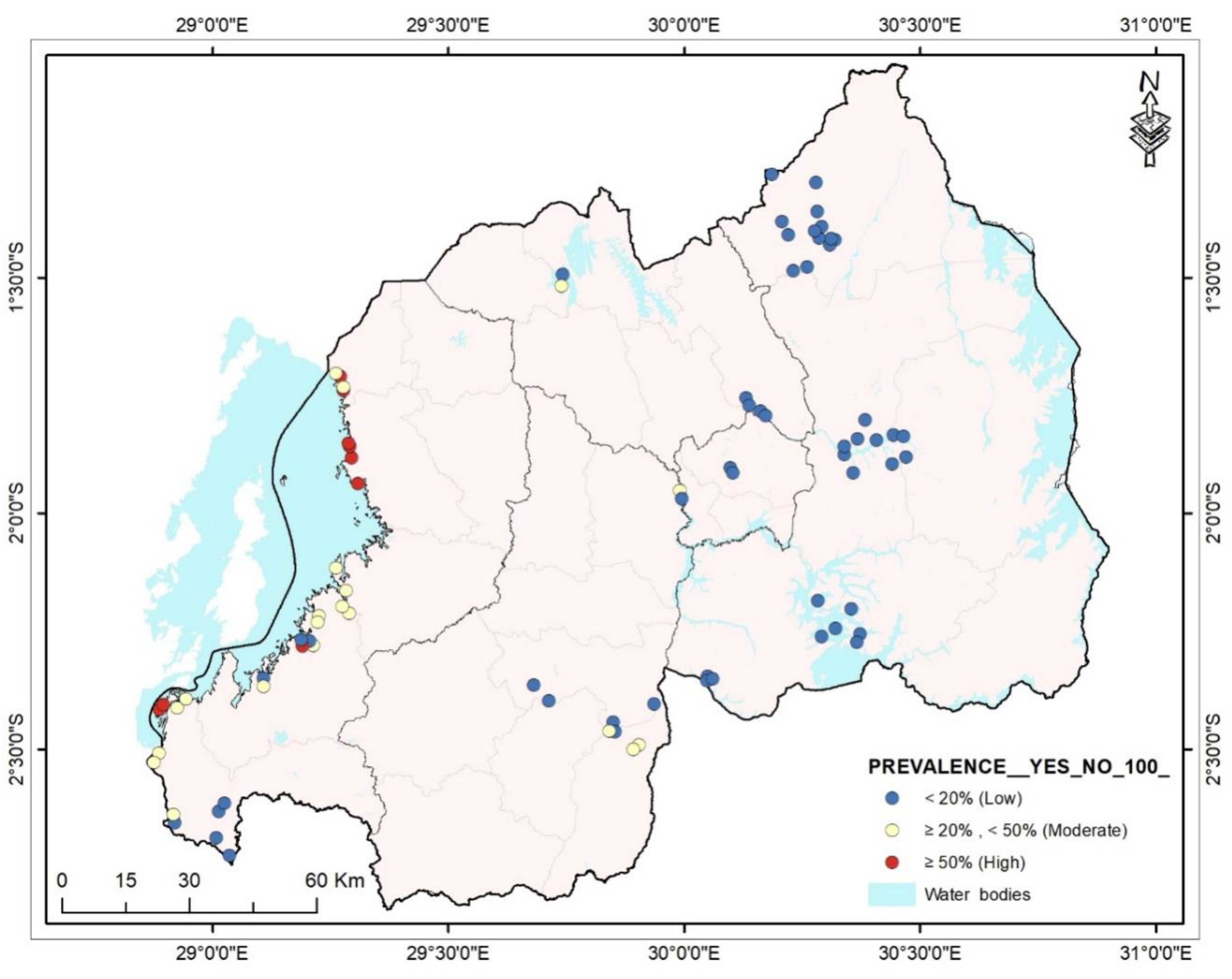
The distributions of intestinal helminthiases and schistosomiasis were determined using a map. Mapping was performed at the lowest administrative level, the village. Depicted in Map 1 by using WHO parasites prevalence categories, most of the villages in the Western province had moderate and high prevalence. The villages of Kigali and the Eastern province had a low prevalence.
Figure 1.
Distribution of intestinal helminthiases and schistosomiasis among preschool-aged children in Rwanda.
Figure 1.
Distribution of intestinal helminthiases and schistosomiasis among preschool-aged children in Rwanda.
3.4. Factors Associated with Intestinal Helminths and Schistosoma Infections
The bivariate analysis, the results of which are illustrated in
Table 3, revealed ten variables associated with intestinal parasites and schistosomiasis. These included age (P <0.001), residence (P <0.001), parents’ occupation (P <0.001), child caregiver during the day (P <0.001), fetching water into the lake or water body (P = 0.023), bathing in the lake (P <0.001), land surface temperature (P <0.001), soil type (P = 0.020), rainfall (P <0.001), and land cover (P <0.001).
However, after running multivariable logistic regression for all these variables, only six were independently associated with intestinal helminthiases and schistosomiasis among preschool-aged children (PSAC).
As illustrated in
Table 4, PSAC aged more than 24 months are 1.553 times higher odds of getting intestinal helminthiases and schistosomiasis compared to PSAC aged 24 months and less (aOR = 1.553; 95% CI:1.231-1.960). PSAC residing in Western province are 5.702 times higher odds of getting intestinal helminthiases and schistosomiasis compared to those living in Kigali city (aOR = 5.702; 95% CI: 2.024-16.068). Children whose parents are self-employed are 2.492 times higher odds of getting intestinal helminthiases and schistosomiasis compared to those whose parents are farmers (aOR = 2.492; 95% CI: 1.397-4.444). Children whose parents fetch water in the lake or water body are 1.829 times higher odds of getting intestinal helminthiases and schistosomiasis compared to those whose parents do not fetch water in the lake or water body (aOR = 1.829; 95% CI: 1.413-2.366). Children living in regions with high land surface temperatures (LST) are 0.248 times lower odds of getting intestinal helminthiases and schistosomiasis compared to those living in regions with very low LST (aOR = 0.248; 95% CI: 0.144-0.428). PSAC living in regions reserved for agriculture, vegetation, and waterbodies were 1.828, 1.809, and 3.452 times higher respectively to get intestinal helminthiases and schistosomiasis compared to PSAC living in built up regions ((aOR = 1.828; 95% CI: 1.041-3.210) for agriculture, (aOR = 1.809; 95% CI: 1.015-3.224) for vegetation, (aOR = 3.452; 95% CI: 1.565-7.612) for water bodies). Children living in regions with clay soil are 0.732 times lower odds to get intestinal helminthiases and schistosomiasis compared to children living regions with silt soil (aOR = 0.732; 95% CI: 0.565-0.948). Finally, children living in regions of high and moderate rainfall are 3.05 and 2.82 times higher respectively to get intestinal helminthiases and schistosomiasis compared with children living in regions of low rainfall.
4. Discussion
This study revealed that one in five preschool-aged children was infected with at least one parasite (intestinal helminthiasis or schistosomiasis). This prevalence of 20.3% was relatively low compared to previous findings in Rwanda in different settings, including 45.2% in 2014 [
34] and 44.8% in 2019 [
18] and in other countries such as Colombia (60%) [
35], but still higher than the WHO target of eliminating intestinal helminthiases and schistosomiasis as public health problems by 2030 [
36].
In this study, the age of the child was significantly associated with intestinal helminthiases and schistosomiasis, and the risk of infection increased with the age of the child. Previous studies in different countries showed similar results [
14,
20,
23,
27,
37,
38,
39]. The older the child, the greater the risk of infection by intestinal helminths, and Schistosoma will be; until reaching a certain age, the infection declines in adults (except for hookworms). As children grow, they are exposed to different activities that may be favorable for parasite infestation and growth, such as drinking untreated water [
15,
18,
19,
20], playing with bare feet [
15] or swimming, not washing their hands with potable water and soap [
19,
20,
21], eating unwashed fruits and vegetables [
40], and sucking their fingers unwashed. In addition, there is slow development of acquired immunity against these parasites.
This study reported that children from families that fetch water in the lake or open water bodies were positively associated with intestinal helminthiases and schistosomiasis. Fetching water in lakes or other open water bodies reveals the lack of access to potable water that contribute to high level of intestinal parasites as reported in other studies [
14,
37,
41].
This study reported that parents ‘occupation was positively associated with intestinal helminthiasis and schistosomiasis. Similar results were provided by other researchers in Ghana [
42] and Mexico [
43]. PSAC whose parents were self-employed, had a higher risk of developing intestinal helminthiases and schistosomiasis. Most self-employed parents do not spend enough time with their children to provide them with the necessary health information.
Our study reported that children living in the western province were 5.7 times odds higher (aOR = 5.70 [95% CI: 2.024-16.068] to be infected by intestinal helminthiases and schistosomiasis compared to those living in the City of Kigali, while children living in other provinces had relatively lower risks of infection. A study carried out in Rutsiro district, one region of the current study, among children under two years old, showed a high prevalence (48.6%) of soil-transmitted helminths [
18]. Additionally, Ruberanziza et al. reported a high prevalence of intestinal helminthiases in the western province and suggested that a high population density combined with poor sanitation levels contributed to the high prevalence of intestinal helminthiases [
44]. Other factors such as the inaccessibility to safe water (75% coverage of water distribution in the Western province) [
45], heavy rain that sometimes leads to flood and leakage of toilets, and domestic and recreational usage of Kivu Lake water may contribute to the high burden of intestinal helminthiases and schistosoma infection.
The current study considered environmental factors such as land surface temperature, rainfall, soil type, and land cover because intestinal helminths and schistosoma in their life cycles have developmental stages accomplished in the human body and other stages accomplished in the environment. Land surface temperature (LST) is well known because
A. lumbricoides,
T. trichiura, and ankylostoma have thermal thresholds outside of which the survival of the infective stages in the soil declines [
46]. Rainfall increases humidity and soil moisture, thereby helping parasites develop and influence infection transmission [
47,
48].
One interesting finding is that land cover is a predictor of intestinal helminthiases and schistosomiasis. Preschool-aged children living in regions reserved for wetlands and waterbodies were 3.452 times higher to become infected than those living in built-up regions. The different aspects found in developing countries are believed to contribute to this. There is always no potable water supply in regions near wetlands and water bodies, especially in rural areas. The soil near wetlands and water bodies has a high humidity content and soil moisture that favors parasite growth, multiplication, and transmission [
47]. People living near wetlands and waterbodies are almost in the lowest economic category, and studies have reported poverty as a predictor of intestinal parasites and schistosomiasis [
40,
49]. When latrines are present in the regions near wetlands and waterbodies, there is a high infiltration rate and parasites reach the water table, resulting in contaminated water sources. This is also justified by runoff and floods [
50] that may contaminate the water sources accessed by people in the neighborhood, such as those living in the Kivu Lake neighborhood in our case. In a study conducted in Brazil, Vieira stated that when proximity to water is combined with humidity, warm climate, sandy soil, and unimproved sanitation, it generates favorable situations for larval growth and persistence in the environment [
51].
Children living in regions reserved for agricultural activities have a higher risk of getting intestinal helminthiases and schistosomiasis. This may be related to the absence of latrines in these areas. For instance, rice farmers do not have mobile latrines to use while they are cultivating, and the parents and children who are together with them practice open defecation.
This study reported that clay soil was negatively associated with intestinal parasites compared with silt soil (aOR = 0.732). The Sharad Suyakant Malavade study carried out in Salvador found the same results. This may be because clay soils do not provide sufficient aeration for parasites eggs development and have low water permeability [
52].
Preschool-aged children living in regions of moderate and high rainfall were approximately three times more likely to be infected by intestinal helminthiases and schistosomiasis than those living in regions of low rainfall (aOR=1.674 and 1.692, respectively, for moderate and high rainfall regions). This positive association between parasites and rainfall was previously talked about in other research [
46,
53,
54]. Rainfall increases humidity and soil moisture and helps helminths and schistosoma develop and influence infection transmission [
47,
48]. This study showed that intestinal helminths (soil-transmitted helminths) are more prevalent in regions of high rainfall, mostly located in the western province of Rwanda.
Children who were cared for by their siblings during the day in this study had a higher risk of being infected by intestinal helminthiases and schistosomiasis compared with those cared for by their mothers (aOR = 1.206 and 1.254 for children cared for by their siblings and brothers, respectively). The high prevalence of infection in children cared for by their siblings may result from practices performed by older children who care for preschool-aged children, such as drinking untreated water [
15,
18,
19,
20], playing together with bare feet [
15], not washing their hands with potable water and soap [
19,
20,
21], and eating unwashed fruits and vegetables [
40]. These PSAC are exposed to parasites because they do not receive appropriate day care. Normally, in Rwanda, PSAC should be cared for in Early Childhood Development (ECD) centers established at the village level of administration. ECD helps children in their early years (three to five) by providing comprehensive access to learning, good nutrition, hygiene, and protection. Children in ECD are cared for by trained mothers who are able to protect them from harm.
5. Conclusions
The prevalence of intestinal helminthiases and schistosomiasis among preschool-aged children in Rwanda was 20.3%, which is moderate, although the Western province had a high prevalence in this study (37%). The risk of developing intestinal helminthiases and schistosomiasis increases with increasing age. It is necessary to educate children as they grow up and their caregivers on behaviors related to preventing the transmission of parasites so that they develop healthier behaviors. Fetching water into the lake or water body, a sign of the lack of safe water supply, and bathing into the lakes or water bodies exposes children to intestinal helminthiases and schistosomiasis. Environmental factors, such as land cover, land surface temperature (LST), and soil type, influenced the presence of parasites. Western provinces had higher risks compared to other provinces and needed interventions. Deworming activities, water supply, reinforcement of the use of Early Childhood Development (ECDs) centers, relocation of people living in proximity to wetlands and water bodies, and health education are key interventions in preventing preschool-aged children from getting intestinal helminthiases and schistosomiasis infections.
6. Recommendations
According to the results of this study, the prevalence of intestinal helminthiases and schistosomiasis has reduced over the years, and the following recommendations are necessary to reduce the moderate prevalence observed. Deworming activities that are performed in our country are of utmost importance and should be continued to eliminate intestinal helminthiases and schistosomiasis among preschool-aged children. Access to potable water may play a role in parasite reduction. In partnership with different actors, there is a need for water supply in regions of high prevalence, especially in the western province, which has the highest infection burden. Reinforcement of Early Childhood Development (ECD) centers is a key issue in preventing preschool children from being exposed to parasites by their day caregivers. These preschool children are exposed during the time they need to be kept safe through ECDs. The population living in proximity to wetlands and water bodies must be relocated to other places that do not expose them to the risk of intestinal helminthiases and schistosomiasis infections.
Furthermore, health education for all parents will increase their knowledge regarding the prevention of intestinal helminthiases and schistosomiasis in their children. As the majority of children were from households of farmers and living in regions reserved for agricultural activities was positively associated with intestinal helminthiases and schistosomiasis, farmers are recommended to not only avoid open defecation while they are busy in their daily activities but also to adopt WASH practices namely proper hand washing, drinking water storage, drying racks, washing vegetables and fruits before cooking, and eating them.
Author Contributions
Vestine Muhawenamahoro: conceptualization, methodology, data analysis, results presentation, interpretation, discussion, and manuscript writing. Elias Nyandwi: Onceptualization, methodology, and results presentation. Nafisa M. K. Elehamer: Mentorship. Emmanuel Nkurunziza: data analysis. Tuyizere Ndera Mucyo: Data collection, analysis, and visualization. Thaddee Nshimiyimana: manuscript writing (review and editing). Michael Fissehaye Habtu: co-supervisor. Nadine Rujeni: mentoring and supervision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This research was authorized by the University of Rwanda, College of Health Sciences and Medicine (CMHS) Institutional Review Board (IRB), which had the following reference number: CMHS/IRB/273/2023.
Data Availability Statement
Data used in this study are available when needed.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Braseth AL, Elliott DE, Ince MN. Parasitic Infections of the Gastrointestinal Track and Liver. Gastroenterol Clin North Am. 2021;50(2):361–81. [CrossRef]
- USAID. Working to Protect Neglected tropical diseases. USAID web [Internet]. 2022;(August). Available from: https://www.neglecteddiseases.gov/wp-content/uploads/2022/09/USAID-NTD-Fact-Sheet-August-2022.pdf.
- Karshima, SN. Prevalence and distribution of soil-transmitted helminth infections in Nigerian children: A systematic review and meta-analysis. Infect Dis Poverty. 2018;7(1):1–14. [CrossRef]
- Uniting to combat NTDs. Kigali Declaration on neglected tropical diseases: Commitment to end ntds. 2020;1–5. Available from: https://unitingtocombatntds.org/en/the-kigali-declaration/the-declaration/.
- Anteneh T, Giday A, Alano A, Awoke A, W/Chirkos A, Dibisa N, et al. Intestinal Parasitosis. 2008;11, 14,.
- Institute of Health Metrics and evaluation (IHME). Intestinal nematode infections— Level 3 cause. Glob Burd Dis [Internet]. 2019;393. Available from: https://www.healthdata.org/results/gbd_summaries/2019/intestinal-nematode-infections-level-3-cause.
- Institute for Health Metrics and Evaluation. Schistosomiasis — Level 3 cause. Glob Burd Dis [Internet]. 2021;393. Available from: http://www.healthdata.org/results/gbd_summaries/2019/schistosomiasis-level-3-cause.
- Ugbomeh A, Goodhead D, Green A, Onwuteaka J. Prevalence of Human Intestinal Nematode Parasites in Three Rural Communities of the Niger Delta, Nigeria. Asian J Biol. 2018;6(3):1–10. [CrossRef]
- Mulambalah CS, Ruto J. Prevalence and infection intensity of geohelminthiases among school children as an environmental health indicator to guide preventive activities in Nandi County, Kenya. Trop J Med Res. 2016;131–7. [CrossRef]
- Rodríguez-Guardado A, Pozo E, Fernandez-García R, Amo-Fernandez J, Nozal-Gancedo T. [Hookworm as cause of iron deficiency anemia in the prison population]. Rev española Sanid Penit. 2013;15(2):63–5.
- Zemene T, Shiferaw MB. Prevalence of intestinal parasitic infections in children under the age of 5 years attending the Debre Birhan referral hospital, North Shoa, Ethiopia. BMC Res Notes. 2018;11(1):1–10. [CrossRef]
- World Health Organization (WHO). Weekly epidemiological record Relevé épidémiologique hebdomadaire. 2022;(48):621–32. Available from: https://apps.who.int/iris/bitstream/handle/10665/364997/WER9748-eng-fre.pdf?sequence=1&isAllowed=y.
- Hajissa K, Islam MA, Sanyang AM, Mohamed Z. Prevalence of intestinal protozoan parasites among school children in africa: A systematic review and meta-analysis. PLoS Negl Trop Dis [Internet]. 2022;16(2):1–20. [CrossRef]
- Habib A, Andrianonimiadana L, Rakotondrainipiana M, Andriantsalama P, Randriamparany R, Randremanana RV, et al. High prevalence of intestinal parasite infestations among stunted and control children aged 2 to 5 years old in two neighborhoods of antananarivo, madagascar. PLoS Negl Trop Dis. 2021;15(4):1–22. [CrossRef]
- Tsegaye B, Yoseph A, Beyene H. Prevalence and factors associated with intestinal parasites among children of age 6 to 59 months in, Boricha district, South Ethiopia, in 2018. 2020;1–7. [CrossRef]
- Rujeni N, Morona D, Ruberanziza E, Mazigo HD. Schistosomiasis and soil-transmitted helminthiasis in Rwanda: An update on their epidemiology and control. Infect Dis Poverty. 2017;6(1):1–11. [CrossRef]
- Marcelline U, Noella U, Tharcisse M, Corine K, Josephat M, Anson BJ. The Impact of Malaria and Gastrointestinal Helminthiasis Co-infection on The Impact of Malaria and Gastrointestinal Helminthiasis Co-infection on Anaemia and Severe Malaria among Children in Bugesera District, Rwanda. 2016;(January).
- Butera E, Mukabutera A, Nsereko E, Munyanshongore C, Rujeni N, Mwikarago IE, et al. two years of age in a rural area of Rutsiro district, Rwanda – a cross-sectional study. 2019;8688:1–9. [CrossRef]
- Mohammed J, Shiferaw A, Zeleke A, Eshetu Y, Gebeyehu Z, Ayehu A, et al. Prevalence and Associated Risk Factors of Intestinal Parasites among Diarrheic Under-Five Children Attending Bahir Dar and Han Health Centers, Northwest Ethiopia: A Cross-Sectional Study. J Parasitol Res. 2022;2022. [CrossRef]
- Boonjaraspinyo S, Boonmars T, Ekobol N, Artchayasawat A, Sriraj P. Prevalence and Associated Risk Factors of Intestinal Parasitic Infections: A Population-Based Study in Phra Lap Sub-District, Mueang Khon Kaen District, Khon Kaen Province, Northeastern Thailand. 2023.
- Yin A, Lee Y, Dlamini S, Maphalala G, Liao C, Fan C. Epidemiologic Investigation of Intestinal Parasite Infection and Associated Risk Factors among Primary Schoolchildren in the Manzini and Lubombo Provinces, the Kingdom of Eswatini. 2022;2022. [CrossRef]
- Asires A, Wubie M, Reta A. Prevalence and Associated Factors of Intestinal Parasitic Infections among Food Handlers at Prison, East and West Gojjam, Ethiopia. 2019;2019. [CrossRef]
- Deka S, Kalita D, Hazarika NK. Prevalence and Risk Factors of Intestinal Parasitic Infection in Under - Five Children With Malnutrition: A Hospital Based Cross - Sectional Study. 2022.
- Tigabu A, Taye S, Aynalem M, Adane K. Prevalence and associated factors of intestinal parasitic infections among patients attending Shahura Health Center, Northwest Ethiopia. BMC Res Notes [Internet]. 2019;12(1):1–8. [CrossRef]
- Mehraj V, Hatcher J, Akhtar S, Rafique G, Beg MA. Prevalence and Factors Associated with Intestinal Parasitic Infection among Children in an Urban Slum of Karachi. 2008;3(11). [CrossRef]
- Khan W, Rahman H, Rafiq N, Kabir M, Salim M, Escalante PDLR. Saudi Journal of Biological Sciences Risk factors associated with intestinal pathogenic parasites in schoolchildren. Saudi J Biol Sci [Internet]. 2022;29(4):2782–6. [CrossRef]
- Al-fakih AA, Al-wrafi EA, Al-motawkil AAAA, Shabalah AA, Aqeel AF, Mahdi MA, et al. Prevalence of Intestinal Parasitic Infections and Associated Risk Factors Among Schoolchildren in Ibb Governorate, Southwest Yemen: A Cross-Sectional Study. 2022;(September):325–33. [CrossRef]
- Mushimiye S. A. L and Bizimana J. Prevalence And Influencers Of Intestinal Protozoa Infection Among School Children At Kigim. J Heal Sci Nurs. 2017;2(10):67–91.
- Rujeni N, Bayingana JB, Nyandwi E, Ntakarutimana A, Kagabo J, Rutayisire R, et al. Prevalence Mapping of Schistosoma mansoni Among Pre-school Age Children in Rwanda. Front Pediatr. 2022;10(June):1–7. [CrossRef]
- Deka, MA. Predictive Risk Mapping of Schistosomiasis in Madagascar Using Ecological Niche Modeling and Precision Mapping. Trop Med Infect Dis. 2022;7(2). [CrossRef]
- Nelwan, ML. Risk factors of schistosomiasis. 2023. Available from: https://ssrn.com/abstract=3722691.
- United Nations. The Sustainable Development Goals Report. 2022;32. Available from: https://unstats.un.org/sdgs/report/2022/The-Sustainable-Development-Goals-Report-2022.pdf.
- Gomes ECS, Marcelino JMR, Cavalcante KRLJ, Nascimento WRC. Quality control of the slides by Kato-Katz method for the parasitological diagnosis of schistosomiasis infection by Schistosoma mansoni. 2017;(April):110–4. [CrossRef]
- Rwanda MoH. RWANDA Neglected Tropical DISEASES Strategic Plan 2019-2024. MoH Website [Internet]. 2019;1:42. Available from: https://moh.gov.rw/fileadmin/templates/Strategic_Plans/RWANDA_NTD_STRATEGIC_PLAN_2019-2024-compressed.pdf.
- Gileydi M, Antonio J, Javier N, Id RD, Alejandro G. Prevalence and associated risk factors of Intestinal parasites in rural high-mountain communities of the Valle del Cauca — Colombia. 2020;1–15.
- Abela-Ridder B, Biswas G, Mbabazi PS, Craven M, Gerber A, Hartenstein L, et al. Ending the neglect to attain the sustainable development goals: a road map for neglected tropical diseases 2021–2030. Who. 2020. 196 p.
- Doni NY, Gürses G, Şimşek Z, Zeyrek FY. Prevalence and associated risk factors of intestinal parasites among children of farm workers in the southeastern anatolian region of Turkey. Ann Agric Environ Med. 2015;22(3):438–42. [CrossRef]
- Nhambirre OL, Cossa-Moiane I, Bauhofer AFL, Chissaque A, Lobo ML, Matos O, et al. Intestinal Parasites in Children up to 14 Years Old Hospitalized with Diarrhea in Mozambique, 2014–2019. Pathogens. 2022;11(3):2014–9. [CrossRef]
- Aleka Y, G/egziabher S, Tamir W, Birhane M, Alemu A. Prevalence and Associated Risk Factors of Intestinal Parasitic Infection among Under five Children in University of Gondar Hospital, Gondar, Northwest Ethiopia. Biomed Res Ther. 2015;2(8):347–53. [CrossRef]
- Bharti B, Bharti S, Khurana S. Worm Infestation: Diagnosis, Treatment and Prevention. Indian J Pediatr. 2018;85(11):1017–24. [CrossRef]
- Gupta R, Rayamajhee B, Sherchan SP, Rai G, Mukhiya RK, Khanal B, et al. Prevalence of intestinal parasitosis and associated risk factors among school children of Saptari district, Nepal: A cross-sectional study. Trop Med Health. 2020;48(1). [CrossRef]
- Martinez, AJ. Free-Living Amebas: Natural History, Prevention, Diagnosis, Pathology, and Treatment of Disease. Free Amebas Nat Hist Prev Diagnosis, Pathol Treat Dis. 2019;1–10.
- Quihui L, Valencia ME, Crompton DWT, Phillips S, Hagan P, Morales G, et al. Role of the employment status and education of mothers in the prevalence of intestinal parasitic infections in Mexican rural schoolchildren. BMC Public Health. 2006;6:1–8. [CrossRef]
- Ruberanziza E, Owada K, Clark NJ, Umulisa I, Ortu G, Lancaster W, et al. Mapping Soil-Transmitted Helminth Parasite Infection in Rwanda: Estimating Endemicity and Identifying At-Risk Populations. 2019. [CrossRef]
- National Institute of Statistics of Rwanda (NISR). The Fifth Rwanda Population and Housing Census, Main Indicators Report. 2023. 1–14 p.
- Scholte RGC, Schur N, Bavia ME, Carvalho EM. Spatial analysis and risk mapping of soil-transmitted helminth infections in Brazil, using Bayesian geostatistical models. 2013;8(1):97–110. [CrossRef]
- S. Brooker, Michael E. ghh Systems and Remote Sensing in the Epidemiology and Control of Human Helminth Infections. 2000.
- Brooker S, Singhasivanon P, Waikagul J, Supavej S, Kojima S, Takeuchi T, et al. Mapping soil-transmitted helminths in Southeast Asia and implications for parasite control. Southeast Asian J Trop Med Public Health. 2003;34(1):24–36.
- Zeme LL, Kitesa K, Mulisa G, Feyisa CD. Prevalence of Intestinal Parasitic Infection and Associated Factors among Adama Science and Technology University Student Adama Town, Oromia, Ethiopia. 2022;4(3):375–85.
- Fuhrimann S, Winkler MS, Kabatereine NB. Risk of Intestinal Parasitic Infections in People with Different Exposures to Wastewater and Fecal Sludge in Kampala, Uganda: A Cross-Sectional Study. 2016;1–19.
- Vieira P, Maciel S, Castro LS, Murat PG, German M, Junior H, et al. Enteroparasites in Riverside Settlements in the Pantanal Wetlands Ecosystem. 2018;2018.
- Malavade, SS. Assessment of soil transmitted helminth infection (STHI) in school children, risk factors, interactions and environmental control in El Salvador. Diss Abstr Int Sect B Sci Eng [Internet]. 2016;77(1-B(E)):No Pagination Specified. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=psyc13&AN=2016-26526-277%0Ahttp://sfx.library.cdc.gov/cdc?sid=OVID:psycdb&id=pmid:&id=&issn=0419-4217&isbn=978-1339010823&volume=77&issue=1-B%28E%29&spage=No&pages=No+Pagination+Specifie.
- Campbell SJ, Nery S V., Wardell R, D’Este CA, Gray DJ, McCarthy JS, et al. Water, Sanitation and Hygiene (WASH) and environmental risk factors for soil-transmitted helminth intensity of infection in Timor-Leste, using real time PCR. PLoS Negl Trop Dis. 2017;11(3):1–20. [CrossRef]
- Soares Magalhães RJ, Salamat MS, Leonardo L, Gray DJ, Carabin H, Halton K, et al. Mapping the Risk of Soil-Transmitted Helminthic Infections in the Philippines. PLoS Negl Trop Dis. 2015;9(9):3. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).