1. Introduction
Multiple primary malignant (MPM) tumors represent a small percentage of the total number of oncological cases. This percentage is inversely proportional to the number of primary malignant tumors [
1].
Multiple primary cancers can involve either metachronous or synchronous development [
2], Warren and Gates being the ones who made this division of multiple primary tumors .[
3]
Synchronous primary malignancy (SPM) are characterized by the concurrent occurrence of multiple primary tumors in the same patient, the second primary cancer is diagnosed within 6 months of the primary cancer.
Metachronous primary tumors (MPM) are secondary primary tumors that develop in a different organ or location from the primary cancer, the second tumor may be detected 6 months afterwards [
4].
Beyond this definition, more than two primary malignancies occurring at different times was defined as metachronous multiple primaries [
2].
The presence of synchronous or metachronous tumors can pose challenges in diagnosis, staging, and treatment planning. It requires a comprehensive evaluation to determine the extent of disease and appropriate management strategies.
We must also consider the possibility of secondary tumor metastases, which will increase the complexity of managing these patients, particularly in terms of diagnostic strategies and, most importantly, therapeutic approaches.
2. Materials and Methods
On February 9, 2024, a 67-year-old female patient was admitted to the Urology Department of the Mures County Clinical Hospital for a slightly altered general condition, lack of diuresis for over 24 hours.
The patient smoked for about 30 years, but she gave up this habit 10 years ago.
The patient's past medical history was extremely generous.
In 2014, the patient presented malignant melanoma, the tumor at the skin level being <0.75 and for which surgical excision was performed within oncological safety limits. The pathological result was invasive malignant melanoma, superficial spreading subtype (
Figure 1,
Figure 2 and
Figure 3.)
In 2017, the patient was diagnosed with stage IA2 cervical cancer, for which internal radiation therapy was practiced using the Varian GammaMedPlus iX technique, followed in the same year by a radical hysterectomy. The pathological assessment revealed the presence of cervical squamous cell carcinoma (
Figure 4).
Enhanced computed tomography (CT) follow-up of the abdomen and pelvis, performed annually until 2019, did not show any local recurrences or distant metastases. Subsequently, the patient did not return for oncological follow-up surveillance protocol.
In 2021, the patient had multiple episodes of rectal bleeding that were initially overlooked. Later that year, a colonoscopy and subsequent biopsy confirmed the diagnosis of sigmoid colon adenocarcinoma (
Figure 5). A partial colectomy was performed for stage II sigmoid cancer, followed by 5 sessions of adjuvant chemotherapy (fluoropyrimidine-based).
In 2022, the patient presented an episode of obstructive anuria. The CT scan performed showed bilateral ureterohydronephrosis due to bilateral stenosis of the last part of the ureter, interpreted as the consequence of brachytherapy for cervical cancer.
The same CT examination highlighted the presence of tumors located in the liver and which were interpreted as liver metastases. The replacement of the bilateral ureteral stents 7 Ch was practiced for a period of 12 months and was followed by the normalization of the renal tests.
In 2023, the metastasectomy of the liver tumor was performed. The pathological examination revealed a liver metastasis of a tumor with a digestive starting point (colon) (
Figure 6). The patient did not receive (refuse) any further adjuvant oncological treatment.
3. Results
On February 9, 2024, the patient presented with a slightly altered general condition, anuria, altered renal samples (creatinine=5.8 mg/dl, K=7.1 mmol/l), BMI=32
The ultrasound evaluation showed bilateral ureterohydronephrosis despite the correct positioning of both stents. Considering the interval that has passed since the insertion of the stents, the decision was to change them.
Cystoscopy was performed under local anesthesia, which revealed the presence of 3 tumor formations at the bladder level: 1 located at the level of the bladder trigone (approximately 10/5 mm, one located at the level of the right lateral wall and a formation located in the left ureteral orifice (10/10 mm) (
Figure 7).
New bilateral ureteral stents were inserted.
After 3 days in spinal anesthesia, transurethral resection of bladder tumor (TURB) was performed. Intraoperatively, the appearance of the tumor located at the left ureteral orifice was that of a tumor with a starting point from the left ureter (upper urinary tract cell carcinoma).
At 2 days postoperatively (post TURB) contrast CT was performed (creatinine val-ues=1.39mg/dl) of the head, thorax, abdomen, and pelvis.
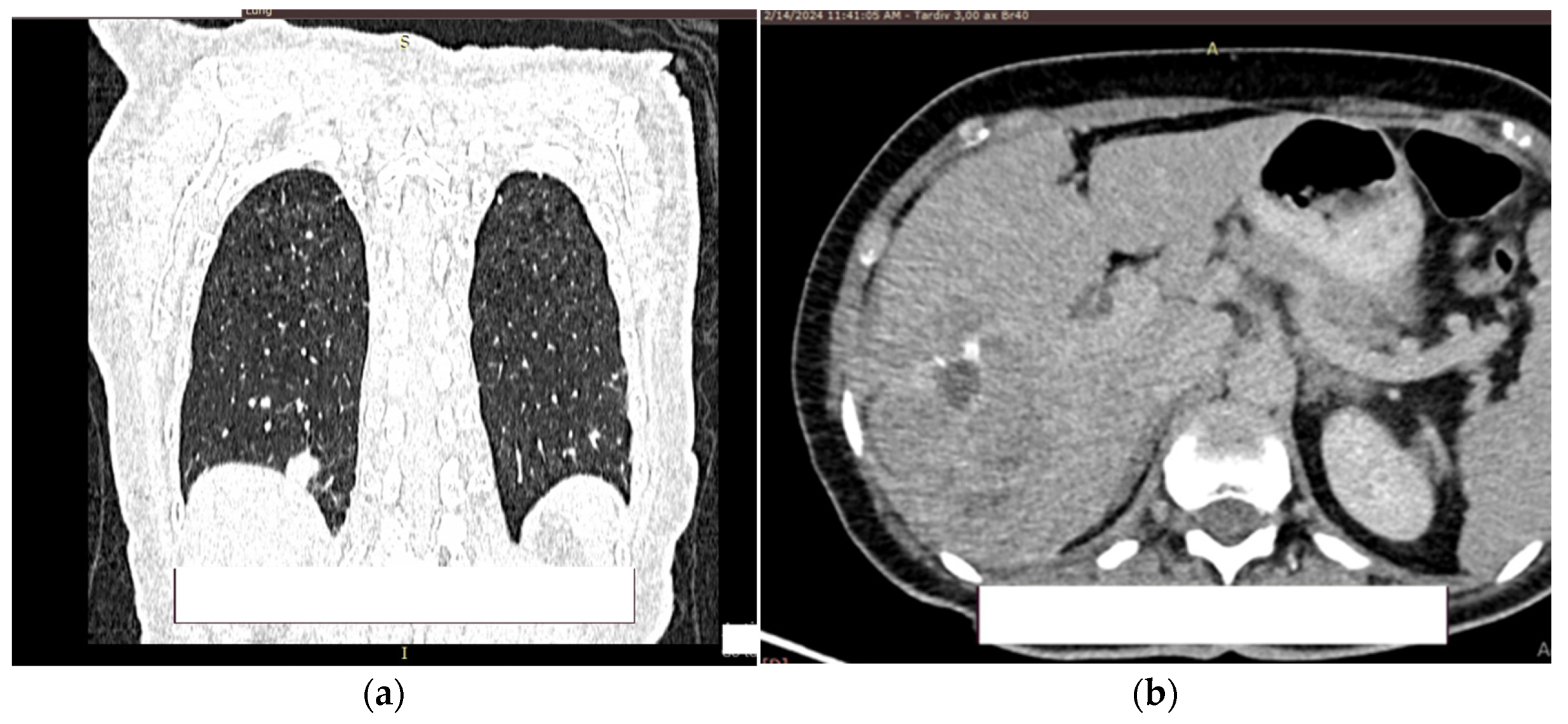
At the level of the pulmonary parenchyma, the CT examination shows multiple pulmonary nodules distributed diffusely, bilaterally with a metastases CT aspect, the largest located in the posterobasal part of the right lung with a 14 mm diameter (
Figure 8a).
No solid tumor masses were observed in the digestive tract; only intestinal fluid stasis and some hydroaeric levels were present. At the level of the right lobe of the liver, a poly nodular conglomerate with a 98/68 mm maximum diameter in the coronal plane, natively hypodense nodules and postcontrast with the presence of metal clips from the previous liver surgery (
Figure 8b). It was unclear whether this finding represents a secondary liver lesion or is a result of the previous liver metastasectomy.
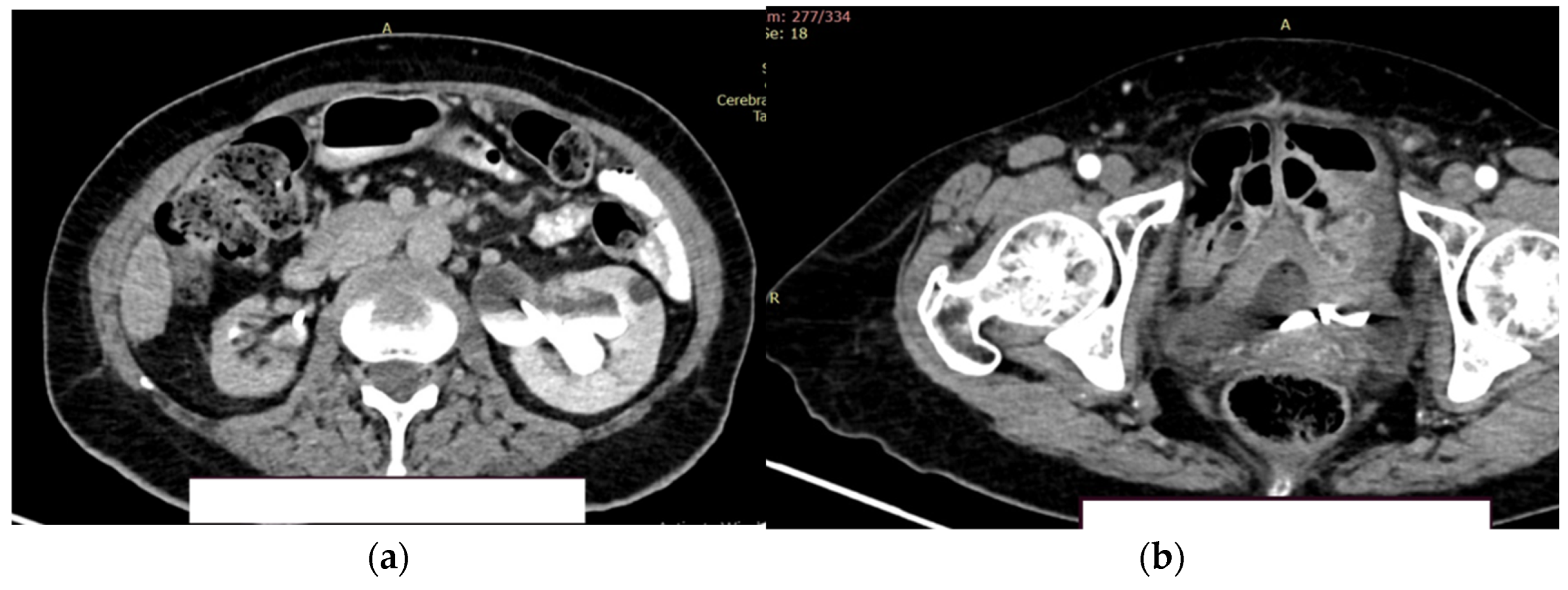
The right kidney is hypotrophic, with the presence of ureteral stent, without stones, without stasis and presence of secretion. Left kidney is with normal position of the stent, II/III grade hydronephrosis, secretion and excretion present. A retroperitoneal lymph node, measuring up to 10 mm, was observed, with some calcification. (
Figure 9a).
In the urinary bladder, concentrically thickened walls are described. It cannot be assessed if the distal, intravesical portion of the ureter present tumors formation (
Figure 9b).
The pathological result of the bladder resection showed an infiltrative adenocarcinoma of the bladder without being able to specify if it is a primary bladder adenocarcinoma or a metastatic colorectal adenocarcinoma (
Figure 10).
The future therapeutic strategy of this case is difficult because there are many variables that must be considered. The decision will belong to the oncology committee, a decision that may or may not be accepted by the patient.
4. Discussion
Considering the patient's medical history and current status, some questions arise regarding the future therapeutic attitude:
What are the possible risk factors involved in the occurrence of multiple primary tumors? Are there carcinogenic factors that can be managed?
Is it a primary bladder adenocarcinoma tumor or metastatic colorectal adenocarcinoma?
Can this secondary cancer be treated with curative intent?
Is there a possibility for the early diagnosis of these multiple primary tumors?
4.1. Risk factors
The possible factors involved can be viral infection, smoking, genetics, and treatment-related factors.
4.1.1. Viral factors
Viral infection can be a risk factor for second primary cancer. The main viruses that can be associated with different types of cancer are human papilloma virus (HPV), Epstein-Barr virus (EBV), Hepatitis B and C virus, Human Herpesvirus 8 (HHV-8), Human T-cell lymphotropic virus type 1 (HTLV-1) [
5].
4.2. Human papilloma virus (HPV)
HPV represents one of the main risk factors for gynecological cancers (cervical, vaginal, vulvar) [
6]. Besides these gynecological cancers, HPV can cause also anal, oropharyngeal, and penile cancer [
7].
Prophylactic vaccination against HPV, screening and treatment of pre-cancer lesions are effective ways to prevent cervical cancer and are cost-effective [
8,
9]. Besides these gynecological cancers, HPV can cause also anal, oropharyngeal, penile cancer [
10,
11].
4.3. Epstein-Barr virus (EBV)
EBV is considered a risk factor for multiple primary tumors such as Hodgkin’s lymphoma, Burkitt's Lymphoma, nasopharyngeal cancer, gastric cancer, breast cancer [
12,
13]. An EBV prophylactic vaccine that induces neutralizing antibodies holds great promise for prevention of EBV associated diseases [
14]. Unfortunately, in 2024 a vaccine against Epstein–Barr virus is not yet available.
4.4. Human herpesvirus 8 (HHV-8)
HHV-8 is involved in lymphoma or nasopharyngeal cancer. Despite our increased understanding of HHV-8 pathobiology, the exact mechanisms by which HHV-8 infection causes Kaposi's sarcoma and lymphoma remain unclear [
15,
16,
17]. There is no systematic progress toward developing a HHV-8 vaccine [
18].
4.5. Hepatitis B (HBV) and C (HCV)
These viruses are associated in the majority of cases with hepatocellular carcinoma but also other types of tumors (biliary tract cancers, pancreatic cancer, stomach, colorectal and oral cavity cancer) [
19,
20]. This association occurs especially with HBV because HBV can be integrated into the host genome, leading to changes in genomic function or chromosomal instability [
21,
22].
Effective vaccines are available for preventing viral hepatitis B. Effective treatment is also available for people with chronic hepatitis B virus infection. Unlike hepatitis A and B, there is currently no vaccine to prevent hepatitis C infection, but Hepatitis C is treated using direct-acting antiviral (DAA) oral medication [
23,
24].
4.6. Human T-cell leukemia virus-1 (HTLV-1)
Even if this oncovirus causes only fatal T-cell leukemia, without being a risk factor for other cancers, the discovery of the first pathogenic human retrovirus (HTLV-1) by Gallo in 1979 represented the turning point in demonstrating the oncogenic capacity of other viruses or bacteria [
25,
26]. There are numerous vaccination research experiments to prevent or control HTLV-1 infection, but no vaccine has been approved by the FDA [
27].
4.1.2. Smoking
Smoking increased the overall risk of cancer [
28]. All the study demonstrated that cigarette smoking is associated with a significantly increased risk of mortality in patients with adenocarcinoma of the colon (CRC). but also in the case of bladder tumors [
29,
30]. The smoking status can plausibly be considered in the risk stratification of CRC, and smoking cessation can be incorporated into comprehensive treatment planning for patients with CRC or bladder cancer [
31].
The effect of smoking on melanoma outcomes remains debatable. Arafa and colleagues [
32], in a study on current and heavy smoking, demonstrated that this habit is associated with a higher risk of squamous cell carcinoma (SCC) but a decreased risk of malignant melanoma, while former smoking was not linked to skin cancer risk. In a case-control study published by Sondermeijer et al. [
33], a strong inverse association between cigarette smoking and melanoma risk in men was presented.
Smoking is also a risk factor for cervical cancer. Sugawara et al. [
34] concluded that there is convincing evidence that cigarette smoking increases the risk of cervical cancer among women. Furthermore, Su et al. [
35], in a meta-analysis, provided evidence that passive smoking is associated with an increased risk of cervical cancer
Quitting smoking is very important for cancer survival and to prevent SPM or MPM [
36].
4.1.3. Genetics
Lynch syndrome is a mutation of DNA repair genes including MLH1, MSH2, MSH6, PMS2, and EPCAM gene, which can cause many cancers at a young age. The Lynch syndrome has increased risks of gastrointestinal (especially nonpolyposis colorectal cancer), liver, kidney, brain, and skin cancers [
37]. In addition to these tumors, Lynch syndrome is associated with a significant increase in the relative risk of bladder cancer [
38].
BRCA gene mutations (especially BRCA1 and BRCA2 genes) [
39] are primarily involved in breast and gynecological cancers but are also a risk factor for pancreatic or prostate cancers.
Multiple endocrine neoplasia (MEN1 and MEN2), Li-Fraumeni syndrome, hereditary diffuse gastric cancer syndrome, Peutz-Jeghers syndrome, and PTEN Hamartoma tumor syndrome represent other genetic malformations that can cause SPM or MPM [
40].
4.2. Primary bladder adenocarcinoma tumor vs metastatic colorectal adenocarcinoma
Primary bladder adenocarcinoma (PBA) is a rare tumor accounting for less than 1% of all malignant vesical tumors [
54]. PBA is very aggressive, most of the time patients present locally advanced stages or distant metastasis. Therefore, overall survival is worse [
55]. Differentiation between metastatic colonic adenocarcinoma (MCA) and PBA it is almost impossible based only on pathological features, but almost a quarter of secondary tumors of the urinary bladder are represented by MCA [
56]. For the differentiation of PBA from MCA, antibodies were used, especially β-catenin, e-cadherin, but also CK7 and CDX-2 [
57,
58]. However, much larger studies are needed than those that currently exist for their validation for diagnostic purposes. Considering the patient's medical history, the differentiation between PBA and MCA is impossible in our case.
4.3. Can this secondary cancer be treated with curative intent?
The bladder adenocarcinoma has a poorer clinical outcome than urothelial carcinoma [
59] firstly due to the superior aggressiveness of bladder adenocarcinoma and secondly due to the presentation of these patients in more advanced stages compared to those with urothelial carcinoma [
60].
For a small number of patients in the non-muscle-invasive tumor stage, the endoscopic intervention of transurethral resection of the bladder tumor can represent the therapeutic solution [
61]. Most patients with PBA have muscle-invasive disease upon admission, for which the treatment of choice is radical cystectomy with pelvic lymphadenectomy [
62]. In case of MCA, the only treatment is the palliative one.
5. Conclusions
The future therapeutic strategy of this case is difficult because there are many variables that must be considered.
Identification and assessment of risk factors such as viral infection, radiotherapy, chemotherapy, smoking, genetics are pivotal in understanding and managing multiple primary malignant tumors. Personalized prevention strategies, screening programs may facilitate the early detection of this tumors, synchronous or metachronous one.
The difference between primary bladder adenocarcinoma tumor and metastatic colorectal adenocarcinoma will make the difference between curative and palliative treatment options.
The use of multicancer early detection (MCED) blood tests for early diagnosis appears promising. However, additional research is needed to standardize these techniques for cancer detection.
Author Contributions
Conceptualization, D.P.H., C.T.M., N.C., and O.B.F.; methodology, D.P.H., and O.B.F.; software, R.G.; validation, M.D.V, M.A.B., and O.S.C.; formal analysis, R.G.; investigation, D.P.H., G.R., M.A.B., A.L, B.L, and O.S.C.; resources, G.R.; writing—original draft preparation, D.P.H., M.D.V, M.A.B., and O.B.F; writing—review and editing, M.O.K.I,.; visualization, D.P.H.; supervision, D.P.H., N.C., and O.B.F.; project administration, D.P.H., and O.B.F; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Clinical County Hospital Mures,, 540136 Târgu Mures,, Romania (13754/09.09.2924) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from the subject involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rosso S, De Angelis R, Ciccolallo L, Carrani E, Soerjomataram I, Grande E, et al. Multiple tumours in survival estimates. Eur J Cancer. 2009 Apr;45(6):1080–94. [CrossRef]
- Coyte A, Morrison DS, McLoone P. Second primary cancer risk - the impact of applying different definitions of multiple primaries: results from a retrospective population-based cancer registry study. BMC Cancer. 2014 Apr 18;14:272. [CrossRef]
- Zhai C, Cai Y, Lou F, Liu Z, Xie J, Zhou X, et al. Multiple Primary Malignant Tumors - A Clinical Analysis of 15,321 Patients with Malignancies at a Single Center in China. J Cancer. 2018;9(16):2795–801. [CrossRef]
- Tanjak P, Suktitipat B, Vorasan N, Juengwiwattanakitti P, Thiengtrong B, Songjang C, et al. Risks and cancer associations of metachronous and synchronous multiple primary cancers: a 25-year retrospective study. BMC Cancer. 2021 Sep 23;21(1):1045. [CrossRef]
- Liao, JB. Liao JB. Viruses and human cancer. Yale J Biol Med. 2006 Dec;79(3–4):115–22. [CrossRef]
- Furumoto H, Irahara M. Human papilloma virus (HPV) and cervical cancer. J Med Invest. 2002 Aug;49(3–4):124–33. [CrossRef]
- Sasidharanpillai S, Ravishankar N, Kamath V, Bhat PV, Bhatt P, Arunkumar G. Prevalence of Human Papillomavirus (HPV) DNA among Men with Oropharyngeal and Anogenital Cancers: A Systematic Review and Meta-Analysis. Asian Pac J Cancer Prev. 2021 May 1;22(5):1351–64. [CrossRef]
- Centers for Disease Control and Prevention (CDC). FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010 May 28;59(20):626–9.
- Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Penny ME, Aranda C, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 2011 Feb 3;364(5):401–11. [CrossRef]
- Schlenker B, Schneede P. The Role of Human Papilloma Virus in Penile Cancer Prevention and New Therapeutic Agents. Eur Urol Focus. 2019 Jan;5(1):42–5.
- Chaitanya NCSK, Allam NSJ, Gandhi Babu DB, Waghray S, Badam RK, Lavanya R. Systematic meta-analysis on association of human papilloma virus and oral cancer. J Cancer Res Ther. 2016;12(2):969–74. [CrossRef]
- Patel PD, Alghareeb R, Hussain A, Maheshwari MV, Khalid N. The Association of Epstein-Barr Virus With Cancer. Cureus. 2022 Jun;14(6):e26314. [CrossRef]
- Fang WL, Chen MH, Huang KH, Lin CH, Chao Y, Lo SS, et al. The Clinicopathological Features and Genetic Alterations in Epstein-Barr Virus-Associated Gastric Cancer Patients after Curative Surgery. Cancers (Basel). 2020 Jun 10;12(6):1517. [CrossRef]
- Cui X, Snapper CM. Epstein Barr Virus: Development of Vaccines and Immune Cell Therapy for EBV-Associated Diseases. Front Immunol. 2021;12:734471. [CrossRef]
- Kedes DH, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. The seroepidemiology of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat Med. 1996 Aug;2(8):918–24.
- Gantt S, Casper C. Human herpesvirus 8-associated neoplasms: the roles of viral replication and antiviral treatment. Curr Opin Infect Dis. 2011 Aug;24(4):295–301.
- Gill J, Bourboulia D, Wilkinson J, Hayes P, Cope A, Marcelin AG, et al. Prospective study of the effects of antiretroviral therapy on Kaposi sarcoma--associated herpesvirus infection in patients with and without Kaposi sarcoma. J Acquir Immune Defic Syndr. 2002 Dec 1;31(4):384–90.
- Mulama DH, Mutsvunguma LZ, Totonchy J, Ye P, Foley J, Escalante GM, et al. A multivalent Kaposi sarcoma-associated herpesvirus-like particle vaccine capable of eliciting high titers of neutralizing antibodies in immunized rabbits. Vaccine. 2019 Jul 9;37(30):4184–94.
- Wang Y, Yuan Y, Gu D. Hepatitis B and C virus infections and the risk of biliary tract cancers: a meta-analysis of observational studies. Infect Agent Cancer. 2022 Aug 27;17(1):45.
- Song C, Lv J, Liu Y, Chen JG, Ge Z, Zhu J, et al. Associations Between Hepatitis B Virus Infection and Risk of All Cancer Types. JAMA Netw Open. 2019 Jun 5;2(6):e195718. [CrossRef]
- El-Serag, HB. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012 May;142(6):1264-1273.e1. [CrossRef]
- Takada S, Kaneniwa N, Tsuchida N, Koike K. Cytoplasmic retention of the p53 tumor suppressor gene product is observed in the hepatitis B virus X gene-transfected cells. Oncogene. 1997 Oct 16;15(16):1895–901.
- Chen F, Nagy K, Chavez D, Willis S, McBride R, Giang E, et al. Antibody Responses to Immunization With HCV Envelope Glycoproteins as a Baseline for B-Cell-Based Vaccine Development. Gastroenterology. 2020 Mar;158(4):1058-1071.e6. [CrossRef]
- Jazwinski AB, Muir AJ. Direct-acting antiviral medications for chronic hepatitis C virus infection. Gastroenterol Hepatol (N Y). 2011 Mar;7(3):154–62.
- Gallo, RC. Gallo RC. The discovery of the first human retrovirus: HTLV-1 and HTLV-2. Retrovirology. 2005 Mar 2;2:17. [CrossRef]
- Tagaya Y, Gallo RC. The Exceptional Oncogenicity of HTLV-1. Front Microbiol. 2017;8:1425. [CrossRef]
- Seighali N, Shafiee A, Rafiee MA, Aminzade D, Mozhgani SH. Human T-cell lymphotropic virus type 1 (HTLV-1) proposed vaccines: a systematic review of preclinical and clinical studies. BMC Infect Dis. 2023 May 11;23(1):320.
- Jacob L, Freyn M, Kalder M, Dinas K, Kostev K. Impact of tobacco smoking on the risk of developing 25 different cancers in the UK: a retrospective study of 422,010 patients followed for up to 30 years. Oncotarget. 2018 Apr 3;9(25):17420–9. [CrossRef]
- Huang YM, Wei PL, Ho CH, Yeh CC. Cigarette Smoking Associated with Colorectal Cancer Survival: A Nationwide, Population-Based Cohort Study. J Clin Med. 2022 Feb 9;11(4):913.
- Brennan P, Bogillot O, Cordier S, Greiser E, Schill W, Vineis P, et al. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies. Int J Cancer. 2000 Apr 15;86(2):289–94.
- Gram IT, Park SY, Wilkens LR, Haiman CA, Le Marchand L. Smoking-Related Risks of Colorectal Cancer by Anatomical Subsite and Sex. Am J Epidemiol. 2020 Jun 1;189(6):543–53. [CrossRef]
- Arafa A, Mostafa A, Navarini AA, Dong JY. The association between smoking and risk of skin cancer: a meta-analysis of cohort studies. Cancer Causes Control. 2020 Aug;31(8):787–94.
- Sondermeijer L, Lamboo LGE, de Waal AC, Galesloot TE, Kiemeney LALM, van Rossum M, et al. Cigarette Smoking and the Risk of Cutaneous Melanoma: A Case-Control Study. Dermatology. 2020;236(3):228–36. [CrossRef]
- Sugawara Y, Tsuji I, Mizoue T, Inoue M, Sawada N, Matsuo K, et al. Cigarette smoking and cervical cancer risk: an evaluation based on a systematic review and meta-analysis among Japanese women. Japanese Journal of Clinical Oncology. 2019 Jan 1;49(1):77–86. [CrossRef]
- Su B, Qin W, Xue F, Wei X, Guan Q, Jiang W, et al. The relation of passive smoking with cervical cancer: A systematic review and meta-analysis. Medicine. 2018 Nov;97(46):e13061.
- Tabuchi T, Ito Y, Ioka A, Nakayama T, Miyashiro I, Tsukuma H. Tobacco smoking and the risk of subsequent primary cancer among cancer survivors: a retrospective cohort study. Ann Oncol. 2013 Oct;24(10):2699–704. [CrossRef]
- Biller LH, Syngal S, Yurgelun MB. Recent advances in Lynch syndrome. Fam Cancer. 2019 Apr;18(2):211–9.
- Nassour AJ, Jain A, Hui N, Siopis G, Symons J, Woo H. Relative Risk of Bladder and Kidney Cancer in Lynch Syndrome: Systematic Review and Meta-Analysis. Cancers. 2023 Jan 13;15(2):506. [CrossRef]
- Pilarski, R. Pilarski R. The Role of BRCA Testing in Hereditary Pancreatic and Prostate Cancer Families. Am Soc Clin Oncol Educ Book. 2019 Jan;39:79–86.
- Garutti M, Foffano L, Mazzeo R, Michelotti A, Da Ros L, Viel A, et al. Hereditary Cancer Syndromes: A Comprehensive Review with a Visual Tool. Genes. 2023 Apr 30;14(5):1025. [CrossRef]
- Liu Y, Hou HA, Qiu H, Tang CH. Is the risk of second primary malignancy increased in multiple myeloma in the novel therapy era? A population-based, retrospective cohort study in Taiwan. Sci Rep. 2020 Sep 1;10(1):14393.
- Xu Y, Wang H, Zhou S, Yu M, Wang X, Fu K, et al. Risk of second malignant neoplasms after cyclophosphamide-based chemotherapy with or without radiotherapy for non-Hodgkin lymphoma. Leuk Lymphoma. 2013 Jul;54(7):1396–404.
- Travis LB, Holowaty EJ, Bergfeldt K, Lynch CF, Kohler BA, Wiklund T, et al. Risk of leukemia after platinum-based chemotherapy for ovarian cancer. N Engl J Med. 1999 Feb 4;340(5):351–7. [CrossRef]
- Thai AA, Lim AM, Solomon BJ, Rischin D. Biology and Treatment Advances in Cutaneous Squamous Cell Carcinoma. Cancers. 2021 Nov 11;13(22):5645.
- Yakkala PA, Penumallu NR, Shafi S, Kamal A. Prospects of Topoisomerase Inhibitors as Promising Anti-Cancer Agents. Pharmaceuticals (Basel). 2023 Oct 13;16(10):1456. [CrossRef]
- Lomov NA, Viushkov VS, Ulianov SV, Gavrilov AA, Alexeyevsky DA, Artemov AV, et al. Recurrent Translocations in Topoisomerase Inhibitor-Related Leukemia Are Determined by the Features of DNA Breaks Rather Than by the Proximity of the Translocating Genes. Int J Mol Sci. 2022 Aug 29;23(17):9824. [CrossRef]
- Dracham CB, Shankar A, Madan R. Radiation induced secondary malignancies: a review article. Radiat Oncol J. 2018 Jun;36(2):85–94. [CrossRef]
- Berrington de Gonzalez A, Gilbert E, Curtis R, Inskip P, Kleinerman R, Morton L, et al. Second solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose-response relationship. Int J Radiat Oncol Biol Phys. 2013 Jun 1;86(2):224–33. [CrossRef]
- Little, MP. Little MP. Cancer and non-cancer effects in Japanese atomic bomb survivors. J Radiol Prot. 2009 Jun;29(2A):A43-59.
- Mullenders L, Atkinson M, Paretzke H, Sabatier L, Bouffler S. Assessing cancer risks of low-dose radiation. Nat Rev Cancer. 2009 Aug;9(8):596–604.
- Hall, EJ. Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006 May 1;65(1):1–7.
- Chaturvedi AK, Engels EA, Gilbert ES, Chen BE, Storm H, Lynch CF, et al. Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J Natl Cancer Inst. 2007 Nov 7;99(21):1634–43.
- Creutzberg CL, Nout RA, Lybeert MLM, Wárlám-Rodenhuis CC, Jobsen JJ, Mens JWM, et al. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2011 Nov 15;81(4):e631-638. [CrossRef]
- Thomas AA, Stephenson AJ, Campbell SC, Jones JS, Hansel DE. Clinicopathologic features and utility of immunohistochemical markers in signet-ring cell adenocarcinoma of the bladder. Hum Pathol. 2009 Jan;40(1):108–16. [CrossRef]
- Grignon DJ, Ro JY, Ayala AG, Johnson DE, Ordóñez NG. Primary adenocarcinoma of the urinary bladder. A clinicopathologic analysis of 72 cases. Cancer. 1991 Apr 15;67(8):2165–72.
- Raspollini MR, Nesi G, Baroni G, Girardi LR, Taddei GL. Immunohistochemistry in the differential diagnosis between primary and secondary intestinal adenocarcinoma of the urinary bladder. Appl Immunohistochem Mol Morphol. 2005 Dec;13(4):358–62. [CrossRef]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997 Mar 21;275(5307):1787–90.
- Elston MS, Gill AJ, Conaglen JV, Clarkson A, Cook RJ, Little NS, et al. Nuclear accumulation of e-cadherin correlates with loss of cytoplasmic membrane staining and invasion in pituitary adenomas. J Clin Endocrinol Metab. 2009 Apr;94(4):1436–42.
- Lughezzani G, Sun M, Jeldres C, Alasker A, Budäus L, Shariat SF, et al. Adenocarcinoma versus urothelial carcinoma of the urinary bladder: comparison between pathologic stage at radical cystectomy and cancer-specific mortality. Urology. 2010 Feb;75(2):376–81. [CrossRef]
- Black PC, Brown GA, Dinney CPN. The impact of variant histology on the outcome of bladder cancer treated with curative intent. Urol Oncol. 2009;27(1):3–7. [CrossRef]
- Porten SP, Willis D, Kamat AM. Variant histology: role in management and prognosis of nonmuscle invasive bladder cancer. Curr Opin Urol. 2014 Sep;24(5):517–23.
- Williams CR, Chavda K. En Bloc Robot-assisted Laparoscopic Partial Cystectomy, Urachal Resection, and Pelvic Lymphadenectomy for Urachal Adenocarcinoma. Rev Urol. 2015;17(1):46–9.
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA A Cancer J Clinicians. 2023 Jan;73(1):17–48.
- World Health Organization (WHO). Cancer. WHO; 2024. Accessed February 1, 2024. https://www.who.int/news-room/fact-sheets/ detail/cancer.
- Hofmarcher T, Lindgren P, Wilking N, Jönsson B. The cost of cancer in Europe 2018. European Journal of Cancer. 2020 Apr;129:41–9. [CrossRef]
- McGarvey N, Gitlin M, Fadli E, Chung KC. Increased healthcare costs by later stage cancer diagnosis. BMC Health Serv Res. 2022 Sep 13;22(1):1155. [CrossRef]
- Loud JT, Murphy J. Cancer Screening and Early Detection in the 21 st Century. Seminars in Oncology Nursing. 2017 May;33(2):121–8.
- Schroll MM, Quinn E, Pritchard D, Chang A, Garner Amanti K, Perez O, et al. Perspectives on Clinical Adoption Barriers to Blood-Based Multi-Cancer Early Detection Tests across Stakeholders. JPM. 2024 Jun 1;14(6):593.
- Connal S, Cameron JM, Sala A, Brennan PM, Palmer DS, Palmer JD, et al. Liquid biopsies: the future of cancer early detection. J Transl Med. 2023 Feb 11;21(1):118. [CrossRef]
- Mariotto AB, Enewold L, Zhao J, Zeruto CA, Yabroff KR. Medical Care Costs Associated with Cancer Survivorship in the United States. Cancer Epidemiology, Biomarkers & Prevention. 2020 Jul 1;29(7):1304–12.
- Liu MC, Oxnard GR, Klein EA, Swanton C, Seiden MV, Liu MC, et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Annals of Oncology. 2020 Jun;31(6):745–59.
- Brito-Rocha T, Constâncio V, Henrique R, Jerónimo C. Shifting the Cancer Screening Paradigm: The Rising Potential of Blood-Based Multi-Cancer Early Detection Tests. Cells. 2023 Mar 18;12(6):935. [CrossRef]
- Poulet G, Massias J, Taly V. Liquid Biopsy: General Concepts. Acta Cytologica. 2019;63(6):449–55. [CrossRef]
- Chen X, Gole J, Gore A, He Q, Lu M, Min J, et al. Non-invasive early detection of cancer four years before conventional diagnosis using a blood test. Nat Commun. 2020 Jul 21;11(1):3475. [CrossRef]
- Schrag D, Beer TM, McDonnell CH, Nadauld L, Dilaveri CA, Reid R, et al. Blood-based tests for multicancer early detection (PATHFINDER): a prospective cohort study. The Lancet. 2023 Oct;402(10409):1251–60. [CrossRef]
- Klein EA, Richards D, Cohn A, Tummala M, Lapham R, Cosgrove D, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Annals of Oncology. 2021 Sep;32(9):1167–77. [CrossRef]
- Lone SN, Nisar S, Masoodi T, Singh M, Rizwan A, Hashem S, et al. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol Cancer. 2022 Mar 18;21(1):79. [CrossRef]
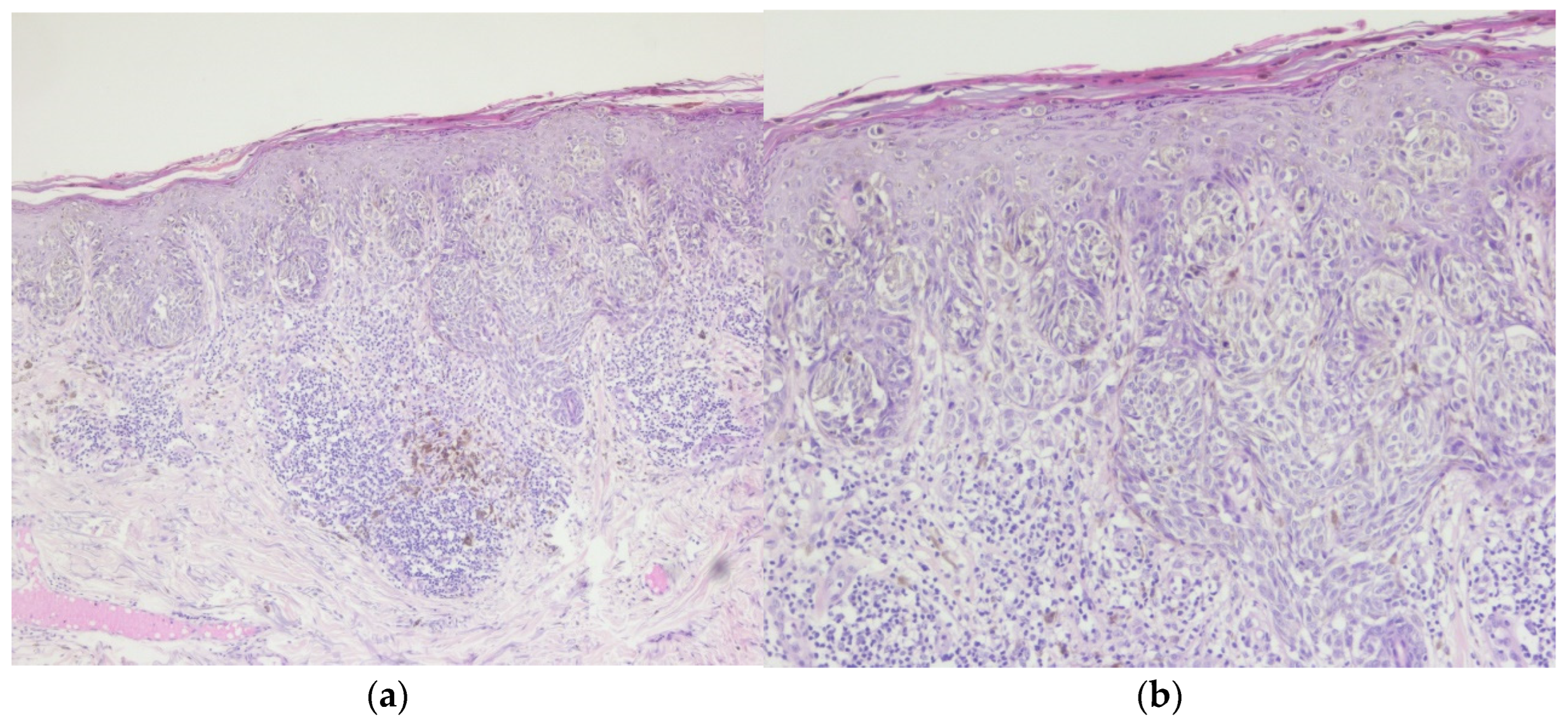
Figure 1.
Invasive malignant melanoma, superficial spreading subtype: (a) Hematoxylin and eosin staining (H&E), 5x magnification (b) Superficial spreading melanoma with haphazardly distributed atypical melanocytes present as single cells and nests at all levels of the epidermis., H&E, 10x magnification.
Figure 1.
Invasive malignant melanoma, superficial spreading subtype: (a) Hematoxylin and eosin staining (H&E), 5x magnification (b) Superficial spreading melanoma with haphazardly distributed atypical melanocytes present as single cells and nests at all levels of the epidermis., H&E, 10x magnification.
Figure 2.
Invasive malignant melanoma: (a) SOX10 immunostain highlights nuclear positivity of malignant melanocytes, 5x magnification; (b) S100 immunostain highlights positivity of malignant melanocytes, 5x magnification.
Figure 2.
Invasive malignant melanoma: (a) SOX10 immunostain highlights nuclear positivity of malignant melanocytes, 5x magnification; (b) S100 immunostain highlights positivity of malignant melanocytes, 5x magnification.
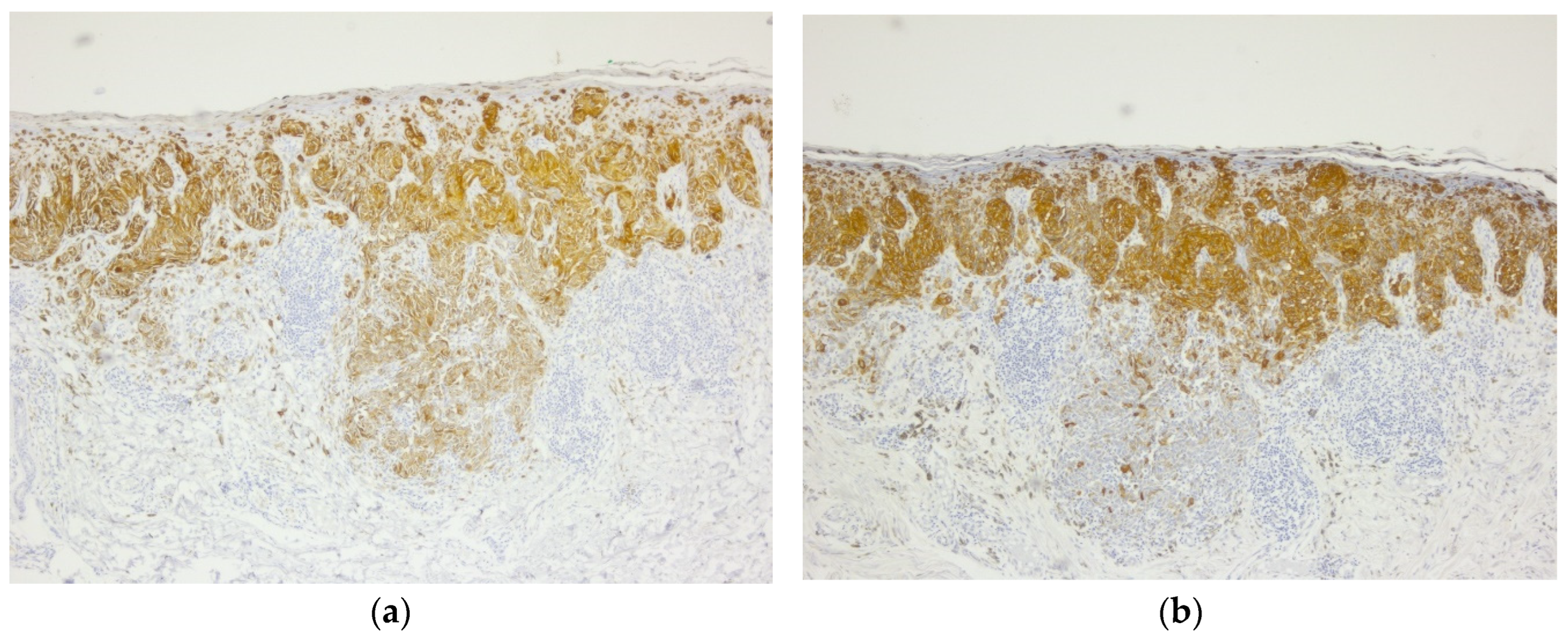
Figure 3.
Invasive malignant melanoma: (a) Melan A immunohistochemical stain, a marker of melanocytic differentiation, highlights intraepithelial pagetoid spread as well as malignant melanocytes at the epi-thelial-connective tissue interface and in the superficial connective tissue, 5x magnification; (b) HMB45 immunostain highlights cytoplasmic positivity of all melanocytes, including deep dermal nests of atypical melanocytes, 5x.
Figure 3.
Invasive malignant melanoma: (a) Melan A immunohistochemical stain, a marker of melanocytic differentiation, highlights intraepithelial pagetoid spread as well as malignant melanocytes at the epi-thelial-connective tissue interface and in the superficial connective tissue, 5x magnification; (b) HMB45 immunostain highlights cytoplasmic positivity of all melanocytes, including deep dermal nests of atypical melanocytes, 5x.
Figure 4.
Squamous cell carcinoma of the cervix: (a) Non-keratinizing squamous cell carcinoma, squamous cells in islands infiltrating deeper tissue with individual cell keratinization but lack epithelial pearls, H&E, 5x magnification; (b) Squamous cell carcinoma of the cervix, non-keratinizing type. Malignant squamous cells have abundant eosinophilic cytoplasm, distinct cell borders, and individual cell keratinization, H&E, 10x magnification.
Figure 4.
Squamous cell carcinoma of the cervix: (a) Non-keratinizing squamous cell carcinoma, squamous cells in islands infiltrating deeper tissue with individual cell keratinization but lack epithelial pearls, H&E, 5x magnification; (b) Squamous cell carcinoma of the cervix, non-keratinizing type. Malignant squamous cells have abundant eosinophilic cytoplasm, distinct cell borders, and individual cell keratinization, H&E, 10x magnification.
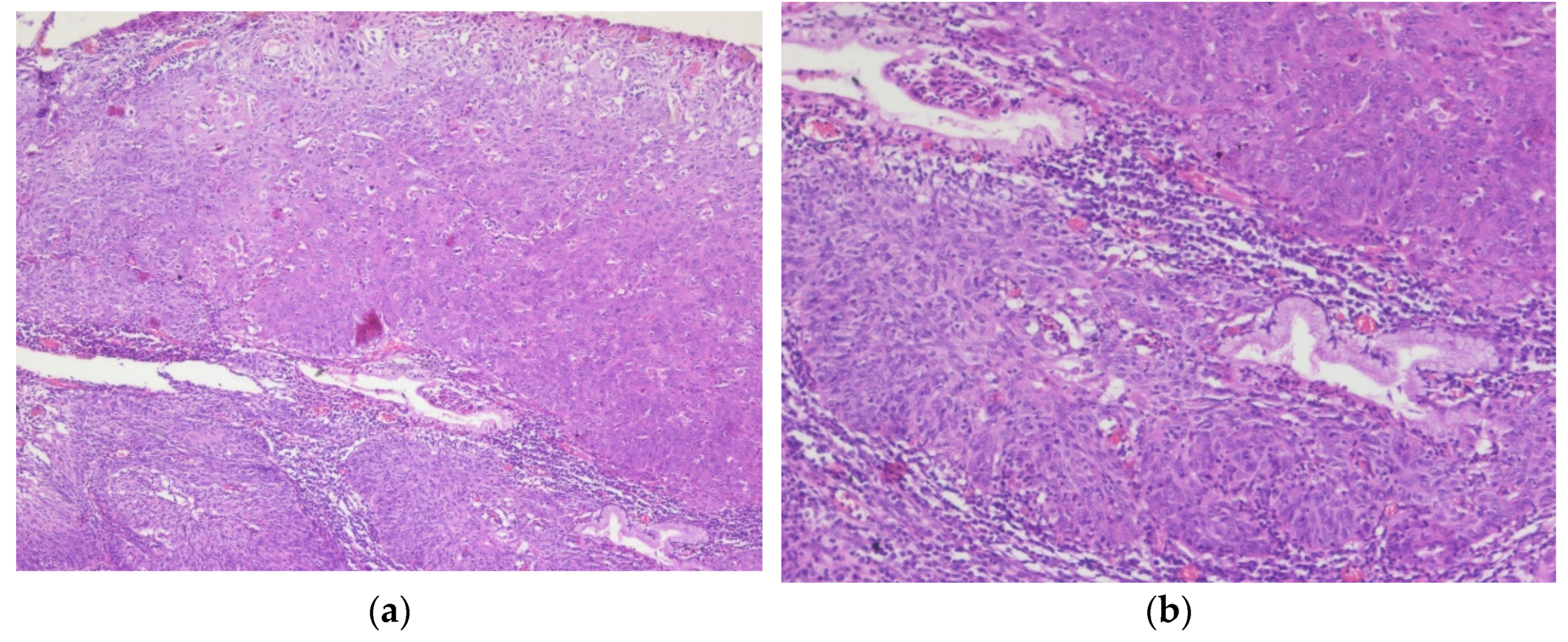
Figure 5.
Sigmoid colon adenocarcinoma: (a) Central comedonecrosis: necrotic debris inside the neoplastic gland, H&E, 10x magnification; (b) Hematoxylin and eosin (H&E) stained sigmoid colon showing grade two, moderately differentiated adenocarcinoma, 5x magnification.
Figure 5.
Sigmoid colon adenocarcinoma: (a) Central comedonecrosis: necrotic debris inside the neoplastic gland, H&E, 10x magnification; (b) Hematoxylin and eosin (H&E) stained sigmoid colon showing grade two, moderately differentiated adenocarcinoma, 5x magnification.
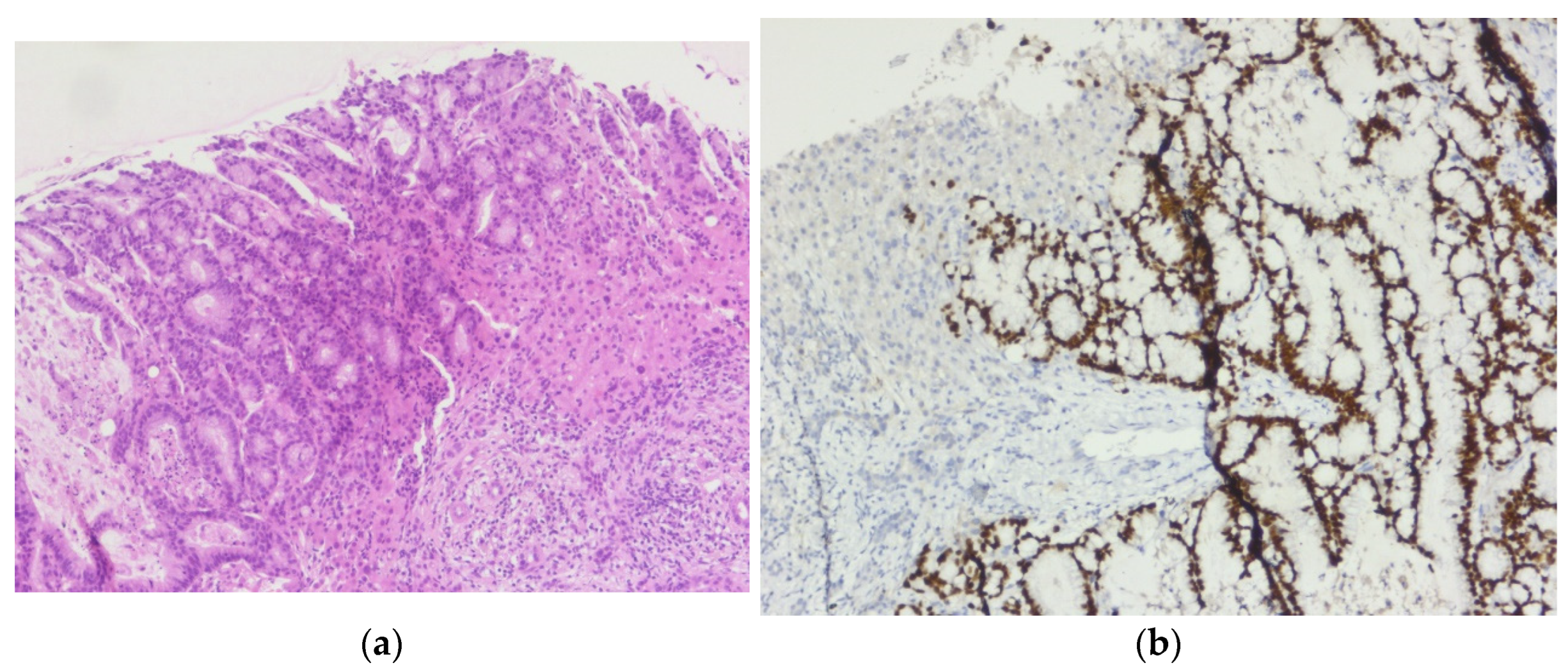
Figure 6.
Liver metastasis of the sigmoid colon cancer: (a) Tumor proliferation composed of irregular, crowded glands, lined by a stratified columnar epithelium with marked cytonuclear atypia, with hyperchromatic and elongated nuclei, H&E, 10x magnification; (b) CDX-2 immunostain highlights positivity within the tumor cells, 10x magnification
Figure 6.
Liver metastasis of the sigmoid colon cancer: (a) Tumor proliferation composed of irregular, crowded glands, lined by a stratified columnar epithelium with marked cytonuclear atypia, with hyperchromatic and elongated nuclei, H&E, 10x magnification; (b) CDX-2 immunostain highlights positivity within the tumor cells, 10x magnification
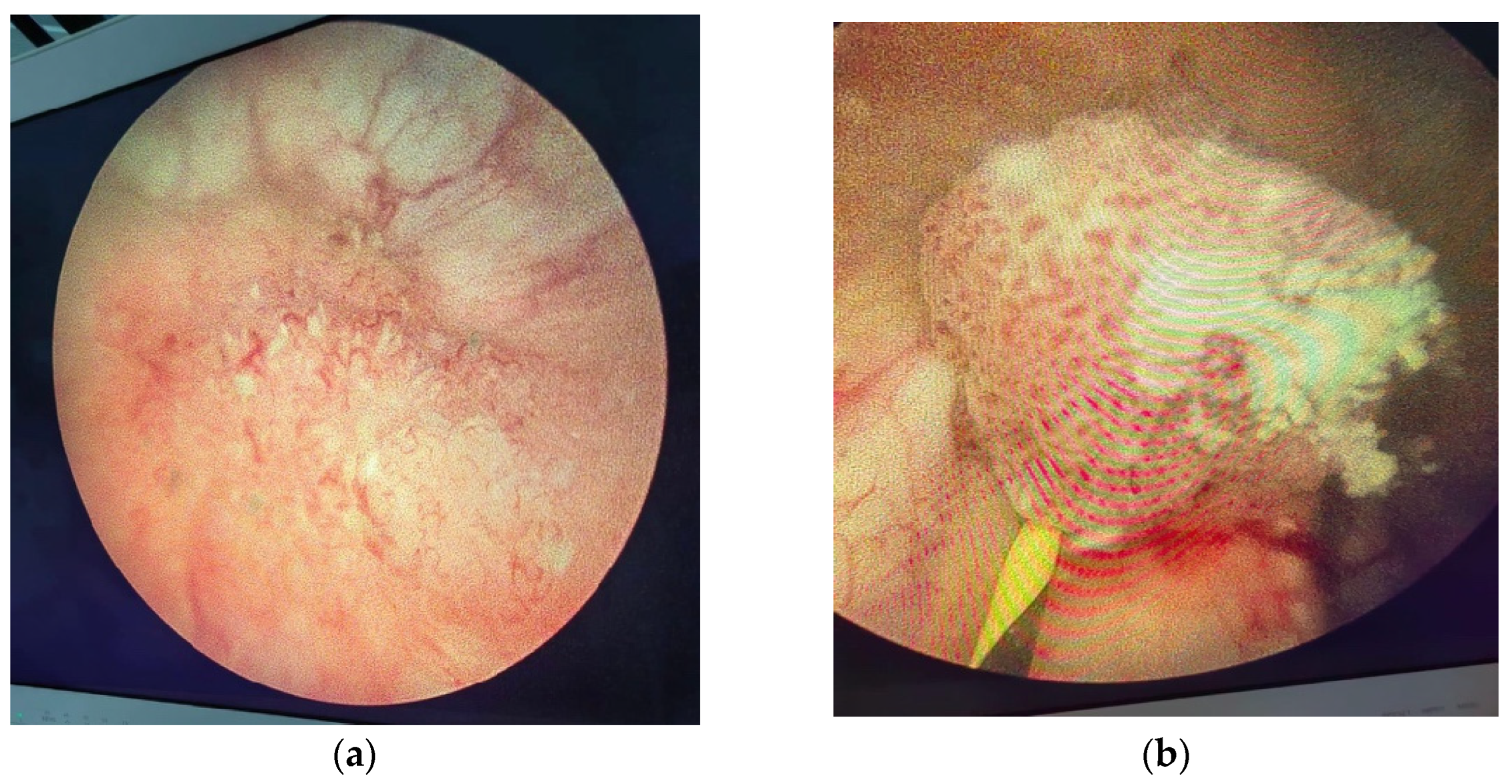
Figure 7.
Endoscopic aspect of bladder tumor: (a) Bladder tumor located at the level of the right lateral wall; (b) Bladder tumor located in the left ureteral orifice.
Figure 7.
Endoscopic aspect of bladder tumor: (a) Bladder tumor located at the level of the right lateral wall; (b) Bladder tumor located in the left ureteral orifice.
Figure 8.
Contrast CT aspect. If there are multiple panels, they should be listed as: (a) Description of what is contained in the first panel; (b) Description of what is contained in the second panel. Figures should be placed in the main text near to the first time they are cited.
Figure 8.
Contrast CT aspect. If there are multiple panels, they should be listed as: (a) Description of what is contained in the first panel; (b) Description of what is contained in the second panel. Figures should be placed in the main text near to the first time they are cited.
Figure 9.
Contrast CT aspect. (a) The right kidney is hypotrophic, with a ureteral stent in place, no stasis, and secretion present. The left kidney also has a ureteral stent, with grade II/III hydronephrosis, and both secretion and excretion are present; (b) The walls of the urinary bladder are concentrically thickened. It is unclear whether the distal intravesical portion of the ureter has any tumor formation.
Figure 9.
Contrast CT aspect. (a) The right kidney is hypotrophic, with a ureteral stent in place, no stasis, and secretion present. The left kidney also has a ureteral stent, with grade II/III hydronephrosis, and both secretion and excretion are present; (b) The walls of the urinary bladder are concentrically thickened. It is unclear whether the distal intravesical portion of the ureter has any tumor formation.
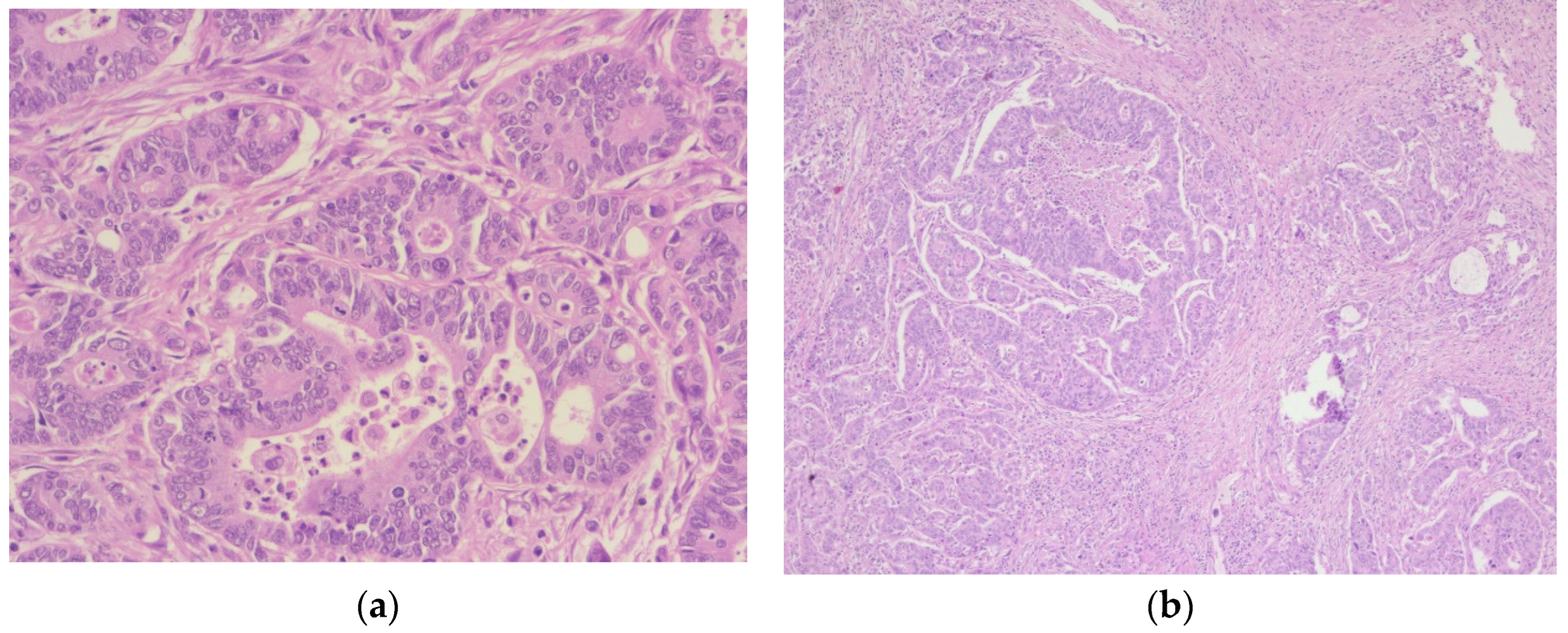
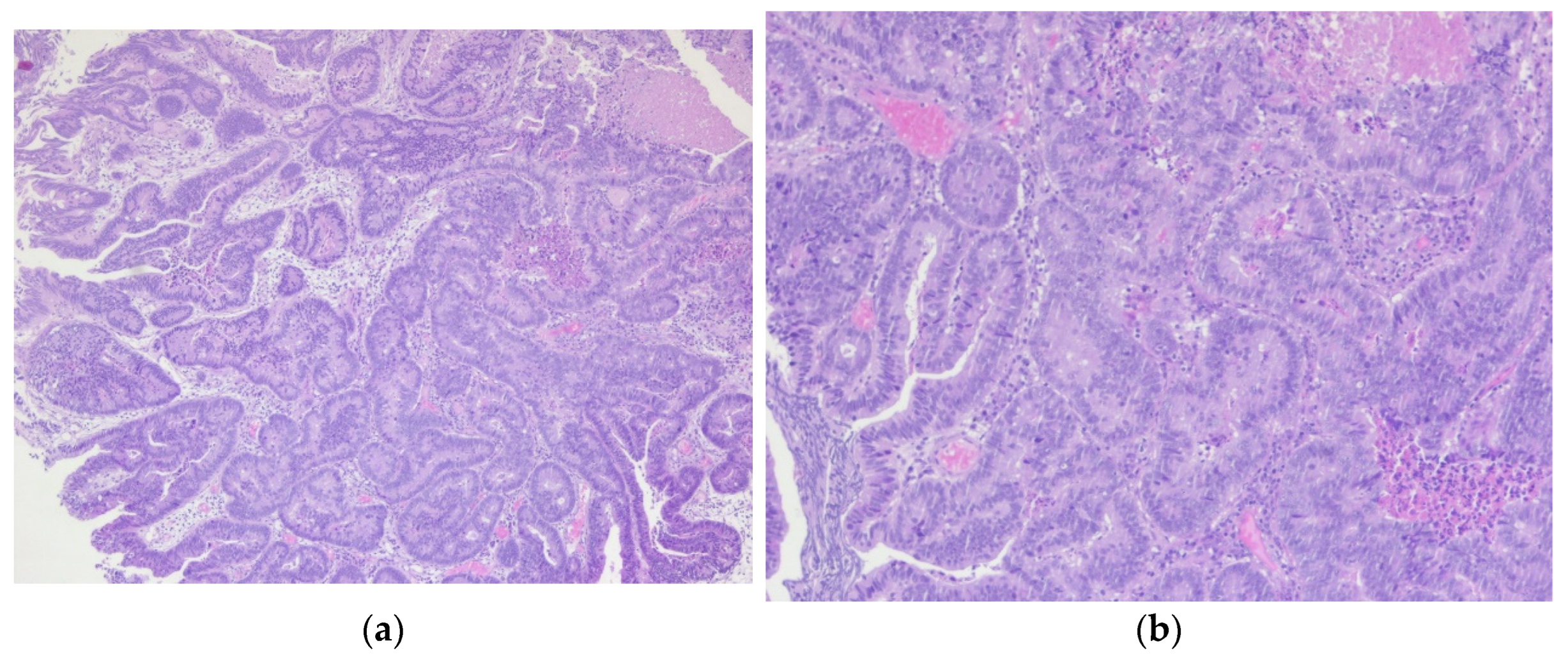
Figure 10.
Adenocarcinoma of the urinary bladder: (a) The tumor proliferation has a glandular architecture. The appearance is highly suggestive of an infiltrative adenocarcinoma, H&E, 5x magnification; (b) The tumor proliferation has a glandular architecture; the glands possess a pseudostratified epithelium with pleomorphic, crowded nuclei, and loss of polarity, H&E, 10x magnification.
Figure 10.
Adenocarcinoma of the urinary bladder: (a) The tumor proliferation has a glandular architecture. The appearance is highly suggestive of an infiltrative adenocarcinoma, H&E, 5x magnification; (b) The tumor proliferation has a glandular architecture; the glands possess a pseudostratified epithelium with pleomorphic, crowded nuclei, and loss of polarity, H&E, 10x magnification.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).