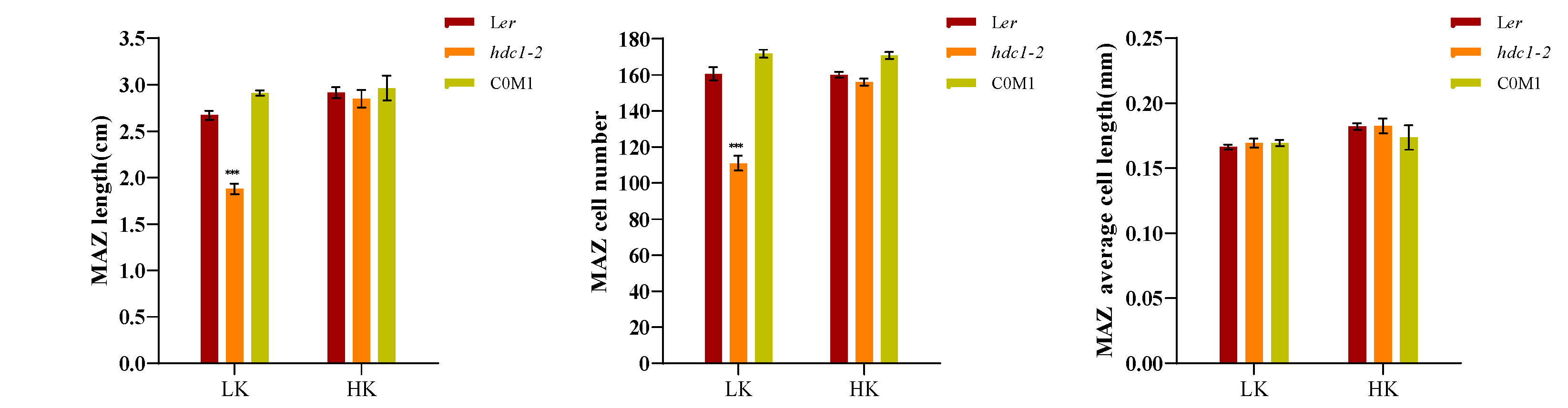1. Introduction
Potassium (K+) is the predominant inorganic cation in plant cells and plays a crucial role in fundamental physiological processes and stress responses [1]. It is involved in enzyme activation, protein synthesis, photosynthesis, and the regulation of water uptake and transport within plants. Additionally, K+ contributes to the maintenance of turgor pressure in plant cells [2,3]. In the soil, the concentration of K+ is relatively low and fluctuates greatly, presenting a challenge for plants to maintain optimal K+ levels for growth and development [4]. However, plants have evolved ability to absorb K+ efficiently from their surroundings and maintain relatively high levels within their cytosol [5]. In depth analysis of the molecular mechanisms of plant response and adaptation to limited K+ in soil can guide the cultivation of crops with higher K+ utilization efficiency and support sustainable agriculture.
The customary K+ concentration in plant cells is generally higher than that observed in the soil of most arable fields [6]. Consequently, most research endeavors have focused on how plants respond to low K+ (LK) environments. Plants have developed sophisticated mechanisms to sense changes in external K+ levels. K+ homeostasis is regulated by a diverse array of K+ channels and transport proteins, facilitating plant adaptation to K+ deficiency [7,8]. Ca2+-CBLs-CIPKs pathways play key roles in connecting K+ status with the activity of channels and transporters [9]. Specifically, to deal with K+ deficiency, the CBL1/9-CIPK1/9/23 complex enhances the uptake of K+ ions from the environment by activating the AKT1 potassium ion channel and HAK5 potassium ion transporter on the plasma membrane through phosphorylation. Concurrently, the CBL2/3-CIPK3/9/23/26 complex can activate the TPK1/3/5 potassium ion channel on the vacuolar membrane, thereby releasing vacuolar K+ into the cytoplasm [10–13]. HAB1/ABC1/ABC2/PP2CA phosphatases in the ABA signaling pathway can dephosphorylate the vacuolar membrane CBL2/3 and influence its degradation under high K+ (HK) conditions, serving a negative regulatory role in the plant response to LK stress [14]. Moreover, the target of the rapamycin complex (TORC) can negatively regulate the activity of CBL2/3-CIPKs, which in turn can inhibit TORC. The two coordinate plant response and adaptation to K nutrient status in the environment [9]. However, the understanding of the upstream regulatory networks of Ca2+-CBL-CIPK pathway and TORC module in LK responses remains limited.
Auxin, a vital hormone regulating plant growth and development and acts on the distribution of plant roots [15]. In the root of Arabidopsis thaliana, auxin is symmetrically distributed in the root cap and meristem, and the concentration of auxin in each tissue is coordinated by inflow and efflux [16]. The auxin efflux carriers formed by PIN-FORMED (PIN) proteins and the auxin influx carriers formed by AUXIN RESISTANT 1 (AUX1) protein in the plasma membrane are essential for polar auxin transport [17–20]. Research has shown an intriguing interplay between the K+ and auxin signaling pathways. K+ deficiency at the root tip can inhibit auxin transport, leading to uneven auxin distribution in the central cylindrical cells of the upper part of the root tip, further accumulation inhibits primary root growth [21–23].
In eukaryotes, multiple post-translational modifications of histones in chromatin, such as acetylation, methylation, phosphorylation, and ubiquitination, can dynamically and reversibly regulate chromatin structure, thereby regulating the expression patterns of genes wrapped around core histones [24,25]. Increasing evidence highlights histone modification plays essential roles in plant response to environmental stress. For example, H3K27me and H3K27ac mediate transcriptional dynamics and contribute to root development or nitrogen metabolic processes in wheat [26]. Histone deacetylase 6 (HDA6), a class I member of the histone deacetylase (HDAC) family, physically interacts with ABI5 to decrease apple drought tolerance [27]. HDA19 was involved in both root cell elongation and expression regulation of some phosphate starvation response genes under Pi-deficiency conditions [28]. Histone Deacetylase Complex 1 (HDC1) is a component of histone deacetylation complex. A previous study showed that HDC1-mediated histone H3 deacetylation represses the transcriptional activation of genes involved in Pi starvation responses in Arabidopsis [29]. Knockout/overexpression of HDC1 increases/decreases the sensitivity of plants to abscisic acid (ABA) and to salt stress [30], and the recent work reveals that HDC1 responds to salt stress via a dual mechanism involving both histone deacetylation and histone methylation [31]. However, the function and mechanism of HDC1 in response to other stresses such as low potassium and low nitrogen still need to be revealed.
In the present study, we used a HDC1 mutant line hdc1-2 to investigate the role of histone acetylation in primary root growth in response to K+ deficiency in Arabidopsis thaliana. HDC1 regulates the root growth under LK stress by regulating PIN-mediated polar auxin transport. We found that the inhibition of hdc1-1 on primary root growth was increased under K+ deficiency compared with WT plants. Auxin signal transduction is necessary for root growth and development. Taken together, our results suggest that HDC1 is a negative regulator of primary root growth under K+ deficiency.
2. Results
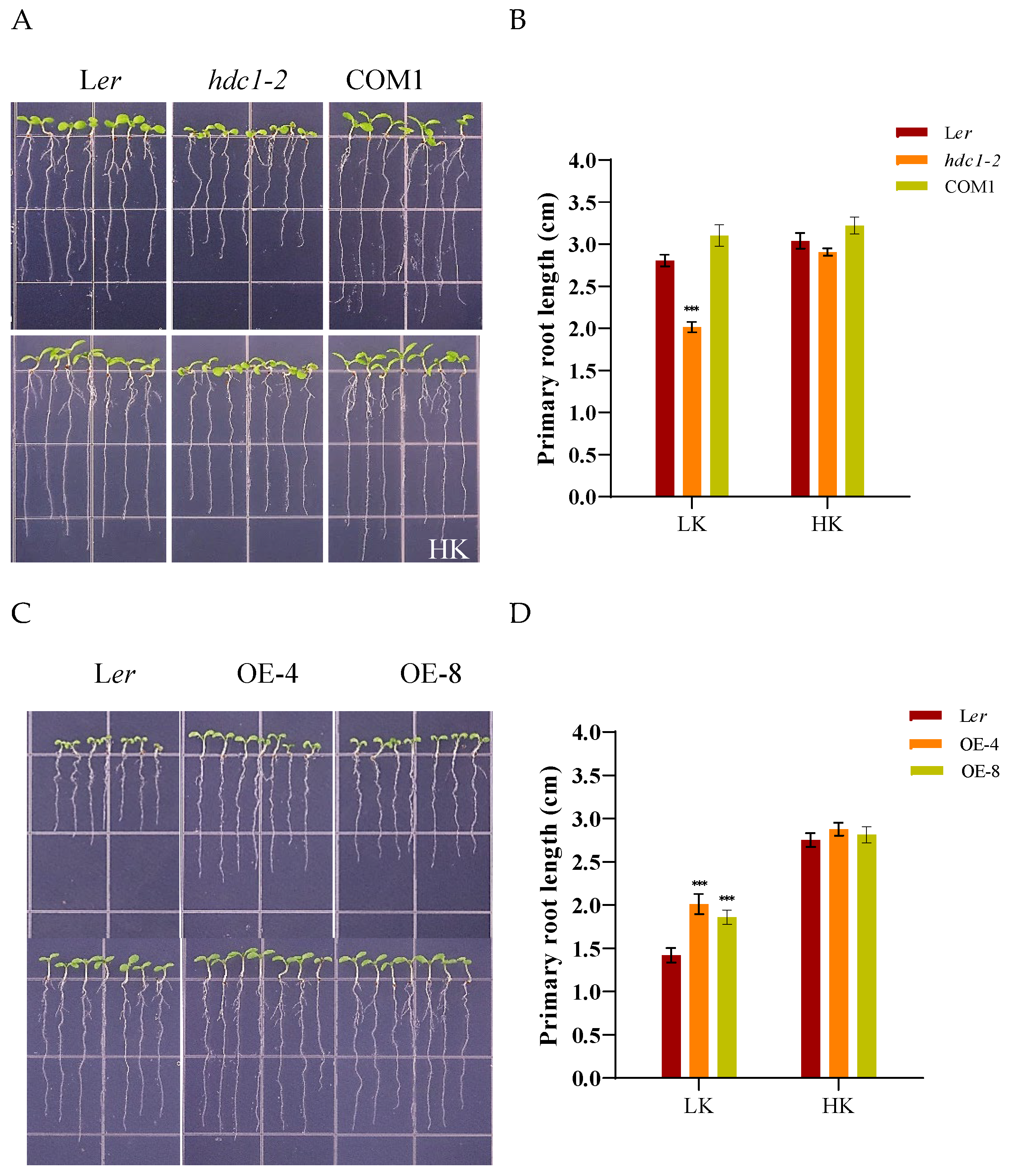
2.1. The Primary Root Growth of hdc1-2 Mutant is Inhibited under low-K+ Conditions
To investigate the role of HDC1 in root growth under low K
+ stress, we observed the growth of a homozygous mutant
hdc1-2, which has been generated through EMS mutagenesis in the L
er ecotype of
Arabidopsis and verified through complementary transformation experiments, under various K
+ conditions. When plants were germinated and cultivated on low-K
+ (LK, 50 μM) medium for 7 days, the
hdc1-2 mutant exhibited significantly greater inhibition of primary root growth compared to L
er and the complementation line (COM1) plants (
Figure 1A,B). However, under high-K
+ (HK, 5 mM) medium,
hdc1-2 showed a marginally shorter primary root compared to L
er and COM1 (
Figure 1A,B). These observations suggest that the short root phenotype of the
hdc1-2 mutant under LK stress is attributable to the loss of
HDC1 function.
To further corroborate this conclusion, we generated over-expression lines of
ProHDC1:HDC1. After 7 days of growth on LK medium, the primary root length of these two over-expression lines (OE-4 and OE-8) was significantly longer than that of the wild-type (L
er) (
Figure 1C,D). This phenotype indicates that
HDC1 is involved in root growth regulation under LK conditions.
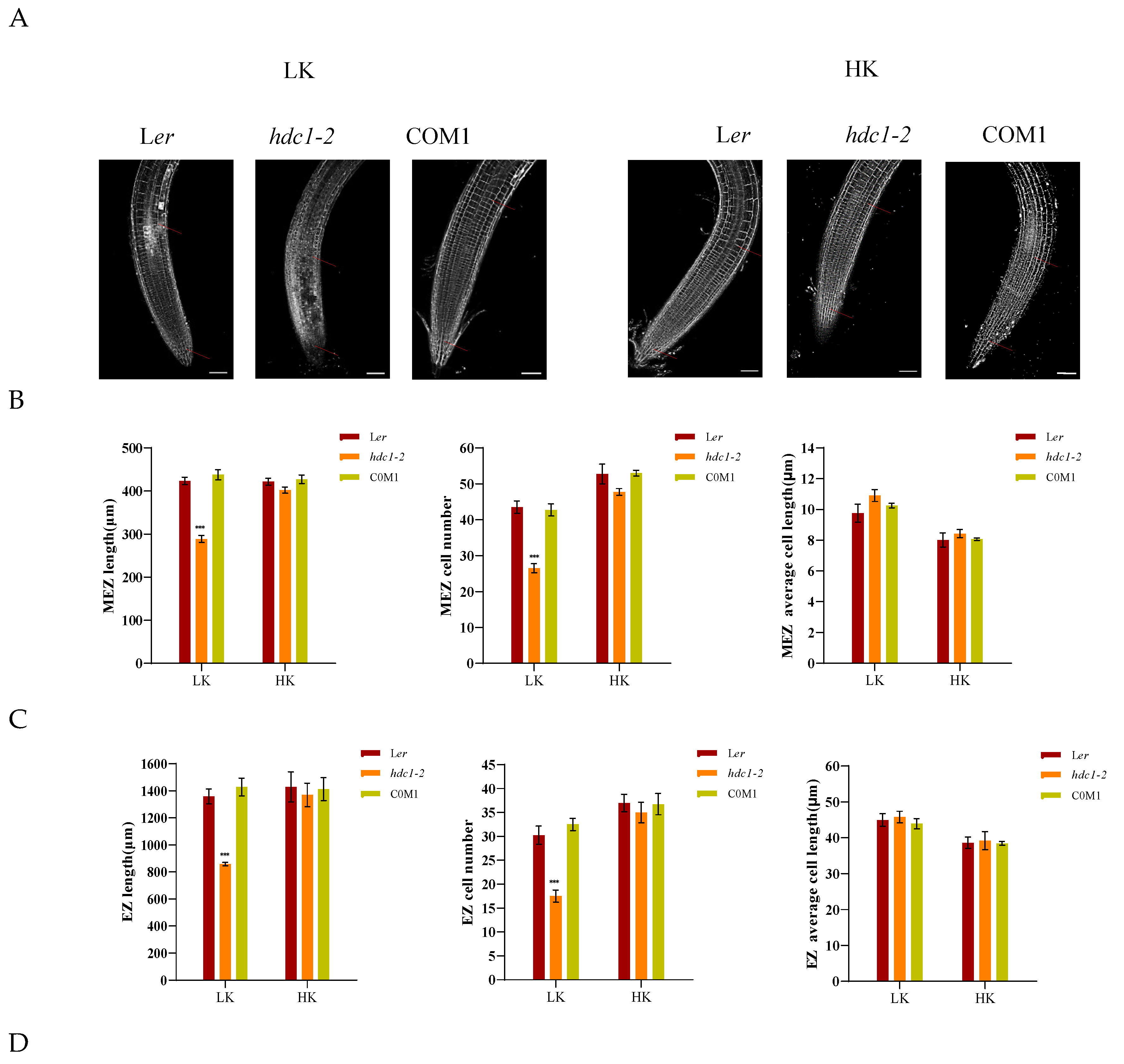
2.2. Root Meristem Activity Is Reduced in hdc1-2 Mutant under low-K+ Conditions
A dynamic balance between cell division and cell differentiation ensures continuous root growth [32,33], and root developmental plasticity relies on changes in the apical meristem’s activity [34].To elucidate the underlying mechanism responsible for the short primary roots of
hdc1-2, we observed and measured the different zones within the primary roots, specifically the meristem zone (MEZ), the elongation zone (EZ), and the maturation zone (MAZ). Under HK conditions, no significant difference in root growth was found between L
er and
hdc1-2 (
Figure 2). However, under LK conditions, all the tested zones were shorter in
hdc1-2 than in L
er, due to a reduction in cell numbers rather than cell length in mutant roots (
Figure 2B–D). This suggested that under LK conditions, the meristem activity of
hdc1-2 may be impaired (
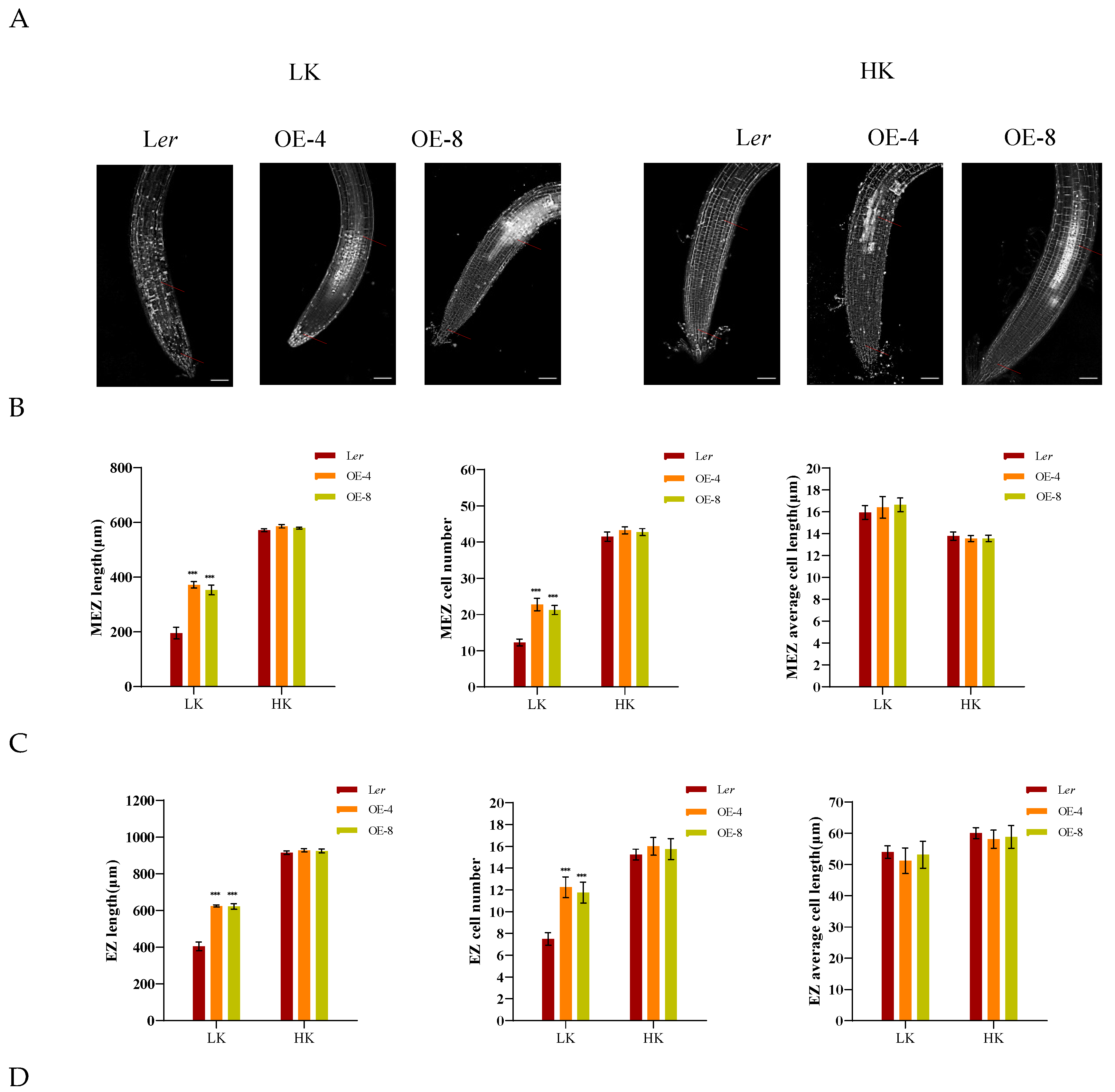
Figure 2A), which leads to a decrease in the number of root cells. At the same time, observation and measurement were conducted on different regions of the main root of overexpressed (OE) lines. Under LK conditions, due to an increase in the number of cells in the root, all test regions of the OE-4 and OE-8 transgenic plants were longer than L
er, but there was no difference under HK conditions (
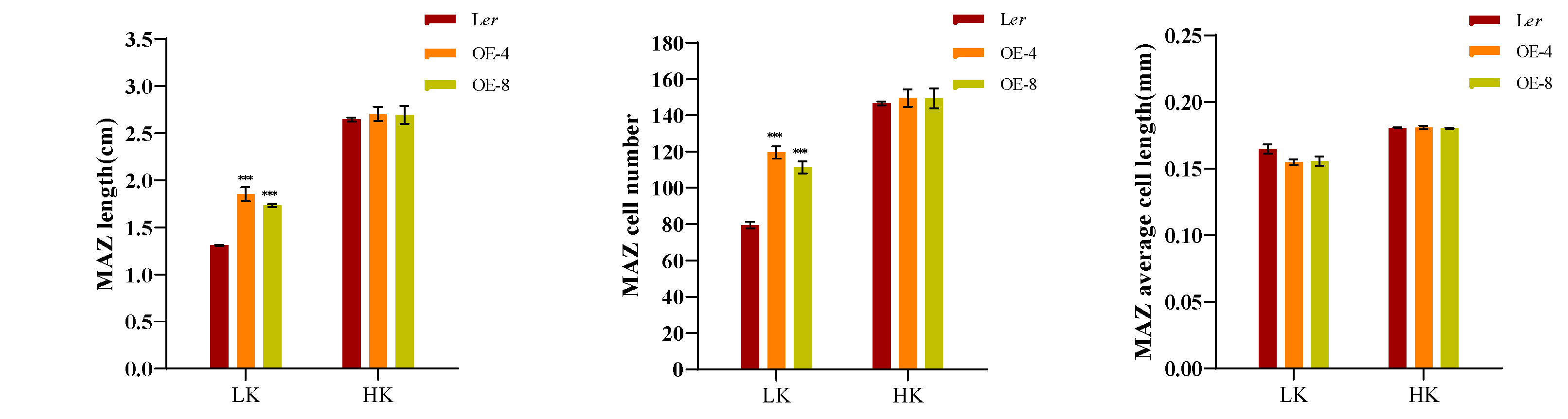
Figure 3B–D). These results further indicate the regulation of root meristem activity by HDC1.
2.3. Expression Pattern of HDC1 and Its Responses to K+ Availability
The phenotypes of impaired root growth and cell division suggested a potential role of HDC1 in root meristem responses to K
+ deficiency. Consequently, we examined the transcript abundance of
HDC1 in the roots under LK and HK conditions. As shown in
Figure 4A, the expression level of
HDC1 remained unaffected by K
+ deficiency in comparison to plants grown under sufficient K
+. To verify and further analyze the HDC1 expression pattern and its response to K
+ availability, we generated transgenic plants (
ProHDC1:GUS) carrying the marker gene GUS driven by the native
HDC1 promoter in L
er ecotype. GUS staining revealed that HDC1 was ubiquitously expressed in
Arabidopsis at the vegetative seedling stage, with GUS activity more pronounced in the root tip than in other parts of the root (
Figure 4B), consistent with a previous study in Col-0 ecotype [29] and a potential role of HDC1 in root meristem responses to K
+ deficiency. Subsequently, randomly selected
ProHDC1:GUS lines were subjected to LK or HK treatment for 7 days. Samples were collected for staining observation. As demonstrated in
Figure 4C, strong GUS activity at root tips could not be differentiated between LK and HK treatments.
2.4. The Root Growth Phenotype of hdc1-2 under low-k Conditions Is Controlled by Auxin Signaling
Previous research has uncovered that auxin plays a crucial role in regulating cell division, elongation, and differentiation, as well as in the root’s response to environmental stimuli [35,36]. Specifically, studies have demonstrated that the root growth phenotype under low K
+ conditions is regulated by auxin signaling in
Arabidopsis [37]. Given that L
er and
hdc1-2 exhibited different root growth phenotypes under LK conditions. We hypothesized that auxin signaling may be involved in this root growth phenotype. To investigate whether the short-root phenotype of
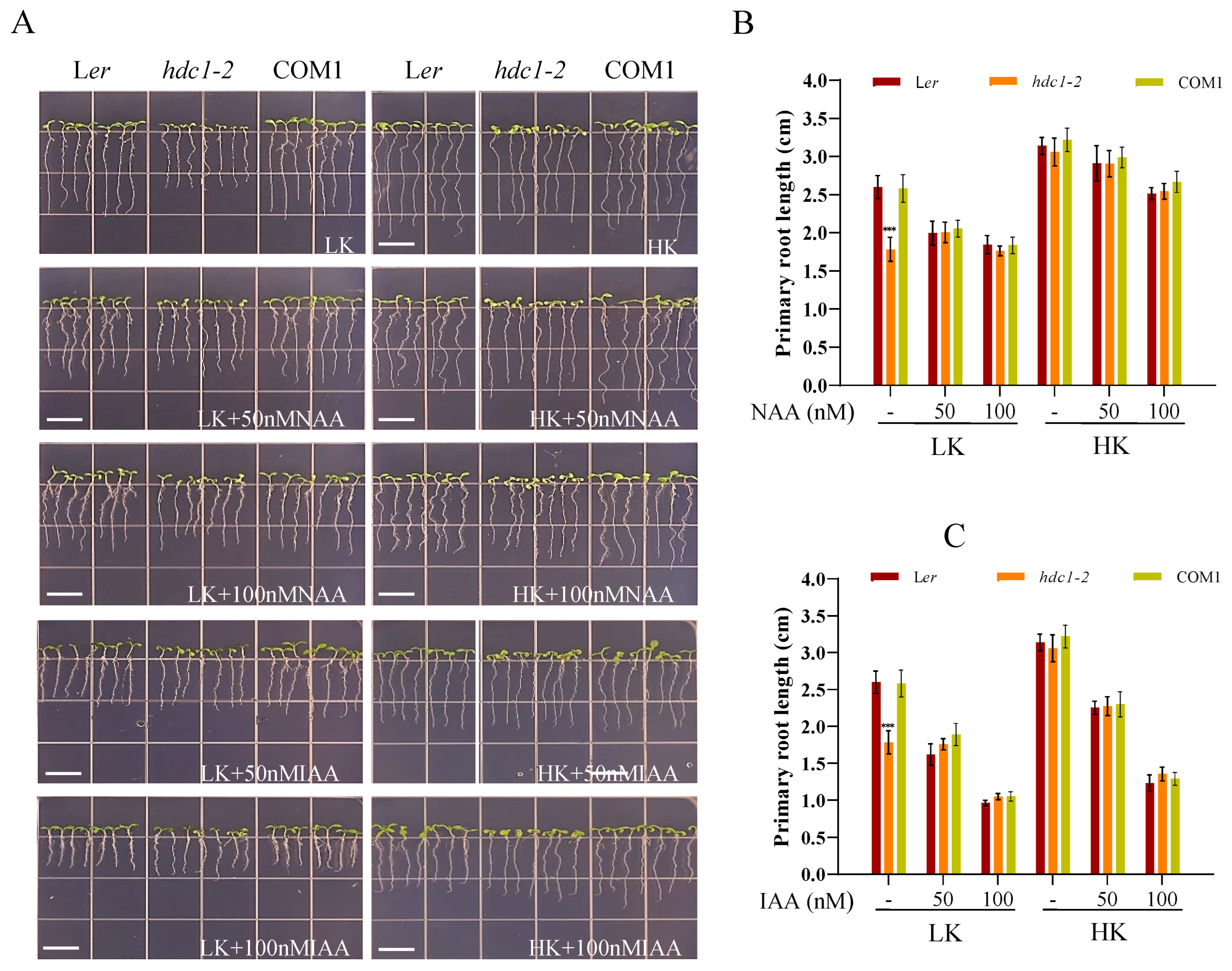
hdc1-2 was due to the reduction of auxin levels, we supplemented the medium with NAA (1- naphthalene acetic acid, and the root growth phenotype was indeed affected. Under LK medium, the addition of NAA inhibited the primary root growth of L
er and COM1, and no significant difference in primary root length was observed among L
er, COM1, and
hdc1-2, as the root growth of
hdc1-2 remained largely unaltered after NAA treatment (
Figure 5). This observation suggests that the sensitivity of
hdc1-2 to NAA/IAA is diminished under LK conditions. Correspondingly, the overexpression lines (OE-4 and OE-8) maintained longer primary root length than L
er despite the inhibitory effect of NAA addition Under LK medium. Similar phenotypes were also observed under exogenous IAA treatment (
Figure 6). These findings suggest that HDC1 positively regulates the sensitivity of
Arabidopsis primary roots to auxin under LK conditions.
Auxin export vector proteins PIN1, PIN2, PIN3 and input vector protein AUX1 mediate rapid auxin signaling in roots [38–40]. To further investigate whether the auxin transport pathway is involved in the growth retardation phenotype of
hdc1-2 under LK condition, we examined the expression of
PIN1,
PIN2,
PIN3 and
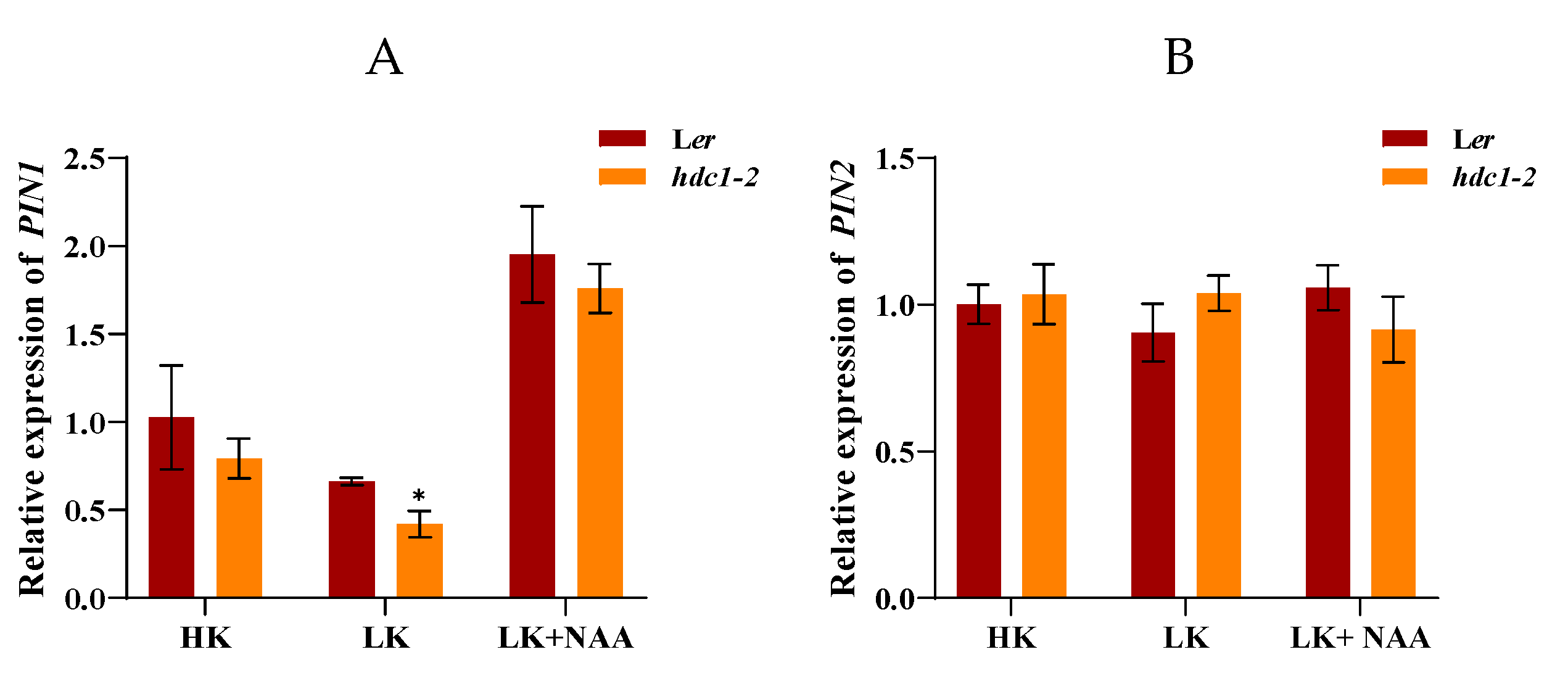
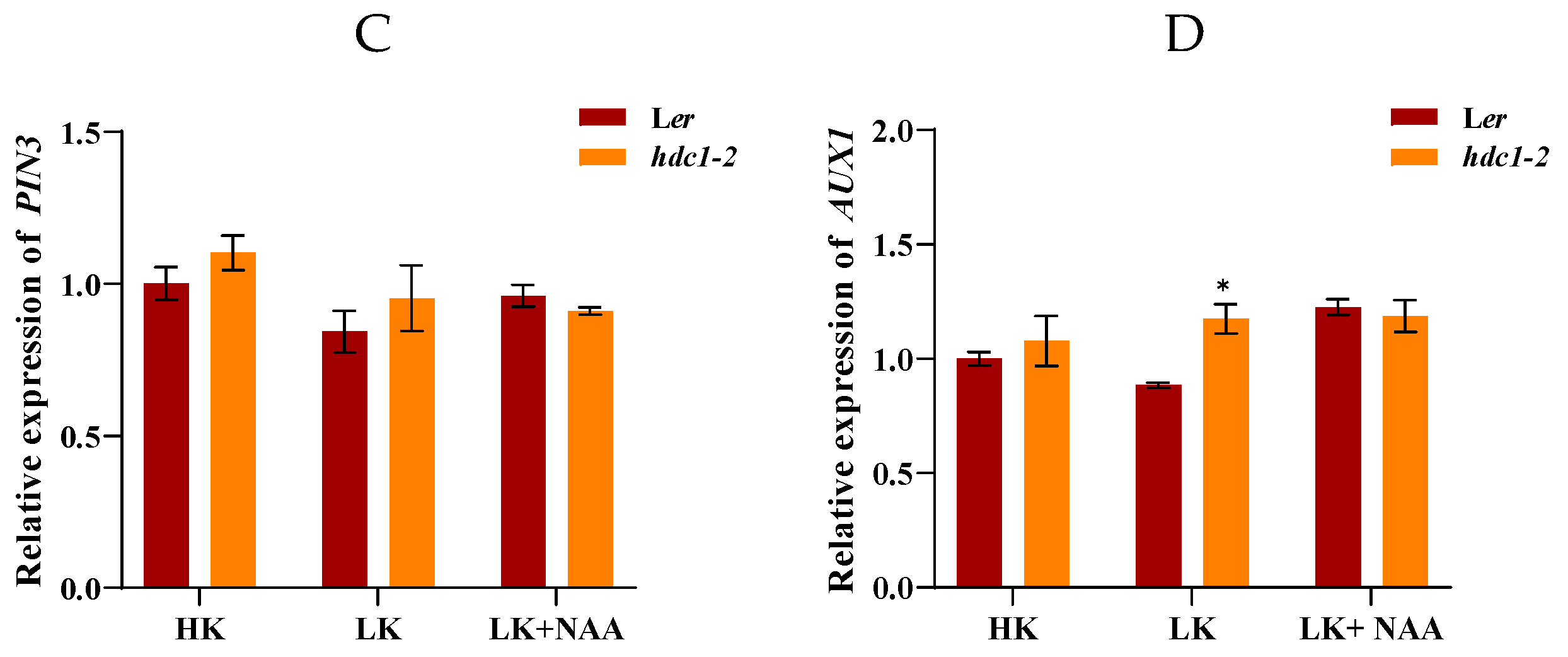
AUX1. RT-qPCR results indicate that the expression of
PIN1 was upregulated and the expression of
AUX1 was significantly downregulated in
hdc1-2 under LK condition (
Figure 7). Upon application of exogenous NAA, a significant increase in the expression of
PIN1 in
hdc1-2 was observed, suggesting that exogenous NAA can partially restore the inhibited auxin transport levels in LK treatment (
Figure 7A). Considering the reduced sensitivity of
hdc1-2 to NAA/IAA under LK conditions, it can infer that HDC1 regulates root growth both by influencing the PIN1 and AUX1 mediated auxin transport and subsequently auxin sensation.
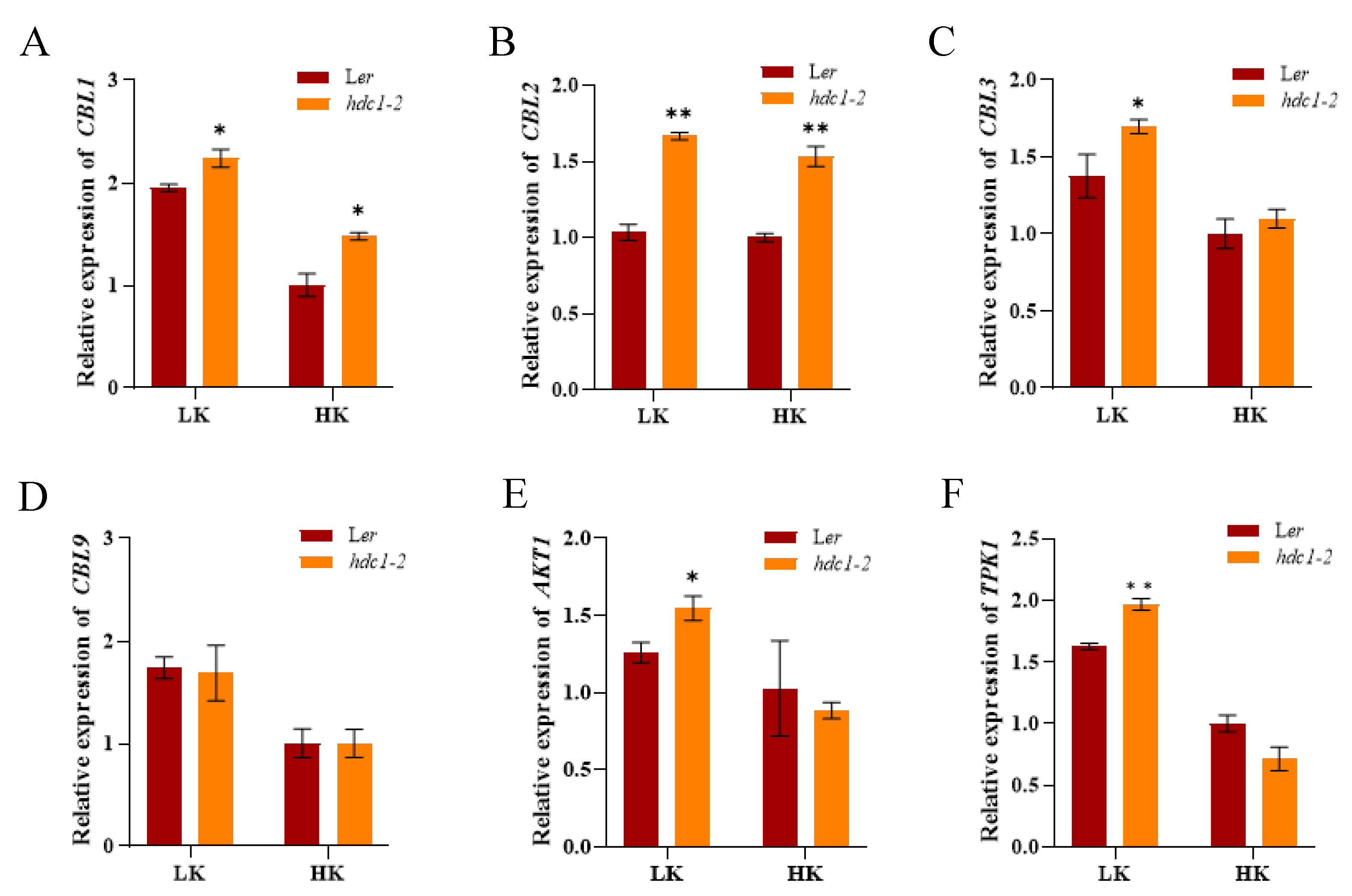
2.5. HDC1 Negatively Regulates the Expression of CBL-CIPK Module Genes
It is well-established that the dual CBL-CIPK pathways regulating K
+ channels and transporters in response to changes in K
+ status in the environment. Given that HDC1 is a component of HDAC complexes, and its knockout promotes histone acetylation and gene expression [30]. We examined the transcript levels of genes in “CBL-CIPK-channel” modules responding to external K
+, and found that their expression was induced by LK (
Figure 8), consistent with the previous reports. There were no differences in transcript level of
CBL9 between
hdc1-2 and L
er although the expression of
CBL9 was induced by LK stress like other genes (
Figure 8B). However, the expression of
CBL1 and
CBL2 was considerably higher in the
hdc1-2 than in L
er both under both HK and LK condition (
Figure 8A,C). Additionally, the expression of
CBL3,
AKT1 and
TPK1 was also significantly increased in
hdc1-2 under LK stress (
Figure 8D–F). These results indicate that HDC1 acts upstream of “CBL-CIPK-channel” modules under K
+ deficiency.
3. Discussion
Histone acetylation/deacetylation, mediates by histone acetyltransferases (HATs) and histone deacetylases (HDACs), is a reversible epigenetic switch regulating gene expression in plants. Studies have demonstrated that HDC1 is a shared subunit of the HDA6 and HDA19 HDAC complexes [42]. HDC1 enables multiple protein interactions in HDAC complexes, participates in the regulation of flowering time, fruit growth, abscisic acid sensitivity, as well as responses to salt and low phosphorus stress [29–31,43]. Plant roots perceive abiotic stresses in the soil and adapt their architecture accordingly to maintain survival and development [44,45].To better characterize the involvement of HDC1 in the nutritional stress response of plants, we investigated the effect of potassium supplement on primary root growth in mutant and over-expression lines of
HDC1 in
Arabidopsis. Our findings indicate that
HDC1 is essential for the maintenance of primary root growth under LK conditions in
Arabidopsis (
Figure 1). Further analysis demonstrates that
HDC1 is crucial for the preservation of meristem activity and cell division under LK conditions (
Figure 2 and
Figure 3), thereby exerting a regulatory function on the primary root growth. This is supported by the observation that HDC1 exhibits strong expression in root tip (
Figure 4). A previous study showed that HDC1 is post-translationally regulated in response to Pi deficiency. Our in-planta promoter-GUS activity assay results revealed that strong GUS activity at root tips could not be differentiated between LK and HK treatments, consistent with the root-specific RT-qPCR analysis results of
HDC1 (
Figure 4). Considering these findings collectively, we propose that HDC1 is also post-translationally regulated in response to K
+ deficiency.
The plasticity of the plant root system is largely related to the phytohormone metabolism and signaling. For example, Higher CuSO4 concentrations inhibited primary root elongation of
Arabidopsis seedlings by modulating auxin distribution through PIN1, but not PIN2 or AUX1 [46]. A subsequent study demonstrated that high concentrations of glucose also reduced the size of the root meristem zone by repressing PIN1 accumulation and, thereby, reducing auxin levels [47]. Previous research has indicated a link between LK perception and auxin signaling [37]. Later research has shown that the AKT1-mediated LK response is primarily through PIN1 proteins [48]. In the current study, we found that HDC1 positively regulates the sensitivity of root growth to auxin because the root growth of
hdc1-2 was almost unchanged and
HDC1-overexpression lines still have longer primary roots than L
er after NAA treatment under LK (
Figure 5 and
Figure 6). HDC1 may regulate root growth both by affecting auxin transport and subsequently auxin sensation since the expression of
PIN1 and
AUX1 were significantly altered (
Figure 7).
Plants mainly rely on two strategies to cope with low potassium stress in the environment: the CBL1/9-CIPKs complex and the CBL2/3-CIPKs complex. The CBL1/9-CIPK1/9/23 complex activates potassium channel AKT1 and potassium transporter HAK5 in plasma membrane in response to low K
+ stress. The tonoplast-localized CBL2 and CBL3 interact with four CIPKs that, in turn, propel K
+ remobilization from the vacuole store through the activating of TPK-type K
+ channels [49,50]. Previous research has demonstrated that “CBL-CIPK-channel” modules respond to external K
+ by altering their protein abundance, which occurs at both the transcriptional and post-translational level [14]. However, limited information is available regarding the upstream regulators of “CBL-CIPK-channel” modules in plants. Our organ-specific RT-qPCR analyses unraveled that HDC1 negatively regulated the expression of
CBL1, CBL2,
CBL3,
AKT1 and
TPK1 (
Figure 8). This observation aligns with the role of HDC1-mediated histone deacetylation in regulating chromatin structure and gene expression. A recent study reported that HDC1 attenuates salt stress responses by moderating the salt-induced H3K9/14 hyperacetylation and transcriptional activation of stress-induced genes [31]. Considering that “CBL-CIPK-channel” modules were induced by LK, it is plausible that HDC1 also attenuates LK responses via deacetylation of LK-inducible CBL-CIPK-K
+ channel-related genes.
HDACs play essential roles in the regulation of plant growth and development as well as in response to environmental changes. In addition to catalyzing histone deacetylation, some HDACs also deacetylate non-histone proteins and thereby regulate multiple pathways [41]. HDC1 in plants is encoded by a single copy gene and functions as one of the components of HDACs. Previous studies have showed that HDC1 mediates histone H3 deacetylation to inhibit LPR and ALMT1 transcription and regulate root system development in Arabidopsis under Pi starvation [29]. The present results indicate that HDC1 regulates the auxin-dependent primary root growth and the expression of CBLs–CIPKs pathway genes. Considering the findings that CBLs-CIPKs pathway modulates auxin transporters and coordinates auxin-mediated root responses to abiotic stresses [51,52]. We hypothesized that HDC1 mediates histone H3 deacetylation to inhibit CBLs-CIPKs signaling pathways, thus modulating auxin transporters and regulating auxin-mediated root responses to LK stress.
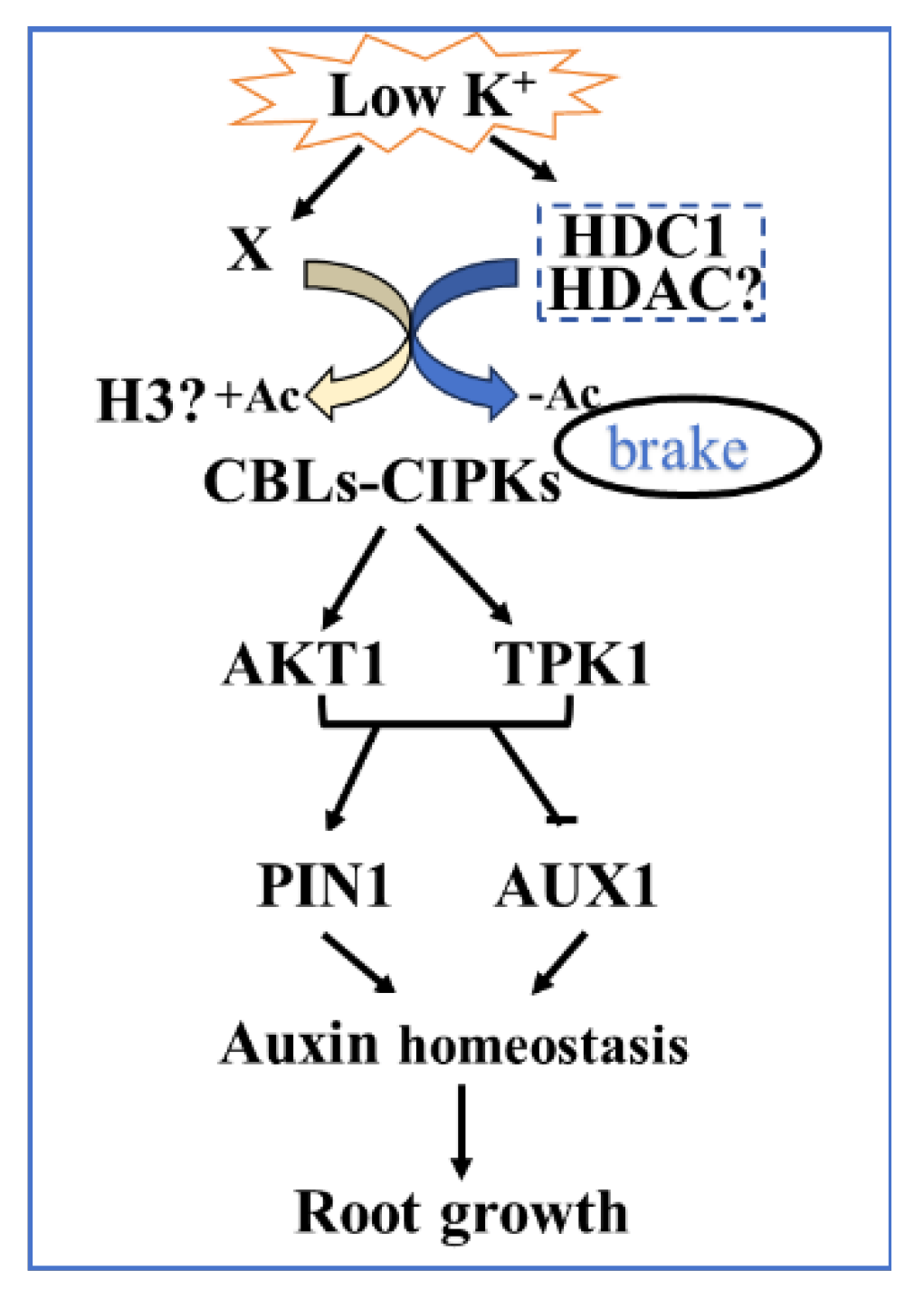
In conclusion, our findings establish a novel molecular pathway of HDC1-controlled root growth and highlights the molecular link between CBLs-CIPKs pathway and auxin signaling in response to K
+ deficiency. We propose that HDC1 pulls the brake on LK responses via deacetylation of LK-inducible “CBL-CIPK-K
+ channel” modules to counteract LK-induced histone hyperacetylation (
Figure 9). This mechanism reinforces the role of HDC1 as a multi-functional scaffolding protein interact with HDACs. Future research should investigate the histone acetylation levels of CBLs–CIPKs pathway and auxin pathway gene sites and identify the HDC1 interactor in response to LK stress.
4. Materials and Methods
4.1. Plant Materials
All Arabidopsis thaliana stocks were in the Landsberg erecta (Ler) background. hdc1-2 was segregated from ag-11 hdc1-2 double mutant, which was obtained by EMS mutagenesis with ag-11. HDC1 full-length genomic sequence was constructed into pGWB616 driven by the HDC1 native promoter and transformed into Ler and hdc1-2 to obtain overexpression lines (OE) and complementation lines (COM). Arabidopsis transformation with Agrobacterium (strain GV3101) was conducted using the floral dip method [50].
4.2. Phenotype Analyses and Plant Growth Conditions
Seed were surface sterilized with a mixed solutions of 75% alcohol and washed with sterilized distilled water, and then incubated in dark at 4 °C for 7 days. The seeds were then placed on HK (5 mM) or LK (50 μM) medium containing 1% (w/v) sucrose and 0.9% (w/v) agar, and grown at 22°C under constant illumination at 60 μmol/m2 /s for 7d. HK medium and LK medium were modified from MS (pH 5.8). NH4NO3 and KNO3 were removed, and KH2PO4 was replaced by H3PO4. The medium contained 1.5 mM MgSO4.7H2O, 85 μL H3PO4, 2.99 mM Ca (NO3)2.4H2O, 0.1 mM MnSO4.4H2O, 5 μM KI, 0.1 μM CuSO4.5H2O, 0.1 mM H3BO3, 0.1 μM CoCl2.6H2O, 0.03 mM ZnSO4.7H2O, 10 μM Na2MoO4.2H2O, 0.1 mM FeSO4.7H2O, and 0.1 mM Na2EDTA [53]. Potassium came almost entirely from agar in the LK medium. For preparing HK medium, KCl was added until the final potassium concentration was 5 mM.
For seed harvesting and hybridization, Arabidopsis plants were cultured in the potting soil mixture (rich soil: vermiculite ¼ 2:1, v/v) and kept in growth chambers at 22°C with illumination at 120 μmol/m2 /s for a 16 h daily light period. The relative humidity of the growth chamber remained at ~70% (+5%).
4.3. Root Growth Phenotype Analyses
The seeds were germinated and grown vertically on HK or LK medium for 7 days and NAA or IAA was added after the medium was autoclaved at 115°C for 15 min and cooled to 60°C. NAA (1-naphthaleneacetic acid) was ordered from Sigma. Photographs were taken at the indicated time points. Root growth in the phenotype analyses represents the length of primary root growth after the seedlings were transplanted. Data were derived from three biological replicates in each experiment.
4.4. Confocal Microscopy and Meristem Cell Number Counting
For primary root tip observation, seedlings directly germinated on HK medium and LK medium for 7 days were imaged by Canon EOS R6 and measured the lengths using Digimizer software. The plants were immersed in propidium iodide solution (10 mg/mL) for 1 min, and washed with deionized water for 2 min. The roots were then observed using a confocal laser scanning microscope (Zeiss LSM710). The PI staining excitation and emission wavelengths were 536 and 617 nm, respectively. The meristem cell number was counted based on the files of cortex cells from the quiescent center to the first elongated cell.
4.5. Histochemical GUS Staining
1158 bp was selected as the promoter of HDC1 and cloned into pGWB162 with GUS coding region. The ProHDC1: GUS construct was then transferred to Ler through Agrobacterium mediated transformation. About 3 independent transgenic lines expressing ProHDC1: GUS were analyzed. Seven-day-old GUS reporter transgenic seedlings were transferred to the HK or LK medium. The tissues or hand-cut sections the transgenic plants were incubated in commercial GUS staining solution at 37℃ overnight and distained in 75% ethanol. Images were taken directly or under the stereomicroscope.
4.6. RT-qPCR Analysis
Total RNA was extracted from roots of 7-day-old seedlings by using TRIzol reagent (Invitrogen) and then treated with DNase I (RNase Free, Takara) to eliminate genomic DNA contamination. 1 μg of total RNA was reverse transcribed using NovoScript Plus All-in-one 1st Strand cDNA Synthesis Super Mix (Novoprotein). The quantitative real-time PCR analysis was performed on the Bio-Rad CFX-96 Real-time PCR system (Bio-Rad) using 2×ChamQ Universal SYBR qPCR Master Mix (Vazyme). The relative expression levels were calculated using the 2
−ΔΔCT method and Actin2 was used as an internal control. Three biological replicates were performed for each sample. The primers used for qPCR analysis are listed in Supplemental
Table S1.
4.7. Accession Numbers
Sequence data from this article can be found in TAIR (
www.Arabidopsis.org) under the following accession numbers:
HDC1 (At5g08450),
PIN1(At1G73590),
PIN2 (At5G08720),
PIN3 (At1G70940),
AUX1(At2G38120),
CBL1(At4g17625),
CBL9(At5g47100),
CBL2(At5G55990),
CBL3(At4G26570),
AKT1(At2G26650),
TPK1(At5G55630).
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Table S1: Primers used in this study.
Author Contributions
X.K. and H.C. designed and performed the experiments, conducted data analyses, and wrote the manuscript draft. J.X. and J.Z. participated in the phenotyping and molecular biology experiments; Q.L. assisted with figure preparation and Arabidopsis planting. Y.S. and C.H. participated in the phenotyping and revised the manuscript. R.W. helped edit the manuscript. W.L. and Z.H. conceived the project, supervised the study, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: This research was funded by the Natural Science Foundation of Hunan Province (2024JJ5196), the Scientific Research Fund of Hunan Provincial Education Department (23A0189), the National Natural Science Foundation of China (30900099), the National Key Research and Development Program of China (2018YFD1000404).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Sardans, J.; Peñuelas, J. Potassium control of plant functions: Ecological and agricultural implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Dreyer, I.; Uozumi, N. Potassium channels in plant cells. FEBS Journal 2011, 278, 4293–4303. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.M.; Ghosh, T.K.; Kabir, A.H. , Abdelrahman, M., Khan, M.A.R., Tran, L.S.P. Potassium in plant physiological adaptation to abiotic stresses. Plant Physiology and Biochemistry 2022, 186, 279–289. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.H. Regulation of potassium transport and signaling in plants. Current opinion in plant biology 2017, 39, 123–128. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.F.; Wu, W.H. Potassium and phosphorus transport and signaling in plants. Journal of Integrative Plant Biology 2021, 63, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.J.; Wang, C.; Li, K.; Luan, S. The CBL–CIPK calcium signaling network: unified paradigm from 20 years of discoveries. Trends in Plant Science 2020, 25, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, W.H. Potassium transport and signaling in higher plants. Annual review of plant biology 2013, 64, 451–476. [Google Scholar] [CrossRef] [PubMed]
- Sassi, A.; Khan, I.; Véry, A.A.; Sentenac, H. Molecular biology of K+ transport across the plant cell membrane: What do we learn from Arabidopsis and rice model plants? Biochimie et Physiologie Moléculaire des Plantes 2012, 47–54. [Google Scholar]
- Li, K.L.; Xue, H.; Tang, R.J.; Luan, S. TORC pathway intersects with a calcium sensor kinase network to regulate potassium sensing in Arabidopsis. Proceedings of the National Academy of Sciences 2023, 120, e2316011120. [Google Scholar] [CrossRef]
- Ragel, P.; Ródenas, R.; García-Martín, E.; Andrés, Z.; Villalta, I.; Nieves-Cordones, M.; Rubio, F. The CBL-interacting protein kinase CIPK23 regulates HAK5-mediated high-affinity K+ uptake in Arabidopsis roots. Plant Physiology 2015, 169, 2863–2873. [Google Scholar]
- Kaya, C.; Uğurlar, F.; Adamakis, I.D.S. Molecular mechanisms of CBL-CIPK signaling pathway in plant abiotic stress tolerance and hormone crosstalk. International Journal of Molecular Sciences 2024, 25, 5043. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.D.; Chen, L.Q.; Wang, Y.; Liu, L.L.; He, L.; Wu, W.H. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 2006, 125, 1347–1360. [Google Scholar] [CrossRef]
- Lara, A.; Ródenas, R.; Andrés, Z.; Martínez, V.; Quintero, F. J.; Nieves-Cordones, M.; Rubio, F. Arabidopsis K+ transporter HAK5-mediated high-affinity root K+ uptake is regulated by protein kinases CIPK1 and CIPK9. Journal of experimental botany 2020, 71, 5053–5060. [Google Scholar] [CrossRef] [PubMed]
- Li, K.L.; Tang, R.J.; Wang, C.; Luan, S. Potassium nutrient status drives posttranslational regulation of a low-K response network in Arabidopsis. Nature Communications 2023, 14, 360. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, T.P.; Steinmacher, D. Plant growth regulation in cell and tissue culture in vitro. Plants 2024, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Band, L.R.; Wells, D.M.; Fozard, J.A.; Ghetiu, T.; French, A.P.; Pound, M.P.; Bennett, M.J. Systems analysis of auxin transport in the Arabidopsis root apex. The Plant Cell 2014, 26, 862–875. [Google Scholar] [CrossRef] [PubMed]
- Grieneisen, V. A.; Xu, J.; Marée, A. F.; Hogeweg, P.; Scheres, B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 2007, 449, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xia, J.; Hong, J.; Zhang, C.; Wei, H.; Ying, W.; Sun, L. Structural insights into auxin recognition and efflux by Arabidopsis PIN1. Nature 2022, 609, 611–615. [Google Scholar] [CrossRef]
- Su, N.; Zhu, A.; Tao, X.; Ding, Z. J.; Chang, S.; Ye, F.; Guo, J. Structures and mechanisms of the Arabidopsis auxin transporter PIN3. Nature 2022, 609, 616–621. [Google Scholar] [CrossRef]
- Wiśniewska, J.; Kęsy, J.; Mucha, N.; Tyburski, J. Auxin resistant 1 gene (AUX1) mediates auxin effect on Arabidopsis thaliana callus growth by regulating its content and distribution pattern. Journal of Plant Physiology 2024, 293, 154168. [Google Scholar] [CrossRef]
- Shin, R.; Burch, A.Y.; Huppert, K.A.; Tiwari, S.B.; Murphy, A.S.; Guilfoyle, T.J.; Schachtman, D.P. The Arabidopsis transcription factor MYB77 modulates auxin signal transduction. The Plant Cell 2007, 19, 2440–2453. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, R.; Zhao, S.; Lu, C.; Zhu, Z.; Li, H. Transporter NRT1. 5/NPF7. 3 suppresses primary root growth under low K+ stress by regulating the degradation of PIN-FORMED2. BMC Plant Biology 2022, 22, 330. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Agullo, F.; Rigas, S.; Desbrosses, G.; Dolan, L.; Hatzopoulos, P.; Grabov, A. Potassium carrier TRH1 is required for auxin transport in Arabidopsis roots. Plant Journal for Cell & Molecular Biology 2004, 40, 523–535. [Google Scholar]
- Over, R.S.; Michaels, S.D. Open and Closed: The Roles of linker histones in plants and animals. Molecular Plant 2014, 7, 481–491. [Google Scholar] [CrossRef]
- Hergeth, S.P.; Schneider, R. The H1 linker histones: multifunctional proteins beyond the nucleosomal core particle. EMBO Reports 2015, 16, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jin, Z.; Cui, F.; Zhao, L.; Zhang, X.; Chen, J.; Xiao, J. Epigenetic modifications regulate cultivar-specific root development and metabolic adaptation to nitrogen availability in wheat. Nature Communications 2023, 14, 8238. [Google Scholar] [CrossRef]
- Li, W.; Deng, M.; Wang, S.; Wang, C.; Guo, M.; Song, Y.; Xu, J. HISTONE DEACETYLASE 6 interaction with ABSCISIC ACID-INSENSITIVE 5 decreases apple drought tolerance. Plant Physiology 2023, 193, 2711–2733. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wu, K,; Schmidt, W. The histone deacetylase HDA19 controls root cell elongation and modulates a subset of phosphate starvation responses in Arabidopsis. Scientific reports 2015, 5, 15708. [Google Scholar] [CrossRef]
- Xu, J.M.; Wang, Z.Q.; Wang, J.Y.; Li, P.F.; Jin, J.F.; Chen, W.W.; Yang, J. L. Low phosphate represses histone deacetylase complex1 to regulate root system architecture remodeling in Arabidopsis. New Phytologist 2020, 225, 1732–1745. [Google Scholar] [CrossRef]
- Perrella, G.; Lopez-Vernaza, M.A.; Carr, C.; Sani, E.; Gosselé, V.; Verduyn, C.; Amtmann, A. Histone deacetylase complex1 expression level titrates plant growth and abscisic acid sensitivity in Arabidopsis. The Plant Cell 2013, 25, 3491–3505. [Google Scholar] [CrossRef]
- Perrella, G.; Fasano, C.; Donald, N.A.; Daddiego, L.; Fang, W.; Martignago, D.; Amtmann, A. Histone Deacetylase Complex 1 and histone 1 epigenetically moderate stress responsiveness of Arabidopsis thaliana seedlings. New Phytologist 2024, 241, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Salvi, E.; Rutten, J.P.; Di Mambro, R.; Polverari, L.; Licursi, V.; Negri, R.; Ten Tusscher, K. A self-organized PLT/Auxin/ARR-B network controls the dynamics of root zonation development in Arabidopsis thaliana. Developmental cell 2020, 53, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Di Mambro, R.; Sabatini, S,; Dello Ioio, R. Patterning the axes: a lesson from the root. Plants 2018, 8, 8. [CrossRef] [PubMed]
- Scintu, D.; Scacchi, E.; Cazzaniga, F.; Vinciarelli, F.; De Vivo, M.; Shtin, M.; Dello Ioio, R. microRNA165 and 166 modulate response of the Arabidopsis root apical meristem to salt stress. Communications biology 2023, 6, 834. [Google Scholar] [CrossRef]
- Marhava, P.; Bassukas, A.E.L.; Zourelidou, M.; Kolb, M.; Moret, B.; Fastner, A.; Hardtke, C.S. A molecular rheostat adjusts auxin flux to promote root protophloem differentiation. Nature 2018, 558, 297–300. [Google Scholar] [CrossRef]
- Hu, Y.; Omary, M.; Hu, Y.; Doron, O.; Hoermayer, L.; Chen, Q.; Shani, E. Cell kinetics of auxin transport and activity in Arabidopsis root growth and skewing. Nature Communications 2021, 12, 1657. [Google Scholar] [CrossRef]
- Cao, Y.; Glass, A.D.; Crawford, N.M. Ammonium inhibition of Arabidopsis root growth can be reversed by potassium and by auxin resistance mutations aux1, axr1, and axr2. Plant Physiology 1993, 102, 983–989. [Google Scholar] [CrossRef]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef]
- Dindas, J.; Scherzer, S.; Roelfsema, M.R.G.; von Meyer, K.; Müller, H.M.; Al-Rasheid, K.A.S.; Hedrich, R. AUX1-mediated root hair auxin influx governs SCFTIR1/AFB-type Ca2+ signaling. Nature Communications 2018, 9, 1174. [Google Scholar] [CrossRef]
- Schroeder, M.M.; Gomez, M.Y.; McLain, N.; Gachomo, E.W. Bradyrhizobium japonicum IRAT FA3 alters Arabidopsis thaliana root architecture via regulation of auxin efflux transporters PIN2, PIN3, PIN7 and ABCB19. Molecular Plant-microbe Interactions 2022, 35, 215–229. [Google Scholar] [CrossRef]
- Cui, X.; Dard, A.; Reichheld, J.P.; Zhou, D.X. Multifaceted functions of histone deacetylases in stress response. Trends in Plant Science. 2023, 28, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.Q.; Chen, Q.; Lin, R.N.; Li, Y.Q.; Li, L.; Chen, S.; He, X.J. The HDA19 histone deacetylase complex is involved in the regulation of flowering time in a photoperiod-dependent manner. The Plant Journal 2019, 98, 448–464. [Google Scholar] [CrossRef]
- Ning, Y.Q.; Chen, Q.; Lin, R.N.; Li, Y.Q.; Li, L.; Chen, S.; He, X.J. The HDA19 histone deacetylase complex is involved in the regulation of flowering time in a photoperiod-dependent manner. The Plant Journal 2019, 98, 448–464. [Google Scholar] [CrossRef]
- Lamers, J.; Van Der Meer, T.; Testerink, C. How plants sense and respond to stressful environments. Plant Physiology 2020, 182, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Karlova, R.; Boer, D.; Hayes, S.; Testerink, C. Root plasticity under abiotic stress. Plant Physiology 2021, 187, 1057–1070. [Google Scholar] [CrossRef]
- Yuan, H.M.; Xu, H.H.; Liu, W.C.; Lu, Y.T. Copper regulates primary root elongation through PIN1-mediated auxin redistribution. Plant Signaling & Hehavior 2013, 8, 766–778. [Google Scholar]
- Yuan, T.T.; Xu, H.H.; Zhang, K.X.; Guo, T.T.; Lu, Y.T. Glucose inhibits root meristem growth via ABA INSENSITIVE 5, which represses PIN1 accumulation and auxin activity in Arabidopsis. Plant Cell & Environment 2014, 37, 1338–1350. [Google Scholar]
- Li, J.; Wu, W.H.; Wang, Y. Potassium channel AKT1 is involved in the auxin-mediated root growth inhibition in Arabidopsis response to low K+ stress. Journal of Integrative Plant Biology 2017, 59, 895–909. [Google Scholar] [CrossRef]
- Tang, R.J.; Zhao, F.G.; Yang, Y.; Wang, C.; Li, K.; Kleist, T.J.; Luan, S. A calcium signalling network activates vacuolar K+ remobilization to enable plant adaptation to low-K environments. Nature Plants 2020, 6, 384–393. [Google Scholar] [CrossRef]
- Niu, F.; Cui, X.; Yang, B.; Wang, R.; Zhao, P.; Zhao, X.; Jiang, Y.Q. WRKY6 transcription factor modulates root potassium acquisition through promoting expression of AKT1 in Arabidopsis. The Plant Journal 2024, 118, 1652–1667. [Google Scholar] [CrossRef]
- Zeeshan, M.; Qiu, C.W.; Naz, S.; Cao, F.; Wu, F. Genome-wide discovery of miRNAs with differential expression patterns in responses to salinity in the two contrasting wheat cultivars. International Journal of Molecular Sciences 2021, 22, 12556. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wu, X.; Wang, P.; Zhu, L.; Liu, Y.; Tang, Y.; Chen, J. Halophyte Nitraria billardieri CIPK25 mitigates salinity-induced cell damage by alleviating H2O2 accumulation. Frontiers in Plant Science 2022, 13, 961651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Huang, P.P.; Ji, Y.; Wang, S.; Wang, S.S.; Li, Z.; Wang, Y. KUP9 maintains root meristem activity by regulating K+ and auxin homeostasis in response to low K. EMBO reports 2020, 21, e50164. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Primary root growth in the hdc1-2 was inhibited under low-K+ conditions. (A) Phenotypes of wild-type (Ler), hdc1-2 mutant (hdc1-2), and the hdc1-2/ProHDC1:HDC1 complementation line (COM1). Seeds were germinated and grown on low-K+ (LK,50 μM) or high-K+ (HK, 5 mM) medium for 7 days. Scale bar, 1 cm; (B) Primary root lengths of the plants tested in (A). Data are means + SE (n = 21, individual plants). Student’s t-test (***P < 0.001) was used to analyze the statistical significance; (C) Phenotypes of wild-type (Ler) and Ler/ProHDC1:HDC1 overexpression lines (OE-4 and OE-8). Seeds were germinated and grown on low-K+ (LK,50 μM) or high-K+ (HK, 5 mM) medium for 7 days. Scale bar, 1 cm; (D) Primary root lengths of plants tested in (C). Data are means + SE (n = 21, individual plants). Student’s t-test (***P < 0.001) was used to analyze statistical significance.
Figure 1.
Primary root growth in the hdc1-2 was inhibited under low-K+ conditions. (A) Phenotypes of wild-type (Ler), hdc1-2 mutant (hdc1-2), and the hdc1-2/ProHDC1:HDC1 complementation line (COM1). Seeds were germinated and grown on low-K+ (LK,50 μM) or high-K+ (HK, 5 mM) medium for 7 days. Scale bar, 1 cm; (B) Primary root lengths of the plants tested in (A). Data are means + SE (n = 21, individual plants). Student’s t-test (***P < 0.001) was used to analyze the statistical significance; (C) Phenotypes of wild-type (Ler) and Ler/ProHDC1:HDC1 overexpression lines (OE-4 and OE-8). Seeds were germinated and grown on low-K+ (LK,50 μM) or high-K+ (HK, 5 mM) medium for 7 days. Scale bar, 1 cm; (D) Primary root lengths of plants tested in (C). Data are means + SE (n = 21, individual plants). Student’s t-test (***P < 0.001) was used to analyze statistical significance.
Figure 2.
Inhibition of meristem cell proliferation in the hdc1-2 mutant leads to a short-root phenotype under low-K+ conditions. (A) Root meristem zones of wild-type (Ler), hdc1-2mutant (hdc1-2), and the c16s/ProHDC1:HDC1 complementation line (COM1). Seeds were germinated and grown on LK or HK medium for 7 days. The meristem zone lengths are marked with red lines. Scale bars, 50 μm; (B-D) represent different zone lengths, zone cell numbers, and cell lengths of the indicated seedlings grown on LK or HK medium for 7 days. MEZ, meristem zone; EZ, elongation zone; MAZ, matur ation zone. Data are means + E (n = 6, individual plants). Student’s t-test (***P < 0.001) was used to analyze statistical significance.
Figure 2.
Inhibition of meristem cell proliferation in the hdc1-2 mutant leads to a short-root phenotype under low-K+ conditions. (A) Root meristem zones of wild-type (Ler), hdc1-2mutant (hdc1-2), and the c16s/ProHDC1:HDC1 complementation line (COM1). Seeds were germinated and grown on LK or HK medium for 7 days. The meristem zone lengths are marked with red lines. Scale bars, 50 μm; (B-D) represent different zone lengths, zone cell numbers, and cell lengths of the indicated seedlings grown on LK or HK medium for 7 days. MEZ, meristem zone; EZ, elongation zone; MAZ, matur ation zone. Data are means + E (n = 6, individual plants). Student’s t-test (***P < 0.001) was used to analyze statistical significance.
Figure 3.
Promotion of meristem cell proliferation in the HDC1 overexpression lines leads to the long-root phenotype under low-K+ conditions. (A) Root meristem zones of wild-type (Ler), Ler/ProHDC1:HDC1 overexpression lines (OE-4 and OE-8). Seeds were germinated and grown on LK or HK medium for 7 days. The meristem zone lengths are marked with red lines. Scale bars, 50 μm; (B-D) represent different zone lengths, different zone cell numbers, and cell length of the indicated seedlings germinated and grown on LK or HK medium for 7 days. MEZ, meristem zone; EZ, elongation zone; MAZ, maturation zone. Data are means + E (n = 6, individual plants). Student’s t-test (***P < 0.001) was used to analyze statistical significance.
Figure 3.
Promotion of meristem cell proliferation in the HDC1 overexpression lines leads to the long-root phenotype under low-K+ conditions. (A) Root meristem zones of wild-type (Ler), Ler/ProHDC1:HDC1 overexpression lines (OE-4 and OE-8). Seeds were germinated and grown on LK or HK medium for 7 days. The meristem zone lengths are marked with red lines. Scale bars, 50 μm; (B-D) represent different zone lengths, different zone cell numbers, and cell length of the indicated seedlings germinated and grown on LK or HK medium for 7 days. MEZ, meristem zone; EZ, elongation zone; MAZ, maturation zone. Data are means + E (n = 6, individual plants). Student’s t-test (***P < 0.001) was used to analyze statistical significance.
Figure 4.
Expression patterns of HDC1 in response to K+ availability in Arabidopsis thaliana. (A) Quantitative reverse transcription polymerase chain reaction (RT-qPCR) analysis of HDC1 expression in roots in response to K+ availability. Wild- type (Ler) seeds germinate and grow on LK or HK medium for 7 days. Expression levels were normalized to the expression of an internal control, AtACT2. Data are means + SD (n = 3); (B) β-Glucuronidase (GUS) activity assay of HDC1 expression. Bars, 100 µm (root tip and mature zone); 1 cm (seedling); (C) GUS staining in the root tip of ProHDC1: GUS transgenic plants. Seeds germinate and grow on LK or HK medium for 7 days.
Figure 4.
Expression patterns of HDC1 in response to K+ availability in Arabidopsis thaliana. (A) Quantitative reverse transcription polymerase chain reaction (RT-qPCR) analysis of HDC1 expression in roots in response to K+ availability. Wild- type (Ler) seeds germinate and grow on LK or HK medium for 7 days. Expression levels were normalized to the expression of an internal control, AtACT2. Data are means + SD (n = 3); (B) β-Glucuronidase (GUS) activity assay of HDC1 expression. Bars, 100 µm (root tip and mature zone); 1 cm (seedling); (C) GUS staining in the root tip of ProHDC1: GUS transgenic plants. Seeds germinate and grow on LK or HK medium for 7 days.
Figure 5.
Partial restoration of primary root growth in hdc1-2 mutant with low-K+ inhibition by exogenous application of NAA and IAA. (A) Phenotypes of various plants. Seeds were germinated and grown on LK or HK medium with or without NAA or IAA for 7 days. Scale bar, 1 cm; (B, C) Primary root length of the plants tested in (A). Data are means + SE (n = 21, individual plants). Student’s t-test (***P < 0.001) was used to analyze statistical significance.
Figure 5.
Partial restoration of primary root growth in hdc1-2 mutant with low-K+ inhibition by exogenous application of NAA and IAA. (A) Phenotypes of various plants. Seeds were germinated and grown on LK or HK medium with or without NAA or IAA for 7 days. Scale bar, 1 cm; (B, C) Primary root length of the plants tested in (A). Data are means + SE (n = 21, individual plants). Student’s t-test (***P < 0.001) was used to analyze statistical significance.
Figure 6.
Inhibition of primary root growth in overexpression lines by exogenous application of NAA and IAA under LK stress. (A) Phenotypes of various plants. Seeds were germinated and grown on LK or HK medium with or without NAA or IAA for 7 days. Scale bar, 1 cm; (B, C) Primary root length of the plants tested in (A). Data are means + SE (n = 21, individual plants). Student’s t-test (***P < 0.001) was used to analyze statistical significance.
Figure 6.
Inhibition of primary root growth in overexpression lines by exogenous application of NAA and IAA under LK stress. (A) Phenotypes of various plants. Seeds were germinated and grown on LK or HK medium with or without NAA or IAA for 7 days. Scale bar, 1 cm; (B, C) Primary root length of the plants tested in (A). Data are means + SE (n = 21, individual plants). Student’s t-test (***P < 0.001) was used to analyze statistical significance.
Figure 7.
Expression of PIN1, PIN2, PIN3 and AUX1 under different treatments. Seeds were germinated and grown on different medium for 7 days. NAA concentration is 100nM. Data are means + SE (n = 3, biological replicates).
Figure 7.
Expression of PIN1, PIN2, PIN3 and AUX1 under different treatments. Seeds were germinated and grown on different medium for 7 days. NAA concentration is 100nM. Data are means + SE (n = 3, biological replicates).
Figure 8.
HDC1 affects the expression of “CBL-CIPK-channel” module genes in Arabidopsis. (A-F) Transcript levels of CBL1, CBL2, CBL3, CBL9, AKT1 and TPK1 in WT (Ler), hdc1-2 mutant under LK and HK conditions. Seeds were germinated and grown on LK or HK medium for 7 days. Transcript levels were normalized to those of Actin2/8. Data are means + E (n = 3, individual plants). Student’s t-test (*, P < 0.05; **, P < 0.01) was used to determine the statistical significance of the differences from the wild-type (Ler) under each condition.
Figure 8.
HDC1 affects the expression of “CBL-CIPK-channel” module genes in Arabidopsis. (A-F) Transcript levels of CBL1, CBL2, CBL3, CBL9, AKT1 and TPK1 in WT (Ler), hdc1-2 mutant under LK and HK conditions. Seeds were germinated and grown on LK or HK medium for 7 days. Transcript levels were normalized to those of Actin2/8. Data are means + E (n = 3, individual plants). Student’s t-test (*, P < 0.05; **, P < 0.01) was used to determine the statistical significance of the differences from the wild-type (Ler) under each condition.
Figure 9.
A working model of HDC1 in root growth regulation in response to LK stress.
Figure 9.
A working model of HDC1 in root growth regulation in response to LK stress.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).