Submitted:
14 October 2024
Posted:
15 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Physiological Process of Longitudinal Growth
2.1. The Growth Plate
2.2. Bone Structure
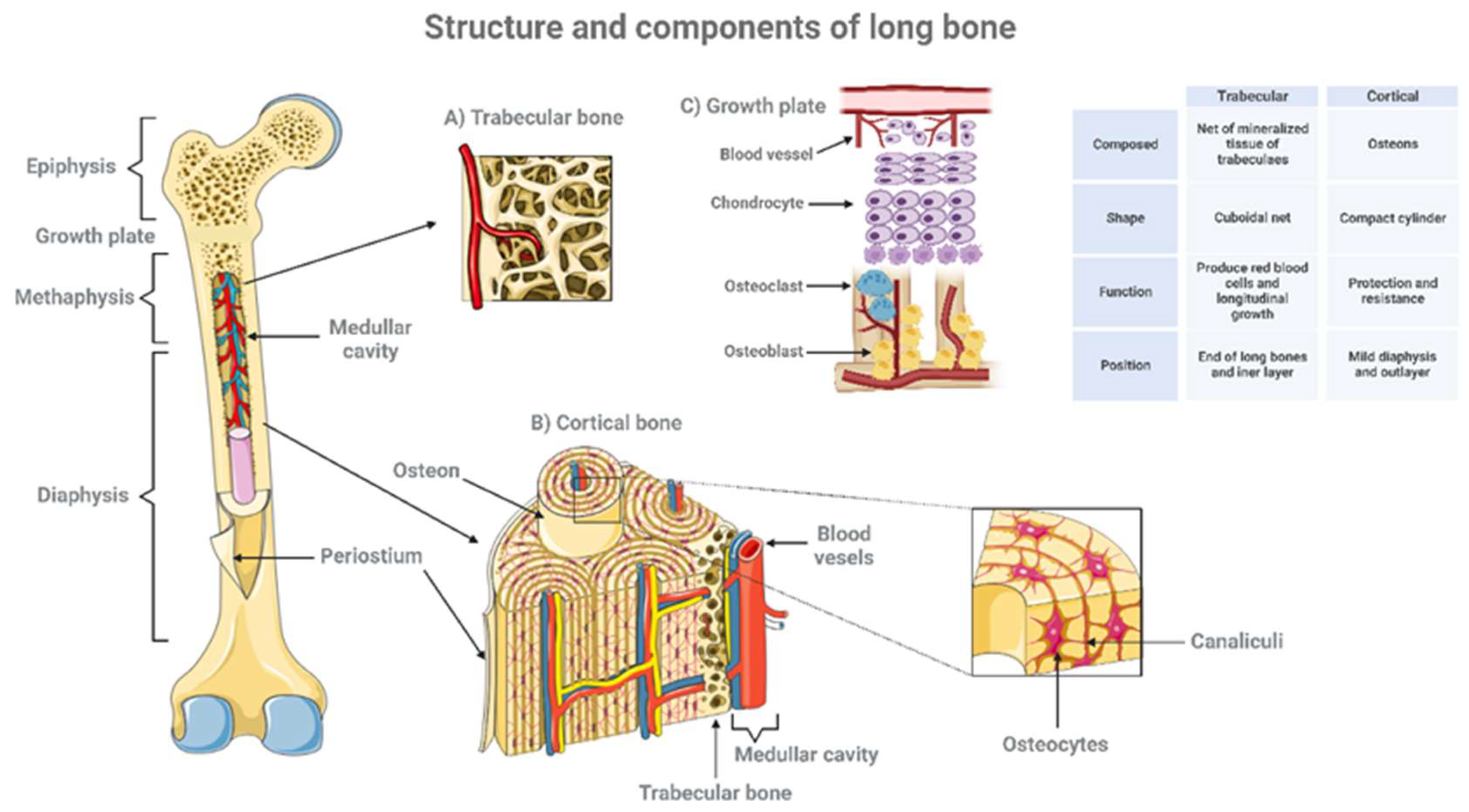
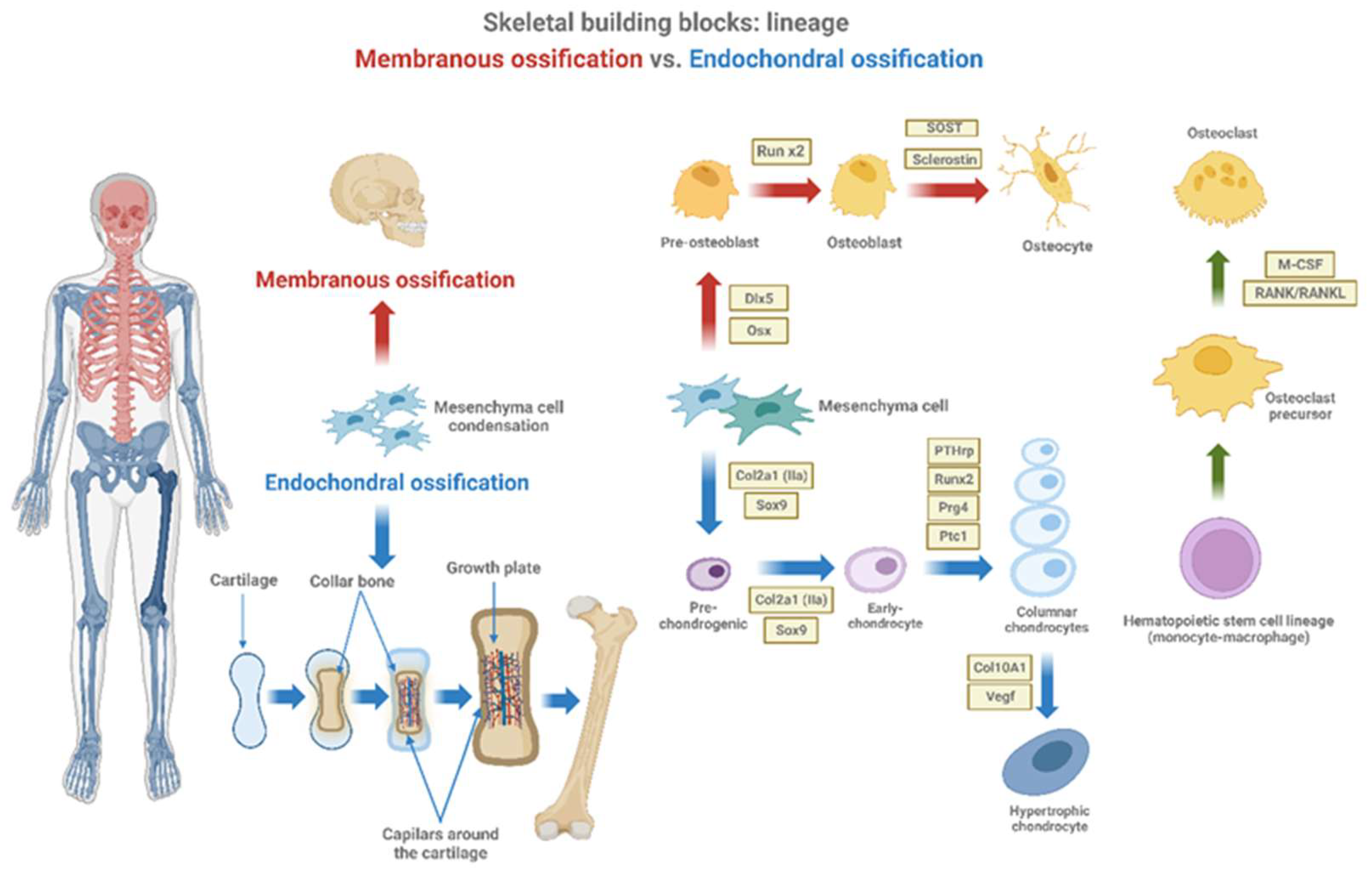
2.3. Endochondral Ossification, by Which the Long Bones Are Form
2.4. Skeleton Development and Growth Rely on the Coordinated Interaction of Cartilage and Bone Cells
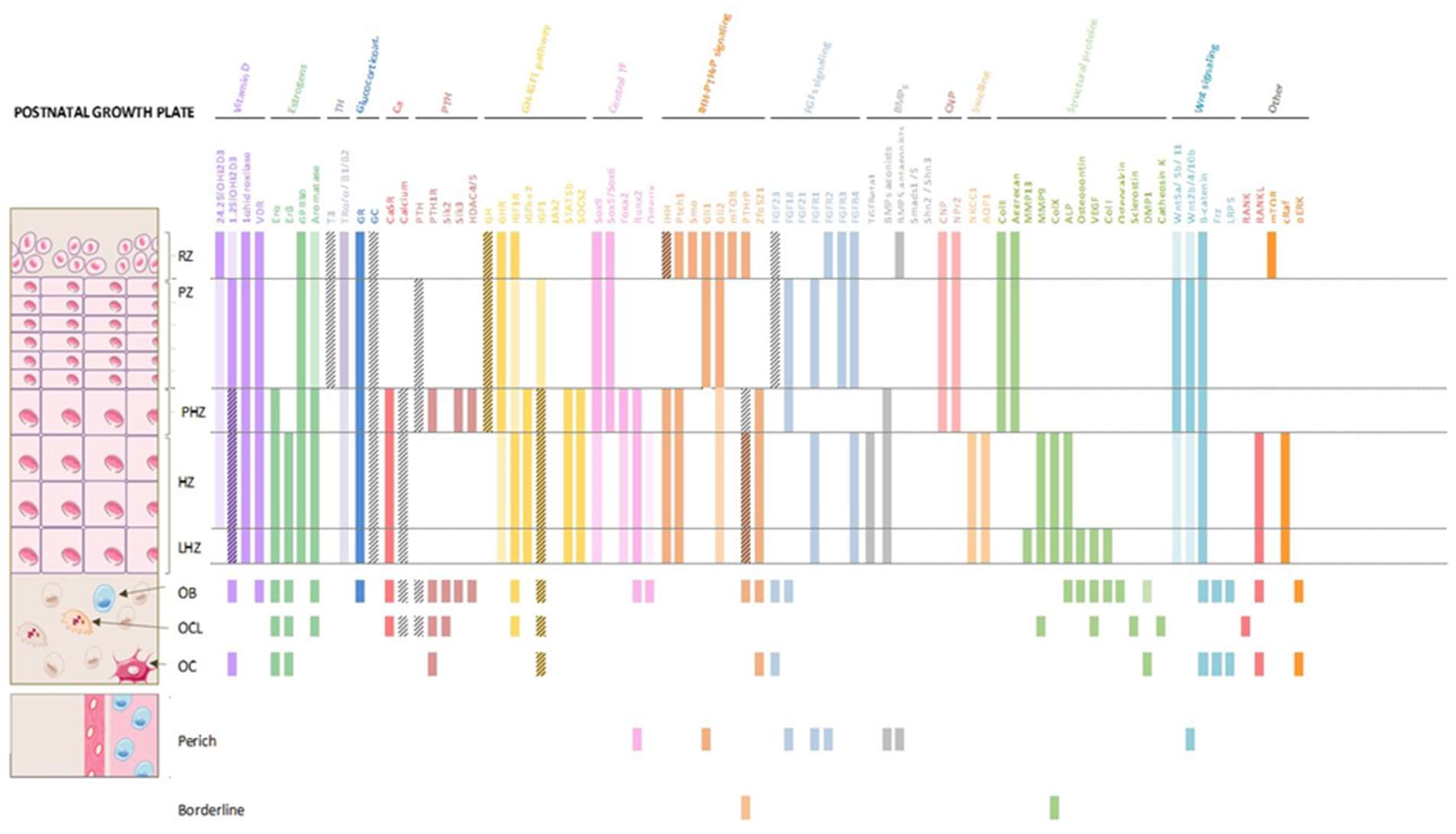
3. Defects in Interaction Lead to Growth Disorders
4. What We Already Know about Clinical Conditions That Associate Growth Retardation
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nichols J. UpToDate. 2022. p. 1–50 Normal growth patterns in infants and prepubertal children.
- Polidori, N.; Castorani, V.; Mohn, A.; Chiarelli, F. Deciphering short stature in children. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 69–79. [CrossRef]
- Graber EG. MSD Manual, Professional Version. 2023. Physical Growth of Infants and Children.
- Bogarín, R.; Richmond, E.; Rogol, A.D. A new approach to the diagnosis of short stature. Minerva Pediatr. 2020, 72, 250–262. [CrossRef]
- Chiarelli, F.; Primavera, M.; Mastromauro, C. Evaluation and management of a child with short stature. Minerva Pediatr. 2021, 72, 452–461. [CrossRef]
- Blumer, M.J. Bone tissue and histological and molecular events during development of the long bones. Ann. Anat. - Anat. Anz. 2021, 235, 151704. [CrossRef]
- Furdock, R.J.; Sanders, J.O.; Cooperman, D.R.; Liu, R.W. Using Skeletal Maturity in Pediatric Orthopaedics: A Primer. J. Pediatr. Orthop. 2022, 42, e793–e800. [CrossRef]
- Ağırdil, Y. The growth plate: a physiologic overview. EFORT Open Rev. 2020, 5, 498–507. [CrossRef]
- Csukasi, F.; Bosakova, M.; Barta, T.; Martin, J.H.; Arcedo, J.; Barad, M.; Rico-Llanos, G.A.; Zieba, J.; Becerra, J.; Krejci, P.; et al. Skeletal diseases caused by mutations in PTH1R show aberrant differentiation of skeletal progenitors due to dysregulation of DEPTOR. Front. Cell Dev. Biol. 2023, 10, 963389. [CrossRef]
- Coates P. Bone turnover markers. Aust Fam Physician. 2013 May;42(5):285–7.
- Capulli, M.; Paone, R.; Rucci, N. Osteoblast and osteocyte: Games without frontiers. Arch. Biochem. Biophys. 2014, 561, 3–12. [CrossRef]
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [CrossRef]
- Dallas, S.L.; Bonewald, L.F. Dynamics of the transition from osteoblast to osteocyte. Ann. N. Y. Acad. Sci. 2010, 1192, 437–443. [CrossRef]
- Helfrich MH. Osteoclast diseases. Microsc Res Tech. 2003 Aug 15;61(6):514–32.
- Ren, X.; Zhou, Q.; Foulad, D.; Tiffany, A.S.; Dewey, M.J.; Bischoff, D.; Miller, T.A.; Reid, R.R.; He, T.-C.; Yamaguchi, D.T.; et al. Osteoprotegerin reduces osteoclast resorption activity without affecting osteogenesis on nanoparticulate mineralized collagen scaffolds. Sci. Adv. 2019, 5, eaaw4991. [CrossRef]
- Šromová, V.; Sobola, D.; Kaspar, P. A Brief Review of Bone Cell Function and Importance. Cells 2023, 12, 2576. [CrossRef]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [CrossRef]
- Hannink G, Arts JJC. Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration? Injury. 2011 Sep;42:S22–5.
- Mackie, E.J.; Ahmed, Y.A.; Tatarczuch, L.; Chen, K.-S.; Mirams, M. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 2008, 40, 46–62. [CrossRef]
- Deng D, Liu X, Huang W, Yuan S, Liu G, Ai S, et al. Osteoclasts control endochondral ossification via regulating acetyl-CoA availability. Bone Res. 2024 Aug 28;12(1):49.
- Mackie, E.J.; Tatarczuch, L.; Mirams, M. The skeleton: a multi-functional complex organ. The growth plate chondrocyte and endochondral ossification. J. Endocrinol. 2011, 211, 109–121. [CrossRef]
- Hall, B.K. Evolutionary Consequences of Skeletal Differentiation. Am. Zoöl. 1975, 15, 329–350. [CrossRef]
- Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16(3):381–90.
- Bonewald LF. The amazing osteocyte. Journal of Bone and Mineral Research. 2011 Feb 1;26(2):229–38.
- Xu, J.; Yu, L.; Liu, F.; Wan, L.; Deng, Z. The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: a review. Front. Immunol. 2023, 14, 1222129. [CrossRef]
- Takegahara N, Kim H, Choi Y. RANKL biology. Bone. 2022 Jun;159:116353.
- Zhou, P.; Zheng, T.; Zhao, B. Cytokine-mediated immunomodulation of osteoclastogenesis. Bone 2022, 164, 116540–116540. [CrossRef]
- Chen, X.; Macica, C.M.; Nasiri, A.; Broadus, A.E. Regulation of articular chondrocyte proliferation and differentiation by indian hedgehog and parathyroid hormone–related protein in mice. Arthritis Rheum. 2008, 58, 3788–3797. [CrossRef]
- Dreyer, C.H.; Kjaergaard, K.; Ding, M.; Qin, L. Vascular endothelial growth factor for in vivo bone formation: A systematic review. J. Orthop. Transl. 2020, 24, 46–57. [CrossRef]
- Grosso, A.; Lunger, A.; Burger, M.G.; Briquez, P.S.; Mai, F.; Hubbell, J.A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. VEGF dose controls the coupling of angiogenesis and osteogenesis in engineered bone. npj Regen. Med. 2023, 8, 1–15. [CrossRef]
- Street, J.; Bao, M.; DeGuzman, L.; Bunting, S.; Peale, F.V., Jr.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [CrossRef]
- Yang, L.; Tsang, K.Y.; Tang, H.C.; Chan, D.; Cheah, K.S.E. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc. Natl. Acad. Sci. 2014, 111, 12097–12102. [CrossRef]
- Zhu, S.; Chen, W.; Masson, A.; Li, Y.-P. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov. 2024, 10, 1–39. [CrossRef]
- Chan, W.C.W.; Tan, Z.; To, M.K.T.; Chan, D. Regulation and Role of Transcription Factors in Osteogenesis. Int. J. Mol. Sci. 2021, 22, 5445. [CrossRef]
- Nagata, K.; Hojo, H.; Chang, S.H.; Okada, H.; Yano, F.; Chijimatsu, R.; Omata, Y.; Mori, D.; Makii, Y.; Kawata, M.; et al. Runx2 and Runx3 differentially regulate articular chondrocytes during surgically induced osteoarthritis development. Nat. Commun. 2022, 13, 1–17. [CrossRef]
- Mollentze, J.; Durandt, C.; Pepper, M.S. An In Vitro and In Vivo Comparison of Osteogenic Differentiation of Human Mesenchymal Stromal/Stem Cells. Stem Cells Int. 2021, 2021, 1–23. [CrossRef]
- Xu J, Li Z, Hou Y, Fang W. Review Article Potential mechanisms underlying the Runx2 induced osteogenesis of bone marrow mesenchymal stem cells [Internet]. Vol. 7, Am J Transl Res. 2015. Available from: www.ajtr.org.
- Qin, X.; Jiang, Q.; Komori, H.; Sakane, C.; Fukuyama, R.; Matsuo, Y.; Ito, K.; Miyazaki, T.; Komori, T. Runt-related transcription factor-2 (Runx2) is required for bone matrix protein gene expression in committed osteoblasts in mice. J. Bone Miner. Res. 2021, 36, 2081–2095. [CrossRef]
- Liu, Q.; Li, M.; Wang, S.; Xiao, Z.; Xiong, Y.; Wang, G. Recent Advances of Osterix Transcription Factor in Osteoblast Differentiation and Bone Formation. Front. Cell Dev. Biol. 2020, 8, 601224. [CrossRef]
- van Bezooijen, R.L.; Roelen, B.A.; Visser, A.; van der Wee-Pals, L.; de Wilt, E.; Karperien, M.; Hamersma, H.; Papapoulos, S.E.; Dijke, P.T.; Löwik, C.W. Sclerostin Is an Osteocyte-expressed Negative Regulator of Bone Formation, But Not a Classical BMP Antagonist. J. Exp. Med. 2004, 199, 805–814. [CrossRef]
- Carpenter, K.A.; Alkhatib, D.O.; Dulion, B.A.; Guirado, E.; Patel, S.; Chen, Y.; George, A.; Ross, R.D. Sclerostin antibody improves alveolar bone quality in the Hyp mouse model of X-linked hypophosphatemia (XLH). Int. J. Oral Sci. 2023, 15, 1–10. [CrossRef]
- Maurizi, A.; Rucci, N. The Osteoclast in Bone Metastasis: Player and Target. Cancers 2018, 10, 218. [CrossRef]
- Marcadet, L.; Bouredji, Z.; Argaw, A.; Frenette, J. The Roles of RANK/RANKL/OPG in Cardiac, Skeletal, and Smooth Muscles in Health and Disease. Front. Cell Dev. Biol. 2022, 10, 903657. [CrossRef]
- Zhou, X.; von der Mark, K.; Henry, S.; Norton, W.; Adams, H.; de Crombrugghe, B. Chondrocytes Transdifferentiate into Osteoblasts in Endochondral Bone during Development, Postnatal Growth and Fracture Healing in Mice. PLOS Genet. 2014, 10, e1004820. [CrossRef]
- Long, F.; Chung, U.-I.; Ohba, S.; McMahon, J.; Kronenberg, H.M.; McMahon, A.P. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development 2004, 131, 1309–1318. [CrossRef]
- Greene, C.A.; Green, C.R.; Sherwin, T. Transdifferentiation of chondrocytes into neuron-like cells induced by neuronal lineage specifying growth factors. Cell Biol. Int. 2014, 39, 185–191. [CrossRef]
- Fakhry, M.; Roszkowska, M.; Briolay, A.; Bougault, C.; Guignandon, A.; Diaz-Hernandez, J.I.; Diaz-Hernandez, M.; Pikula, S.; Buchet, R.; Hamade, E.; et al. TNAP stimulates vascular smooth muscle cell trans-differentiation into chondrocytes through calcium deposition and BMP-2 activation: Possible implication in atherosclerotic plaque stability. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2017, 1863, 643–653. [CrossRef]
- Kobayashi, T.; Soegiarto, D.W.; Yang, Y.; Lanske, B.; Schipani, E.; McMahon, A.P.; Kronenberg, H.M. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J. Clin. Investig. 2005, 115, 1734–1742. [CrossRef]
- Esbrit, P. The role of parathyroid hormone related protein (PTHrP) in bone metabolism: from basic to clinical research. Rev. de Osteoporos. y Metab. Miner. 2023, 15, 1–5. [CrossRef]
- Lanske, B.; Amling, M.; Neff, L.; Guiducci, J.; Baron, R.; Kronenberg, H.M. Ablation of the PTHrP gene or the PTH/PTHrP receptor gene leads to distinct abnormalities in bone development. J. Clin. Investig. 1999, 104, 399–407. [CrossRef]
- Li, X.; Han, Y.; Li, G.; Zhang, Y.; Wang, J.; Feng, C. Role of Wnt signaling pathway in joint development and cartilage degeneration. Front. Cell Dev. Biol. 2023, 11, 1181619. [CrossRef]
- Yuan, J.; Guo, L.; Wang, J.; Zhou, Z.; Wu, C. α-parvin controls chondrocyte column formation and regulates long bone development. Bone Res. 2023, 11, 1–12. [CrossRef]
- Wang, B.; Zhou, X.; Price, C.; Li, W.; Pan, J.; Wang, L. Quantifying load-induced solute transport and solute-matrix interaction within the osteocyte lacunar-canalicular system. J. Bone Miner. Res. 2013, 28, 1075–1086. [CrossRef]
- Hopkins, T.; Wright, K.T.; Kuiper, N.J.; Roberts, S.; Jermin, P.; Gallacher, P.; Kuiper, J.H. An In Vitro System to Study the Effect of Subchondral Bone Health on Articular Cartilage Repair in Humans. Cells 2021, 10, 1903. [CrossRef]
- Fuente R, Gil-Peña H, Claramunt-Taberner D, Hernández O, Fernández-Iglesias A, Alonso-Durán L, et al. X-linked hypophosphatemia and growth. Vol. 18, Reviews in Endocrine and Metabolic Disorders. Springer New York LLC; 2017. p. 107–15.
- Wöhrle, S.; Henninger, C.; Bonny, O.; Thuery, A.; Beluch, N.; E Hynes, N.; Guagnano, V.; Sellers, W.R.; Hofmann, F.; Kneissel, M.; et al. Pharmacological inhibition of fibroblast growth factor (FGF) receptor signaling ameliorates FGF23-mediated hypophosphatemic rickets. J. Bone Miner. Res. 2013, 28, 899–911. [CrossRef]
- Fernández-Iglesias, .; López, J.M.; Santos, F. Growth plate alterations in chronic kidney disease. Pediatr. Nephrol. 2020, 35, 367–374. [CrossRef]
- Voller, T.; Cameron, P.; Watson, J.; Phadnis, J. The growth plate: anatomy and disorders. Orthop. Trauma 2020, 34, 135–140. [CrossRef]
- Kornak, U.; Mundlos, S. Genetic Disorders of the Skeleton: A Developmental Approach. Am. J. Hum. Genet. 2003, 73, 447–474. [CrossRef]
- Wang, Y.; Cheng, Z.; ElAlieh, H.Z.; Nakamura, E.; Nguyen, M.-T.; Mackem, S.; Clemens, T.L.; Bikle, D.D.; Chang, W. IGF-1R signaling in chondrocytes modulates growth plate development by interacting with the PTHrP/Ihh pathway. J. Bone Miner. Res. 2011, 26, 1437–1446. [CrossRef]
- Savage MO. Insulin-Like Growth Factors, Nutrition and Growth. In: R. Shamir, D. Turck, M. Phillip, editors. World Review of Nutrition and Dietetics [Internet]. 2013 [cited 2023 Nov 27]. p. 52–9. Available from: . [CrossRef]
- Kim, S.-M.; Sultana, F.; Korkmaz, F.; Rojekar, S.; Pallapati, A.; Ryu, V.; Lizneva, D.; Yuen, T.; Rosen, C.J.; Zaidi, M. Neuroendocrinology of bone. Pituitary 2024, 1–17. [CrossRef]
- Zhang, M.; Xuan, S.; Bouxsein, M.L.; von Stechow, D.; Akeno, N.; Faugere, M.C.; Malluche, H.; Zhao, G.; Rosen, C.J.; Efstratiadis, A.; et al. Osteoblast-specific Knockout of the Insulin-like Growth Factor (IGF) Receptor Gene Reveals an Essential Role of IGF Signaling in Bone Matrix Mineralization. J. Biol. Chem. 2002, 277, 44005–44012. [CrossRef]
- Saltarelli MA, Quarta A, Chiarelli F. Growth plate extracellular matrix defects and short stature in children. Ann Pediatr Endocrinol Metab. 2022 Dec 31;27(4):247–55.
- Witkowska-Sędek, E.; Pyrżak, B. Chronic inflammation and the growth hormone/insulin-like growth factor-1 axis. Central Eur. J. Immunol. 2020, 45, 469–475. [CrossRef]
- MacRae, V.; Wong, S.; Farquharson, C.; Ahmed, S. Cytokine actions in growth disorders associated with pediatric chronic inflammatory diseases (review).. Int. J. Mol. Med. 2006, 18, 1011–1018. [CrossRef]
- Bechtold, S.; Simon, D. Growth abnormalities in children and adolescents with juvenile idiopathic arthritis. Rheumatol. Int. 2014, 34, 1483–1488. [CrossRef]
- Choukair D, Hügel U, Sander A, Uhlmann L, Tönshoff B. Inhibition of IGF-I–related intracellular signaling pathways by proinflammatory cytokines in growth plate chondrocytes. Pediatr Res. 2014 Sep 18;76(3):245–51.
- Witkowska-Sędek, E.; Pyrżak, B. Chronic inflammation and the growth hormone/insulin-like growth factor-1 axis. Central Eur. J. Immunol. 2020, 45, 469–475. [CrossRef]
- Fazeli, P.K.; Klibanski, A. Determinants of GH resistance in malnutrition. J. Endocrinol. 2013, 220, R57–R65. [CrossRef]
- Soendergaard, C.; Young, J.A.; Kopchick, J.J. Growth Hormone Resistance—Special Focus on Inflammatory Bowel Disease. Int. J. Mol. Sci. 2017, 18, 1019. [CrossRef]
- Cirillo, F.; Lazzeroni, P.; Sartori, C.; Street, M.E. Inflammatory Diseases and Growth: Effects on the GH–IGF Axis and on Growth Plate. Int. J. Mol. Sci. 2017, 18, 1878. [CrossRef]
- Patel, L. Growth and Chronic Disease. 2007, 65, 129–136. [CrossRef]
- Amaro, F.; Chiarelli, F. Growth and Puberty in Children with Inflammatory Bowel Diseases. Biomedicines 2020, 8, 458. [CrossRef]
- Gupta, N.; Lustig, R.H.; Kohn, M.A.; McCracken, M.; Vittinghoff, E. Sex differences in statural growth impairment in Crohnʼs disease: Role of IGF-1. Inflamm. Bowel Dis. 2011, 17, 2318–2325. [CrossRef]
- Gaspari, S.; Marcovecchio, M.L.; Breda, L.; Chiarelli, F. Growth in juvenile idiopathic arthritis: the role of inflammation.. 2011, 29, 104–10.
- Fernandez-Vojvodich, P.; Palmblad, K.; Karimian, E.; Andersson, U.; Sävendahl, L. Pro-Inflammatory Cytokines Produced by Growth Plate Chondrocytes May Act Locally to Modulate Longitudinal Bone Growth. Horm. Res. Paediatr. 2012, 77, 180–187. [CrossRef]
- Fernandez-Vojvodich, P.; Zaman, F.; Sävendahl, L. Interleukin-6 acts locally on the growth plate to impair bone growth. Ann. Rheum. Dis. 2013, 72, e24–e24. [CrossRef]
- Nakajima, S.; Naruto, T.; Miyamae, T.; Imagawa, T.; Mori, M.; Nishimaki, S.; Yokota, S. Interleukin-6 inhibits early differentiation of ATDC5 chondrogenic progenitor cells. Cytokine 2009, 47, 91–97. [CrossRef]
- D’angelo, D.M.; Di Donato, G.; Breda, L.; Chiarelli, F. Growth and puberty in children with juvenile idiopathic arthritis. Pediatr. Rheumatol. 2021, 19, 1–13. [CrossRef]
- Mårtensson, K.; Chrysis, D.; Sävendahl, L. Interleukin-1β and TNF-α Act in Synergy to Inhibit Longitudinal Growth in Fetal Rat Metatarsal Bones. J. Bone Miner. Res. 2004, 19, 1805–1812. [CrossRef]
- Fernandez-Vojvodich, P.; Palmblad, K.; Karimian, E.; Andersson, U.; Sävendahl, L. Pro-Inflammatory Cytokines Produced by Growth Plate Chondrocytes May Act Locally to Modulate Longitudinal Bone Growth. Horm. Res. Paediatr. 2012, 77, 180–187. [CrossRef]
- Sederquist, B.; Fernandez-Vojvodich, P.; Zaman, F.; Sävendahl, L. RECENT RESEARCH ON THE GROWTH PLATE: Impact of inflammatory cytokines on longitudinal bone growth. J. Mol. Endocrinol. 2014, 53, T35–T44. [CrossRef]
- Zhao Y, Meng C, Wang Y, Huang H, Liu W, Zhang JF, et al. IL-1β inhibits β-Klotho expression and FGF19 signaling in hepatocytes. American Journal of Physiology-Endocrinology and Metabolism. 2016 Feb 15;310(4):E289–300.
- Zhao, Y.; Xiao, X.; Frank, S.J.; Lin, H.Y.; Xia, Y. Distinct mechanisms of induction of hepatic growth hormone resistance by endogenous IL-6, TNF-α, and IL-1β. Am. J. Physiol. Metab. 2014, 307, E186–E198. [CrossRef]
- E MacRae, V.; Farquharson, C.; Ahmed, S.F. The restricted potential for recovery of growth plate chondrogenesis and longitudinal bone growth following exposure to pro-inflammatory cytokines. J. Endocrinol. 2006, 189, 319–328. [CrossRef]
- Cirillo, F.; Catellani, C.; Lazzeroni, P.; Sartori, C.; Street, M.E. The Role of MicroRNAs in Influencing Body Growth and Development. Horm. Res. Paediatr. 2020, 93, 7–15. [CrossRef]
- Fedorczak, A.; Lewiński, A.; Stawerska, R. Involvement of Sirtuin 1 in the Growth Hormone/Insulin-like Growth Factor 1 Signal Transduction and Its Impact on Growth Processes in Children. Int. J. Mol. Sci. 2023, 24, 15406. [CrossRef]
- Catellani, C.; Ravegnini, G.; Sartori, C.; Angelini, S.; Street, M.E. GH and IGF System: The Regulatory Role of miRNAs and lncRNAs in Cancer. Front. Endocrinol. 2021, 12. [CrossRef]
- Shah A, Hashmi MF, Aeddula NR. StatPearls [Internet]. 2024. Chronic Kidney Disease-Mineral Bone Disorder (CKD-MBD).
- Cirillo, L.; De Chiara, L.; Innocenti, S.; Errichiello, C.; Romagnani, P.; Becherucci, F. Chronic kidney disease in children: an update. Clin. Kidney J. 2023, 16, 1600–1611. [CrossRef]
- Molinos, I.; Santos, F.; Carbajo-Perez, E.; Garcia, E.; Rodriguez, J.; Garcia-Alvarez, O.; Gil, H.; Ordoñez, F.; Loredo, V.; Mallada, L. Catch-up growth follows an abnormal pattern in experimental renal insufficiency and growth hormone treatment normalizes it. Kidney Int. 2006, 70, 1955–1961. [CrossRef]
- Hu, B.; Li, H.; Zhang, X. A Balanced Act: The Effects of GH–GHR–IGF1 Axis on Mitochondrial Function. Front. Cell Dev. Biol. 2021, 9. [CrossRef]
- Chen, N.; Wu, R.W.; Lam, Y.; Chan, W.C.; Chan, D. Hypertrophic chondrocytes at the junction of musculoskeletal structures. Bone Rep. 2023, 19, 101698. [CrossRef]
- Hallett, S.A.; Ono, W.; Ono, N. Growth Plate Chondrocytes: Skeletal Development, Growth and Beyond. Int. J. Mol. Sci. 2019, 20, 6009. [CrossRef]
- Ikegawa, K.; Hasegawa, Y. Fracture risk, underlying pathophysiology, and bone quality assessment in patients with Turner syndrome. Front. Endocrinol. 2022, 13, 967857. [CrossRef]
- Papadopoulou, A.; Bountouvi, E. Skeletal defects and bone metabolism in Noonan, Costello and cardio-facio-cutaneous syndromes. Front. Endocrinol. 2023, 14, 1231828. [CrossRef]
- LaCombe, J.M.; Roper, R.J. Skeletal dynamics of Down syndrome: A developing perspective. Bone 2019, 133, 115215–115215. [CrossRef]
- Homans, J.F.; Tromp, I.N.; Colo, D.; Schlösser, T.P.C.; Kruyt, M.C.; Deeney, V.F.X.; Crowley, T.B.; McDonald-McGinn, D.M.; Castelein, R.M. Orthopaedic manifestations within the 22q11.2 Deletion syndrome: A systematic review. Am. J. Med Genet. Part A 2017, 176, 2104–2120. [CrossRef]
- Funato, N. Craniofacial Phenotypes and Genetics of DiGeorge Syndrome. J. Dev. Biol. 2022, 10, 18. [CrossRef]
- Piché J, Van Vliet PP, Pucéat M, Andelfinger G. The expanding phenotypes of cohesinopathies: one ring to rule them all! Cell Cycle. 2019 Nov 2;18(21):2828–48.
- Kline, A.D.; Moss, J.F.; Selicorni, A.; Bisgaard, A.-M.; Deardorff, M.A.; Gillett, P.M.; Ishman, S.L.; Kerr, L.M.; Levin, A.V.; Mulder, P.A.; et al. Diagnosis and management of Cornelia de Lange syndrome: first international consensus statement. Nat. Rev. Genet. 2018, 19, 649–666. [CrossRef]
- Ishida, M. New developments in Silver-Russell syndrome and implications for clinical practice. Epigenomics 2016, 8, 563–580. [CrossRef]
- M Saal H, D Harbison M, Netchine I. GeneReviews® [Internet]. 2024. Silver-Russell Syndrome. [Cited 2024 Sep 10]. https://www.ncbi.nlm.nih.gov/books/NBK1324/.
- Pauli, R.M. Achondroplasia: a comprehensive clinical review. Orphanet J. Rare Dis. 2019, 14, 1–49. [CrossRef]
- Hypochondroplasia. In: Atlas of Genetic Diagnosis and Counseling. New York, NY: Springer US; 2012. p. 1105–12.
- B Bober M, A Bellus G, M Nikkel S, E Tiller G. GeneReviews® [Internet]. 2020. Hypochondroplasia. [Cited 2024 Sep 10]. https://www.ncbi.nlm.nih.gov/sites/books/NBK1477/.
- Fukami, M.; Seki, A.; Ogata, T. SHOX Haploinsufficiency as a Cause of Syndromic and Nonsyndromic Short Stature. Mol. Syndr. 2016, 7, 3–11. [CrossRef]
- Charoenngam, N.; Nasr, A.; Shirvani, A.; Holick, M.F. Hereditary Metabolic Bone Diseases: A Review of Pathogenesis, Diagnosis and Management. Genes 2022, 13, 1880. [CrossRef]
- Besio, R.; Chow, C.; Tonelli, F.; Marini, J.C.; Forlino, A. Bone biology: insights from osteogenesis imperfecta and related rare fragility syndromes. FEBS J. 2019, 286, 3033–3056. [CrossRef]
- Subramanian S., Anastasopoulou C., Krishnan Viswanathan V. StatPearls [Internet]. 2023. Osteogenesis Imperfecta.
- Nakamura-Utsunomiya, A. Bone Biomarkers in Mucopolysaccharidoses. Int. J. Mol. Sci. 2021, 22, 12651. [CrossRef]
- Melbouci, M.; Mason, R.W.; Suzuki, Y.; Fukao, T.; Orii, T.; Tomatsu, S. Growth impairment in mucopolysaccharidoses. Mol. Genet. Metab. 2018, 124, 1–10. [CrossRef]
- Jiang, Z.; Byers, S.; Casal, M.L.; Smith, L.J. Failures of Endochondral Ossification in the Mucopolysaccharidoses. Curr. Osteoporos. Rep. 2020, 18, 759–773. [CrossRef]
- Fuente, R.; García-Bengoa, M.; Fernández-Iglesias, .; Gil-Peña, H.; Santos, F.; López, J.M. Cellular and Molecular Alterations Underlying Abnormal Bone Growth in X-Linked Hypophosphatemia. Int. J. Mol. Sci. 2022, 23, 934. [CrossRef]
- Faienza, M.F.; D'Amato, E.; Natale, M.P.; Grano, M.; Chiarito, M.; Brunetti, G.; D'Amato, G. Metabolic Bone Disease of Prematurity: Diagnosis and Management. Front. Pediatr. 2019, 7, 143. [CrossRef]
- Cho, W.K.; Suh, B.-K. Catch-up growth and catch-up fat in children born small for gestational age. Korean J. Pediatr. 2016, 59, 1–7. [CrossRef]
- Bandeira, F.; de Oliveira, L.B.; Caldeira, R.B.; Toscano, L.S. Skeletal consequences of heart failure. Women's Heal. 2022, 18. [CrossRef]
- Trost, S.; Tesfaye, N.; Harindhanavudhi, T. The interplay between bone and heart health as reflected in medication effects: A narrative review. Women's Heal. 2023, 19. [CrossRef]
- Alonso-Gonzalez R, Massarella D, Swan L. Skeletal system in adult congenital heart disease. International Journal of Cardiology Congenital Heart Disease. 2023 Sep;13:100460.
- Ma, Y.; Qiu, S.; Zhou, R. Osteoporosis in Patients With Respiratory Diseases. Front. Physiol. 2022, 13, 939253. [CrossRef]
- Zhang, L.; Sun, Y. Muscle-Bone Crosstalk in Chronic Obstructive Pulmonary Disease. Front. Endocrinol. 2021, 12. [CrossRef]
- Danford, C.J.; Trivedi, H.D.; Bonder, A. Bone Health in Patients With Liver Diseases. J. Clin. Densitom. 2020, 23, 212–222. [CrossRef]
- Xia, S.; Qin, X.; Wang, J.; Ren, H. Advancements in the pathogenesis of hepatic osteodystrophy and the potential therapeutic of mesenchymal stromal cells. Stem Cell Res. Ther. 2023, 14, 1–12. [CrossRef]
- Saeki, C.; Saito, M.; Tsubota, A. Association of chronic liver disease with bone diseases and muscle weakness. J. Bone Miner. Metab. 2024, 42, 399–412. [CrossRef]
- Harris, L.; Senagore, P.; Young, V.B.; McCabe, L.R. Inflammatory bowel disease causes reversible suppression of osteoblast and chondrocyte function in mice. Am. J. Physiol. Liver Physiol. 2009, 296, G1020–G1029. [CrossRef]
- Gordon, H.; Burisch, J.; Ellul, P.; Katsanos, K.; Allocca, M.; Bamias, G.; Acosta, M.B.-D.; Braithwaite, T.; Greuter, T.; Harwood, C.; et al. ECCO Guidelines on Extraintestinal Manifestations in Inflammatory Bowel Disease. J. Crohn’s Colitis 2023, 18, 1–37. [CrossRef]
- Yang, H.R. Updates on bone health in children with gastrointestinal diseases. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 10–14. [CrossRef]
- Braga CBM, Bizari L, Suen VMM, Marchini JS, Paula FJA de, Cunha SF de C da. Bone mineral density in short bowel syndrome: correlation with BMI and serum vitamins C, E and K. Arch Endocrinol Metab. 2015 Jun;59(3):252–8.
- Santos, F.; Díaz-Anadón, L.; A Ordóñez, F.; Haffner, D. Bone Disease in CKD in Children. Calcif. Tissue Int. 2021, 108, 423–438. [CrossRef]
- Lalayiannis, A.D.; Soeiro, E.M.D.; Moysés, R.M.A.; Shroff, R. Chronic kidney disease mineral bone disorder in childhood and young adulthood: a ‘growing’ understanding. Pediatr. Nephrol. 2023, 39, 723–739. [CrossRef]
- Zhang, H.; Yang, F.; Cao, Z.; Xu, Y.; Wang, M. The influence of iron on bone metabolism disorders. Osteoporos. Int. 2023, 35, 243–253. [CrossRef]
- Toxqui, L.; Vaquero, M.P. Chronic Iron Deficiency as an Emerging Risk Factor for Osteoporosis: A Hypothesis. Nutrients 2015, 7, 2324–2344. [CrossRef]
- Kaltsas, G.; Makras, P. Skeletal Diseases in Cushing’s Syndrome: Osteoporosis versus Arthropathy. Neuroendocrinology 2010, 92, 60–64. [CrossRef]
- Leszczyńska, D.; Szatko, A.; Papierska, L.; Zgliczyński, W.; Glinicki, P. Musculoskeletal complications of Cushing syndrome. Rheumatology 2023, 61, 197–208. [CrossRef]
- Wang, D.; Dang, C.-X.; Hao, Y.-X.; Yu, X.; Liu, P.-F.; Li, J.-S. Relationship between osteoporosis and Cushing syndrome based on bioinformatics. Medicine 2022, 101, e31283. [CrossRef]
- Loxton P, Narayan K, Munns CF, Craig ME. Bone Mineral Density and Type 1 Diabetes in Children and Adolescents: A Meta-analysis. Diabetes Care. 2021 Aug 1;44(8):1898–905.
- Hofbauer, L.C.; Busse, B.; Eastell, R.; Ferrari, S.; Frost, M.; Müller, R.; Burden, A.M.; Rivadeneira, F.; Napoli, N.; Rauner, M. Bone fragility in diabetes: novel concepts and clinical implications. Lancet Diabetes Endocrinol. 2022, 10, 207–220. [CrossRef]
- Parthasarathy, L.; Khadilkar, V.; Chiplonkar, S.; Khadilkar, A. Longitudinal growth in children and adolescents with type 1 diabetes. Indian Pediatr. 2016, 53, 990–992. [CrossRef]
- Csonka, V.; Varjú, C.; Lendvay, M. Diabetes mellitus-related musculoskeletal disorders: Unveiling the cluster of diseases. Prim. Care Diabetes 2023, 17, 548–553. [CrossRef]
- Zhu, S.; Pang, Y.; Xu, J.; Chen, X.; Zhang, C.; Wu, B.; Gao, J. Endocrine Regulation on Bone by Thyroid. Front. Endocrinol. 2022, 13, 873820. [CrossRef]
- Williams, G.R.; Bassett, J.H.D. Thyroid diseases and bone health. J. Endocrinol. Investig. 2017, 41, 99–109. [CrossRef]
- Sidhu, K.; Ali, B.; Burt, L.A.; Boyd, S.K.; Khan, A. Spectrum of microarchitectural bone disease in inborn errors of metabolism: a cross-sectional, observational study. Orphanet J. Rare Dis. 2020, 15, 1–13. [CrossRef]
- Langeveld, M.; Hollak, C.E.M. Bone health in patients with inborn errors of metabolism. Rev. Endocr. Metab. Disord. 2018, 19, 81–92. [CrossRef]
- Kalra, S.; Grimer, R.J.; Spooner, D.; Carter, S.R.; Tillman, R.M.; Abudu, A. Radiation-induced sarcomas of bone. J. Bone Jt. Surgery. Br. Vol. 2007, 89, 808–813. [CrossRef]
- Hua, J.; Huang, J.; Li, G.; Lin, S.; Cui, L. Glucocorticoid induced bone disorders in children: Research progress in treatment mechanisms. Front. Endocrinol. 2023, 14. [CrossRef]
- A Alfaedi, S.; Kubbara, M.F.; A Alaithan, A.; Alhudhaif, H.M.; A Al Abdullah, A.; Sahool, H.M.; AL Jawad, M.S.; A Almatar, M.; Alnakhli, I.R.; A Altawili, M. Beneath the Surface: Exploring Hidden Threats of Long-Term Corticosteroid Therapy to Bone Density. Cureus 2024, 16, e55109. [CrossRef]
- Cooper, M.S. Glucocorticoids in bone and joint disease: the good, the bad and the uncertain. Clin. Med. 2012, 12, 261–265. [CrossRef]
- Dixit, M.; Poudel, S.B.; Yakar, S. Effects of GH/IGF axis on bone and cartilage. Mol. Cell. Endocrinol. 2020, 519, 111052–111052. [CrossRef]
- Lindsey, R.C.; Mohan, S. Skeletal effects of growth hormone and insulin-like growth factor-I therapy. Mol. Cell. Endocrinol. 2015, 432, 44–55. [CrossRef]
- Oichi T, Kodama J, Wilson K, Tian H, Imamura Kawasawa Y, Usami Y, et al. Nutrient-regulated dynamics of chondroprogenitors in the postnatal murine growth plate. Bone Res. 2023 Apr 21;11(1):20.
- Prentice A, Schoenmakers I, Ann Laskey M, de Bono S, Ginty F, Goldberg GR. Symposium on ‘Nutrition and health in children and adolescents’ Session 1: Nutrition in growth and development Nutrition and bone growth and development. Proceedings of the Nutrition Society. 2006 Nov 21;65(04):348–60.
- Kelly, R.R.; McDonald, L.T.; Jensen, N.R.; Sidles, S.J.; LaRue, A.C. Impacts of Psychological Stress on Osteoporosis: Clinical Implications and Treatment Interactions. Front. Psychiatry 2019, 10, 200. [CrossRef]
- Legroux-Gerot, I.; Vignau, J.; Collier, F.; Cortet, B. Bone loss associated with anorexia nervosa. Jt. Bone Spine 2005, 72, 489–495. [CrossRef]
- Mollard, E.; Bilek, L.; Waltman, N. Emerging evidence on the link between depressive symptoms and bone loss in postmenopausal women. Int. J. Women's Heal. 2017, ume 10, 1–9. [CrossRef]
- Wang, S.-T.; Ni, G.-X. Depression in Osteoarthritis: Current Understanding. Neuropsychiatr. Dis. Treat. 2022, ume 18, 375–389. [CrossRef]
- Rajkumar V, Waseem M. StatPearls [Internet]. . 2023. Familial Short Stature. [Cited 2024 September 10]. https://www.ncbi.nlm.nih.gov/books/NBK559123/.
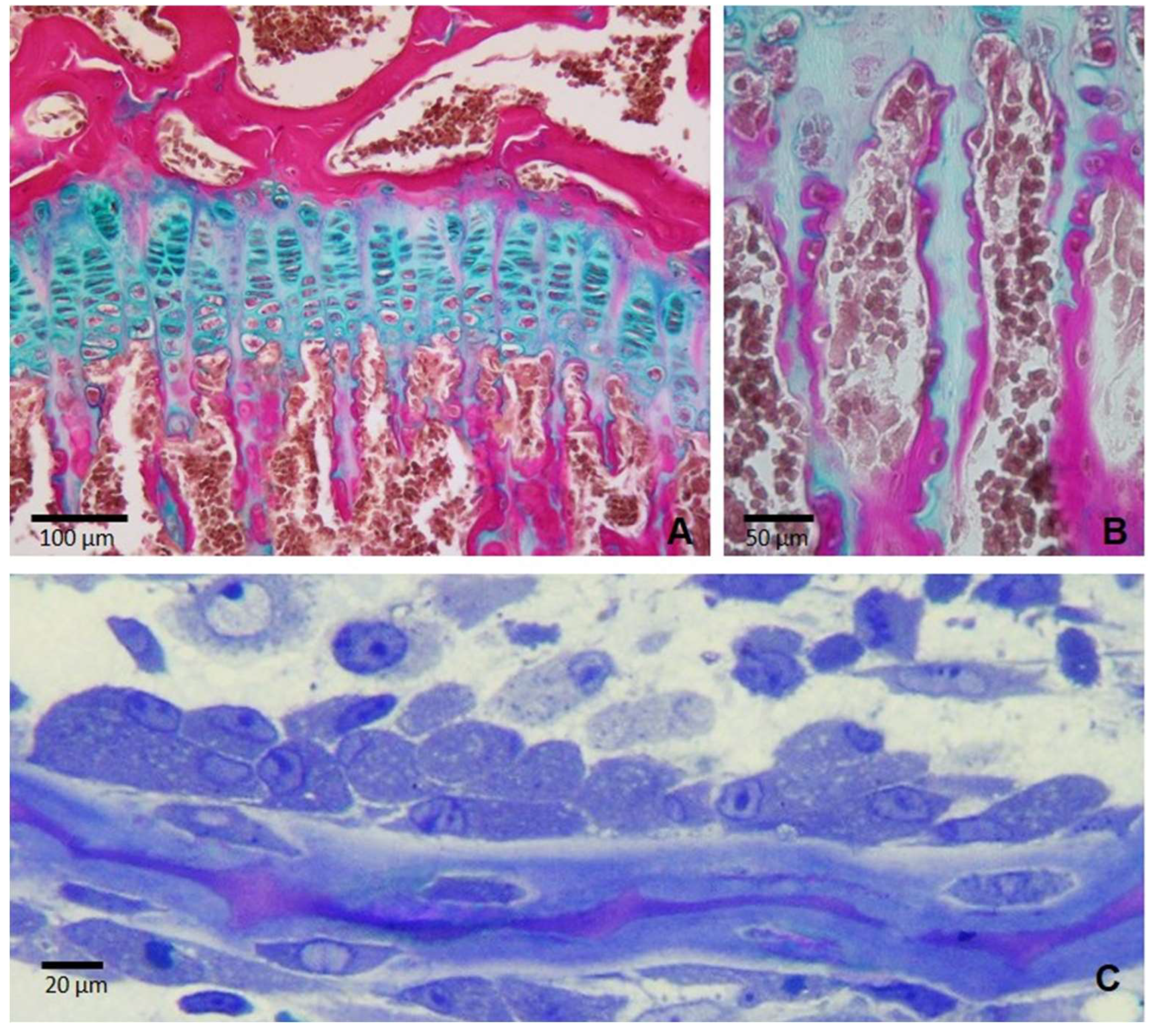
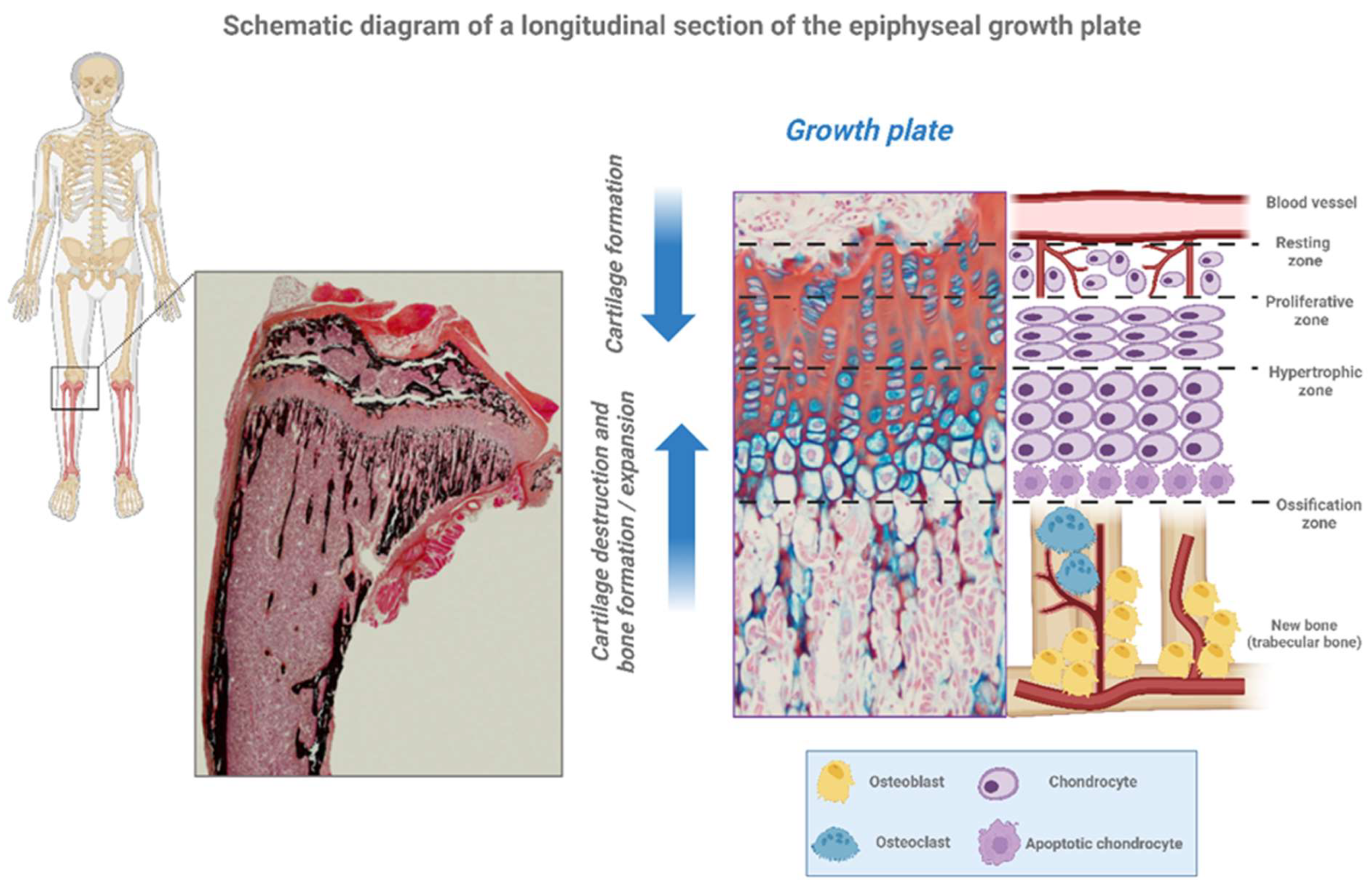
| Epiphyseal Growth Plate | Description |
|---|---|
| Resting Zone (RZ) | Quiescent chondrocytes. These resting cells can be moved to the zone of proliferation. |
| Proliferative Zone (PZ) | Chondrocytes are arranged in columns and multiply. These cells, undergo rapid mitosis by growth hormone (GH) effect. |
| Pre-Hypertrophic Zone (PHZ) | Matrix synthesis is started |
| Hypertrophic Zone (HZ) | Chondrocytes stop proliferation and change their phenotype to hypertrophic by synthesis of proteins and swelling. |
|
Calcification Zone (CZ) |
The terminally hypertrophic chondrocytes undergo apoptosis and differentiation, and the cartilaginous mold welcomes the osteoblasts so that they become osteoclasts and thus induce the formation of new bone tissue. Simultaneously, vascular invasion occurs. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





