Introduction
Skin cancer is a significant concern for healthcare services [
1]. In 2022, there were nearly 1.2 million reported cases of nonmelanoma skin cancer (NMSC) worldwide compared with 331,722 cases of melanoma [
2]. This pathology takes up a substantial fraction of dermatological and plastic surgical outpatient visits and surgeries [
3]. After skin cancer removal, when healing by primary closure is not feasible, reconstruction with skin grafts or flaps is performed [
4]. Skin grafts are a common and effective solution in surgical procedures, providing prompt coverage for wounds. This surgical technique may be preferred in certain cases where a suitable donor area is not available for flap harvesting or in patients, particularly the elderly, where lengthy procedures are best avoided [
5]. This results in a satisfactory clinical outcome. However, we should not forget that one of the possible complications of skin graft reconstruction is graft failure, which can lead to the formation of ulcers. Infection, hematoma, seroma, inadequate vascular supply, factors related to the patient's own pathological history or incorrect postoperative management of the graft may be the causes of this complication.
To promote a complete healing and recovery after surgery, it is crucial for the clinician to monitor the patient's skin graft dressing. The use of topical antiseptic dye agents in the skin graft management is of fundamental importance in enabling a successful healing of the wound after surgery [
6]. The therapeutic efficacy of certain antiseptic dyes in treating skin grafts has been highlighted in the past [
6,
7]. Evaluating and selecting the most appropriate antiseptic solution is necessary for both convenience and effectiveness.
This study will assist healthcare professionals in the selection of the most appropriate antiseptic solution among Fluorescein solution, Eosin solution and Povidone-Iodine solution for the post-operative care of skin grafts after skin cancer removal so that optimal reconstructive results and patient satisfaction can be achieved while minimizing complications.
Materials and Methods
A single-center prospective randomized controlled trial was conducted on patients undergoing excision of skin cancer and subsequent reconstruction with skin graft at the Plastic Surgery Unit of San Gallicano Dermatological Institute, IRCCS, Rome, from 2022 to 2024. One hundred eighty-two patients were included and divided into three randomized groups according to the type of antiseptic received: 63 patients received Fluorescein solution, 60 patients received Eosin solution and 59 patients received Povidone-Iodine solution. Skin grafts were harvested from the inguinal crease or the medial brachial region. Care was taken to ensure that the donor skin closely matched the color, thickness, degree of actinic damage, and texture of the skin surrounding the defect [
8]. The graft was then used to reconstruct the loss of substance following the skin cancer excision and, according to the normal treatment protocol [
9,
10,
11], an external tie-over dressing of Triticum Vulgare extract impregnated gauze and sterile gauze was applied and medicated intraoperatively. The grafted area was evaluated after removal of the tie-over dressing, after 6-8 postoperative days. The patient was asked to keep the surgical area clean and dry, to avoid wetting the dressing and, after tie-over dressing removal, to clean the wound carefully as described below.
As per normal treatment protocol, patients were seen in our outpatient clinic at three times:
(T0) At the tie-over dressing removal (after 6-8 postoperative days) we evaluated the grafted area. In particular, the following data were evaluated: the graft rooting percentage (>90%, 50-90%, <50%), the presence of complications such as infection, hematoma, seroma. We took photographs of the grafted area and its size was determined. Cleansing of the graft with the preparation assigned to the patient was performed and instructions were provided for subsequent applications. The patient was asked to cleanse the wound daily by application of one drop of product per square centimeter of graft area and cover the wound with sterile gauze.
(T1) One week after removal of the tie-over dressing, grafted areas were re-examined.
(T2) We calculated the total healing time meaning time until complete re-epithelialization of the grafted area.
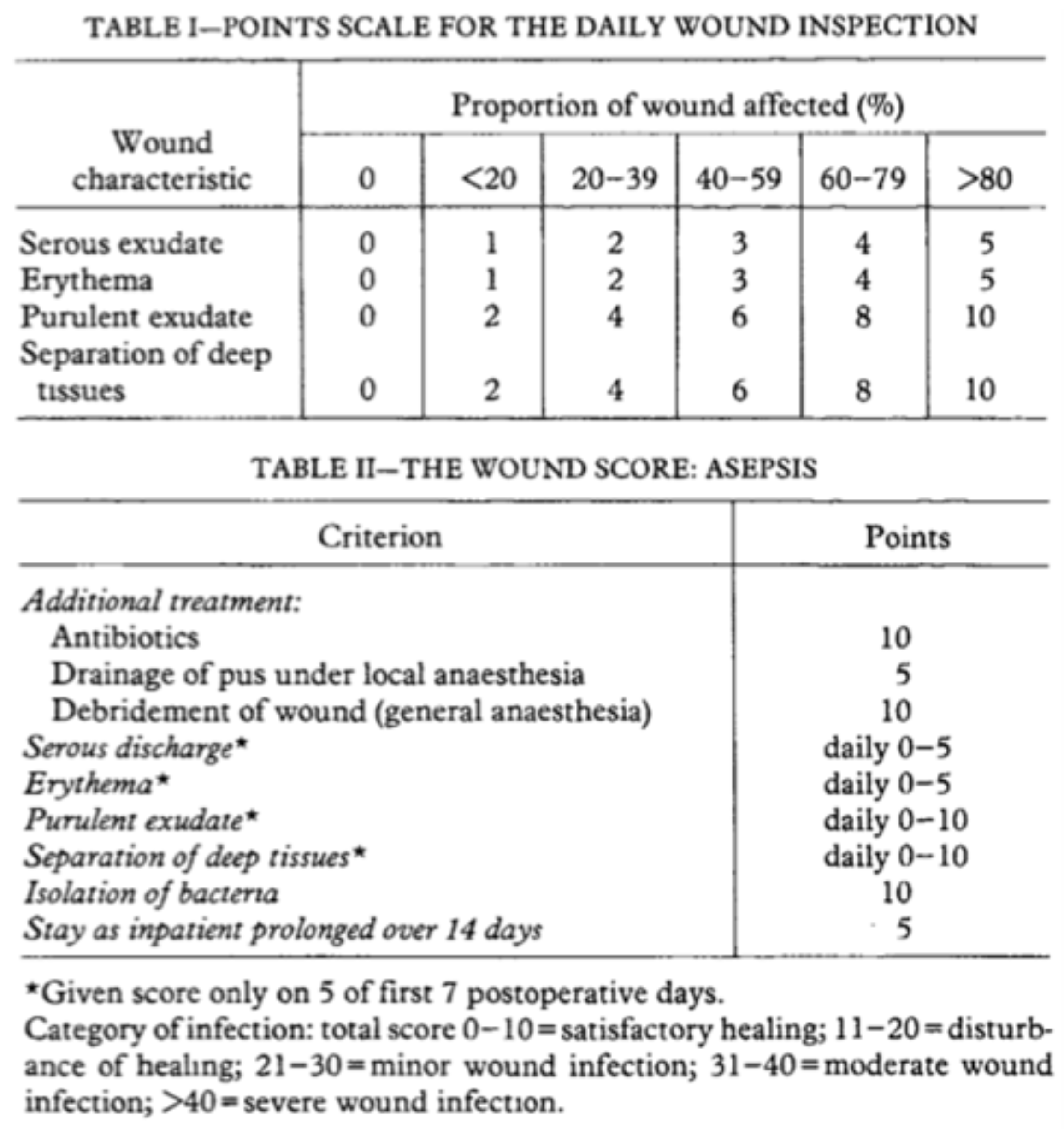
The ASEPSIS SCORE [
12] was used to assess the condition of the skin graft at each dressing to evaluate the efficacy of the antiseptic solution. ASEPSIS score is a reliable tool that objectively evaluates surgical wounds over time. The literature clearly supports the validity, reliability, and usefulness of the ASEPSIS scale in everyday clinical practice.
Points are given for the need of Additional treatment, the presence of Serous discharge, Erythema, Purulent exudate, and Separation of the deep tissues, the Isolation of bacteria, and the duration of inpatient Stay (ASEPSIS) [
12,
13]. A lower score indicates a better healing process. It is important to note that if a complication occurs (for example: an infection requiring antibiotic treatment), the ASEPSIS score must be recalculated by adding additional points (
Figure 1), as specified in the Italian validation of the score [
14].
The primary endpoint of this study is the achievement of an ASEPSIS score value of less than 10 points, for patients receiving Fluorescein 2% solution compared with the two comparison arms. The study collected comprehensive data, including age, diagnosis, and comorbidities such as smoking, diabetes, and vasculopathy. The procedures performed in the study were in concordance with the ethical standards of the institutional and/or national research committee (CE RS 1537/21) and the 1964 Declaration of Helsinki and its subsequent amendments or comparable ethical standards.
Statistical Analysis
We calculated the descriptive statistics for all variables of interest: in particular, we reported continuous variables with their means, standard deviations (±SD), medians and min-max intervals and categorical variables with absolute and percentage frequencies. The Kolmogorov-Smirnov normality test was calculated for all the continuous variables. The Kruskal-Wallis test was used to explore differences between continuous variables and, since we were also interested in paired treatment comparisons, the Bonferroni correction was applied. We assessed relationships between categorical variables using the Chi2 test.
Univariate logistic regression models were used to identify variables that might play a role in the risk of ASEPSIS scale >10.
We performed multivariate logistic regression models with the predictor variables that were significant in the univariate analyses. All statistical analyses were performed with the aid of SPSS version 29 statistical software (SPSS inc., Chicago IL, USA).
Results
The characteristics of the population studied are shown in
Table 1. The groups were homogeneous regarding age, smoking habits and comorbidity. Postoperative complications (infection, hematoma, necrosis, seroma, and need for reintervention) were not significantly different between groups. Comparisons of povidone iodine vs. 2%-Fluorescein (p-value 0.8883), povidone iodine vs. eosin (p-value 0.8062), and 2%-Fluorescein vs. eosin (p-value 0.5387) also showed no significant differences (Bonferroni correction applied).
We found no significant association between the ASEPSIS score and treatment received, even when stratified by "presence of at least 1 comorbidity", "smoking status", "presence of diabetes", and "presence of vasculopathy".
Thereafter, the results of the univariate logistic regression showed that treatment type, or at least one comorbidity, were not meaningful predictors of an ASEPSIS >10. This was confirmed by multivariate logistic regression.
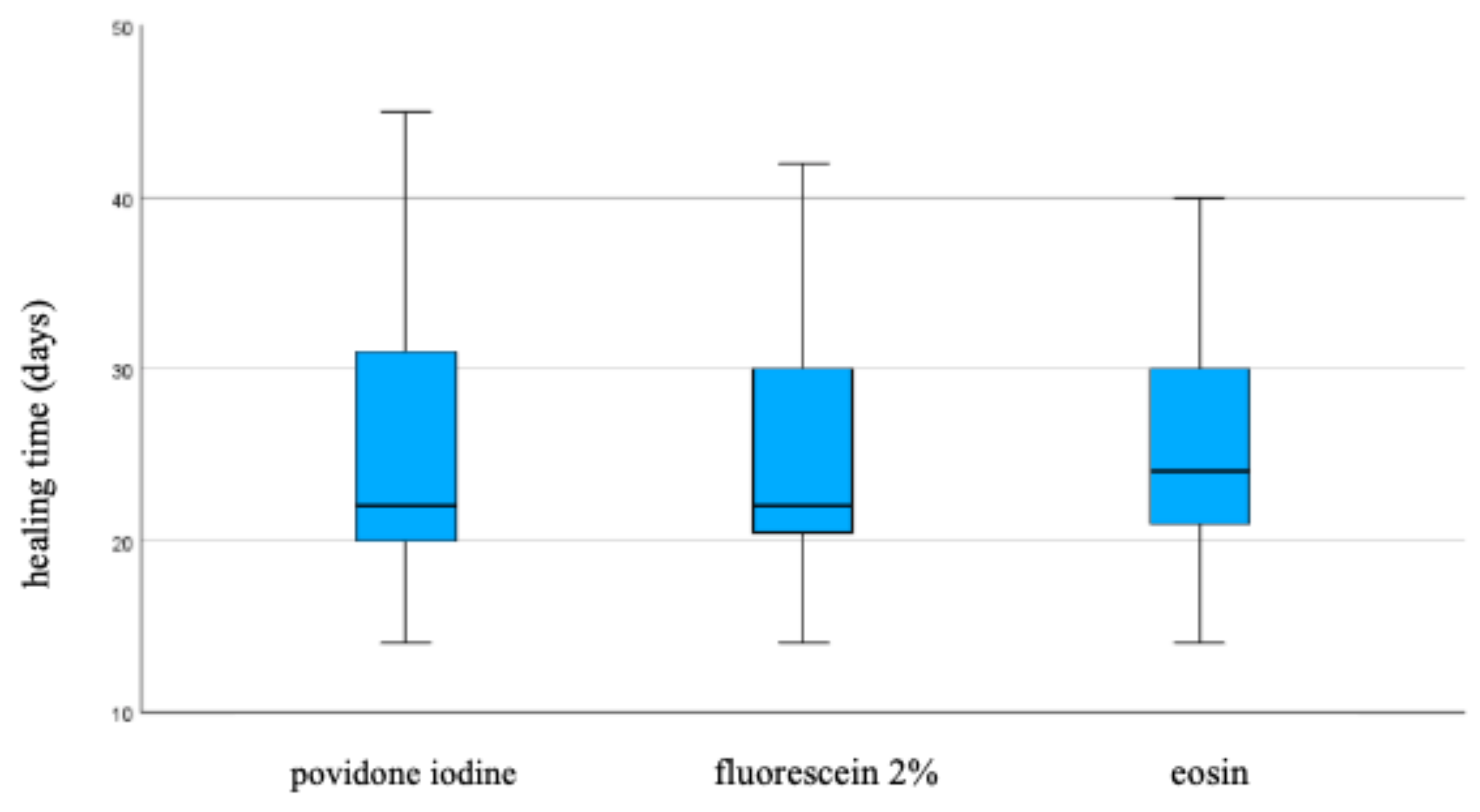
The three treatments do not differ significantly regarding the healing time, even when stratified by "site" and "median size". (
Figure 2)
Discussion
Postoperative wound management is a crucial aspect of surgical treatment, considering the patient's physical well-being and healthcare economics. Our study compares three types of antiseptic dye agents, examining the role of 2%-Fluorescein antiseptic solution, and fills a gap in current research. In the past, publications related to 2%-Fluorescein antiseptic solution have been conducted for postprocedural scar prevention care [
15] or the treatment of pathologies such as psoriasis and diabetic foot.
For skin graft management, the efficacy of liposomal povidone iodine hydrogel has been demonstrated to significantly enhance antiseptic efficacy, wound epithelialization, healing quality, when compared with chlorhexidine gauze, with no clinically relevant topical or systemic adverse effects reported [
7]. Eosin is a highly effective and affordable antiseptic compound that is widely used in both hospital and home settings for the treatment of skin wounds. However, there is no definite data on its efficacy on re-epithelialization plus it leaves a persistent red color on the skin, which can transfer to gauze and clothing. Patients may find it less appealing, but the benefits outweigh this minor inconvenience [
16]. Fluorescein is a non-toxic dye used as a contrast agent in medicine and as a tracer for water flows [
17]. The fluorescein solution used in our study has been manufactured containing a topical 2% fluorescein solution combined with allantoin [
18], Melaleuca alternifolia, and Uncaria tomentosa. These additional active ingredients are not present in common eosin formulations making the product under review competitive to treat ulcers without greatly elevating its cost. Fluorescein is a non-bromate precursor of eosin with bacteriostatic and regenerative properties, mainly used in exudative cutaneous afflictions; allantoin has a significant hydrating and healing effect, Melaleuca alternifolia has an additional antiseptic action [
19], and Uncaria tomentosa is a natural anti-inflammatory agent [
20,
21]. The 2%-Fluorescein lotion is widely used as a natural (non-drug) adjuvant in all skin conditions characterized by exudation, and maceration with possible microbial (bacterial or fungal) overlap in association with other specific therapies (topical or systemic). Since 2%-Fluorescein antiseptic solution does not contain any antibiotics, it does not present the problem of creating resistance in the strains of bacteria that are present in the wound. Allergic sensitization and anaphylactic events are not reported. In the literature, we could not trace reports of local or systemic side effects of Fluorescein by intradermal administration except itch and pain on the injection site, dependent on the fluorescein preparation used. Despite the potential risk of cardiovascular, neurologic, allergic, and other systemic adverse reactions, dermal use of fluorescein for in vivo study of skin is concluded as being widely safe [
22,
23].
Validated scores should be relied upon for the assessment of wound healing, rather than personal opinions. ASEPSIS SCORE was used to obtain this result.
2%-Fluorescein antiseptic solution is similar in efficacy to the other two disinfectants but, compared with eosin, has the added benefit of not interfering with the clinician's ability to assess the healing process due to its color, is cost-effective, and contains natural adjuvants that may aid in the healing process, making it a suitable option for patients.
According to these results, it appears that 2%-Fluorescein antiseptic solution may be a viable medical antiseptic solution for the treatment of surgical wounds.
Conclusion
Our comparative study marks a significant contribution to the scientific literature, offering valuable insights for the management of patients undergoing surgical excision and reconstruction with skin graft or other reconstructive options.
Although the results of our experimental study were not in line with the predefined objectives, the positive outcomes associated with the use of 2%-Fluorescein antiseptic solution suggest that this type of antiseptic can be used, eliminating the drawback of others and adding a stimulus to healing, given its active ingredients. Therefore, it would be interesting to enroll a larger number of patients to confirm, with more statistical data, that the 2%-Fluorescein antiseptic solution is safe, effective, and valuable in the management of post-operative wounds, as in our study.
IRB approval status
Reviewed and approved by IRB; approval CE RS 1537/21
Conflicts of Interest
None declared.
References
- Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012 May;166(5):1069–80. [CrossRef]
- The Global Cancer Observatory. Melanoma of Skin. December 2020 [cited 2024 Apr 11]. https://gco.iarc.who.int/media/globocan/factsheets/cancers/16melanoma-of-skin-fact-sheet.pdf.
- Mamsen FPW, Kiilerich CH, Hesselfeldt-Nielsen J, Saltvig I, Remvig CLN, Trøstrup H, et al. Risk Stratification of Local Flaps and Skin Grafting in Skin Cancer-Related Facial Reconstruction: A Retrospective Single-Center Study of 607 Patients. J Pers Med. 2022 Dec 15;12(12):2067. [CrossRef]
- Kianian S, Zhao K, Kaur J, Lu KW, Rathi S, Ghosh K, et al. Autologous Skin Grafts, versus Tissue-engineered Skin Constructs: A Systematic Review and Meta-analysis. Plast Reconstr Surg Glob Open. 2023 Jun 27;11(6):e5100. [CrossRef]
- Skin grafts: Do we need to suture? J Am Acad Dermatol. 2010 Mar 1;62(3):AB144.
- Mann-Salinas EA, Joyner DD, Guymon CH, Ward CL, Rathbone CR, Jones JA, et al. Comparison of Decontamination Methods for Human Skin Grafts. J Burn Care Res Off Publ Am Burn Assoc. 2015;36(6):636–40. [CrossRef]
- Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, Pyon JK, Wa CTC, Villa MA. Povidone iodine in wound healing: A review of current concepts and practices. Int J Surg Lond Engl. 2017 Aug;44:260–8. [CrossRef]
- Ramsey ML, Walker B, Patel BC. Full-Thickness Skin Grafts. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited 2024 Apr 14]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK532875/.
- Blair VP, Brown JB. THE USE AND USES OF LARGE SPLIT SKIN GRAFTS OF INTERMEDIATE THICKNESS. Plast Reconstr Surg. 1968 Jul;42(1):65.
- Seymour FK, Giele HP. Tie-overs under pressure. Br J Plast Surg. 2003 Jul;56(5):494–7. [CrossRef]
- Wolf Y, Kalish E, Badani E, Friedman N, Hauben DJ. Rubber foam and staples: do they secure skin grafts? A model analysis and proposal of pressure enhancement techniques. Ann Plast Surg. 1998 Feb;40(2):149–55. [CrossRef]
- Wilson AP, Treasure T, Sturridge MF, Grüneberg RN. A scoring method (ASEPSIS) for postoperative wound infections for use in clinical trials of antibiotic prophylaxis. Lancet Lond Engl. 1986 Feb 8;1(8476):311–3. [CrossRef]
- Broex ECJ, van Asselt ADI, Bruggeman CA, van Tiel FH. Surgical site infections: how high are the costs? J Hosp Infect. 2009 Jul;72(3):193–201. [CrossRef]
- Terzoni di S, Destrebecq A, Teresa A, Bellotti GC, Carelli L, Matteini di M, et al. La scala ASEPSIS: validazione italiana di uno strumento per la valutazione delle caratteristiche del sito chirurgico [Internet]. Fnopi L’infermiere. 2015 [cited 2024 Apr 11].
- Efficacy and safety of 2% fluorescein solution for post- procedural scar prevention care | Journal of Applied Cosmetology. 2023 Dec 5 [cited 2024 Apr 13]; Available from: https://scientificeditorial.com/index.php/JAC/article/view/Efficacy-and-safety-of-2-fluorescein-solution-for-post-procedura.
- Romano I, Ayadi F, Rizzello L, Summa M, Bertorelli R, Pompa PP, et al. Controlled antiseptic/eosin release from chitosan-based hydrogel modified fibrous substrates. Carbohydr Polym. 2015 Oct 20;131:306–14. [CrossRef]
- Romanchuk KG. Fluorescein. Physiochemical factors affecting its fluorescence. Surv Ophthalmol. 1982;26(5):269–83. [CrossRef]
- An investigation into multifaceted mechanisms of action of allantoin in wound healing. J Am Acad Dermatol. 2017 Jun 1;76(6):AB40.
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (Tea Tree) Oil: a Review of Antimicrobial and Other Medicinal Properties. Clin Microbiol Rev. 2006 Jan;19(1):50–62. [CrossRef]
- Akesson C, Lindgren H, Pero RW, Leanderson T, Ivars F. An extract of Uncaria tomentosa inhibiting cell division and NF-kappa B activity without inducing cell death. Int Immunopharmacol. 2003 Dec;3(13–14):1889–900. [CrossRef]
- Sandoval-Chacón M, Thompson JH, Zhang XJ, Liu X, Mannick EE, Sadowska-Krowicka H, et al. Antiinflammatory actions of cat’s claw: the role of NF-kappaB. Aliment Pharmacol Ther. 1998 Dec;12(12):1279–89.
- Mullins JM. Overview of fluorophores. Methods Mol Biol Clifton NJ. 1994;34:107–16. [CrossRef]
- O’goshi K ichiro, Serup J. Safety of sodium fluorescein for in vivo study of skin. Skin Res Technol Off J Int Soc Bioeng Skin ISBS Int Soc Digit Imaging Skin ISDIS Int Soc Skin Imaging ISSI. 2006 Aug;12(3):155–61. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).