Submitted:
14 October 2024
Posted:
15 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Functionalization of WS2 Nanoparticles
| Material produced | Drug conjugation | Method | Physicochemical properties alteration | Reference |
|---|---|---|---|---|
| WS2 - BSA | - | 1) WS2 production by ultrasound-assisted liquid phase exfoliation 2) BSA electrostatic adsorption on the surface by stirring |
Improved stability in saline, water and DMEM Zeta potential: BSA = −27.4 mV WS2 = 20.8 mV WS2-BSA = −19.8 mV |
[33] |
| Fe (III)-WS2 -PVP | DOX Drug loading: 41% (Fe (III)-WS2 -PVP: DOX weight ratio: n/s) Drug release: 7.5 (pH 7.4; 6 h) 12.7% (pH 7.4; 48 h) 37% (pH 6.0; 6 h) 48.2% (pH 6.0; 48 h) |
1) Fe (III)- WS2 -PVP formation by hydrothermal reaction 2) DOX adsorption on Fe (III)- WS2 -PVP |
Increased biodegradation rate Improved stability in saline, water and DMEM |
[34] |
| WS2 – PEG |
- | Covalent bond formation between WS2 and PEG | Improved stability in saline Improved photothermal properties: broad NIR absorption band 700-1000 nm |
[28] |
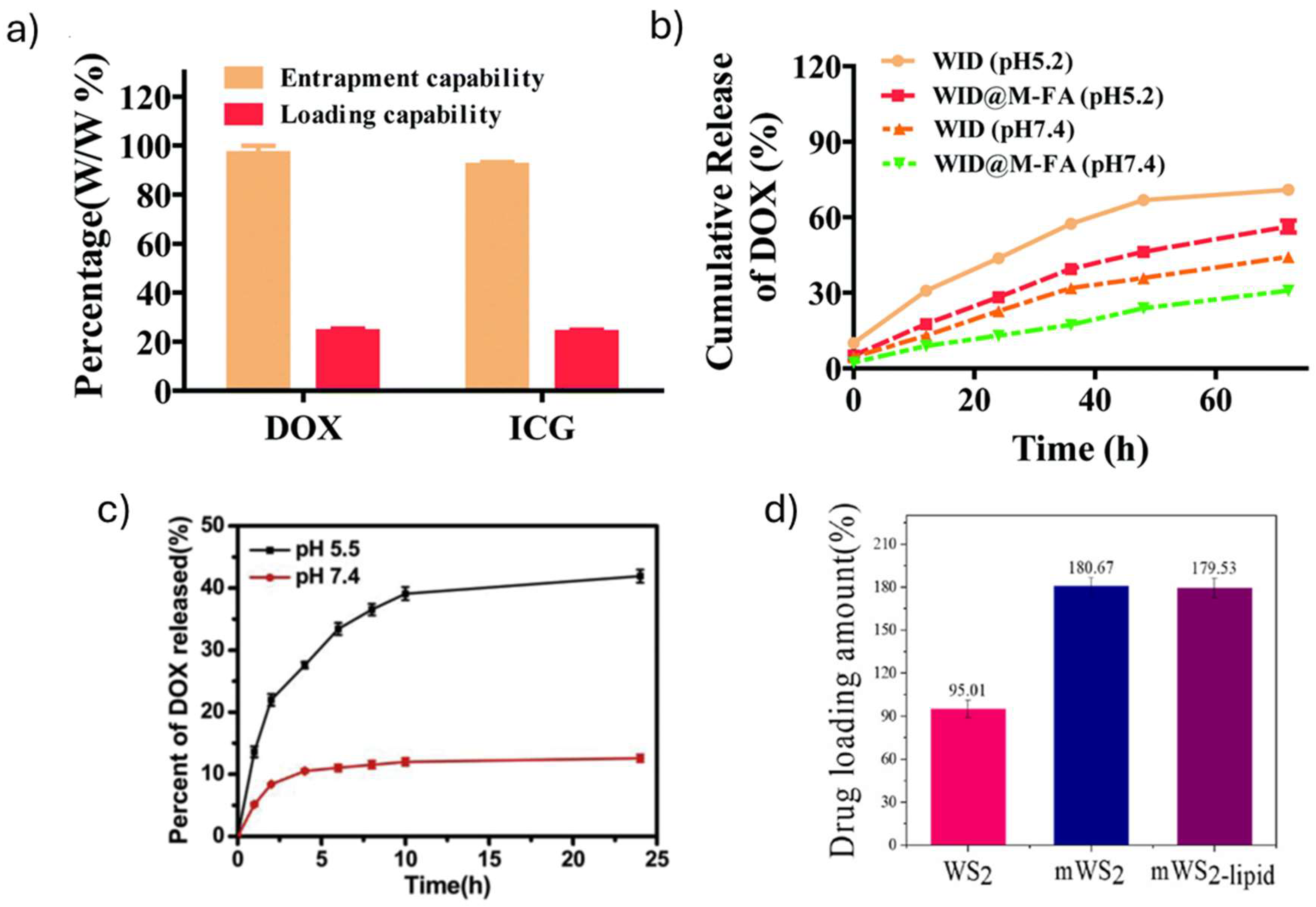
| WID-M-FA | DOX loading: 24.2% (WID-M-FA: DOX weight ratio: n/s) DOX release: 12 % (pH 7.4; 20 h) 30.9% (pH 7.4; 72 h) 24.2% (pH 5.2; 20 h) 56.4% (pH 5.2; 72 h) ICG loading: 22.9% (WID-M-FA: ICG weight ratio: n/s) ICG release: 14.3 % (pH 7.4; 20 h) 19.5% (pH 7.4; 72 h) 27.1% (pH 5.2; 20 h) 35.2% (pH 5.2; 72 h) |
1) LA-PEG2000-NH2 adsorption on WS2 surface 2) WI formation through non-covalent bond formation between ICG (I) and WS2-PEG 3) WID formation trough non-covalent bond formation between WI and DOX (D) 4) WID-M formation trough physical extrusion of WID and erythrocyte vesicles 5) WID-M-FA formation trough DSPE- PEG2000-FA adsorption on WID-M membrane |
Improved water stability and biocompatibility. Improved photothermal properties: higher absorption at 808 nm. Zeta potential: WS2 = -16.5 mV WS2-PEG = -7.4 mV DOX = -2.8 mV ICG = -5.5 mV M = -9.6 mV WID = -14.2 mV WID-M = -24.9 mV WID-M-FA = -30.9 mV |
[40] |
| N-WS2 | - | N-WS2 formation by hydrothermal reaction | Improved stability in water and biocompatibility Improved absorption in the NIR region |
[38] |
| WS2-IO-MS-PEG/DOX | DOX Drug loading: 13.5% (WS2-IO-MS-PEG: DOX weight ratio: n/s, pH 8) Drug release: 10.6 % (pH 7.4; 5 h) 12.5% (pH 7.4; 24 h) 11.9% (pH 5.5; 5 h) 42.5% (pH 5.5; 24 h) |
1) Covalent bond formation between DMSA-modified IONPs and WS2 2) Covalent bond formation between SiO2 and WS2-IO 3) PEG adsorption on WS2-IO-MS surface 4) DOX adsorption on WS2-IO-MS-PEG |
Improved stability in water, saline and serum |
[32] |
| PEG-WS2:Gd3+ | - | 1) Covalent bond formation between WS2 and Gd3+ 2) C18PMH-PEG adsorption on WS2:Gd3+ surface |
Improved stability in water, saline, PBS, 1640-Medium and FBS | [39] |
| WS2 -lipid | DOX Drug loading: 87% (WS2 -lipid: DOX weight ratio 1:5, pH 7.4) Drug release: 13.3% (pH 7.4; 8 h) 32.1% (pH 7.4; 168 h) 22.3% (pH 5; 8 h) 43.2% (pH 5;168 h) |
1) Liposomes formation by membrane hydration methods 2) WS2 -lipid formation by liposomes adsorption on WS2 surface 3) DOX adsorption on WS2 -lipid |
Improved stability in distilled water, PBS and RPMI-1640 medium containing 10% fetal bovine serum Zeta potential: WS2 = -42.9 mV Liposome = -25.87 mV WS2 -lipid = -33.77 mV |
[35] |
| mWS2-lipid | DOX Drug loading: 179.53% (WS2 -lipid: DOX weight ratio 1:2) Drug release: 12.5% (pH 7.4; 8 h) 33% (pH 7.4; 168 h) 23% (pH 5; 8 h) 48% (pH 5;168 h) |
1) mWS2 formation by solvothermal reaction 2) Liposome adsorption on mWS2 3) DOX adsorption on mWS2-lipid |
Improved stability in water, PBS and DMEM Zeta potential: WS2 = −42.96 mV mWS2 = −46.25 mV mWS2-lipid = −24.74 mV Lipid = -24.66 mV Superparamagnetic properties |
[36] |
| WS2/Au-lipid-DOX | DOX Drug loading: 84.54% (WS2/Au-lipid: DOX weight ratio n/s, pH 7.4) Drug release: 12.5% (pH 7.4; 8 h) 24% (pH 7.4; 168 h) 17% (pH 5; 8 h) 42.5% (pH 5; 168 h) |
1) WS2/Au was synthesized using Na3C6H5O7 reduction method 2) WS2/Au-lipid by magnetic stirring 3) DOX adsorption on WS2/Au-lipid |
Improved stability in water, PBS and DMEM Zeta potential: WS2 = −42.19 mV WS2/Au = −40.61 mV WS2/Au-lipid = −44.72 mV Lipid = -36.79 mV |
[37] |
3. Drug Loading and Release
4. In Vitro Biocompatibility Studies
5. In Vivo Biocompatibility Studies
6. In Vitro Photothermal Therapy Studies
7. In Vivo Photothermal Therapy Studies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Institute, N.C. Global Cancer Research. 2022. Available online: https://www.cancer.gov/research/areas/global-health.
- Sermeus, A., et al., Advances in radiotherapy and targeted therapies for rectal cancer. World Journal of Gastroenterology: WJG, 2014. 20(1): p. 1. [CrossRef]
- Farzam, O.R., et al., Nanoparticles for imaging-guided photothermal therapy of colorectal cancer. Heliyon, 2023. [CrossRef]
- Rodrigues, J.A. and J.H. Correia, Photodynamic therapy for colorectal cancer: An update and a look to the future. International Journal of Molecular Sciences, 2023. 24(15): p. 12204. [CrossRef]
- Han, H.S. and K.Y. Choi, Advances in nanomaterial-mediated photothermal cancer therapies: toward clinical applications. Biomedicines, 2021. 9(3): p. 305. [CrossRef]
- Silva, F.A., et al., 2D Nanomaterials and Their Drug Conjugates for Phototherapy and Magnetic Hyperthermia Therapy of Cancer and Infections. Small, 2023: p. 2306137. [CrossRef]
- Tang, P., et al., Thermochromism-induced temperature self-regulation and alternating photothermal nanohelix clusters for synergistic tumor chemo/photothermal therapy. Biomaterials, 2019. 188: p. 12-23. [CrossRef]
- Murugan, C., et al., Two-dimensional cancer theranostic nanomaterials: Synthesis, surface functionalization and applications in photothermal therapy. Journal of Controlled Release, 2019. 299: p. 1-20. [CrossRef]
- Singh, S., et al., Graphene nanomaterials: The wondering material from synthesis to applications. Sensors International, 2022. 3: p. 100190. [CrossRef]
- Zhu, Y., et al., Graphene and graphene oxide: synthesis, properties, and applications. Advanced materials, 2010. 22(35): p. 3906-3924. [CrossRef]
- Yang, K., et al., Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano letters, 2010. 10(9): p. 3318-3323. [CrossRef]
- Silva, F.A., et al., UV-C driven reduction of nanographene oxide opens path for new applications in phototherapy. Colloids and Surfaces B: Biointerfaces, 2024. 233: p. 113594. [CrossRef]
- Murali, A., et al., Emerging 2D nanomaterials for biomedical applications. Materials Today, 2021. 50: p. 276-302. [CrossRef]
- Hu, T., et al., Two-dimensional nanomaterials: fascinating materials in biomedical field. Science Bulletin, 2019. 64(22): p. 1707-1727. [CrossRef]
- Liu, J., et al., Gold nanostars decorated MnO2 nanosheets for magnetic resonance imaging and photothermal erasion of lung cancer cell. Materials Today Communications, 2018. 16: p. 97-104. [CrossRef]
- Guan, X., et al., 2D MXene nanomaterials: Synthesis, mechanism, and multifunctional applications in microwave absorption. Small Structures, 2022. 3(10): p. 2200102. [CrossRef]
- Derakhshi, M., et al., Two-dimensional nanomaterials beyond graphene for biomedical applications. Journal of Functional Biomaterials, 2022. 13(1): p. 27. [CrossRef]
- Khandelwal, A., et al., Phosphorene–the two-dimensional black phosphorous: Properties, synthesis and applications. Materials Science and Engineering: B, 2017. 221: p. 17-34. [CrossRef]
- Mo, S., et al., Two-dimensional antibacterial Pd@ Ag nanosheets with a synergetic effect of plasmonic heating and Ag+ release. Journal of materials chemistry B, 2015. 3(30): p. 6255-6260. [CrossRef]
- Liu, J., et al., Heat/pH-boosted release of 5-fluorouracil and albumin-bound paclitaxel from Cu-doped layered double hydroxide nanomedicine for synergistical chemo-photo-therapy of breast cancer. Journal of Controlled Release, 2021. 335: p. 49-58. [CrossRef]
- Rahman, M.T., et al., Two-dimensional transition metal dichalcogenides and their composites for lab-based sensing applications: Recent progress and future outlook. Sensors and Actuators A: Physical, 2021. 318: p. 112517. [CrossRef]
- Zhou, X., H. Sun, and X. Bai, Two-dimensional transition metal dichalcogenides: synthesis, biomedical applications and biosafety evaluation. Frontiers in Bioengineering and Biotechnology, 2020. 8: p. 236. [CrossRef]
- Gong, L., et al., Two-dimensional transition metal dichalcogenide nanomaterials for combination cancer therapy. Journal of materials chemistry B, 2017. 5(10): p. 1873-1895. [CrossRef]
- Chou, S.S., et al., Chemically exfoliated MoS2 as near-infrared photothermal agents. Angewandte Chemie International Edition, 2013. 52(15): p. 4160-4164. [CrossRef]
- Brent, J.R., N. Savjani, and P. O’Brien, Synthetic approaches to two-dimensional transition metal dichalcogenide nanosheets. Progress in Materials Science, 2017. 89: p. 411-478. [CrossRef]
- Wang, S., et al., Synthesis and biocompatibility of two-dimensional biomaterials. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019. 583: p. 124004. [CrossRef]
- Meivita, M.P., et al., WS2/Polyethylene Glycol Nanostructures for Ultra-Efficient MCF-7 Cancer Cell Ablation and Electrothermal Therapy. ACS omega, 2022. 7(27): p. 23075-23082. [CrossRef]
- Cheng, L., et al., PEGylated WS2 nanosheets as a multifunctional theranostic agent for in vivo dual-modal CT/photoacoustic imaging guided photothermal therapy. Advanced materials, 2014. 26(12): p. 1886-1893. [CrossRef]
- Gazzi, A., et al., Photodynamic therapy based on graphene and MXene in cancer theranostics. Frontiers in Bioengineering and Biotechnology, 2019. 7: p. 295. [CrossRef]
- Silva, F.A.L.S., et al., 2D Nanomaterials and Their Drug Conjugates for Phototherapy and Magnetic Hyperthermia Therapy of Cancer and Infections. Small, 2023. [CrossRef]
- Robinson, J.T., et al., Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. Journal of the American Chemical Society, 2011. 133(17): p. 6825-6831. [CrossRef]
- Yang, G., et al., Two-dimensional magnetic WS2@ Fe3O4 nanocomposite with mesoporous silica coating for drug delivery and imaging-guided therapy of cancer. Biomaterials, 2015. 60: p. 62-71. [CrossRef]
- Yi, H., et al., Liquid exfoliated biocompatible WS 2@ BSA nanosheets with enhanced theranostic capacity. Biomaterials Science, 2021. 9(1): p. 148-156. [CrossRef]
- Wu, C., et al., Biodegradable Fe (III)@ WS2-PVP nanocapsules for redox reaction and TME-enhanced nanocatalytic, photothermal, and chemotherapy. Advanced Functional Materials, 2019. 29(26): p. 1901722. [CrossRef]
- Xie, M., et al., WS 2 nanosheets functionalized by biomimetic lipids with enhanced dispersibility for photothermal and chemo combination therapy. Journal of Materials Chemistry B, 2020. 8(11): p. 2331-2342. [CrossRef]
- Xie, M., et al., Magnetic WS2 nanosheets functionalized by biomimetic lipids with enhanced dispersibility for combined photothermal and chemotherapy therapy. Journal of Drug Delivery Science and Technology, 2023. 86: p. 104744. [CrossRef]
- Li, J., et al., Construction of WS2/Au-lipid drug delivery system for multiple combined therapy of tumor. Journal of Drug Delivery Science and Technology, 2022. 76: p. 103747. [CrossRef]
- Liu, Q., et al., Stable metallic 1T-WS 2 ultrathin nanosheets as a promising agent for near-infrared photothermal ablation cancer therapy. Nano Research, 2015. 8: p. 3982-3991. [CrossRef]
- Cheng, L., et al., Bottom-up synthesis of metal-ion-doped WS2 nanoflakes for cancer theranostics. ACS nano, 2015. 9(11): p. 11090-11101. [CrossRef]
- Long, Y., et al., PEGylated WS 2 nanodrug system with erythrocyte membrane coating for chemo/photothermal therapy of cervical cancer. Biomaterials Science, 2020. 8(18): p. 5088-5105. [CrossRef]
- Zhang, H., et al., Recent advances of two-dimensional materials in smart drug delivery nano-systems. Bioactive Materials, 2020. 5(4): p. 1071-1086. [CrossRef]
- Martín, C., et al., Biocompatibility and biodegradability of 2D materials: graphene and beyond. Chemical communications, 2019. 55(39): p. 5540-5546. [CrossRef]
- Iso, I., 10993–5: 2009 Biological evaluation of medical devices—part 5: tests for in vitro cytotoxicity. International Organization for Standardization, Geneva, 2009. 34.
| 2D material | Particle size (nm) | Culture conditions and cell viability | 2DnMat location | Additional outcomes | Reference |
|---|---|---|---|---|---|
| WS2 – BSA | 150-200 | WS2 –BSA (HeLa1, 2, 24, 36, 48 h incubation) >80% (6.25 ppm) >70% (12.5 ppm) >70% (25 ppm) >80% (50 ppm) Concentrations tested: 6.25-50 ppm |
- | - | [33] |
| Fe (III)- WS2 -PVP | 108 | Fe (III)- WS2 -PVP (HT29, 24 h incubation) > 80% (100 µg mL−1) |
- | - | [34] |
| WS2 – PEG | 94 | WS2 – PEG (4T1, 24 h incubation) > 90% (0.1 mg mL−1) WS2 – PEG (HeLa, 24 h incubation) > 90% (0.1 mg mL−1) WS2 – PEG (293T, 24 h incubation) > 90% (0.1 mg mL−1) Concentrations tested: 0.006-0.1 mg mL−1 |
- | LDH release (4T1, HeLa, 293T, 24 h incubation): 0% (100 μg mL−1) ROS generation (4T1, HeLa, 293T, 24 h incubation): 7% (50, 100 μg mL−1) Concentrations tested: 13–100 μg mL−1 |
[28] |
| WS2-IO-MS-PEG/DOX | 90 | WS2-IO-MS-PEG/DOX (4T1, 24 h incubation) > 70% (0.781 µg mL−1) > 60% (1.563 µg mL−1) > 60% (3.125 µg mL−1) > 60% (6.25 µg mL−1) > 30% (12.5 µg mL−1) > 20% (25 µg mL−1) Concentrations tested:0.781-0.25 µg mL−1 |
- | - | [32] |
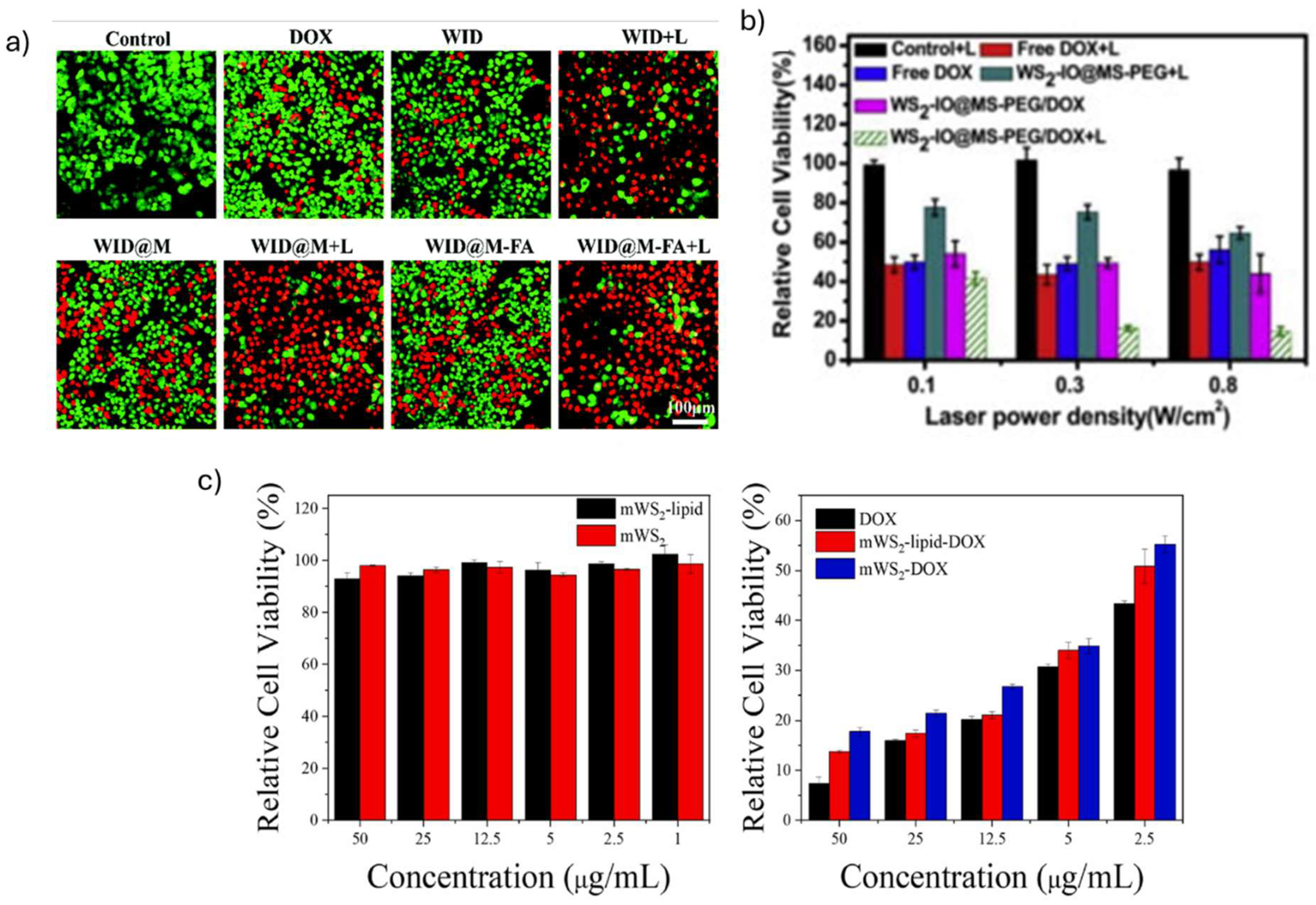
| WID-M-FA | 162 | WI-M-FA (HeLa, 24 h incubation) 89% (12.5 µg mL−1) 89% (25 µg mL−1) 75.9% (50 µg mL−1) 68.5% (100 µg mL−1) Concentrations tested: 12.5-100 µg mL−1 |
Observed in lysosomes (4 h incubation) |
1% hemolysis (100 μg mL−1, 4 h incubation with RBCs) | [40] |
| N- WS2 | 150 | N- WS2 (Hela, 24 h incubation) >90% (15 µg mL−1) >88% (120 µg mL−1) N- WS2 (MDA-MB-231, 24 h incubation) >90% (15 µg mL−1) >90% (120 µg mL−1) N- WS2 (HepG2, 24 h incubation) >90% (15 µg mL−1) >90% (120 µg mL−1) Concentrations tested: 0.469-120 µg mL−1 |
- | - | [38] |
| PEG-WS2:Gd3+ | 90-100 | PEG-WS2:Gd3+ (4T1, 24, 48 h incubation) >90% (50 µg mL−1) Concentrations tested: 1.56-100 µg mL−1 |
- | - | [39] |
| WS2 -lipid-DOX | 222.58 |
WS2 -lipid (MCF-7, 24 h incubation) > 90% (100 µg mL−1) Concentrations tested: 2.5-200 µg mL−1 WS2 -lipid-DOX (MCF-7, 24 h incubation) > 80% (1, 2.5, 5 µg mL−1) > 40% (12. 5 µg mL−1) > 30% (25 µg mL−1) > 20% (50 µg mL−1) Concentrations tested: 1-50 µg mL−1 |
Observed in cytoplasm (MCF-7, 4 h incubation) | - | [35] |
| mWS2-lipid | 318.07 | mWS2-lipid (MCF-7, 4 h incubation) >90% Concentrations tested: 1-50 µg mL−1 mWS2-lipid-DOX (MCF-7, 4 h incubation) 50% (2.5 µg mL−1) 34% (5 µg mL−1) 22% (12.5 µg mL−1) 17% (25 µg mL−1) 14% (50 µg mL−1) Concentrations tested: 2.5-50 µg mL−1 |
Observed in cytoplasm (MCF-7, 24 h incubation) | - | [36] |
| WS2/Au-lipid-DOX | 196 | WS2/Au-lipid (MCF-7, 4 h incubation) >90% (50 µg mL−1) Concentrations tested: 2-50 µg mL−1 WS2/Au-lipid-DOX (MCF-7, 4 h incubation) >68% (2.5 µg mL−1) >50% (5, 12.5 µg mL−1) >41% (25 µg mL−1) >33% (50 µg mL−1) Concentrations tested: 2.5-50 µg mL−1 |
Observed in cytoplasm (MCF-7, 4 h incubation) | - | [37] |
| 2D material | Animal model | Animal survival | Treatment Conditions | Main Results | Reference |
|---|---|---|---|---|---|
| WS2 – BSA | Zebrafish embryos | 120 h: >80% |
Zebrafish embryos were incubated in E3 medium with WS2 – BSA Concentrations tested: 0-50 ppm |
120 h: Hatching rate: 50% (0 ppm) 27.5% (6.25 ppm) 32.5% (12.5 ppm) 45% (25 ppm) 48.5% (50 ppm) |
[33] |
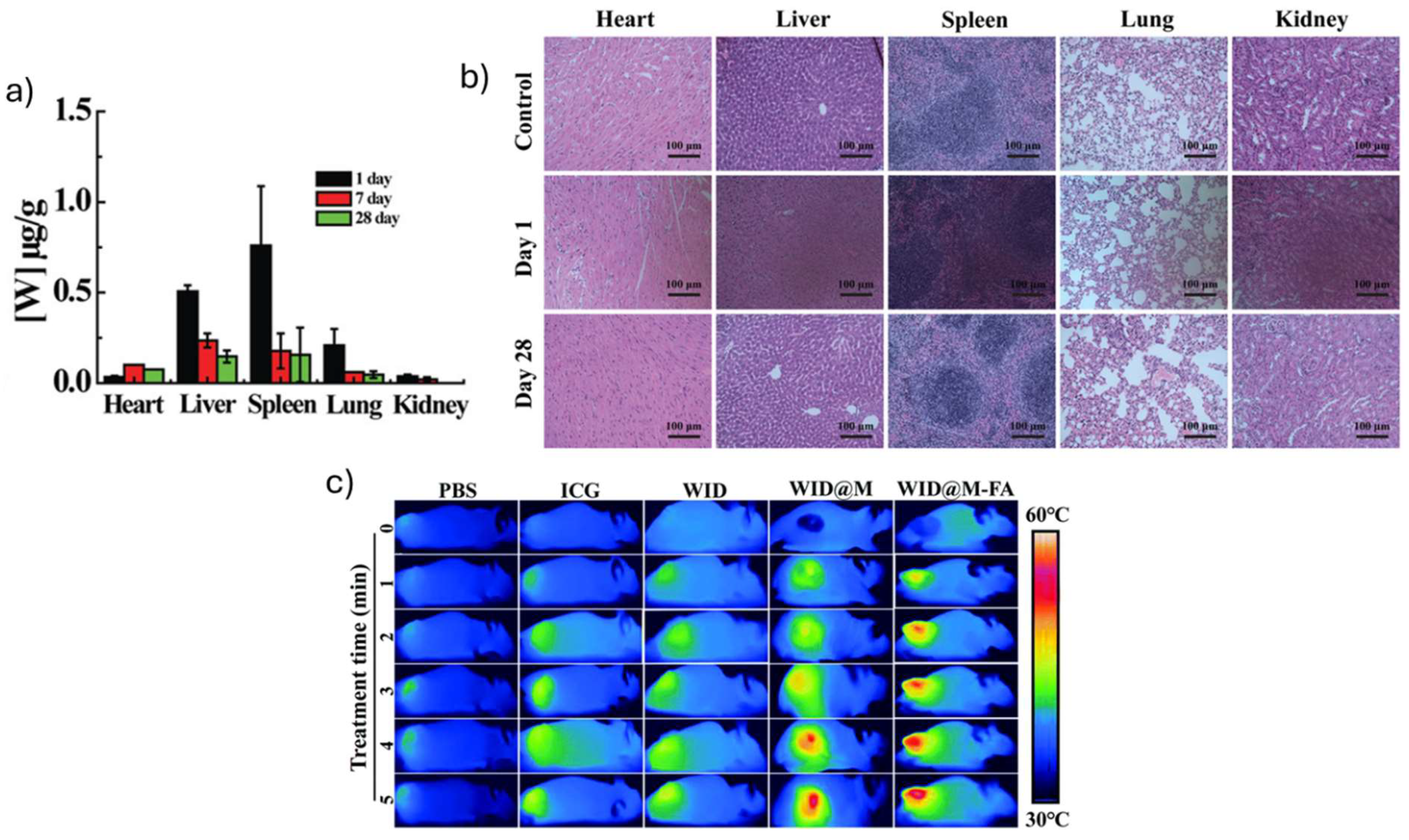
| Fe (III)- WS2 -PVP | HT29 colorectal carcinoma bearing KM mice | - | i.v. administration (100 µg mL−1) Heart: 1 day: 0,03 µg g-1 7 days: 0,11 µg g-1 Liver: 1 day: 0,52 µg g-1 7 days: 0,25 µg g-1 Spleen: 1 day: 0,77 µg g-1 7 days: 0,18 µg g-1 Lung: 1 day: 0,22 µg g-1 7 days: 0,07 µg g-1 Kidney: 1 day: 0,07 µg g-1 7 days: 0,02 µg g-1 |
28 days: no changes on heart, liver, spleen, lung and kidney tissues | [34] |
| WS2 – PEG | 4T1 tumor bearing Balb/C mice | - | i.t administration (2 mg kg−1, 30 min) i.v. administration (20 mg kg−1, 24 h) No changes on body weight 28 days: serum biochemistry markers on normal variation ranges (ALT, ALP, AST, BUN levels, WBC, RBC, HCT, Hgb, MCV, MCH, MCHC, and platelets) |
45 days: no changes on liver, spleen, kidney, heart and lung | [28] |
| WS2-IO-MS-PEG/DOX | 4T1 tumor bearing Balb/C mice | - | i.v. administration (WS2 = 8.4 mg kg-1, DOX = 7 mg kg-1, 24 h) Circulation half-life = 4.77 h Heart: 24 h: 1.18% ID g−1 Liver: 24 h: 25.88% ID g−1 Spleen: 24 h: 38.82% ID g−1 Lung: 24 h: 5.29% ID g−1 Kidney: 24 h: 4.71% ID g−1 Stomach: 24 h: 2.35% ID g−1 Intestine: 24 h: 1.76% ID g−1 Muscle: 24 h: 1.76% ID g−1 Tumor: 24 h: 8.24% ID g−1 |
24 h: high accumulation on liver spleen and tumor | [32] |
| WID-M-FA | HeLA tumor-bearing Balb/c mice | - | i.v. administration (DOX = 2 mg kg-1, ICG = 5 mg kg-1, 24 h) 18 days: serum biochemistry markers on normal variation ranges (RBC, RDW, MCHC, MCV, PLT, WBC, ALT, AST, CRE, BUN) |
18 days: no changes on liver, spleen, kidney, heart and lung | [40] |
| N- WS2 |
HeLa tumor-bearing female NOD/SCID mice | - | i.t administration (1.2 mg mL-1, 40 µL, 24 h) 16 days: serum biochemistry markers on normal variation ranges (WBC, RBC, Hb, HCT, PLT, MCV, MCHC, MCH) |
48 h: no changes in liver and lung tissues | [38] |
| PEG-WS2:Gd3+ | 4T1 tumor-bearing Balb/c mice | - | i.v. administration (2 mg mL-1, 200 µL, 24 h) Tumor: 24h: 11.8% ID g−1 Liver: 24 h: 50.9% ID g−1 Spleen: 24 h: 94.5% ID g−1 |
T1-MR signal (a.u.): 200 (before i.v. injection) 500 (after i.v. injection) |
[39] |
| 2D material | Irradiation method | Energy (W cm−2) |
Time of irradiation (min) | Culture conditions and cell viability | Reference |
|---|---|---|---|---|---|
| WS2 – BSA | Laser (808 nm) |
1.5 | 5 | Tmax = 44.5 ˚C (1.5 W cm−2, 5 min) WS2 – BSA (HeLa, 24 h incubation) 65% (6.25, 12.5 ppm) 40% (25 ppm) 35% (50 ppm) |
[33] |
| Fe (III)- WS2 -PVP | Laser (808 nm) |
1 | 5 | Tmax = 46 ˚C (1 W cm−2, 5 min) DOX-Fe (III)-WS2-PVP (HT29, 24 h incubation) 5.2% (250 µg mL−1) |
[34] |
| WS2 – PEG | Laser (808 nm) |
0.1, 0.3, 0.5, 0.8 | 5 | WS2 – PEG (4T1, 6 h incubation) 100% (0.1 W cm−2, 0.1 mg mL−1 ) 72.7% (0.3 W cm−2, 0.1 mg mL−1 ) 45.5% (0.5 W cm−2, 0.1 mg mL−1 ) 7.3% (0.8 W cm−2, 0.1 mg mL−1 ) |
[28] |
| WS2-IO-MS-PEG/DOX | Laser (808 nm) |
0.1, 0.3, 0.8 | 20 | WS2-IO-MS-PEG/DOX (4T1, 24 h incubation) 41.3% (0.1 W cm−2, 50 µg mL−1 DOX) 16% (0.3 W cm−2, 50 µg mL−1 DOX) 14.7% (0.8 W cm−2, 50 µg mL−1 DOX) |
[32] |
| WID-M-FA | Laser (808 nm) |
1 | 5 | Tmax = 60 ˚C (1 W cm−2, 5 min) WID-M-FA (HeLa, 24 h incubation) 18.5% (1 µg mL−1 DOX and 10 µg mL−1 ICG ) |
[40] |
| N- WS2 | Laser (808 nm) |
0.3, 0.45, 0.6, 0.75 | 10 | Tmax = 50 ˚C (0.6 W cm−2, 4 min) N- WS2 (HeLa, 6 h incubation) 89.4% (0.3 W cm−2, 120 µg mL−1) 71.2% (0.45 W cm−2, 120 µg mL−1) 40.4% (0.6 W cm−2, 120 µg mL−1) 30.8% (0.75 W cm−2, 120 µg mL−1) Concentrations tested: 15-120 µg mL−1 |
[38] |
| PEG-WS2:Gd3+ | Laser (808 nm) |
0.8 | 5 | PEG-WS2:Gd3+ (4T1,12 h incubation) 75.5% (6.25 µg mL−1) 51.1% (12.5 µg mL−1) 28.9% (25 µg mL−1) 4.4% (50 µg mL−1) |
[39] |
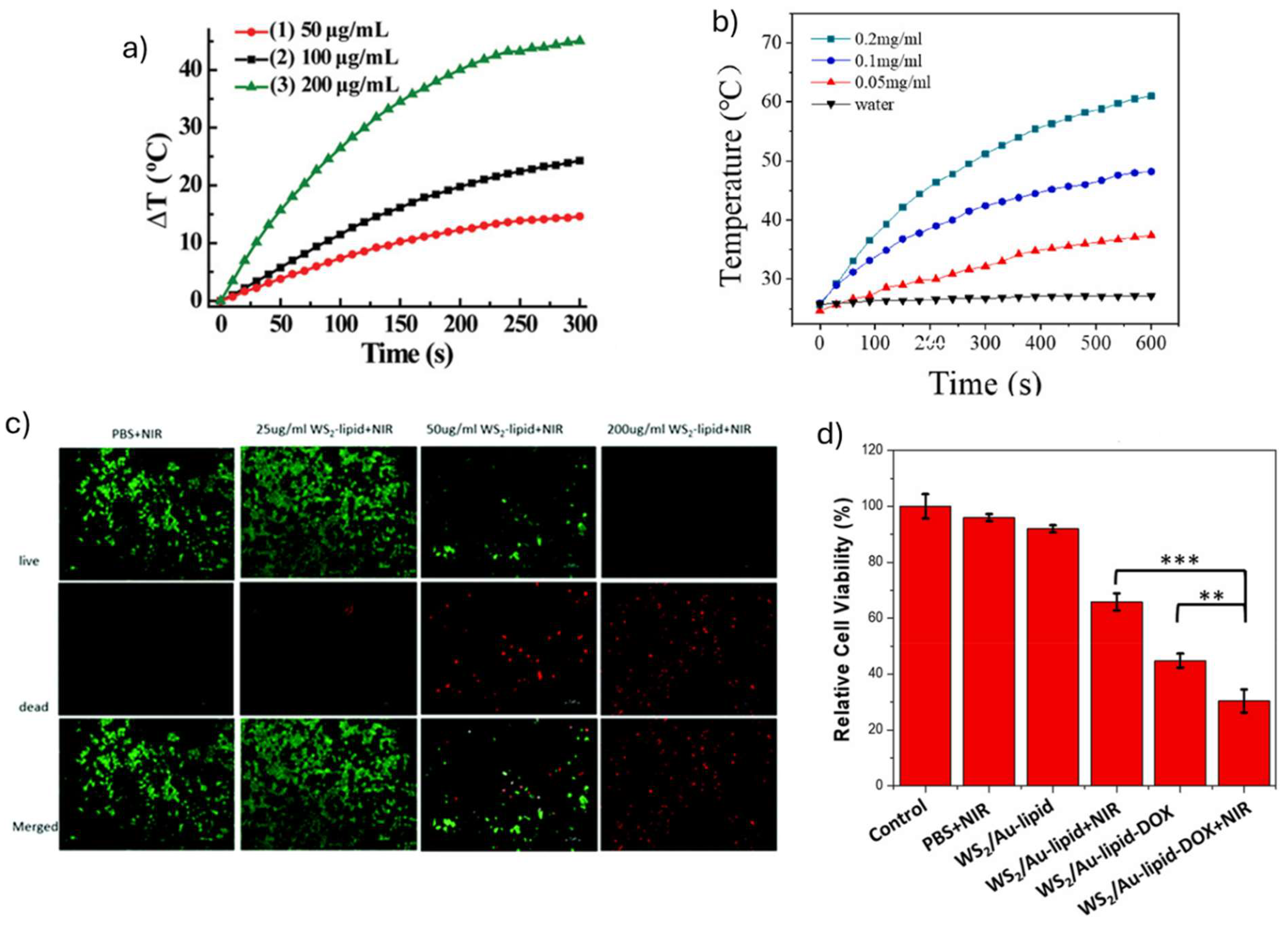
| WS2 -lipid | Laser (808 nm) |
2 | 10 | Tmax = 60 ˚C (2 W cm−2, 10 min) WS2 -lipid (MCF-7, 24 h incubation). 78% (25 µg mL−1) 43% (50 µg mL−1 ) 18 % (100 µg mL−1 ) 9% (200 µg mL−1 ) WS2 -lipid-DOX (MCF-7, 24 h incubation) 20% (50 µg mL−1 DOX) |
[35] |
| mWS2-lipid | Laser (808 nm) |
2 | 10 | Tmax = 68.1 ˚C (1.5 W cm−2, 10 min) mWS2-lipid (MCF-7, 24 h incubation). 77% (12.5 µg mL−1 ) 58% (25 µg mL−1 ) 30% (50 µg mL−1 ) 16% (100 µg mL−1 ) mWS2-lipid-DOX (MCF-7, 24 h incubation) 27% (50 μg mL-1 DOX) |
[36] |
|
WS2/Au-lipid-DOX |
Laser (808 nm) |
2 |
10 |
Tmax = 75 ˚C (1.5 W cm−2, 10 min) WS2/Au-lipid (MCF-7, 24 h incubation). 54% (12.5 µg mL−1 ) 29% (25 µg mL−1 ) 18% (50 µg mL−1 ) 7% (100 µg mL−1 ) WS2/Au-lipid-DOX (MCF-7, 4 h incubation) 30% (12.5 μg mL-1 DOX) |
[37] |
| 2D material | Energy (W cm−2) |
Time (min) | Animal model | Tumor growth | Additional outcomes | Reference |
|---|---|---|---|---|---|---|
| Fe (III)- WS2 -PVP-DOX | 1 | 5 | HT29 colorectal carcinoma bearing Balb/c mice | i.v. administration (250 µg mL−1) Tumors volume decreased during the study (15% compared to control, 28 days after irradiation) |
- | [34] |
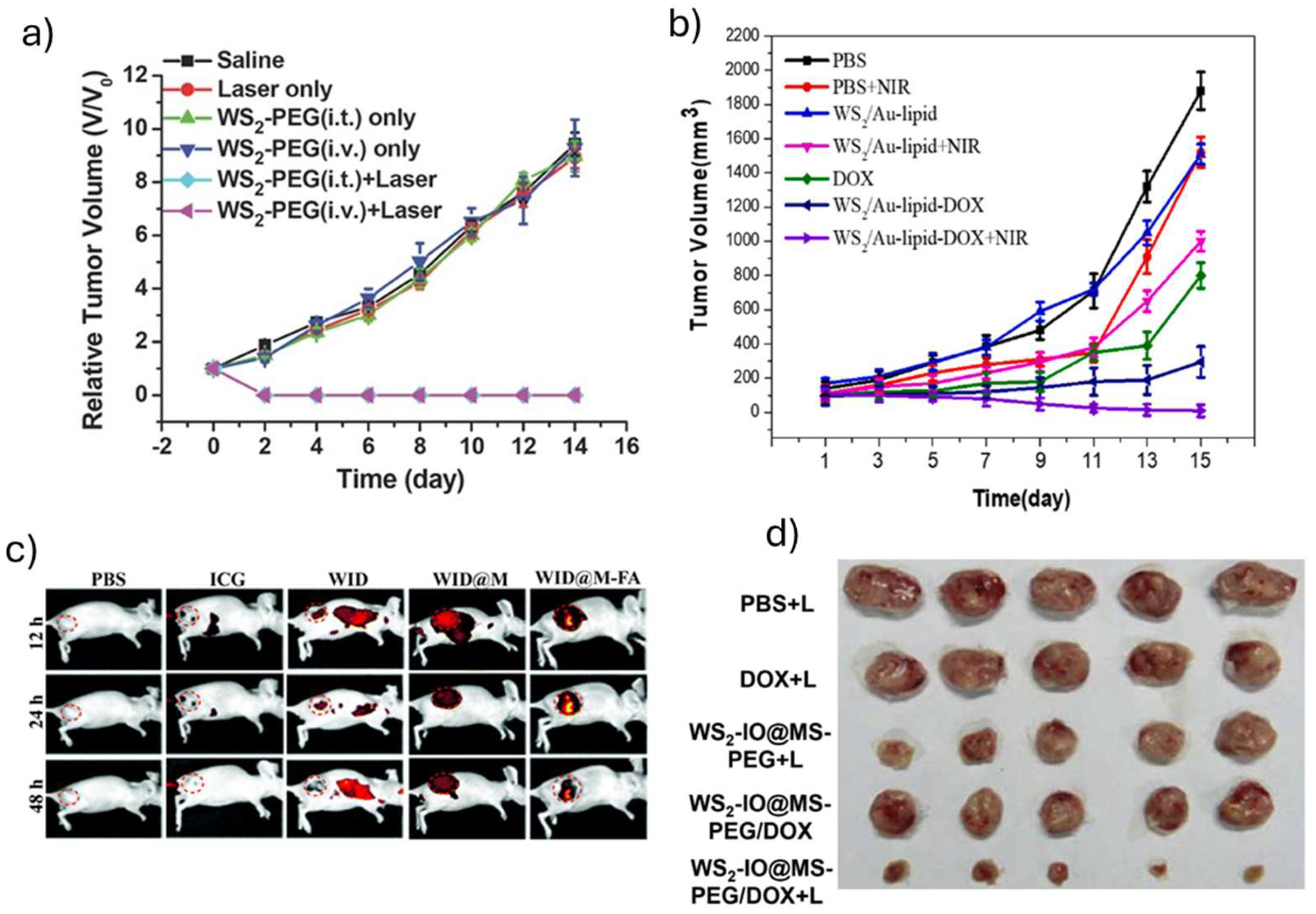
| WS2 – PEG | 0.8 | 5 | 4T1 tumor bearing Balb/C mice | i.t administration (2 mg kg−1, 30 min incubation) i.v. administration (20 mg kg−1, 24 h incubation) Tumors were eliminated after 2 days of irradiation No tumor regrowth during the study (14 days after irradiation) |
- | [28] |
| WS2-IO-MS-PEG/DOX | 0.55 | 10 | 4T1 tumor bearing Balb/C mice | i.v. administration (WS2 = 8.4 mg kg-1, DOX = 7 mg kg-1, 24 h) Tumors volume decreased during the study (10% compared to control, 14 days after irradiation) Tumor mass decreased after 14 days |
- | [32] |
| WID-M-FA | 1 | 5 | HeLA tumor bearing Balb/C mice | i.v. administration (DOX = 2 mg kg-1, ICG = 5 mg kg-1, 24 h) Tumor was eliminated after 18 days of irradiation No changes on body weight |
- | [40] |
| N-WS2 | 0.6 | 10 | HeLa tumor-bearing female NOD/SCID mice | i.t administration (1.2 mg mL-1, 40 µL, 0 h incubation) Tumor was eliminated after 4 days of irradiation No tumor regrowth during the study (14 days after irradiation) |
No changes on body weight (14 d after irradiation) Significant damage in tumor tissue (2 d after irradiation) Serum biochemistry markers on normal ranges (6, 16 d after irradiation, WBC, RBC, Hgb, HCT, platelets, MCV, MCH, MCHC) |
[38] |
| PEG-WS2:Gd3+ | 0.5 | 10 | 4T1 tumor-bearing Balb/c mice | i.v. administration (20 mg kg-1, 24 h) Tumor was eliminated 12 days after irradiation |
- | [39] |
| WS2-lipid | 2 | 2 | 4T1 breast tumor-bearing ICR mice | i.v. administration (1 mg mL-1, 24 h) |
- | [35] |
| mWS2-lipid | 1.5 | 5 | 4T1 tumor-bearing Balb/c mice | i.v. administration (2 mg mL-1, 24 h) Tumors volume decreased during the study (50% compared to control, 7 days after irradiation) |
No changes in body weight |
[36] |
| WS2/Au-lipid | 1.5 | 5 | 4T1 breast tumor-bearing female Balb/c mice | i.v. administration (DOX = 150 μg), 24 h) Tumor was eliminated after 11 days of irradiation |
No changes in body weight |
[37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





