Submitted:
15 October 2024
Posted:
15 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. FT-IR Spectra
2.2. Microstructure
2.3. TGA Analysis
2.4. DSC Analysis
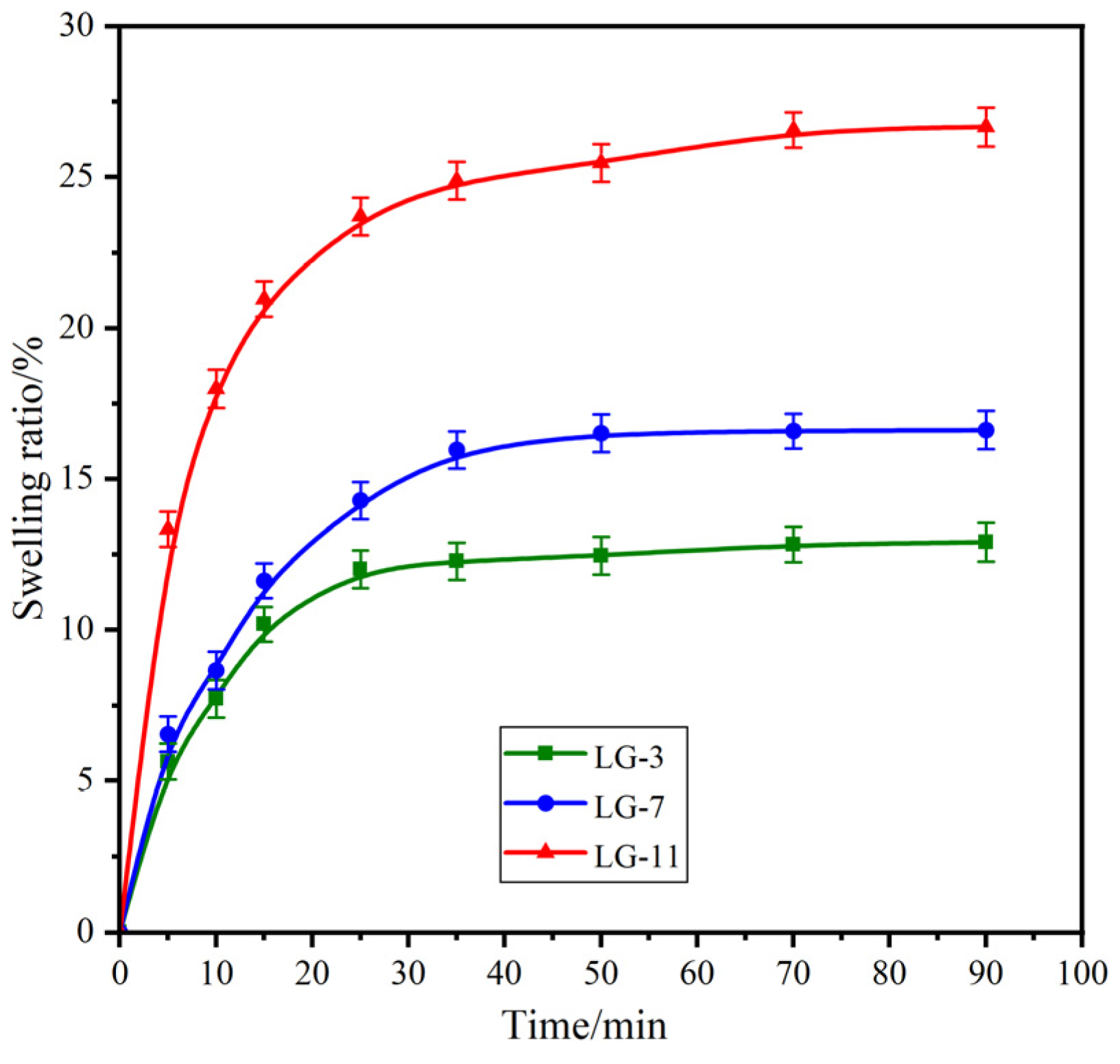
2.5. Swelling Performance
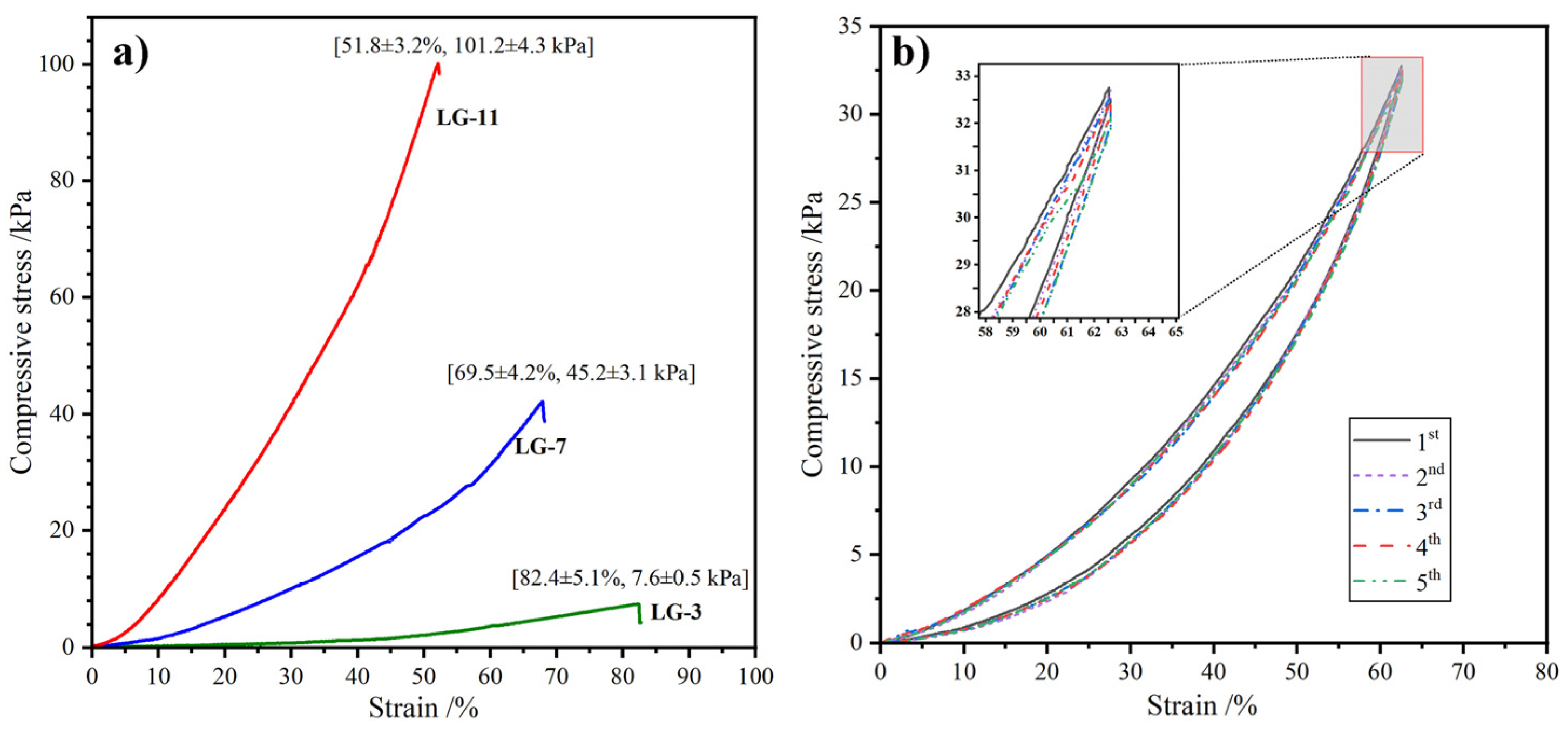
2.6. Compression Performance
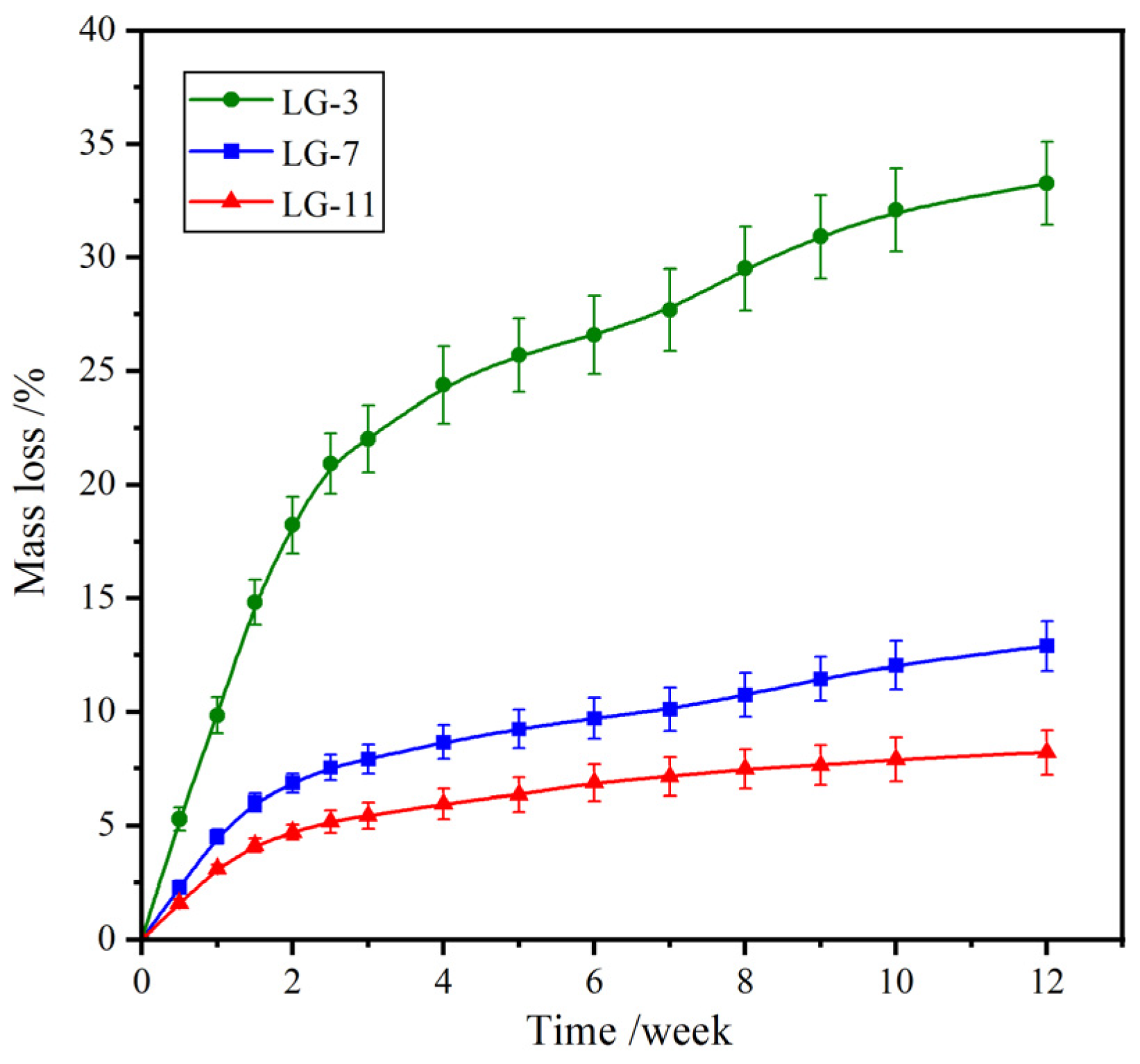
2.7. Biodegradability
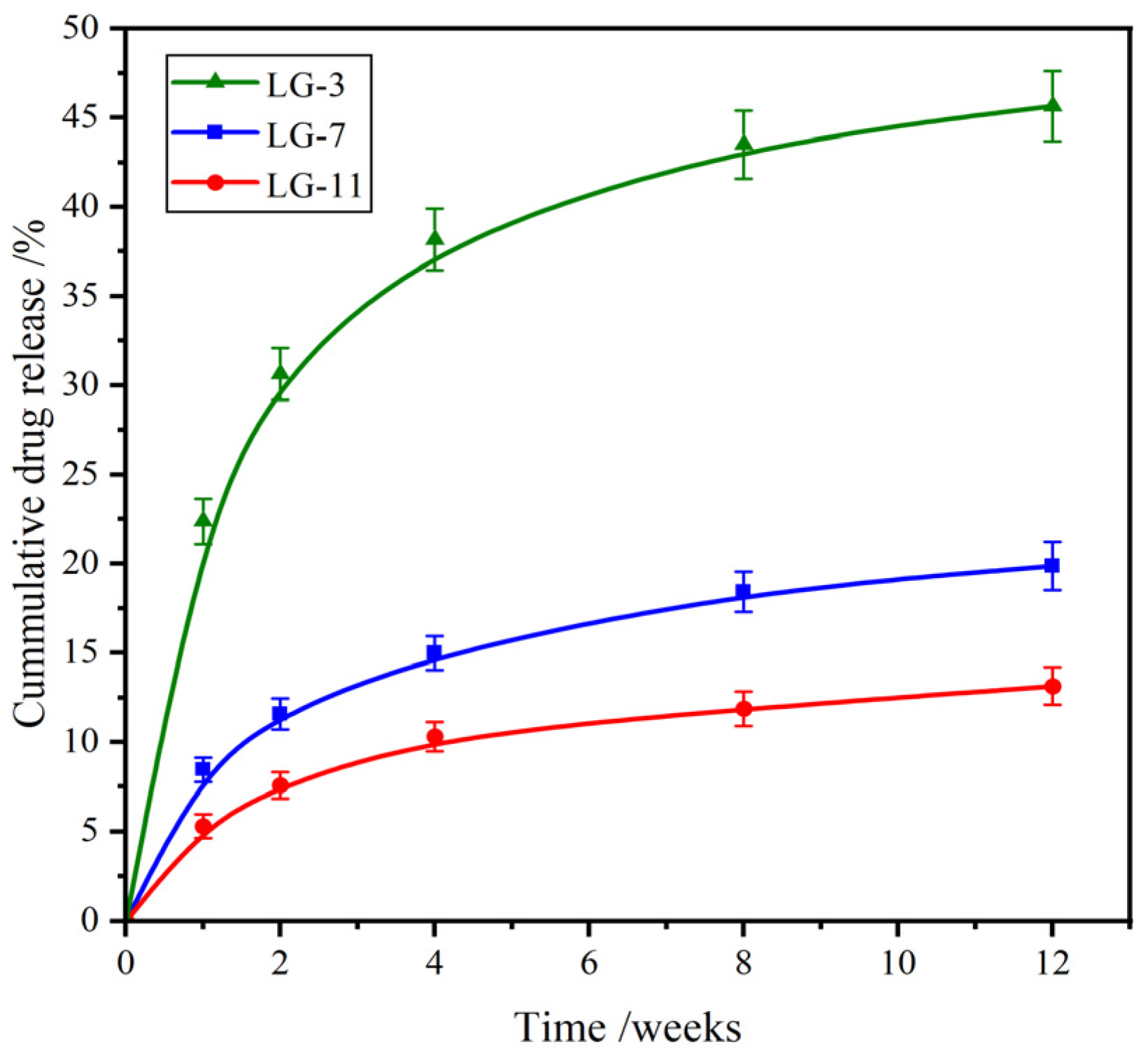
2.8. Drug Release Performance
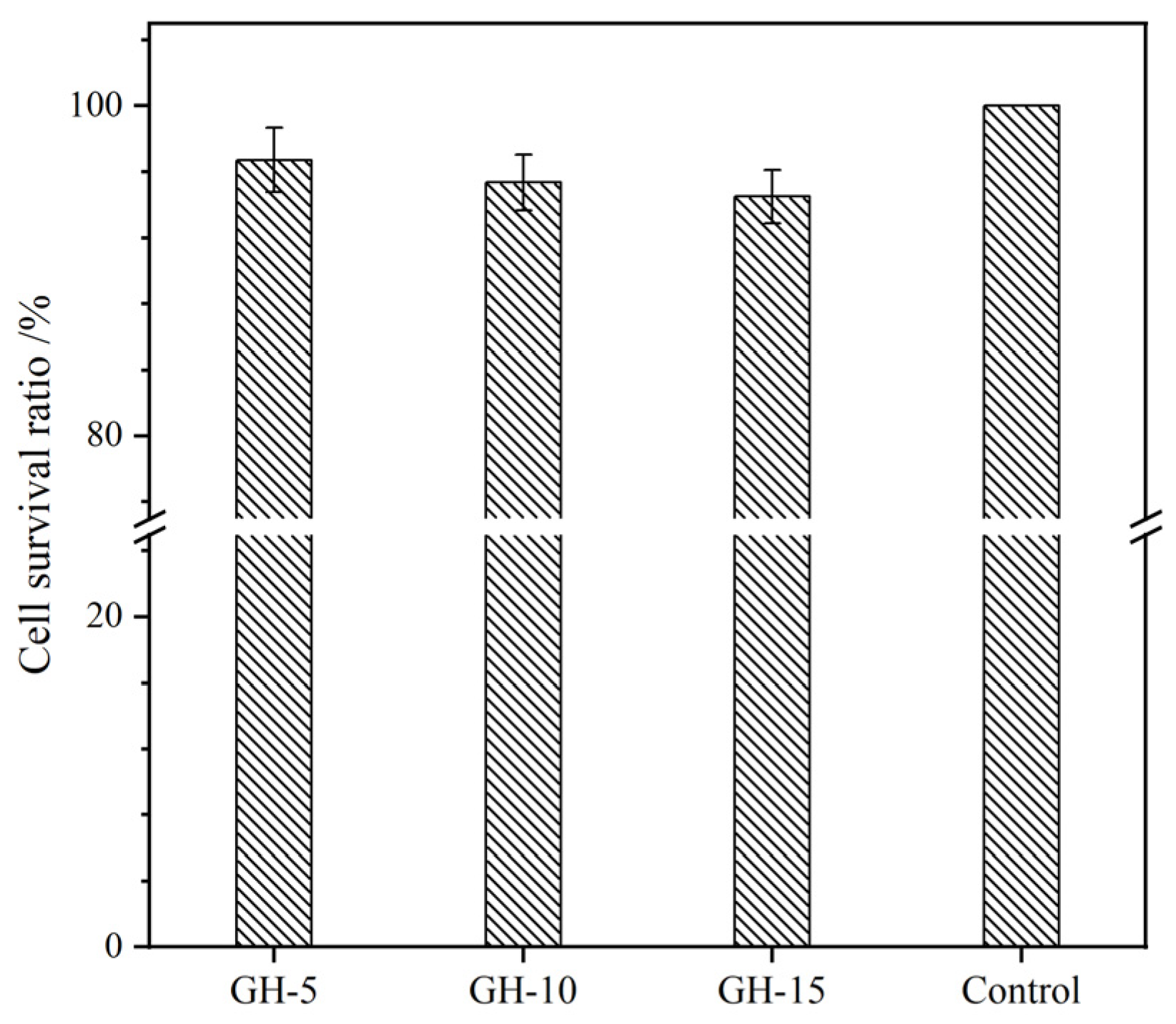
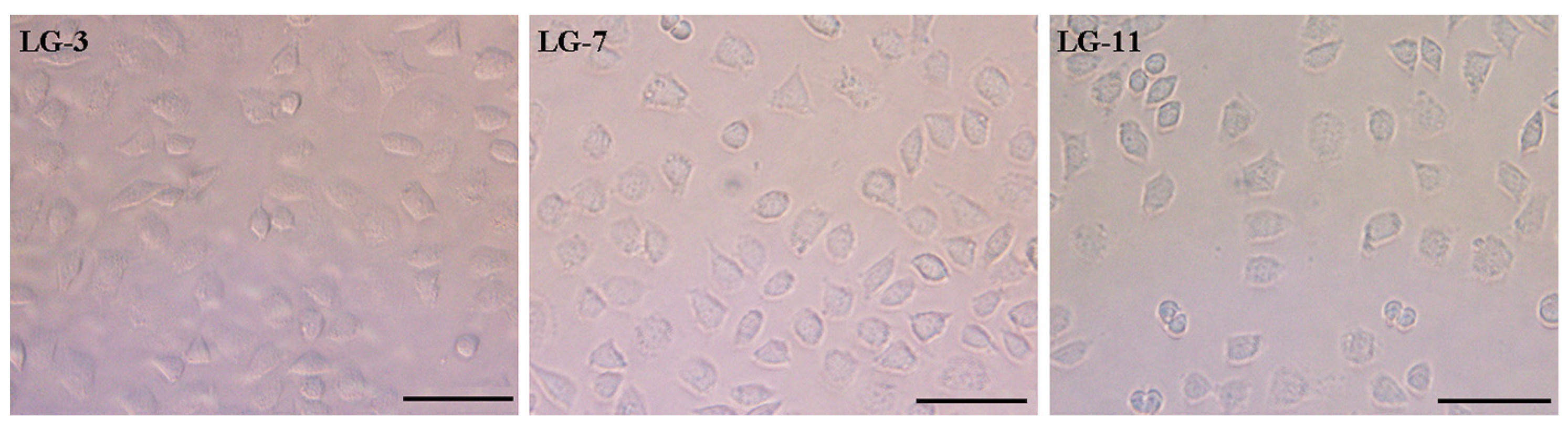
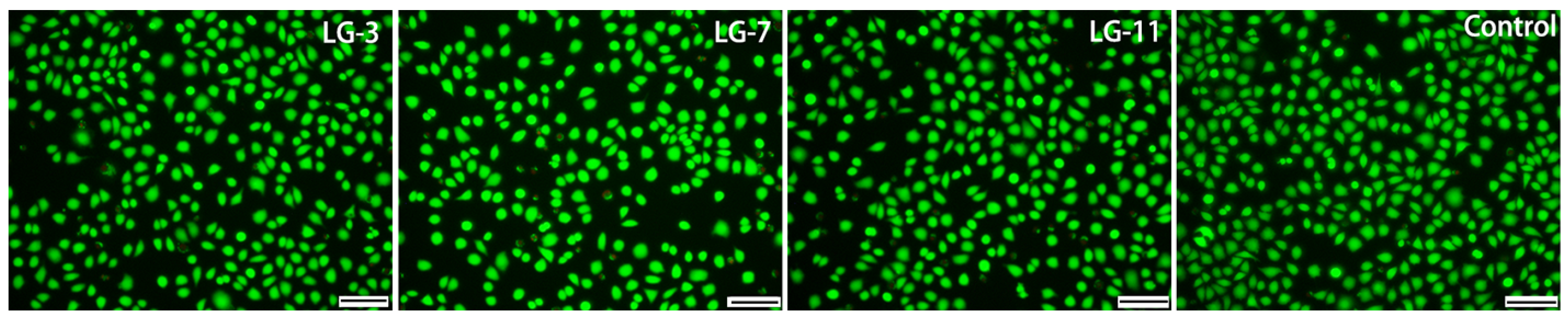
2.9. Cytocompatibility Assay
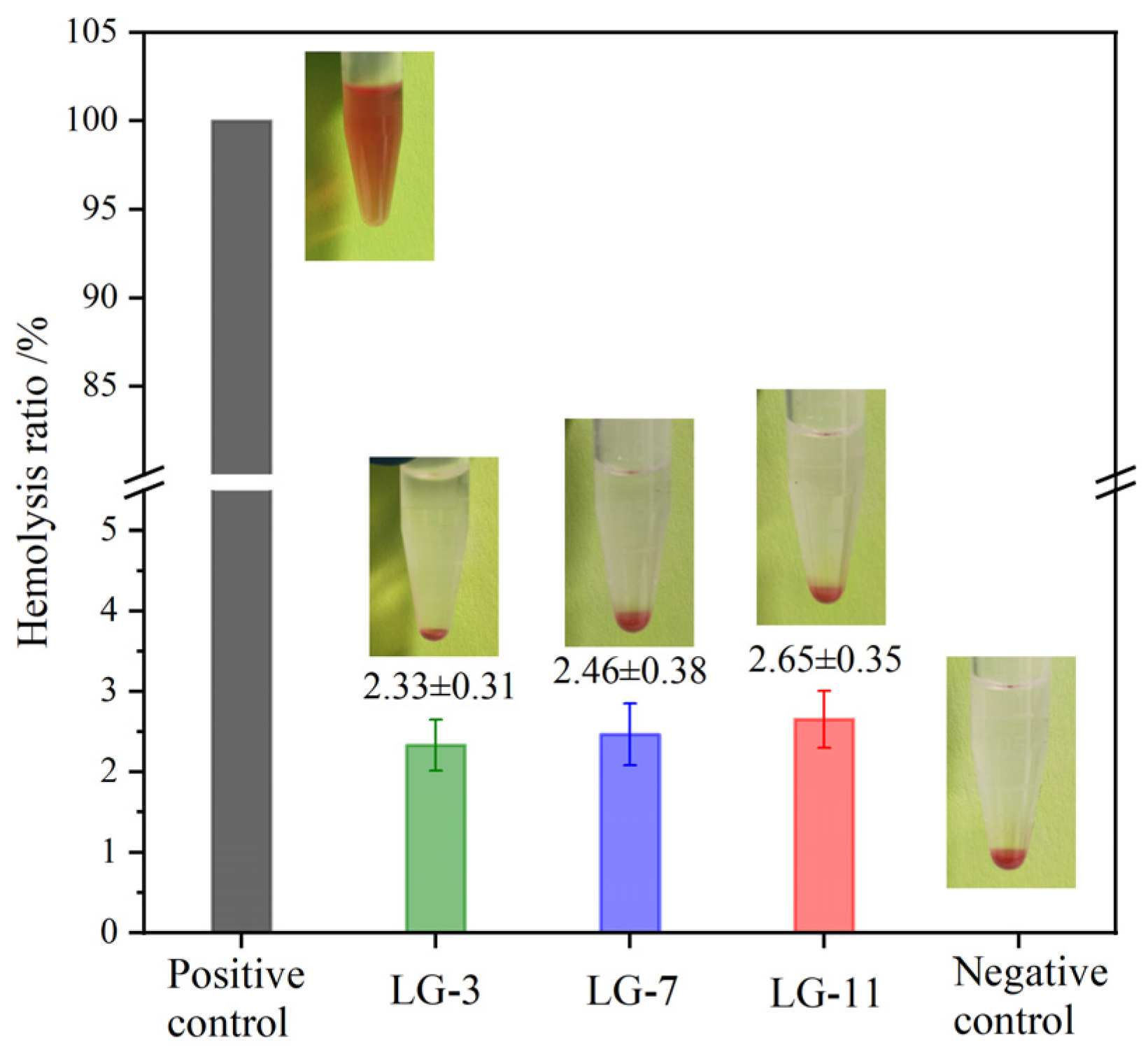
2.10. Hemolysis Assay
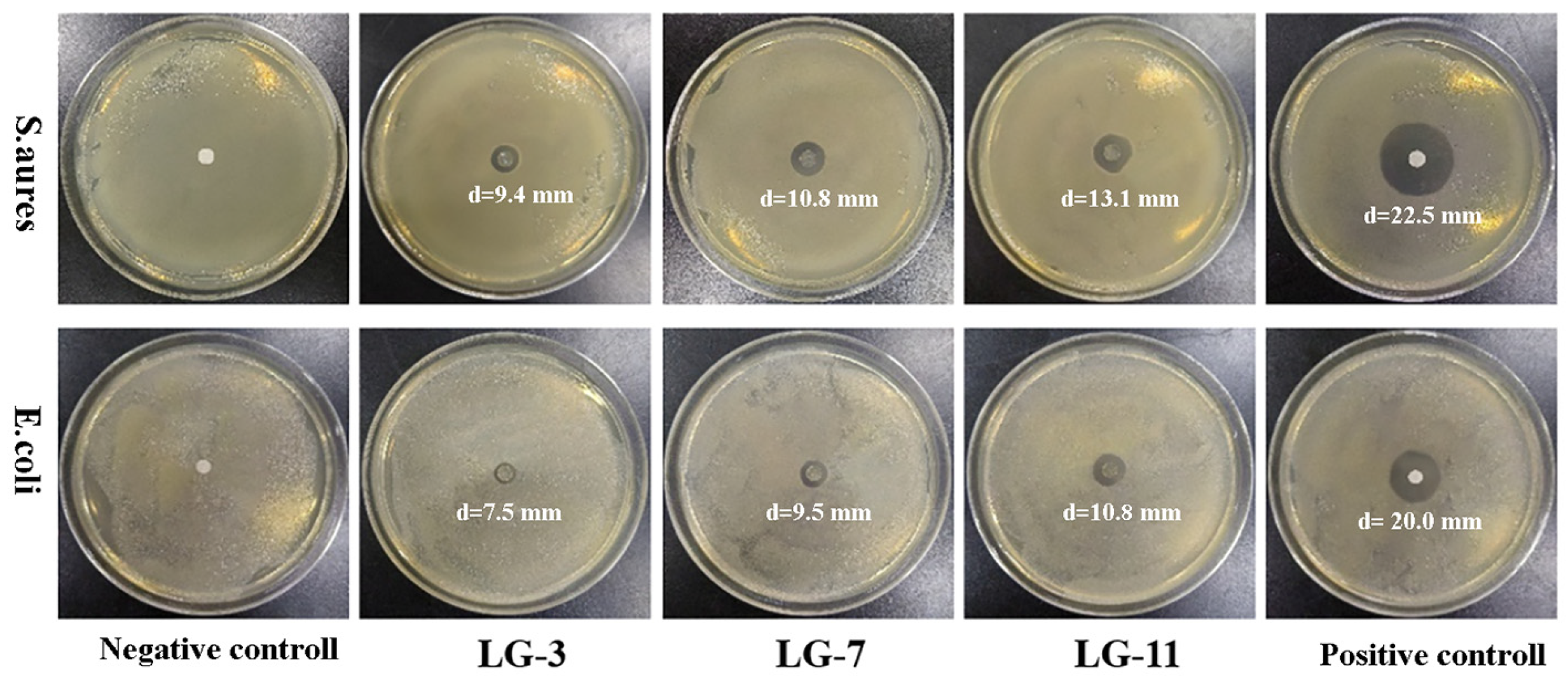
2.11. Antibacterial Property
3. Materials and Methods
3.1. Materials
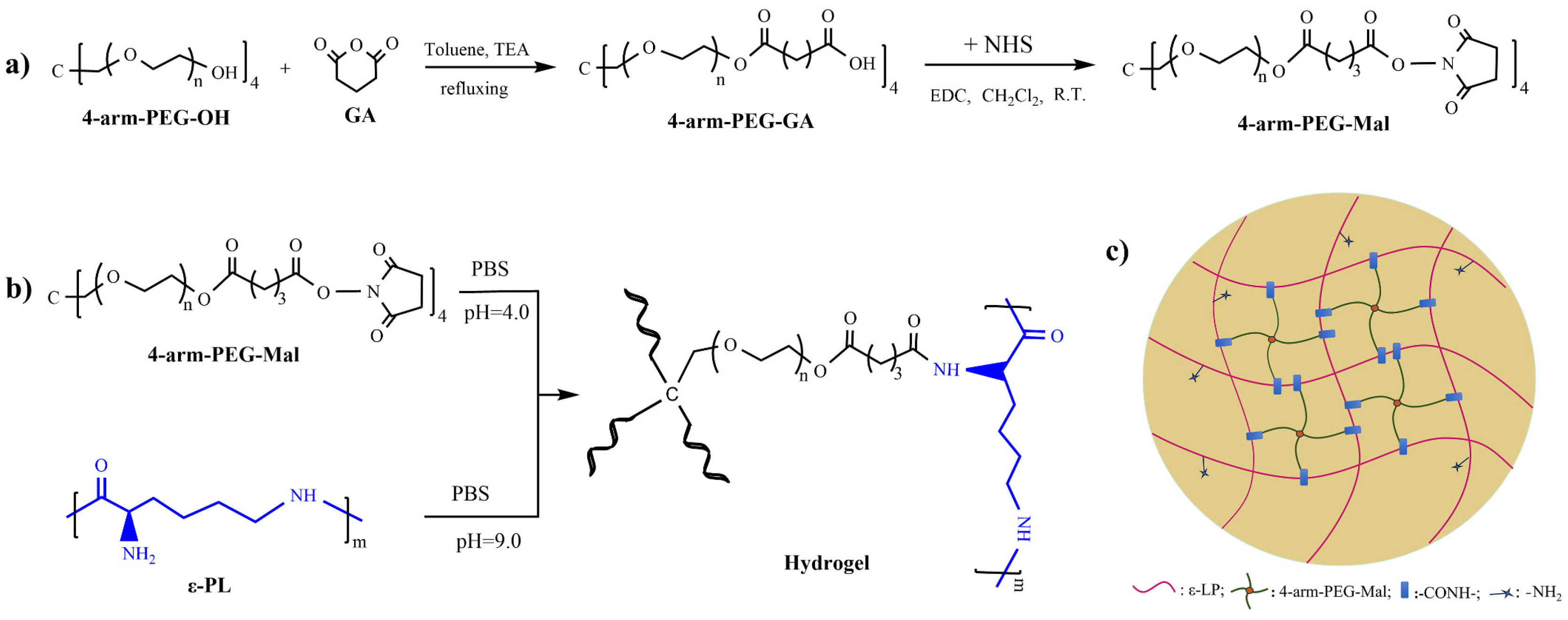
3.2. Synthesis of 4–Arm–PEG–Mal
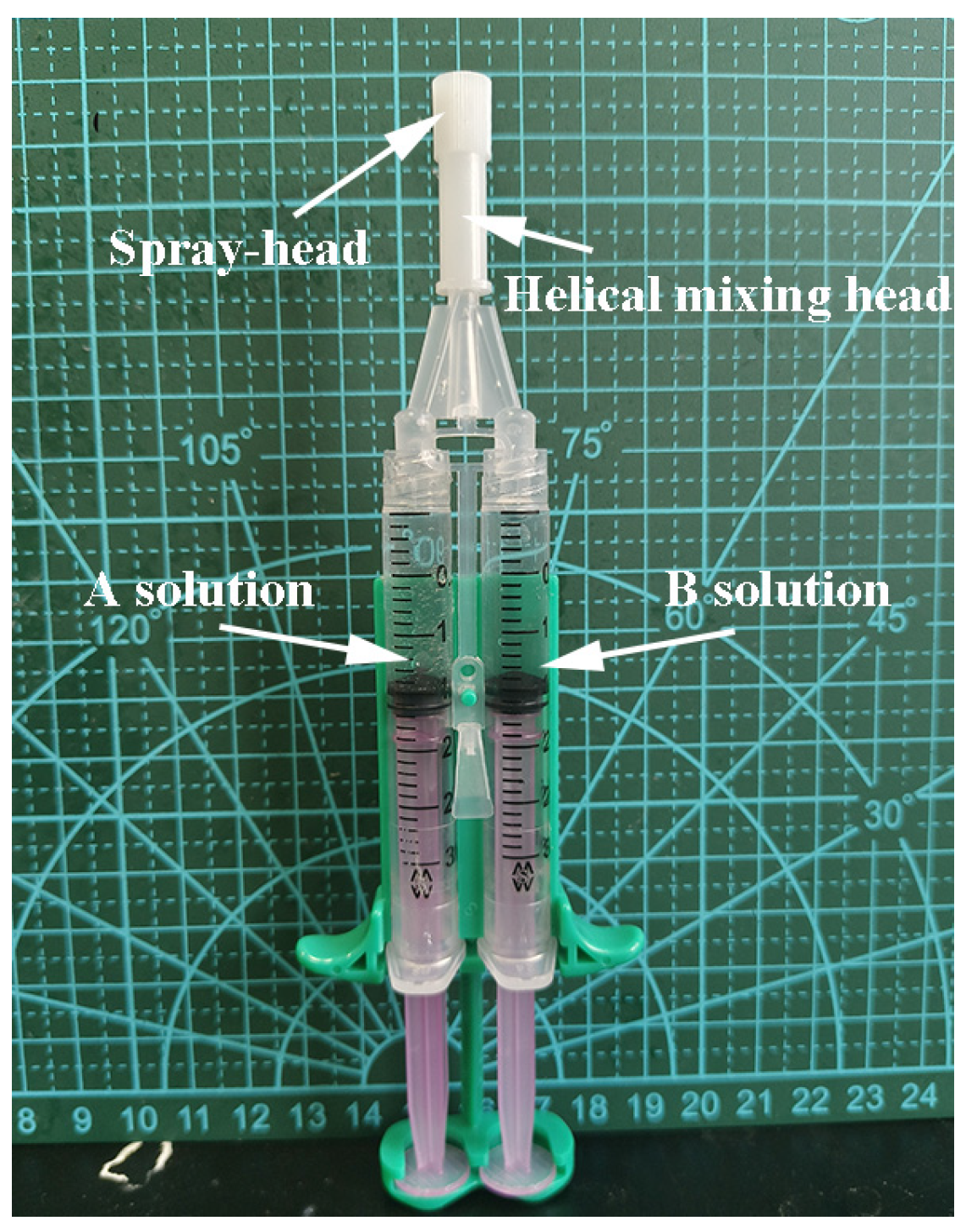
3.3. Preparation of PEG/ε–PL Hydrogels
3.4. Instruments and Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: a review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-based hydrogels applied in drug delivery: an overview. Gels. 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Dreiss, C.A. Hydrogel design strategies for drug delivery. Curr. Opin. Colloid. In. 2020, 48, 1–17. [Google Scholar] [CrossRef]
- Jose, G.; Shalumon, K.T.; Chen, J.P. Natural polymers based hydrogels for cell culture applications. Curr. Med. Chem. 2020, 27, 2734–2776. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a biomaterial for bone tissue engineering: a review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef] [PubMed]
- Elangwe, C.N.; Morozkina, S.N.; Olekhnovich, R.O.; Polyakova, V.O.; Krasichkov, A.; Yablonskiy, P.K.; Uspenskaya, M.V. Pullulan-based hydrogels in wound healing and skin tissue engineering applications: a review. Int. J. Mol. Sci. 2023, 24, 4962. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Stojanović, G.M.; Abdullah, M.F.B.; Dolatshahi-Pirouz, A.; Marei, H.E.; Ashammakhi, N.; Hasan, A. Fundamental properties of smart hydrogels for tissue engineering applications: a review. Int. J. Biol. Macromol. 2024, 254, 127882. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional hydrogels as wound dressing to enhance wound healing. ACS nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Farazin, A.; Shirazi, F.A.; Shafiei, M. Natural biomacromolecule-based antimicrobial hydrogel for rapid wound healing: a review. Int. J. Biol. Macromol. 2023, 244, 125454. [Google Scholar] [CrossRef]
- Qi, C.; Li, A.; Wu, B.; Wang, P. Multi-sensitive Au NCs/5-FU@Carr-LA composite hydrogels for targeted multimodal anti-tumor therapy. Molecules 2024, 29, 4051. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, J. Smart stimuli-responsive chitosan hydrogel for drug delivery: a review. Int. J. Biol. Macromol. 2023, 235, 123902. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F.; Rafael, D.; Schwartz, S. Stimuli-responsive hydrogels for cancer treatment: the role of pH, light, ionic strength and magnetic field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef] [PubMed]
- Kaith, B.S.; Singh, A.; Sharma, A.K.; Sud, D. Hydrogels: synthesis, classification, properties and potential applications-a brief review. J. Polym. Environ. 2021, 29, 3827–3841. [Google Scholar] [CrossRef]
- Liu, J.; Su, C.; Chen, Y.; Tian, S.; Lu, C.; Huang, W.; Lv, Q. Current understanding of the applications of photocrosslinked hydrogels in biomedical engineering. Gels. 2022, 8, 216. [Google Scholar] [CrossRef]
- Lin, R.Z.; Chen, Y.C.; Moreno-Luna, R.; Khademhosseini, A.; Melero-Martin, J.M. Transdermal regulation of vascular network bioengineering using a photopolymerizable methacrylated gelatin hydrogel. Biomaterials. 2013, 34, 6785–6796. [Google Scholar] [CrossRef]
- Zustiak, S.P.; Leach, J.B. Characterization of protein release from hydrolytically degradable poly(ethylene glycol) hydrogels. Biotechnol. Bioeng. 2015, 108, 197–206. [Google Scholar] [CrossRef]
- Kim, I.; Choi, J.S.; Lee, S.; Byeon, H.J.; Lee, E.S.; Shin, B.S.; Choi, H.; Lee, K.C.; Youn, Y.S. In situ facile-forming PEG cross-linked albumin hydrogels loaded with an apoptotic TRAIL protein. J. Control. Release. 2015, 214, 30–39. [Google Scholar] [CrossRef]
- Ibrahim, M.; Ramadan, E.; Elsadek, N.E.; Emam, S.E.; Shimizu, T.; Ando, H.; Ishida, T. Polyethylene glycol (PEG): the nature, immunogenicity, and role in the hypersensitivity of PEGylated products. J. Control. Release. 2022, 351, 215–230. [Google Scholar] [CrossRef]
- Fu, Y.; Ding, Y.; Zhang, L.; Zhang, Y.; Liu, J.; Yu, P. Poly ethylene glycol (PEG)-related controllable and sustainable antidiabetic drug delivery systems. Eur. J. Med. Chem. 2021, 217, 113372. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhao, S.; Zhang, X.; Wei, G.; Su, Z. Recent advances in peptide engineering of PEG hydrogels: strategies, functional regulation, and biomedical applications. Macromol. Mater. Eng. 2022, 307, 2200385. [Google Scholar] [CrossRef]
- Gao, Y.; Joshi, M.; Zhao, Z.; Mitragotri, S. PEGylated therapeutics in the clinic. Bioeng. Transl. Med. 2024, 9, e10600. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.R.; Patil, P.; Yu, F.; Gupta, M.K.; Duvall, C.L. Enhanced stem cell retention and antioxidative protection with injectable, ROS-degradable PEG hydrogels. Biomaterials 2020, 263, 120377. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Cunha, G.M.; Chen, K.M.; Chen, F.; Le, P.; Han, J.H.; Mahajan, L.A.; Myung, D. In situ-forming collagen hydrogel crosslinked via multi-functional PEG as a matrix therapy for corneal defects. Sci. Rep. 2020, 10, 16671. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Jiang, T.; Zhou, C.; He, Y.; Jiang, A.; Yang, G.; Lu, C. An adenine-modified chitosan cross-linked 8arm-PEG-CHO hydrogel for promoting infected wound healing. Mater. Chem. Front. 2024, 8, 2525–2538. [Google Scholar] [CrossRef]
- Shih, I.L.; Shen, M.H.; Van, Y.T. Microbial synthesis of poly (ε-lysine) and its various applications. Bioresour. Technol. 2006, 97, 1148–1159. [Google Scholar] [CrossRef]
- Patil, N.A.; Kandasubramanian, B. Functionalized polylysine biomaterials for advanced medical applications: a review. Eur. Polym. J. 2021, 146, 110248. [Google Scholar] [CrossRef]
- Chen, Y.; Miao, W.; Li, X.; Xu, Y.; Gao, H.; Zheng, B. The structure, prties, synthesis method and antimicrobial mechanism of ε-polylysine with the preservative effects for aquatic products. Trends. Food. Sci. Tech. 2023, 139, 104131. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Hu, Y.; Qin, J.; Yu, B. Design and optimization of ε-poly-l-lysine with specific functions for diverse applications. Int. J. Biol. Macromol. 2024, 262, 129513. [Google Scholar] [CrossRef]
- Dima, S.; Lee, Y.Y.; Watanabe, I.; Chang, W.J.; Pan, Y.H.; Teng, N.C. Antibacterial effect of the natural polymer ε-polylysine against oral pathogens associated with periodontitis and caries. Polymers 2020, 12, 1218. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Fauzi, M.B. Antibacterial polylysine˗ containing hydrogels for hemostatic and wound healing applications: preparation methods, current advances and future perspectives. Biomater. Sci. 2024, 12, 3293–3320. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Xue, Q.; Chen, J.; Li, Y.; Shao, Z. Structure, stability, rheology, and texture properties of ε-polylysine-whey protein complexes. J. Dairy Sci. 2022, 105, 3746–3757. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.; Lan, W.Q.; Xie, J. Preparation and antimicrobial mechanism of Maillard reaction products derived from ε-polylysine and chitooligosaccharides. Biochem. Bioph. Res. Co. 2023, 650, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, Q.; Zhao, X.; Sun, Y.; Lin, X.; Zhang, X.; Wang, T.; Yang, T.; Jiang, X.; Li, J.; Cao, Z.; Cai, T.; Liu, W.; Zhang, H.; Bai, J.; Yao, Q. Honeycomb-like biomimetic scaffold by functionalized antibacterial hydrogel and biodegradable porous Mg alloy for osteochondral regeneration. Front. Bioeng. Biotech. 2024, 12, 1417742. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Kong, N.; Hou, Z.; Men, L.; Yang, P.; Wang, Z. Sponge-Like Porous Polyvinyl Alcohol/Chitosan-Based Hydrogel with Integrated Cushioning, pH-Indicating and Antibacterial Functions. Inter. J. Biol. Macromol. 2024, 272, 132904. [Google Scholar] [CrossRef]
- Liao, W.; Liu, X.; Zhao, Q.; Lu, Z.; Feng, A.; Sun, X. Physicochemical, antibacterial and food preservation properties of active packaging films based on chitosan/ε-polylysine-grafted bacterial cellulose. Inter. J. Biol. Macromol. 2023, 253, 127231. [Google Scholar] [CrossRef]
- Yang, B.; Yin, S.; Bian, X.; Liu, C.; Liu, X.; Yan, Y.; Zhang, C.; Zhang, H.; Hou, Z. Preparation and properties of monomethoxyl polyethylene glycol grafted O-carboxymethyl chitosan for edible, fresh-keeping packaging materials. Food. Packaging. Shelf. 2022, 33, 100874. [Google Scholar] [CrossRef]
- Lee, J.H.; Go, A.K.; Oh, S.H.; Lee, K.E.; Yuk, S.H. Tissue anti-adhesion potential of ibuprofen-loaded PLLA–PEG diblock copolymer films. Biomaterials. 2005, 26, 671–678. [Google Scholar] [CrossRef]
- Barrioni, B.R.; Carvalho, S.M.D.; Orèfice, R.L.; Oliveira, A.A.R.D.; Pereira, M.D.M. Synthesis and characterization of biodegradable polyurethane films based on HDI with hydrolyzable crosslinked bonds and a homogeneous structure for biomedical applications. Mater. Sci. Eng. C 2015, 52, 22–30. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, L.; Qu, L.; Zhang, R.; Qin, Z.; Zhang, H.; Wei, J.; Xu, J.; Hou, Z. Cross-linked poly(ester urethane)/starch composite films with high starch content as sustainable food-packaging materials: influence of cross-link density. Int. J. Biol. Macromol. 2024, 256, 128441. [Google Scholar] [CrossRef]
- Liu, X.; Duan, L.; Gao, G. Rapidly self-recoverable and fatigue-resistant hydrogels toughened by chemical crosslinking and hydrophobic association. Eur. Polym. J. 2017, 89, 185–194. [Google Scholar] [CrossRef]
- Sahraro, M.; Barikani, M.; Daemi, H.; Baei, P. Anti-fatigue, highly resilient photocrosslinkable gellan gum hydrogels reinforced by flexible nanoparticulate polyurethane multi-crosslinkers. Int. J. Biol. Macromol. 2021, 183, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.R.; Wells, A.D. Ceftibuten: clinical use and effectiveness in the treatment of bacterial infections. Int. J. Antimicrob. Ag. 2001, 17, 145–152. [Google Scholar]
- Mårild, S.; Jodal, U.; Sandberg, T. Ceftibuten versus trimethoprim-sulfamethoxazole for oral treatment of febrile urinary tract infection in children. Pediatr. Nephrol. 2009, 24, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Niu, G.; Zhang, H.; Zhu, Y.; Zhang, C.; Kong, F.; Xu, J.; Hou, Z. Enhanced hemocompatibility and antibacterial activity of biodegradable poly(ester-urethane) modified with quercetin and phosphorylcholine for durable blood-contacting applications. J. Mater. Chem. B 2023, 11, 5846. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Zhang, Y.P.; Zhang, W.F.; Chen, X.G. Biocompatibility and characteristics of injectable chitosan-based thermosensitive hydrogel for drug delivery. Carbohyd. Polym. 2011, 83, 1643–1651. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, J.; Zhao, J.; Wang, S. Injectable wound dressing based on carboxymethyl chitosan triple-network hydrogel for effective wound antibacterial and hemostasis. Int. J. Biol. Macromol. 2023, 225, 1235–1245. [Google Scholar] [CrossRef]
- Tan, Z.; Shi, Y.; Xing, B.; Hou, Y.; Ciu, J.; Jia, S. The antimicrobial effects and mechanism of ε-poly-lysine against Staphylococcus aureus. Bioresour. Bioprocess. 2019, 6, 11. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, S.; Dhiman, A. Design of antibiotic containing hydrogel wound dressings: biomedical properties and histological study of wound healing. Int. J. Pharmaceut. 2013, 457, 82–91. [Google Scholar] [CrossRef]
- Li, J.; Kao, W.J. Synthesis of polyethylene glycol (PEG) derivatives and PEGylated-peptide biopolymer conjugates. Biomacromolecules 2003, 4, 1055–1067. [Google Scholar] [CrossRef]
- Hou, P.; Zhang, N.; Wu, R.; Xu, W.; Hou, Z. Photo-cross-linked biodegradable hydrogels based on n-arm-poly(ethylene glycol), poly(ε-caprolactone) and/or methacrylic acid for controlled drug release. J. Biomater. Appl. 2017, 32, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Rekha, S.; Anila, E.H. In vitro cytotoxicity studies of surface modified CaS nanoparticles on L929 cell lines using MTT assay. Mater. Lett. 2019, 236, 637–639. [Google Scholar] [CrossRef]
- Lan, X.; Luo, T.; Zhong, Z.; Huang, D.; Liang, C.; Liu, Y.; Wang, H.; Tang, Y. Green cross-linking of gelatin/tea polyphenol/ε-poly (L-lysine) electrospun nanofibrous membrane for edible and bioactive food packaging. Food Packaging Shelf 2022, 34, 100970. [Google Scholar] [CrossRef]
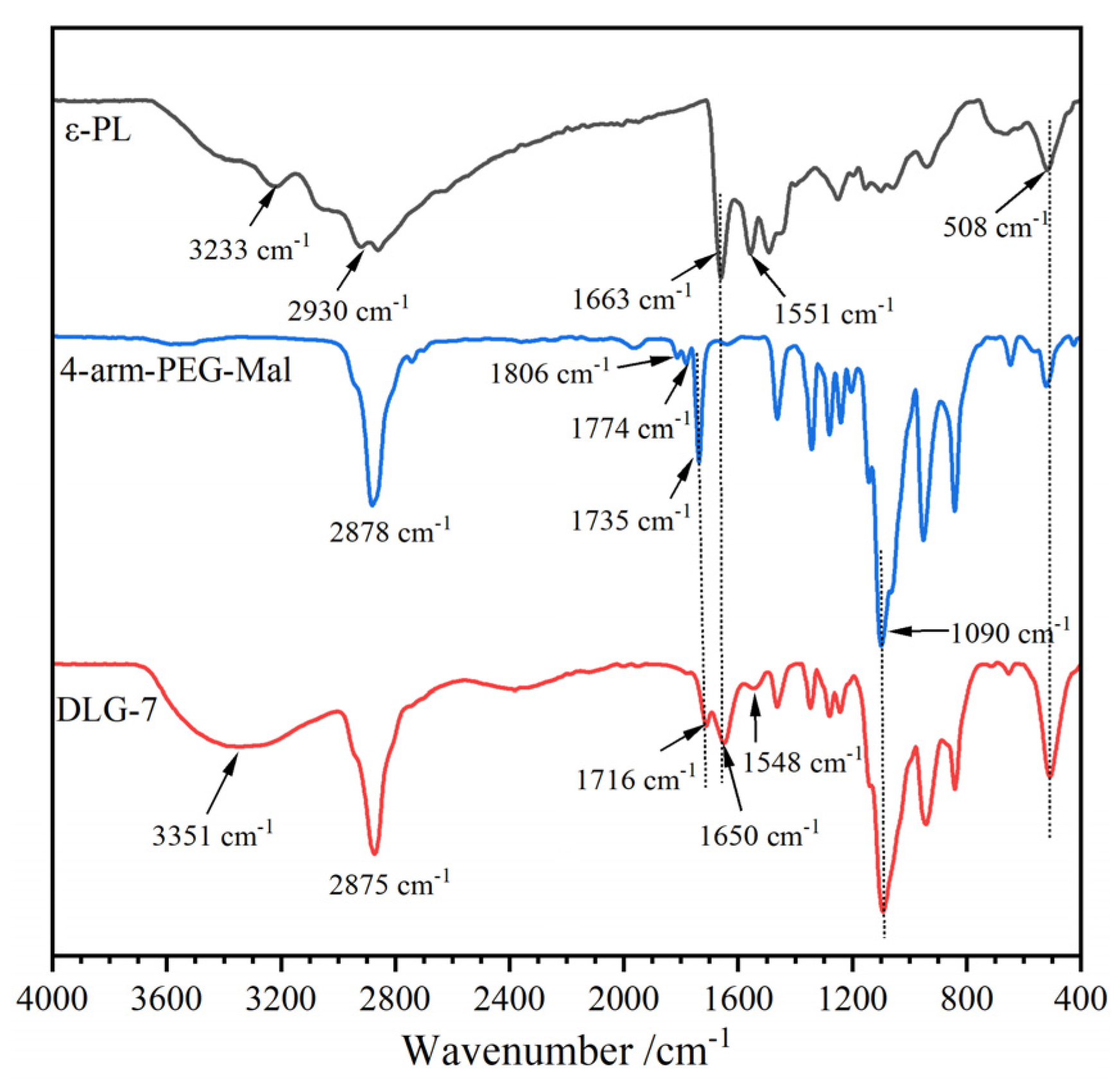
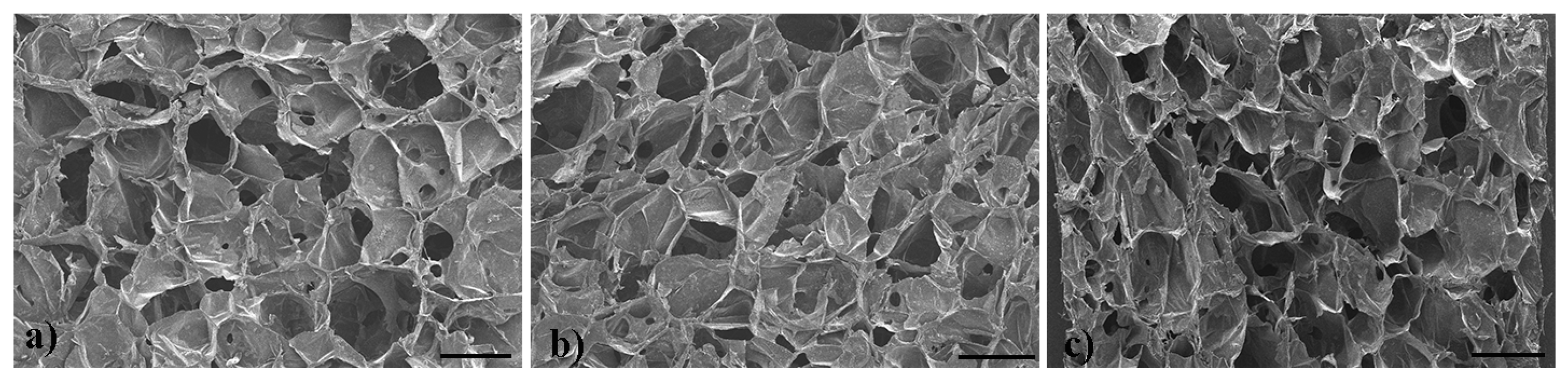
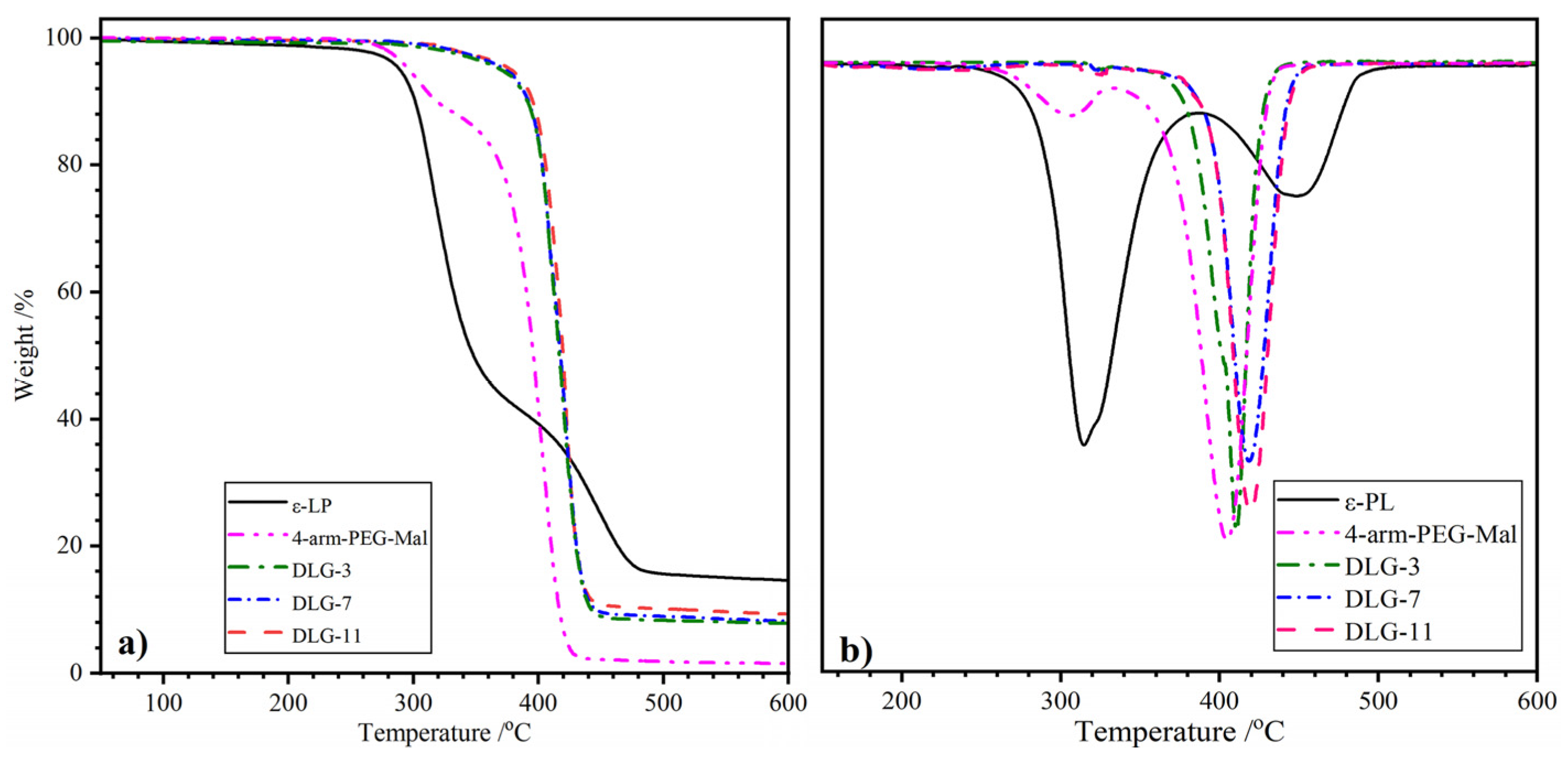
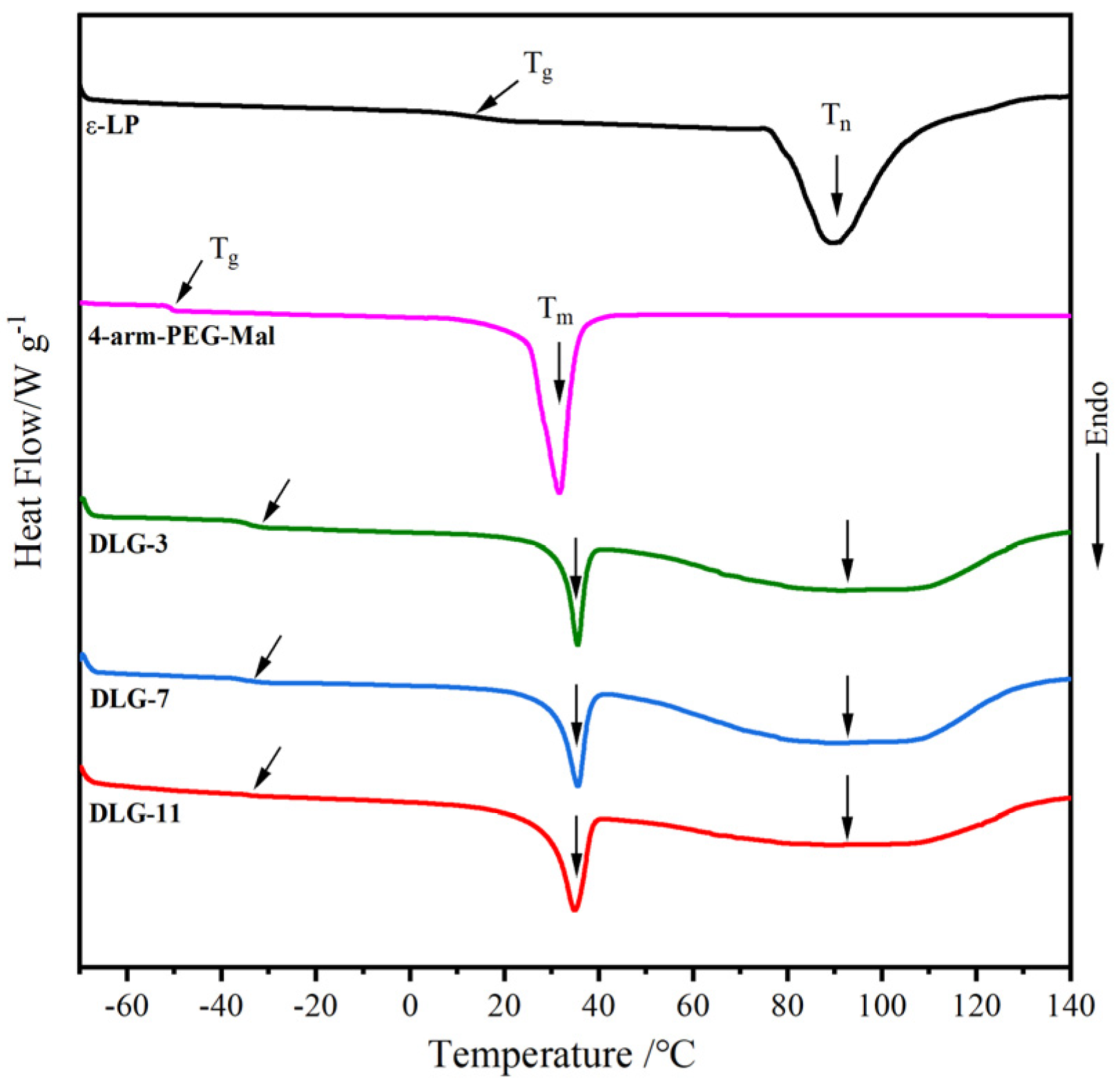
| Samples | T5%/°C | T1/°C | T2/°C | Wr/% |
|---|---|---|---|---|
| ε–PL | 289.3 | 315.0 | 449.6 | 14.6 |
| 4–arm–PEG–Mal | 295.4 | 306.1 | 405.6 | 1.6 |
| DLG–3 | 377.8 | / | 411.2 | 8.2 |
| DLG–7 | 371.8 | / | 418.6 | 7.9 |
| DLG–11 | 370.3 | / | 421.1 | 7.8 |
| Samples | Tg /°C | Tm /°C | Tn | ΔHm /J·g–1 | ΔHn /J·g–1 |
|---|---|---|---|---|---|
| ε–PL | 13.4 | – | 89.3 | – | 43.9 |
| 4–arm–PEG–Mal | –49.6 | 31.7 | – | 28. 1 | – |
| LG–3 | –33.9 | 45.6 | 97.1 | 15.2 | 25.2 |
| LG–7 | –33.9 | 45.6 | 97.4 | 14.7 | 26.2 |
| LG–11 | –33.8 | 45.5 | 96.9 | 14.6 | 25.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





