1. Introduction
The merits of conventional penile venous surgery (CPVS) in treating erectile dysfunction (ED) have been disputed for centuries [
1,
2,
3]. This method is rooted in the conventional penile venous anatomy, as illustrated by Sage Da Vinci's penile illustration in medieval times [
4]. Despite prevailing in medical literature for six centuries, this anatomy has significant omissions and is not sustainable. Consequently, its derived penile venous surgery has been unsuccessful and considered experimental, even though it has been revisited in popularity multiple times since its prototype in 1895 [
5].
Moreover, the CPVS approach follows traditional surgery principles, utilizing electrocautery for rapid bleeding control, which can catastrophically damage the delicate sinusoids of corpora cavernosa (CC) [
6,
7]. Given that CC sinusoids can only avoid electrocautery damage if intracorporeal pressure exceeds 125 mmHg [
8], the feasibility of reaching this pressure level physiologically in a CC affected by ED is questionable. Implies performing CPVS, for those whose intracorporeal pressure exceeds 125 mmHg physiologically, must be overtreated. Unsurprisingly, CPVS is notorious for causing irreversible numbness and penile deformity due to nerve injury and fibrosis of the traumatized fibrous-layering tissues because of the unavailability of the cleverest electrocautery device for targeting offensive veins exclusively at no expense of influencing nearby neighboring tissues, such as arterial nervous, lymphatic and interstitial tissues thus far. [
9].
In contrast, since 1986, we have utilized a method of penile venous stripping (PVS) to confine every corporeal drop of blood in the corpora cavernosa (CC), resulting in negligible blood loss [
10]. Importantly, neither electrocautery nor a suction apparatus is required throughout the entire procedure, helping to prevent inadvertent damage to innocent penile tissues but offensive leakage veins, which results in excellent outcomes across various age groups, conspicuously for adolescent-onset ED males [
11]. Our method secured a patent from the United States Patent and Trade Office (USPTO) on August 14, 2012 [
12]. This innovation is based on the De Novo penile fibro-vascular assembly, which obviates the necessity for electrocautery or suction apparatus. The CC is primarily responsible for erectile function. According to Pascal's Law, pressure remains undiminished throughout an enclosed system when pressure is applied. Therefore, if the CC functions appropriately as an enclosed system, there is no veno-occlusive dysfunction (VOD).
Penile length and size vary among individuals, and it is expected to encounter patients suffering from dysmorphia in daily practice [
13]. Although some marketers claim that extenders, vacuum devices, pills, and even lotions can increase penis size, none have reported sustainable increases thus far. Surgical strategies for penile augmentation have also been controversial due to the lack of techniques for penile enhancement free of complications. Varied surgical approaches have been used; however, no sustainable method has resulted in factual girth enlargement or elongation of the corpora cavernosa (CC). Additionally, various minimally invasive procedures prompt many patients to resort to penile injections of autologous fat cells or tissue cells, hyaluronic acid, and even inorganic materials such as paraffin and jelly, all of which have the potential for complications [
14].
Recently, some surgeons have resorted to ligamentolysis with V-Y plasty to elongate the exposed penile portion at the expense of compromising penile stability without elongating the entire CC factually [
15]. As a result, many complications can arise, such as disfigurement, downward-directed penile shaft either with or without erection, loss of erection quality and penile stability, infection, scarring, and lumpiness [
16]. From cycling bedside to bench-side research on the human penis, an innovative penile venous anatomy was recently elucidated [
17]. Therefore, considering practice is the only criterion for testing truth, we would like to conduct a retrospective report on an innovative surgical strategy combining factual penile girth enhancement and erection restoration over the decade.
2. Methods
Following its approval by the institutional review board of China Medical University, we conducted a retrospective study. Conspicuously, all patients recommend close friends with categorical requests, although this practice is above contemporary consensus. From January 2013 to May 2023, refractory impotence and dysmorphia prompted 31 men aged 24 to 61 to seek penile venous stripping (PVS) and factual penile girth enhancement (FPGE) with autologous venous walls as the inner tunica albuginea and either venous wall or Surgiform acting as the outer tunica.
2.1. Primary Diagnostic Workup
Multiplanar investigations were conducted, and no isolated contributable factor was found elsewhere before consulting our institute. Therefore, all patients were primarily diagnosed with dual cavernosography in which a pilot cavernosograpy disclosed the penile venous anatomy (
Figure 1A, B), a PGE-1 test was performed in-between, and a pharmaco-cavernosography proved a veno-occlusive dysfunction (VOD) (Figure C). In addition, Doppler sonography was utilized if there was no full erection during the PGE-1 test (n=5). Finally, 18 patients underwent PVS and FPGE surgery on an ambulatory basis, and the remaining 13 men were unaffordable for surgery.
2.2. Penile Venous Stripping and Vein Harvest
Under acupuncture-assisted local anesthesia, neither electrocautery nor suction apparatus is required in the entire procedure. In conducting penile venous stripping (PVS), the procedure began with a circumferential incision and circumcision, if indicated (n=7). After hydro dissection, tissues superficial to Colles' fascia were degloved to the penile base (
Figure 2A). A milking maneuver is helpful in vascular differentiation. Distally, venous stripping was performed at the level of the retro-coronal sulcus, where the number of veinlets could reach up to 32 (
Figure 2B). The deep dorsal vein (DDV) was thoroughly stripped after double ligation of every emissary vein 1 cm apart with a 6-0 nylon suture, as close to the tunica albuginea as possible and proximally to the penile base. During this procedure, a pull-through maneuver of the DDV was conducted step-by-step. Its trunk serves as a guide when Buck's fascia intermittently opened over the emissary's vein as it was dissected along the shaft. The DDV was passed from opening to opening during the entire venous stripping procedure to minimize tissue damage instead of making a complete opening on Buck's fascia (
Figure 2B).
Additionally, 6-8 circumflex veins were explicitly managed. Similarly, two cavernosal veins were stripped after every emissary vein was fixed firmly at the tunica level with a 6-0 nylon suture. Segmental ligation was performed on four para-arterial veins. Eventually, the absence of an engorged vein indicated the completion of venous stripping while palms force-squeezed the entire corpora cavernosa (CC). Consequently, the CC was free from veno-occlusive dysfunction (VOD). Then, a 4 cm pubic longitudinal incision was created to relay the DDV and CVs (
Figure 2C). The DDV trunk was carefully skeletonized (
Figure 2D). The CV's trunk was meticulously identified and freed (
Figure 2E), then passed underneath the pubic tissues (
Figure 2F). Double ligation of every emissary vein was performed following the principle. There were 8-9 and 7-8 significant tributaries of the DDV and CVs, respectively, until the infra-pubic angle was encountered. An encircling gauge was applied to the pendulous penis to prevent oozing, and bilateral spermatic cords (n=8) were hooked out through the pubic incisions (
Figure 2G). The internal spermatic veins were patched (
Figure 2H). All venous tissues were harvested for factual penile girth enhancement (FPGE). Two incisions were repaired with fine sutures, respectively (
Figure 2I). The autologous vein was also individually obtained from the cephalic (n=4) or basilic (n=3) veins.
2.3. Factual Penile Enhancement
In the FPGE, a meticulous longitudinal incision was made on the tunica albuginea along the 3 and 9 O'clock positions of the pendulous portion bilaterally (
Figure 3A, B). Then, using 6-0 nylon, the two tunic defects were fashioned with a 70.0x30.0 mm rectangle venous strip using watertight techniques and covered with Surgiform (SF, n=8) or autologous venous walls (AVW, n=10) as the outer layer bilaterally (
Figure 3C, D). Measurements of penile girth and glans diameter were obtained preoperatively, immediately after surgery (
Figure 4), and six months postoperatively with a repeat cavernosography (
Figure 5) conducted if available. Radiopacity during erection restoration was compared to that of the femoral cortex and the penile crus on preoperative and postoperative cavernosograms. Additionally, the abridged 5-item version of the International Index of Erectile Function (IIEF-5) score system and the Erection Hardness Scale (EHS) were utilized to confirm yearly preoperative and postoperative follow-up improvement. Statistically, the Wilcoxon signed rank test, and Fisher's exact test was used as necessary.
3. Results
In the AVW group, we needed more data on two patients, including a 52-year-old international male. Ten months postoperatively, this patient reported insufficient rigidity inconsistently, which was attributed to severe sinusoidal fibrosis induced by PGE-1 injections administered over the previous 1.5 years. The patient's surgery lasted 15 hours with negligible blood loss. Remarkably, we must grant him the ability to endure a long-distance flight of a dozen hours on the fourth postoperative day. However, we excluded him from further analysis due to the inconsistent data and loss of follow-up in the subsequent year.
Table 1 provides a comprehensive overview of the remaining 18 operated patients and control group (n=11) men who were unaffordable and eventually just followed up on the Internet. The average follow-up period ranged from 0.2 to 10 years, with an average of 5.3±1.5 years, and operation times varied from 6.0 to 13.5 hours with a median of 8.8 hours, averaging 7.2±1.6 hours. Regarding erection rigidity, 22.2% (4/18) reported satisfactory rigidity attained 1 to two years postoperatively in the AVW group, compared to just one month postoperatively in the SF group.
Consequently, we favored the SF and pooled data of the two groups to expand the sample size. Although one year was indifferent postoperatively between AVW and SF, there was a significant improvement (both P<0.01) in IIEF-5 and EHS scores (9.7±2.8 vs. 20.8±2.3; 1.7±0.6 vs. 3.2±0.2, respectively). Thus, the EHS improved on at least one scale. The diameter of the glans and distal penile shaft increased from 28.0±2.3 mm and 28.3±2.1 mm (n=16) to 35.3±2.2 mm and 36.3±2.1 mm, respectively. Immediate postoperative Cavernosography revealed that the diameter of the CC did not change compared to preoperative measurements (
Figure 4). However, the girth enhancement was evident after six months postoperatively (
Figure 5). The intracorporeal retention and improved erection quality were unexceptional in the AVW group. Two patients reported poor sleep patterns resulting from painful nocturnal penile erections postoperatively, which lasted for three weeks. One patient in the SF group presented with a self-limited infection, which was treated with an oral antibiotic.
4. Discussion
Anatomy is fundamental to surgery; our PVS (penile venous stripping) represents a prime example of translation medicine, moving from bench-side research to bedside application. Over time, it has been developed and refined since 1986 [
10], conspicuously rooted in innovative studies of penile erection-related venous anatomy [
18]. Each specific vein within the penile anatomy has emissaries to corpora cavernosa, including the deep dorsal veins (DDV), cavernosal veins (CVs), and para-arterial veins. The relationship of these veins can be likened to the connection between rattan node roots and a yam vine, where multiple smaller yams can grow if the root remains intact, albeit at the expense of the primary plant unbaling to nurse, the giant yam. Agricultural principles inspired our PVS approach. Similarly, FPGE (fibro-vascular surgery) is grounded in De Novo penile fibro-vascular anatomy.
Physicians strive to adhere to best practices and respect patient autonomy. While we provide no strict therapeutic algorithms, we perform these procedures upon patient requests. A surgeon with a deep understanding of how to confine every drop of blood within the corpora cavernosa, gained through rigorous, precise training, can confidently handle these techniques. This skill is akin to mastering microsurgical techniques for small animals. Once a surgeon becomes less daunting, reducing the temptation of using electrocautery. Human existence spans 300,000 years [
19], suggesting that penile fibro-vascular anatomy and erectile dysfunction (ED) have a similarly long history. While medicine has made significant strides thanks to advancing knowledge and technology, understanding penile anatomy may not have progressed at the same pace. Despite comprehensive interpretations of every organ system in the human body, including the human genome [
20], the portrayal of the penis remains reminiscent of medieval depictions [
21]. Illustrated by Da Vinci, the penile anatomy is depicted as a single 360 circumferential tunica circling the corpora cavernosa (CC), housing a deep dorsal vein (DDV) flanked by paired dorsal arteries [
22]. According to this model, a solitary DDV is tasked with draining the CC of blood.
Recent studies have challenged this traditional understanding, revealing that while the DDV serves as the common drainage vein for both the corpora cavernosa (CC) and corpus spongiosum, a pair of the cavernosal veins (CVs) and two sets of para-arterial veins (PAVs) are responsible for draining the corresponding corpus cavernosum bilaterally [
23]. As a result, specific emissary veins are present in the DDVs, CVs, and PAVs. A 300-degree skeletal longitudinal tunica surrounds a 360-degree smooth circumferential tunica, collectively encircling the CC. The absence of a robust outer tunica capable of counteracting the expansion of corporeal sinusoids compromises veno-occlusive function. An analog of the human body trunk, the penile CC features a skeletal-like component that encases the smooth viscera.
The advancement of surgical techniques for penile morphology, erection restoration, and various enhancement surgeries have been ongoing for decades, yet they have primarily been based on outdated anatomical models. Early surgeries dating back to 1965 [
24] targeted structures that were only thoroughly understood much later, around 1991 [
25], highlighting the lack of anatomical foundation at the time. While subsequent innovations have improved surgical outcomes, questions remain about the sustainability and timelines of these approaches [
26]. One intriguing aspect is the potential for hypertrophy in the smooth muscle fibers of the corpus cavernosum (CC) sinusoidal wall, akin to the hypertrophy observed in uterine smooth muscle during pregnancy. In this study, the CC smooth muscle fibers can be hypertrophied as CC diameter is apparent enlargement in those cavernosograms after six months postoperatively (
Figure 5) but not in the immediate postoperative ones (
Figure 4). CC augmentation could lead to hypertrophy of the smooth muscle fibers and subsequently enhance erectile function. However, conclusive evidence is needed, and further research is required to validate this hypothesis. Challenges persist in accurately assessing patients and obtaining reliable data, particularly penile dimensions measurement. Thus, patient reports and X-ray findings underscore the need for standardized methodologies and rigorous research protocols to ensure reliable data collection and interpretation. Overall, while there is potential for further advancements in penile surgery techniques, including the potential for CC smooth muscle hypertrophy in an exclusive physiological manner and progression of operated penile erection-related veins [
27,
28], comprehensive research and evidence-based approaches are essential to validate these hypotheses and improve patient outcomes.
It is so common for males, even a 61-year-old man in this study, to experience psychological distress due to penile dysmorphia and erectile dysfunction (ED). As a result, many seek interventions such as penile augmentation and penile vascular surgery despite ongoing controversies surrounding the effectiveness of these procedures, particularly in terms of corpus cavernosum (CC) augmentation and lengthening. This study focused on patients who underwent factual CC girth augmentation, inspired, and penile venous stripping, excluding penile lengthening (n=8) due to its longer operation time, conspicuously penile length influenced by pubic fat. This study focuses on corporeal expansion, where the penile shaft skin is free from fat cells. The physiological approach to CC girth enhancement, inspired by the De Novo penile fibro-vascular assembly [
29], represents a novel strategy that warrants further investigation and dissemination despite the limited sample size.
Recent research has shed light on the mechanisms of penile erection, highlighting the critical role of smooth muscle relaxation in the CC [
30]. Additionally, a comprehensive list of contributors to ED has been established, including hormonal imbalance, arterial insufficiency, neurologic deficits, adverse drug effects, psychogenic factors, and cavernosal disintegration [
31,
32]. Vasculogenic ED is the primary contributor to the above list. Given that venous leakage plays a significant role in ED males without comorbidity and in those whose ED is a consequence of heavy cigarette smoking [
33,34], is the VOD inadequately ousted from the erection pathophysiology list? Recently, several hemodynamic experiments have been conducted on human cadavers.
Consequently, penile veins are the principal component for determining erection rigidity since the power of Reductio ad absurdum is categorically seamless because only corpses can be free from psychogenic influence. The factor VOD sits atop the pathophysiology list, and penile venous stripping is viable for a more significant percentage of ED patients [35]. The combined surgery is performed under acupuncture-assisted local anesthesia, with no need for electrocautery or suction apparatus throughout the entire procedure. Given that postoperative patients can fly across either the Pacific or Atlantic Ocean, concern about the surgical trauma of the practice may be overstated. Similarly, postoperative complications are avoidable based on the De Novo penile fibro-vascular assembly. However, it is essential to note that this study is retrospective and has a small sample size. Consequently, limitations exist, and further research is warranted.
5. Conclusions
The novel combination strategy of factual penile girth enhancement and erection restoration, based on the De Novo penile fibro-vascular assembly, appears feasible. However, further studies with larger patient cohorts and more extended follow-up periods are warranted to fully evaluate its effectiveness and long-term outcomes.
Authors Contribution
Chung CH made surgery partially and assisting surgery, patient identification, and in-depth discussion; Chang KS contributed to the concept, design, and editing, data acquisition; Chen HS worked on the above issues, including acquisition and helping with the editing of data acquisition; Hsieh YY worked on some of the above matters, including acquisition and helping with the editing; Chang YH assisted in data acquisition and editing; Hsu GL conducted PVSS surgery, patient follow-up, and therapy; study concept, data collection, data interpretation, authoring the full manuscripts, In addition, the inventor of USPTO patent; Tsai MH completed the IRB application, conspicuously materializing the education of medical students; Chueh JSC edited the paper and led the team. All authors read and approved the last version of this manuscript.
Consent
Written informed consent was obtained before every procedure; neither photo nor imaging violates confidentiality.
Disclosure
All authors follow research ethics and are qualified for research courses.
Acknowledgments
We express our gratitude to Director Chih-Chung Lu at National Taiwan University, Ms. Hsiu-Chen Lu at China Medical University, Ying-Hui Chen, and Kuo-Wei Cheng for their invaluable contributions in preparing the illustrations and photos for this manuscript. The data for this research article was anonymized and taken from the decades-old repository of clinical records.
Conflicts of Interest
None of the contributing authors has any conflict of interest, including specific commercial interests, relationships, and affiliations relevant to the subject makers or materials discussed in the manuscript. The authors received no financial support for this article's research authorship or publication.
References
- Wooten, J.S. Ligation of the Dorsal Vein of the Penis as a Cure for Atonic Impotence. Texas Medical Journal 1902, 18, 325–328. [Google Scholar]
- Wespes, E.; Schulman, C.C. Venous leakage: surgical treatment of a curable cause of impotence. Journal of. Urology 1985, 133, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Montague, D.K.; Barada, J.H.; Belker, A.M.; Levine, L.A.; Nadig, P.W.; Roehrborn, C.G.; et al. Clinical guidelines panel on erectile dysfunction: summary report on the treatment of organic erectile dysfunction. The American Urological Association. Journal of. Urology 1996, 156, 2007–2011. [Google Scholar] [CrossRef] [PubMed]
- Keele, K.D. Leonardo da Vinci’s Influence on Renaissance Anatomy. Medical History 1964, 8, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Lowsley, O.S.; Rueda, A. Further experience with an operation for the cure of certain types of impotence. Journal of International College of. Surgeons 1953, 19, 69–77. [Google Scholar]
- Polk, H.C., Jr; Cheadle, W.G.; Franklin, G.A. Principles of operative surgery. In: Townsend ED Jr CM, ed. Sabiston Textbook of Surgery. Philadelphia: WB Saunders; 2001:163-188.
- Tsai, V.F.; Chang, H.C.; Liu, S.P.; Kuo, Y.C.; Chen, J.H.; Jaw, F.S.; et al. Determination of human penile electrical resistance and implication on safety for electrosurgery of penis. The Journal of Sexual Medicine 2010, 7, 2891–2898. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Huang, Y.P.; Tsai, M.H.; Chen, H.S.; Huang, P.C.; Lin, C.W.; et al. Tunical Outer Layer Plays an Essential Role in Penile Veno-occlusive Mechanism Evidenced from Electrocautery Effects to the Corpora Cavernosa in Defrosted Human Cadavers. Urology 2015, 86, 1129–1135. [Google Scholar] [CrossRef]
- Berardinucci, D.; Morales, A.; Heaton, J.P.; Fenemore, J.; Bloom, S. Surgical treatment of penile veno-occlusive dysfunction: is it justified? Urology 1996, 47, 88–92. [Google Scholar] [CrossRef]
- Huang, P.C.; Hsu, G.L. Vascular Surgery for Erectile Dysfunction. In Encyclopedia of Reproduction (Second Edition) (Skinner MK ed), 2018; pp. 427–436, Academic Press, Oxford.
- Chang, K.S.; Chang, Y.K.; Chung, C.H.; Hsu, G.L.; Chueh, J.S. Emergent Penile Venous Stripping for Treating Adolescent Impotence. Life 2024, 14, 762. [Google Scholar] [CrossRef]
- Hsu, G.L. Physiological Approach to Penile Venous Stripping Surgical Procedure for Patients with Erectile Dysfunction (Patent No: US 8,240,313 B2). http://www.google.com/patents/US20110271966.
- Spyropoulos, E.; Christoforidis, C.; Borousas, D.; Mavrikos, S.; Bourounis, M.; Athanasiadis, S. Augmentation phalloplasty surgery for penile dysmorphophobia in young adults: considerations regarding patient selection, outcome evaluation and techniques applied. European Urology 2005, 48, 121–127. [Google Scholar] [CrossRef]
- Ahmed, U.; Freeman, A.; Kirkham, A.; Ralph, D.J.; Minhas, S.; Muneer, A. Self-injection of foreign materials into the penis. Annals the Royal College Surgeon. England 2017, 99, e78–e82. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Gillis, J. A review of penile elongation surgery. Translation Andrology and Urology 2017, 6, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Gao, Z.R.; Dai, X.; Kuang, L.; Zhang, M.; Li, Q.; et al. Complications and management of penile augmentation with hyaluronic acid injection. Asian Journal Andrology 2021, 23, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Hsu, G.L.; Chang, H.C.; Molodysky, E.; Hsu, C.Y.; Tsai, M.H.; Yin, J.H. The penile fibro-vascular assembly is the last remaining independent vascular compartment to be elucidated in the entire human body. 22nd International Society of Sexual Medicine World Webinar Meeting, Zorgniotti-Newman prize-winning paper. 2021.
- Hsu, G.L.; Hsieh, C.H.; Wen, H.S.; Chen, Y.C.; Chen, S.C.; Mok, M.S. Penile venous anatomy: an additional description and its clinical implication. Journal. Andrology 2003, 24, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Hublin, J.J.; Ben-Ncer, A.; Bailey, S.E.; Freidline, S.E.; Neubauer, S.; Skinner, M.M.; et al. New fossils from Jebel Irhoud, Morocco and the pan-African origin of Homo sapiens. Nature 2017, 546, 289–292. [Google Scholar] [CrossRef]
- A comprehensive genetic linkage map of the human genome. NIH/CEPH Collaborative Mapping Group. Science 1992, 258, 67–86. [CrossRef]
- Standring, S.; Neil, R.; Borley, N.R.; Henry, G. eds. Gray's anatomy: the anatomical basis of clinical practice (40th ed.) 2008; London: Churchill Livingstone. ISBN 978-0-8089-2371-8.
- Putz, R.; Pabsteds, R. Pelvic diaphragm [floor]: male and female external genitalia. In: Putz R, Pabsteds R, eds. Sobotta Atlas of Human Anatomy. Philadelphia: Lippincott Williams & Wilkins; 2001. p. 222-239.
- Hsu, G.L.; Huang, Y.P.; Tsai, M.H.; Chang, H.C.; Liu, S.P.; Molodysky, E.; Hsu, M.C.Y. The venous drainage of the corpora cavernosa in the human penis. Arab Journal Urology 2013, 11, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Nesbit, R.M. Congenital curvature of the phallus: report of three cases with description of corrective operation. Journal of Urology 1965, 93, 230–232. [Google Scholar] [CrossRef]
- Hsu, G.L.; Brock, G.; Martinez-Pineiro, L.; Nunes, L.; von Heyden, B.; Lue, F.T. The three-dimensional structure of the human tunica albuginea: anatomical and ultrastructural level. International Journal Impotence Research 1992, 4, 117–129. [Google Scholar]
- Cormio, L.; Mancini, V.; Massenio, P.; d'Altilia, N.; Selvaggio, O.; Fino, G.D.; et al. Combined Plaque Incision, Buccal Mucosa Grafting, and Additional Tunica Albuginea Plication for Peyronie's Disease. Sexual Medicine Epub 2018 Dec 11. 2019, 7, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Liu, S.P.; Hsu, G.L.; Chen, H.S.; Molodysky, E.; Chen, Y.H.; et al. Advances in our understanding of mammalian penile evolution, human penile anatomy and human erection physiology: Clinical implications for physicians and surgeons. Medical Science Monitor 2012, 18, RA118–RA125. [Google Scholar] [PubMed]
- Hsu, G.L. Erection Abnormality. In M. K. Skinner (Ed.), Encyclopedia of Reproduction. 2018; vol. 1, pp. 382–390. Academic Press: Elsevier. [CrossRef]
- Schultheiss, D.; Stief, C.G. Physiology and pathophysiology of erection: consequences for present medical therapy of erectile dysfunction. Andrologia 1999, 31, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.C.; Lue, T.F. Physiology of penile erection and pathophysiology of erectile dysfunction. Urologic Clinics of North America 2005, 32, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.M.; Mehringer, C.M.; Rajfer, J. Anatomy of penile venous drainage in potent and impotent men during cavernosography. The Journal of Urology 1989, 141, 1353–1356. [Google Scholar] [CrossRef]
- Elhanbly, S.; Abdel-Gaber, S.; Fathy, H.; El-Bayoumi, Y.; Wald, M.; Niederberger, C.S. Erectile dysfunction in smoker: a penile dynamic and vascular study. Journal of Andrology 2004, 25, 991–995. [Google Scholar] [CrossRef]
- Hsu, G.L.; Hung, Y.P.; Tsai, M.H.; Hsieh, C.H.; Chen, H.S.; Molodysky, E.; et al. Penile veins are the principal component in erectile rigidity: a study of penile venous stripping on defrosted human cadavers. Journal of Andrology 2012, 33, 1176–1185. [Google Scholar] [CrossRef]
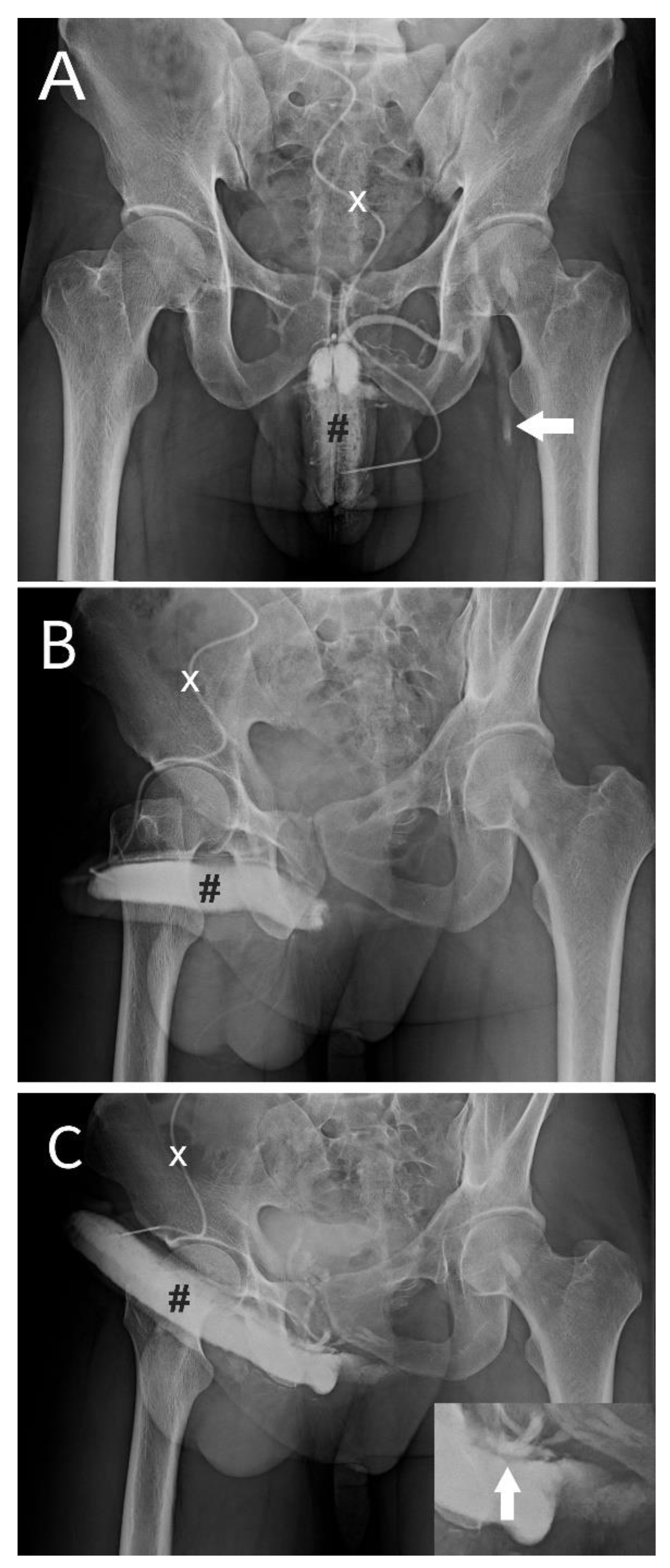
Figure 1.
Dual cavernosography disclosing veno-occlusive dysfunction is suitable for venous stripping in a 33-year-old patient. A) The pilot cavernosogram was obtained via an anterior-posterior view. After a 19G scalp needle (white cross) was inserted in the corpora cavernosa (black pump), at the same time, a 10-mL diluted iohexol solution was injected intracavernously, resulting in the immediate opacification of the erection-related vein. Note the corporeal drop of blood drained into the left femoral vein (white arrow). B) A 30-degree oblique view of a further 10-ml solution was injected. Note the venous drainage was too fast to opacify the left penile crus. C) A pharmaco-cavernosogram was obtained 10 min after 20 mg of prostaglandin E1 (test) was intracavernous injected (white star). D) The bulking of drainage veins was prominent despite a rigid erection attained (white arrow, inserted film). Thus, a veno-occlusive dysfunction was documented.
Figure 1.
Dual cavernosography disclosing veno-occlusive dysfunction is suitable for venous stripping in a 33-year-old patient. A) The pilot cavernosogram was obtained via an anterior-posterior view. After a 19G scalp needle (white cross) was inserted in the corpora cavernosa (black pump), at the same time, a 10-mL diluted iohexol solution was injected intracavernously, resulting in the immediate opacification of the erection-related vein. Note the corporeal drop of blood drained into the left femoral vein (white arrow). B) A 30-degree oblique view of a further 10-ml solution was injected. Note the venous drainage was too fast to opacify the left penile crus. C) A pharmaco-cavernosogram was obtained 10 min after 20 mg of prostaglandin E1 (test) was intracavernous injected (white star). D) The bulking of drainage veins was prominent despite a rigid erection attained (white arrow, inserted film). Thus, a veno-occlusive dysfunction was documented.
Figure 2.
Photos of the ongoing penile venous stripping in a 61-year-old patient. A) A circumferential incision was made to access the offensive veins, and the DDV trunk was severed 3 cm proximally to the retro coronal sulcus (all-white arrow). The visibility of the deep dorsal vein (DDV) was enhanced by a milking manipulation, mimicking a squeeze applied to a balloon of the corpora cavernosa. B) There were 5-7 significant tributaries of the DDV (white curve arrow), which were treated one by one to the retro-coronal sulcus (white arrow). Note the proximal stump was freed from three emissary veins (black arrow). C) The venous plexus of DDV (arrow) and CV (dotted arrow) was stripped by 4–5 pull-through maneuvers until the pubic level was encountered. A 4 cm pubic wound was made, and the distal end was clamped with a mosquito hemostat (black arrow), which benefited the tracing of the DDV trunk (white arrow). D) Following the DDV system and passing underneath; likewise, the CVs were managed (curved arrow). E). Differentiation between the DDV (white arrow) and CVs (curved arrow) could be readily made. F) There were 8-9 and 7-8 significant tributaries of the DDV (white arrow) and CVs (white curved arrow), which should be administrated till the infra-pubic angle. Two venous stumps were severed with scissors and harvested for FPE. G) An encircling gauge was performed on the pendulous penis to prevent oozing (black star), then the left spermatic cords (white curved arrow) and right one (white arrow) were hooked out in sequence. H). Isolation was made of significant branches of the internal spermatic vein (white arrow), plenty of venous tissues were collected and harvested for FPE, and then two wounds were fashioned with fine sutures (I).
Figure 2.
Photos of the ongoing penile venous stripping in a 61-year-old patient. A) A circumferential incision was made to access the offensive veins, and the DDV trunk was severed 3 cm proximally to the retro coronal sulcus (all-white arrow). The visibility of the deep dorsal vein (DDV) was enhanced by a milking manipulation, mimicking a squeeze applied to a balloon of the corpora cavernosa. B) There were 5-7 significant tributaries of the DDV (white curve arrow), which were treated one by one to the retro-coronal sulcus (white arrow). Note the proximal stump was freed from three emissary veins (black arrow). C) The venous plexus of DDV (arrow) and CV (dotted arrow) was stripped by 4–5 pull-through maneuvers until the pubic level was encountered. A 4 cm pubic wound was made, and the distal end was clamped with a mosquito hemostat (black arrow), which benefited the tracing of the DDV trunk (white arrow). D) Following the DDV system and passing underneath; likewise, the CVs were managed (curved arrow). E). Differentiation between the DDV (white arrow) and CVs (curved arrow) could be readily made. F) There were 8-9 and 7-8 significant tributaries of the DDV (white arrow) and CVs (white curved arrow), which should be administrated till the infra-pubic angle. Two venous stumps were severed with scissors and harvested for FPE. G) An encircling gauge was performed on the pendulous penis to prevent oozing (black star), then the left spermatic cords (white curved arrow) and right one (white arrow) were hooked out in sequence. H). Isolation was made of significant branches of the internal spermatic vein (white arrow), plenty of venous tissues were collected and harvested for FPE, and then two wounds were fashioned with fine sutures (I).
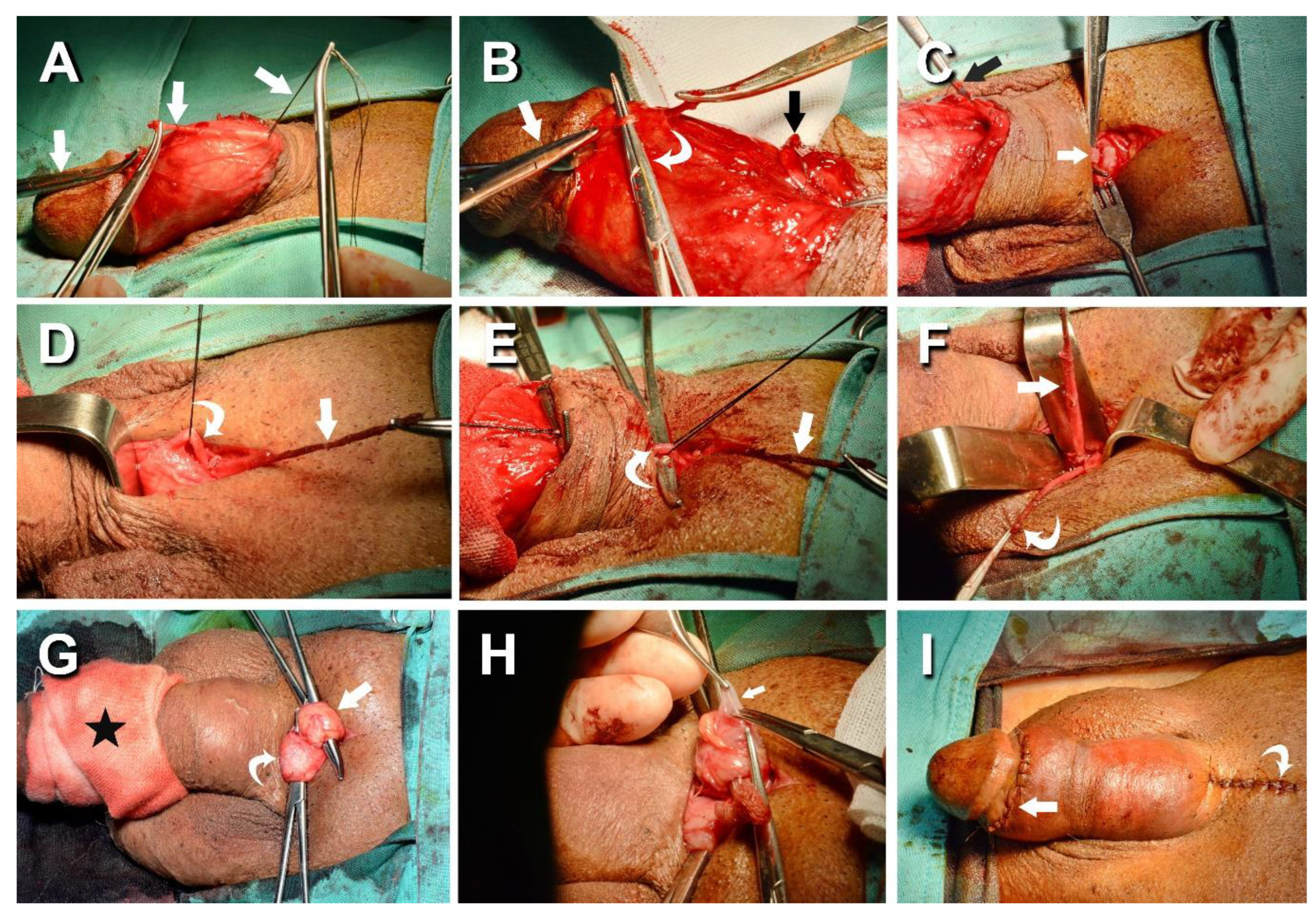
Figure 3.
An illustration of the factual penile enhancement. A) Lateral aspect of the penile shaft, following the penile venous stripping of one deep dorsal vein (DDV), two cavernosal veins (CVs) form the retro coronal sulcus to the infra pubic angle, and Buck’s fascia is opened along the pendulous portion. Next, a 6-8 cm longitudinal incision is performed at the 3 and 9 O’clock positions (red dotted lines). B) A cross-section view of the middle penis denotes the opening line of the tunica albuginea (Red). It shows the bi-layered tunica albuginea with a 360-degree inner circular is incompletely encased by a 300-degree outer longitudinal tunica. C) After harvesting the venous material, the tunica defect was water-tight-suture with a 70.0x30.0mm rectangle venous strip (Red) to the 3 and 9 O’clock positions, respectively. Then, a more prominent venous strip covered the area (Blue) with a 6-0 nylon interrupt suture. D) A cross-section view of the middle penis shows the well-water-tight suture of the autologous vein as the inner tunica (red) is covered by another venous strip (blue). Note the penis glans and shaft augmentation.
Figure 3.
An illustration of the factual penile enhancement. A) Lateral aspect of the penile shaft, following the penile venous stripping of one deep dorsal vein (DDV), two cavernosal veins (CVs) form the retro coronal sulcus to the infra pubic angle, and Buck’s fascia is opened along the pendulous portion. Next, a 6-8 cm longitudinal incision is performed at the 3 and 9 O’clock positions (red dotted lines). B) A cross-section view of the middle penis denotes the opening line of the tunica albuginea (Red). It shows the bi-layered tunica albuginea with a 360-degree inner circular is incompletely encased by a 300-degree outer longitudinal tunica. C) After harvesting the venous material, the tunica defect was water-tight-suture with a 70.0x30.0mm rectangle venous strip (Red) to the 3 and 9 O’clock positions, respectively. Then, a more prominent venous strip covered the area (Blue) with a 6-0 nylon interrupt suture. D) A cross-section view of the middle penis shows the well-water-tight suture of the autologous vein as the inner tunica (red) is covered by another venous strip (blue). Note the penis glans and shaft augmentation.
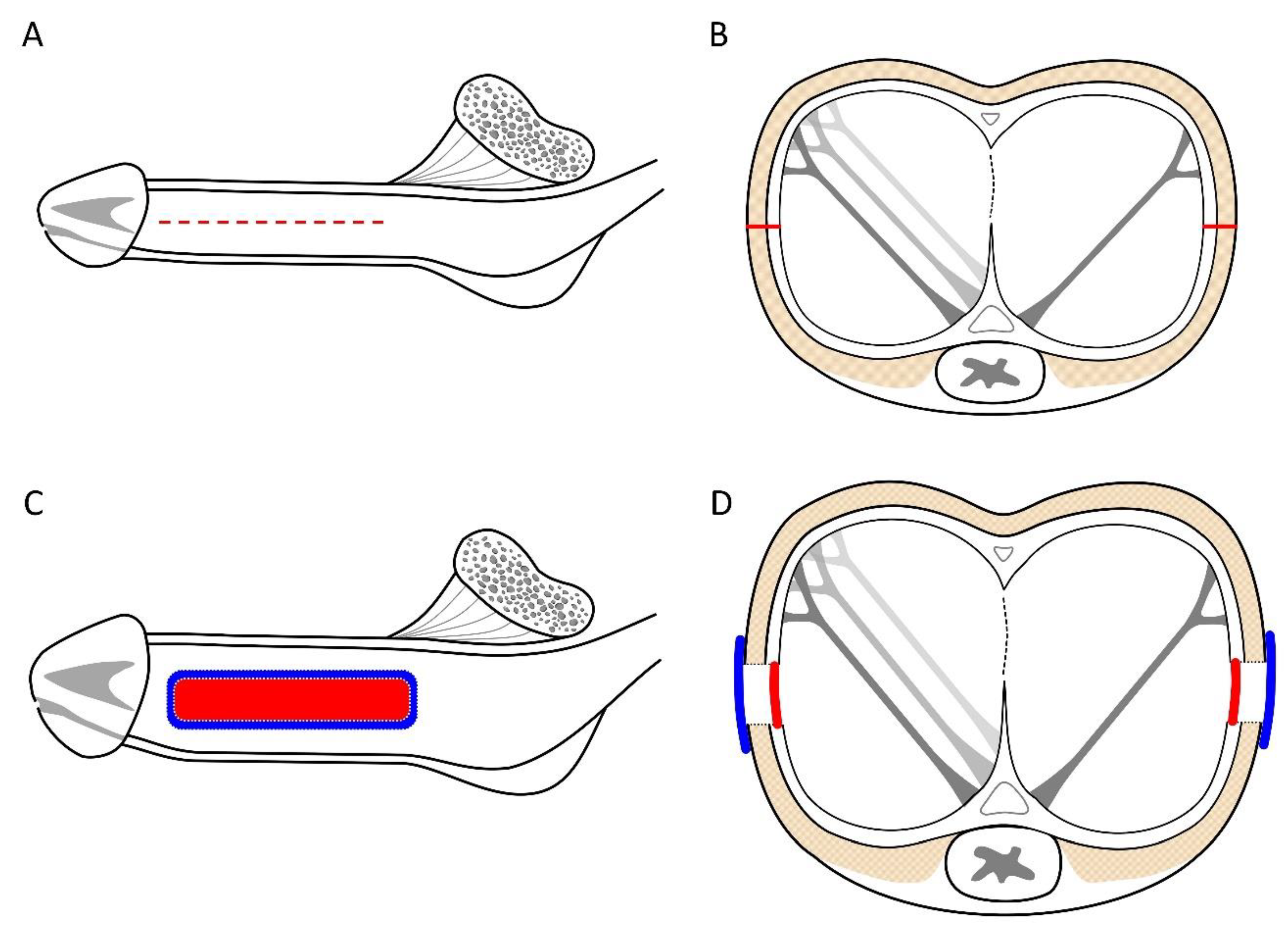
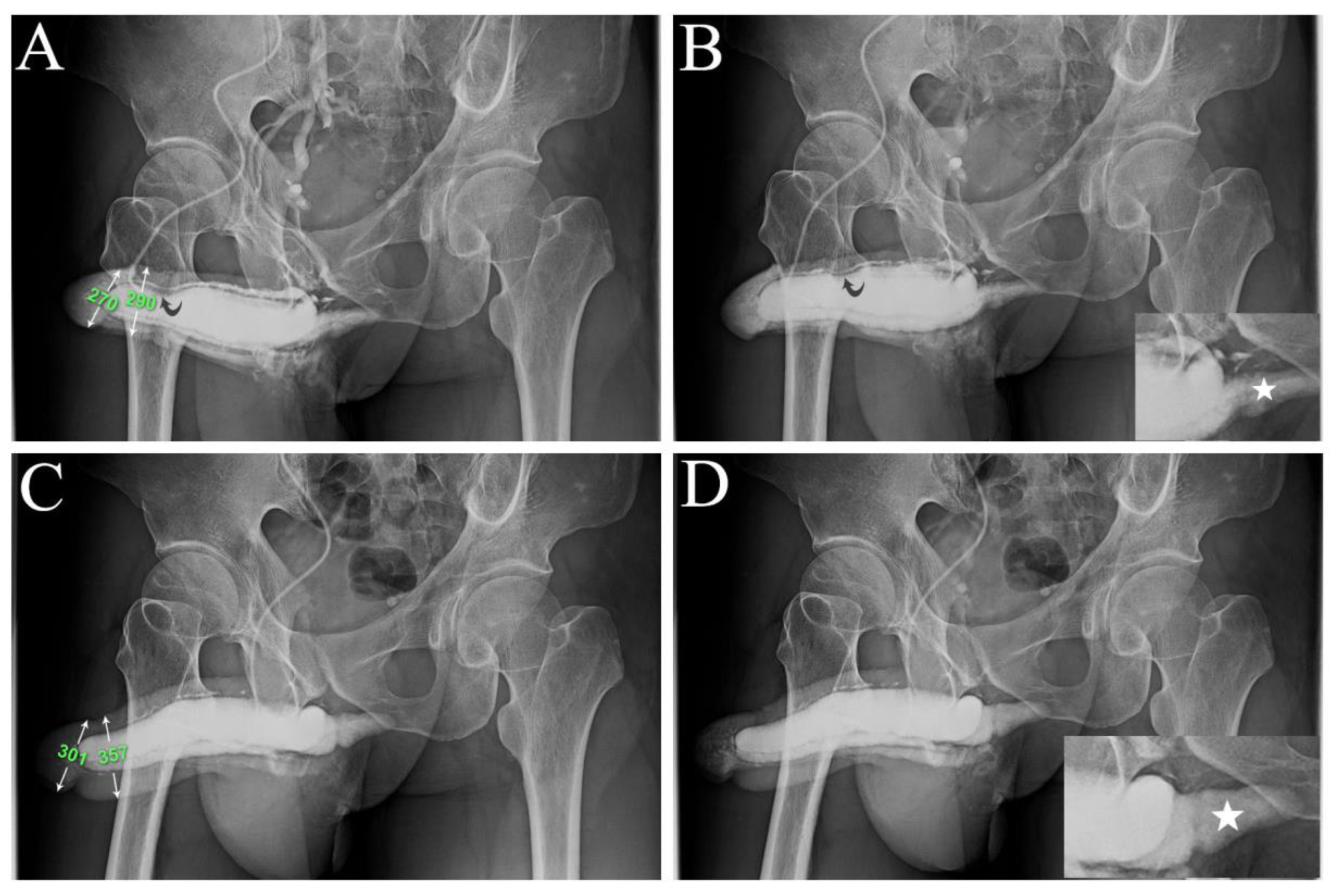
Figure 4.
A representative example is a 1973-born patient who underwent this combined surgery strategy. A) A preoperative anterior-posterior view of the pilot cavernosogram shows the intracavernous contract medium draining back to the periprostatic plexus, internal pudendal, and internal iliac veins immediately. The diameter of the glans penis and distal penile shaft is 27.0 mm and 29.0 mm, respectively. Note a deformity area is also seen (black arrow). B A further 15 mL solution was intravenously injected; the saddle region (black arrow) remained unchanged, and the penile crus was slim (white star in the film inserted). C) An immediate postoperative cavernosogram disclosed the excellent modeling of the saddle region, and the diameter of the glans and penile shaft increased to 30.0 mm and 35.7 mm, respectively. A further 15 mL solution was intravenously injected to confirm intracorporeal retention and a readily inflated penile crus (white star inserted photo).
Figure 4.
A representative example is a 1973-born patient who underwent this combined surgery strategy. A) A preoperative anterior-posterior view of the pilot cavernosogram shows the intracavernous contract medium draining back to the periprostatic plexus, internal pudendal, and internal iliac veins immediately. The diameter of the glans penis and distal penile shaft is 27.0 mm and 29.0 mm, respectively. Note a deformity area is also seen (black arrow). B A further 15 mL solution was intravenously injected; the saddle region (black arrow) remained unchanged, and the penile crus was slim (white star in the film inserted). C) An immediate postoperative cavernosogram disclosed the excellent modeling of the saddle region, and the diameter of the glans and penile shaft increased to 30.0 mm and 35.7 mm, respectively. A further 15 mL solution was intravenously injected to confirm intracorporeal retention and a readily inflated penile crus (white star inserted photo).
Figure 5.
A representative example is a 1966-born patient who underwent this combined surgery strategy. A) A preoperative anterior-posterior view of the pilot cavernosogram shows the intracavernous contract medium draining back to the periprostatic plexus, internal pudendal, and internal iliac veins immediately. Note the radiopacity of the penile crura is just equivalent to that of the femoral cortex, and the diameter of the corpora cavernosa (CC) is 13.7 mm. B) Six months postoperatively, a parallel cavernosogram shows the marked enhancement of intracorporeal retention and radiopacity between the penile crura and the femoral cortex. At that same time, the diameter escalates to 24.3 mm. C) A 30-degree right oblique view of the pilot cavernosogram shows the intracavernous contract medium draining back to the periprostatic plexus, internal pudendal, and internal iliac veins immediately. Note that the radiopacity of the penile crura is just equivalent to that of the femoral cortex. Meanwhile, the diameter of the glans penis and penile shaft is 23.2 mm and 23.1 mm, respectively. D) Six months postoperatively, a parallel cavernosogram shows the marked enhancement of intracorporeal retention, radiopacity between the penile crura and that of the femoral cortex, and conspicuously significant penile girth augmentation. Additionally, the diameter of the glans penis and penile shaft escalated to 34.6 mm and 34.5 mm, respectively.
Figure 5.
A representative example is a 1966-born patient who underwent this combined surgery strategy. A) A preoperative anterior-posterior view of the pilot cavernosogram shows the intracavernous contract medium draining back to the periprostatic plexus, internal pudendal, and internal iliac veins immediately. Note the radiopacity of the penile crura is just equivalent to that of the femoral cortex, and the diameter of the corpora cavernosa (CC) is 13.7 mm. B) Six months postoperatively, a parallel cavernosogram shows the marked enhancement of intracorporeal retention and radiopacity between the penile crura and the femoral cortex. At that same time, the diameter escalates to 24.3 mm. C) A 30-degree right oblique view of the pilot cavernosogram shows the intracavernous contract medium draining back to the periprostatic plexus, internal pudendal, and internal iliac veins immediately. Note that the radiopacity of the penile crura is just equivalent to that of the femoral cortex. Meanwhile, the diameter of the glans penis and penile shaft is 23.2 mm and 23.1 mm, respectively. D) Six months postoperatively, a parallel cavernosogram shows the marked enhancement of intracorporeal retention, radiopacity between the penile crura and that of the femoral cortex, and conspicuously significant penile girth augmentation. Additionally, the diameter of the glans penis and penile shaft escalated to 34.6 mm and 34.5 mm, respectively.
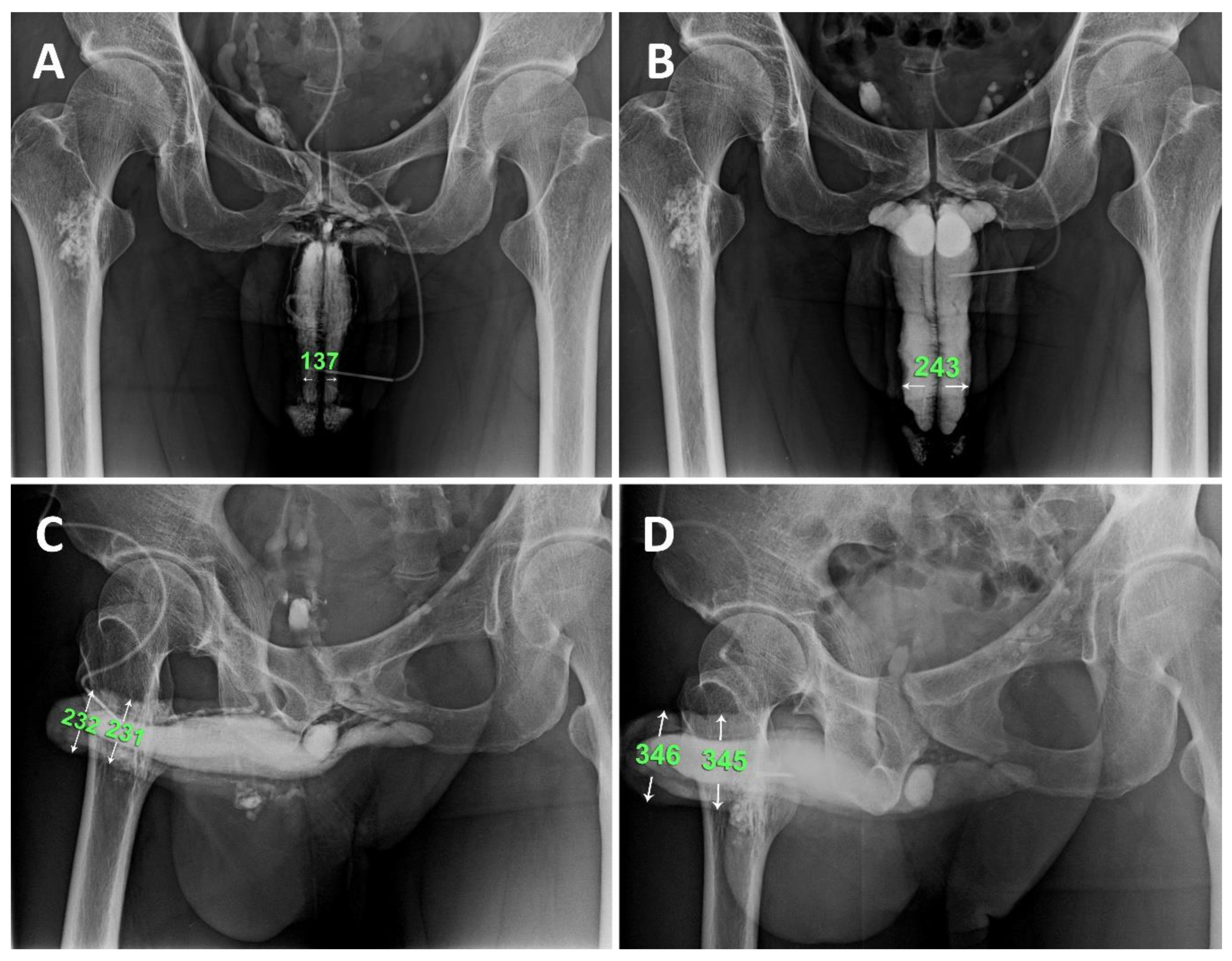
Table 1.
Summary of 16 patients who underwent combining erection restoration and factual penile enhancement in the recent decade.
Table 1.
Summary of 16 patients who underwent combining erection restoration and factual penile enhancement in the recent decade.
| Grouping |
Patients |
IIEF-5 score |
Patients using
PDE-5 inhibitor |
Penile
Prothesis |
Diameter (MM)↑
Glans penis Penile saft |
|
| |
Number |
Age |
Pre-op |
post-op
(1 year) |
|
Post-op
(2 years) |
Pre-op |
Post-op |
|
Pre-op Post-op Pre-op Post-op |
|
| AVW a
|
8 |
24-39 |
9.6±2.3 |
18.3±2.3 |
|
20.8±2.3 |
8 |
2 |
1 |
28.1±2.0 34.9±2.3 27.8±2.2 35.8±2.2 |
|
| SF b
|
8 |
25-61 |
9.8±2.2 |
20.3±1.8 |
|
20.7±2.1 |
8 |
1 |
0 |
27.9±2.3 35.4±2.1 28.3±2.0 36.8±2.0 |
|
| Total |
16 28.8 |
9.7±2.8 19.6±2.1 20.8±2.3 |
16 |
|
1 |
28.0±2.3 35.3±2.2 28.2±2.1 36.5±2.1 |
|
| |
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).