1. Introduction
In December 2019, there was an outbreak of a new infectious disease in Wuhan, capital of the Hubei province (China) caused by a new type of coronavirus: severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and later named “coronavirus disease-2019” (COVID-19) [
1]. In the month of February 2020, the Chinese Center for Disease Control and Prevention (China CDC) provided important information to the international community about the SARS-CoV-2 infection: “The COVID-19 epidemic had spread very rapidly and it took only 30 days (from December 31, 2019 to February 11, 2020) to spread from Hubei Province to the rest of mainland China” [
2]. On 11 March 2020, the World Health Organization (WHO) declared a public health emergency of international concern, but the virus spread rapidly causing a global pandemic. COVID-19 exhibits highly heterogeneous clinical manifestations, ranging from an asymptomatic or paucisymptomatic process to a life-threatening condition characterized by features of interstitial pneumonia, acute respiratory distress syndrome (ARDS), and severe multi-organ failure [
3]. It is transmitted from human-to-human in a variety of ways (direct contact or indirect contact (fomites), small airborne droplets or aerosols), but primarily by large inhaled droplets from coughing or sneezing [
4,
5]. Although the first data available on COVID-19 suggests possible animal-to-human transmission, probably from bats, epidemiological data have increasingly shown that the virus is transmitted from human-to-human, through droplets or direct contact [
6,
7].
The China CDC reported the first description of the demographic characteristics of the COVID-19 outbreak of the novel coronavirus SARS-CoV-2 in February 2020. Of the total 44,672 confirmed cases of COVID-19 in China, only 2% were patients aged 0-19 years [
2]. Italian data, published on March 2020, reported that only 1.2% of 22,512 Italian cases of COVID-19 were children [
8]. Of the total 4,226 COVID-19 cases reported in the United States as of March 2020, only 5% of cases occurred in patients aged 0-19 years [
9]. According to a multicenter retrospective observational cohort study of 8,886 pediatric patients (0 to 18 years) diagnosed with COVID-19, the most common age group was over 10 years (57.0%). The proportion of disease was 8.4% in children less than 1 year of age, 20.7% in the 1-6 age group, and 13.9% in the 6-10 age group [
10]. However, some children may experience a post-infectious inflammatory process temporally related to Covid-19 named “multisystem inflammatory syndrome” (MIS-C) [
11,
12,
13].
From the early stages of the pandemic, it was observed that there was a significant difference in the severity of COVID-19 in different age groups: elderly patients infected with SARS-CoV-2 are at high risk for ARDS, complications, and death, while children with COVID-19 showed milder cases and a better prognosis than adults, and death was relatively rare [
14,
15,
16,
17,
18,
19,
20]. The results found in a recent meta-analysis, after analyzing a large population group of patients (611,583 subjects) with COVID-19 from several national and regional registries (China, Italy, Spain, the United Kingdom and New York State), confirm that age has a determining effect on the mortality of COVID-19 patients. According to age, mortality in patients younger than 50 years was 0.3-1.1%, and increased exponentially with age: 3.0% (50 to 59 years), 9.5% (60 to 69 years), 22.8% (70 to 79 years) and 29.6% (over 80 years) [
21]. In some patients, COVID-19 infection is often accompanied by an aggressive inflammatory response with the release of large amounts of pro-inflammatory cytokines, a so-called "cytokine storm." In addition, several studies suggested that cytokine storm correlates with lung injury, multi-organ failure, and poor prognosis in severe COVID-19 [
22,
23,
24].
The important discovery that vitamin D can act on various cell types through the vitamin D receptor (VDR) opened a wide field for investigation of its role in human health. As a result, VDRs (nuclear transcription factors) have been detected in almost all immune cells, and their activation would regulate the expression of many genes involved in both the innate and adaptive immune systems. Therefore, vitamin D status in COVID-19 could play an important immunomodulatory role by regulating the pathophysiological manifestations of the cytokine storm (anti-inflammatory agent). However, it remains to be seen whether vitamin D supplementation is associated with a potentially favorable outcome of COVID-19 [
25,
26].
The objective of this review is to provide a comprehensive literature review (narrative review) of (a) recent data on vitamin D and its association with COVID-19 and cytokine storm, (b) clinical evidence between COVID-19 and/or MIS-C and vitamin D and finally, (c) evaluate the efficacy of vitamin D supplementation in pediatric patients with COVID-19. This review is based on an electronic search of the literature performed by two independent researchers in the PubMed database of the U.S. National Library of Medicine published between January 2020 and August 2024. The following specific keywords (Medical Subject Headings) were used alone or in combination for the search: children, COVID-19, MIS-C, SARS-CoV-2 infection, vitamin D or vitamin D deficiency/insufficiency and vitamin D supplementation.
2. The COVID-19 Cytokine Storm
The normal response to a viral infection requires activation of the inflammatory pathways of the immune system. The inflammatory response to the invading virus results in the activation of transcription factors that induce the expression of genes encoding various pro-inflammatory cytokines. Cytokines are produced by different immune cells, both innate immune system (macrophages, dendritic cells, natural killer cells) and adaptive immune system (T and B-lymphocytes). The clinical findings are mainly attributed to the action of the pro-inflammatory: interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor-alfa (TNF-α) and interferon-gamma (IFN-γ) This increase in cytokines results in the influx of several immune cells (macrophages, neutrophils, T cells, etc.) from the circulation to the site of infection, with destructive effects on human tissues (damage of vascular barrier and hemodynamic instability, diffuse alveolar damage, multi-organ failure, etc.) and, if left untreated, death [
27].
In other words, COVID-19 infection is usually accompanied by an exaggerated inflammatory response with the release of a large number of pro-inflammatory cytokines in an event known as "cytokine storm," which has been directly correlated with lung injury, multi-organ failure, and adverse prognosis [
22,
23,
28]. Few studies provide data on cytokines in children with severe/critical COVID-19 cases, but the most frequently reported elevated cytokines were IL-6 and IFN-γ [
29].
Several studies have reported that IL-6 is the cytokine that is most often significantly increased in severe/critical cases compared to mild/moderate cases of COVID-19, in both adults and children [
5,
22,
23,
29]. In fact, the optimal results of clinical trials with tocilizumab (an IL-6 inhibitor) in patients with severe and critical COVID-19 led to its approval by the U.S. Food and Drug Administration (FDA) and its inclusion in the current Guidance for the Management of Acute COVID-19 in Children [
30].
3. Immune Modulatory Activity of Vitamin D.
The widespread expression of VDR in immune cells, including antigen-presenting cells (such as dendritic cells and macrophages), as well as T cells and B cells, suggests that vitamin D plays a critical role in the regulation of both the innate and adaptive immune systems. Therefore, vitamin D deficiency may compromise the integrity of the immune system and lead to inappropriate immune responses [
31,
32,
33,
34].
Vitamin D is an important regulator of innate immune responses. For example, by modulating the gene expression of potent antimicrobial peptides such as cathelicidin and β-defensin, vitamin D enhances the antimicrobial properties of immune cells (monocytes, neutrophils and natural killer cells) and barrier epithelia, which could reduce viral replication rates. Furthermore, vitamin D has important effects on immune cells, increasing their production of anti-inflammatory cytokines: IL-4 and IL-10, and inhibiting their production of inflammatory cytokines: IL-1, IL-2, IL-6, IL-8, IL-12 and IFN-γ. In this way, it helps prevent an excessive innate response and the resulting tissue damage (systemic inflammation and/or septic shock).
Vitamin D also plays a role in the regulation of adaptive immunity. Vitamin D modulates the activation and differentiation of naïve CD4+ lymphocytes in the lymph nodes, resulting in a shift from a T-helper 1 (Th1) to a Th2 phenotype. This change implies inhibition of pro-inflammatory cytokine production: IL-2, IFN-γ, and TNF-α, and an increased production of anti-inflammatory cytokines (IL-4, IL-5, and IL-10). Similarly, vitamin D affects differentiation to the Th17 phenotype, leading to a decrease in the production of inflammatory cytokines (IL-17 and IL-21), and facilitates the induction of T regulatory cells (Tregs) with increased production of anti-inflammatory cytokines: IL-10 and transforming growth factor beta (TGF-β) [
35,
36,
37].
In other words, in COVID-19, a link between vitamin D deficiency and the cytokine storm could be established in view of the anti-inflammatory effect of vitamin D. In fact, several studies shown that vitamin D can reduce the risk and/or the gravity of certain inflammatory reactions [
38]. In severe COVID-19 has been observed high levels of pro-inflammatory cytokines, such as IL-1, IL-6, IL-8, IL-12, IL-17, TNF-α and IFN-γ. It appears that vitamin D, through its immunomodulatory role in both innate and adaptive immunity, could potentially prevent or reduce complications associated with the cytokine storm caused by SARS-CoV-2 infection by increasing the levels of anti-inflammatory cytokines (IL-4, IL-5, IL-10), as well as by reducing the levels of pro-inflammatory cytokines [
39,
40]. In this context, it would be useful to measure the basal level of vitamin D as soon as a diagnosis of SARS-CoV-2 infection has been made. Sequential measurements should also be made during the course of the disease. Whether vitamin D supplementation is associated with a potentially favorable outcome of COVID-19 remains to be seen.
In short, immunological dysregulation would be the main consequence of hypovitaminosis D. For example, patients with SARS-CoV-2 infection have a tendency to develop a hyperinflammatory state and even an overactive pathological immune response. Uncorrected vitamin D deficiency can lead to a "cytokine storm", increasing the risk of systemic complications (ARDS, multi organ failure, etc.) and death [
41]. In addition, some authors have shown an association between serum vitamin D levels and lymphocyte count in children with COVID-19. This suggests that serum vitamin D concentration may play a role in lymphocyte count, which is very important for the immune system [
18].
4. Effect of Pandemic Confinement on Vitamin D Status
Vitamin D is mainly produced by the synthesis of the pro-vitamin dehydrocholesterol in the skin under the influence of ultraviolet radiation (type B). The resulting vitamin D is biologically inert and requires double hepatic and renal hydroxylation to become active, also known as "calcitriol" [
25,
26]. Inadequate exposure to sunlight is therefore one of the main causes of vitamin D deficiency.
Following the rapid spread of COVID-19 and the recommendations of the WHO, many countries adopted restrictive measures to contain the spread of the virus (total closure of schools, universities, public places and all shops except supermarkets, grocery stores and pharmacies). In other words, a series of social distancing measures were implemented. Travel and movement were restricted and citizens were asked to stay at home to reduce transmission. In children, for example, confinement meant replacing face-to-face schooling with online education and reducing time spent outdoors. In other words, the COVID-19 pandemic forced confinement in all age groups, leading to a significant reduction in time exposed to sunlight and consequently an increased risk of vitamin D deficiency.
Epidemiological studies conducted in China [
42,
43], Poland [
44], Türkiye [
45] and Italy [
46] have reported that the COVID-19 pandemic-related confinement changed vitamin D status in pediatric age.
The first observational study to compare vitamin D status in children before and after pandemic-related confinement was conducted in Guangzhou, China [
42]. Their results showed an increase in the proportion of children aged 3-6 years with vitamin D deficiency, which was associated with reduced sunlight exposure. However, another study conducted in Hong Kong found evidence of a progressive decline in serum vitamin D levels in infants and young children aged 2-24 months over the course of social distancing and home confinement during the COVID-19 pandemic [
43].
In Warsaw, Poland, mean vitamin D levels in the pediatric population aged 1 month to 18 years were reported to be significantly lower during the COVID-19 pandemic (March 2020 to February 2021) than before the pandemic (January 2019 to February 2020), resulting in an increase in the proportion of children with vitamin D deficiency [
44]. In addition, the characteristic pre-pandemic seasonal variability of serum vitamin D levels in relation to changes in sunlight exposure was not observed during the pandemic period. Similar results were also obtained in Izmir (Türkiye). That is, vitamin D levels in schoolchildren (6-12 years) and adolescents (12-18 years) were significantly lower in the pandemic period (April 2019-March 2020) than in the pre-pandemic period (April 2020-April 2021) [
45]. Likewise, in Naples, Italy, the incidence of hypovitaminosis D was generally higher during the pandemic period (March 2020 to March 2021) than in the pre-pandemic period (March 2019 to March 2020), with higher incidence in preschoolers (1-5 years) and schoolchildren (6-12 years). In this study, no significant differences in serum vitamin D levels were observed in infants (0-12 months) between the pre- and post-COVID periods [
46]. Finally, it should be noted that a recent systematic review and meta-analysis study [
47] of a large cohort of children and adolescents found significant decreases in vitamin D levels during the pandemic in all pediatric age groups (<18 years) compared with pre-pandemic levels. However, severe vitamin D deficiency was only observed in preschoolers and schoolchildren, and this condition was not observed in infants (less than 1 year of age), probably because of the recommended vitamin D supplementation in this age group [
48].
In contrast, in other studies of late adolescents (aged 18-19 years) living in southern Switzerland [
49] or children and adolescents (aged 0-18 years) living on the island of Sardinia, Italy [
50], the authors reported that vitamin D concentrations remained in the sufficient range during the pandemic period and did not differ significantly from those observed in the pre-pandemic period. Note that these differences could be due to geographical and/or cultural heterogeneity, national differences in severity/duration of confinement, variability in study periods, pediatrician vitamin D supplementation, intake of vitamin D fortified foods, etc.
5. Clinical Manifestations of COVID-19 in Children
The incubation period of SARS-CoV-2, which causes COVID-19, is 3 to 7 days. Infectious sources are predominantly respiratory secretions, although SARS-CoV-2 RNA can be isolated from saliva, blood, feces, and urine. A number of systematic reviews and meta-analyses have described the demographic characteristics, clinical symptoms, laboratory findings and radiological features of COVID-19 [
7,
16,
17,
19,
20,
51]. Based on these data, criteria for the severity of COVID-19 infection in children were established, including the following clinical types: asymptomatic infection and mild, moderate, severe or critical infection [
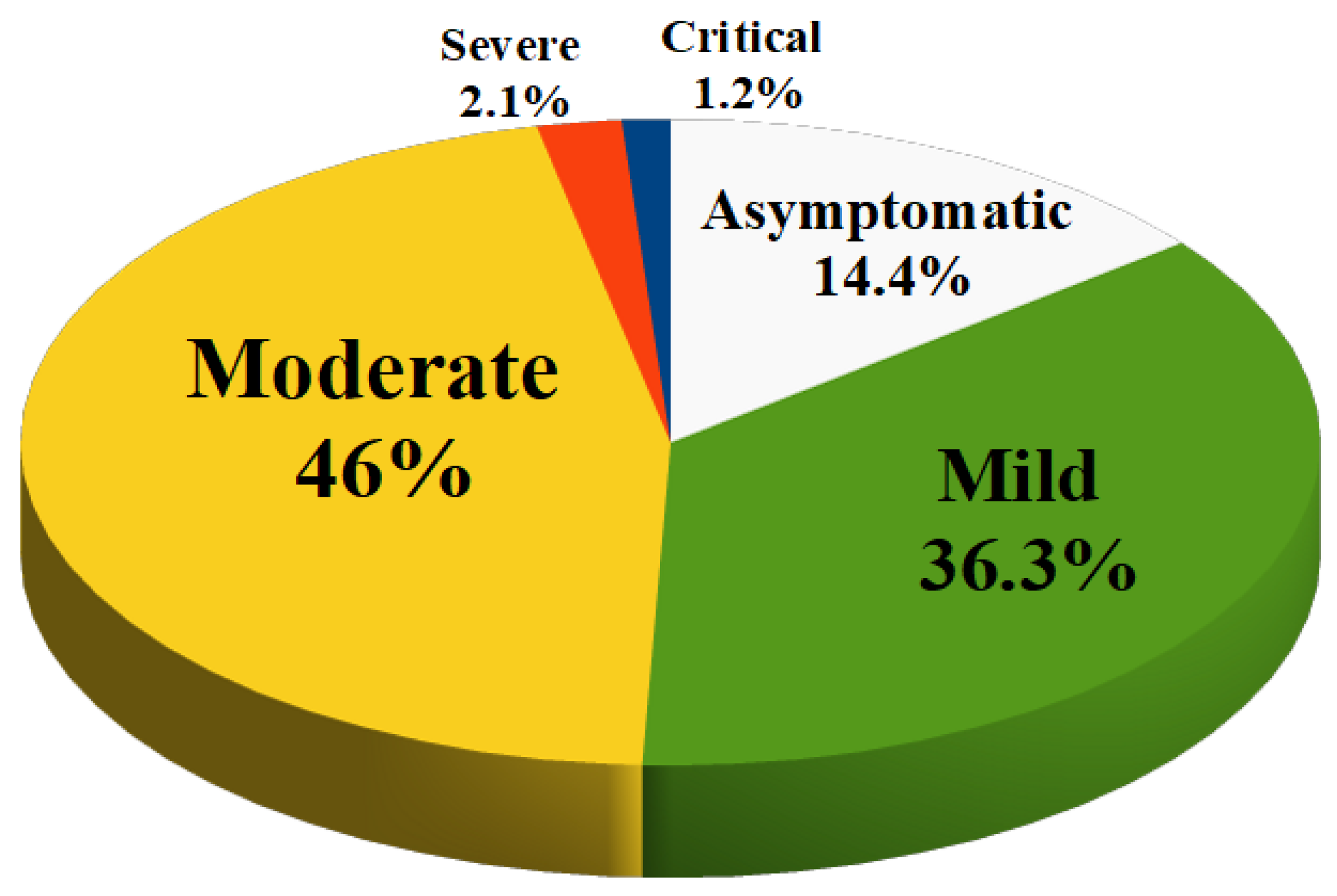
52].
The prevalence of asymptomatic infection was 14.2% of children (no clinical symptoms and signs, normal chest imaging, but SARS-CoV-2 infection detected by positive nucleic acid test).
Mild infection was present in 36.3% of children (symptoms of acute upper respiratory tract infection such as fever, fatigue, myalgia, cough, sore throat, runny nose and sneezing; some children may have only gastrointestinal symptoms such as diarrhea, vomiting and abdominal pain). There are no auscultatory abnormalities on physical examination.
Moderate infection (features of viral bronchitis and pneumonia such as fever, productive cough, wheezing, or wet crackles without tachypnea and hypoxemia) occurred in 46% of children. In some cases, there may be no clinical symptoms and signs, but a chest computed tomography scan may reveal subclinical pulmonary lesions.
Severe infections occurred in 2.1% of children (the disease usually progresses in about 1 week and dyspnea occurs with central cyanosis and oxygen saturation less than 92%).
Finally, 1.2% of cases had a critical infection (children can rapidly progress to acute respiratory distress syndrome or respiratory failure). Multiple organ dysfunction such as circulatory shock, encephalopathy, heart failure, disseminated coagulopathy and acute renal failure can be life-threatening).
Figure 1 is a summary of the severity of disease in children with COVID-19. This means that about 95% of the children diagnosed with COVID-19 had an asymptomatic, mild or moderate disease and had a good prognosis with recovery within 1 to 2 weeks. However, the first retrospective study on the epidemiological characteristics of COVID-19 in children was reported by the Chinese CDC in March 2020. This study showed that young children, especially infants, are more susceptible to COVID-19 infection. In fact, the proportion of severe and critical disease was 10.6% in children aged <1 year at diagnosis, 1-5 years (7.3%), 6-10 years (4.2%), 11-15 years (4.1%), and 16-17 years (3.0%) [
20].
The most common hematological abnormality in patients with mild COVID-19 was a decreased neutrophil or lymphocyte count, according to laboratory findings reported in several meta-analyses. Biochemical abnormalities were characterized by an increase in the levels of creatine kinase-MB, lactate dehydrogenase (LDH) and liver enzymes: aspartate aminotransferase and alanine aminotransferase. In addition, increased levels of procalcitonin (PCT), D-dimer and C-reactive protein (CRP) were found among laboratory abnormalities related to inflammation/coagulation. Elevated creatine kinase-MB levels may be an indication of cardiac damage and monitoring of cardiac troponin levels is recommended in hospitalized patients. The higher frequency of elevated PCT levels compared with CRP levels suggests the possibility of viral and bacterial co-infection [
16,
17,
51].
As mentioned above, less than 5% of children with COVID-19 are at risk of developing severe disease; therefore, accumulated data on children with severe COVID-19 are limited. However, leukocyte indices do not appear to be reliable indicators of disease severity in children, according to a pooled analysis and review. In addition, children with severe COVID-19 tended to have elevated levels of LDH, CRP, PCT, D-dimer and serum ferritin, similar to what has been reported in adult patients with COVID-19. Few studies provide data on cytokines in children with severe/critical COVID-19. However, the most commonly reported elevated cytokines were IL-6, IL-8, TNF-α and IFN-γ [
51,
52,
53,
54,
55].
Chest computed tomography findings of COVID-19 in pediatric patients vary according to the severity of the disease. A recent meta-analysis showed that 41% of cases had normal imaging. The most common lung abnormality was ground-glass opacities (36%) and, to a lesser extent, local (26%) or bilateral (28%) patchy opacities [
14,
16].
6. Multisystem Inflammatory Syndrome
Initial reports indicated that children had mild or asymptomatic COVID-19 and were hospitalized and died at lower rates than adults did [
14,
19,
20,
56]. However, within weeks of the COVID-19 pandemic declaration, clinicians in Western Europe and the United States reported clusters of previously healthy children presenting with cardiovascular shock, fever, and Kawasaki disease-like features in the setting of recent SARS-CoV-2 infection or exposure [
11,
57,
58]. This condition has been referred to as the "multi-system inflammatory syndrome temporally associated with COVID-19" (MIS-C). According to the Centers for Disease Control and Prevention (CDC) and WHO, the initial definition of MIS-C included persistent fever (lasting more than 4 days), laboratory data showing inflammation and multiple organ dysfunction associated with recent SARS-CoV-2 infection or exposure within the previous 2-6 weeks, and no other likely explanation. The majority of MIS-C patients were school-aged or adolescents (aged 5-13 years), and available data suggest that the incidence of MIS-C was approximately 3 per 10,000 persons aged <21 years infected with SARS-CoV-2. This means that MIS-C is likely to be a post-infectious inflammatory process that is temporally related to Covid-19. [
11,
12,
13,
57,
60,
61]
According to published descriptive studies and systematic reviews covering the largest cohorts of MIS-C patients to date, it is characterized by persistent fever and multi organ dysfunction (at least four organs systems are involved), including cardiovascular (hypotension, myocardial dysfunction, pericarditis, coronary artery dilation or aneurysm, myocarditis, etc.), mucocutaneous (skin rash, conjunctival injection), respiratory (pneumonia, acute respiratory distress syndrome, pleural effusion, etc.) and gastrointestinal system (abdominal pain, vomiting or diarrhea). It also includes, to a lesser extent, neurologic (headache, meningism, encephalopathy, etc.), nephrological (renal failure) and hepatological (hepatitis or hepatomegaly) symptoms, and a history of previous asymptomatic or mild COVID-19 or exposure to a person with confirmed COVID-19. Their laboratory tests revealed hematology abnormalities (neutrophilia, lymphopenia, and thrombocytopenia), as well as significant increases in inflammation/coagulation markers (erythrocyte sedimentation rate, CRP, PCT, ferritin, fibrinogen, and D-dimer) and elevated cardiac troponin levels. [
13,
59,
60,
61,
62]. Furthermore, these patients had a "cytokine storm" characterized by increased IL-1, IL-6, IL-8, IL-17, TNFα, and IFN-γ [
40,
63,
64]. Although the pathophysiology of MIS-C is still unknown [
63], it is thought to be an autoimmune vasculitis in which high levels of anti-SARS-CoV-2 IgG antibodies are associated with a pro-inflammatory cytokine storm. This leads to diffuse endothelial damage by immune complexes and activation of complement pathways [
3,
64].
More than 80% of MIS-C patients required intensive care for respiratory support and/or hemodynamic instability, but recovered with no long-term sequelae and a mortality rate of 1-2%. [
13,
57,
60,
65,
66]. There appears to be a close association between MIS-C and SARS-CoV-2 infection, and MIS-C would therefore be considered a post-infectious manifestation, probably due to an abnormal immune response in most cases.
7. Clinical Evidence between COVID-19 and Vitamin D
Regression models showed that the northernmost countries in the Northern Hemisphere had relatively high COVID-19 mortality (May 2020). That is, there appears to be an association between COVID-19 deaths and countries based on latitude (countries with latitudes below 35° North had low mortality rates). This suggests a possible role for vitamin D in determining COVID-19 outcomes, as people living in regions with latitudes above 35°N would receive less exposure to ultraviolet B radiation (an important source of cutaneous vitamin D synthesis) [
67,
68]. On the other hand, epidemiologic studies have reported that vitamin D deficiency or insufficiency increases susceptibility to infections, such as acute respiratory infections, and that vitamin D supplementation reduces them [
41,
69]. There is little evidence that vitamin D can protect against SARS-CoV-2 infection through antiviral activity alone, but it may be very important in preventing the cytokine storm and subsequent acute respiratory distress syndrome or MIS-C, which is often the cause of death [
27,
36,
70].
Based on these premises, several studies were conducted to investigate the clinical significance of vitamin D deficiency in both adults and children with COVID-19. According to a systematic review and meta-analysis conducted in adults, including studies from different European (United Kingdom, Italy and Spain) and Asian (Iran, China, Republic of Korea and Saudi Arabia) countries, vitamin D deficiency was identified as an independent risk factor for COVID-19 (patients with vitamin D deficiency are more susceptible to SARS-CoV-2 infection). In addition, vitamin D deficiency was associated with more severe lung injury, acute respiratory distress and risk of death in elderly COVID-19 patients, probably in relation with an immunological imbalance and hyper-inflammatory state [
71].
Few studies, mostly observational, have evaluated the relationship between vitamin D levels and clinical severity and inflammatory markers in pediatric SARS-CoV-2 patients because of the milder clinical course of COVID-19. However, several studies have reported similar results in the pediatric population despite differences in methods and statistical approaches. Most of them indicated that vitamin D status seems to have an impact on the outcome of COVID-19 also in children, as low vitamin D levels were associated with a worse prognosis. In fact, serum vitamin D levels were lower in both MIS-C and COVID-19 patients compared to control groups (healthy children). Furthermore, some authors concluded that vitamin D levels could be valuable in the prediction of severe forms of MIS-C and that correction of its abnormal levels in severe MIS-C could have an influence on its evolution [
34,
72,
73,
74,
75,
76,
77].
These findings were supported by a meta-analysis of pediatric patients with COVID-19, which included studies from Germany, Romania, the United States, Italy, and Türkiye. Indeed, vitamin D-deficient children and adolescents had a higher risk of contracting SARS-CoV-2 and a worse prognosis compared to those with normal vitamin D levels [
78]. Additionally, multivariable logistic regression analyses found that vitamin D deficiency and D-dimer and fibrinogen levels are independent predictive biomarkers of moderate to severe clinical outcome [
79,
80].
8. Why Is COVID19 Less Severe in Children?
The majority of children infected with COVID-19 are asymptomatic or present with mild or moderate clinical manifestations; namely, symptoms of an acute upper respiratory tract infection or only gastrointestinal symptoms. In the cases with pneumonia, they presented with fever and a dry or productive cough, but did not have obvious hypoxemia or respiratory distress [
10]. From the early stages of the pandemic, it was observed that children had mild or asymptomatic COVID-19 and had lower rates of hospitalization and death than adults had. In fact, since the beginning of the pandemic, age has been identified as an independent predictor of mortality in patients with COVID-19 [
14,
15,
16,
17,
18,
19,
20,
21,
56,
81].
Several hypotheses (
Table 1) have been proposed to explain the difference in the severity of COVID-19 between children and adults. These hypotheses are based on: (a) factors that would increase the risk in adults, or (b) factors that would protect children, or (c) vitamin D status. In reality, these factors are a reflection of the differences in physiological background between children and adults with their clinical consequences [
82,
83,
84].
(a) Factors That Increase the Risk in Adults
Viral entry into cells. Angiotensin converting enzyme 2 (ACE2) receptors are functional receptors of SARS-CoV-2 that are predominantly expressed in the respiratory epithelium (nasopharynx, oropharynx, pneumocytes, etc.) and facilitate viral entry into cells. The expression of the ACE2 gene in the airway epithelium and the affinity of ACE2 receptors for SARS-CoV-2 increases with age and is lower in children than in adults, which would reduce viral entry in children and therefore result in a milder infection.
Endothelial damage and hypercoagulable state. Endothelial damage and an excessive susceptibility to clotting increase with age. SARS-CoV-2 can infect endothelial cells and cause vasculitis, resulting in activation of coagulation pathways and thrombotic complications such as myocardial infarction and stroke in COVID-19. The endothelium in children is "pre-damaged" to a lesser extent than in adults, and the coagulation system is also different, making children less prone to abnormal clotting.
Comorbidities. Children have a lower prevalence of comorbidities associated with severe COVID-19 in adults (obesity, diabetes mellitus, hypertension, immunosuppression, chronic kidney disease, chronic obstructive pulmonary disease, cardiovascular disease, etc.), probably related to endothelial damage.
Intensity of viral exposure. Adults are at higher risk of exposure to SARS-CoV2 due to their work responsibilities (workplace) and social relationships (shopping, travel, etc.), and viral load influences the severity of COVID-19. Therefore, a lower intensity of viral exposure may be another factor favoring less severe disease in children. In addition, since children are usually infected by an adult, they are infected with a second or third generation SARS-CoV-2, and subsequent generations of the virus have reduced pathogenicity in comparison to the first generation virus.
Inmunosenescence. Aging is associated with a gradual decline in the function of the innate and adaptive immune systems, which is likely to contribute to the reduced clearance of SARS-CoV-2 [
81]. That is, age-related functional defects in the immune system could result in a failure to control viral replication, which could lead to poor outcome.
(b) Factors That Protect Children
"Trained" innate immunity. Children infected with SARS-CoV-2 often have co-infections with other viruses (including less pathogenic coronaviruses). Frequent recurrent and concurrent viral infections may interfere with the replication of SARS-CoV-2 by inducing "trained immunity" through epigenetic changes and metabolic reprogramming in innate immune cells, making them more effective in the clearance of SARS-CoV-2.
Off-target effects of live vaccines. Many live vaccines have off-target (non-specific) immunomodulatory effects beyond protection against their target disease. The inclusion of live attenuated vaccines, such as measles, mumps and rubella vaccines and oral polio vaccine, in the childhood immunization schedule would protect against other viral infections by inducing "trained immunity" and/or activating heterologous lymphocytes. This could contribute to differences in the severity of COVID-19.
Age-related differences in immune responses. Children have a stronger innate immune response to cytoplasmic viral pathogens (e.g. SARS-CoV-2) than adults do, with higher natural killer T cell numbers. There is an early type I interferon response (during the incubation period of SARS-CoV-2) and consequently a more rapid activation of host defenses that would lead to more effective containment/elimination of the virus. In terms of adaptive immunity, children also have a higher proportion of T helper 1 lymphocytes (CD4+ T cells), cytotoxic lymphocytes (CD8+ T cells) and activated B lymphocytes than adults, which are essential against viral infections. In addition, it appears that children are less able to generate the pro-inflammatory cytokines storm (lower pro-inflammatory cytokine response); these play an important role in the pathogenesis of severe COVID-19.
(c) Vitamin D Status
Finally, it should be emphasized that vitamin D is an important regulator (genomic pathway) of both the innate and adaptive immune responses and could therefore play a critical role in the host immune response to SARS-CoV-2 infection. As mentioned above, the mechanism by which vitamin D would play a protective role in COVID-19 would be due to its antiviral activity and, in particular, its anti-inflammatory activity, which would moderate the exaggerated inflammatory response (cytokine storm). Clinical evidence suggests that vitamin D status appears to influence the outcome of COVID-19 in both adults and children, as low vitamin D levels are associated with a worse prognosis.
9. Vitamin D Supplementation in COVID-19
Indeed, low vitamin D is a recognized risk factor for COVID-19 [
73,
79,
85]. Considering that the severe course of COVID-19 is characterized by an overproduction of pro-inflammatory cytokines ("cytokine storm") and that vitamin D has an anti-inflammatory effect and also protects against acute respiratory infections in general [
41,
69], several clinical trials and observational studies have been conducted to evaluate the efficacy of vitamin D supplementation in improving the clinical conditions of patients with COVID-19.
Randomized controlled trials have been conducted in adult patients and have shown that high-dose oral cholecalciferol (60,000 IU daily for 7 days or a single dose of 600,000 IU) is safe and reduces inflammatory biomarkers, sequential organ failure, and mortality in vitamin D-deficient COVID-19 [
86,
87]. In addition, a multicenter randomized clinical trial conducted in Saudi Arabia showed that oral supplementation with 5000 IU of cholecalciferol daily for 2 weeks reduced cough recovery time and taste sensory loss in patients with suboptimal vitamin D status and mild to moderate COVID-19 symptoms, and this dose is recommended as adjuvant therapy for COVID-19 patients [
88].
The most recent reviews and meta-analyses of randomized clinical trials suggest that cholecalciferol supplementation may reduce hospital length of stay and intensive care unit admission rates in patients infected with SARS-CoV-2. However, the level of evidence is relatively low [
3,
89,
90,
91]. This is most likely due to the high heterogeneity of the studies analyzed (different study designs, differences in treatment protocols and diagnosis of COVID-19 infection, patients with different severity of disease, vitamin D supplementation in different forms and dosages, etc.). That is, despite some positive findings, their results should be interpreted with caution (the evidence to support the use of vitamin D supplementation as an adjuvant treatment for SARS-CoV-2 infection is still uncertain). Large-scale randomized controlled trials are needed to confirm the potential benefits of vitamin supplementation in both the prevention and treatment of COVID-19.
At present, the available literature on the efficacy and safety of vitamin D supplementation in pediatric patients with COVID-19 is virtually nonexistent. A recent open-label, randomized, controlled, single-blind clinical trial evaluated the efficacy and safety of vitamin D supplementation (intervention: 2,000 IU/day in children aged 1 to 17 years for 7-14 days versus control) in children with COVID-19 requiring hospitalization and supplemental oxygen, without excluding patients with comorbidities (obesity, cancer, and chronic diseases). None of the patients receiving vitamin D had adverse effects. In children with vitamin D supplementation, a decrease in baseline CRP was observed (there was no difference in D-dimer and fibrinogen) and seemed to be able to reduce the risk of COVID-19 clinical deterioration and death. The trial was stopped for ethical reasons, since none of the patients in either group had normal vitamin D status after receiving the results of the baseline vitamin D status [
92]. That is, despite some positive findings, their results should be interpreted with caution because the evidence supporting vitamin D supplementation as an adjuvant treatment for SARS-CoV-2 infection is still uncertain.
10. Conclusions
COVID-19 is caused by infection with a novel coronavirus (SARS-CoV-2). The first outbreak of COVID-19 occurred in December 2019, when a cluster of unexplained pneumonia cases was reported in Wuhan, China. The infection continued to spread rapidly around the world, and the WHO declared the COVID-19 pandemic a global health emergency in March 2020.
The clinical manifestations of COVID-19 are highly heterogeneous, ranging from asymptomatic disease to life-threatening conditions (interstitial pneumonia, ARDS or severe multi-organ failure). The normal response to a viral infection requires activation of the inflammatory pathways of the immune system, but COVID-19 infection can generally be associated with an exaggerated release of pro-inflammatory cytokines (cytokine storm), which has been directly correlated with lung injury, multi-organ failure and adverse prognosis. However, some children may experience a post-infectious inflammatory process (MIS-C) associated with COVID-19, probably due to an abnormal immune response. Most of these patients require intensive care for respiratory support and/or hemodynamic instability, but they recover without sequelae and with minimal mortality.
From the early stages of the pandemic, it was observed that most children diagnosed with COVID-19 had asymptomatic, mild or moderate disease and a good prognosis, while older patients infected with SARS-CoV-2 were at high risk of complications and death. Various hypotheses have been proposed for the age-related difference in severity of COVID-19, including both factors that would increase risk in adults and factors that would protect children, but there is unlikely to be a single explanation. However, it appears that the immune system of children is better adapted to clear SARS-CoV-2, and indeed most children ultimately have mild or asymptomatic disease.
The COVID-19 pandemic forced home confinement in all age groups, resulting in a significant reduction in sunlight exposure and, consequently, an increased risk of developing vitamin D deficiency. In fact, epidemiologic studies conducted in several countries have reported that confinement associated with the COVID-19 pandemic altered vitamin D status in children. Mean vitamin D levels in the pediatric population were significantly lower during the COVID-19 pandemic than before the pandemic.
Vitamin D, through its immunomodulatory role in both innate and adaptive immunity, could potentially prevent or reduce complications associated with the cytokine storm caused by SARS-CoV-2 infection by reducing levels of pro-inflammatory cytokines and increasing levels of anti-inflammatory cytokines. Available data would support a role for vitamin D in reducing complications associated with uncontrolled inflammation or cytokine storm induced by COVID-19. Despite limited clinical data, vitamin D status also appears to affect COVID-19 outcome in children, as low vitamin D levels have been associated with worse prognosis.
Although there is no strong evidence to support systematic vitamin D supplementation in patients with SARS-CoV-2 infection, accumulated experience from clinical trials and observational studies suggests that vitamin D supplementation may play a helpful role in the evolution of COVID-19. At present, the available literature on the efficacy and safety of vitamin D supplementation in pediatric COVID-19 patients is practically non-existent. Large-scale randomized controlled trials are needed to confirm the potential benefits of vitamin supplementation in both the prevention and treatment of COVID-19.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article (none declared).
Contribution Statement
TDT and FGV participated in study design, data collection and analysis. Both authors participated in manuscript preparation and approved its final version.
Informed consent
Not applicable.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
Not applicable (this article does not contain any studies with human participants or animals performed by any of the authors).
Disclosure of potential conflicts of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article (none declared).
List of Abbreviation
ARDS: acute respiratory distress syndrome
China CDC: Chinese Center for Disease Control and Prevention
COVID-19: Coronavirus disease-2019
CRP: C-reactive protein.
IFN-γ: interferon-gamma
IL: interleukin
LDH: lactate dehydrogenase
PCT: procalcitonin
SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2
TGF-β: growth factor beta
Th1: CD4+ type 1 T helper
Th2: CD4+ type 2 T helper
Th17: CD4+ type 17 T helper
TNF-α: tumor necrosis factor-alfa
Treg: CD4+ T regulatory cells
VDR: vitamin D receptor
WHO: World Health Organization
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [CrossRef]
- Zhang Y. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) — China, 2020. Chinese Journal of Epidemiology (by The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team). 2020, 2, 113-122.
- Rizzi, M.; Avellis, V.; Messina, A.; Germano, C.; Tavella, E.; Dodaro, V.; Vitale, R.; Revelli, A.; Zola, P.; Picone, S.; et al. Vitamin D Supplementation in Neonatal and Infant MIS-C Following COVID-19 Infection. Int. J. Mol. Sci. 2024, 25, 3712. [CrossRef]
- Zhao, S.; Zhuang, Z.; Ran, J.; Lin, J.; Yang, G.; Yang, L.; He, D. The association between domestic train transportation and novel coronavirus (2019-nCoV) outbreak in China from 2019 to 2020: A data-driven correlational report. Travel Med. Infect. Dis. 2020, 33, 101568–101568. [CrossRef]
- Chung, Y.-S.; Lam, C.-Y.; Tan, P.-H.; Tsang, H.-F.; Wong, S.-C.C. Comprehensive Review of COVID-19: Epidemiology, Pathogenesis, Advancement in Diagnostic and Detection Techniques, and Post-Pandemic Treatment Strategies. Int. J. Mol. Sci. 2024, 25, 8155. [CrossRef]
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med 2020, 382, 1199-1207.
- Isoldi S, Mallardo S, Marcellino A, Bloise S, Dilillo A, Iorfida D, et al. The comprehensive clinic, laboratory, and instrumental evaluation of children with COVID-19: A 6-months prospective study. J Med Virol. 2021, 93, 3122-3132.
- Livingston, E.; Bucher, K. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA 2020, 323, 1335. [CrossRef]
- Bialek, S.; Boundy, E.; Bowen, V.; Chow, N.; Cohn, A.; Dowling, N.; Ellington, S.; Gierke, R.; Hall, A.; MacNeil, J.; et al. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) — United States, February 12–March 16, 2020. Mmwr-Morbidity Mortal. Wkly. Rep. 2020, 69, 343–346. [CrossRef]
- Duman, M.; Şık, N.; Tekşam, .; Akça, H.; Kurt, F.; Çağlar, A.A.; Yıldız, L.A.; Taşar, M.A.; Fidancı, .; Yayla, B.C.C.; et al. COVID-19 Disease in Presenting to the Pediatric Emergency Department: A Multicenter Study of 8886 Cases. Am. J. Emerg. Med. 2022, 59, 133–140. [CrossRef]
- Godfred-Cato S, Bryant B, Leung J, Oster ME, Conklin L, Abrams J, et al. COVID-19-Associated Multisystem Inflammatory Syndrome in Children-United States, March-July 2020. MMWR Morb Mortal Wkly Rep 2020, 69, 1074-1080.
- Payne AB, Gilani Z, Godfred-Cato S, Belay ED, Feldstein LR, Patel MM, et al. MIS-C Incidence Authorship Group. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw Open. 2021, 4, e2116420.
- Miller AD, Zambrano LD, Yousaf AR, Abrams JY, Meng L, Wu MJ, et al. Multisystem Inflammatory Syndrome in Children—United States, February 2020–July 2021. Clin Infect Dis. 2022, 75, e1165-e1175.
- Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. Coronavirus Study Team.SARS-CoV-2 Infection in Children. N Engl J Med. 2020, 382, 1663-1665.
- Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J 2020, 39, 355–368.
- Cui, X.; Zhao, Z.; Zhang, T.; Guo, W.; Guo, W.; Zheng, J.; Zhang, J.; Dong, C.; Na, R.; Zheng, L.; et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J. Med Virol. 2020, 93, 1057–1069. [CrossRef]
- de Souza, T.H.; Nadal, J.A.; Nogueira, R.J.N.; Pereira, R.M.; Brandão, M.B. Clinical manifestations of children with COVID-19: A systematic review. Pediatr. Pulmonol. 2020, 55, 1892–1899. [CrossRef]
- Ozden, A.; Doneray, H.; Erdeniz, E.H.; Altinkaynak, K.; Igan, H. Clinical and Laboratory Findings by Serum Vitamin D Levels in Children with COVID-19. Eurasian J. Med. 2022, 54, 285–291. [CrossRef]
- Ludvigsson, J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020, 109, 1088–1095. [CrossRef]
- Dong, Y.; Mo, X.; Hu, Y.; Qi, X.; Jiang, F.; Jiang, Z.; Tong, S. Epidemiology of COVID-19 Among Children in China. Pediatrics 2020, 145, e20200702. [CrossRef]
- Bonanad, C.; García-Blas, S.; Tarazona-Santabalbina, F.; Sanchis, J.; Bertomeu-González, V.; Fácila, L.; Ariza, A.; Núñez, J.; Cordero, A. The Effect of Age on Mortality in Patients With COVID-19: A Meta-Analysis With 611,583 Subjects. J. Am. Med. Dir. Assoc. 2020, 21, 915–918. [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [CrossRef]
- Ragab, D.; Eldin, H.S.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [CrossRef]
- Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, et al. Risk factors for severe and critically ill COVID-19 patients: A review Allergy. 2021, 76, 428-455.
- Hossein-Nezhad, A.; Holick, M.F. Vitamin D for Health: A Global Perspective. Mayo Clin. Proc. 2013, 88, 720–755. [CrossRef]
- Palermo, N.E.; Holick, M.F. Vitamin D, bone health, and other health benefits in pediatric patients. J. Pediatr. Rehabilitation Med. 2014, 7, 179–192. [CrossRef]
- Rhodes JM, Subramanian S, Laird E, Griffin G, Kenny RA. Perspective: Vitamin D deficiency and COVID-19 severity – plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J Intern Med. 2021, 289, 97-115.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020, 395, 497–506.
- Sun, D.; Li, H.; Lu, X.-X.; Xiao, H.; Ren, J.; Zhang, F.-R.; Liu, Z.-S. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J. Pediatr. 2020, 16, 251–259. [CrossRef]
- National Institutes of Health. 2022. COVID-19 Treatment Guidelines; Anti-SARS-CoV-2 Antibody Products. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 17 January 2022).
- Cyprian, F.; Lefkou, E.; Varoudi, K.; Girardi, G. Immunomodulatory Effects of Vitamin D in Pregnancy and Beyond. Front. Immunol. 2019, 10, 2739. [CrossRef]
- Koivisto O, Hanel A, Carlberg C. Key vitamin D target genes with function s in the mmune system. Nutrients. 2020, 12, 1140.
- Bilezikian J, Bikle D, Hewison M, Lazaretti-Castro M, Formenti AM, Gupta A, et al. Mechanisms in endocrinology: Vitamn D and COVID-19. Eur J Endocrinol. 2020, 183, R133-R.147.
- Ekemen-Keleş Y, Yılmaz D, Taşar S, Üstündağ G, Şahin A, Tuz AE, et al. Can Serum 25-Hydroxy Vitamin D Levels Predict the Severity of Multisystem Inflammatory Syndrome in Children and COVID-19? J Clin Res Pediatr Endocrinol. 2023, 15, 190-198.
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97. [CrossRef]
- Kalia, V.; Studzinski, G.P.; Sarkar, S. Role of vitamin D in regulating COVID-19 severity—An immunological perspective. J. Leukoc. Biol. 2021, 110, 809–819. [CrossRef]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 17, 9784. [CrossRef]
- Panfili, F.M.; Roversi, M.; D’argenio, P.; Rossi, P.; Cappa, M.; Fintini, D. Possible role of vitamin D in Covid-19 infection in pediatric population. J. Endocrinol. Investig. 2020, 44, 27–35. [CrossRef]
- Greiller, C.L.; Martineau, A.R. Modulation of the Immune Response to Respiratory Viruses by Vitamin D. Nutrients 2015, 7, 4240–4270. [CrossRef]
- Carter MJ, Fish M, Jennings A, Doores KJ, Wellman P, Seow J, et al. Shankar-Hari: Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. 2020, 26, 1701–1707.
- Wimalawansa, S.J. Infections and Autoimmunity—The Immune System and Vitamin D: A Systematic Review. Nutrients 2023, 15, 3842. [CrossRef]
- Yu L, Ke HJ, Che D, Luo SL, Guo Y, Wu JL. Effect of Pandemic-Related Confinement on Vitamin D Status among Children Aged 0–6 Years in Guangzhou, China: A Cross-Sectional Study. Risk Manag. Healthc. Policy. 2020, 13, 2669–2675.
- Wong, R.S.; Tung, K.T.S.; So, H.-K.; Wong, W.H.S.; Wong, S.Y.; Tsang, H.W.; Tung, J.Y.L.; Chua, G.T.; Ho, M.H.K.; Wong, I.C.K.; et al. Impact of COVID-19 Pandemic on Serum Vitamin D Level among Infants and Toddlers: An Interrupted Time Series Analysis and before-and-after Comparison. Nutrients 2021, 13, 1270. [CrossRef]
- Rustecka, A.; Maret, J.; Drab, A.; Leszczyńska, M.; Tomaszewska, A.; Lipińska-Opałka, A.; Będzichowska, A.; Kalicki, B.; Kubiak, J.Z. The Impact of COVID-19 Pandemic during 2020–2021 on the Vitamin D Serum Levels in the Paediatric Population in Warsaw, Poland. Nutrients 2021, 13, 1990. [CrossRef]
- Beyazgül, G.; Bağ, .; Yurtseven, I.; Coşkunol, F.; Başer, S.; Çiçek, D.; Kanberoğlu, G..; Çelik, F.; Nalbantoğlu, .; Özkan, B. How Vitamin D Levels of Children Changed During COVID-19 Pandemic: A Comparison of Pre-pandemic and Pandemic Periods. J. Clin. Res. Pediatr. Endocrinol. 2022, 14, 188–195. [CrossRef]
- Mosca, C.; Colucci, A.; Savoia, F.; Calì, C.; Del Bene, M.; Ranucci, G.; Maglione, A.; Pepe, A.; Morelli, A.; Vajro, P.; et al. Vitamin D Levels in the Pre- and Post-COVID-19 Pandemic Periods and Related Confinement at Pediatric Age. Nutrients 2023, 15, 2089. [CrossRef]
- Cui, X.; Zhai, Y.; Wang, S.; Ding, K.; Yang, Z.; Tian, Y.; Huo, T. Effect of the COVID-19 Pandemic on Serum Vitamin D Levels in People Under Age 18 Years: A Systematic Review and Meta-Analysis. Med Sci. Monit. 2022, 28, e935823-1–e935823-9. [CrossRef]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. Horm. Res. Paediatr. 2016, 85, 83–106. [CrossRef]
- Meoli, M.; Muggli, F.; Lava, S.A.; Bianchetti, M.G.; Agostoni, C.; Kocher, C.; Bührer, T.W.; Ciliberti, L.; Simonetti, G.D.; Milani, G.P. Vitamin D Status in Adolescents during COVID-19 Pandemic: A Cross-Sectional Comparative Study. Nutrients 2021, 13, 1467. [CrossRef]
- Antonucci, R.; Vacca, N.; Biasia, B.; Locci, C.; Dore, M.P.; Pes, G.M.; Bitti, A. Impact of COVID-19 Pandemic and Associated Restrictions on Vitamin D Status in a Large Cohort of Italian Children and Adolescents. Medicina 2023, 60, 65. [CrossRef]
- Henry, B.M.; Benoit, S.W.; de Oliveira, M.H.S.; Hsieh, W.C.; Benoit, J.; Ballout, R.A.; Plebani, M.; Lippi, G. Laboratory abnormalities in children with mild and severe coronavirus disease 2019 (COVID-19): A pooled analysis and review. Clin. Biochem. 2020, 81, 1–8. [CrossRef]
- Fang F, Chen Y, Zhao D, Liu T, Huang Y, Qiu L, et al. Recommendations for the diagnosis, prevention and control of coronavirus disease-19 in children-The Chinese perspectives. Front Pediatr. 2020, 8, 553394.
- Henry BM, Santos de Oliveira MH, Benoit S, Plebania M, Giuseppe Lippia G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med 2020, 58, 1021–1028.
- Henry, B.M.; Lippi, G.; Plebani, M. Laboratory abnormalities in children with novel coronavirus disease 2019. cclm 2020, 58, 1135–1138. [CrossRef]
- Moreno-Galarraga L, Urretavizcaya-Martínez M, Alegría Echauri J, García Howard M, Ruperez García E, Aguilera-Albesa S, et al. SARS-CoV-2 infection in children requiring hospitalization: the experience of Navarra, Spain. World J Pediatr. 2020, 16, 614-622.
- Liu, W.; Zhang, Q.; Chen, J.; Xiang, R.; Song, H.; Shu, S.; Chen, L.; Liang, L.; Zhou, J.; You, L.; et al. Detection of Covid-19 in Children in Early January 2020 in Wuhan, China. New Engl. J. Med. 2020, 382, 1370–1371. [CrossRef]
- Dufort, E.M.; Koumans, E.H.; Chow, E.; Rosenthal, E.M.; Muse, A.; Rowlands, J.; Barranco, M.A.; Maxted, A.M.; Rosenberg, E.S.; Easton, D.; et al. Multisystem Inflammatory Syndrome in Children in New York State. N. Engl. J. Med. 2020, 383, 347–358. [CrossRef]
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020, 395, 1607–1608. [CrossRef]
- Esposito S, Principi N. Multisystem Inflammatory Syndrome in Children Related to SARS-CoV-2. Pediatric Drugs. 2021, 23, 119–129.
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [CrossRef]
- Dionne A, Son MBF, Randolph AG. An Update on Multisystem Inflammatory Syndrome in Children Related to SARS-CoV-2. Pediatr. Infect. Dis. J. 2022, 41, e6–e9.
- García-Salido A, de Carlos Vicente JC, Belda Hofheinz S, Balcells Ramírez J, Slöcker Barrio M, Leóz Gordillo I, et al. Severe manifestations of SARS-CoV-2 in children and adolescents: from COVID-19 pneumonia to multisystem inflammatory syndrome: a multicentre study in pediatric intensive care units in Spain. Crit Care. 2020, 24, 666.
- Brodsky NN, Ramaswamy A, Lucas CL. The Mystery of MIS-C Post-SARS-CoV-2 Infection Trends Microbiol. 2020, 28, 956-958.
- Waltuch, T.; Gill, P.; Zinns, L.E.; Whitney, R.; Tokarski, J.; Tsung, J.W.; Sanders, J.E. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am. J. Emerg. Med. 2020, 38, 2246.e3–2246.e6. [CrossRef]
- Blat, AM, Randolph AG, Severe COVID-19 and Multisystem Inflammatory Syndrome in Children in Children and Adolescents. Crit. Care Clin. 2022, 38, 571–586.
- Yilmaz, D.; Keles, Y.E.; Emiroglu, M.; Duramaz, B.B.; Ugur, C.; Kocabas, B.A.; Celik, T.; Ozdemir, H.; Bayturan, S.; Turel, O.; et al. Evaluation of 601 children with multisystem inflammatory syndrome (Turk MISC study). Eur. J. Pediatr. 2023, 182, 5531–5542. [CrossRef]
- Rhodes, J.M.; Subramanian, S.; Laird, E.; Kenny, R.A. Editorial: low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment. Pharmacol. Ther. 2020, 51, 1434–1437. [CrossRef]
- Lansiaux, .; Pébaÿ, P.P.; Picard, J.-L.; Forget, J. Covid-19 and vit-d: Disease mortality negatively correlates with sunlight exposure. Spat. Spatio-temporal Epidemiology 2020, 35, 100362–100362. [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [CrossRef]
- Sacco, K.; Castagnoli, R.; Vakkilainen, S.; Liu, C.; Delmonte, O.M.; Oguz, C.; Kaplan, I.M.; Alehashemi, S.; Burbelo, P.D.; Bhuyan, F.; et al. Immunopathological signatures in multisystem inflammatory syndrome in children and pediatric COVID-19. Nat. Med. 2022, 28, 1050–1062. [CrossRef]
- Rafique, Z.; Gasecka, A.; Chirico, F.; Gawel, W.; Hernik, J.; Kaminska, H.; Filipiak, K.J.; Jaguszewski, M.J.; Szarpak, L. A systematic review and meta-analysis of effect of vitamin D levels on the incidence of COVID-19. Cardiol. J. 2021, 28, 647–654. [CrossRef]
- Feketea, G.; Vlacha, V.; Bocsan, I.C.; Vassilopoulou, E.; Stanciu, L.A.; Zdrenghea, M. Vitamin D in Corona Virus Disease 2019 (COVID-19) Related Multisystem Inflammatory Syndrome in Children (MIS-C). Front. Immunol. 2021, 12, 648546. [CrossRef]
- Yılmaz, K.; ¸Sen, V. Is Vitamin D Deficiency a Risk Factor for COVID-19 in Children? Pediatr. Pulmonol. 2020, 55, 3595–3601.
- del Giudice, M.M.; Indolfi, C.; Dinardo, G.; Decimo, F.; Decimo, A.; Klain, A. Vitamin D status can affect COVID-19 outcomes also in pediatric population. PharmaNutrition 2022, 22, 100319–100319. [CrossRef]
- Mamishi S, Olfat M, Pourakbari B, Eshaghi H, Abdolsalehi MR, Shahbabaie MA, et al. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in children: update and new insights from the second report of an Iranian referral hospital. Epidemiol Infect 2022, 150, 179.
- Rivera, D.T.; Misra, A.; Sanil, Y.; Sabzghabaei, N.; Safa, R.; Garcia, R.U. Vitamin D and morbidity in children with Multisystem inflammatory syndrome related to Covid-19. Prog. Pediatr. Cardiol. 2022, 66, 101507–101507. [CrossRef]
- Petrovic, D.; Benzon, B.; Srsen, S.; Polic, B.; Novogradec, A.V.; Milic, P.; Markic, J. The Impact of Vitamin D Levels on Clinical Manifestations of Multisystem Inflammatory Syndrome in Children: A Cross-Sectional Study. Life 2023, 13, 674. [CrossRef]
- Shah, K.; Varna, V.P.; Pandya, A.; Saxena, D. Low vitamin D levels and prognosis in a COVID-19 pediatric population: a systematic review. Qjm: Int. J. Med. 2021, 114, 447–453. [CrossRef]
- Bayramoğlu, E.; Akkoç, G.; Ağbaş, A.; Akgün, .; Yurdakul, K.; Duru, H.N.S.; Elevli, M. The association between vitamin D levels and the clinical severity and inflammation markers in pediatric COVID-19 patients: single-center experience from a pandemic hospital. Eur. J. Pediatr. 2021, 180, 2699–2705. [CrossRef]
- Bagiu, I.C.; Scurtu, I.L.; Horhat, D.I.; Mot, I.C.; Horhat, R.M.; Bagiu, R.V.; Capraru, I.D.; Diaconu, M.M.; Adam, O.; Ciornei, B.; et al. COVID-19 Inflammatory Markers and Vitamin D Relationship in Pediatric Patients. Life 2022, 13, 91. [CrossRef]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 28;395(10229):1054-1062. [CrossRef]
- Wong LSY, Loo EXL, Kang AYH, Lau HX, Tambyah PA, Tham EH. Age-Related Differences in Immunological Responses to SARS-CoV-2. J Allergy Clin Immunol Pract. 2020, 8, 3251-3258.
- Zimmermann P, Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child 2021, 106, 429–439.
- Kapustova, L.; Petrovicova, O.; Banovcin, P.; Antosova, M.; Bobcakova, A.; Urbancikova, I.; Rennerova, Z.; Jesenak, M. COVID-19 and the Differences in Physiological Background Between Children and Adults and Their Clinical Consequences. Physiol. Res. 2021, 70, S209–S225. [CrossRef]
- Alpcan, A.; Tursun, S.; Kandur, Y. Vitamin D levels in children with COVID-19: a report from Turkey. Epidemiology Infect. 2021, 149, e180. [CrossRef]
- Rastogi, A.; Bhansali, A.; Khare, N.; Suri, V.; Yaddanapudi, N.; Sachdeva, N.; Puri, G.D.; Malhotra, P. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgrad. Med J. 2020, 98, 87–90. [CrossRef]
- Singh, A.; Rastogi, A.; Puri, G.D.; Ganesh, V.; Naik, N.B.; Kajal, K.; Kahlon, S.; Soni, S.L.; Kaloria, N.; Saini, K.; et al. Therapeutic high-dose vitamin D for vitamin D-deficient severe COVID-19 disease: randomized, double-blind, placebo-controlled study (SHADE-S). J. Public Heal. 2024, 46, 256–266. [CrossRef]
- Sabico, S.; Enani, M.A.; Sheshah, E.; Aljohani, N.J.; Aldisi, D.A.; Alotaibi, N.H.; Alshingetti, N.; Alomar, S.Y.; Alnaami, A.M.; Amer, O.E.; et al. Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial. Nutrients 2021, 13, 2170. [CrossRef]
- Sîrbu, A.C.; Sabin, O.; Bocșan, I.C.; Vesa, .C.; Buzoianu, A.D. The Effect of Vitamin D Supplementation on the Length of Hospitalisation, Intensive Care Unit Admission, and Mortality in COVID-19—A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3470. [CrossRef]
- Sinopoli A, Sciurti A, Isonne C, Santoro MM, Baccolini V. The Efficacy of Multivitamin, Vitamin A, Vitamin B, Vitamin C, and Vitamin D Supplements in the Prevention and Management of COVID-19 and Long-COVID: An Updated Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients 2024, 16, 1345.
- Sobczak, M.; Pawliczak, R. Effect of Vitamin D3 Supplementation on Severe COVID-19: A Meta-Analysis of Randomized Clinical Trials. Nutrients 2024, 16, 1402. [CrossRef]
- Zurita-Cruz, J.; Fonseca-Tenorio, J.; Villasís-Keever, M.; López-Alarcón, M.; Parra-Ortega, I.; López-Martínez, B.; Miranda-Novales, G. Efficacy and safety of vitamin D supplementation in hospitalized COVID-19 pediatric patients: A randomized controlled trial. Front. Pediatr. 2022, 10, 943529. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).