Submitted:
15 October 2024
Posted:
15 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Colonization of B. bassiana in the Tomato Seedlings
2.2. Effect of B. bassiana Colonization on Growth of Tomato Seedling Under Simulated Drought Conditions in Hydroponics
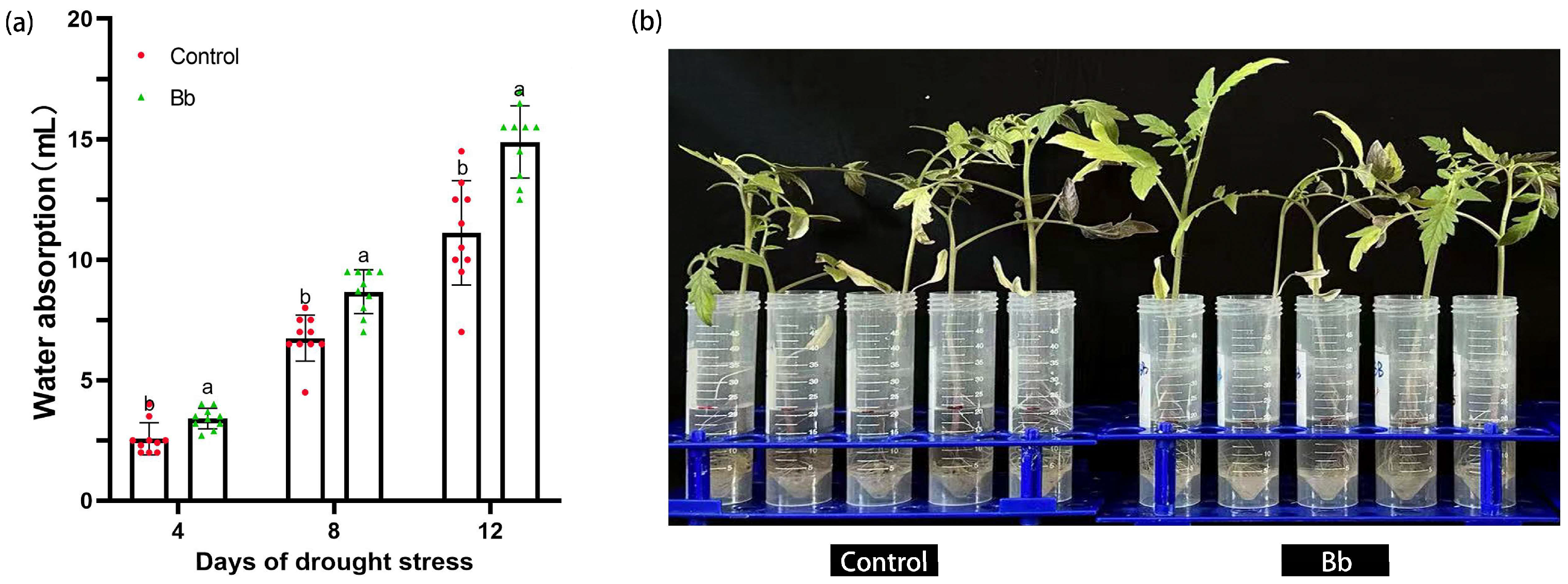
2.3. Effect of B. bassiana Treatment on Water Absorption
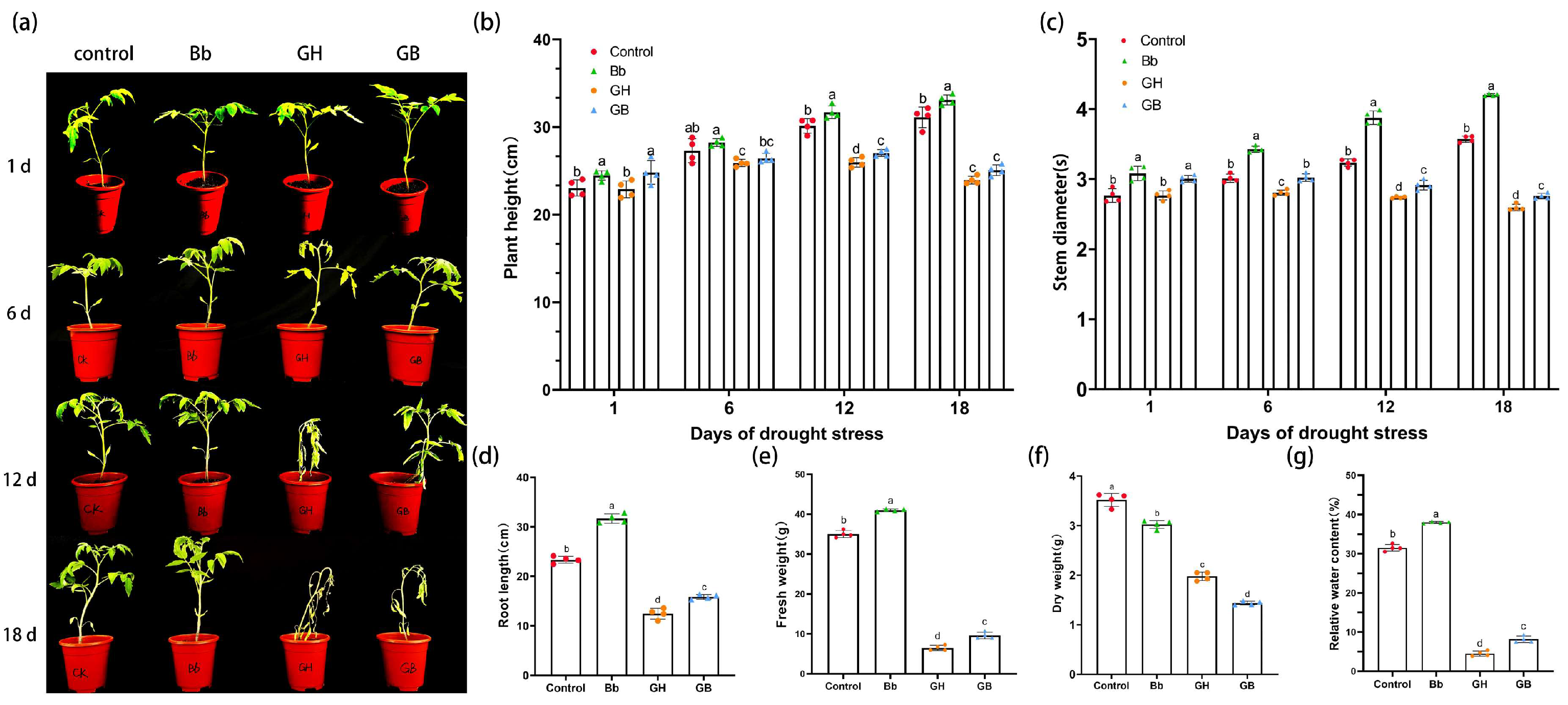
2.4. Effect of B. bassiana Colonization on Tomato Seedling Growth Indicators Under Drought Stress in the Pot Experiment
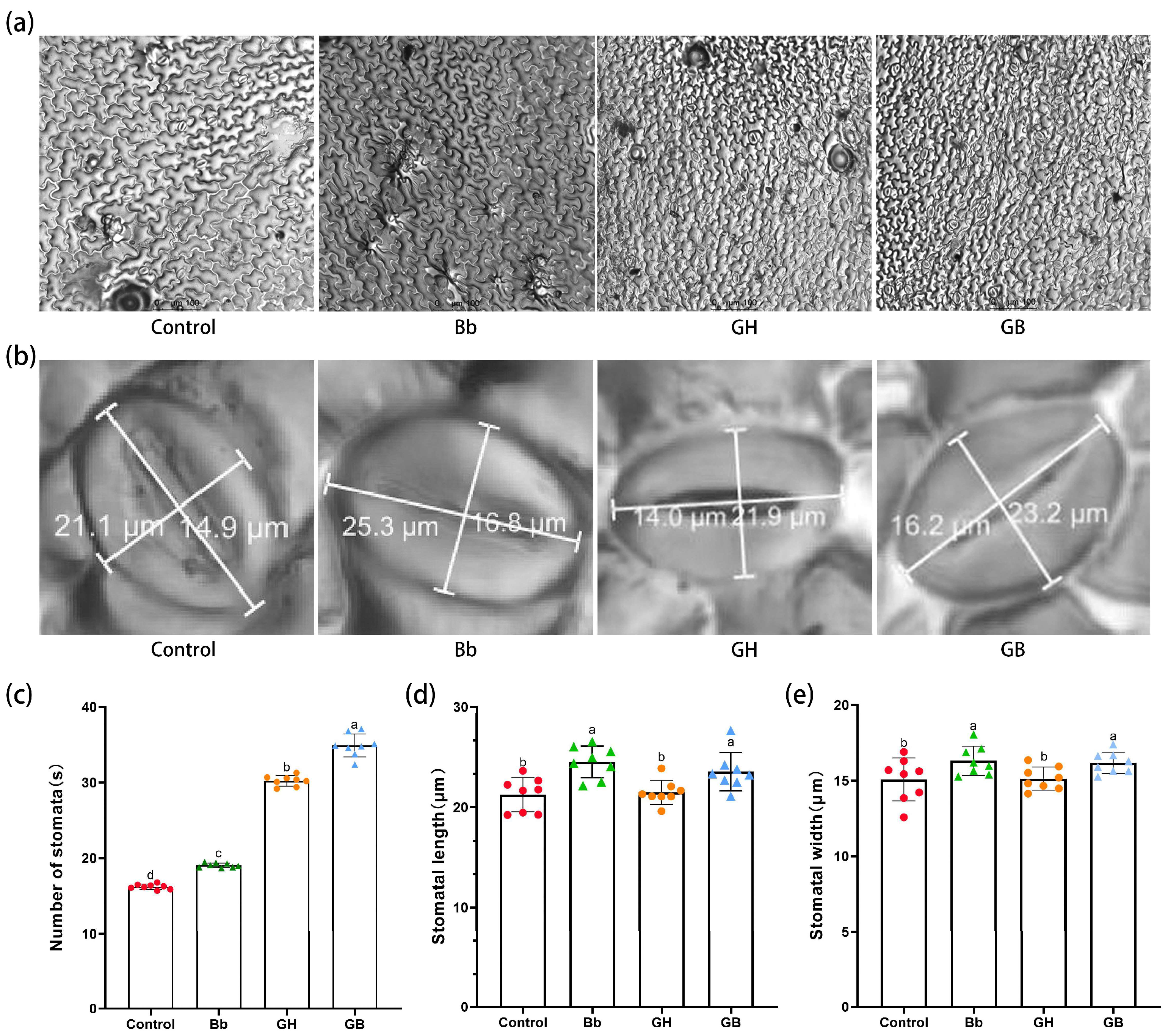
2.5. Effect of B. bassiana Treatment on Stomata Morphology in Tomato Leaves Under Drought Stress
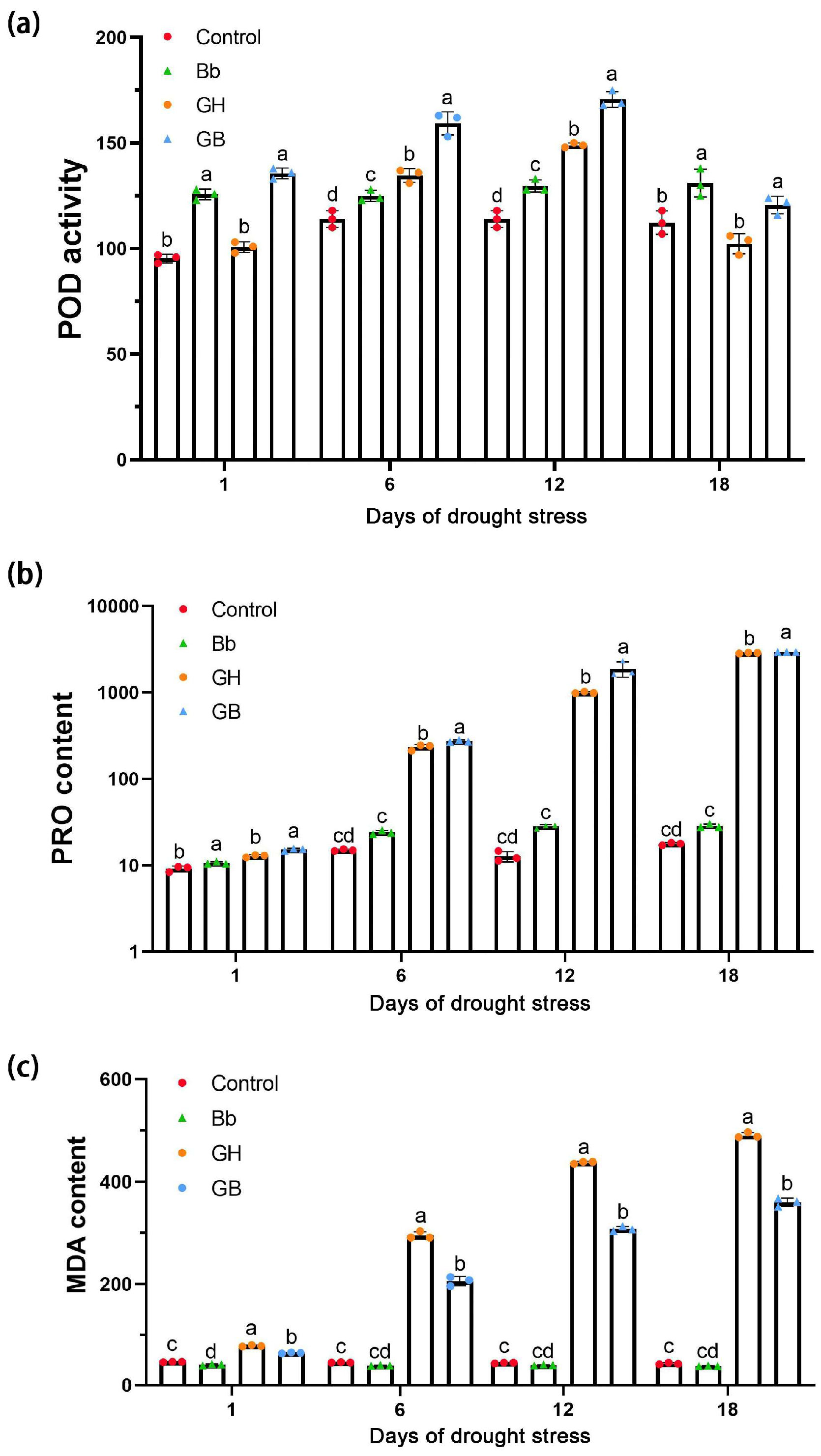
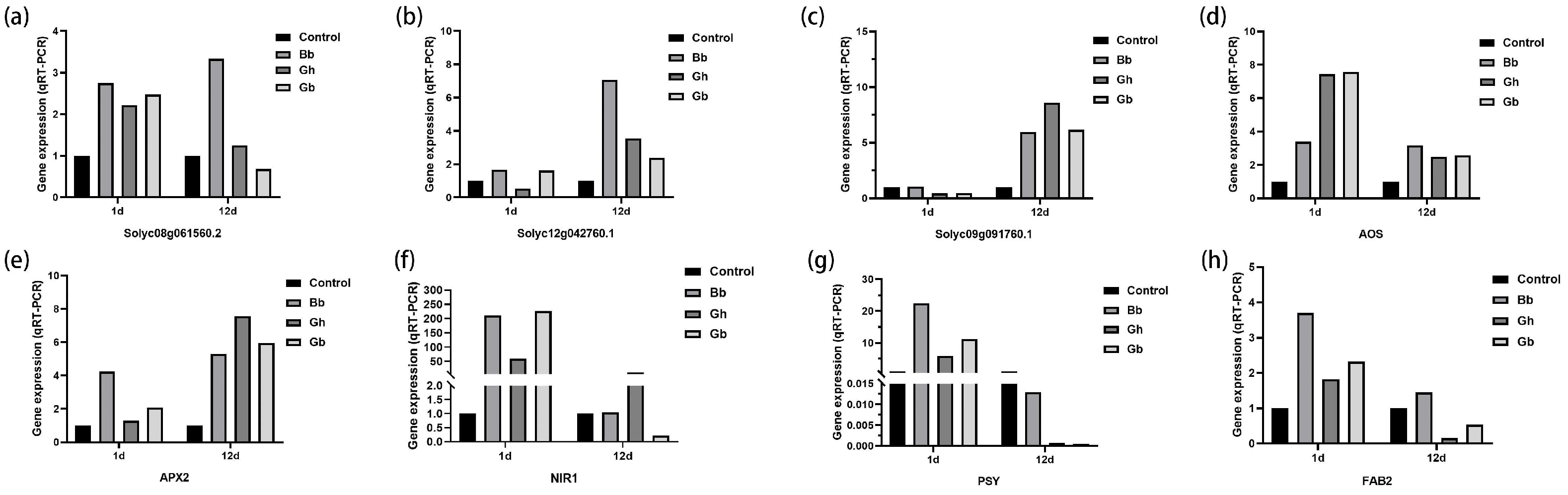
2.6. Analysis of Physicochemical Indexes in the Tomato Seedlings
2.7. Analysis of Physicochemical Indexes in the Tomato Seedlings
3. Discussion
4. Materials and Methods
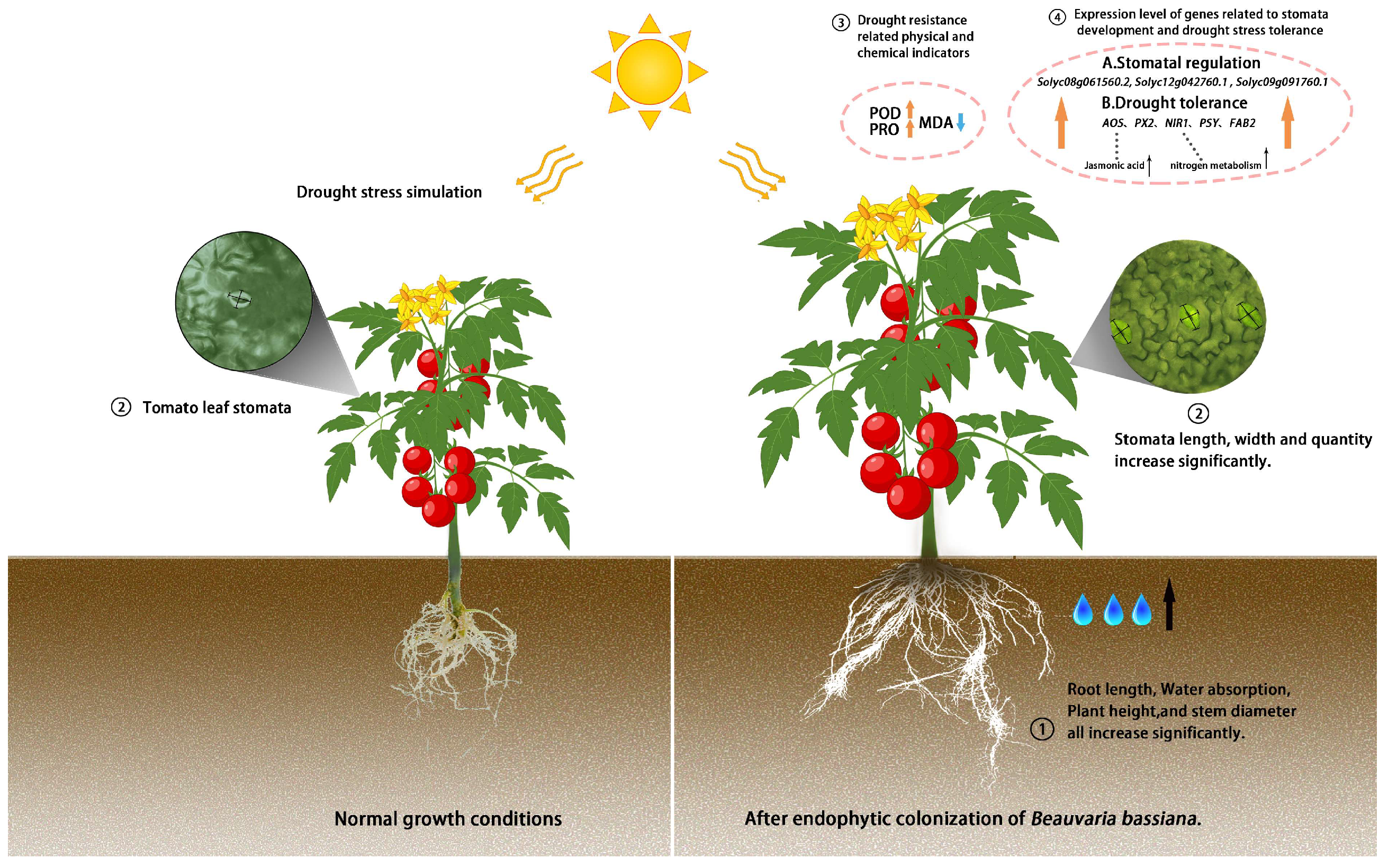
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Institutional Review Board Statement
Informed Consent Statement
Data availability statement
References
- Zytynska, S. Embracing the complexity of plant-microbe-insect interactions under a changing climate for sustainable agriculture. Curr Opin Insect Sci. 2021, 44, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.; Ansari, W.; Soumia, P.; Yadav, A.; Jaiswal, D.; Kumar, S.; Singh, A.; Singh, M.; Verma, J. Biotechnological Interventions in Tomato (Solanum lycopersicum) for Drought Stress Tolerance: Achievements and Future Prospects. BioTech. 2022, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Eti, F.; Ahmed, R.; Gupta, S.; Jhan, P.; Islam, T.; Bhuiyan, M.; Rubel, M.; Khayer, A. Drought-responsive genes in tomato: meta-analysis of gene expression using machine learning. Scientific reports. 2023, 13, 19374. [Google Scholar] [CrossRef]
- González, E. Drought Stress Tolerance in Plants. International journal of molecular sciences. 2023, 24, 6562. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Applied microbiology and biotechnology. 2019, 103, 3327–3340. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Shurigin, V.; Hashem, A.; Abd, A.; Endophytic Bacteria Improve Plant Growth. Symbiotic Performance of Chickpea (Cicer arietinum L.) and Induce Suppression of Root Rot Caused by Fusarium solani under Salt Stress. Front Microbiology. 2017, 28, 1887. [Google Scholar]
- Bing, L.; Lewis, L. Suppression of Ostrinia nubilalis (Hübner)(Lepidoptera: Pyralidae) by endophytic Beauveria bassiana (Balsamo) Vuillemin. Environmental entomology. 1991, 20, 1207. [Google Scholar] [CrossRef]
- Půža, V.; Tarasco, E. Interactions between Entomopathogenic Fungi and Entomopathogenic Nematodes. Microorganisms. 2023, 8, 163. [Google Scholar] [CrossRef]
- Bamisile, B. S.; Dash, C. K.; Akutse, K. S.; Qasim, M.; Ramos Aguila, L. C.; Wang, F.; Keppanan, R.; Wang, L. Endophytic Beauveria bassiana in Foliar-Treated Citrus limon Plants Acting as a Growth Suppressor to Three Successive Generations of Diaphorina citri Kuwayama (Hemiptera: Liviidae). Insects. 2019, 10, 176. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Zhang, J.; Liu, X.; Ju, Z.; Shi, H.; Mendoza-Mendoza, A.; Zhou, W. ; Dual role of endophytic entomopathogenic fungi: induce plant growth and control tomato leafminer Phthorimaea absoluta. Pest Manag Sci. 2023, 79, 4557. [Google Scholar] [CrossRef]
- Sui, L.; Lu, Y.; Yang, H.; et al. Endophytic Beauveria bassiana promotes sunflower growth and confers resistance against sclerotinia disease. BioControl. 2024. [Google Scholar] [CrossRef]
- Deb, L.; Dutta, P. Antagonistic potential of Beauveria bassiana (Balsamo) Vuillemin against Pythium myriotylum causing damping off of tomato. Indian Phytopathology. 2021, 74, 715. [Google Scholar] [CrossRef]
- Sui, L.; Lu, Y.; Zhou, L.; Li, N.; Li, Q.; Zhang, Z. Endophytic Beauveria bassiana promotes plant biomass growth and suppresses pathogen damage by directional recruitment. Front Microbiol. 2023. 16, 1227269. [CrossRef]
- Ozturk, M.; Turkyilmaz, U.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol Plant. 2021, 172, 1321. [Google Scholar] [CrossRef] [PubMed]
- Parrotta, L.; Aloisi, I.; Faleri, C.; Romi, M.; Del Duca, S.; Cai, G. Chronic heat stress affects the photosynthetic apparatus of Solanum lycopersicum L. cv Micro-Tom. Plant physiology and biochemistry. 2020, 154, 463. [Google Scholar] [CrossRef]
- Zuo, H.; Chen, J.; Lv, Z.; Shao, C.; Chen, Z.; Zhou, Y.; Shen, C. Tea-Derived Polyphenols Enhance Drought Resistance of Tea Plants (Camellia sinensis) by Alleviating Jasmonate-Isoleucine Pathway and Flavonoid Metabolism Flow. International journal of molecular sciences. 2024, 25, 3817. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, J.; Liu, W.; Liu, L.; Sheng, Y.; Sun, Y.; Li, Y.; Scheller, H. V.; Jiang, M.; Hou, X.; Ni, L.; Zhang, A. Phosphorylation of a NAC Transcription Factor by a Calcium/Calmodulin-Dependent Protein Kinase Regulates Abscisic Acid-Induced Antioxidant Defense in Maize. Plant physiology. 2016, 171, 1651–1664. [Google Scholar] [CrossRef]
- Ramachandra, R.; Chaitanya, K.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol. 2004, 161, 1189. [Google Scholar]
- Alhaithloul, H. Impact of Combined Heat and Drought Stress on the Potential Growth Responses of the Desert Grass Artemisia sieberi alba: Relation to Biochemical and Molecular Adaptation. Plants (Basel, Switzerland). 2019, 8, 416. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, W.; Sheng, L.; Yang, X.; Mao, H.; Sun, S.; Chen, Z. Integrated transcriptome and metabolomics analyses revealed key functional genes in Canna indica under Cr stress. Scientific reports. 2024, 14, 14090. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, F.; Li, S.; Feng, Y.; Yang, J.; Zhang, N.; Si, H. Calcium-Dependent Protein Kinase 28 Maintains Potato Photosynthesis and Its Tolerance under Water Deficiency and Osmotic Stress. International journal of molecular sciences. 2022, 23, 8795. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Parrotta, L.; Romi, M.; Del, D.; Cai, G. Tomato Biodiversity and Drought Tolerance: A Multilevel Review. Int J Mol Sci. 2023, 24, 10044. [Google Scholar] [CrossRef] [PubMed]
- Kuzhuppillymyal, L.; Tamez, P.; Gomez, R.; Rodriguez, M.; Ek-Ramos, M. Endophytic Beauveria bassiana promotes drought tolerance and early flowering in corn. World J Microbiol Biotechnol. 2020, 36, 47. [Google Scholar] [CrossRef] [PubMed]
- Aimone, C.; Giauque, H.; Hawkes, C. Fungal symbionts generate water-saver and water-spender plant drought strategies via diverse effects on host gene expression. Phytobiomes Journal. 2023, 7, 2471–2906. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Wang, L.; Xie, W.; Song, L.; Zhang, H.; Bi, H.; Zheng, Y.; Zhang, Y.; Zhang, X.; Li, Y.; Lv, Z. Analysis of 22-year Drought Characteristics in Heilongjiang Province Based on Temperature Vegetation Drought Index. Computational intelligence and neuroscience. 2022, 1003243. [Google Scholar] [CrossRef]
- Shah, S.; Tiwari, A.; Song, X.; Talchabahdel, R.; Habiyakare, T.; Adhikari, A. Drought index predictability for historical and future periods across the Southern plain of Nepal Himalaya. Environ Monit Assess. 2022, 194, 642. [Google Scholar] [CrossRef]
- Sun, X.; Xiong, H.; Jiang, C.; Zhang, D.; Yang, Z.; Huang, Y.; Zhu, W.; Ma, S.; Duan, J.; Wang, X.; Liu, W.; Guo, H.; Li, G.; Qi, J.; Liang, C.; Zhang, Z.; Li, J.; Zhang, H.; Han, L.; Zhou, Y.; Peng, Y.; Li, Z. Natural variation of DROT1 confers drought adaptation in upland rice. Nat Commun. 2022, 13, 4265. [Google Scholar] [CrossRef]
- Ren, XL.; Zhang, P.; Chen, X.; Jia, ZK. Impacts of ridge-furrow rainfall concentration systems and mulches on corn growth and yield in the semiarid region of China. Journal of the Science of Food and Agriculture. 2016, 96, 3882–3889. [Google Scholar] [CrossRef]
- Robinson, D.; Jones, S.; Lebron, I.; Reinsch, S.; Domínguez, M.; Smith, A.; Jones, D.; Marshall, M.; Emmett, B. Experimental evidence for drought induced alternative stable states of soil moisture. Scientific Reports. 2016, 6, 20018. [Google Scholar] [CrossRef]
- Wang, J.; Fu, B. Z.; Li, S. X.; Wang, X.; Song, W. X.; Ye, Y. N.; Hu, P. F.; Wang, T. R. Effects of exogenous melatonin on growth and physiological characteristics of Agropyron mongolicum seedlings under drought stress. The journal of applied ecology. 2023, 34, 2947–2957. [Google Scholar]
- Zhao, M.; Li, J.; Shi, X.; Sanaullah, M.; Quan, Y.; Guo, D.; Wang, L.; Wang, S. Effects of exogenous plant regulators on growth and development of "Kyoho" grape under salt alkali stress. Frontiers in Plant Science. 2023, 14, 1274684. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Tang, Y.; Yuan, Y.; Sun, J.; Zhong, M.; Guo, S. The role of 24-epibrassinolide in the regulation of photosynthetic characteristics and nitrogen metabolism of tomato seedlings under a combined low temperature and weak light stress. Plant Physiology Biochemistry. 2016, 107, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Ma, X.; Hussain, Q.; Asim, M.; Iqbal, A.; Ren, X.; Shah, S.; Chen, K.; Shi, Y. Application of 2,4-Epibrassinolide Improves Drought Tolerance in Tobacco through Physiological and Biochemical Mechanisms. Biology (Basel). 2022, 11, 1192. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, Z.; Sun, Y.; Wang, E.; Tian, C.; Sun, Y.; Liu, J.; Sun, C.; Tian, L. Current Studies of the Effects of Drought Stress on Root Exudates and Rhizosphere Microbiomes of Crop Plant Species. Int. J. Mol. Sci. 2022, 23, 2374. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, K.; Sun, H.; Zhu, L.; Zhang, Y.; Wang, G.; Li, A.; Bai, Z.; Liu, L.; Li, C. Root Cortical Senescence Enhances Drought Tolerance in Cotton. Plant, cell & environment 2024. [Google Scholar] [CrossRef]
- Xu, L.; He, J.; Meng, Y.; Zheng, Y.; Lu, B.; Zhang, J.; Zhou, Y. Enhancing drought resistance in Pinus tabuliformis seedlings through root symbiotic fungi inoculation. Frontiers in Plant Science. 2024, 15, 1446437. [Google Scholar] [CrossRef]
- Javed, J.; Rauf, M.; Arif, M.; Hamayun, M.; Gul, H.; Ud-Din, A.; Ud-Din, J.; Sohail, M.; Rahman, M.; Lee, I. Endophytic Fungal Consortia Enhance Basal Drought-Tolerance in Moringa oleifera by Upregulating the Antioxidant Enzyme (APX) through Heat Shock Factors. Antioxidants (Basel). 2022, 11, 1669. [Google Scholar] [CrossRef] [PubMed]
- Santra, H.; Banerjee, D. Production, Optimization, Characterization and Drought Stress Resistance by β-Glucan-Rich Heteropolysaccharide From an Endophytic Fungi Colletotrichum alatae LCS1 Isolated From Clubmoss (Lycopodium clavatum). Front Fungal Biology. 2022, 2, 796010. [Google Scholar] [CrossRef]
- Zhu, S.; Feng, X.; Liu, Y.; Jin, D.; Luo, X.; Fan, Y. Expression of a viral ecdysteroid UDP-glucosyltransferase enhanced the insecticidal activity of the insect pathogenic fungus Beauveria bassiana. Pest Manag Sci. 2024, 80, 4915–4923. [Google Scholar] [CrossRef]
- Basso, V.; Pinheiro, D.; Toldi, M.; Gonçalves, K.; Vicenço, B. Beauveria bassiana submerged spores for control of two-spotted spider mite (Tetranychus urticae Koch (Acari: Tetranychidae)): production, stability, and virulence. Archieves of Microbiology. 2023, 206, 23. [Google Scholar] [CrossRef]
- Aak, A.; Hage, M.; Rukke, B. Biological control of Cimex lectularius with Beauveria bassiana: Effects of substrate, dosage, application strategy, and bed bug physiology. Pest Management Science. 2023, 79, 4599–4606. [Google Scholar] [CrossRef] [PubMed]
- Daza, F.; Roman, G.; Rodriguez, M.; Vargas, I.; Heano, H.; Cereda, M.; Mulet, R. Spores of Beauveria bassiana and Trichoderma lignorum as a bioinsecticide for the control of Atta cephalotes. Biological Research. 2019, 52, 51. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Wang, B. Entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae play roles of maize (Zea mays) growth promoter. Scientific Reports. 2022, 12, 15706. [Google Scholar] [CrossRef] [PubMed]
- Raad, M.; Glare, T.; Brochero, H.; Müller, C.; Rostás, M. Transcriptional Reprogramming of Arabidopsis thaliana DefencePathways by the Entomopathogen Beauveria bassiana Correlates With Resistance Against a Fungal Pathogen but Not Against Insects. Frontiers Microbiology. 2019, 10, 615. [Google Scholar]
- Sui, L.; Lu, Y.; Xu, M.; Liu, J.; Zhao, Y.; Li, Q.; Zhang, Z. Insect hypovirulence-associated mycovirus confers entomopathogenic fungi with enhanced resistance against phytopathogens. Virulence. 2024, 15, 2401978. [Google Scholar] [CrossRef]
- Idrees, A.; Afzal, A.; Qadir, Z.; Li, J. Bioassays of Beauveria bassiana Isolates against the Fall Armyworm, Spodoptera frugiperda. Journal of Fungi(Basel). 2022, 8, 717. [Google Scholar] [CrossRef]
- Sui, L.; Lu, Y.; Zhu, H.; Wan, T.; Li, Q.; Zhang, Z. Endophytic blastospores of Beauveria bassiana provide high resistance against plant disease caused by Botrytis cinerea. Fungal Biol. 2022, 126, 528–533. [Google Scholar] [CrossRef]
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New phytologist. 2007, 175, 81–93. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, X.; Huang, S.; Deng, J.; Li, X.; Luo, Z.; Zhang, Y. Pest management via endophytic colonization of tobacco seedlings by the insect fungal pathogen Beauveria bassiana. Pest Manag Sci. 2021, 77, 2007–2018. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. Journal of experimental botany. 2008, 59, 3317–3325. [Google Scholar] [CrossRef]
- Zu, X.; Lu, Y.; Wang, Q.; et al. Increased Drought Resistance 1 Mutation Increases Drought Tolerance of Upland Rice by Altering Physiological and Morphological Traits and Limiting ROS Levels. Plant Cell Physiol. 2021, 62, 1168–1184. [Google Scholar] [CrossRef] [PubMed]
- Živanović, B.; Milić Komić, S.; Tosti, T.; Vidović, M.; Prokić, L.; Veljović, J. Leaf Soluble Sugars and Free Amino Acids as Important Components of Abscisic Acid-Mediated Drought Response in Tomato Plants. Plants(Basel). 2020, 9, 1147. [Google Scholar]
- Zou, Y. N. , Wu, Q. S., Huang, Y. M., Ni, Q. D., He, X. H. Mycorrhizal-mediated lower proline accumulation in Poncirus trifoliata under water deficit derives from the integration of inhibition of proline synthesis with increase of proline degradation. PloS one. 2013, 8, e80568. [Google Scholar] [CrossRef]
- Gurrieri, L.; Merico, M.; Trost, P.; Forlani, G.; Sparla, F. Impact of Drought on Soluble Sugars and Free Proline Content in Selected Arabidopsis Mutants. Biology (Basel). 2020, 9, 367. [Google Scholar] [CrossRef]
- Pouris, J.; Levizou, E.; Karatassiou, M.; Meletiou-Christou, M.; Rhizopoulou, S. The Influence of the Partitioning of Sugars, Starch, and Free Proline in Various Organs of Cyclamen graecum on the Biology of the Species and Its Resistance to Abiotic Stressors. Plants (Basel.). 2022, 11, 1254. [Google Scholar] [CrossRef]
- Tu, Y.; Jiang, A.; Gan, L.; Hossain, M.; Zhang, J.; Peng, B.; Xiong, Y.; Song, Z.; Cai, D.; Xu, W.; Zhang, J.; He, Y. Genome duplication improves rice root resistance to salt stress. Rice. 2014, 7, 15. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, G.; Bai, X.; Ye, Z.; Zhang, S.; Xie, K.; Sun, F.; Zhang, C.; Xi, Y. Regulatory Network of Preharvest Sprouting Resistance Revealed by Integrative Analysis of mRNA, Noncoding RNA, and DNA Methylation in Wheat. Journal of agricultural and food chemistry. 2021, 69, 4018–4035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, B.; Lin, Q.; Riley, I. .; Ding, S.; Chen, L.; Cui, J.; Yang, L.; Li, H. Colonization of Beauveria bassiana 08F04 in root-zone soil and its biocontrol of cereal cyst nematode (Heterodera filipjevi). PLoS One 2020, 15, e0232770. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Javed, R.; Adeel, M.; Rizwan, M.; Yang, Y. PEG 6000-Stimulated Drought Stress Improves the Attributes of In Vitro Growth, Steviol Glycosides Production, and Antioxidant Activities in Stevia rebaudiana Bertoni. Plants (Basel). 2020, 9, 1552. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, Y.; Li, Z.; Dong, F.; Chen, L. Two grasses differ in their absorptive root physiological traits and rooting depth under drought in an alpine steppe. Annals of botany. 2024, mcae151. [Google Scholar] [CrossRef]
- Jordan, W.; Ritchie, J. Influence of soil water stress on evaporation, root absorption, and internal water status of cotton. Plant Physiol. 1971, 48, 783. [Google Scholar] [CrossRef]
- Vidović, M.; Ćuković, K. Isolation of high-quality RNA from recalcitrant leaves of variegated and resurrection plants. 3 Biotech. 2020, 10, 286. [Google Scholar] [CrossRef]
- Zhou, R.; Song, Y.; Xue, X.; Xue, R.; Jiang, H.; Zhou, Y.; Qi, X.; Wang, Y. Differential Transcription Profiling Reveals the MicroRNAs Involved in Alleviating Damage to Photosynthesis under Drought Stress during the Grain Filling Stage in Wheat. International journal of molecular sciences. 2024, 25, 5518. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jin, M.; Xu, L.; Xi, H.; Wang, B.; Du, S.; Liu, H.; Wen, Y. Effects of ketoprofen on rice seedlings: Insights from photosynthesis, antioxidative stress, gene expression patterns, and integrated biomarker response analysis. Environmental pollution (Barking, Essex : 1987). 2020, 263, 114533. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, J.; Han, Z.; Han, Z.; Li, S.; Zhang, J.; Ma, H.; Han, Y. Screening of differentially expressed microRNAs and target genes in two potato varieties under nitrogen stress. BMC plant biology. 2022, 22, 478. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Meng, C.; Yuan, Y.; Nath, U. K.; Zhao, Y.; Wang, Z.; Yang, S.; Li, L.; Niu, L.; Yao, Q.; Wei, F.; Zhang, X. CaPSY1 gene plays likely the key role in carotenoid metabolism of pepper (Capsicum annuum) at ripening. Functional plant biology : FPB. 2021, 48, 141–155. [Google Scholar] [CrossRef]
- Park, M.; Lee, H.; Kim, I.; Kim, H. Characterization of fab2 T-DNA insertion mutants in terms of fatty acid composition and plant phenotype. Plant signaling & behavior. 2023, 18, 2213937. [Google Scholar]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.). 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, X.; Dong, X.; Liu, Z.; Shi, Z.; Jiang, Y.; Qi, M.; Xu, T.; Li, T. Repression of ARF10 by microRNA160 plays an important role in the mediation of leaf water loss. Plant molecular biology. 2016, 92, 313–336. [Google Scholar] [CrossRef]
- Egea, I.; Albaladejo, I.; Meco, V.; Morales, B.; Sevilla, A.; Bolarin, M.; Flores, F. The drought-tolerant Solanum pennellii regulates leaf water loss and induces genes involved in amino acid and ethylene/jasmonate metabolism under dehydration. Scientific reports. 2018, 8, 2791. [Google Scholar] [CrossRef]
- Bokhale, M.; Mwaba, I.; Allie, F. Real-time PCR data for reference candidate gene selection in tomato infected with Tomato curly stunt virus. Data in Brief. 2020, 31, 105750. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.; Ottosen, C.; Zhou, R. Exogenous Melatonin Alters Stomatal Regulation in Tomato Seedlings Subjected to Combined Heat and Drought Stress through Mechanisms Distinct from ABA Signaling. Plants (Basel). 2023, 12, 1156. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




