Submitted:
15 October 2024
Posted:
16 October 2024
You are already at the latest version
Abstract
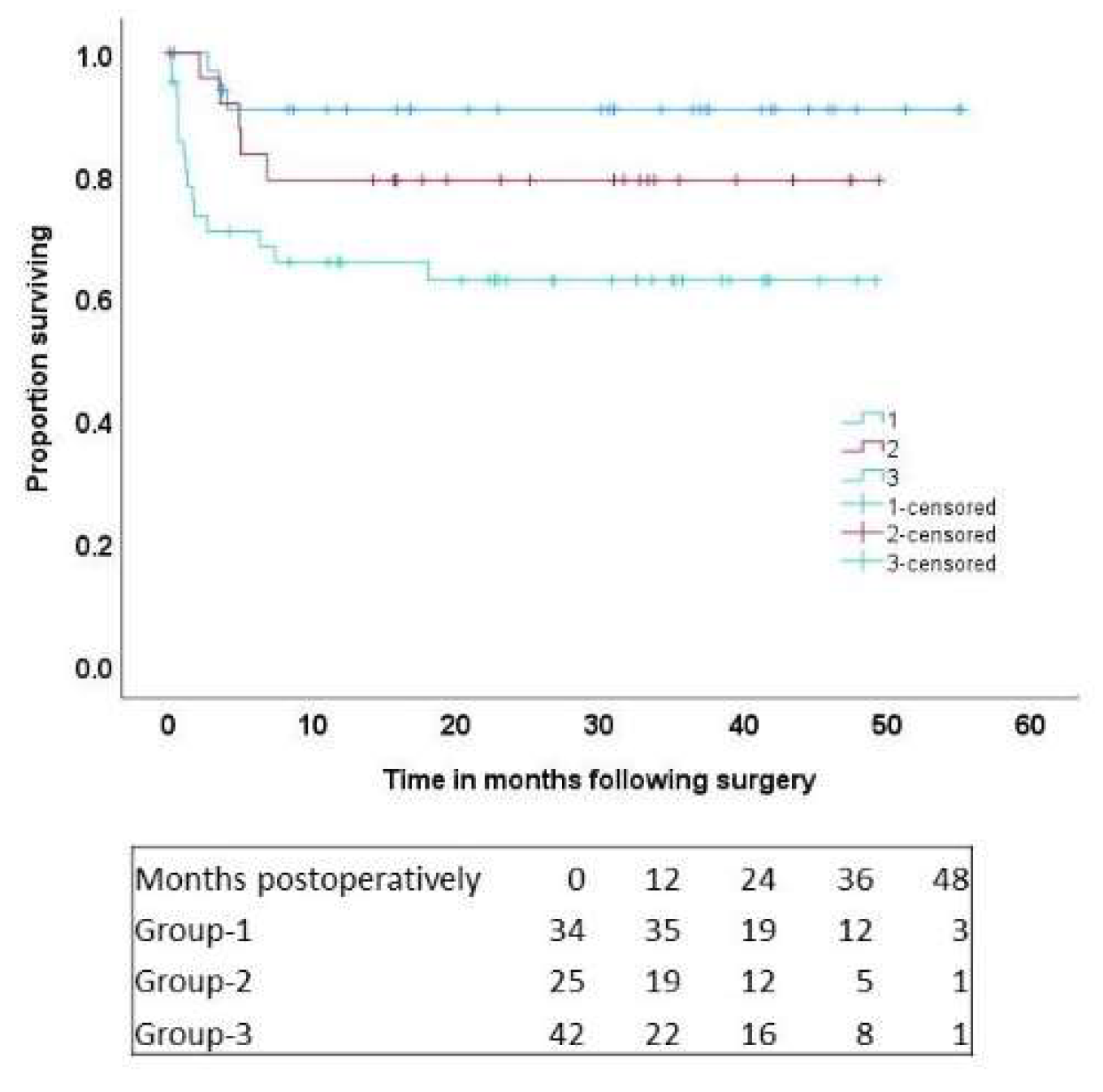
Objectives: Cardiogenic shock [CS] is associated with a high-mortality. Suitable patients maybe successfully bridged using newer intravascular micro-axial left-ventricular assist devices [M-LVAD] for recovery or determination of definitive therapy. Methods: Between January-2020 and July-2024, 107 patients underwent placement of M-LVAD for CS. The cohort was divided into 4 groups based on their destination; group-1: 34 patients [32%] receiving transplant; group-2: 25 patients [23%] receiving durable LVAD; group-3: 42 patients [39%] bridging from post-cardiotomy CS [PCCS]; and group-4: 6 patients [5.6%] bridging decision/recovery [these were excluded from analysis]. Multivariable logistic-regression [MLR] and Cox-regression [MCR] models identified predictors of early-hospital and late mortality, with data reported as odds ratios [ORs], and hazard ratios [HRs], respectively. P<0.05 was statistically significant. Results: Complications included device-malfunction [n=6, 6%], gastrointestinal bleed [n=9, 9%], stroke [n=11, 11%]; long-term hemodialysis [n=21, 21%]. Early-hospital mortality included 13 patients [13%]: 2 in group-1, 1 in group-2 and 10 in group-3 [p=0.02]. In the MLR model, the category of cardiogenic shock requiring M-LVAD placement was statistically significant [OR=4.7 (0.9-24), P=0.05]. Patients were followed for up to 4.5-years, and 23 deaths occurred; group-1: 3 patients, group-2: 5 patients, and group-3: 15 patients [p=0.019]. At 4.5-years, actuarial survival was 90.7±5.1% in group-1, 79.2±8.3% in group-2, and 62.8%±7.7% in group-3 [P=0.01]. In the MCR model, M-LVAD category [HR=3.63 (1.03-12.9) P=0.04], and long-term postoperative dialysis [HR=3.9 (1.6-9) P=0.002], emerged as statistically significant predictors of long-term mortality. Conclusions: In cardiogenic shock, our mid-term outcomes demonstrate good survival with M-LVADs as bridge to transplant and durable LVAD, and reasonable survival as bridge to recovery following cardiotomy, reduced ECMO usage and early ambulation/rehabilitation.
Keywords:
1. Introduction:
2. Methods:
2.1. Technique of M-LVAD Insertion:

3. Results:
3.1. Postoperative Results:
3.2. Predictors of Perioperative Mortality:
3.3. Long-Term Results:
3.4. Predictors of Long-Term Mortality:
3.4.1. Cox Regression:
4. Discussion:
4.1. Limitations:
5. Conclusions
Funding
Conflicts of Interest
References
- Kakuturu J, Dhamija A, Chan E, et al. Mortality and cost of post-cardiotomy extracorporeal support in the United States. Perfusion. 2022 Aug 5;2676591221117355. [CrossRef]
- DeArmas IAS, Holifield L, Janowiak LM, et al. The use of veno-arterial extracorporeal membrane oxygenation in the octogenarian population: A single-center experience. Perfusion. 2022 Jun 29;2676591221111506. [CrossRef]
- Mahesh B, Williams L, Punjabi PP, Katsaridis S. Novel strategy for improved outcomes of extra-corporeal membrane oxygenation as a treatment for refractory post cardiotomy cardiogenic shock in the current era: a refreshing new perspective. Perfusion. 2021 Jun 11:2676591211023304. [CrossRef]
- Ramzy D, Anderson M, Batsides G, et al. Early Outcomes of the First 200 US Patients Treated with Impella-5.5: A Novel Temporary Left Ventricular Assist Device. Innovations (Phila). 2021 Jul-Aug;16(4):365-372. [CrossRef]
- Kleinbaum D, Klein M. Survival Analysis: A Self-Learning Text. 1st ed. New York: Springer; 1996.
- Saha A, Kurlansky P, Ning Y, et al. Early venoarterial extracorporeal membrane oxygenation improves outcomes in post-cardiotomy shock. J Artif Organs 2021;24:7-14.
- Lorusso R, Whitman G, Milojevic M, et al. 2020 EACTS/ELSO/STS/AATS expert consensus on post-cardiotomy extracorporeal life support in adult patients. J Thorac Cardiovasc Surg 2021;161:1287-1331.
- Usman AA, Spelde AE, Olia SE, et al. First-in-man successful use of the SPECTRUM percutaneous dual-stage right ventricle and right atrium to pulmonary artery ventricular assist device. J Card Surg. 2022;37:3403-7.
- Roscoe A, Zochios V. Echocardiography in weaning right ventricular mechanical circulatory support: are we measuring the right stuff? J Cardiothorac Vasc Anesth 2022;36:362-6.
- Ortoleva JP, Alfadhel A, Dalia AA. Invasive hemodynamic and physiological considerations in patients undergoing extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth 2021;35:2549-51.
- Coco VL, Lorusso R, Raffa GM, et al. clinical complications during VA-ECMO in post cardiotomy and non-post cardiotomy shock: Still the Achilles’ heel. J Thorac Dis 2018. [CrossRef]
- Pawale A, Schwartz Y, Itagaki S, et al. Selective implantation of durable left ventricular assist devices as primary therapy for refractory shock. J Thorac Cardiovasc Surg 2018;155:1059-68.
- Soleimani B, Brehm C, Campbell DC, Conte JV. A bridge to many? J Thorac Cardiovasc Surg 2017; 1-2. Invited editorial commentary.
- Tarabichi S, Ikegami H, Russo MJ, Lee LY, Lemaire A. The role of the axillary Impella-5.0 device on patients with acute cardiogenic shock. J Cardiothorac Surg. 2020, 15, 218. [Google Scholar] [CrossRef] [PubMed]
- Gill G, Rowe G, Chen Q, et al. Bridging with surgically placed microaxial left ventricular assist devices: A High-volume center experience. Eur J Cardiothorac Surg 2023, 63, ezad116. [Google Scholar] [CrossRef] [PubMed]
- Schumer ED, Bai Y, Kotkar K, et al. Surgically implanted endovascular, micro axial left ventricular assist device: A single institution study. J thorac Cardiovasc Tech 2024, 23, 63–71. [Google Scholar]
| Variable | Group-1 [n=34] | Group-2 [n=25] | Group-3 [n=42] | P-value |
| Means±SD | Means±SD | Means±SD | ||
| Age [years] | 53±12.4 | 57.5±13 | 63.3±12.4 | 0.002 |
| Body mass index | 28.5±3.9 | 29.1±5.3 | 31.4±6.1 | 0.045 |
| Number [%] | Number [%] | Number [%] | ||
| Female | 2 [5.9] | 3 [12] | 4 [9.5] | 0.69 |
| RVAD | 0 | 5 [20] | 10 [24] | 0.009 |
| ECMO to M-LVAD | 3 [9] | 6 [24] | 11 [26] | 0.043 |
| Group-1 [n=34] | Group-2 [n=25] | Group-3 [n=42] | P-value | |
| Means±SD | Means±SD | Means±SD | ||
| Days on Impella support | 27± 21 | 20± 14 | 14.5± 11 | 0.003 |
| Intensive care unit days | 38.7± 26 | 53±30.5 | 27.8± 25 | 0.002 |
| Number [%] | Number [%] | Number [%] | ||
| Axillary hematoma | 5 [14.7] | 3 [12.5] | 2 [4.8] | 0.32 |
| Device malfunction | 2 [5.9] | 2 [8] | 2 [4.8] | 0.87 |
| Gastrointestinal bleed | 2 [5.9] | 1 [4] | 6 [14.3] | 0.3 |
| Stroke | 1 [2.9] | 2 [8] | 8 [19] | 0.07 |
| Right ventricular assist device | 0 | 5 [20] | 10 [27.8] | 0.004 |
| Dialysis | 4 [11.8] | 5 [20] | 12 [28.6] | 0.195 |
| Hospital mortality | 2 [5.9] | 1 [4] | 10 [23.8] | 0.021 |
| Odds Ratio [OR] | OR 95% confidence intervals | P-value | |
| Right ventricular assist device | 1.3 | 0.28-6 | 0.74 |
| M-LVAD category | 4.7 | 0.9-24 | 0.05 |
| covariate | Hazard Ratios [HR] | HR 95% confidence interval |
p-value |
| M-LVAD category | 3.63 | 1.03-12.9 | 0.04 |
| Postoperative long-term hemodialysis | 3.9 | 1.6-9 | 0.002 |
| Gastrointestinal bleeding | 1.5 | 0.5-4.5 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




