1. Introduction
X-Ray Photoelectron Spectroscopy (XPS) is a powerful spectroscopy to identify and quantify elements on the extreme surface of a material [
1]. However, when heavy elements (fourth period and beyond) are present, much care must be put on the spectral analysis since the number of photoelectron and Auger peaks is large and spectral superpositions may arise. The problem increases if more than one heavy element is present. To understand if a superposition between a photoelectron and an Auger peak exists, changing the incident radiation energy is helpful since, in a binding energy (BE) scale, the photoelectron peak keeps the same position while the Auger peak changes its position. The Auger electrons are ejected with kinetic energies (KE) which are typical of the element (and respective involved electron transitions) and independent of the X-ray energy (E
rad), whereas the photoelectron kinetic energy depends on both the element (and the energy level from which it originates) and the X-radiation energy [
1].
Zinc and iron co-exist in several synthesized or natural systems. As an example of the first ones, we can cite the biocompatible Fe-Zn alloys [
2] or the Ni-Zn-Fe layered double hydroxide as an electrode active material for high-performance supercapacitors [
3], and, as an example of the second type, the sphalerite mineral having the general composition Zn
1-xFe
xS with 0≤x≤0.4 [
4,
5].
In some of the systems where Zn and Fe coexist, the quantitative proportion may be very unbalanced. In those systems, where a huge amount of Zn exists and the amount of iron is just residual, a misidentification of iron may occur.
In this paper, by means of the use of two different X-ray energies – 1486.6 eV, corresponding to the Al Kα emission and 1253.6 eV, corresponding to the Mg Kα one – we show that an Auger structure of Zn rarely reported in most popular databases [
6] exists in the BE range corresponding to Fe 2p photoelectron, when the Al Kα radiation is used.
2. Experimental
2.1. Materials
Sphalerite was acquired to Richard Tayler Minerals (
http://richardtayler.co.uk/) kindly furnished by Zita Martins, Professor at Instituto Superior Técnico. ZnO was ZnO Puratronic purchased to Alfa Aesar, grade 99.9995 % metals basis. An iron containing sample, Fe
4(Fe(CN)
6)
3 was also analysed. Iron (II, III) Hexacyanidoferrate (Prussian blue), ≥30% (ICP), was purchased to Sigma-Aldrich.
2.2. Materials Characterization
XPS was carried out using a Kratos XSAM800 spectrometer (Kratos Analytical Ltd, Manchester, UK) with an X-ray double anode. Both Al Kα and Mg Kα non-monochromatic radiations were used. A pass energy of 20 eV and a power of 120 W (12 kV and 10 mA) were used. Detailed spectra for the most intense regions of the main expected elements (Zn 2p, Fe 2p, S 2p and O 1s), as well as for C 1s detected as the main contaminant element, were fitted with Shirley backgrounds and pseudo-Voigt profiles (Gaussian-Lorentzian products) using the freeware XPSPeak 4.1. Since the samples have an insulating character, the position of the C 1s peak corresponding to the carbonaceous contamination was set to 285 eV for binding energy/kinetic energy correction due to positive charge accumulation. The sensitivity factors applied to the quantitative results were provided by the software Vision 2 for Windows, Version 2.2.9, from KRATOS. The same software was used for data acquisition with a step of 0.1 eV.
3. Results and Discussion
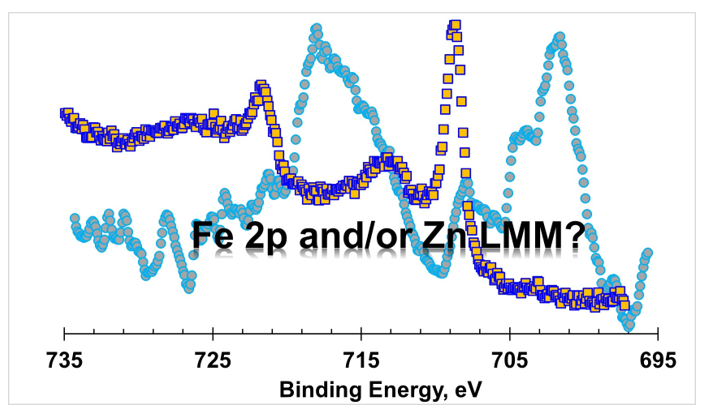
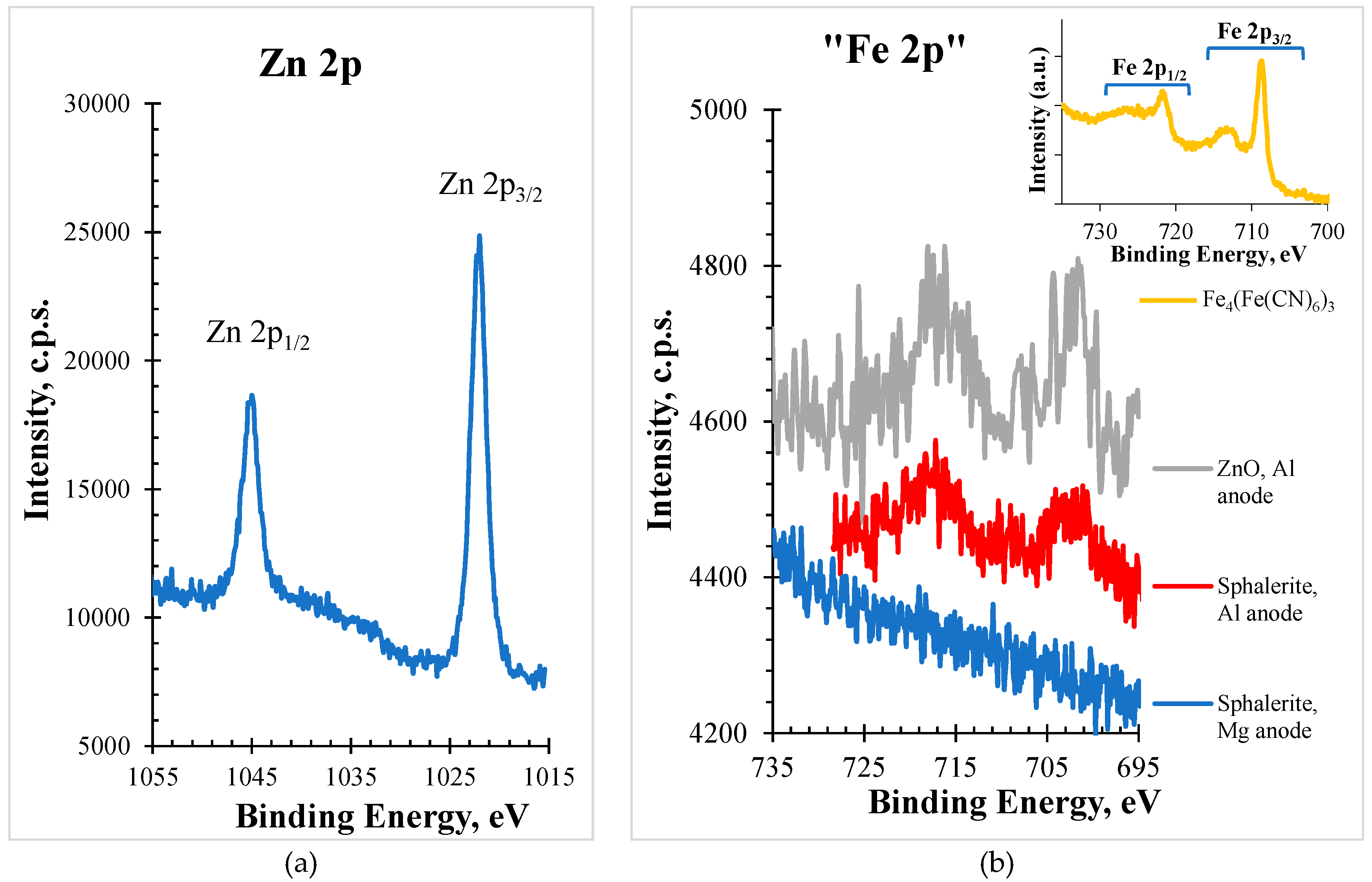
In
Figure 1, XPS spectra for the Zn 2p and Fe 2p regions (red line), acquired with Al anode, of the sphalerite sample are shown. As an insert, in the right upper corner, it is shown a typical Fe 2p region in an iron containing sample, (Fe
4(Fe(CN)
6)
3).
The simple visual inspection of the shape and position of the structure appearing in the binding energy range corresponding to the Fe 2p region allowed to suspect that, at least a part of, the structure was not assignable to the existence of iron in the sample. To assess this suspicion, a region ranging from 695 to 735 eV was extracted from the wide spectrum acquired (also with Al Kα) for a sample which does not contain iron, a ZnO sample. The structure detected is included in
Figure 1 (gray line). Even with a worse statistic, it compares rather well with the sphalerite spectrum (red line).
The best candidate to be responsible for this structure (at least a part of it in the case of sphalerite sample) is a zinc Auger structure. To confirm this assumption, profiting from the fact that the used equipment possesses two different anodes, as described above, the Fe 2p region for the sphalerite sample was also acquired with the Mg Kα source. In a binding energy scale, different incident energies will not affect the photoelectron peak position, provided that the composition of the surface is homogeneous within the probed depth; but an Auger peak will appear at a different position (in this case, with Al and Mg anodes, it will be detected 233 eV apart). As shown in
Figure 1 (blue line), no structure appears confirming that this sphalerite sample has no iron or has an amount less than 0.1% atomic percentage, below the XPS detection limit [
1].
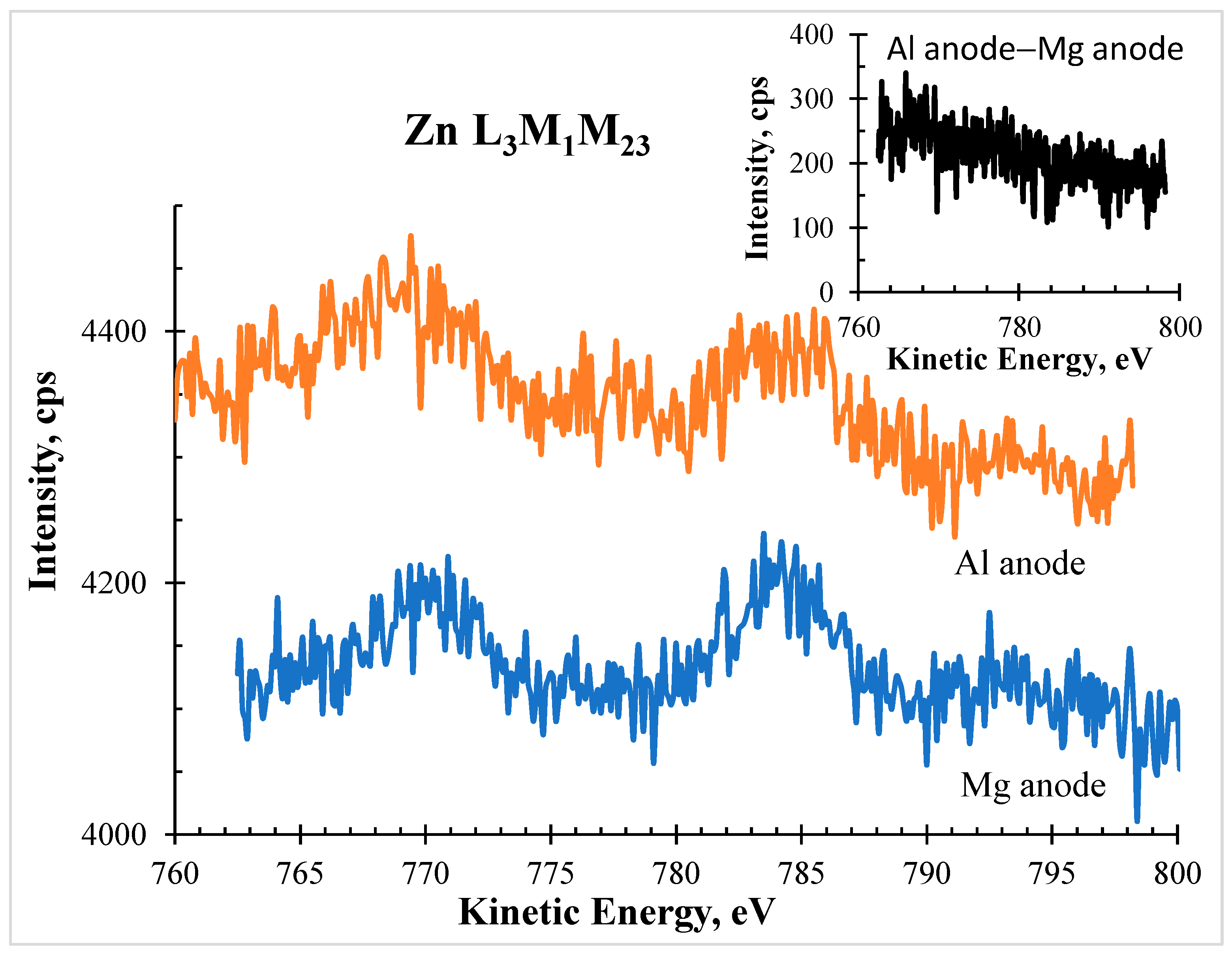
Moreover, to prove that the obtained structure (red line in
Figure 1) was in fact a zinc Auger structure, a detailed spectrum in the same kinetic energy range (from BE=695-233=462 eV to BE=735-233=502 eV, which corresponds to KE = ]751 eV, 791 eV[) was run using the Mg anode.
Figure 2 shows the comparison of the spectra obtained in the corresponding kinetic energy. As an insert, the difference between both is also shown, confirming that they are very similar and that for the Al anode spectrum no detectable photoelectron signal from Fe 2p is contributing.
The next step was the identification of this Auger structure. In common databases, no Auger line for zinc was reported. However, in an old paper [
7] on Auger calculated transition energies listed by energy and element, an Auger L
3M
1M
23 at a kinetic energy (KE) of 777.5 eV is reported. In another paper, a little bit more recent and more detailed, Larkins [
8] reports a computed value of KE=773.7 eV for L
3M
1M
2,
1P
1 and a KE=789.4 eV for the following Auger structures: (L
3M
1M
3,
3P
2), (L
3M
1M
3,
3P
1) and (L
3M
1M
2,
3P
0). They are undoubtedly in the same range of kinetic energies where our spectral features are detected, having the generic identification Zn L
3M
1M
23. The complete match between KEs was not expected because the computed values, besides the approximations made, correspond to pure elements (in the gaseous or solid phases) and, therefore, for salts of the metal it is not expected to have exactly the same structure, as it happens for other metallic elements such as silver [
9], for instance. Anyway, two different salts, the sulphide and the oxide, gave very similar Auger structures as shown in
Figure 1.
Finally, since this short paper is mainly an alert to XPS users, specially for those using the Al Kα radiation, the quantitative analysis is also important. In the case of the sphalerite here studied, the total area of Zn 2p region was 52785 cps.eV and the area of its Auger structure that exists in Fe 2p BE region - as demonstrated above - was 2178 cps.eV. The sensitivity factor for Zn 2p photoelectron, ejected by Al Kα, is 8.384 and the one for Fe 2p is 4.436. In a sample with identical atomic amounts of zinc and iron, the area of Fe 2p is 5.589/2.957=1.89 times lower than the area of the Zn 2p peak. Since the area ratio photoelectron/Auger for Zn is 52785/2178=24.2, the area ratio between the real Fe 2p signal area and the Zn Auger peak area superposed with it will be (52785/1.89)/2178)=12.8. Computing the atomic ratio Fe/Zn, instead of obtaining 1, as it should be, the value obtained would be ((52785/1.89+2178)×1.89)/ 52785=1.078. Therefore, even in a sample where zinc and iron coexist in the same atomic amount, the quantitative error can be up to around 8%. Therefore, the impact on the quantitative analysis may be important when in (Zn,Fe)-based samples the Zn is the major component. The extreme case is the one where no iron exists but could exist, as it is the case in the sphalerite analysed here. In the literature, making a non-exhaustive search, immediately a paper is found where the displayed Fe 2p region does not correspond at all to the profile of a Fe 2p region [
10]. Since the referred paper deals with the synthesis of an organic/inorganic hybrid composite catalyst by coupling Fe
3O
4@MIL-100 (Fe) (FM) with ZnS nanoparticles, it is obvious that the assignments and conclusions reached in the paper are very doubtful, to say the least. Specially a Fe 2p
3/2 peak at 701.5 eV is not reported anywhere else.
4. Conclusions
The XPS analysis of a sphalerite sample using two different X-ray radiations – Al Kα and Mg Kα – showed that a spectral feature appearing in the binding energy range corresponding to the Fe 2p region when the Al Kα is used, disappears when the Mg Kα radiation is used. The study of the spectral region corresponding to the same kinetic energy range in a spectrum acquired with Mg Kα radiation proved that the obtained feature corresponds to the Zn L3M1M23 Auger structure. Therefore, when analysing a sample where the coexistence of zinc and iron is expected, it is preferable to avoid the use of the Al Kα radiation. If this cannot be accomplished, anyway, much care should be used to analyse data both from the point of view of assignments and quantitative analysis. The analysis of other iron regions (2s, 3s or 3p), although much less intense than Fe 2p and for that reason much harder to detect when in residual amounts, could help to confirm or discard its presence, bearing in mind that other superpositions may also exist in these regions.
Author Contributions
Conceptualization, A.M.d.R. and A.M.F.; Methodology, A.M.d.R. and A.M.F.; Validation, A.M.d.R. and A.M.F.; Formal Analysis, A.M.d.R. and A.M.F.; Investigation, A.M.d.R. and A.M.F.; Data Curation, A.M.d.R. and A.M.F.; Writing – Original Draft Preparation, A.M.d.R. and A.M.F.; Writing – Review & Editing, A.M.d.R. and A.M.F.
Acknowledgments
This work was funded by FCT (Portugal) - Fundação para a Ciência e a Tecnologia, I.P. - through projects UIDB/04565/2020 and UIDP/04565/2020 of iBB and the project LA/P/0140/2020 of i4HB. A.M.F. wishes to thank IST-ID for the Scientific Employment contract IST-ID/131/2018. The authors also acknowledge Professor Zita Martins (University of Lisbon) for providing the Sphalerite which triggered this study.
Conflicts of Interest
No conflicts of interest to declare.
References
- Surface Analysis by Auger and X-Ray Photoelectron Spectroscopy, Ed. by D. Briggs and J.T. Grant, IMPublications and SurfaceSpectra Limited, West Sussex and Manchester, UK, 2003.
- H. Kabir, K. Munir, C. Wen, Y. Li, Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: Biomechanical and biocorrosion perspectives, Bioactive Materials 6(3) (2021) 836-879. [CrossRef]
- Amr. Elgendy, N.M. El Basiony, F. El-Taib Heakal, A.E. Elkholy, Mesoporous Ni-Zn-Fe layered double hydroxide as an efficient binder-free electrode active material for high-performance supercapacitors, Journal of Power Sources 466 (2020) 228294. [CrossRef]
- D.J. Vaughan, U. Becker, K. Wright, Sulphide mineral surfaces: theory and experiment, Int. J. Miner. Process. 51 (1997) 1-14. [CrossRef]
- S.L. Harmer, L.V. Goncharova, R. Kolarova, W.N. Lennard, M.A. Muñoz-Márquez, I.V. Mitchell, H.W. Nesbitt, Surface structure of sphalerite studied by medium energy ion scattering and XPS, Surface Science 601 (2007) 352–361. [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database Number 20, National Institute of Standards and Technology, Gaithersburg MD, 20899 (2000). [CrossRef]
- W. Goghlan, R.E. Clausing, Auger catalog calculated transition energies listed by energy and element, Atomic Data and Nuclear Data Tables, 5(4) (1973) 317-469. Page 342. [CrossRef]
- F.P. Larkins, Semiempirical Auger-electron energies for elements 10 ≤ Z ≤ 100, Atomic Data and Nuclear Data Tables 20 (4) (1977) 311-387. Page 345. [CrossRef]
- A.M. Ferraria, A.P. Carapeto, A.M. Botelho do Rego X-ray photoelectron spectroscopy: Silver salts revisited, Vacuum, 86 (2012) 1988-1991. [CrossRef]
- K. Zhang, J. Zhang, X. He, Y. Zhao, A. Zada, A. Peng, K. Qi, Fe3O4@MIL-100(Fe) modified ZnS nanoparticles with enhanced sonocatalytic degradation of tetracycline antibiotic in water, Ultrasonics Sonochemistry 95 (2023) 106409. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).