Submitted:
15 October 2024
Posted:
16 October 2024
You are already at the latest version
Abstract
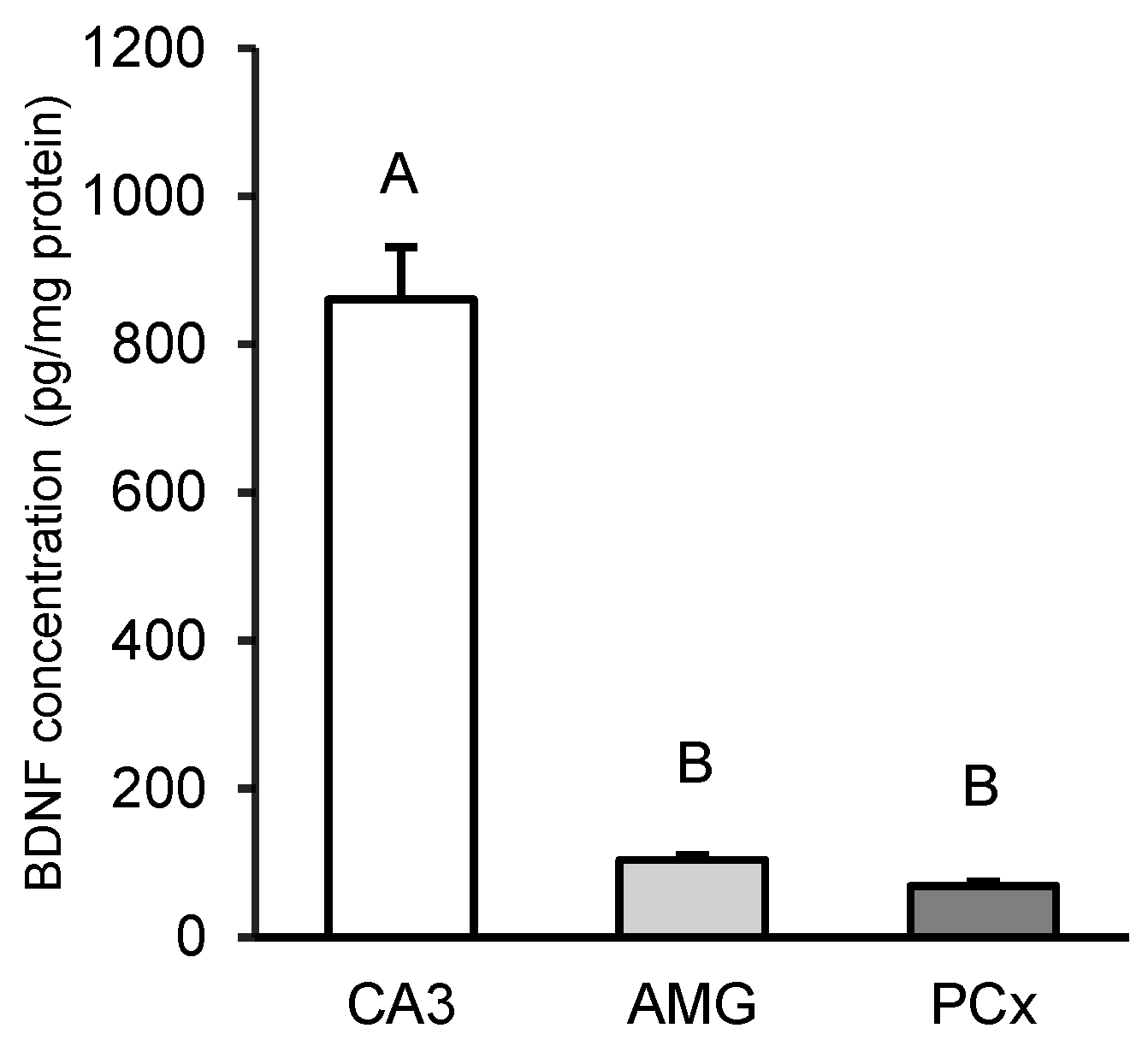
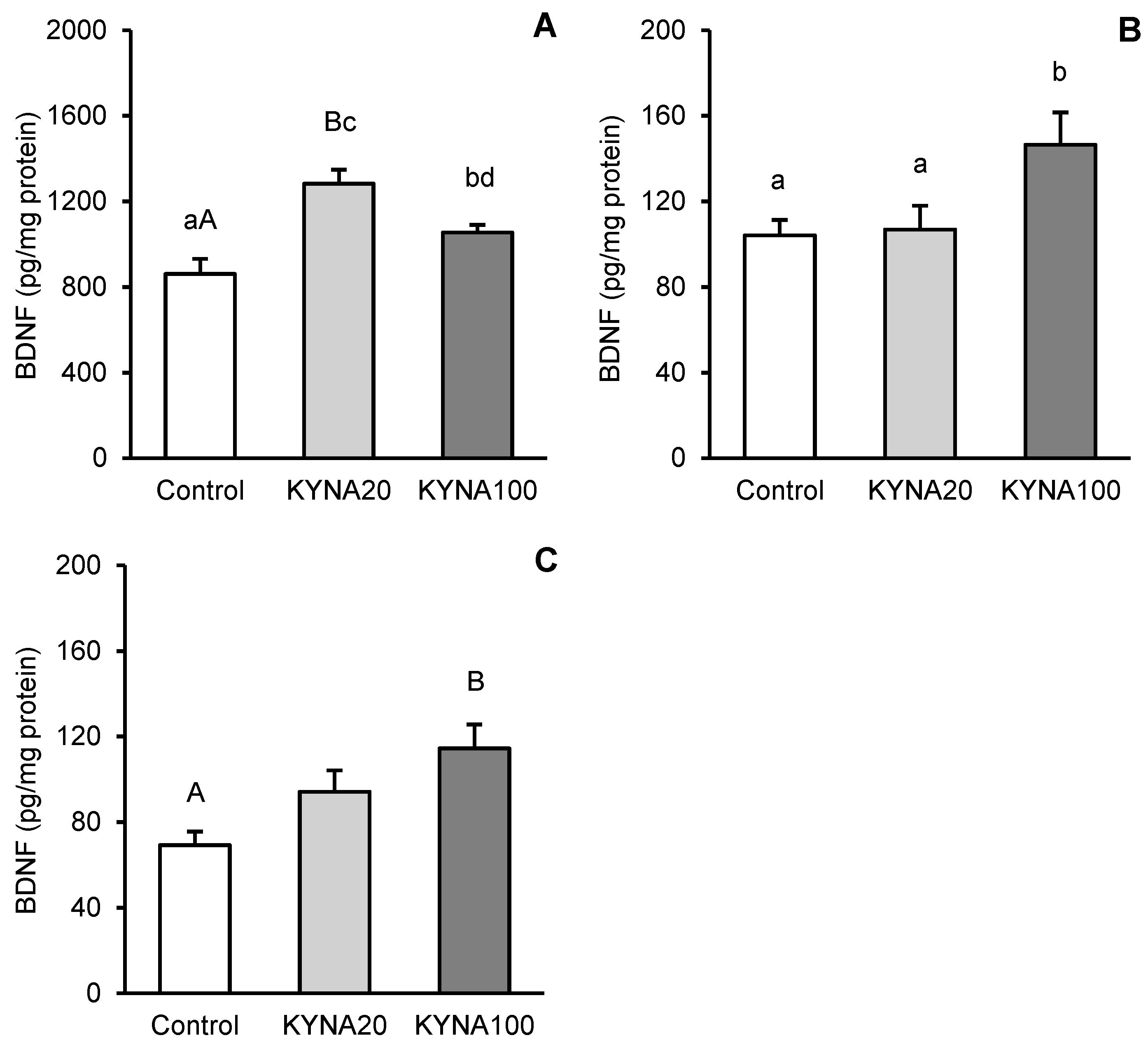
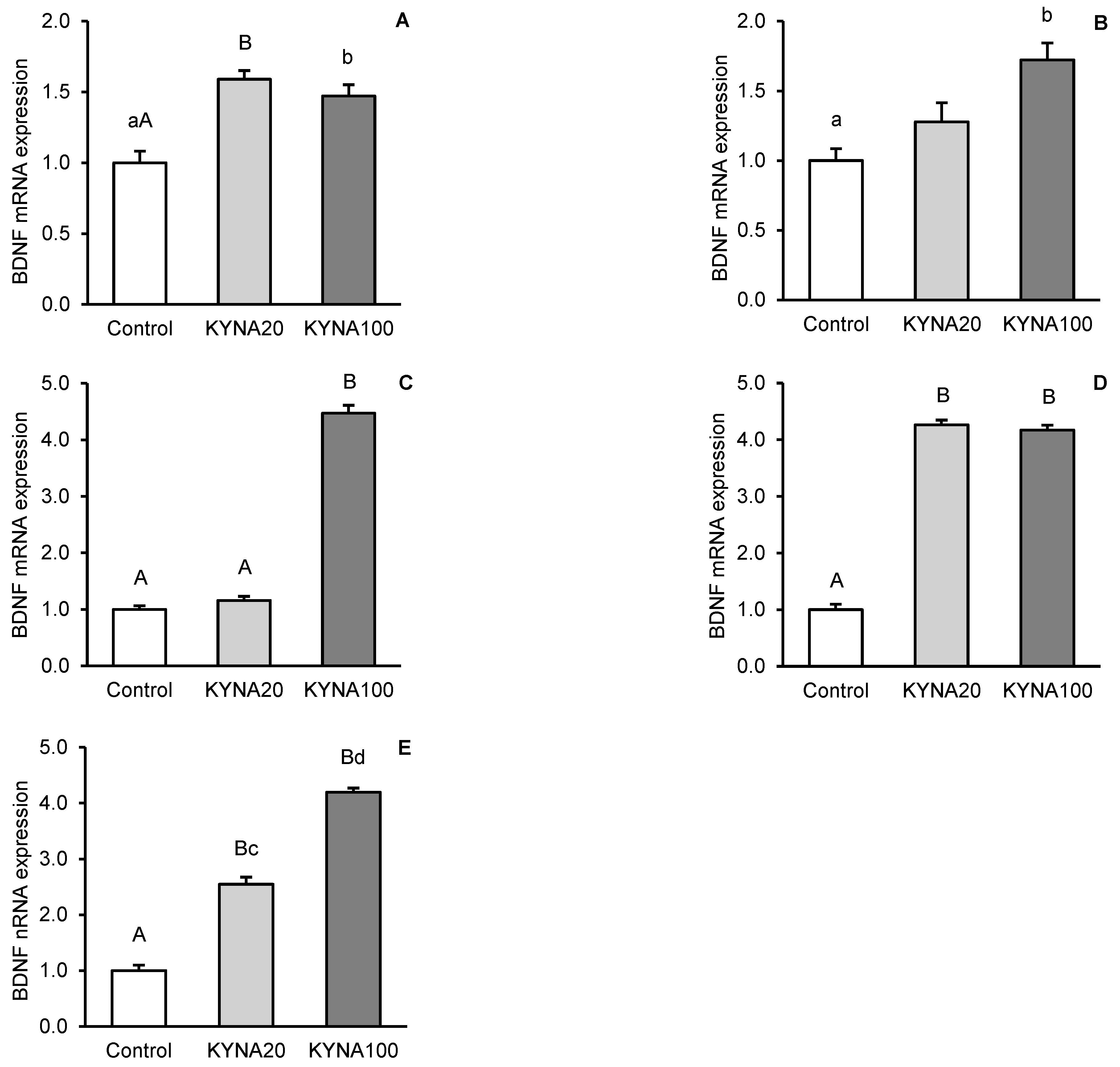
Fluctuations in kynurenic acid (KYNA) and brain-derived neurotrophic factor (BDNF) levels in the brain reflect its neurological status. The aim of the study was to investigate the effect of transiently elevated KYNA concentration in the cerebroventricular circulation on the expression of BDNF and its high-affinity tropomyosin-related kinase receptor B (TrkB) in specific structures of the sheep brain. Intracerebroventricularly cannulated anestrous sheep were subjected to a series of four 30-min infusions of KYNA: 4×5 μg/60 μL/30 min (KYNA20, n=6) and 4×25 μg/60 μL/30 min (KYNA100, n=6) or a control infusion (n=6), at 30-min intervals. Sections of the hippocampal CA3 field, amygdala (AMG), prefrontal cortex (PCx), and the hypothalamic medial-basal (MBH) and preoptic (POA) areas were dissected from the brain immediately after the experiment. The highest concentration of BDNF protein was found in the CA3 field (P<0.01), which was 8-fold higher than in the AMG and 12-fold higher than that in the PCx (MBH and POA were not analyzed). The most pronounced BDNF mRNA expression was observed in the MBH followed by the PCx, POA, AMG and CA3, while the highest abundance of TrkB mRNA was recorded in the AMG followed by the MBH, PCx, CA3 and POA. KYNA increased (P<0.05-P<0.01) BDNF protein levels and the expression of its gene in the brain structures examined, with the effect varying by dose and brain region. KYNA, particularly at the KYNA100 dose, also increased (P<0.01) TrkB gene expression, except for the AMG, where the lower KYNA20 dose was more effective (P<0.01). These findings suggest a positive relationships between KYNA levels in the cerebroventricular circulation and BDNF-TrkB expression in specific brain regions in a sheep model. This indicates that a transient increase in CSF KYNA concentration can potentially restore BDNF production, whose deficiency underlies numerous neurological disorders.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animal Management
2.2. Third Ventricle (IIIv) Cannulation
2.3. Experimental Design and Tissue Collection
2.3. Tissue BDNF Concentration Assay
2.4. Relative mRNA Abundance
2.5. Statistical Analysis
| GENE | PRIMERS (5’ – 3’) | GENBANK ACC. NO. |
AMPLICON SIZE |
|---|---|---|---|
| BDNF | F: CGTTGGCTGACACTTTTGAA R: CGCAGCATCCAGGTAATTTT |
XM_012143442.1 | 188 |
| TRKB | F: TGTCTGAGCTGATCCTGGTG R: TATCTGCAGGTTTGCCAGTG |
XM_012117231.2 | 155 |
| GAPDH | F: GGGTCATCATCTCTGCACCT R: GGTCATAAGTCCCTCCACGA |
NM_001190390.1 | 131 |
| PPIC | F: TGGAAAAGTCGTGCCCAAGA R: TGCTTATACCACCAGTGCCA |
XM_004008676.1 | 158 |
3. Results
3.1. Tissue BDNF Concentration
3.2. Relative Abundance of BDNF and TrkB mRNA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Ethical Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bothwell, M. NGF, BDNF, NT3, and NT4. Handb. Exp. Pharmacol. 2014, 220, 3–15. [Google Scholar] [PubMed]
- Gibon, J.; Barker, P.A. Neurotrophins and proneurotrophins: Focus on synaptic activity and plasticity in the brain. Neuroscientist 2017, 23, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Lin, C.C.; Huang, T.L. Brain-derived neurotrophic factor and mental disorders. Biomed. J. 2020, 43, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Lledo, P.M.; Alonso, M.; Grubb, M.S. Adult neurogenesis and functional plasticity in neuronal circuits. Nature Rev. Neurosci. 2006, 7, 179–193. [Google Scholar] [CrossRef]
- Vilar, M.; Mira, H. Regulation of neurogenesis by neurotrophins during adulthood: Expected and unexpected roles. Front. Neurosci. 2016, 10, 26. [Google Scholar] [CrossRef]
- Gould, E. How widespread is adult neurogenesis in mammals? Nat. Rev. Neurosci. 2007, 8, 481–488. [Google Scholar] [CrossRef]
- Lee, D.A.; Blackshaw, S. Functional implications of hypothalamic neurogenesis in the adult mammalian brain. Int. J. Devel. Neurosci. 2012, 30, 615–621. [Google Scholar] [CrossRef]
- Cameron, H.A.; Glover, L.R. Adult neurogenesis: Beyond learning and memory. Ann. Rev. Psychol. 2015, 66, 53–81. [Google Scholar] [CrossRef]
- McEwen, B.S.; Nasca, C.; Gray, J.D. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacol. Rev. 2016, 41, 3–23. [Google Scholar] [CrossRef]
- Pawluski, J.L.; Lambert, K.G.; Kinsley, C.H. Neuroplasticity in the maternal hippocampus: Relation to cognition and effects of repeated stress. Horm. Behav. 2016, 77, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Forlenza, O.V.; Miranda, A.S.; Guimar, I.; Talib, L.L.; Diniz, B.S.; Gattaz, W.F.; Teixeira, A.L. Decreased neurotrophic support is associated with cognitive decline in non-demented subjects. J. Alzheimers Dis. 2015, 46, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Katoh-Semba, R.; Takeuchi, I.K.; Semba, R.; Kato, K. Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brain. J. Neurochem. 1997, 69, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Dieni, S.; Matsumoto, T.; Dekkers, M.; Rauskolb, S.; Ionescu, M.S.; Deogracias, R.; Gundelfinger, E.D.; Kojima, M.; Nestel, S.; Frotscher, M.; Barde, Y.A. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J. Cell. Biol. 2012, 196, 775–788. [Google Scholar] [CrossRef]
- Edelmann, E.; Lessmann, V.; Brigadski, T. Pre- and postsynaptic twists in BDNF secretion and action in synaptic plasticity. Neuropharmacology 2014, 76 Pt C, 610–627. [Google Scholar] [CrossRef]
- Hempstead, B. Brain-derived neurotrophic factor: three ligands, many actions. Trans. Am. Clin. Climatol. Assoc. 2015, 126, 9. [Google Scholar]
- Adachi, N.; Numakawa, T.; Richards, M.; Nakajima, S.; Kunugi, H. New insight in expression, transport, and secretion of brain-derived neurotrophic factor: Implications in brain-related diseases. World J. Biol. Chem. 2014, 5, 409–428. [Google Scholar] [CrossRef]
- Diniz, B.S.; Teixeira, A.L. Brain-derived neurotrophic factor and Alzheimer’s disease: Physiopathology and beyond. Neuromol. Med. 2011, 13, 217–222. [Google Scholar] [CrossRef]
- Zuccato, C.; Marullo, M.; Conforti, P.; MacDonald, M.E.; Tartari, M.; Cattaneo, E. Systematic assessment of BDNF and its receptor levels in human cortices affected by Huntington’s disease. Brain Pathol. 2008, 18, 225–238. [Google Scholar] [CrossRef]
- Angelucci, F.; Brenè, S.; Mathé, A.A. BDNF in schizophrenia, depression and corresponding animal models. Mol. Psychiat. 2005, 10, 345–352. [Google Scholar] [CrossRef]
- Cannazza, G.; Chiarugi., A.; Parenti, C.; Zanoli, P.; Baraldi, M. Changes in kynurenic, anthranilic, and quinolinic acid concentrations in rat brain tissue during development. Neurochem. Res. 2001, 26, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Moroni, F.; Cozzi, A.; Sili, M.; Mannaioni, G. Kynurenic acid: a metabolite with multiple actions and multiple targets in brain and periphery. J. Neural Transm. 2012, 119, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Vamos, E.; Pardutz, A.; Klivenyi, P.; Toldi, J.; Vecsei, L. The role of kynurenines in disorders of the central nervous system: Possibilities for neuroprotection. J. Neurol. Sci. 2009, 283, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kozak, R.; Campbell, B.M.; Strick, C.A.; Horner, W.; Hoffmann, W.E.; Kiss, T.; Chapin, D.S.; McGinnis, D.; Abbott, A.L.; Roberts, B.M.; Fonseca, K.; Guanowsky, V.; Young, D.A.; Seymour, P.A.; Dounay, A.; Hajos, M.; Williams, G.V.; Castner, S.A. Reduction of brain kynurenic acid improves cognitive function. J. Neurosci. 2014, 34, 10592–10602. [Google Scholar] [CrossRef]
- Stone, T.W. Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends Pharmacol. Sci. 2000, 21, 149–154. [Google Scholar] [CrossRef]
- Strzetelski, J. IZ PIB–INRA Feeding recommendations for ruminants and feed tables; Kraków, Poland, 2014. (in Polish) [Google Scholar]
- Welento, J.; Szteyn, S.; Milart, Z. Observations on the stereotaxic configuration of the hypothalamus nuclei in the sheep. Anat. Anz. 1969, 124, 1–27. [Google Scholar]
- Traczyk, W.; Przekop, F. Methods of investigation of the function of the hypothalamus and hypophysis in chronic experiments in sheep. Acta Physiol. Pol. 1963, 14, 227–236. [Google Scholar]
- Misztal, T.; Kowalczyk, P.; Młotkowska, P.; Marciniak, E. The effect of allopregnanolone on enzymatic activity of the DNA base excision repair pathway in the sheep hippocampus and amygdala under natural and stressful conditions. Int. J. Mol. Sci. 2020, 21, 7762. [Google Scholar] [CrossRef] [PubMed]
- Roszkowicz-Ostrowska, K.; Młotkowska, P.; Kowalczyk, P.; Marciniak, E.; Barszcz, M.; Misztal, T. Central stimulatory effect of kynurenic acid on BDNF-TrkB signaling and BER enzymatic activity in the hippocampal CA1 field in sheep. Int. J. Mol. Sci. 2023, 24, 136. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, 36. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper – Excel-based tool using pairwise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Veening, J.G.; Barendregt, H.P. The regulation of brain states by neuroactive substances distributed via the cerebrospinal fluid; a review. Cerebrospin. Fluid Res. 2010, 7, 1. [Google Scholar] [CrossRef]
- Yang, L.; Kress, B.T.; Weber, H.J.; Thiyagarajan, M.; Wang, B.; Deane, R.; Benveniste, H.; Iliff, J.J.; Nedergaard, M. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J. Transl. Med. 2013, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Conner, J.M.; Lauterborn, J.C.; Yan, Q.; Gall, C.M.; Varon, S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J. Neurosci. 1997, 17, 2295–2313. [Google Scholar] [CrossRef]
- Yan, Q.; Rosenfeld, R.D.; Matheson, C.R.; Hawkins, N.; Lopez, O.T.; Bennett, L.; Welcher, A.A. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience 1997, 78, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Griego, E.; Galván, E.J. BDNF and Lactate as Modulators of Hippocampal CA3 Network Physiology. Cell. Mol. Neurobiol. 2023, 43, 4007–4022. [Google Scholar] [CrossRef]
- Cherubini, E.; Miles, R. ; The CA3 region of the hippocampus: how is it? What is it for? How does it do it? Front. Cell. Neurosci. 2015, 9, 19. [Google Scholar] [CrossRef]
- Ji, Y.; Lu, Y.; Yang, F.; Shen, W.; Tang, T.T.; Feng, L.; Duan, S.; Lu, B. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat. Neurosci. 2010, 13, 302–309. [Google Scholar] [CrossRef]
- Cheng, P.L.; Song, A.H.; Wong, Y.H.; Wang, S.; Zhang, X.; Poo, M.M. Self-amplifying autocrine actions of BDNF in axon development. Proc. Natl. Acad. Sci. USA 2011, 108, 18430–18435. [Google Scholar] [CrossRef]
- Shelly, M.; Cancedda, L.; Lim, B.K.; Popescu, A.T.; Cheng, P.L.; Gao, H.; Poo, M.M. Semaphorin3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth. Neuron 2011, 71, 433–446. [Google Scholar] [CrossRef]
- Leal, G.; Comprido, D.; Duarte, C.B. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 2014, 76 Pt C, 639–656. [Google Scholar] [CrossRef]
- Fernandez-Garcia, S.; Sancho-Balsells, A.; Longueville, S.; Herve, D.; Gruart, A.; Delgado-Garcia, J.M.; Alberch, J.; Giralt, A. Astrocytic BDNF and TrkB regulate severity and neuronal activity in mouse models of temporal lobe epilepsy. Cell Death Dis. 2020, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Migaud, M.; Batailler, M.; Segura, S.; Duittoz, A.; Franceschini, I.; Pillon, D. Emerging new sites for adult neurogenesis in the mammalian brain: a comparative study between the hypothalamus and the classical neurogenic zones. Europ. J. Neurosci. 2010, 32, 2042–2052. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.J.; Amygdala. Encyclopedia of the Neurological Sciences, Second ed.; Aminoff, M.J., Daroff, R.B., Eds.; Academic Press, 2014; pp. 153–156. [Google Scholar]
- Meis, S.; Endres, T.; Lessmann, V. Neurotrophin signalling in amygdala-dependent cued fear learning. Cell Tissue Res. 2020, 382, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.P.; Robbins, T.W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Gorka, S.M.; Teppen, T.; Radoman, M.; Phan, K.L.; Pandey, S.C. Human plasma BDNF is associated with amygdala-prefrontal cortex functional connectivity and problem drinking behaviors. Internat. J. Neuropsychopharmacol. 2020, 23, 1–11. [Google Scholar] [CrossRef]
- Roussel, S.; Hemsworth, P.H.; Leruste, H.; White, C.; Duvaux-Ponter, C.; Nowak, R.; Boissy, A. Repeated transport and isolation during pregnancy in ewes: effects on the reactivity to humans and to their offspring after lambing. Appl. Anim. Behav. Sci. 2006, 97, 172–189. [Google Scholar] [CrossRef]
- Coulon, M.; Wellman, C.L.; Marjara, I.S.; Janczak, A.M.; Zanella, A.J. Early adverse experience alters dendritic spine density and gene expression in prefrontal cortex and hippocampus in lambs. Psychoneuroendocrinology 2013, 38, 1112–1121. [Google Scholar] [CrossRef]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006, 59, 1116–1127. [Google Scholar] [CrossRef]
- Tripp, A.; Oh, H.; Guilloux, J.P.; Martinowich, K.; Lewis, D.A.; Sibille, E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder, Am. J. Psychiatry 2012, 169, 1194–1202. [Google Scholar]
- Hashimoto, T.; Bergen, S.E.; Nguyen, Q.L.; Xu, B.; Monteggia, L.M.; Pierri, J.N.; Sun, Z.; Sampson, A.R.; Lewis, D.A. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J. Neurosci. 2005, 25, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Forrest, C.M.; Khalil, O.S.; Pisar, M.; Darlington, L.G.; Stone, T.W. Prenatal inhibition of the tryptophan–kynurenine pathway alters synaptic plasticity and protein expression in the rat hippocampus. Brain Res. 2013, 1504, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, C.F.; Chen, X.M.; Chen, S.M.; Mu, R.H.; Liu, B.B.; Luo, L.; Liu, X.L.; Geng, D.; Liu, Q.; Yi, L.T. Activation of hippocampal BDNF signaling is involved in the antidepressant-like effect of the NMDA receptor antagonist 7-chlorokynurenicacid. Brain Res. 2016, 1630, 73–82. [Google Scholar] [CrossRef]
- Chojnacki, C.; Gąsiorowska, A.; Popławski, T.; Konrad, P.; Chojnacki, M.; Fila, M.; Blasiak, J. Beneficial effect of increased tryptophan intake on its metabolism and mental state of the elderly. Nutrients 2023, 15, 847. [Google Scholar] [CrossRef]
- Potter, M.C.; Elmer, G.I.; Bergeron, R.; Albuquerque, E.X.; Guidetti, P.; Wu, H.Q.; Schwarcz, R. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology 2010, 35, 1734–1742. [Google Scholar] [CrossRef]
- DeAngeli, N.E.; Todd, T.P.; Chang, S.E.; Yeh, H.H.; Yeh, P.W.; Bucci, D.J. Exposure to kynurenic acid during adolescence increases sign-tracking and impairs long-term potentiation in adulthood. Front. Behav. Neurosci. 2015, 8, 451. [Google Scholar] [CrossRef]
- Lebrun, B.; Bariohay, B.; Moyse, E.; Jean, A. Brain-derived neurotrophic factor (BDNF) and food intake regulation: A minireview. Auton. Neurosci. 2006, 126-127, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Ameroso, D.; Meng, A.; Chen, S.; Felsted, J.; Dulla, S.G.; Rios, M. Astrocytic BDNF signaling within the ventromedial hypothalamus regulates energy homeostasis. Nat. Metab. 2022, 4, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Parent, A.D.; Perkins, E. The hypothalamus. In Fundamental Neuroscience for Basic and Clinical Applications, 5th Edition; Haines, D.E., Mihailoff, G.A., Eds.; Elsevier: Philadelphia, PA, 2018; pp. 442–456. [Google Scholar]
- Tsuneoka, Y. Molecular neuroanatomy of the mouse medial preoptic area with reference to parental behavior. Anat. Sci. Int. 2019, 94, 39–52. [Google Scholar] [CrossRef]
- Przybył, B.J.; Szlis, M.; Wójcik-Gładysz, A. Brain-derived neurotrophic factor (BDNF) affects the activity of the gonadotrophic axis in sheep. Horm. Behav. 2021, 131, 104980. [Google Scholar] [CrossRef]
- Demitrack, M.A.; Heyes, M.P.; Altemus, M.; Pigott, T.A.; Gold, P.W. Cerebrospinal fluid levels of kynurenine pathway metabolites in patients with eating disorders: relation to clinical and biochemical variable. Biol. Psychiatry 1995, 37, 512–20. [Google Scholar] [CrossRef] [PubMed]
- Elmas, O.; Cenik, P.; Sirinyildiz, F.; Elmas, S.; Sirin, F.B.; Cesur, G. Relationship between cognitive functions, levels of NR2A and NR2B subunits of hippocampal NMDA receptors, serum TGF-β1 level, and oxidative stress in rats fed a high-fat diet. J. Anim. Feed Sci. 2022, 31, 318–327. [Google Scholar] [CrossRef]
- Kokoeva, M.V.; Yin, H.; Flier, J.S. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J. Comp. Neurol. 2007, 505, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Meiri, N. Brain-derived neurotrophic factor is critically involved in thermal-experience-dependent developmental plasticity. J. Neurosci. 2006, 26, 3899–3907. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.M.; Rabin, S.J.; Lipsky, R.H.; Mocchetti, I. Activity-dependent release of brain-derived neurotrophic factor underlies the neuroprotective effect of N-methyl-D-aspartate. J. Biol. Chem. 1998, 273, 29394–29399. [Google Scholar] [CrossRef]
- Autry, A.; Adachi, M.; Nosyreva, E.; Na, E.S.; Los, M.F.; Cheng, P.; Kavalali, E.T.; Monteggia, L.M. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011, 475, 91–95. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, N.; Yang, C.; Li, X.M.; Zhou, Z.; Yang, J.J. Ketamine-induced antidepressant effects are AMPA receptors mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur. Psychiatry 2014, 29, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.E.; Goodman, J.H. Effects of central and peripheral administration of kynurenic acid on hippocampal evoked responses in vivo and in vitro. Neuroscience 1998, 86, 751–764. [Google Scholar] [CrossRef]
- Heyes, M.P.; Quearry, B.J. Quantification of kynurenic acid in cerebrospinal fluid: effects of systemic and central L-kynurenine administration. J. Chromatogr. 1990, 530, 108–111. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





