1. Introduction
The rhythmic flow of spoken English is shaped by the patterned distribution of lexical stress, the relative prominence of syllables within words. By emphasizing specific syllables with greater intensity, length, and pitch, this stress pattern forms a metrical structure that guides speech segmentation and comprehension. In English, lexical stress is unevenly distributed across content words, with a strong bias towards first-syllable stress (85-90%)[
1]. Through statistical learning, this uneven distribution is leveraged by English-learning infants who show a preference for words with first-syllable stress and acquire them earlier in language development than words containing second-syllable stress [
2,
3,
4]. The location of stressed syllables serves as a powerful cue for word segmentation in the continuous stream of speech. Both infants and adults use this cue to identify word boundaries, thereby facilitating word learning [
5,
6,
7,
8]. Furthermore, the ability to perceive, understand, and reproduce the patterns of stress in spoken language (i.e., speech rhythm awareness) may facilitate the acquisition of essential reading skills by acting as a bridge between oral language and written language [
9].
While English exhibits a rhythmic pattern that tends towards isochrony, where the timing between stressed syllables is equal [
10,
11], it does not rigidly adhere to this principle. Research has shown that while the intervals between stressed syllables tend to be more consistent in carefully articulated speech or in poetry, where rhythmic patterns are deliberately emphasized, the timing can be more flexible and less predictable in casual conversation [
12,
13]. Yet, listeners have been found to prefer contexts with regular rhythm [
14], and such contexts have been associated with improved processing of phonological information [
15,
16], syllables [
17], semantic information [
18], and syntax [
19], ultimately leading to enhanced sentence comprehension [
18].
1.1. Neural Correlates of Speech Rhythm
Complementary insights into the neural mechanisms underlying lexical stress perception and speech rhythm expectancy have been provided by Event-Related Potentials (ERPs) and time-frequency representations (TFRs) derived from electroencephalographic (EEG) data. ERPs offer high temporal resolution, precisely tracking neural events as they unfold over time in response to a stimulus, while TFRs reveal the brain’s parallel processing of information, showing multiple neural processes co-occurring and interacting across distinct frequency bands.
Research utilizing ERPs to examine neural responses to unexpected rhythms in spoken language has generally produced consistent findings. For example, studies utilizing violations of rhythm and/or metrical structures [
18,
20,
21,
22,
23,
24], words with correct but unexpected rhythmic/metrical patterns [
25,
26], or pseudowords with unexpected stress patterns [
27] have found increased negativity in the first 400 ms post-stimulus onset for rhythmically anomalous or unexpected words, though some found negativities up to 1000 ms post-stimulus onset [
20,
23,
26]. These negativities are sometimes followed by late positivities between 500-900 ms [
18,
20,
21,
22,
23,
24,
26] which some attribute to task-relevant processing [
20,
21].
TFR analyses provide a window into neural oscillations – the rhythmic fluctuation in brain activity occurring at distinct frequencies. Converging data suggests that theta band oscillations (4-8 Hz) are particularly crucial for extracting prosodic cues related to lexical stress and speech intelligibility [
28]. Additionally, theta oscillations are thought to regulate higher frequency activity, such as gamma oscillations (> 30 Hz). It has been proposed that this theta-gamma cross-frequency coupling ensures that gamma oscillations encode phonemic details in sync with speech rhythm at the syllabic level as dictated by theta oscillations [
29]. Furthermore, research suggests potential roles for alpha (8-13 Hz) and beta (13-30 Hz) oscillations in speech rhythm perception. Alpha oscillations have been linked to attentional demand [
30] and might be involved in the processing of stressed syllables, which act as attentional anchors within the speech stream, thus aiding in parsing and interpreting incoming linguistic information [
15]. Beta oscillations, on the other hand, are thought to be involved in top-down control mechanisms and may contribute to the temporal prediction and anticipation of upcoming speech units [
31]. Overall, these findings thus underscore the complexity of neural dynamics underlying speech rhythm perception and highlight the importance of investigating the contributions of different frequency bands to this process.
1.2. Implicit Prosody
Although traditionally linked to spoken language, prosody has emerged as a potential factor influencing reading abilities, with mounting evidence suggesting a correlation between prosodic sensitivity and individual reading variations. This relationship is further supported by the Implicit Prosody Hypothesis (IPH). The IPH, proposed by Bader [
32] and Fodor [
33,
34], posits that during silent reading, individuals internally generate prosodic representations that mirror the explicit prosody used in similar spoken contexts. In essence, even when reading silently, we unconsciously apply the rhythms, stresses, and intonations we would use if speaking the text aloud. This hypothesis is supported by behavioral studies demonstrating that many features of explicit prosody are evident during silent reading, influencing how we comprehend phrases and sentences [
32,
35]. The significance of prosody in silent reading extends beyond general comprehension. Research on lexical stress, for instance, suggests it may be an inherent component of word processing. Using eye-tracking, [
36] found readers spent more time reading polysyllabic words with two stressed syllables, regardless of word length or frequency. Moreover, readers generate expectations for prosodic representations of words while reading silently. Noun-verb homographs are words containing identical spellings but pronunciations that vary by stress pattern according to their grammatical function (e.g., ABstract as a noun vs. abSTRACT as a verb, where capital letters denote the stressed syllable). Researchers embedded these words in syntactically ambiguous sentences and found readers were slower to read target words when ambiguity resolution required a shift in stress than when it did not [
37,
38]. Furthermore, evidence suggests that internally generated speech rhythm plays a role in the early stages of sentence parsing. When readers were presented with sentences containing ambiguous noun-verb homographs differentiated by lexical stress, they initially assigned stress to maintain the rhythm established by the preceding words in the sentence. It was only when the complete sentence was available to readers that the syntactic information overrode their initial stress assignment for ambiguous words [
39].
While a growing body of behavioral research supports implicit rhythm sensitivity during silent reading, the underlying neural mechanisms remain relatively unexplored compared to the body of research on explicit rhythm sensitivity. The few existing studies present divergent findings. For example, in a pilot study on 8 participants, Magne and colleagues [
40] visually presented sequences of English words that all contained the same stress pattern, either all first-syllable stressed words (i.e., trochaic) or all second-syllable stressed words (i.e., iambic), followed by target words that either matched or mismatched the stress pattern of the preceding sequence. They found increased negativities in the N400 time window for both iambic and trochaic target words when they were unexpected, with larger effects for trochaic targets.
In a similar study with a larger sample, Kriukova and Mani [
41] recorded neural responses while participants read sequences of Dutch disyllabic words. Sequences consisted of either three trochaic words or three iambic words, followed by target words that were metrically consistent or inconsistent with the prime sequences. They did not observe a significant negativity for inconsistent trochaic words, despite a small, non-significant trend in that direction (see
Figure 1B in [
41]). Furthermore, they reported an increased positivity for iambic words. Fotidzis and colleagues (2018) examined neural responses to silently read words that were presented following an auditory rhythmic tone prime that either matched or mismatched the rhythmic structure of targets. They found increased negativities between 300 and 708 ms post-stimulus for words that did not match the rhythm structure of the auditory primes.
Finally, Breen and colleagues [
43] embedded stress-alternating noun-verb homographs (e.g., PERmit vs perMIT) in rhyming couplets with a regular trochaic or iambic metric structure. The homograph word class was either consistent or inconsistent with the stress expectation generated by the couplets. They observed increased early (80-155 ms) and late (325-375 ms) negativities for inconsistent trochaic words and, similar to [
41], increased positivities (365-435 ms) post-stimulus for inconsistent iambic words. In sum, not only do the results of these studies differ from each other, but they also diverge from the spoken language literature in that previous auditory studies have generally found enhanced negativities between 300 and 500 ms post-stimulus for unexpected lexical stress compared to expected lexical stress [
17,
26,
27,
44,
45]. There are many differences between these studies, and though this is also the case for the auditory studies of lexical stress, it is possible that neural responses to implicit lexical stress are more subtle and harder to detect. Further research is warranted to determine factors that contributed to these divergent findings.
1.3. Design of Present Study and Predictions
The goal of the present study was to identify differences in electrophysiological responses when the lexical stress of targets aligned or deviated from the expected rhythmic pattern. Previous research has revealed several distinctions related to the use and processing of trochaic and iambic words. These include a higher prevalence of trochaic than iambic words in English [
1], a developmental trajectory where trochaic words are typically acquired earlier than iambic words [
2,
3,
4], and more varied ERP responses to unexpected iambic compared to unexpected trochaic words [
40,
41,
42,
43]. Considering these findings, we also sought to explore whether brain responses differ between trochaic and iambic words during silent reading.
To this end, we manipulated the stress pattern and metrical expectancy of words in a silent reading task. EEG was recorded while participants silently read five-word sequences. The first four words of each sequence were either all trochaic or all iambic, while the stress pattern of the fifth word in the sequences either matched or did not match the stress pattern of the previous four words. Both ERPs and time-frequency characteristics of the EEG data were analyzed to identify neural correlates of implicit stress sensitivity. Building on previous ERP research manipulating overt [
21,
27,
45,
46] or implicit [
40,
42,
43] speech rhythm cues, we hypothesized increased negativity in the 200-600 ms window following unexpected trochaic patterns. Given the less consistent findings for iambic words, we predicted either a similar but attenuated negativity or potentially an increased positivity for unexpected iambic patterns.
In the time-frequency domain, we proposed that stress pattern expectancy would primarily modulate neural activity in the theta band. More specifically, we anticipated an increased theta activity for expected stress patterns, reflecting enhanced neural synchronization with predictable word metrical structure. This hypothesis is grounded in previous research that has highlighted the role of theta oscillations in encoding and integrating rhythmic information in speech [
28]. Alternatively, given that theta activity increase has also been observed for semantically unexpected words, reflecting the brain’s engagement in control processes [
47], we hypothesize that an increased beta activity may be observed for unexpected stress patterns if they disrupt lexical access, as suggested in previous research [
21,
22]. Interestingly, these predictions align with the ongoing debate regarding the functional significance of negative ERPs associated with rhythmically unexpected words, potentially supporting interpretations of these either reflecting semantic processing difficulty and/or rule-based/predictive sequencing error detection [
27]. Note these potential outcomes are not mutually exclusive, as distinct brain sources have been implicated in theta activity related to rhythmic and semantic aspects of speech, suggesting different neural substrates for expected and unexpected stress patterns.
2. Materials and Methods
2.1. Participants
Twenty college students received course credit for their participation (11 females and 9 males, mean age = 22, SD = 3, age range: 18-28). Data from two participants were ultimately excluded from the analysis due to excessive artifacts in the EEG, leaving 18 participants included in the final analysis. All participants were right-handed, had normal hearing and vision, and were native speakers of English. Written consent was obtained from each participant prior to their participation in the experiment. Approval for the study was granted by the Institutional Review Board of Middle Tennessee State University.
2.2. Stimuli
The English Lexicon Project database [
48] was used to build four types of word sequences varying in stress pattern type and/or expectancy. A total of 500 disyllabic nouns and adjectives were selected. Half (250) were trochaic (i.e., containing first syllable stress), and half (250) were iambic (i.e., containing second syllable stress). These words were then used to build 100 five-word sequences in which the initial four words followed either a trochaic or iambic stress pattern. Metrical expectancy was manipulated by varying the stress pattern of the fifth word (target word) of each sequence. In metrically expected conditions, the stress pattern matched that of the first four words, whereas in metrically unexpected conditions, the stress pattern differed from the preceding four. A total of twenty-five sequences were created for each of the four conditions (trochaic expected, iambic expected, trochaic unexpected, and iambic unexpected). Two counterbalanced lists of the 100-word sequences were used to ensure that target words were presented in both expected and unexpected conditions across participants, with each participant seeing each target word only once. Examples of stimuli in each experimental condition are presented in
Table 1.
The lexical frequency of individual words within each sequence was controlled using the Hyperspace Analogue to Language (HAL) frequency norms [
49] to ensure equivalent frequency between the initial four words and the target word (see
Table 2). To minimize semantic relatedness, words within each sequence were evaluated by two independent judges, unaware of the purpose of the experiment. Additionally, the web-based Latent Semantic Analysis (LSA) tool was used to gauge semantic similarity between the target word and the preceding four words in the sequence. LSA generates a similarity score ranging from 0 (indicating no relation) to 1 (indicating a strong relation). LSA values indicated low semantic similarities for metrically expected (Mean = 0.16, SD = 0.12) and unexpected conditions (Mean = 0.12, SD = 0.09).
2.3. Procedure
Participants were seated in a soundproofed and electronically shielded room. They were positioned approximately one meter in front of a computer monitor. Stimuli were presented in black lowercase letters on a white background using E-prime 2.0 Professional with Network Timing Protocol (Psychology Software Tools, Inc., Pittsburgh, PA, USA). Each word sequence was presented one word at a time for 0.5 seconds, followed by a blank screen for 0.4 seconds. A fixation cross was displayed in between word sequences for 1 second.
Participants viewed a total of 100 unique word sequences broken down into two blocks of 50-word sequences each. The order of trials was randomized within each block, and the order of blocks was counterbalanced across participants. To maintain participants' attention to the stimuli, they were told they were participating in a memory task and should carefully read the word sequences. They were not informed about the manipulation of stress patterns within the sequences. The entire experimental session lasted one hour.
2.4. EEG Acquisition and Preprocessing
Continuous EEG data were recorded at a sampling rate of 500 Hz on a MacBook Pro computer using a 64-channel Hydrocel Geodesic Sensor Net (EGI, Eugene, OR, USA) connected to a Net Amps 300 amplifier. Electrode impedances were kept below 50kΩ, and data were referenced online to Cz. Data preprocessing was performed in MATLAB (The MathWorks, Inc.) using the EEGLAB toolbox [
50]. The raw signal was first down-sampled to 250 Hz for computational efficiency and high-pass filtered at 0.5 Hz. We used the PREP pipeline plugin [
51] to detect bad channels, apply a robust re-reference, and remove 60 Hz line noise. Next, we performed independent component analysis (ICA) using the runica algorithm on a copy of the data down-sampled to 100 Hz and high-pass filtered at 2 Hz to improve the efficiency of the ICA decomposition. The resulting ICA weights were then applied to the original data. The ICLabel function was used to automatically label each component. Any component labeled as indicating eye movements or muscle activity with a probability exceeding 90% was excluded. Finally, we used the artifact subspace reconstruction (ASR) algorithm and a 20-burst detection criteria threshold [
52] to automatically identify and remove portions of the EEG containing large transient artifacts.
2.5. Statistical Analysis
To analyze single-trial ERPs elicited by the critical word (i.e., fifth words of each sequence), EEG epochs were extracted from -0.2 to 1 second relative to the word onset, and epochs were visually inspected and discarded if any remaining artifacts were present. A baseline correction was performed by averaging data from 200 ms to 0 ms pre-stimulus onset and subtracting this average from the rest of the time points. TFRs were computed on epochs from -1 to 2 seconds relative to the critical word. Each epoch was convolved with a Hanning-windowed sinusoidal Morlet wavelet. A total of 13 log-spaced frequencies ranging from 4 Hz to 30 Hz were calculated every 4 ms with an increasing number of wavelet cycles of 3 at 4 Hz and 11.25 at 30 Hz. The maximum wavelet window length was 836 ms at 4 Hz.
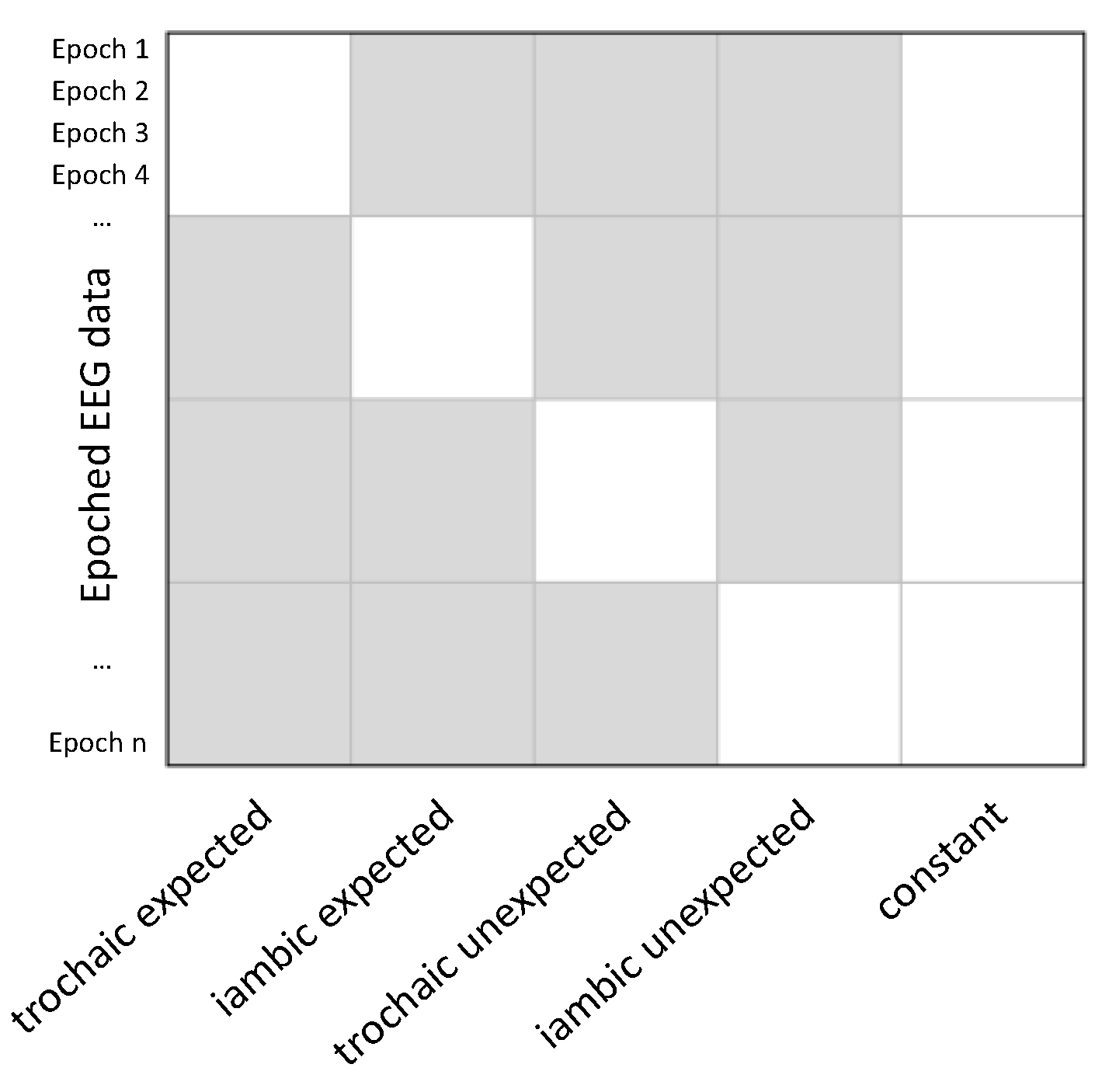
We implemented hierarchical linear modeling with the LIMO EEG plug-in for EEGLAB [
53]. To account for within-subject variability across single trials, a general linear model was first implemented to estimate the beta parameters representing the effect of each experimental condition (i.e., the four combinations of expectancy and stress pattern) using ordinary least squares (OLS) regression. The within-subject model of each single-trial data at each electrode and time point (and frequency bin for TF analysis) has the general form (1) with Y the single trial measurement (voltage amplitude for ERP or relative power for TF),
β0 the intercept,
β1 to
β4 the beta coefficients for each experimental condition to be estimated, X
1 to X
4 the coding for each column corresponding to the type of stimulus in the design matrix (
Figure 1), and
ε the error term.
Then, for group-level analysis, 2 x 2 repeated measures ANOVAs (generalized Hotelling’s T
2) were computed on the beta estimates using robust bootstrapping, with stress pattern and expectancy as within-subject factors. Significant interactions were subsequently resolved using simple contrast analysis. For all analyses, a non-parametric bootstrap spatiotemporal clustering approach was used to correct for multiple comparisons, controlling the family-wise error rate at an alpha level of 0.05 [
54].
3. Results
3.1. ERPs
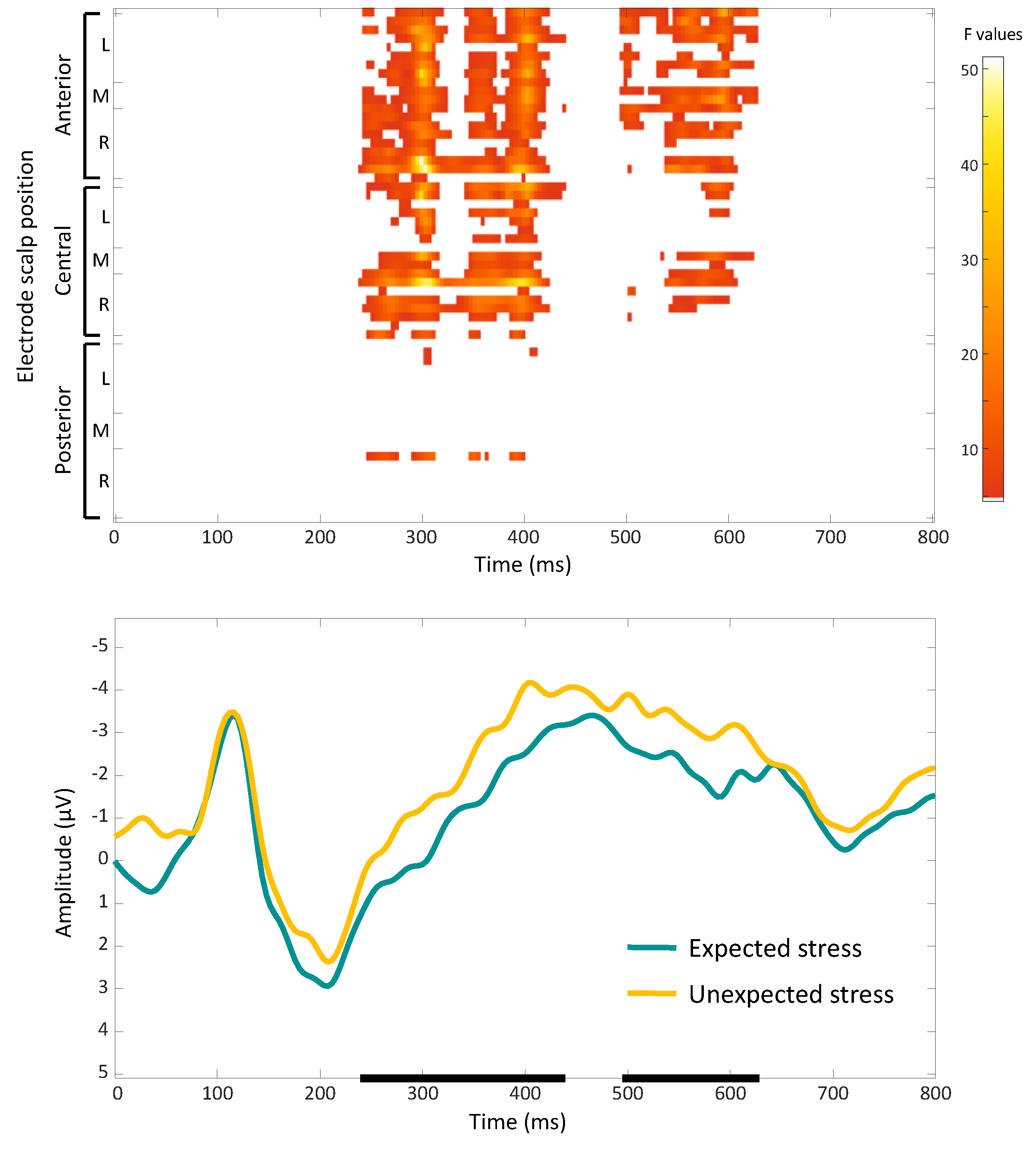
The mixed-model ANOVAs revealed a significant main effect of expectancy but no significant main effect of stress or expectancy and stress interaction. The main effect of expectancy was observed in two clusters covering central and frontal regions and spanning time windows from 240 to 628 ms (cluster 1: from 240 to 440 ms, maximum F = 51.29, corrected
p = 0.002; cluster 2: from 496 to 628 ms, maximum F = 30.53, corrected
p = 0.033). In both clusters, target words with an unexpected stress pattern elicited a more negative deflection compared to those with an expected pattern (
Figure 2).
3.2. TFRs
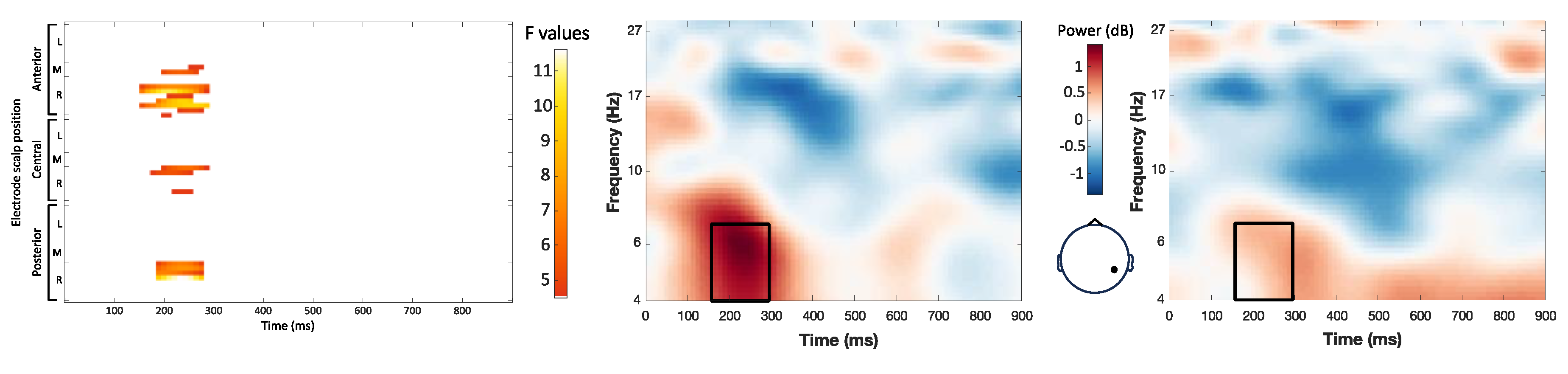
Results of the mixed-model ANOVAs indicated several significant findings associated with the main effects of expectancy and stress pattern, as well as a significant interaction between these two factors. Given that significant clusters emerged within distinct time windows and frequency bands, the main effects and interaction are detailed separately in the following section.
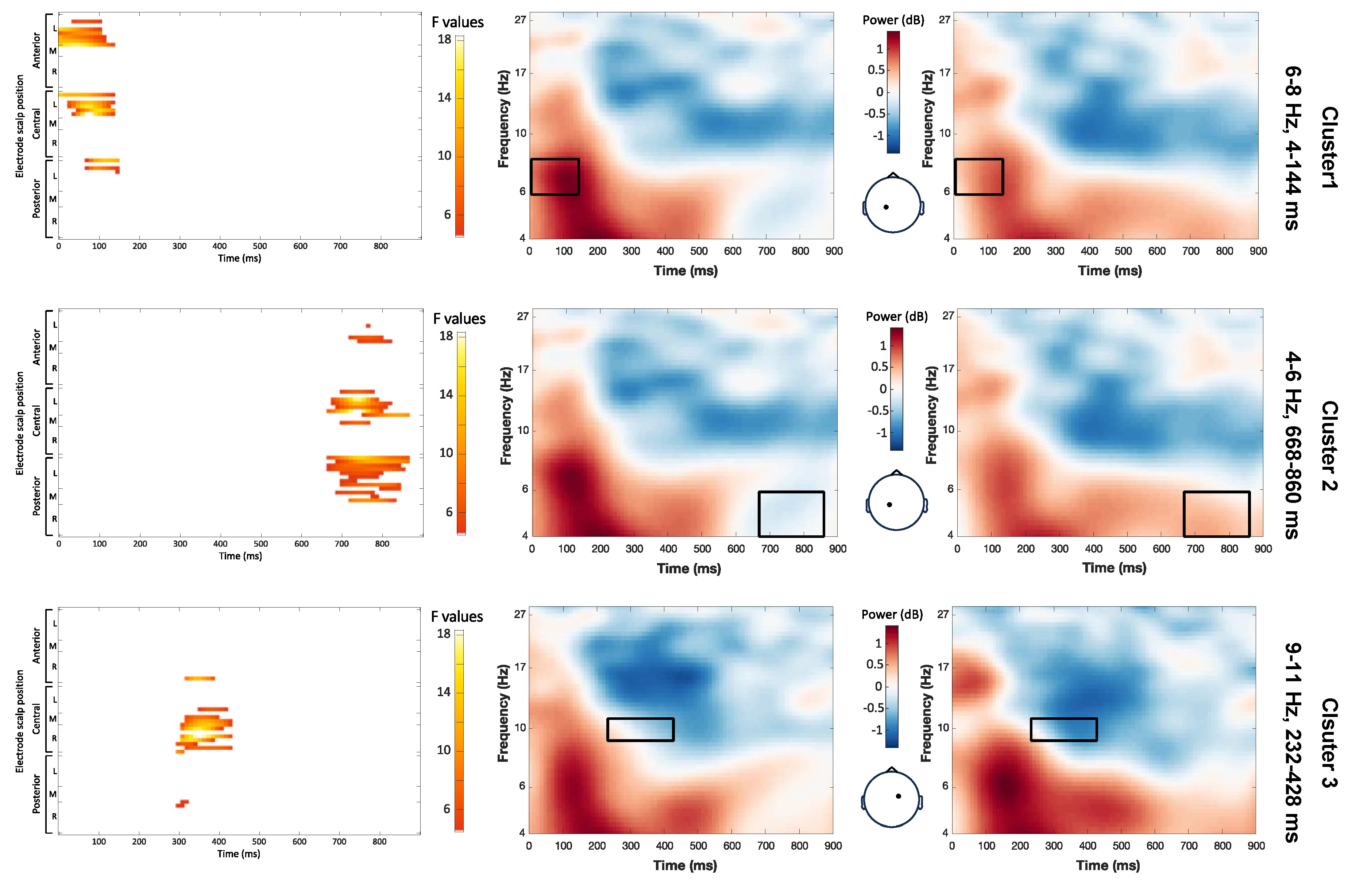
The main effect of expectancy included significant clusters in the theta and alpha bands (
Figure 3). Target words with expected stress patterns showed increased power in the 6-8 Hz frequency range compared to words with unexpected stress patterns (maximum F = 13.96, corrected
p = 0.012). This effect was observed between 4 and 144 ms after word onset and localized to left parietal, central, and frontal scalp regions. Target words with expected stress patterns also showed increased power in the 9-11 Hz range over right central and temporal scalp regions, 232 to 428 ms post word onset (maximum F = 9.55, corrected
p = 0.012). By contrast, target words with unexpected stress patterns displayed higher power in the 4-6 Hz range (maximum F = 18.30, corrected
p = 0.012). This cluster occurred between 668 and 860 ms post-word onset and was more pronounced over the scalp’s left central and posterior regions.
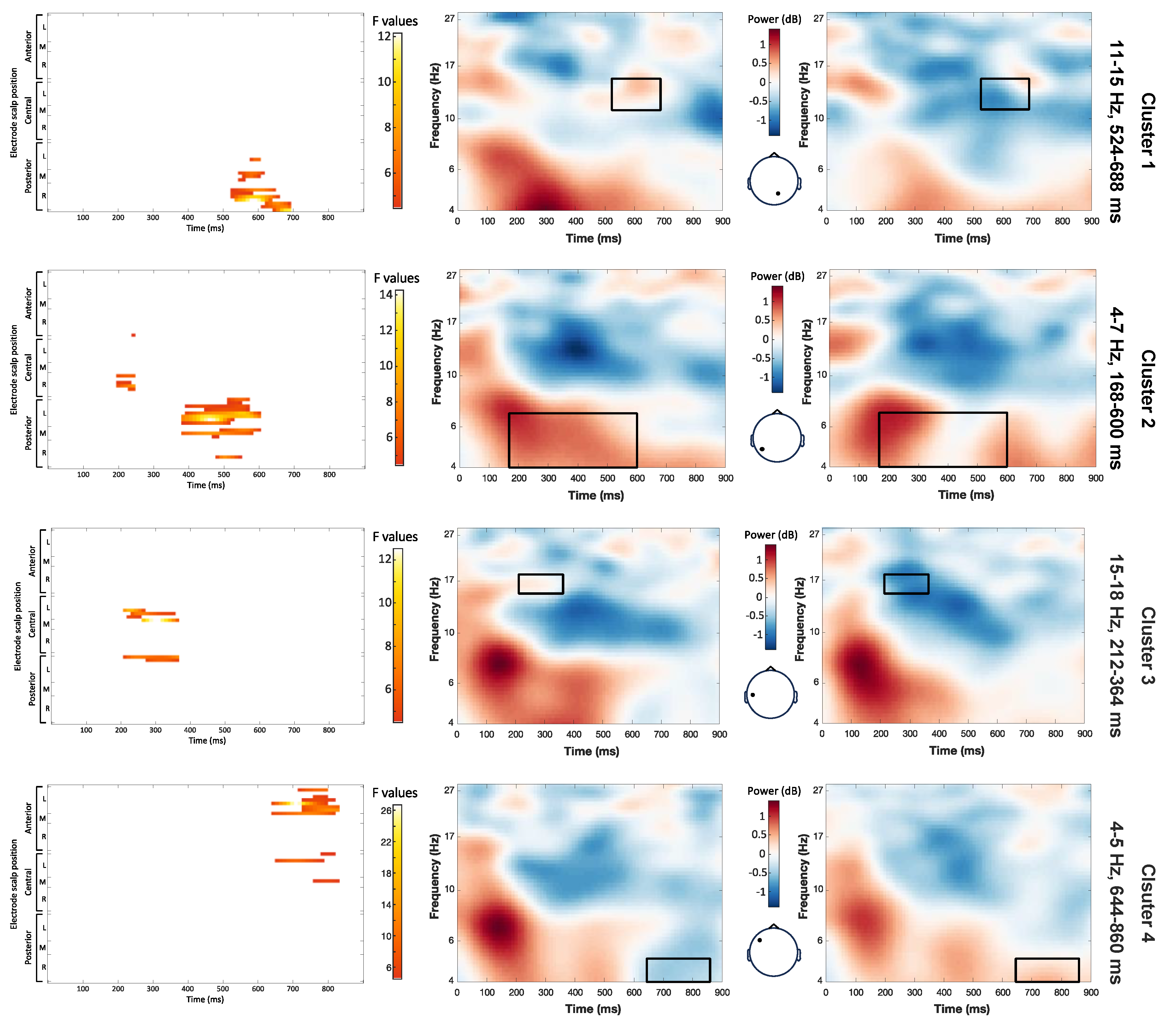
The main effect stress pattern yielded significant clusters in the theta and beta bands (
Figure 4). First, a key difference emerged in the 4-7 Hz frequency range from 168 to 600 ms (maximum F = 14.26, corrected
p = 0.012). Notably, power was higher for target words with a trochaic pattern compared to those with an iambic pattern extending from the posterior left to the central right regions of the scalp. Next, between 212 and 364 ms, there was a significant cluster resulting from a stronger deactivation in the mid beta band (15-18 Hz) for iambic than trochaic target words over left parietal regions (maximum F = 12.52, corrected
p = 0.048). Between 524 and 688 ms, iambic target words also had a stronger low beta (11-15Hz) deactivation than trochaic words over the right parietal and occipital regions (maximum F = 12.14, corrected
p = 0.012). The last cluster showed a significant difference in the low theta range (4-5 Hz), between 644 and 860 ms (maximum F = 26.74, corrected
p = 0.012). In this cluster, power was higher for iambic than trochaic patterns over the left central and frontal regions.
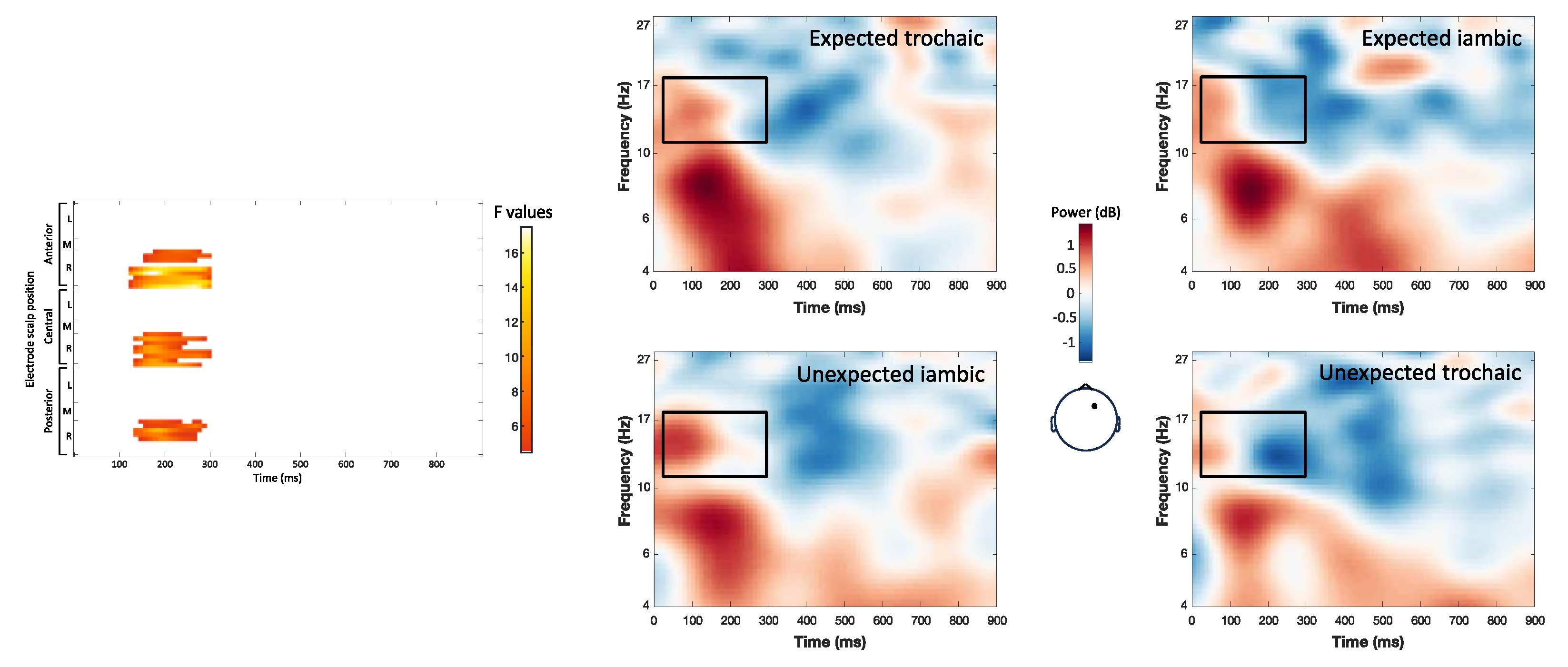
The interaction of word expectancy and stress pattern elicited two distinct clusters of significant effects. A theta band cluster (4-7 Hz, 156-296 ms; maximum F = 11.62, corrected
p = 0.012) showed increased power for expected trochaic compared to expected iambic words over a broad right scalp region (
Figure 5). A low/mid beta band cluster (11-18 Hz, 24-296 ms; maximum F = 17.47, corrected
p = 0.012) revealed increased power for conditions with a preceding trochaic context (regardless of expectancy), compared to an iambic context, over right central and frontal regions. All post hoc contrasts were significant at
p < 0.05 (
Figure 6).
4. Discussion
In this study, we examined the neural correlates of processing implicit speech rhythm cues (specifically, lexical stress) during silent reading. Utilizing robust single-trial analysis of ERPs and TFRs, we investigated whether brain responses differentiated between expected and unexpected lexical stress, given a preceding metric context. Additionally, we explored the potential impact of the type of stress pattern on neural activity by comparing responses to trochaic (more common) and iambic (less common) stress patterns. Our findings revealed several key insights. First, unexpectedly stressed target words, irrespective of stress pattern, elicited an enhanced negative ERP component followed by increased theta power. Words with the expected trochaic stress pattern elicited early increases in both theta and beta power, with trochaic words generally showing higher theta activity than iambic words. Notably, this theta difference appeared earlier for expected trochaic words. Additionally, beta activity was modulated by both stress pattern and metrical expectancy. The following discussion will elaborate on the interpretation of these effects.
4.1. Effect of Metrical Expectancy
Consistent with prior ERP research on the perception of overt speech rhythm cues [
17,
18,
20,
21,
22,
24,
25,
26,
27,
45], we observed an enhanced negative ERP component for target words with unexpected stress patterns. This effect emerged over centro-frontal regions within the first 400 ms post-stimulus, mirroring previous findings. Our results also partially converge with the limited studies on implicit speech rhythm, which reported negativities between 250–500 ms for unexpected trochaic words [
40,
42,
43].
The negative ERP effect elicited by unexpected stress patterns has been attributed to several distinct, yet not mutually exclusive, mechanisms. It may reflect a contingent negative variation (CNV) generated in anticipation of a stressed syllable, and that is sustained when an unexpected unstressed syllable occurs instead [
20,
23]. Alternatively, the effect may represent an enhanced N400 component, typically associated with lexico-semantic processing, potentially indexing increased semantic processing demands due to the unexpected stress pattern [
20,
21,
23,
26]. Finally, the negativity could be a subcomponent of the left anterior negativity (LAN), suggesting a more domain-general error detection mechanism responding to deviations from rule-based predictions [
18,
22,
24,
27]. In the context of the present study, the N400 interpretation seems most compelling, as it is accompanied by a subsequent increase in theta power—a pattern previously associated with semantic processing difficulties in response to unexpected words [
55].
It's worth noting that, in our study, unexpected stress patterns triggered a heightened negative ERP response, regardless of whether the stress was iambic or trochaic in nature. This finding contrasts somewhat with previous studies on implicit prosody, which have shown more varied results in this regard. This variability may stem from several factors, including methodological differences and the complex interplay of cognitive processes involved in implicit prosody processing. For instance, Kriukova and Mani [
41] observed an increased positivity for unexpected iambic stress and small but nonsignificant increased negativity to unexpected trochaic stress, potentially due to their weak metrical context and long interstimulus interval (ISI. These factors may have led to a closure positive shift (CPS), masking the expected negativity, particularly for less frequent iambic words. Furthermore, Breen and colleagues [
43] used stress-alternating noun-verb homographs embedded in the final position of rhyming couplets. Since lexical stress influences not only acoustic properties (such as alterations in duration, intensity, and pitch) but also phonemic structure (e.g., vowel reduction, consonant clarity), manipulating stress in their study may have disrupted the expected rhyming pattern, introducing confounding phonological factors. These factors highlight the challenge of isolating ERP effects specifically related to metrical expectations when other phonological aspects are simultaneously manipulated. Finally, individual differences in language proficiency and sensitivity to less common metrical structures, like iambic stress patterns in English [
1], may contribute to the variability in ERP findings. Previous research supports this notion, linking the amplitude of the negativity evoked by unexpected stress patterns to individual differences in musical rhythm perception skills [
45] and reading comprehension ability in adults [
56]. In conclusion, our findings contribute to the growing literature on implicit prosody processing while underscoring the intricate interplay of metrical expectations, phonological factors, and individual differences in shaping neural responses to unexpected stress patterns during silent reading.
Mirroring the ERP findings, stress expectancy also influenced TFRs. Expected stress patterns elicited increased power in high theta (6-8 Hz) over a broad left parietal to frontal scalp area. This heightened theta activity aligns with previous research demonstrating enhanced theta power during the processing of rhythmically regular speech such as nursery rhymes [
57]. Theta activity, especially over frontal and temporal scalp regions, has been shown to synchronize with rhythmic cues in the speech stream, such as syllable boundaries and stress patterns [
28]. Recent research suggests that theta activity may play a crucial role in processing linguistic structure and acting as a scaffolding that aids with the integration of incoming linguistic information [
29]. Our findings suggest this role may extend to reading. This notion is further supported by evidence of impaired neural tracking of speech rhythm cues in the theta band in individuals with reading deficits [
58,
59]. Additionally, recent research combining eye tracking and EEG indicates a rhythmic pattern in eye movements during natural reading, synchronized with specific brain oscillations, notably in the theta band [
60]. These findings collectively point to the potential significance of theta band activity in the active processing of individual words during reading.
Finally, words with expected stress patterns also elicited an increase in alpha power. As alpha activity is inversely related to attentional engagement [
30], this increase in alpha activity suggests a reduced demand on attentional resources, indicating more efficient processing when word stress patterns match expectations set by the context.
4.2. Effect of Stress Pattern
Both trochaic and iambic words led to increased theta power compared to each other, albeit in different time frames. Notably, the increase in theta power occurred earlier for trochaic words (with stress on the first syllable) between 168-600 ms, compared to iambic words (with stress on the second syllable), where the increase was seen later between 644-860 ms. This finding further suggests that theta activity might be sensitive to stress cues in general, not just a specific type of stress pattern.
In contrast, changes in beta activity were more selective to the type of stress pattern, with a stronger low beta deactivation (i.e., a decrease in beta power relative to baseline) found for iambic words than for trochaic words. Prior research has linked beta deactivation to semantic processing and lexical access. For instance, stronger beta deactivation has been observed for semantically unexpected words during sentence processing and target words following semantically unrelated primes in word pairs [
61]. In the context of language comprehension, modulation of beta activity has been proposed to reflect changes in the neurocognitive network underlying the construction of meaning representations as well as the top-down propagation of the predictions about incoming inputs based on these established representations [
62]. In our study, the stronger beta deactivation for iambic words may thus be attributed to their lower prevalence in English [
1]. Conversely, the trochaic stress pattern, representing approximately 80% of the lexicon, may facilitate word recognition. Additionally, since trochaic words have initial stress, this effect could also reflect the prioritization of stressed syllables at the beginning of words for quicker access to phonological representations and subsequent lexical processing.
4.3. Trochaic Regularity and Beta Oscillations
In the present study, both iambic and trochaic target words were associated with an increase in low beta activity (11-18 Hz) when following a regular trochaic context, and this effect was independent of their stress expectancy. This finding also aligns with the predictive coding hypothesis described earlier [
62], as well as a recently proposed framework for a role of lower beta activity in multimodal temporal predictions [
31]. In that regard, increased beta activity could signal the brain's sensitivity to the established rhythmic structure, aiding the integration of subsequent words into the metrical framework, even if they are unexpected. This may be facilitated by top-down modulation from the metrical framework, where established expectations streamline the processing of incoming words. Additionally, the increased beta activity might reflect enhanced phonological processing due to the clearer rhythmic boundaries provided by the trochaic pattern. Moreover, the stronger beta activity could indicate a heightened attentional focus on words within a trochaic context, potentially due to their salience within the rhythmic structure. Ultimately, the precise interplay of these factors warrants further investigation.
4.4. Limitations and Future Directions
While the present study sheds light on the neural correlates of implicit stress processing, it does not address individual differences in reading skills. Given the established relationship between speech rhythm perception and reading skills [
63,
64,
65], further study is warranted. As suggested by Fotidzis and colleagues [
42], it is possible that individual differences in reading and/or language skills are differentially associated with neural responses to linguistic rhythm during silent reading. Future research investigating the relationship between observed neural markers (e.g., ERP components, theta, delta, and alpha power) and individual variations in reading ability would be informative.
Additionally, future studies should explore the potential relationship between neural tracking of the speech signal (phase alignment of brain oscillations) and the processing of implicit stress patterns. This could provide insights into the underlying mechanisms linking rhythm perception, phonological processing, and reading development, potentially contributing to a deeper understanding of dyslexia and other reading disabilities [
59,
63].
5. Conclusions
This research contributes to our knowledge of the neural responses to lexical stress during a silent reading task. We utilized a conservative, data-driven approach and found responses varied by stress expectancy, suggesting lexical stress was a component of the mental phonological representation of words, even when readers’ attention was not drawn to the prosodic features of the text. This refines our understanding of word-level representations produced by readers during silent reading. Furthermore, the similarity of responses to those in the spoken language literature, both the ERPs and modulations in theta, delta, and alpha bands, suggests overlapping neural networks for the processing of spoken and written language.
Author Contributions
Conceptualization, C.M.; methodology, S.P. and C.M.; software, S.P. and C.M.; validation, S.P.; formal analysis, S.P. and C.M.; investigation, S.P., S.N. and C.M.; resources, C.M.; data curation, S.P.; writing—original draft preparation, S.P. and C.M.; writing—review and editing, S.N.; supervision, S.N.; project administration, C.M.; funding acquisition, C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation (NSF), grant number 1926736 (PI: Magne).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the MTSU Institutional Review Board (protocol number 13-197, initially approved on February 20, 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Cutler, A.; Carter, D.M. The Predominance of Strong Initial Syllables in the English Vocabulary. Computer Speech & Language 1987, 2, 133–142. [Google Scholar] [CrossRef]
- Jusczyk, P.W.; Cutler, A.; Redanz, N.J. Infants’ Preference for the Predominant Stress Patterns of English Words. Child development 1993, 64, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Jusczyk, P.W.; Houston, D.M.; Newsome, M. The Beginnings of Word Segmentation in English-Learning Infants. Cognitive Psychology 1999, 39, 159–207. [Google Scholar] [CrossRef] [PubMed]
- Nazzi, T.; Ramus, F. Perception and Acquisition of Linguistic Rhythm by Infants. Speech Communication 2003, 41, 233–243. [Google Scholar] [CrossRef]
- Butterfield, S.; Cutler, A. PROCEEDINGS OF SPEECH ’88. 1988.
- Cutler, A.; Butterfield, S. Rhythmic Cues to Speech Segmentation: Evidence from Juncture Misperception. Journal of Memory and Language 1992, 31, 218–236. [Google Scholar] [CrossRef]
- Cutler, A.; Norris, D. The Role of Strong Syllables in Segmentation for Lexical Access. Journal of Experimental Psychology: Human Perception and Performance 1988, 14, 113–121. [Google Scholar] [CrossRef]
- Sanders, L.D.; Newport, E.L.; Neville, H.J. Segmenting Nonsense: An Event-Related Potential Index of Perceived Onsets in Continuous Speech. Nat Neurosci 2002, 5, 700–703. [Google Scholar] [CrossRef]
- Tong, S.X.; Lentejas, K.; Deng, Q.; An, N.; Cui, Y. How Prosodic Sensitivity Contributes to Reading Comprehension: A Meta-Analysis. Educ Psychol Rev 2023, 35, 78. [Google Scholar] [CrossRef]
- Liberman, M.; Prince, A. On Stress and Linguistic Rhythm. Linguistic Inquiry 1977, 8, 249–336. [Google Scholar]
- Selkirk, E. The Interaction of Constraints on Prosodic Phrasing. In Prosody: Theory and Experiment: Studies Presented to Gösta Bruce; Horne, M., Ed.; Springer Netherlands: Dordrecht, 2000; pp. 231–261 ISBN 978-94-015-9413-4. Horne, M. (Ed.).
- Dauer, R.M. Stress-Timing and Syllable-Timing Reanalyzed. Journal of Phonetics 1983, 11, 51–62. [Google Scholar] [CrossRef]
- Grabe, E.; Low, E.L. Durational Variability in Speech and the Rhythm Class Hypothesis. In Laboratory Phonology 7; Gussenhoven, C., Warner, N., Eds.; Mouton de Gruyter, 2002; pp. 515–546 ISBN 978-3-11-017086-3.
- Obermeier, C.; Menninghaus, W.; von Koppenfels, M.; Raettig, T.; Schmidt-Kassow, M.; Otterbein, S.; Kotz, S.A. Aesthetic and Emotional Effects of Meter and Rhyme in Poetry. Front. Psychol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Pitt, M.A.; Samuel, A.G. The Use of Rhythm in Attending to Speech. J Exp Psychol Hum Percept Perform 1990, 16, 564–573. [Google Scholar] [CrossRef]
- Quene, H.; Port, R.F. Effects of Timing Regularity and Metrical Expectancy on Spoken-Word Perception. Phonetica 2005, 62, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Henrich, K.; Alter, K.; Wiese, R.; Domahs, U. The Relevance of Rhythmical Alternation in Language Processing: An ERP Study on English Compounds. Brain and Language 2014, 136, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Rothermich, K.; Schmidt-Kassow, M.; Kotz, S.A. Rhythm’s Gonna Get You: Regular Meter Facilitates Semantic Sentence Processing. Neuropsychologia 2012, 50, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Roncaglia-Denissen, M.P.; Schmidt-Kassow, M.; Kotz, S.A. Speech Rhythm Facilitates Syntactic Ambiguity Resolution: ERP Evidence. PLOS ONE 2013, 8, e56000. [Google Scholar] [CrossRef]
- Domahs, U.; Wiese, R.; Bornkessel-Schlesewsky, I.; Schlesewsky, M. The Processing of German Word Stress: Evidence for the Prosodic Hierarchy. Phonology 2008, 25, 1–36. [Google Scholar] [CrossRef]
- Magne, C.; Astesano, C.; Aramaki, M.; Ystad, S.; Kronland-Martinet, R.; Besson, M. Influence of Syllabic Lengthening on Semantic Processing in Spoken French: Behavioral and Electrophysiological Evidence. Cerebral Cortex 2007, 17, 2659–2668. [Google Scholar] [CrossRef]
- Marie, C.; Magne, C.; Besson, M. Musicians and the Metric Structure of Words. Journal of cognitive neuroscience 2011, 23, 294–305. [Google Scholar] [CrossRef]
- McCauley, S.M.; Hestvik, A.; Vogel, I. Perception and Bias in the Processing of Compound versus Phrasal Stress: Evidence from Event-Related Brain Potentials. Lang Speech 2013, 56, 23–44. [Google Scholar] [CrossRef]
- Schmidt-Kassow, M.; Kotz, S.A. Event-Related Brain Potentials Suggest a Late Interaction of Meter and Syntax in the P600. Journal of Cognitive Neuroscience 2009, 21, 1693–1708. [Google Scholar] [CrossRef]
- Böcker, K.B.E.; Bastiaansen, M.C.M.; Vroomen, J.; Brunia, C.H.M.; De Gelder, B. An ERP Correlate of Metrical Stress in Spoken Word Recognition. Psychophysiology 1999, 36, 706–720. [Google Scholar] [CrossRef]
- Bohn, K.; Knaus, J.; Wiese, R.; Domahs, U. The Influence of Rhythmic (Ir)Regularities on Speech Processing: Evidence from an ERP Study on German Phrases. Neuropsychologia 2013, 51, 760–771. [Google Scholar] [CrossRef]
- Rothermich, K.; Schmidt-Kassow, M.; Schwartze, M.; Kotz, S.A. Event-Related Potential Responses to Metric Violations: Rules versus Meaning. NeuroReport 2010, 21, 580. [Google Scholar] [CrossRef]
- Poeppel, D.; Assaneo, M.F. Speech Rhythms and Their Neural Foundations. Nat Rev Neurosci 2020, 21, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Giraud, A.-L.; Poeppel, D. Cortical Oscillations and Speech Processing: Emerging Computational Principles and Operations. Nat Neurosci 2012, 15, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. α-Band Oscillations, Attention, and Controlled Access to Stored Information. Trends Cogn Sci 2012, 16, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Biau, E.; Kotz, S.A. Lower Beta: A Central Coordinator of Temporal Prediction in Multimodal Speech. Front. Hum. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Bader, M. Prosodic Influences on Reading Syntactically Ambiguous Sentences. In Reanalysis in Sentence Processing; Fodor, J.D., Ferreira, F., Eds.; Studies in Theoretical Psycholinguistics; Springer Netherlands: Dordrecht, 1998; pp. 1–46 ISBN 978-94-015-9070-9. Fodor, J.D.; Ferreira, F. (Eds.).
- Fodor, J. Learning To Parse? Journal of Psycholinguistic Research 1998, 27, 285–319. [Google Scholar] [CrossRef]
- Fodor, J. Psycholinguistics Cannot Escape Prosody. Proceedings of the 1st International Conference on Speech Prosody 2002. [Google Scholar]
- Breen, M. Empirical Investigations of the Role of Implicit Prosody in Sentence Processing. Language and Linguistics Compass 2014, 8, 37–50. [Google Scholar] [CrossRef]
- Ashby, J.; Clifton Jr., C. The Prosodic Property of Lexical Stress Affects Eye Movements during Silent Reading. Cognition 2005, 96, B89–B100. [Google Scholar] [CrossRef] [PubMed]
- Breen, M.; Clifton, C. Stress Matters: Effects of Anticipated Lexical Stress on Silent Reading. Journal of Memory and Language 2011, 64, 153–170. [Google Scholar] [CrossRef]
- Breen, M.; Clifton, C. Stress Matters Revisited: A Boundary Change Experiment. Quarterly Journal of Experimental Psychology 2013, 66, 1896–1909. [Google Scholar] [CrossRef]
- Kentner, G. Linguistic Rhythm Guides Parsing Decisions in Written Sentence Comprehension. Cognition 2012, 123, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Magne, C.; Gordon, R.L.; Midha, S. Influence of Metrical Expectancy on Reading Words: An ERP Study. In Proceedings of the Proceedings of the Fifth International Conference on Speech Prosody; 2010; Vol. 100432, pp. 1–4.
- Kriukova, O.; Mani, N. Processing Metrical Information in Silent Reading: An ERP Study. Frontiers in Psychology 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Fotidzis, T.S.; Moon, H.; Steele, J.R.; Magne, C.L. Cross-Modal Priming Effect of Rhythm on Visual Word Recognition and Its Relationships to Music Aptitude and Reading Achievement. Brain Sciences 2018, 8, 210. [Google Scholar] [CrossRef]
- Breen, M.; Fitzroy, A.B.; Oraa Ali, M. Event-Related Potential Evidence of Implicit Metric Structure during Silent Reading. Brain sciences 2019, 9, 192. [Google Scholar] [CrossRef]
- BÖCKER, K.B.E.; BASTIAANSEN, M.C.M.; Vroomen, J.; BRUNIA, C.H.M.; Gelder, B.D.; Bocker, K.B.E.; BASTIAANSEN, M.C.M.; Vroomen, J.; BRUNIA, C.H.M.; Gelder, B.D. An ERP Correlate of Metrical Stress in Spoken Word Recognition. Psychophysiology 1999, 36, 706–720. [Google Scholar] [CrossRef]
- Magne, C.; Jordan, D.K.; Gordon, R.L. Speech Rhythm Sensitivity and Musical Aptitude: ERPs and Individual Differences. Brain and Language 2016, 153–154, 13–19. [Google Scholar] [CrossRef]
- Domahs, U.; Wiese, R.; Bornkessel-Schlesewsky, I.; Schlesewsky, M. The Processing of German Word Stress: Evidence for the Prosodic Hierarchy. Phonology 2008, 25, 1–36. [Google Scholar] [CrossRef]
- Ryskin, R.; Nieuwland, M.S. Prediction during Language Comprehension: What Is Next? Trends in Cognitive Sciences 2023, 27, 1032–1052. [Google Scholar] [CrossRef]
- Balota, D.A.; Yap, M.J.; Hutchison, K.A.; Cortese, M.J.; Kessler, B.; Loftis, B.; Neely, J.H.; Nelson, D.L.; Simpson, G.B.; Treiman, R. The English Lexicon Project. Behavior Research Methods 2007, 39, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Lund, K.; Burgess, C. Producing High-Dimensional Semantic Spaces from Lexical Co-Occurrence. Behavior Research Methods, Instruments, & Computers 1996, 28, 203–208. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. Journal of Neuroscience Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Bigdely-Shamlo, N.; Mullen, T.; Kothe, C.; Su, K.-M.; Robbins, K.A. The PREP Pipeline: Standardized Preprocessing for Large-Scale EEG Analysis. Frontiers in Neuroinformatics 2015, 9. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Hsu, S.-H.; Pion-Tonachini, L.; Jung, T.-P. Evaluation of Artifact Subspace Reconstruction for Automatic Artifact Components Removal in Multi-Channel EEG Recordings. IEEE Transactions on Biomedical Engineering 2020, 67, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Pernet, C.R.; Chauveau, N.; Gaspar, C.; Rousselet, G.A. LIMO EEG: A Toolbox for Hierarchical LInear MOdeling of ElectroEncephaloGraphic Data. Computational Intelligence and Neuroscience 2011, 2011, e831409. [Google Scholar] [CrossRef]
- Pernet, C.R.; Latinus, M.; Nichols, T.E.; Rousselet, G.A. Cluster-Based Computational Methods for Mass Univariate Analyses of Event-Related Brain Potentials/Fields: A Simulation Study. Journal of Neuroscience Methods 2015, 250, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Kutas, M.; Federmeier, K.D. Thirty Years and Counting: Finding Meaning in the N400 Component of the Event-Related Brain Potential (ERP). Annu. Rev. Psychol. 2011, 62, 621–647. [Google Scholar] [CrossRef]
- Jung, S.H. Metrical Stress Sensitivity and Reading Skills in Adults, ProQuest Dissertations & Theses: Ann Arbor, 2017.
- Attaheri, A.; Panayiotou, D.; Phillips, A.; Ní Choisdealbha, Á.; Di Liberto, G.M.; Rocha, S.; Brusini, P.; Mead, N.; Flanagan, S.; Olawole-Scott, H.; et al. Cortical Tracking of Sung Speech in Adults vs Infants: A Developmental Analysis. Front. Neurosci. 2022, 16. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.A.; Nicol, T.; Zecker, S.; Kraus, N. Abnormal Cortical Processing of the Syllable Rate of Speech in Poor Readers. Journal of Neuroscience 2009, 29, 7686–7693. [Google Scholar] [CrossRef] [PubMed]
- Power, A.J.; Colling, L.J.; Mead, N.; Barnes, L.; Goswami, U. Neural Encoding of the Speech Envelope by Children with Developmental Dyslexia. Brain Lang 2016, 160, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Henke, L.; Lewis, A.G.; Meyer, L. Fast and Slow Rhythms of Naturalistic Reading Revealed by Combined Eye-Tracking and Electroencephalography. J. Neurosci. 2023, 43, 4461–4469. [Google Scholar] [CrossRef]
- Brennan, J.; Lignos, C.; Embick, D.; Roberts, T.P.L. Spectro-Temporal Correlates of Lexical Access during Auditory Lexical Decision. Brain and Language 2014, 133, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.G.; Schoffelen, J.-M.; Schriefers, H.; Bastiaansen, M. A Predictive Coding Perspective on Beta Oscillations during Sentence-Level Language Comprehension. Front. Hum. Neurosci. 2016, 10. [Google Scholar] [CrossRef]
- Goswami, U. Language Acquisition and Speech Rhythm Patterns: An Auditory Neuroscience Perspective. R. Soc. open sci. 2022, 9, 211855. [Google Scholar] [CrossRef]
- Thomson, J.; Jarmulowicz, L. Linguistic Rhythm and Literacy; John Benjamins Publishing Company, 2016; ISBN 978-90-272-6755-9.
- Wade-Woolley, L.; Wood, C.; Chan, J.; Weidman, S. Prosodic Competence as the Missing Component of Reading Processes Across Languages: Theory, Evidence and Future Research. Scientific Studies of Reading 2022. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).