Submitted:
16 October 2024
Posted:
17 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
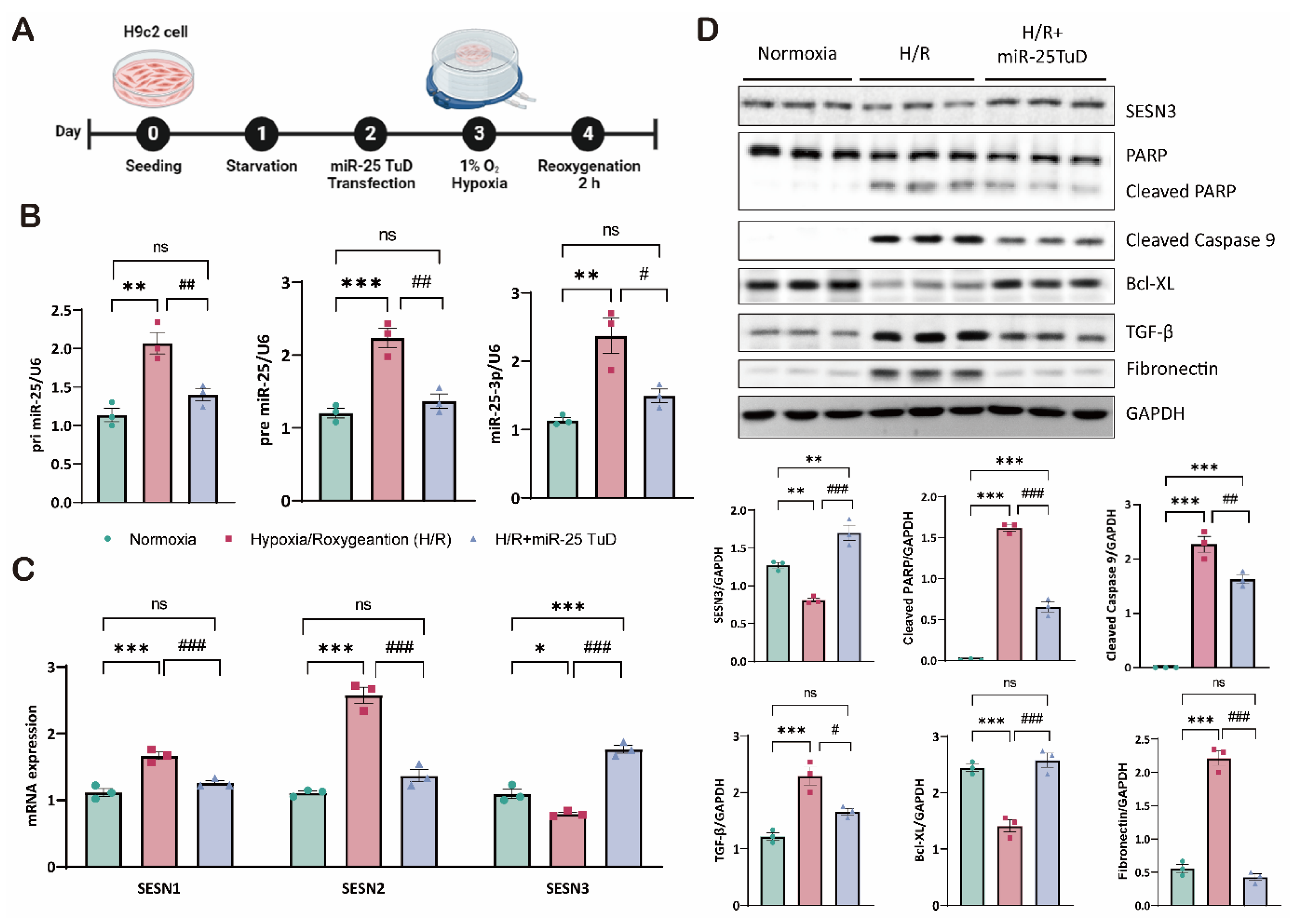
2.1. Cell Culture and Hypoxia/Reoxygenation In Vitro Model
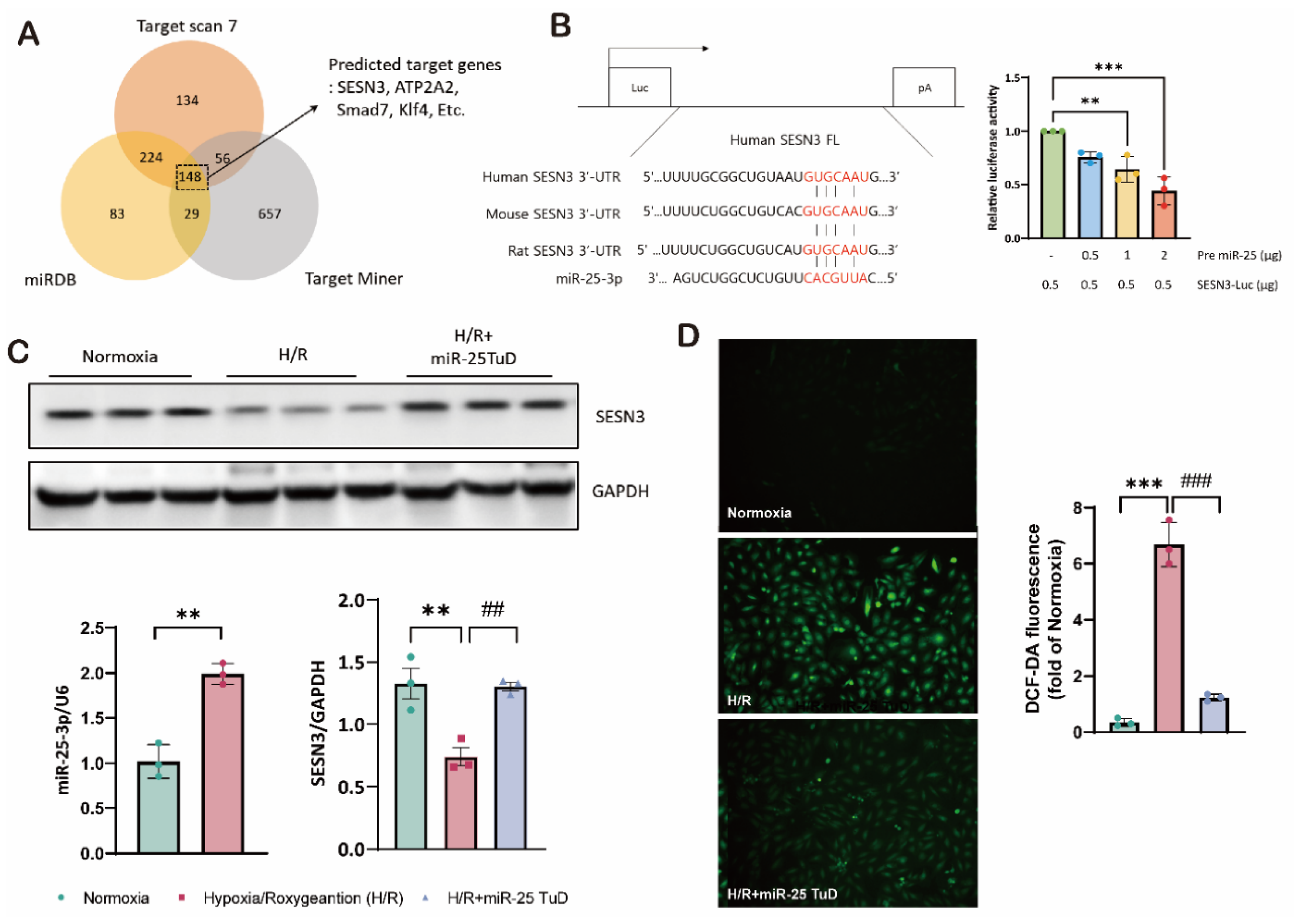
2.2. SESN3 3’-UTR Luciferase Assay
2.3. Measurement of Cellular ROS
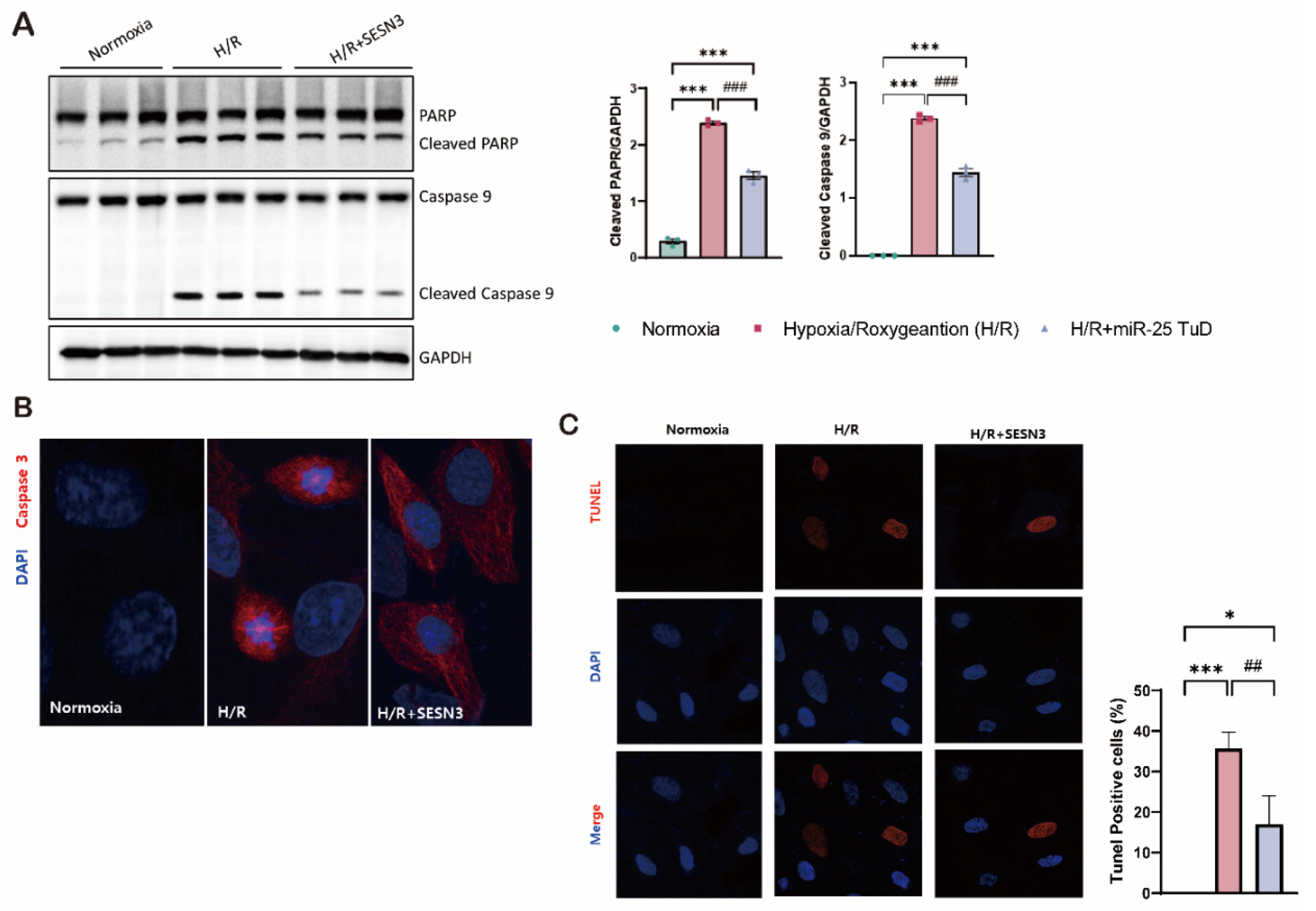
2.4. Immunofluorescence and TdT-Mediated dUTP Nick End-Labeling (TUNEL) Staining
2.5. Animal Care
2.6. Western Blot Analysis
2.7. Quantitative Real-Time PCR
2.8. Myocardial Ischemia-Reperfusion Injury (IRI) Model
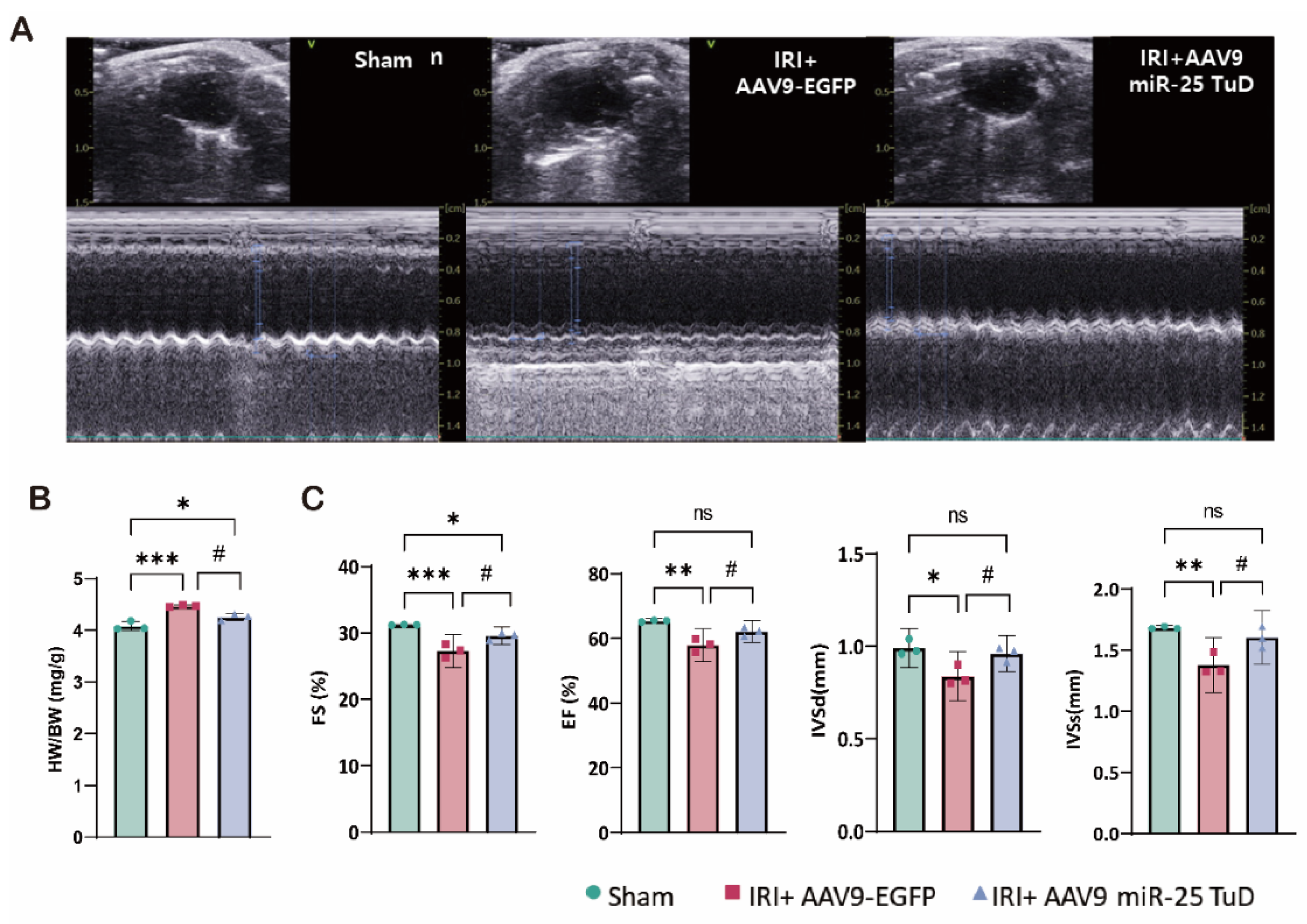
2.9. Evaluation of Heart Function by Echocardiography
2.10. Statistical Analysis
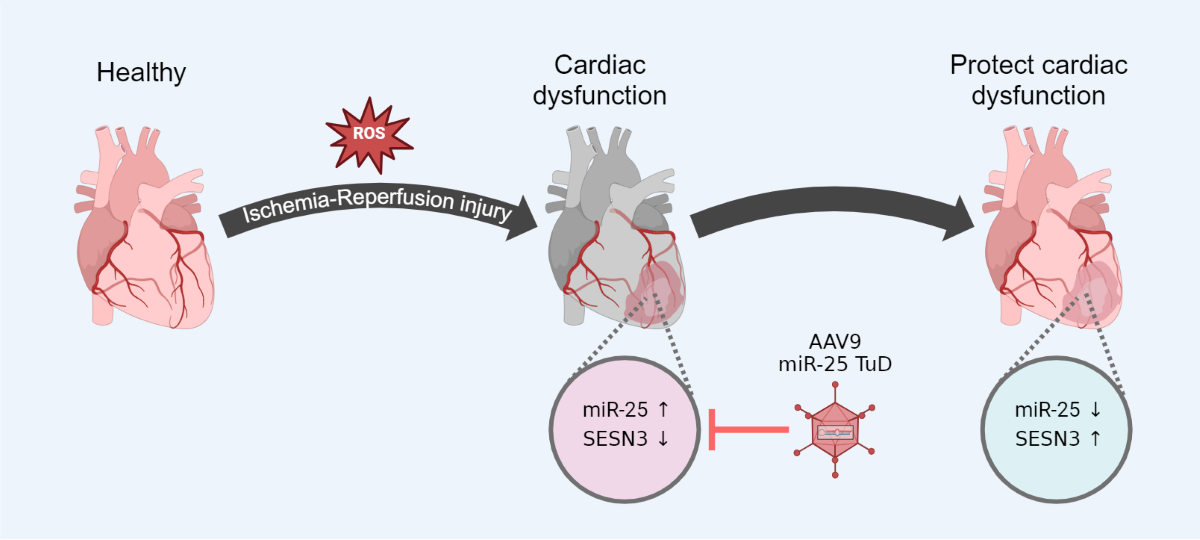
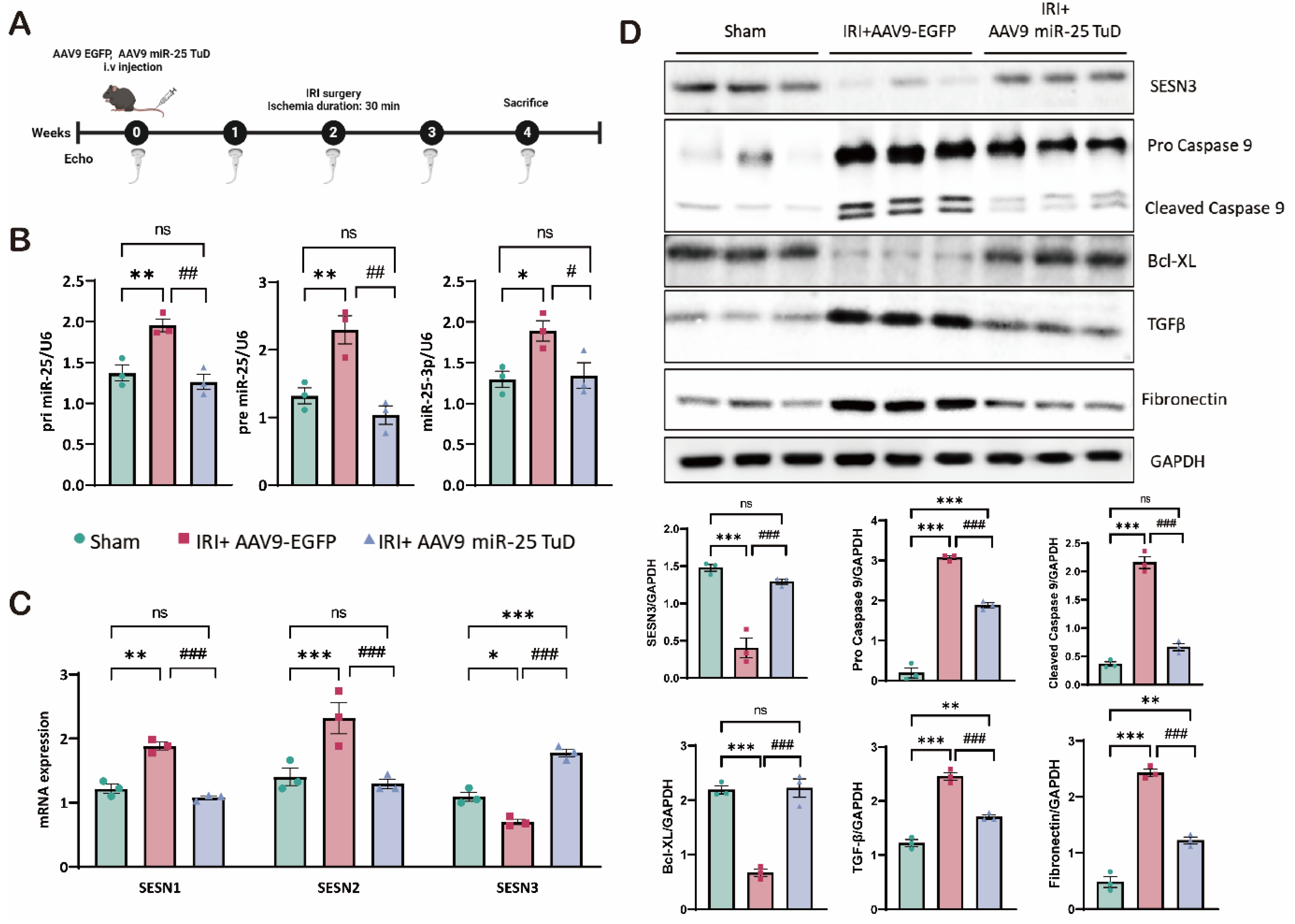
3. Results
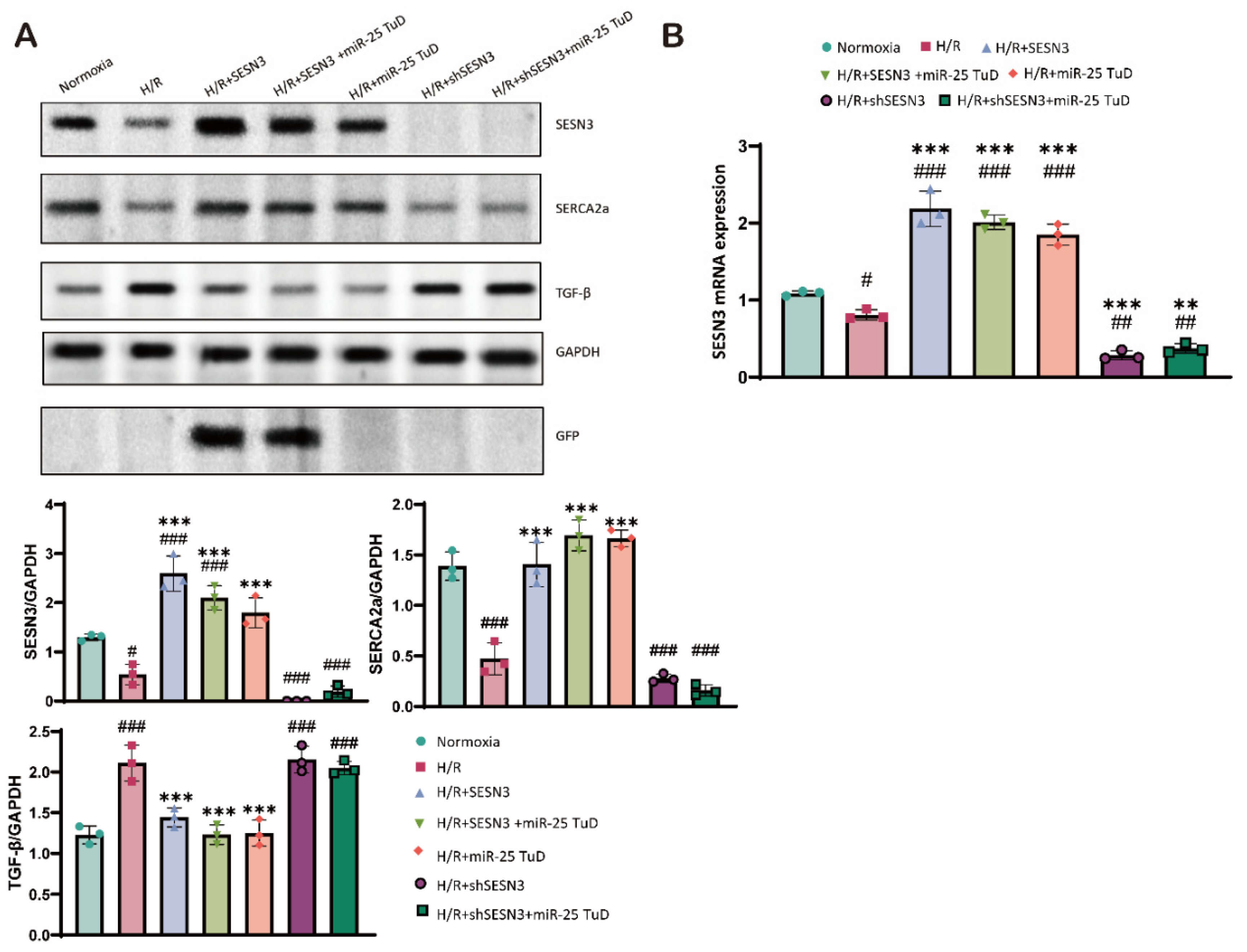
3.1. SESN3 Is Newly Identified Target of miR-25 as a ROS Scavenger
3.2. SESN3 Overexpression Ameliorates ROS-Induced Apoptosis In Vitro
3.6. Knock-Down of SESN3 Neutralizes the Effect of miR-25 TuD Delivery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Ethics Approval
Conflicts of Interest
References
- Del Buono, M.G., Moroni, F., Montone, R.A., Azzalini, L., Sanna, T., and Abbate, A. (2022). Ischemic Cardiomyopathy and Heart Failure After Acute Myocardial Infarction. Curr Cardiol Rep 24, 1505-1515. [CrossRef]
- Panza, J.A., Chrzanowski, L., and Bonow, R.O. (2021). Myocardial Viability Assessment Before Surgical Revascularization in Ischemic Cardiomyopathy: JACC Review Topic of the Week. J Am Coll Cardiol 78, 1068-1077. [CrossRef]
- Cabac-Pogorevici, I., Muk, B., Rustamova, Y., Kalogeropoulos, A., Tzeis, S., and Vardas, P. (2020). Ischaemic cardiomyopathy. Pathophysiological insights, diagnostic management and the roles of revascularisation and device treatment. Gaps and dilemmas in the era of advanced technology. Eur J Heart Fail 22, 789-799. [CrossRef]
- Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., Squadrito, F., Altavilla, D., and Bitto, A. (2017). Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev 2017, 8416763. 10.1155/2017/8416763.
- Li, T., Zhang, X., Jiang, K., Liu, J., and Liu, Z. (2018). Dural effects of oxidative stress on cardiomyogenesis via Gata4 transcription and protein ubiquitination. Cell Death Dis 9, 246. [CrossRef]
- Munzel, T., Camici, G.G., Maack, C., Bonetti, N.R., Fuster, V., and Kovacic, J.C. (2017). Impact of Oxidative Stress on the Heart and Vasculature: Part 2 of a 3-Part Series. J Am Coll Cardiol 70, 212-229. [CrossRef]
- Zhazykbayeva, S., Hassoun, R., Herwig, M., Budde, H., Kovacs, A., Mannherz, H.G., El-Battrawy, I., Toth, A., Schmidt, W.E., Mugge, A., et al. (2023). Oxidative stress and inflammation distinctly drive molecular mechanisms of diastolic dysfunction and remodeling in female and male heart failure with preserved ejection fraction rats. Front Cardiovasc Med 10, 1157398. 10.3389/fcvm.2023.1157398.
- Duan, D., Li, H., Chai, S., Zhang, L., Fan, T., Hu, Z., and Feng, Y. (2024). The relationship between cardiac oxidative stress, inflammatory cytokine response, cardiac pump function, and prognosis post-myocardial infarction. Sci Rep 14, 8985. [CrossRef]
- Li, Q., Ding, J., Xia, B., Liu, K., Zheng, K., Wu, J., Huang, C., Yuan, X., and You, Q. (2024). L-theanine alleviates myocardial ischemia/reperfusion injury by suppressing oxidative stress and apoptosis through activation of the JAK2/STAT3 pathway in mice. Mol Med 30, 98. 10.1186/s10020-024-00865-0.
- Chen, Y., Guo, X., Zeng, Y., Mo, X., Hong, S., He, H., Li, J., Fatima, S., and Liu, Q. (2023). Oxidative stress induces mitochondrial iron overload and ferroptotic cell death. Sci Rep 13, 15515. [CrossRef]
- Cohn, J.N., Ferrari, R., and Sharpe, N. (2000). Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 35, 569-582. [CrossRef]
- Evans, S., Weinheimer, C.J., Kovacs, A., Williams, J.W., Randolph, G.J., Jiang, W., Barger, P.M., and Mann, D.L. (2020). Ischemia reperfusion injury provokes adverse left ventricular remodeling in dysferlin-deficient hearts through a pathway that involves TIRAP dependent signaling. Sci Rep 10, 14129. [CrossRef]
- Hwang, J.W., Park, J.H., Park, B.W., Kim, H., Kim, J.J., Sim, W.S., Mishchenko, N.P., Fedoreyev, S.A., Vasileva, E.A., Ban, K., et al. (2021). Histochrome Attenuates Myocardial Ischemia-Reperfusion Injury by Inhibiting Ferroptosis-Induced Cardiomyocyte Death. Antioxidants (Basel) 10. 10.3390/antiox10101624.
- Pluijmert, N.J., Bart, C.I., Bax, W.H., Quax, P.H.A., and Atsma, D.E. (2020). Effects on cardiac function, remodeling and inflammation following myocardial ischemia-reperfusion injury or unreperfused myocardial infarction in hypercholesterolemic APOE*3-Leiden mice. Sci Rep 10, 16601. [CrossRef]
- van der Bijl, P., Abou, R., Goedemans, L., Gersh, B.J., Holmes, D.R., Jr., Ajmone Marsan, N., Delgado, V., and Bax, J.J. (2020). Left Ventricular Post-Infarct Remodeling: Implications for Systolic Function Improvement and Outcomes in the Modern Era. JACC Heart Fail 8, 131-140. [CrossRef]
- Ikeda, Y., Young, L.H., Scalia, R., Ross, C.R., and Lefer, A.M. (2001). PR-39, a proline/arginine-rich antimicrobial peptide, exerts cardioprotective effects in myocardial ischemia-reperfusion. Cardiovasc Res 49, 69-77. [CrossRef]
- Yang, J., Wang, Z., and Chen, D.L. (2017). Shikonin ameliorates isoproterenol (ISO)-induced myocardial damage through suppressing fibrosis, inflammation, apoptosis and ER stress. Biomed Pharmacother 93, 1343-1357. [CrossRef]
- Glorieux, C., and Calderon, P.B. (2017). Catalase, a remarkable enzyme: targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol Chem 398, 1095-1108. 10.1515/hsz-2017-0131.
- Lai, L., Yan, L., Gao, S., Hu, C.L., Ge, H., Davidow, A., Park, M., Bravo, C., Iwatsubo, K., Ishikawa, Y., et al. (2013). Type 5 adenylyl cyclase increases oxidative stress by transcriptional regulation of manganese superoxide dismutase via the SIRT1/FoxO3a pathway. Circulation 127, 1692-1701. 10.1161/CIRCULATIONAHA.112.001212.
- Dudek, H., Datta, S.R., Franke, T.F., Birnbaum, M.J., Yao, R., Cooper, G.M., Segal, R.A., Kaplan, D.R., and Greenberg, M.E. (1997). Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275, 661-665. 10.1126/science.275.5300.661.
- Kennedy, S.G., Wagner, A.J., Conzen, S.D., Jordan, J., Bellacosa, A., Tsichlis, P.N., and Hay, N. (1997). The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev 11, 701-713. 10.1101/gad.11.6.701.
- Widmann, C., Gibson, S., Jarpe, M.B., and Johnson, G.L. (1999). Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev 79, 143-180. 10.1152/physrev.1999.79.1.143.
- Nguyen, B.Y., Ruiz-Velasco, A., Bui, T., Collins, L., Wang, X., and Liu, W. (2019). Mitochondrial function in the heart: the insight into mechanisms and therapeutic potentials. Br J Pharmacol 176, 4302-4318. 10.1111/bph.14431.
- Hollander, J.M., Thapa, D., and Shepherd, D.L. (2014). Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: influence of cardiac pathologies. Am J Physiol Heart Circ Physiol 307, H1-14. 10.1152/ajpheart.00747.2013.
- Ikeuchi, M., Matsusaka, H., Kang, D., Matsushima, S., Ide, T., Kubota, T., Fujiwara, T., Hamasaki, N., Takeshita, A., Sunagawa, K., et al. (2005). Overexpression of mitochondrial transcription factor a ameliorates mitochondrial deficiencies and cardiac failure after myocardial infarction. Circulation 112, 683-690. 10.1161/CIRCULATIONAHA.104.524835.
- Forman, H.J., and Zhang, H. (2021). Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov 20, 689-709. [CrossRef]
- Rojo, A.I., Salinas, M., Martin, D., Perona, R., and Cuadrado, A. (2004). Regulation of Cu/Zn-superoxide dismutase expression via the phosphatidylinositol 3 kinase/Akt pathway and nuclear factor-kappaB. J Neurosci 24, 7324-7334. 10.1523/JNEUROSCI.2111-04.2004.
- Velasco-Miguel, S., Buckbinder, L., Jean, P., Gelbert, L., Talbott, R., Laidlaw, J., Seizinger, B., and Kley, N. (1999). PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Oncogene 18, 127-137. [CrossRef]
- Peeters, H., Debeer, P., Bairoch, A., Wilquet, V., Huysmans, C., Parthoens, E., Fryns, J.P., Gewillig, M., Nakamura, Y., Niikawa, N., et al. (2003). PA26 is a candidate gene for heterotaxia in humans: identification of a novel PA26-related gene family in human and mouse. Hum Genet 112, 573-580. [CrossRef]
- Kumar, A., Dhiman, D., and Shaha, C. (2020). Sestrins: Darkhorse in the regulation of mitochondrial health and metabolism. Mol Biol Rep 47, 8049-8060. [CrossRef]
- Tao, R., Xiong, X., Liangpunsakul, S., and Dong, X.C. (2015). Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes 64, 1211-1223. 10.2337/db14-0539.
- Cordani, M., Butera, G., Dando, I., Torrens-Mas, M., Butturini, E., Pacchiana, R., Oppici, E., Cavallini, C., Gasperini, S., Tamassia, N., et al. (2018). Mutant p53 blocks SESN1/AMPK/PGC-1alpha/UCP2 axis increasing mitochondrial O(2-). production in cancer cells. Br J Cancer 119, 994-1008. [CrossRef]
- Deng, W., Cha, J., Yuan, J., Haraguchi, H., Bartos, A., Leishman, E., Viollet, B., Bradshaw, H.B., Hirota, Y., and Dey, S.K. (2016). p53 coordinates decidual sestrin 2/AMPK/mTORC1 signaling to govern parturition timing. J Clin Invest 126, 2941-2954. 10.1172/JCI87715.
- Morsch, A., Wisniewski, E., Luciano, T.F., Comin, V.H., Silveira, G.B., Marques, S.O., Thirupathi, A., Silveira Lock, P.C., and De Souza, C.T. (2019). Cigarette smoke exposure induces ROS-mediated autophagy by regulating sestrin, AMPK, and mTOR level in mice. Redox Rep 24, 27-33. [CrossRef]
- Xue, R., Zeng, J., Chen, Y., Chen, C., Tan, W., Zhao, J., Dong, B., Sun, Y., Dong, Y., and Liu, C. (2017). Sestrin 1 ameliorates cardiac hypertrophy via autophagy activation. J Cell Mol Med 21, 1193-1205. 10.1111/jcmm.13052.
- Lear, T.B., Lockwood, K.C., Ouyang, Y., Evankovich, J.W., Larsen, M.B., Lin, B., Liu, Y., and Chen, B.B. (2019). The RING-type E3 ligase RNF186 ubiquitinates Sestrin-2 and thereby controls nutrient sensing. J Biol Chem 294, 16527-16534. [CrossRef]
- Ovchinnikova, E.S., Schmitter, D., Vegter, E.L., Ter Maaten, J.M., Valente, M.A., Liu, L.C., van der Harst, P., Pinto, Y.M., de Boer, R.A., Meyer, S., et al. (2016). Signature of circulating microRNAs in patients with acute heart failure. Eur J Heart Fail 18, 414-423. [CrossRef]
- Sygitowicz, G., Tomaniak, M., Blaszczyk, O., Koltowski, L., Filipiak, K.J., and Sitkiewicz, D. (2015). Circulating microribonucleic acids miR-1, miR-21 and miR-208a in patients with symptomatic heart failure: Preliminary results. Arch Cardiovasc Dis 108, 634-642. [CrossRef]
- Seronde, M.F., Vausort, M., Gayat, E., Goretti, E., Ng, L.L., Squire, I.B., Vodovar, N., Sadoune, M., Samuel, J.L., Thum, T., et al. (2015). Circulating microRNAs and Outcome in Patients with Acute Heart Failure. PLoS One 10, e0142237. 10.1371/journal.pone.0142237.
- Wahlquist, C., Jeong, D., Rojas-Munoz, A., Kho, C., Lee, A., Mitsuyama, S., van Mil, A., Park, W.J., Sluijter, J.P., Doevendans, P.A., et al. (2014). Inhibition of miR-25 improves cardiac contractility in the failing heart. Nature 508, 531-535. [CrossRef]
- Park, W.J., and Oh, J.G. (2013). SERCA2a: a prime target for modulation of cardiac contractility during heart failure. BMB Rep 46, 237-243. 10.5483/bmbrep.2013.46.5.077.
- del Monte, F., Harding, S.E., Schmidt, U., Matsui, T., Kang, Z.B., Dec, G.W., Gwathmey, J.K., Rosenzweig, A., and Hajjar, R.J. (1999). Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation 100, 2308-2311. 10.1161/01.cir.100.23.2308.
- Kim, M., Sujkowski, A., Namkoong, S., Gu, B., Cobb, T., Kim, B., Kowalsky, A.H., Cho, C.S., Semple, I., Ro, S.H., et al. (2020). Sestrins are evolutionarily conserved mediators of exercise benefits. Nat Commun 11, 190. [CrossRef]
- Wang, A.J., Tang, Y., Zhang, J., Wang, B.J., Xiao, M., Lu, G., Li, J., Liu, Q., Guo, Y., and Gu, J. (2022). Cardiac SIRT1 ameliorates doxorubicin-induced cardiotoxicity by targeting sestrin 2. Redox Biol 52, 102310. [CrossRef]
- Haidurov, A., and Budanov, A.V. (2020). Sestrin family - the stem controlling healthy ageing. Mech Ageing Dev 192, 111379. [CrossRef]
- Li, R., Huang, Y., Semple, I., Kim, M., Zhang, Z., and Lee, J.H. (2019). Cardioprotective roles of sestrin 1 and sestrin 2 against doxorubicin cardiotoxicity. Am J Physiol Heart Circ Physiol 317, H39-H48. 10.1152/ajpheart.00008.2019.
- Moris, D., Spartalis, M., Spartalis, E., Karachaliou, G.S., Karaolanis, G.I., Tsourouflis, G., Tsilimigras, D.I., Tzatzaki, E., and Theocharis, S. (2017). The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Ann Transl Med 5, 326. 10.21037/atm.2017.06.27.
- Verma, S., Fedak, P.W., Weisel, R.D., Butany, J., Rao, V., Maitland, A., Li, R.K., Dhillon, B., and Yau, T.M. (2002). Fundamentals of reperfusion injury for the clinical cardiologist. Circulation 105, 2332-2336. 10.1161/01.cir.0000016602.96363.36.
- Lang, A., Grether-Beck, S., Singh, M., Kuck, F., Jakob, S., Kefalas, A., Altinoluk-Hambuchen, S., Graffmann, N., Schneider, M., Lindecke, A., et al. (2016). MicroRNA-15b regulates mitochondrial ROS production and the senescence-associated secretory phenotype through sirtuin 4/SIRT4. Aging (Albany NY) 8, 484-505. 10.18632/aging.100905.
- Caravia, X.M., Fanjul, V., Oliver, E., Roiz-Valle, D., Moran-Alvarez, A., Desdin-Mico, G., Mittelbrunn, M., Cabo, R., Vega, J.A., Rodriguez, F., et al. (2018). The microRNA-29/PGC1alpha regulatory axis is critical for metabolic control of cardiac function. PLoS Biol 16, e2006247. 10.1371/journal.pbio.2006247.
- Dubois-Deruy, E., Cuvelliez, M., Fiedler, J., Charrier, H., Mulder, P., Hebbar, E., Pfanne, A., Beseme, O., Chwastyniak, M., Amouyel, P., et al. (2017). MicroRNAs regulating superoxide dismutase 2 are new circulating biomarkers of heart failure. Sci Rep 7, 14747. [CrossRef]
- Li, Z., Li, H., Liu, B., Luo, J., Qin, X., Gong, M., Shi, B., and Wei, Y. (2020). Inhibition of miR-25 attenuates doxorubicin-induced apoptosis, reactive oxygen species production and DNA damage by targeting PTEN. Int J Med Sci 17, 1415-1427. 10.7150/ijms.41980.
- Liu, Q., Wang, Y., Yang, T., and Wei, W. (2016). Protective effects of miR-25 against hypoxia/reoxygenation-induced fibrosis and apoptosis of H9c2 cells. Int J Mol Med 38, 1225-1234. 10.3892/ijmm.2016.2702.
- Chen, C.C., Jeon, S.M., Bhaskar, P.T., Nogueira, V., Sundararajan, D., Tonic, I., Park, Y., and Hay, N. (2010). FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell 18, 592-604. [CrossRef]
- Budanov, A.V., and Karin, M. (2008). p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134, 451-460. [CrossRef]
- Liu, X., Niu, Y., Yuan, H., Huang, J., and Fu, L. (2015). AMPK binds to Sestrins and mediates the effect of exercise to increase insulin-sensitivity through autophagy. Metabolism 64, 658-665. [CrossRef]
- Hagenbuchner, J., Kuznetsov, A., Hermann, M., Hausott, B., Obexer, P., and Ausserlechner, M.J. (2012). FOXO3-induced reactive oxygen species are regulated by BCL2L11 (Bim) and SESN3. J Cell Sci 125, 1191-1203. 10.1242/jcs.092098.
- Johnson, M.R., Behmoaras, J., Bottolo, L., Krishnan, M.L., Pernhorst, K., Santoscoy, P.L.M., Rossetti, T., Speed, D., Srivastava, P.K., Chadeau-Hyam, M., et al. (2015). Systems genetics identifies Sestrin 3 as a regulator of a proconvulsant gene network in human epileptic hippocampus. Nat Commun 6, 6031. [CrossRef]
- Ge, L., Xu, M., Brant, S.R., Liu, S., Zhu, C., Shang, J., Zhao, Q., and Zhou, F. (2020). Sestrin3 enhances macrophage-mediated generation of T helper 1 and T helper 17 cells in a mouse colitis model. Int Immunol 32, 421-432. [CrossRef]
- Huang, M., Kim, H.G., Zhong, X., Dong, C., Zhang, B., Fang, Z., Zhang, Y., Lu, X., Saxena, R., Liu, Y., et al. (2020). Sestrin 3 Protects Against Diet-Induced Nonalcoholic Steatohepatitis in Mice Through Suppression of Transforming Growth Factor beta Signal Transduction. Hepatology 71, 76-92. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




