Submitted:
16 October 2024
Posted:
17 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
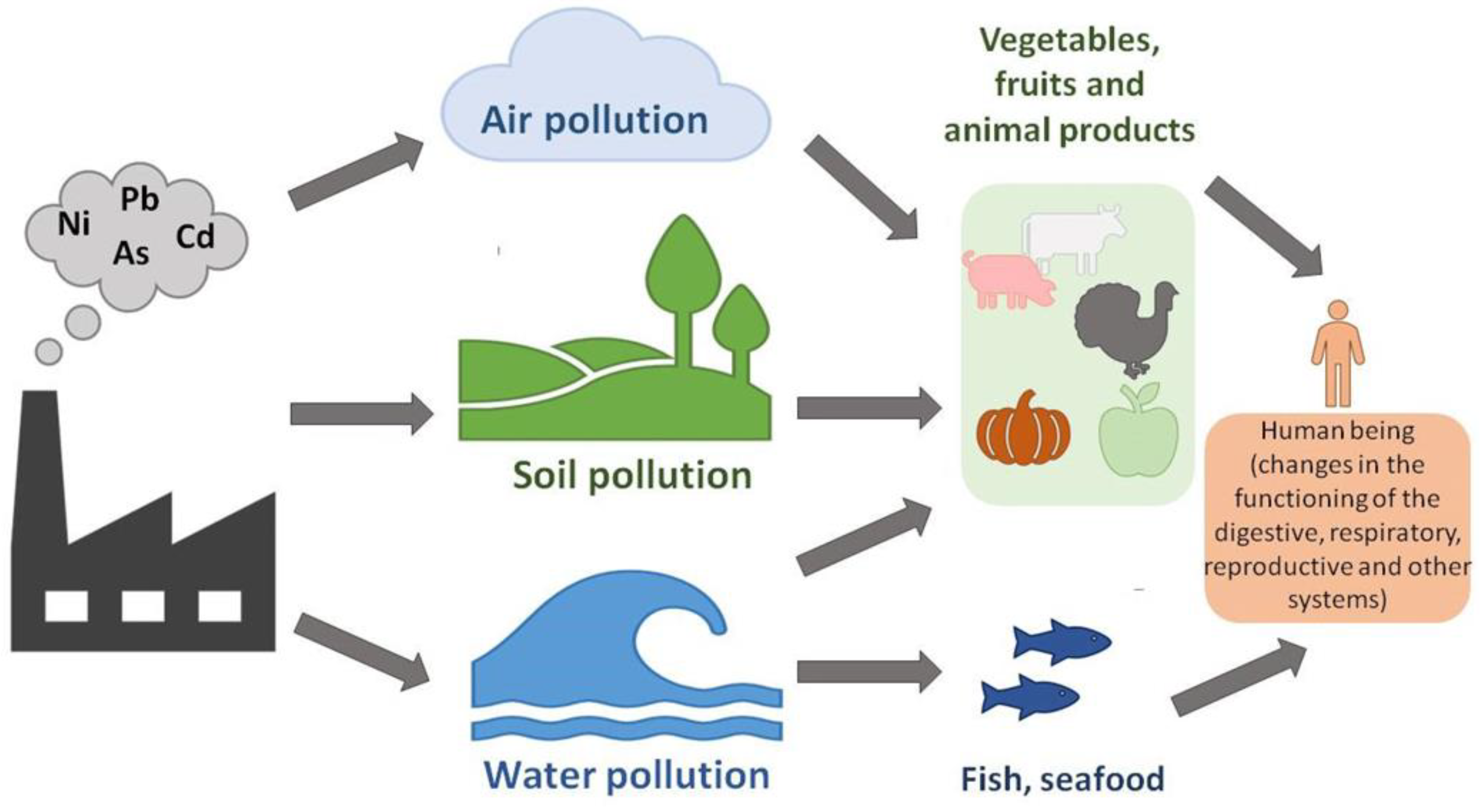
2. The Origin of Cd Pollution
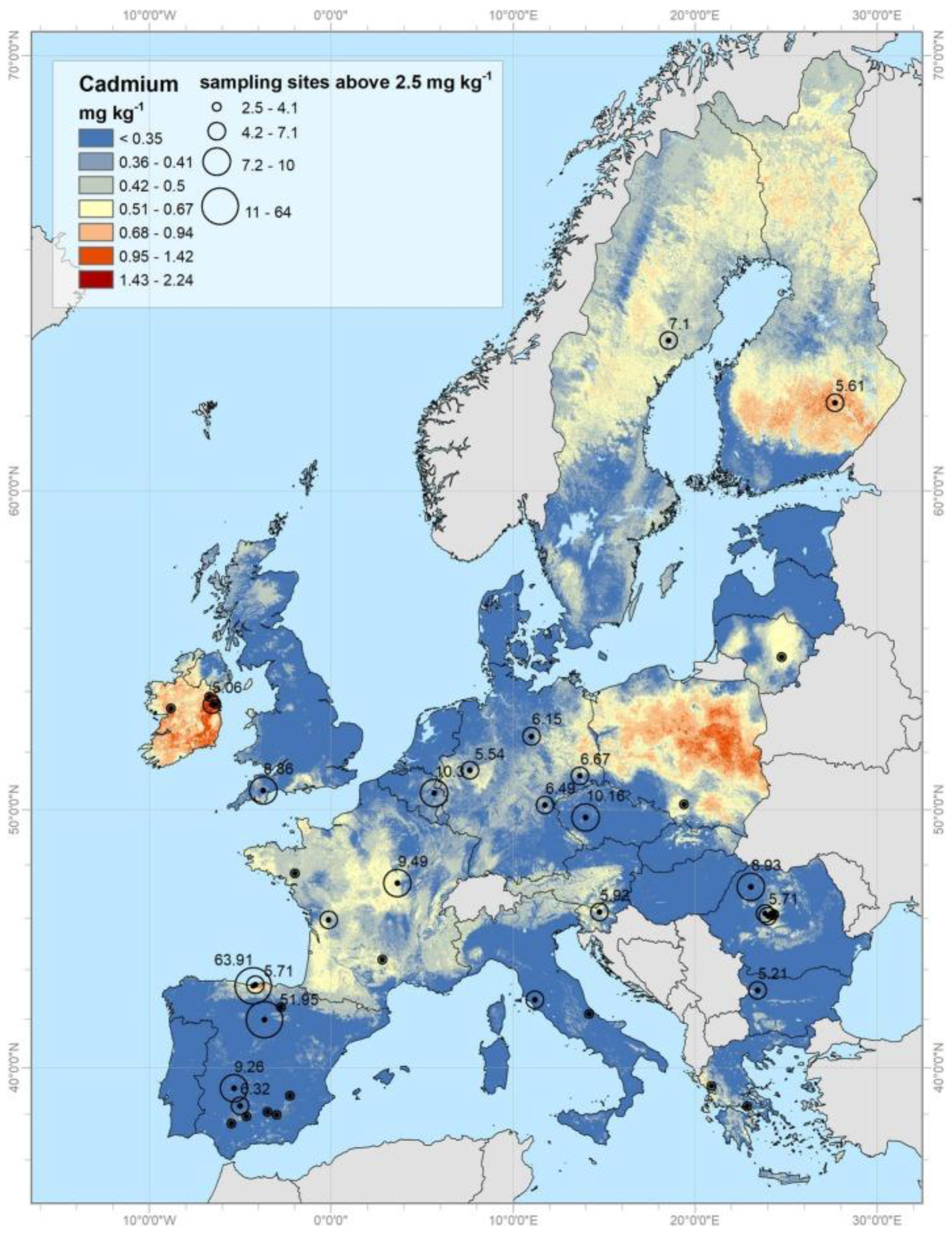
2.1. Natural Sources of Cd
2.1.1. Cd in Soil Water and Groundwater
2.1.2. Air
2.2. Anthropogenic Cd Sources
3. Cd Toxicity on Living Organisms
3.1. Cd Accumulation and Toxicity on Bacteria
3.1.1. Resistance Mechanisms of Bacteria
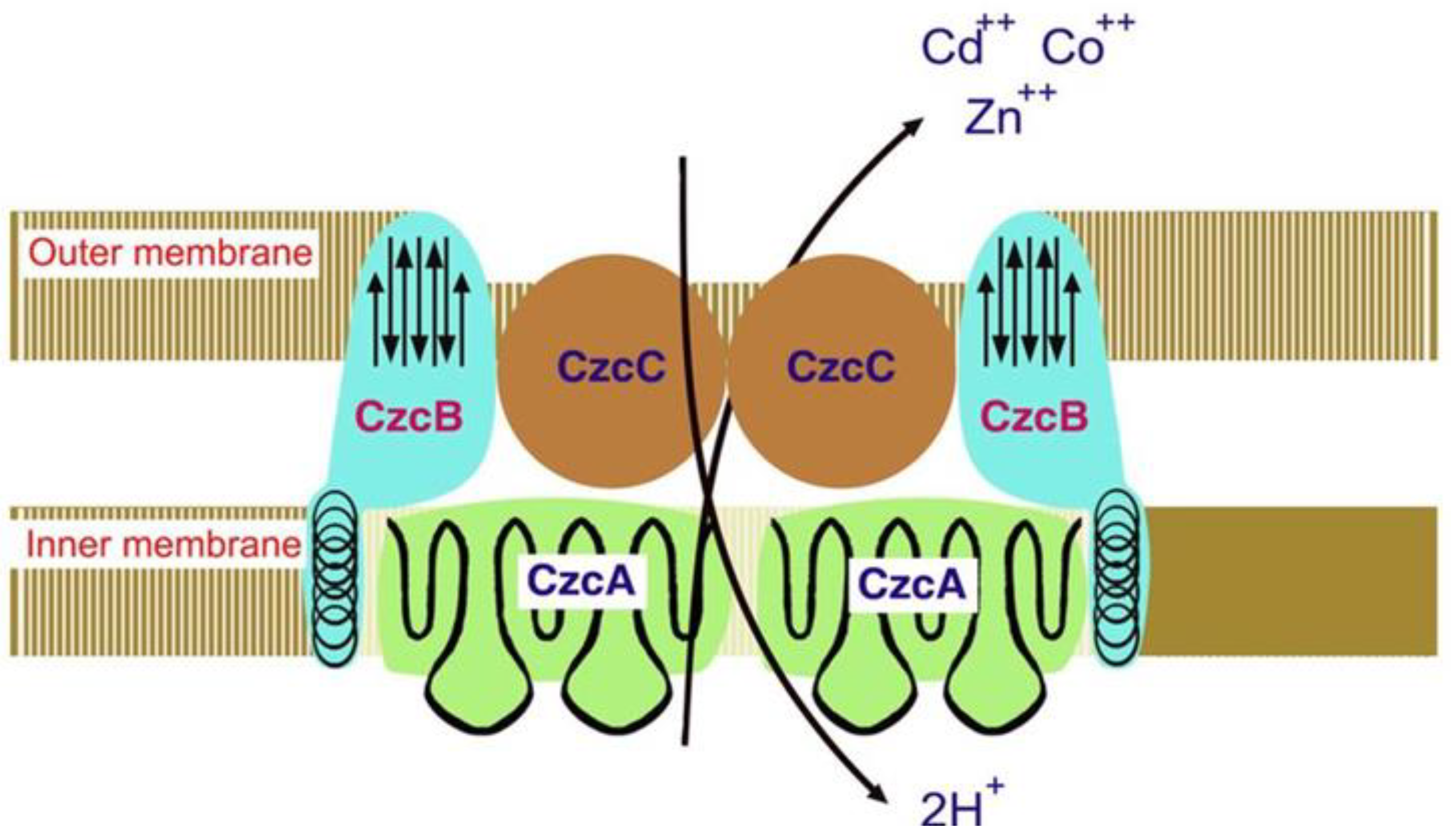
3.1.1.1. Proteins Conducting the Export of Heavy Metals
3.1.1.2. Proteins Facilitating the Transport of Heavy Metals
3.1.1.3. P-Type ATP-Ase
3.1.2. Bacteria in Cd-Contaminated Soil
3.2. Cd Accumulation and Toxicity on Fungi
3.3. Cd Accumulation and Toxicity on Edible Mushrooms
3.4. Cd Accumulation and Toxicity on Plants
3.5. Cd Accumulation and Toxicity on Animals
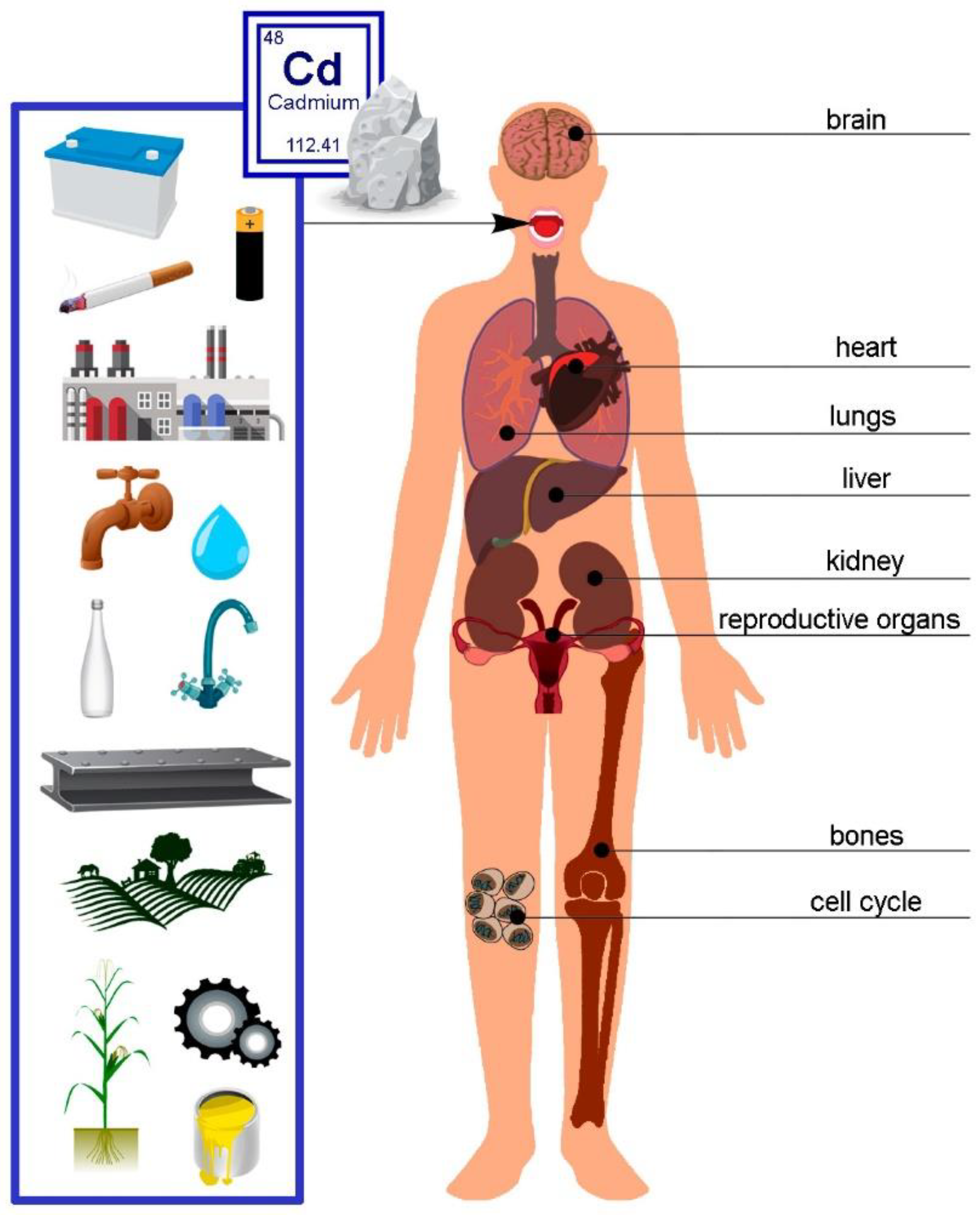
3.6. Cd Accumulation and Toxicity in Humans
3.6.1. Cd Distribution in the Human Body
3.6.1.1. Ingestion
3.6.1.2. Inhalation
3.6.1.3. Permeation
3.6.2. Harmful Effects on Human Organs and Systems
3.6.2.1. Effects on the Blood and Circulatory System
3.6.2.2. Effects on the Reproductive System
3.6.2.3. Effects on the Respiratory System
3.6.2.4. Effects on the Kidney System and Bones
3.6.2.5. Effects on the Central Nervous System
3.6.2.6. Effects on the Immune System
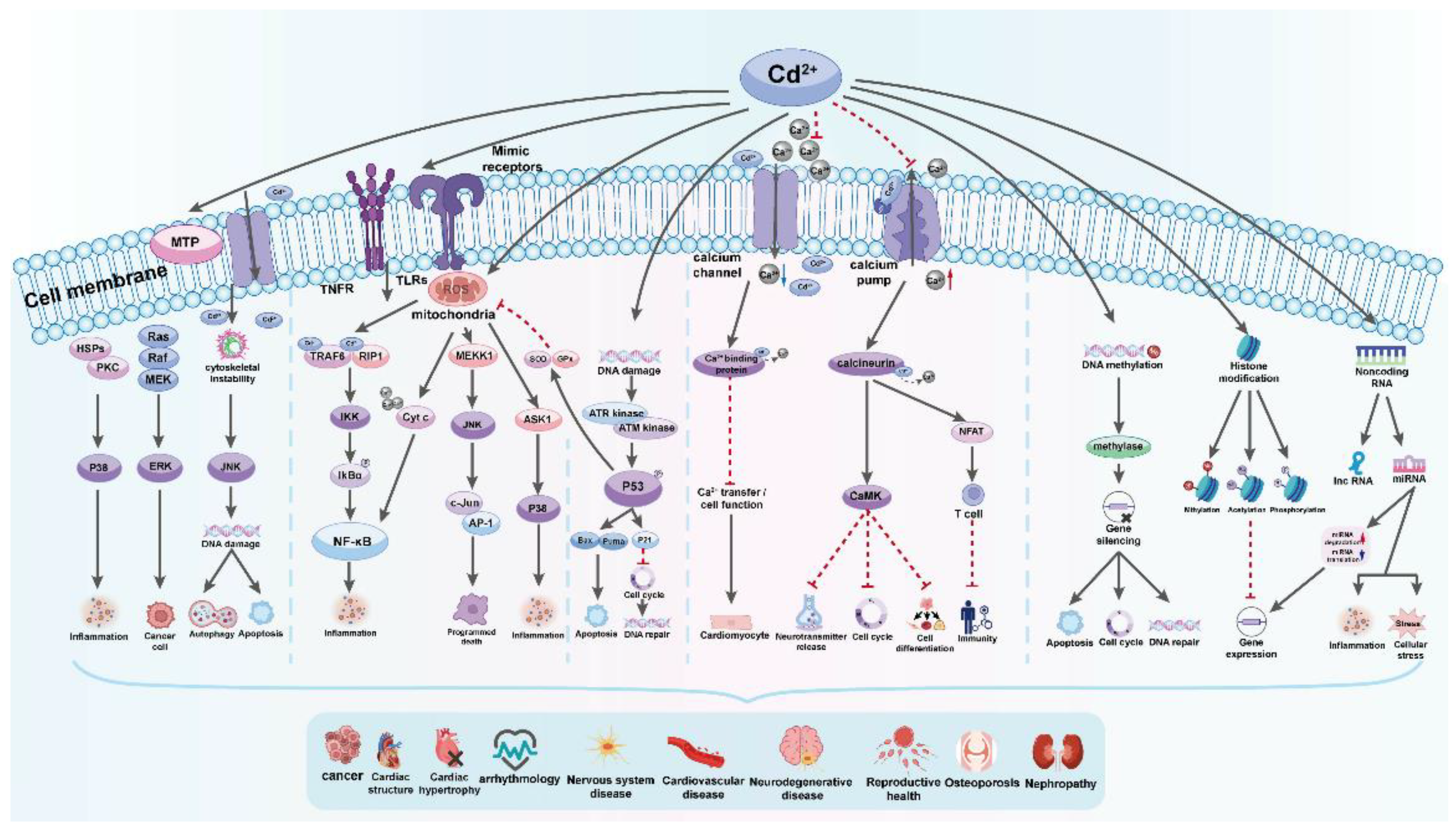
4. Molecular Mechanisms of Cd Toxicity
5. Methods for Cd Removal
5.1. From the Environment
5.1.1. Chemical Precipitation
5.1.2. Adsorption
5.1.3. Ion-Exchange
5.1.4. Membrane Filtration
5.2. From the Human Body
6. Cd Resistance Mechanisms
6.1. Biosorption
6.2. Efflux Transport Systems
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peana, M.; Pelucelli, A.; Chasapis, C.T.; Perlepes, S.P.; Bekiari, V.; Medici, S.; Zoroddu, M.A. Biological Effects of Human Exposure to Environmental Cadmium. Biomolecules 2023, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Thomas O. Llewellyn IC 9380 Cadmium (Materials Flow). The National Institute for Occupational Safety and Health (NIOSH), 1994.
- Goering, P. L.; Waalkes, M. P.; Klaassen, C. D. Toxicology of Cadmium. Toxicology of Metals 1995, 189–214. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, J.; Gao, Y.; Liu, X.; Qu, L.; Zhu, L. Physiologically Based Toxicokinetic and Toxicodynamic (PBTK-TD) Modelling of Cd and Pb Exposure in Adult Zebrafish Danio Rerio: Accumulation and Toxicity. Environmental Pollution 2019, 249, 959–968, Nordberg, G. F. Historical Perspectives on Cadmium Toxicology. Toxicology and Applied Pharmacology 2009, 238 (3), 192–200. https://doi.org/10.1016/j.taap.2009.03.015. [Google Scholar] [CrossRef] [PubMed]
- Fatima, G.; Raza, A. M.; Hadi, N.; Nigam, N.; Mahdi, A. A. Cadmium in Human Diseases: It’s More than Just a Mere Metal. Indian Journal of Clinical Biochemistry 2019, 34, 371–378. [Google Scholar] [CrossRef]
- Nordberg, G. F. Historical Perspectives on Cadmium Toxicology. Toxicology and Applied Pharmacology 2009, 238, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ding, S.; Li, C.; Tang, Y.; Fan, X.; Xu, H.; Daniel C.W., Tsang; Zhang, C. High Cadmium Pollution from Sediments in a Eutrophic Lake Caused by Dissolved Organic Matter Complexation and Reduction of Manganese Oxide. Water Research 2021, 190, 116711–116711. [Google Scholar] [CrossRef]
- Sienko, M. J. Chemistry, Principles and Applications; New York: McGraw-Hill, 1979. [Google Scholar]
- Friberg, L. T.; Carl-Gustaf Elinder; Tord Kjellstrom; Nordberg, G. F. Cadmium and Health; CRC Press, 2019.
- Lindén, K. Andersson, A. Os, A. Cadmium in Organic and Conventional Pig Production. Archives of Environmental Contamination and Toxicology 2001, 40, 425–431. [Google Scholar] [CrossRef]
- Suciu, N. A.; Devivo, R.; Rizzati, N.; Capri, E. Cd Content in Phosphate Fertilizer: Which Potential Risk for the Environment and Human Health? Current Opinion in Environmental Science & Health 2022, 100392. [Google Scholar] [CrossRef]
- Alloway, B. J.; Steinnes, E. Anthropogenic Additions of Cadmium to Soils. Cadmium in Soils and Plants 1999, 97–123. [Google Scholar] [CrossRef]
- Satchanska, G. Toxicity of heavy metals and pesticides on living world. NBU Publishing house, Sofia, Bulgaria. Monograph. 2024 ISBN 978-619-233-322-5.
- Yuan, Z.; Luo, T.; Liu, X.; Hua, H.; Zhuang, Y.; Zhang, X.; Zhang, L.; Zhang, Y.; Xu, W.; Ren, J. Tracing Anthropogenic Cadmium Emissions: From Sources to Pollution. Science of The Total Environment 2019, 676, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, K.; Jiang, S.; Liu, D.; Zhou, H.; Zhong, R.; Zeng, Q.; Cheng, L.; Miao, X.; Tong, Y.; Lu, Q. Association of Co-Exposure to Heavy Metals with Renal Function in a Hypertensive Population. Environment international 2018, 112, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Goncharuk, E.A.; Zagoskina, N.V. Heavy Metals, Their Phytotoxicity, and the Role of Phenolic Antioxidants in Plant Stress Responses with Focus on Cadmium: Review. Molecules 2023, 28, 3921. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Zhang, Y.; Cloquet, C.; Zhu, C.; Fan, H.; Luo, C. Tracing Sources of Pollution in Soils from the Jinding Pb–Zn Mining District in China Using Cadmium and Lead Isotopes. Applied Geochemistry 2015, 52, 147–154. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, X. Multivariate and Geostatistical Analyses of Heavy Metal Pollution from Different Sources among Farmlands in the Poyang Lake Region, China. Journal of Soils and Sediments 2019, 19, 2472–2484. [Google Scholar] [CrossRef]
- Mikhailenko, A.V.; Ruban, D.A.; Ermolaev, V.A.; van Loon, A.J. Cadmium Pollution in the Tourism Environment: A Literature Review. Geosciences 2020, 10, 242. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R. T.; Pichler, T. Cadmium in Soils and Groundwater: A Review. Applied Geochemistry 2019, 108, 104388. [Google Scholar] [CrossRef]
- Hayat, M. T.; Nauman, M.; Nazir, N.; Ali, S.; Bangash, N. Environmental Hazards of Cadmium: Past, Present, and Future. Cadmium Toxicity and Tolerance in Plants 2019, 163–183. [Google Scholar] [CrossRef]
- European Commission. Europa.eu. https://esdac.jrc.ec.europa.eu/search/node/cadmium (accessed 2024-08-21).
- Ballabio, C.; Jones, A.; Panagos, P. Cadmium in Topsoils of the European Union – an Analysis Based on LUCAS Topsoil Database. Science of The Total Environment 2024, 912, 168710. [Google Scholar] [CrossRef]
- Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe.
- Wu, Y.; Yang, X.; Wang, H.; Jia, G.; Wang, T. Relationship between Ambient PM2.5 Exposure and Blood Cadmium Level in Children under 14 Years in Beijing, China. Journal of Hazardous Materials 2021, 403, 123871. [Google Scholar] [CrossRef]
- Scaccabarozzi, D.; Castillo, L.; Aromatisi, A.; Milne, L.; Búllon Castillo, A.; Muñoz-Rojas, M. Soil, Site, and Management Factors Affecting Cadmium Concentrations in Cacao-Growing Soils. Agronomy 2020, 10, 806. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Haider, F.U.; Maqsood, M.F.; Mohy-Ud-Din, W.; Shabaan, M.; Ahmad, M.; Kaleem, M.; Ishfaq, M.; Aslam, Z.; Shahzad, B. Recent Advances in Microbial-Assisted Remediation of Cadmium-Contaminated Soil. Plants 2023, 12, 3147. [Google Scholar] [CrossRef]
- Satchanska, G.; Ivanova, I.; Veneta Groudeva; Pentcheva, E. N.; Kerestedjian, T.; Evgeny Golovinsky. Culture-Dependent Approach for Determining Microbial Diversity in Soils from KCM/AGRIA Region. Comptes Renduе of Academy Bulgarian of Science 2005, 58, 409–416. [Google Scholar]
- Surenbaatar, U.; Lee, S.; Kwon, J.-Y.; Lim, H.; Kim, J.-J.; Kim, Y.-H.; Hong, Y.-S. Bioaccumulation of Lead, Cadmium, and Arsenic in a Mining Area and Its Associated Health Effects. Toxics 2023, 11, 519. [Google Scholar] [CrossRef]
- Satchanska, G. Bioindicatory and biotransformation activity of microorganisms obtained from xenobiotics polluted soils and waters. PHD Thesis, A book. Edd. PSSE, Sofia, Bulgaria. 2022, 146 p. ISBN 978-954-749-126-7.
- Gómez, M.; Grimes, S.; Qian, Y.; Feng, Y.; Fowler, G. Critical and Strategic Metals in Mobile Phones: A Detailed Characterisation of Multigenerational Waste Mobile Phones and the Economic Drivers for Recovery of Metal Value. Journal of Cleaner Production 2023, 419, 138099. [Google Scholar] [CrossRef]
- Igweze, Z. N.; Ekhator, O. C.; Orisakwe, O. E. A Pediatric Health Risk Assessment of Children’s Toys Imported from China into Nigeria. Heliyon 2020, 6. [Google Scholar] [CrossRef]
- Bielak, E.; Marcinkowska, E. Heavy Metals in Leathers, Artificial Leathers, and Textiles in the Context of Quality and Safety of Use. Scientific Reports 2022, 12. [Google Scholar] [CrossRef]
- Kern, M. S.; Boron, M. L.; Weidenhamer, J. D. Buyer Beware: Inexpensive, High Cadmium Jewelry Can Pose Severe Health Risks. Science of The Total Environment 2020, 142926. [Google Scholar] [CrossRef]
- Satchanska, G.; Pentcheva, E. N.; Atanasova, R.; Groudeva, V.; Trifonova, R.; Golovinsky, E. Microbial Diversity in Heavy-Metal Polluted Waters. Biotechnology & Biotechnological Equipment 2005, 19, 61–67. [Google Scholar] [CrossRef]
- Cordoba-Novoa, H. A.; Jeimmy Cáceres-Zambrano; Torres-Rojas, E. Isolation of Native Cadmium-Tolerant Bacteria and Fungi from Cacao (Theobroma Cacao L.) - Cultivated Soils in Central Colombia. Heliyon 2023, 9, e22489–e22489. [Google Scholar] [CrossRef]
- Chellaiah, E. R. Cadmium (Heavy Metals) Bioremediation by Pseudomonas aeruginosa: A Minireview. Applied Water Science 2018, 8. [Google Scholar] [CrossRef]
- Mergeay, M.; Monchy, S.; Vallaeys, T.; Auquier, V.; Benotmane, A.; Bertin, P.; Taghavi, S.; Dunn, J.; van der Lelie, D.; Wattiez, R. Ralstonia Metallidurans, a Bacterium Specifically Adapted to Toxic Metals: Towards a Catalogue of Metal-Responsive Genes. FEMS Microbiology Reviews 2003, 27, (2–3). [Google Scholar] [CrossRef] [PubMed]
- Osman, G. E. H.; Abulreesh, H. H.; Elbanna, K.; Shaaban, M. R.; Samreen, S.; Ahmad, I. Recent Progress in Metal-Microbe Interactions: Prospects in Bioremediation. Journal of Pure and Applied Microbiology 2019, 13, 13–26. [Google Scholar] [CrossRef]
- Salam, L. B.; Obayori, O. S.; Ilori, M. O.; Amund, O. O. Effects of Cadmium Perturbation on the Microbial Community Structure and Heavy Metal Resistome of a Tropical Agricultural Soil. Bioresources and Bioprocessing 2020, 7. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, J.; Liu, X.; Sun, L.; Tian, J.; Wu, N. Cadmium Pollution Impact on the Bacterial Community Structure of Arable Soil and the Isolation of the Cadmium Resistant Bacteria. Frontiers in Microbiology 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Xie, L.; Jin, D.; Mi, B.; Wang, D. H.; Li, X. F.; Dai, X.; Zou, X.; Zhang, Z.; Ma, Y.; Liu, F. Bacterial Community Response to Cadmium Contamination of Agricultural Paddy Soil. Applied Soil Ecology 2019, 139, 100–106. [Google Scholar] [CrossRef]
- Sun, H.; Shao, C.; Jin, Q.; Li, M.; Zhang, Z.; Liang, H.; Lei, H.; Qian, J.; Zhang, Y. Effects of Cadmium Contamination on Bacterial and Fungal Communities in Panax Ginseng-Growing Soil. BMC Microbiology 2022, 22. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Elahi, A.; Bukhari, D. A.; Rehman, A. Cadmium Sources, Toxicity, Resistance and Removal by Microorganisms-A Potential Strategy for Cadmium Eradication. Journal of Saudi Chemical Society 2022, 26. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A. M.; Brewer, T. E.; Benavent-González, A.; Eldridge, D. J.; Bardgett, R. D.; Maestre, F. T.; Singh, B. K.; Fierer, N. A Global Atlas of the Dominant Bacteria Found in Soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef]
- Li, X.; Meng, D.; Li, J.; Yin, H.; Liu, H.; Liu, X.; Cheng, C.; Xiao, Y.; Liu, Z.; Yan, M. Response of Soil Microbial Communities and Microbial Interactions to Long-Term Heavy Metal Contamination. Environmental Pollution 2017, 231, 908–917. [Google Scholar] [CrossRef]
- Hemmat-Jou, M. H.; Safari-Sinegani, A. A.; Mirzaie-Asl, A.; Tahmourespour, A. Analysis of Microbial Communities in Heavy Metals-Contaminated Soils Using the Metagenomic Approach. Ecotoxicology 2018, 27, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, I. V. N.; Megharaj, M.; Krishnamurti, G. S. R.; Bolan, N. S.; Naidu, R. Heavy Metal Toxicity to Bacteria – Are the Existing Growth Media Accurate Enough to Determine Heavy Metal Toxicity? Chemosphere 2013, 90, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Yin, R.; Yu, L.; Zhao, J.; Chen, W.; Yu, R.; Liu, X.; Chen, Y.; Zhang, H.; Chen, W. Screening of Lactic Acid Bacteria with Potential Protective Effects against Cadmium Toxicity. 2015, 54, 23–30. [Google Scholar] [CrossRef]
- Sidhu, G. P. S.; Bali, A. S.; Bhardwaj, R. Use of Fungi in Mitigating Cadmium Toxicity in Plants. 2019, 397–426. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, S.; Chen, L.; Kimotho, R. N.; Chen, M.; Chen, J.; Zhang, J.; Li, X. A Newly-Isolated Cd-Loving Purpureocillium Sp. Strain YZ1 Substantially Alleviates Cd Toxicity to Wheat. Plant and Soil 2021. [Google Scholar] [CrossRef]
- Fazli, M. M.; Soleimani, N.; Mehrasbi, M.; Darabian, S.; Mohammadi, J.; Ramazani, A. Highly Cadmium Tolerant Fungi: Their Tolerance and Removal Potential. Journal of Environmental Health Science and Engineering 2015, 13. [Google Scholar] [CrossRef]
- Dowlati, M.; Sobhi, H. R.; Esrafili, A.; FarzadKia, M.; Yeganeh, M. Heavy Metals Content in Edible Mushrooms: A Systematic Review, Meta-Analysis and Health Risk Assessment. Trends in Food Science & Technology 2021, 109, 527–535. [Google Scholar] [CrossRef]
- Gałgowska, M.; Pietrzak-Fiećko, R. Cadmium and Lead Content in Selected Fungi from Poland and Their Edible Safety Assessment. Molecules 2021, 26, 7289. [Google Scholar] [CrossRef]
- Širić, I.; Kumar, P.; Eid, E.M.; Bachheti, A.; Kos, I.; Bedeković, D.; Mioč, B.; Humar, M. Occurrence and Health Risk Assessment of Cadmium Accumulation in Three Tricholoma Mushroom Species Collected from Wild Habitats of Central and Coastal Croatia. J. Fungi 2022, 8, 685. [Google Scholar] [CrossRef]
- Orywal, K.; Socha, K.; Nowakowski, P.; Zoń, W.; Kaczyński, P.; Mroczko, B.; Łozowicka, B.; Perkowski, M. Health Risk Assessment of Exposure to Toxic Elements Resulting from Consumption of Dried Wild-Grown Mushrooms Available for Sale. PLOS ONE 2021, 16. [Google Scholar] [CrossRef]
- Brima, E.I. Toxic Elements in Different Medicinal Plants and the Impact on Human Health. Int. J. Environ. Res. Public Health 2017, 14, 1209. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, M.; Cuypers, A.; Deckers, J.; Iven, V.; Vandionant, S.; Jozefczak, M.; Hendrix, S. Cadmium and Plant Development: An Agony from Seed to Seed. Int. J. Mol. Sci. 2019, 20, 3971. [Google Scholar] [CrossRef] [PubMed]
- Haider, F. U.; Liqun, C.; Coulter, J. A.; Cheema, S. A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium Toxicity in Plants: Impacts and Remediation Strategies. Ecotoxicology and Environmental Safety 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Nazar, R.; Iqbal, N.; Masood, A.; Khan, M. I. R.; Syeed, S.; Khan, N. A. Cadmium Toxicity in Plants and Role of Mineral Nutrients in Its Alleviation. American Journal of Plant Sciences 2012, 03, 1476–1489. [Google Scholar] [CrossRef]
- Ismael, M. A.; Elyamine, A. M.; Moussa, M. G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in Plants: Uptake, Toxicity, and Its Interactions with Selenium Fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef]
- Shaari, N. E. M.; Tajudin, M. T. F. M.; Khandaker, M. M.; Majrashi, A.; Alenazi, M. M.; Abdullahi, U. A.; Mohd, K. S. Cadmium Toxicity Symptoms and Uptake Mechanism in Plants: A Review. Brazilian Journal of Biology 2024, 84. [Google Scholar] [CrossRef]
- Pan, J.; Li, D.; Shu, Z.; Jiang, X.; Ma, W.; Wang, W.; Zhu, J.; Wang, Y. CsPDC-E1α, a Novel Pyruvate Dehydrogenase Complex E1α Subunit Gene from Camellia Sinensis, Is Induced during Cadmium Inhibiting Pollen Tube Growth. Canadian Journal of Plant Science 2017. [Google Scholar] [CrossRef]
- Nguyen, J.; Patel, A.; Gensburg, A.; Bokhari, R.; Lamar, P.; Edwards, J. Diabetogenic and Obesogenic Effects of Cadmium in Db/Db Mice and Rats at a Clinically Relevant Level of Exposure. Toxics 2022, 10, 107. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, X.; Hao, Z.; Yu, M.; Tang, Y.; Teng, X.; Sun, W.; Kang, L. Cadmium Exposure Caused Cardiotoxicity in Common Carps (Cyprinus Carpio L.): MiR-9-5p, Oxidative Stress, Energetic Impairment, Mitochondrial Division/Fusion Imbalance, Inflammation, and Autophagy. Fish & Shellfish Immunology 2023, 138, 108853–108853. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Buha Djordjevic, A.; Antonijevic, E.; Antonijevic, B.; Stanic, M.; Kotur-Stevuljevic, J.; Spasojevic-Kalimanovska, V.; Jovanovic, M.; Boricic, N.; Wallace, D.; et al. Toxic Effect of Acute Cadmium and Lead Exposure in Rat Blood, Liver, and Kidney. Int. J. Environ. Res. Public Health 2019, 16, 274. [Google Scholar] [CrossRef]
- Cui, J.; Liu, Y.; Hao, Z.; Liu, Y.; Qiu, M.; Kang, L.; Teng, X.; Tang, Y. Cadmium Induced Time-Dependent Kidney Injury in Common Carp via Mitochondrial Pathway: Impaired Mitochondrial Energy Metabolism and Mitochondrion-Dependent Apoptosis. Aquatic Toxicology 2023, 261, 106570–106570. [Google Scholar] [CrossRef] [PubMed]
- Raikwar, M.; Kumar, P.; Singh, M. Toxic Effect of Heavy Metals in Livestock Health. Veterinary World 2008, 28. [Google Scholar] [CrossRef]
- Bąkowska, M.; Pilarczyk, B.; Tomza-Marciniak, A.; Pilarczyk, R.; Udała, J. Cadmium in Selected Organs of Game Animals from Areas with Different Degrees of Industrialisation and Its Intake by Human Consumers. Animals 2024, 14, 305. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, S. Heavy Metals’ Poisoning in Farm Animals. IntechOpen eBooks 2023. [Google Scholar] [CrossRef]
- Satarug, S. Cadmium Sources and Toxicity. Toxics 2019, 7, 25. [Google Scholar] [CrossRef]
- Sethy, K.; Pati, S.; Jena, D.; Panda, S.; Pradhan, S.; Mishra, C. Heavy Metal Toxicity in Animals: A Review. The Pharma Innovation Journal 2020, 9, 134–137. [Google Scholar]
- Dobson, S.; World Health Organization. Cadmium, Environmental Aspects; 1992, ISBN 92 4 157135 7.
- Nordberg, M.; Nordberg, G.F. Metallothionein and Cadmium Toxicology—Historical Review and Commentary. Biomolecules 2022, 12, 360. [Google Scholar] [CrossRef]
- Baldisserotto, B.; Kamunde, C.; Matsuo, A.; Wood, C. M. Acute Waterborne Cadmium Uptake in Rainbow Trout Is Reduced by Dietary Calcium Carbonate. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2004, 137, 363–372. [Google Scholar] [CrossRef]
- Mahjoub, M.; Fadlaoui, S.; El Maadoudi, M.; Smiri, Y. Mercury, Lead, and Cadmium in the Muscles of Five Fish Species from the Mechraâ-Hammadi Dam in Morocco and Health Risks for Their Consumers. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Djedjibegovic, J.; Marjanovic, A.; Tahirovic, D.; Caklovica, K.; Turalic, A.; Lugusic, A.; Omeragic, E.; Sober, M.; Caklovica, F. Heavy Metals in Commercial Fish and Seafood Products and Risk Assessment in Adult Population in Bosnia and Herzegovina. Scientific Reports 2020, 10. [Google Scholar] [CrossRef]
- Browar, A.W.; Koufos, E.B.; Wei, Y.; Leavitt, L.L.; Prozialeck, W.C.; Edwards, J.R. Cadmium Exposure Disrupts Periodontal Bone in Experimental Animals: Implications for Periodontal Disease in Humans. Toxics 2018, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Drapal, J.; Steinhauser, L.; Stastny, K.; Faldyna, M. Cadmium Concentration in Cattle Tissues in the Czech Republic. Veterinární Medicína 2021, 66, 369–375. [Google Scholar] [CrossRef]
- Tahir, I.; Alkheraije, K. A. A Review of Important Heavy Metals Toxicity with Special Emphasis on Nephrotoxicity and Its Management in Cattle. 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Chirinos-Peinado, D.; Castro-Bedriñana, J.; Ríos-Ríos, E.; Mamani-Gamarra, G.; Quijada-Caro, E.; Huacho-Jurado, A.; Nuñez-Rojas, W. Lead and Cadmium Bioaccumulation in Fresh Cow’s Milk in an Intermediate Area of the Central Andes of Peru and Risk to Human Health. Toxics 2022, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Fay, M.J.; Alt, L.A.C.; Ryba, D.; Salamah, R.; Peach, R.; Papaeliou, A.; Zawadzka, S.; Weiss, A.; Patel, N.; Rahman, A.; et al. Cadmium Nephrotoxicity Is Associated with Altered MicroRNA Expression in the Rat Renal Cortex. Toxics 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Smereczański, N.M.; Brzóska, M.M. Current Levels of Environmental Exposure to Cadmium in Industrialized Countries as a Risk Factor for Kidney Damage in the General Population: A Comprehensive Review of Available Data. Int. J. Mol. Sci. 2023, 24, 8413. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Omeljaniuk, W.J.; Nowak, K.; Garley, M.; Nikliński, J. Cadmium Toxicity and Health Effects—A Brief Summary. Molecules 2023, 28, 6620. [Google Scholar] [CrossRef]
- Skipper, A.; Sims, J.N.; Yedjou, C.G.; Tchounwou, P.B. Cadmium Chloride Induces DNA Damage and Apoptosis of Human Liver Carcinoma Cells via Oxidative Stress. Int. J. Environ. Res. Public Health 2016, 13, 88. [Google Scholar] [CrossRef]
- Chavatte, L.; Juan, M.; Mounicou, S.; Leblanc Noblesse, E.; Pays, K.; Nizard, C.; Bulteau, A.-L. Elemental and Molecular Imaging of Human Full Thickness Skin after Exposure to Heavy Metals. Metallomics 2020, 12, 1555–1562. [Google Scholar] [CrossRef]
- Sarkar, A., Ravindran, G. and Krishnamurthy, V. A brief review on the effect of cadmium toxicity: from cellular to organ level. Int J Biotechnol Res 2013, 3(1), 17–36. [Google Scholar]
- Egger, A. E.; Grabmann, G.; Gollmann-Tepeköylü, C.; Pechriggl, E. J.; Artner, C.; Türkcan, A.; Hartinger, C. G.; Fritsch, H.; Keppler, B. K.; Brenner, E.; Grimm, M.; Messner, B.; Bernhard, D. Chemical Imaging and Assessment of Cadmium Distribution in the Human Body. Metallomics 2019, 11, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Satchanska, G. Cadmium Toxicity on Human: A Review. Journal of Balkan Ecology http://en.ecobalk.com/. 2012, 15, 349–357. [Google Scholar]
- Chavez, E.; He, Z. L.; Stoffella, P. J.; Mylavarapu, R. S.; Li, Y. C.; Moyano, B.; Baligar, V. C. Concentration of Cadmium in Cacao Beans and Its Relationship with Soil Cadmium in Southern Ecuador. Science of The Total Environment 2015, 533, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; C. Gobe, G.; A. Vesey, D.; Phelps, K.R. Cadmium and Lead Exposure, Nephrotoxicity, and Mortality. Toxics 2020, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Lin, K.; Williams, D.V.; Liu, Y.; Dai, H.; Cao, F. Cadmium Accumulation in Cereal Crops and Tobacco: A Review. Agronomy 2022, 12, 1952. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Z. Recent Advances in Minimizing Cadmium Accumulation in Wheat. Toxics 2022, 10, 187. [Google Scholar] [CrossRef]
- Kim, K.; Melough, M.M.; Vance, T.M.; Noh, H.; Koo, S.I.; Chun, O.K. Dietary Cadmium Intake and Sources in the US. Nutrients 2019, 11, 2. [Google Scholar] [CrossRef]
- Ai, H.; Wu, D.; Li, C.; Hou, M. Advances in Molecular Mechanisms Underlying Cadmium Uptake and Translocation in Rice. Frontiers in Plant Science 2022, 13. [Google Scholar] [CrossRef]
- Nogawa, K.; Suwazono, Y.; Nishijo, M.; Sakurai, M.; Ishizaki, M.; Morikawa, Y.; Watanabe, Y.; Kido, T.; Nakagawa, H. Increase of Lifetime Cadmium Intake Dose-Dependently Increased All Cause of Mortality in Female Inhabitants of the Cadmium-Polluted Jinzu River Basin, Toyama, Japan. Environmental Research 2018, 164, 379–384. [Google Scholar] [CrossRef]
- Nishijo, M.; Nogawa, K.; Suwazono, Y.; Kido, T.; Sakurai, M.; Nakagawa, H. Lifetime Cadmium Exposure and Mortality for Renal Diseases in Residents of the Cadmium-Polluted Kakehashi River Basin in Japan. Toxics 2020, 8, 81. [Google Scholar] [CrossRef]
- Uetani, M.; Kobayashi, E.; Suwazono, Y.; Kido, T.; Nogawa, K. Cadmium Exposure Aggravates Mortality More in Women than in Men. International journal of environmental health research 2006, 16, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Murillo, F.; Martinez-Yanez, K.; Fortich-Revollo, A.; Paternina-Caicedo, A.; Johnson-Restrepo, B. Biochemical and Hematological Markers in Workers with Chronical Exposure to Lead and Cadmium in Colombia. Toxics 2022, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Conterato, G.M.M.; Bulcão, R.P.; Sobieski, R.; Moro, A. M.; Charão, M.F.; Monteiro, F.; Maria, F.; Moreira, A.; Roehrs, M.; Tonello, R.; Batista, B.L.; Grotto, D.; Barbosa, F.; Garcia, S.C.; Emanuelli, T. Blood Thioredoxin Reductase Activity, Oxidative Stress and Hematological Parameters in Painters and Battery Workers: Relationship with Lead and Cadmium Levels in Blood. 2013, 33, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Moore, M. R. Adverse Health Effects of Chronic Exposure to Low-Level Cadmium in Foodstuffs and Cigarette Smoke. Environmental Health Perspectives 2004, 112, 1099–1103. [Google Scholar] [CrossRef]
- Barregard, L.; Fabricius-Lagging, E.; Lundh, T.; Mölne, J.; Wallin, M.; Olausson, M.; Modigh, C.; Sallsten, G. Cadmium, Mercury, and Lead in Kidney Cortex of Living Kidney Donors: Impact of Different Exposure Sources, Environmental Research 2010, 110, 47–54. [Google Scholar] [CrossRef]
- Gray, N.; Halstead, M.; Gonzalez-Jimenez, N.; Valentin-Blasini, L.; Watson, C.; Pappas, R.S. Analysis of Toxic Metals in Liquid from Electronic Cigarettes. Int. J. Environ. Res. Public Health 2019, 16, 4450. [Google Scholar] [CrossRef]
- Liu, H.; Gao, H.; Long, M.; Fu, H.; Alvarez, P. J. J.; Li, Q.; Zheng, S.; Qu, X.; Zhu, D. Sunlight Promotes Fast Release of Hazardous Cadmium from Widely-Used Commercial Cadmium Pigment. Environmental Science & Technology 2017, 51, 6877–6886. [Google Scholar] [CrossRef]
- Tellez-Plaza, M.; Guallar, E.; Fabsitz, R. R.; Howard, B. V.; Umans, J. G.; Francesconi, K. A.; Goessler, W.; Devereux, R. B.; Navas-Acien, A. Cadmium Exposure and Incident Peripheral Arterial Disease. Circulation. Cardiovascular quality and outcomes 2013, 6, 626–633. [Google Scholar] [CrossRef]
- Qu, F.; Zheng, W. Cadmium Exposure: Mechanisms and Pathways of Toxicity and Implications for Human Health. Toxics 2024, 12, 388. [Google Scholar] [CrossRef]
- Tinkov, A. A.; Filippini, T.; Ajsuvakova, O. P.; Skalnaya, M. G.; Aaseth, J.; Bjørklund, G.; Gatiatulina, E. R.; Popova, E. V.; Nemereshina, O. N.; Huang, P.-T.; Vinceti, M.; Skalny, A. V. Cadmium and Atherosclerosis: A Review of Toxicological Mechanisms and a Meta-Analysis of Epidemiologic Studies. Environmental Research 2018, 162, 240–260. [Google Scholar] [CrossRef]
- Ledda, C.; Cannizzaro, E.; Lovreglio, P.; Vitale, E.; Stufano, A.; Montana, A.; Li Volti, G.; Rapisarda, V. Exposure to Toxic Heavy Metals Can Influence Homocysteine Metabolism? Antioxidants 2020, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Massányi, M.; Satarug, S.; Madeddu, R.; Stawarz, R.; Massányi, P. Evidence for Ovarian and Testicular Toxicities of Cadmium and Detoxification by Natural Substances. Stresses 2022, 2, 1–16. [Google Scholar] [CrossRef]
- Saha, R.; S. Roychoudhury; Varghese, A.; Nandi, P.; Kar, K.; A. Paul Choudhury; Kalita, J. C.; Lukac, N.; P. Massanyi; A. Kolesarova. Cadmium Induced Deterioration of Sperm Quality: Protective Role of Coenzyme Q10 in Rats. CRC Press eBooks 2019, 127–132. [CrossRef]
- Massányi, P.; Massányi, M.; Madeddu, R.; Stawarz, R.; Lukáč, N. Effects of Cadmium, Lead, and Mercury on the Structure and Function of Reproductive Organs. Toxics 2020, 8, 94. [Google Scholar] [CrossRef]
- Dettwiler, M.; Flynn, A.C.; Rigutto-Farebrother, J. Effects of Non-Essential “Toxic” Trace Elements on Pregnancy Outcomes: A Narrative Overview of Recent Literature Syntheses. Int. J. Environ. Res. Public Health 2023, 20, 5536. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, A. Cadmium Toxicity: Effects on Human Reproduction and Fertility. Reviews on Environmental Health 2019, 34, 327–338. [Google Scholar] [CrossRef]
- Marini, H.R.; Micali, A.; Squadrito, G.; Puzzolo, D.; Freni, J.; Antonuccio, P.; Minutoli, L. Nutraceuticals: A New Challenge against Cadmium-Induced Testicular Injury. Nutrients 2022, 14, 663. [Google Scholar] [CrossRef]
- Taylor, B. A.; Heiniger, H. J.; Meier, H. Genetic Analysis of Resistance to Cadmium-Induced Testicular Damage in Mice. Experimental Biology and Medicine 1973, 143, 629–633. [Google Scholar] [CrossRef]
- Xiong, R.; Wu, Q.; Trbojevich, R.; Levan Muskhelishvili; Davis, K.; Bryant, M.; Richter, P.; Cao, X. Disease-Related Responses Induced by Cadmium in an in Vitro Human Airway Tissue Model. Toxicology Letters 2019, 303, 16–27. [Google Scholar] [CrossRef]
- Lampe, B. J.; Park, S. K.; Robins, T.; Mukherjee, B.; Litonjua, A. A.; Amarasiriwardena, C.; Weisskopf, M.; Sparrow, D.; Hu, H. Association between 24-Hour Urinary Cadmium and Pulmonary Function among Community-Exposed Men: The va Normative Aging Study. Environmental Health Perspectives 2008, 116, 1226–1230. [Google Scholar] [CrossRef]
- Sasaki, T.; Hyogo Horiguchi; Matsukawa, T.; Kobayashi, M.; Omori, Y.; Oguma, E.; Atsushi Komatsuda. A Suspected Case of “Itai-Itai Disease” in a Cadmium-Polluted Area in Akita Prefecture, Japan. Environmental Health and Preventive Medicine 2024, 29, 40–40. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Ruangyuttikarn, W.; Nishijo, M.; Ruiz, P. Urinary Cadmium Threshold to Prevent Kidney Disease Development. Toxics 2018, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Afridi, H. I.; Kazi, T. G.; Kazi, N.; Jamali, M. K.; Arain, M. B.; Jalbani, N.; Baig, J. A.; Sarfraz, R. A. Evaluation of Status of Toxic Metals in Biological Samples of Diabetes Mellitus Patients. Diabetes Research and Clinical Practice 2008, 80, 280–288. [Google Scholar] [CrossRef]
- Thomas, L. D. K.; Hodgson, S.; Nieuwenhuijsen, M.; Jarup, L. Early Kidney Damage in a Population Exposed to Cadmium and Other Heavy Metals. Environmental Health Perspectives 2009, 117, 181–184. [Google Scholar] [CrossRef]
- Tobwala, S.; Wang, H.-J.; Carey, J.W.; Banks, W.A.; Ercal, N. Effects of Lead and Cadmium on Brain Endothelial Cell Survival, Monolayer Permeability, and Crucial Oxidative Stress Markers in an in Vitro Model of the Blood-Brain Barrier. Toxics 2014, 2, 258–275. [Google Scholar] [CrossRef]
- Agnihotri, S. K.; Agrawal, U.; Ghosh, I. Brain Most Susceptible to Cadmium Induced Oxidative Stress in Mice. Journal of Trace Elements in Medicine and Biology 2015, 30, 184–193. [Google Scholar] [CrossRef]
- Leal, R. B.; Rieger, D. K.; Peres, T. V.; Lopes, M. W.; Gonçalves, C. A. S. Cadmium Neurotoxicity and Its Role in Brain Disorders. Metal Ion in Stroke 2012, 751–766. [Google Scholar] [CrossRef]
- Zheng, W. Toxicology of Choroid Plexus: Special Reference to Metal-Induced Neurotoxicities. Microscopy Research and Technique 2001, 52, 89–103. [Google Scholar] [CrossRef]
- Oggiano, R.; Pisano, A.; Sabalic, A.; Farace, C.; Fenu, G.; Lintas, S.; Forte, G.; Bocca, B.; Madeddu, R. An Overview on Amyotrophic Lateral Sclerosis and Cadmium. Neurological Sciences 2020, 42, 531–537. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Fiorillo, C.; Carrino, D.; Paternostro, F.; Taddei, N.; Gulisano, M.; Pacini, A.; Becatti, M. Cadmium-Induced Oxidative Stress: Focus on the Central Nervous System. Antioxidants 2020, 9, 492. [Google Scholar] [CrossRef]
- Fern, R.; Black, J. A.; Ransom, B. R.; Waxman, S. G. Cd(2+)-Induced Injury in CNS White Matter. Journal of Neurophysiology 1996, 76, 3264–3273. [Google Scholar] [CrossRef] [PubMed]
- Almazan, G.; Liu, H.-N.; Khorchid, A.; Sundararajan, S.; Martinez-Bermudez, A. K.; Chemtob, S. Exposure of Developing Oligodendrocytes to Cadmium Causes HSP72 Induction, Free Radical Generation, Reduction in Glutathione Levels, and Cell Death. Free radical biology & medicine 2000, 29, 858–869. [Google Scholar] [CrossRef]
- Arruebarrena, M.A.; Hawe, C.T.; Lee, Y.M.; Branco, R.C. Mechanisms of Cadmium Neurotoxicity. Int. J. Mol. Sci. 2023, 24, 16558. [Google Scholar] [CrossRef] [PubMed]
- Viaene, M. K.; Roels, H. A.; Leenders, J.; De Groof, M.; Swerts, L. J.; Lison, D.; Masschelein, R. Cadmium: A Possible Etiological Factor in Peripheral Polyneuropathy. Neurotoxicology 1999, 20, 7–16. [Google Scholar] [PubMed]
- Zhong, Q.; Zhou, W.; Lin, J.; Sun, W.; Qin, Y.; Li, X.; Xu, H. Independent and Combined Associations of Blood Manganese, Cadmium and Lead Exposures with the Systemic Immune-Inflammation Index in Adults. Toxics 2023, 11, 659. [Google Scholar] [CrossRef]
- Mirkov, I.; Popov Aleksandrov, A.; Ninkov, M.; Tucovic, D.; Kulas, J.; Zeljkovic, M.; Popovic, D.; Kataranovski, M. Immunotoxicology of Cadmium: Cells of the Immune System as Targets and Effectors of Cadmium Toxicity. Food and Chemical Toxicology 2021, 149, 112026. [Google Scholar] [CrossRef]
- Thomas, P. T.; Ratajczak, H. V.; Aranyi, C.; Gibbons, R. D.; Fenters, J. D. Evaluation of Host Resistance and Immune Function in Cadmium-Exposed Mice. Toxicology and Applied Pharmacology 1985, 80, 446–456. [Google Scholar] [CrossRef]
- Dakeshita, S.; Kawai, T.; Uemura, H.; Hiyoshi, M.; Oguma, E.; Horiguchi, H.; Kayama, F.; Aoshima, K.; Shirahama, S.; Rokutan, K.; Arisawa, K. Gene Expression Signatures in Peripheral Blood Cells from Japanese Women Exposed to Environmental Cadmium. Toxicology 2009, 257, 25–32. [Google Scholar] [CrossRef]
- Bertin, G.; Averbeck, D. Cadmium: Cellular Effects, Modifications of Biomolecules, Modulation of DNA Repair and Genotoxic Consequences (a Review). Biochimie 2006, 88, 1549–1559. [Google Scholar] [CrossRef]
- Teschke, R. Aluminum, Arsenic, Beryllium, Cadmium, Chromium, Cobalt, Copper, Iron, Lead, Mercury, Molybdenum, Nickel, Platinum, Thallium, Titanium, Vanadium, and Zinc: Molecular Aspects in Experimental Liver Injury. Int. J. Mol. Sci. 2022, 23, 12213. [Google Scholar] [CrossRef]
- Luevano, J.; Damodaran, C. A Review of Molecular Events of Cadmium-Induced Carcinogenesis. Journal of Environmental Pathology, Toxicology and Oncology 2014, 33, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Templeton, D. M.; Liu, Y. Multiple Roles of Cadmium in Cell Death and Survival. Chemico-Biological Interactions 2010, 188, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.; Carmona, A. Neurotoxicity of Environmental Metal Toxicants: Special Issue. Toxics 2022, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Đukić-Ćosić, D.; Baralić, K.; Javorac, D.; Djordjevic, A. B.; Bulat, Z. An Overview of Molecular Mechanisms in Cadmium Toxicity. Current Opinion in Toxicology 2020, 19, 56–62. [Google Scholar] [CrossRef]
- Carmona, A.; Roudeau, S.; Ortega, R. Molecular Mechanisms of Environmental Metal Neurotoxicity: A Focus on the Interactions of Metals with Synapse Structure and Function. Toxics 2021, 9, 198. [Google Scholar] [CrossRef]
- Hansen, K. B.; Yi, F.; Perszyk, R. E.; Furukawa, H.; Wollmuth, L. P.; Gibb, A. J.; Traynelis, S. F. Structure, Function, and Allosteric Modulation of NMDA Receptors. The Journal of General Physiology 2018, 150, 1081–1105. [Google Scholar] [CrossRef]
- Forcella, M.; Lau, P.; Oldani, M.; Melchioretto, P.; Bogni, A.; Gribaldo, L.; Fusi, P.; Urani, C. Neuronal Specific and Non-Specific Responses to Cadmium Possibly Involved in Neurodegeneration: A Toxicogenomics Study in a Human Neuronal Cell Model. NeuroToxicology 2020, 76, 162–173. [Google Scholar] [CrossRef]
- Qu, F.; Zheng, W. Cadmium Exposure: Mechanisms and Pathways of Toxicity and Implications for Human Health. Toxics 2024, 12, 388. [Google Scholar] [CrossRef]
- Satchanska, G. Growing Environmental Bacterium Biofilms in PEO Cryogels for Environmental Biotechnology Application. IntechOpen eBooks 2022. [Google Scholar] [CrossRef]
- Chwastowski, J.; Bradło, D.; Żukowski, W. Adsorption of Cadmium, Manganese and Lead Ions from Aqueous Solutions Using Spent Coffee Grounds and Biochar Produced by Its Pyrolysis in the Fluidized Bed Reactor. Materials 2020, 13, 2782. [Google Scholar] [CrossRef]
- Satchanska, G., Topalova, Y., Dimkov, R., Petrov, P., Tsvetanov, C., Selenska-Pobell, S., Gorbovskaya, A., Golovinsky, E. Phenol biodegradation by two xenobiotics-tolerant bacteria immobilized in polyethylene oxide cryogels. Comptes Rendus de L'Academie Bulgare des Sciences 2009, 62, 957–964, http://www.proceedings.bas.bg/.
- Alfadaly, R.A.; Elsayed, A.; Hassan, R.Y.A.; Noureldeen, A.; Darwish, H.; Gebreil, A.S. Microbial Sensing and Removal of Heavy Metals: Bioelectrochemical Detection and Removal of Chromium(VI) and Cadmium(II). Molecules 2021, 26, 2549. [Google Scholar] [CrossRef] [PubMed]
- Vicas, S.I.; Laslo, V.; Timar, A.V.; Balta, C.; Herman, H.; Ciceu, A.; Gharbia, S.; Rosu, M.; Mladin, B.; Chiana, L.; et al. Nano Selenium—Enriched Probiotics as Functional Food Products against Cadmium Liver Toxicity. Materials 2021, 14, 2257. [Google Scholar] [CrossRef] [PubMed]
- George, F.; Mahieux, S.; Daniel, C.; Titécat, M.; Beauval, N.; Houcke, I.; Neut, C.; Allorge, D.; Borges, F.; Jan, G.; et al. Assessment of Pb(II), Cd(II), and Al(III) Removal Capacity of Bacteria from Food and Gut Ecological Niches: Insights into Biodiversity to Limit Intestinal Biodisponibility of Toxic Metals. Microorganisms 2021, 9, 456. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Tian, F.; Zhao, J.; Zhang, H.; Narbad, A.; Chen, W. Oral Administration of Probiotics Inhibits Absorption of the Heavy Metal Cadmium by Protecting the Intestinal Barrier. Applied and Environmental Microbiology 2016, 82, 4429–4440. [Google Scholar] [CrossRef]
- Capriglione, F.; Maiuolo, J.; Celano, M.; Damante, G.; Russo, D.; Bulotta, S.; Maggisano, V. Quercetin Protects Human Thyroid Cells against Cadmium Toxicity. Int. J. Mol. Sci. 2021, 22, 6849. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, B.; Ke, T.; Li, S.; Yang, X.; Aschner, M.; Chen, P. Mechanisms of Metal-Induced Mitochondrial Dysfunction in Neurological Disorders. Toxics 2021, 9, 142. [Google Scholar] [CrossRef]
- Smereczański, N.M.; Brzóska, M.M.; Rogalska, J.; Hutsch, T. The Protective Potential of Aronia melanocarpa L. Berry Extract against Cadmium-Induced Kidney Damage: A Study in an Animal Model of Human Environmental Exposure to This Toxic Element. Int. J. Mol. Sci. 2023, 24, 11647. [Google Scholar] [CrossRef]
- Al-Baqami, N.M.; Hamza, R.Z. Protective Effect of Resveratrol against Hepatotoxicity of Cadmium in Male Rats: Antioxidant and Histopathological Approaches. Coatings 2021, 11, 594. [Google Scholar] [CrossRef]
- Grosicki, A.; Małagocki, P.; Kycko, A.; Monkiewicz, J.; Korol, W. Magnesium Supplements Affect Selected Cadmium Toxic Actions and Uptake of Repeated Doses of Cadmium. Bulletin of the Veterinary Institute in Pulawy 2015, 59, 541–546. [Google Scholar] [CrossRef]
- Kong, Z.; Liu, C.; Opeyemi Joshua Olatunji. Asperuloside Attenuates Cadmium-Induced Toxicity by Inhibiting Oxidative Stress, Inflammation, Fibrosis and Apoptosis in Rats. Scientific Reports 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Satchanska, G., Selenska-Pobell S. Bacterial diversity in heavy metal polluted soil sample KCM-B collected near Pb-Zn smelter KCM, Bulgaria. Acta Microbiologica Bulgarica 2023, 37, 350–358. [Google Scholar] [CrossRef]
- Wei, W.; Smith, N.; Wu, X.; Kim, H.; Seravalli, J.; Khalimonchuk, O.; Lee, J. YCF1-Mediated Cadmium Resistance in Yeast Is Dependent on Copper Metabolism and Antioxidant Enzymes. Antioxidants & Redox Signaling 2014, 21, 1475–1489. [Google Scholar] [CrossRef]
- Camacho-Chab, J.C.; Castañeda-Chávez, M.D.R.; Chan-Bacab, M.J.; Aguila-Ramírez, R.N.; Galaviz-Villa, I.; Bartolo-Pérez, P.; Lango-Reynoso, F.; Tabasco-Novelo, C.; Gaylarde, C.; Ortega-Morales, B.O. Biosorption of Cadmium by Non-Toxic Extracellular Polymeric Substances (EPS) Synthesized by Bacteria from Marine Intertidal Biofilms. Int. J. Environ. Res. Public Health 2018, 15, 314. [Google Scholar] [CrossRef]
- Satchanska, G.; Topalova, Y.; Ivanov, I.; Golovinsky, E. Xenobiotic Biotransformation Potential Of Pseudomonas Rhodesiae KCM-R5 and Bacillus Subtilis KCM-RG5, Tolerant to Heavy Metals and Phenol Derivatives. Biotechnology & Biotechnological Equipment 2006, 20, 97–102. [Google Scholar] [CrossRef]
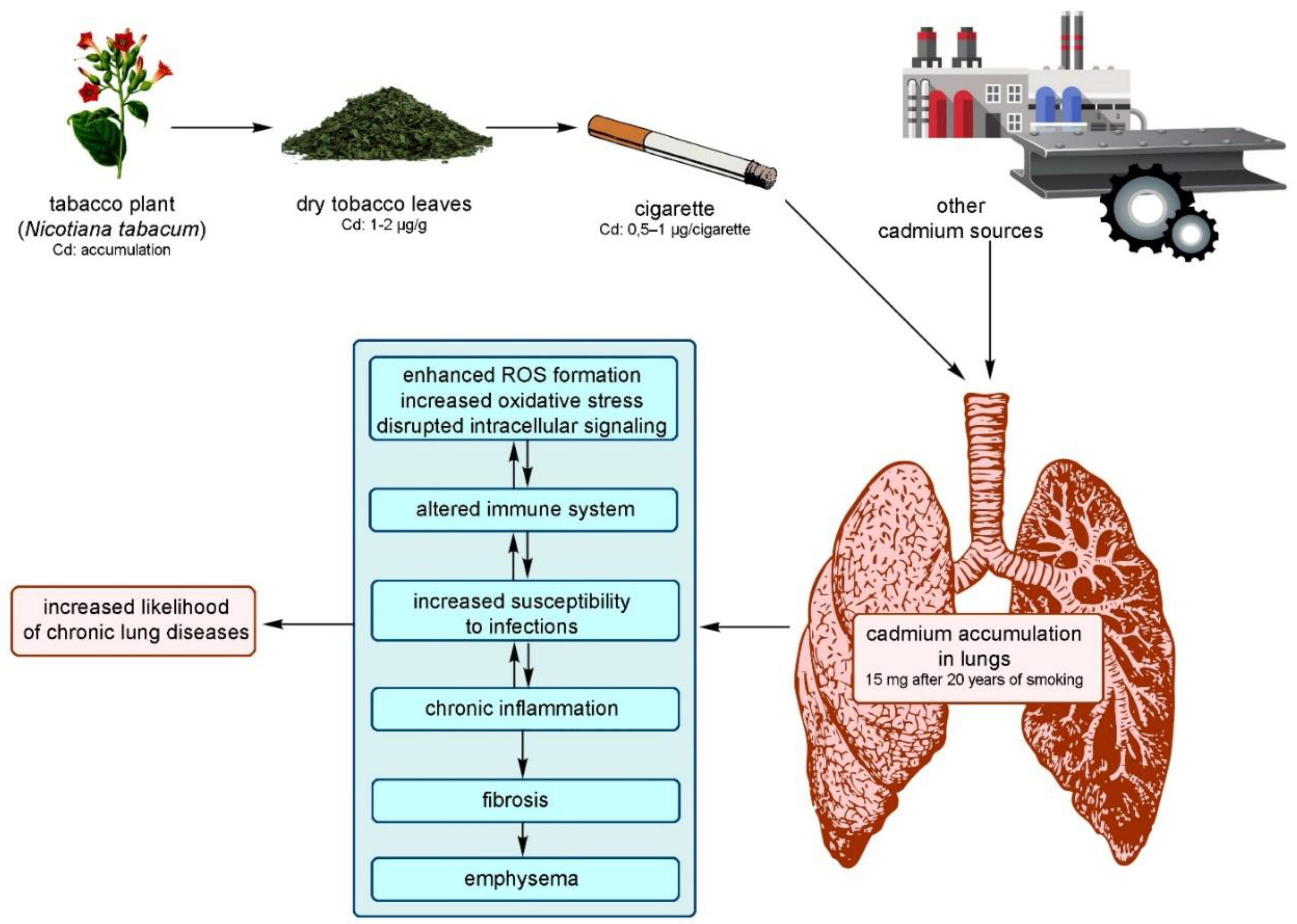
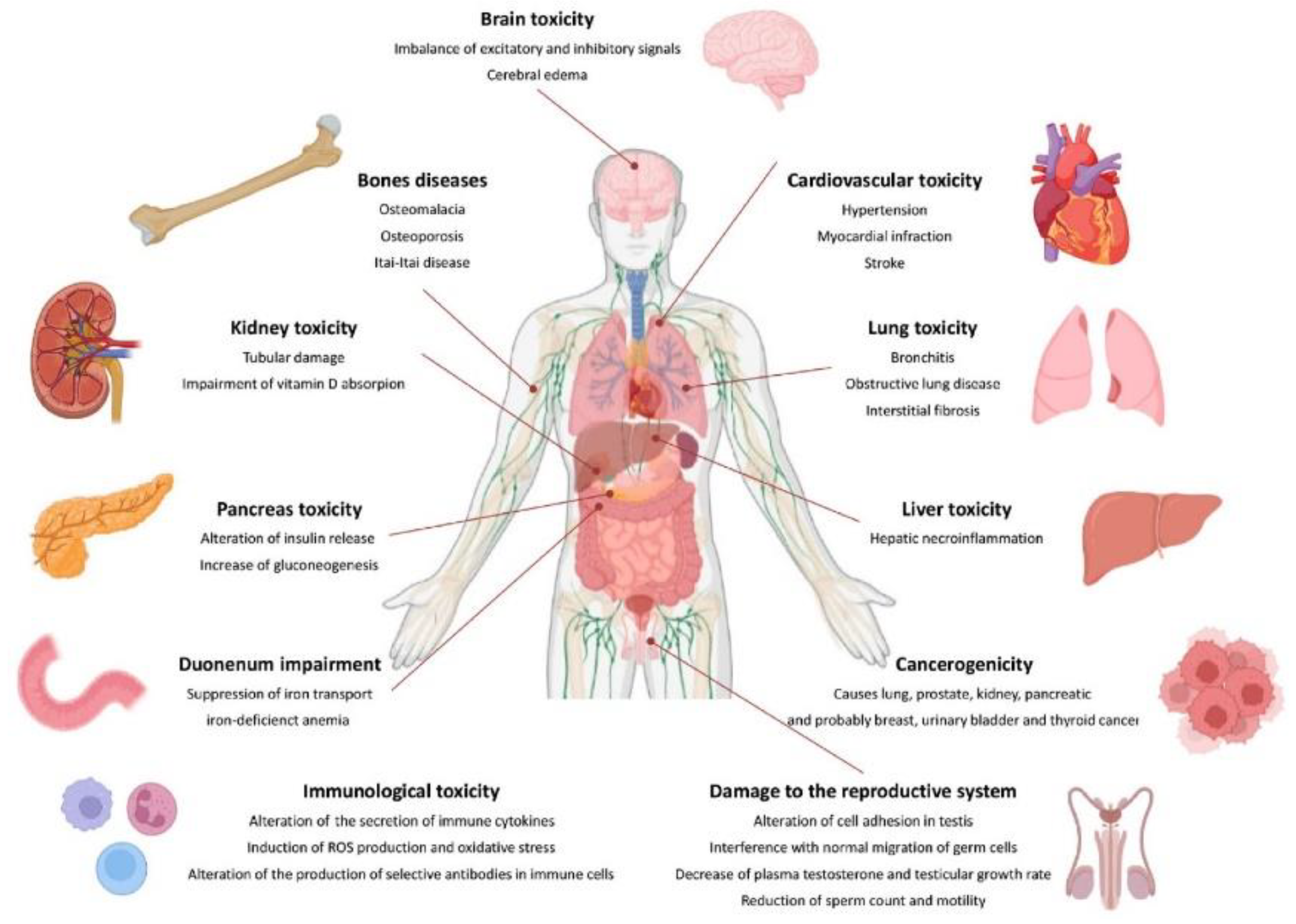
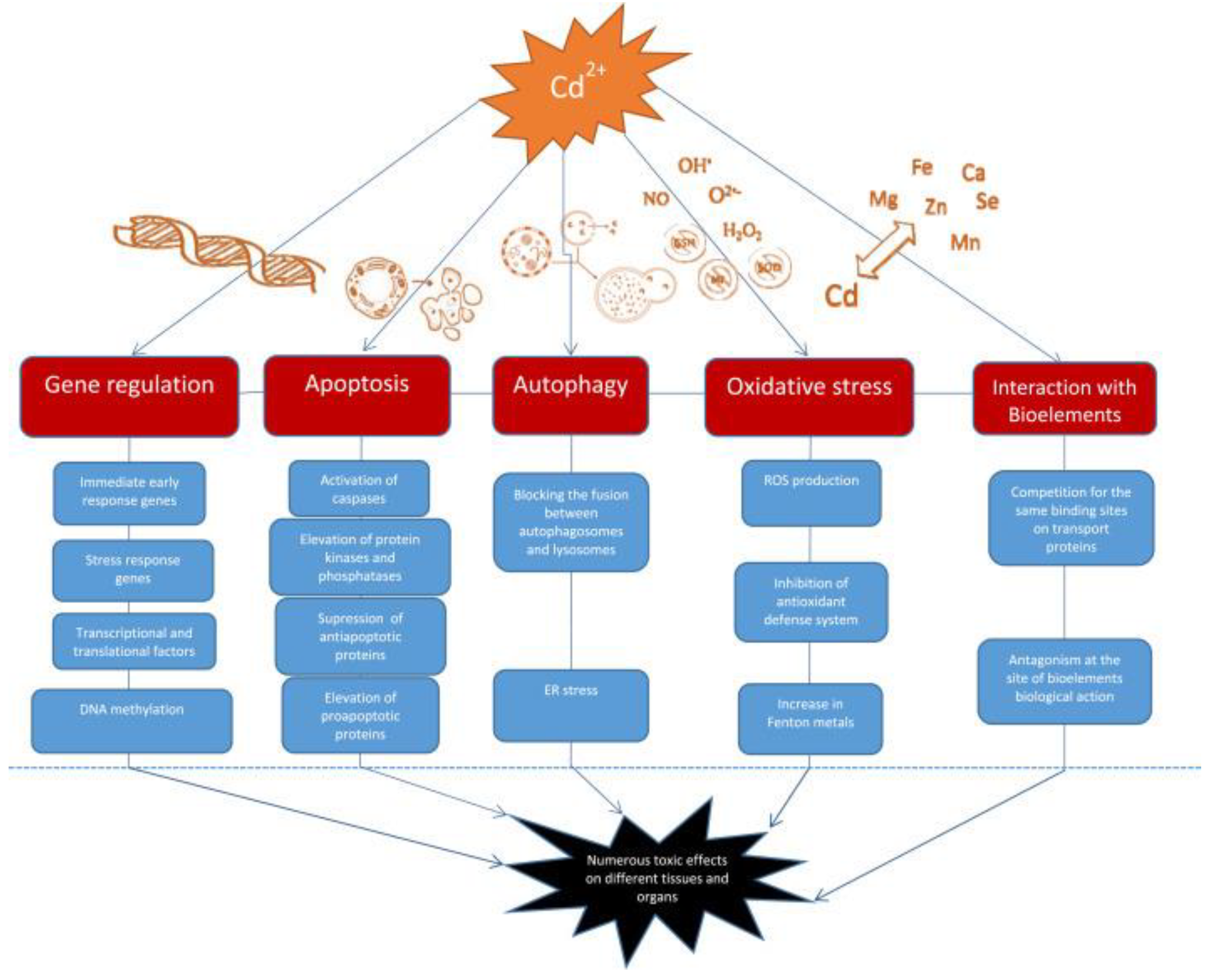
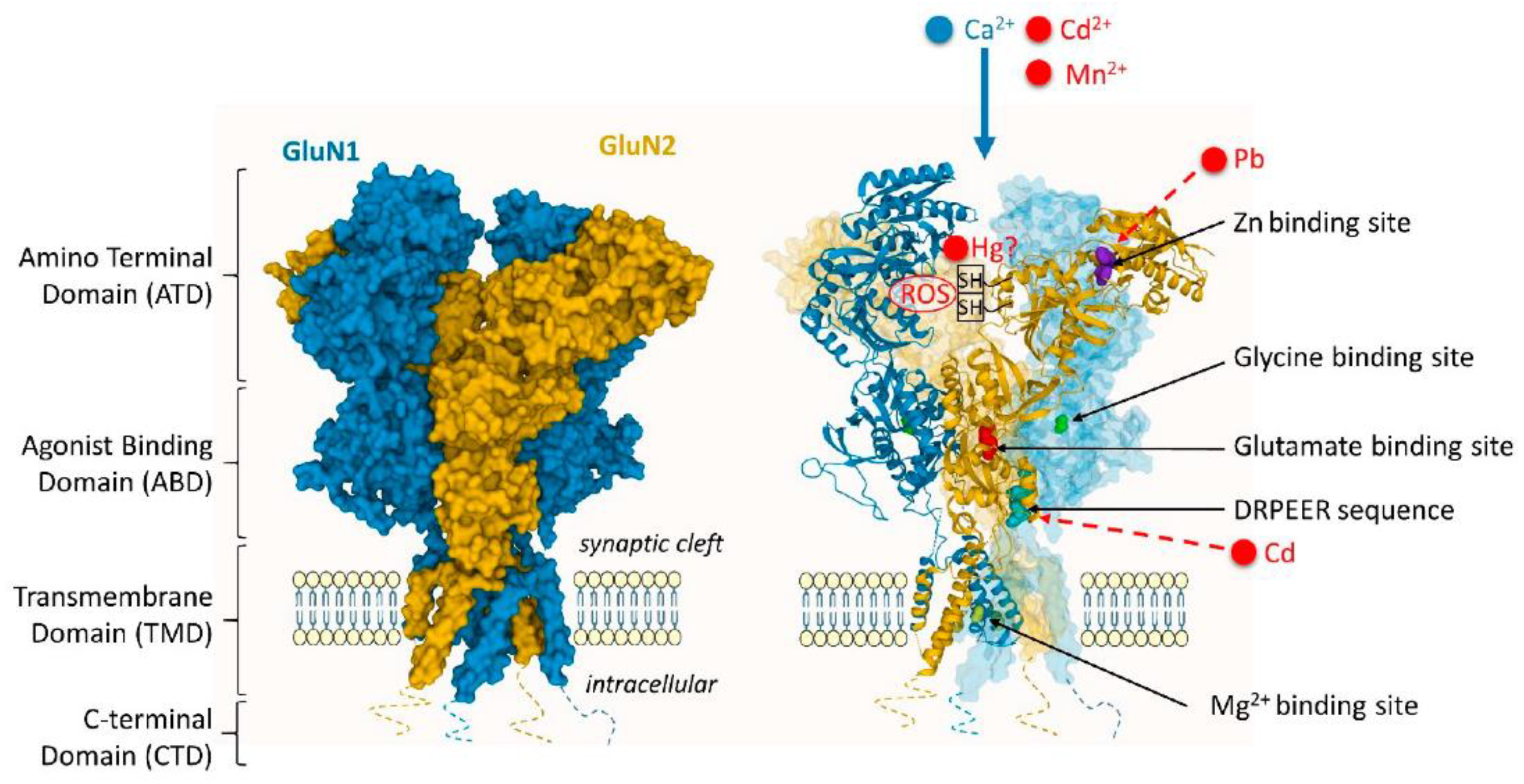
| Atomic number | 48 |
| Atomic weight | 112.41 u |
| Atomic radius | 155 pm |
| Electronic configuration | [Kr]4d105s2 |
| Melting point | 321.07 °C |
| Boiling point | 767.3 °C |
| Density at 20 °C | 8.65 g/cm3 |
| Reduction potential Cd2+ + 2e− → Cd(s) | −0.40 E° |
| Heat of fusion | 6.21 kJ/mol |
| Heat of vaporization | 99.6 kJ/mol |
| Electronegativity (Pauling scale) | 1.69 |
| First ionization energy | 867.8 kJ/mol |
| Second ionization energy | 1631.4 kJ/mol |
| Organism | Cd+2 resistance (mM) |
Cd+2 removal (%) uptake (mM/g) |
|---|---|---|
| Klebsiella pneumoniae | 13.3 | 57.4 |
| Escherichia coli P4 | 10.6 | 56 |
| Salmonella enterica 43C | 13.3 | 22 |
| Bacillus sp. | – | 50 |
| Rhodobacter sphaeroides | – | 30.7 |
| Microbacterium oxydans CM3 | – | 43 |
| Candida tropicalis | 25.0 | 92 |
| Pichia hampshirensis 4Aer | 24.0 | 28 |
| Candida tropicalis 3Aer | 25.1 | 31 |
| Trametes versicolor | 5.0 | 0.300 |
| Trichosporon ashii | 10 | 78 |
| Pichia kudriavzevii | 15 | 61 |
| Species | Organ | Cd Concentration |
Exposure Duration |
Effect |
|---|---|---|---|---|
| Allium cepa | Root tip | 50–200 µM | 2 h + 24 h recovery |
Micronucleus formation Chromosomal aberrations |
| Root tip | 25 µM | 48 h | ||
| Root tip | 25 µM | 48 h | % tail DNA ↑ | |
| Arabidopsis thaliana | Root tip | 0.125–2.5 mg L−1 | 5 d | Altered expression DNA repair genes |
| Root | 1.25–4 mg L−1 | 5 d | Altered RAPD profile Altered expression DNA repair genes |
|
| Leaf | 0.5–5 mg L−1 | 16 d | Altered AFLP profile | |
| Leaf | 0.25–8 mg L−1 | 15 d | Microsatellite instability Altered RAPD profile |
|
| Leaf | 5 µM | 72 h | Altered expression DNA repair genes | |
| Brassica chinensis | Leaf | 15–120 mg kg−1 soil | 30 d | Altered RAPD profile |
| Brassica oleracea | Root | 2.5–20 mg kg−1 soil | 3–56 d | Altered % tail intensity |
| Capsicum annuum | Root tip | 20–100 ppm | 24 h | Chromosomal aberrations |
| Leaf | 20–100 ppm | 24 h | Altered RAPD profile | |
| Hordeum vulgare | Root tip | 75–225 µM | 7 d | Altered RAPD profile (GTS ↓) |
| Leaf | 5 µM | 15 d | DNA damage ↑ | |
| Ipomoea aquatica | Entire seedling | 15–120 mg kg−1 soil | 21 d | Altered RAPD profile (GTS ↓) |
| Lactuca sativa | Root tip | 25 µM | 48 h | Chromosomal aberrations Micronucleus formation % DNA damage ↑ |
| Lathyrus sativus | Root tip | 5–50 µM | 3–7 d | Chromosomal aberrations Micronucleus formation |
| Leucaena leucocephala | Leaf | 50 mg L−1 | 15 d | Altered RAPD profile |
| Nicotiana tabacum | Root and leaf | 10–15 µM | 7 d | % tail DNA ↑ |
| Oryza sativa | Root tip | 50–200 µM | 48–96 h | Altered SRAP profile (GTS ↓) |
| Sphagnum palustre | Shoot | 0.1–10 µM | 24–48 h | Altered ISSR profile (GTS ↓) |
| Species | Organ | Cd Concentration |
Exposure Duration |
Effect |
|---|---|---|---|---|
| Allium cepa | Root tip | 50–200 µM | 2 h + 24 h recovery |
Mitotic index ↓ |
| Root tip | 25 µM | 48 h | Mitotic index ↓ | |
| Arabidopsis thaliana | Root tip | 0.125–2.5 mg L−1 | 5 d | 2C ↓, 4C ↑, 8C ↑ Altered cell cycle phase distribution Altered expression cell cycle-related genes |
| Root | 1.25–4 mg L−1 | 5 d | 2C ↓, 4C ↑ Altered expression of cell cycle-related genes |
|
| Leaf | 5 µM | 3–12 d | Endoreduplication factor ↓ Epidermal cell number and cell surface area ↓ Altered expression of cell-cycle related genes |
|
| Capsicum annuum | Root tip | 20–100 ppm | 24 h | Mitotic index ↓ |
| Lactuca sativa | Root tip | 25 µM | 48 h | Mitotic index ↓ |
| Lathyrus sativus | Root tip | 5–50 µM | 3–7 d | Mitotic index ↓ |
| Oryza sativa | Root | 200 µM | 7 d | Cortex cell length in the elongation zone ↓ Cortex cell number in the elongation zone ↓ Altered expression of cell cycle-related genes |
| Sorghum bicolor | Root tip | 50–200 µM | 5 d | Inhibition of S phase progression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





