Submitted:
16 October 2024
Posted:
18 October 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
- (1)
- Subject between 18 and 55 years old with clinical, instrumental, and microbiological diagnosis of chronic prostatitis, according to indications of the EAU and history of symptomatology related to the diagnosis of chronic bacterial prostatitis for more than 6 months [4].
- (2)
- Isolation of uropathogens, according to EAU indications, in microbiological samples from Meares-Stamy tests or semen culture [4].
- (3)
- NIH-CPSI >9 and change in IPSS and SF-36 questionnaires (no significant cut-off is indicated), in line with EAU recommendations [4].
- (4)
- Subjects who can follow the study and give their consent to enroll in the study.
2.3. Drugs
- − 1 capsule/day containing 24 billion live cells of Lactobacillus Casei DG® (Enterolactis® plus), supported by Alfasigma S.p.A. - Via Ragazzi del ‘99, 5 - Bologna (Italy).
- − 1 capsule/day in packaging identical to probiotic with the same color, weight, smell and taste, but without bacteria, supported by Alfasigma S.p.A. - Via Ragazzi del ‘99, 5 - Bologna (Italy).
2.4. Experimental Protocol
2.5. Questionnaires
- (1)
- International Prostatic Symptoms Score (IPSS) consists of 8 questions used to screen, diagnose and monitor symptoms linked to benign prostatic hyperplasia. The answers are given points on a scale of 0 to 5. The IPSS values were classified as mild (scores 0–7), moderate (scores 8–19), and severe (scores 20–35) non-neurologic lower urinary tract symptoms, specifically incomplete bladder emptying, frequency, intermittency, urgency, weak stream, straining to void, and nocturia [6].
- (2)
- NIH-Chronic Prostatitis Symptom Index (NIH-CPSI), consisting of 9 items and used to assess the symptom severity of prostatitis and the effectiveness of treatment. NIH-CPSI includes three subscales with a total score ranging from 0 to 43: pain or discomfort (4 items with a total score ranging from 0 to 21), urinary symptoms (2 items with a total score ranging from 0 to 10), impact on the quality of life (3 items with a total score ranging from 0 to 12 points). The scores will also be higher as the symptoms get more severe [7].
- (3)
- International Index of Erectile Function (IIEF-5), consisting of 5 questions indicating the presence of erectile dysfunction, each of which can be scored from 0 or 1 (representing the worst) to 5 (representing the best). The final score ranges from 1 to 25 points. Erectile dysfunction is classified based on the overall score as severe (score 0 to 7), moderate to severe (score 8 to 11), mild to moderate (score 12 to 16), mild (score 17 to 21) and absent (score 22 to 25) [8].
- (4)
- The 36-Item Short Form Health Survey (SF-36), consisting of 36 questions and used to assess quality of life about pathology and the effectiveness of treatment. Quality of life is defined as the subjective perception of one's own well-being within socio-cultural context or as the satisfaction of desires and pleasures. Questions are summarized in two component summary scores, the Physical Component Summary (PCS) and the Mental Component Summary (MCS) scores representing eight concepts of health: physical functioning (PF), bodily pain (BP), role limitations due to physical health problems (RP), role limitations due to personal or emotional problems (RE), general mental health (MH), social functioning (SF), energy/fatigue or vitality (VIT), and general health perceptions (GH). A higher score represents better health while a low score corresponds to a lower quality of life [9,10].
- (5)
- Zung’s Self-Rating Anxiety Scale (Zung SAS) consists of a 20-item question scale that rates the four common characteristics of anxiety, both psychological and somatic. Responses are given on a 4-point scale which range from 1 (none, or a little of the time) to 4 (most, or all of the time). Items include both negative and positive experiences. The final score ranges from 20 to 80 points. Anxiety is classified as normal (score 0 to 44), moderate (score 45 to 59) and severe (score 60 to 80) [11].
- (6)
- Zung’s Self-Rating Depression Scale (Zung SDS) consists of a 20-item question scale that rates the four common characteristics of depression. Items tap psychological and physiological symptoms: 10 express negative experiences, and 10 express positive experiences. Responses are given on a 4-point scale ranging from 1 (none, or a little of the time) to 4 (most, or all of the time). Total raw scores range from 20 to 80. Depression is classified as normal (score 20 to 49), mild (score 50 to 59), moderate (score 60 to 69) and severe (score 70 to 80) [12].
2.6. Meares-Stamey Test
2.7. Fecal Samples
2.8. Microbiological Identification Tests
2.9. Admission (T0)
2.10. Follow-Up Visits (T1, T2, T3)
2.11. Outcomes
2.12. Statistical Analysis and Ethical Considerations
3. Results
3.1. Population
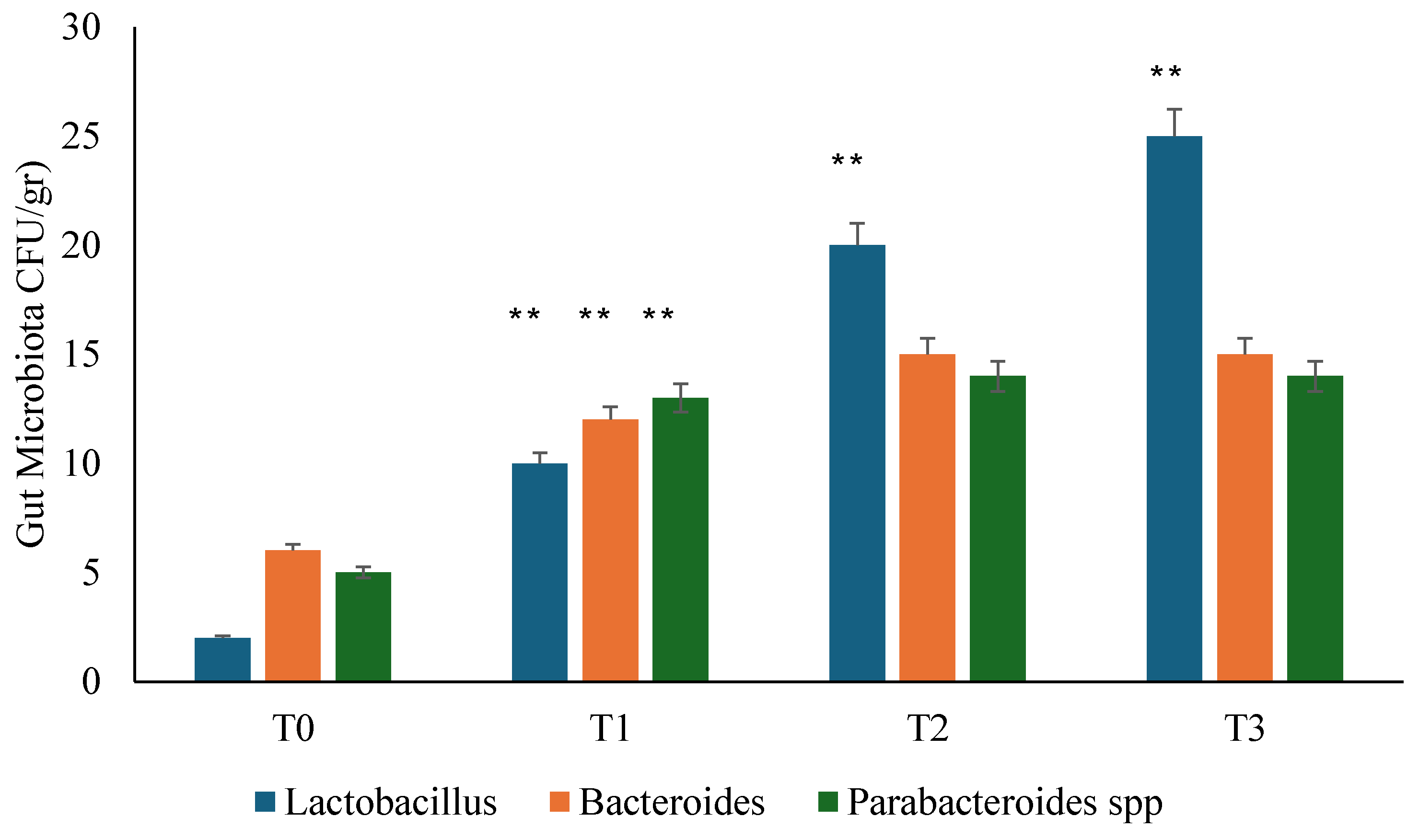
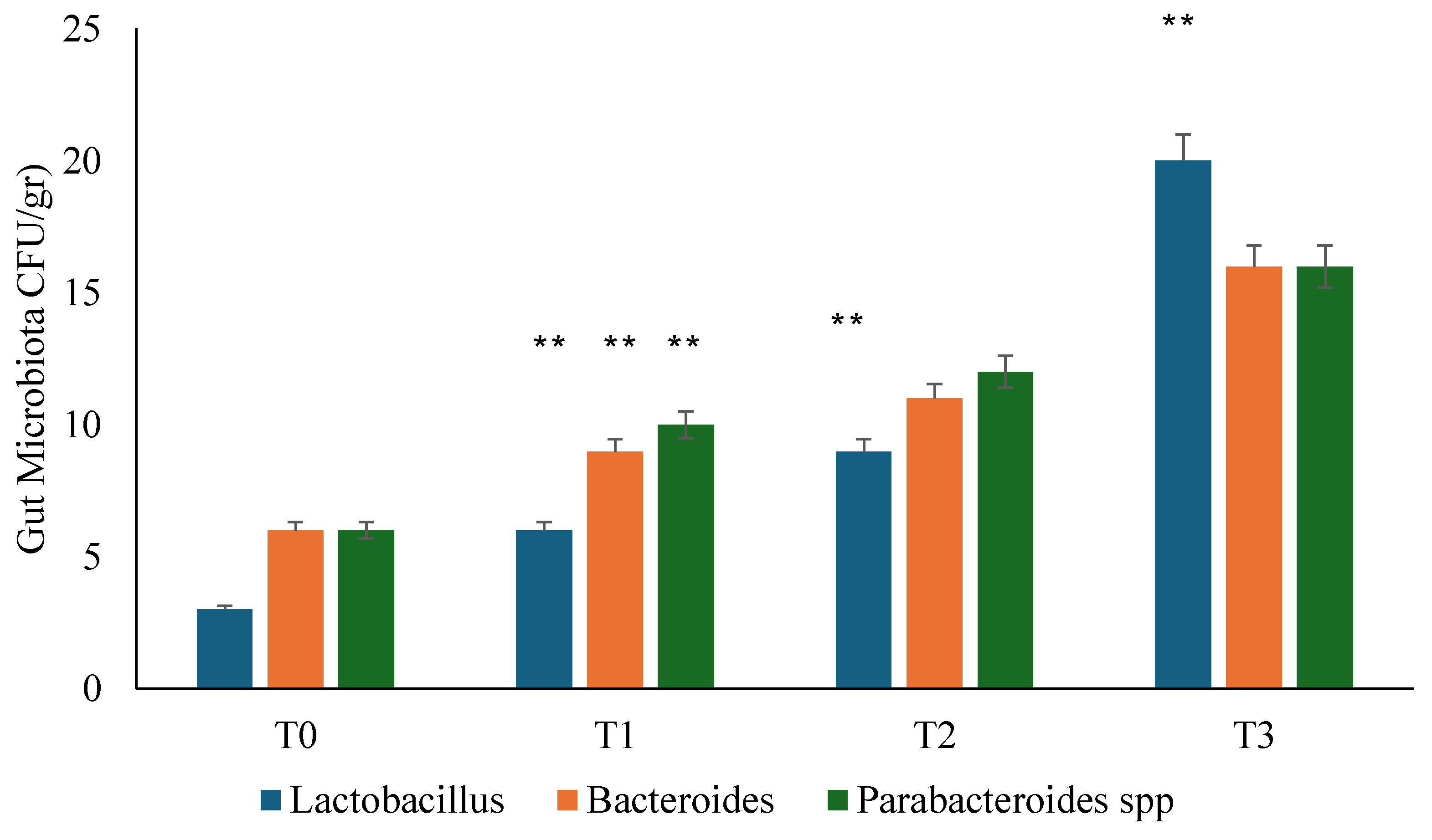
3.2. Gut Microbiota Analysis
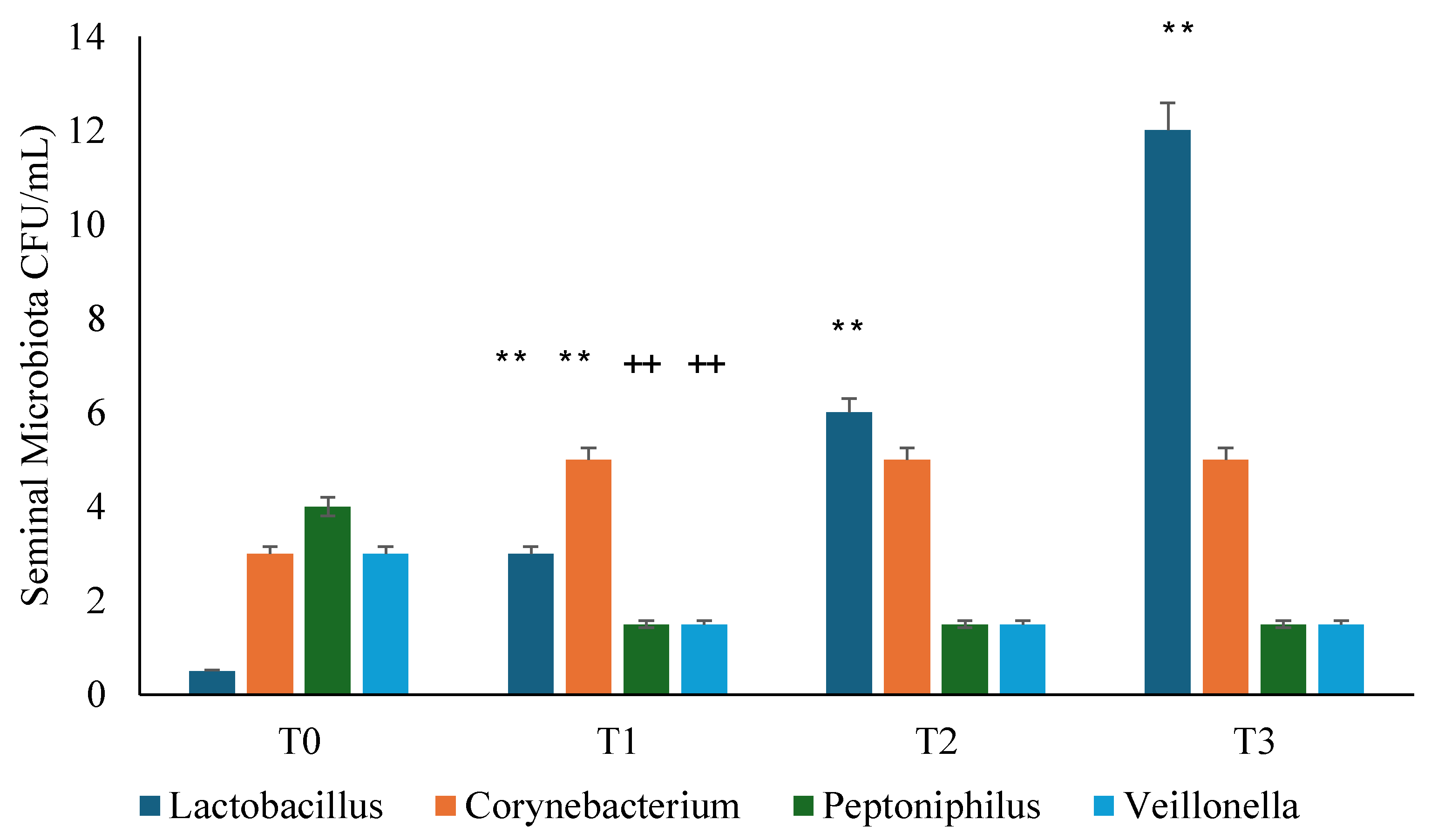
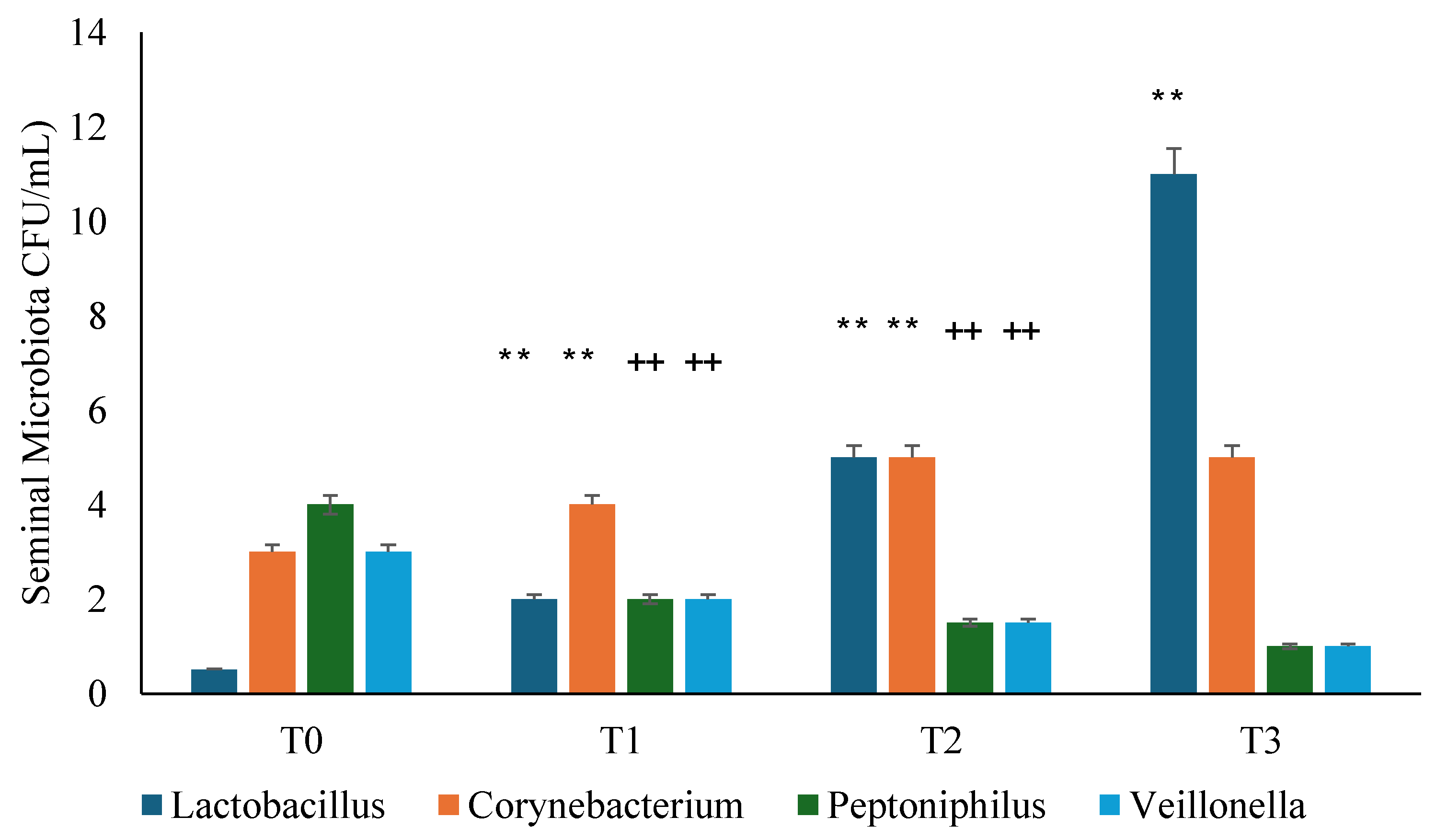
3.3. Seminal Microbiota Analysis
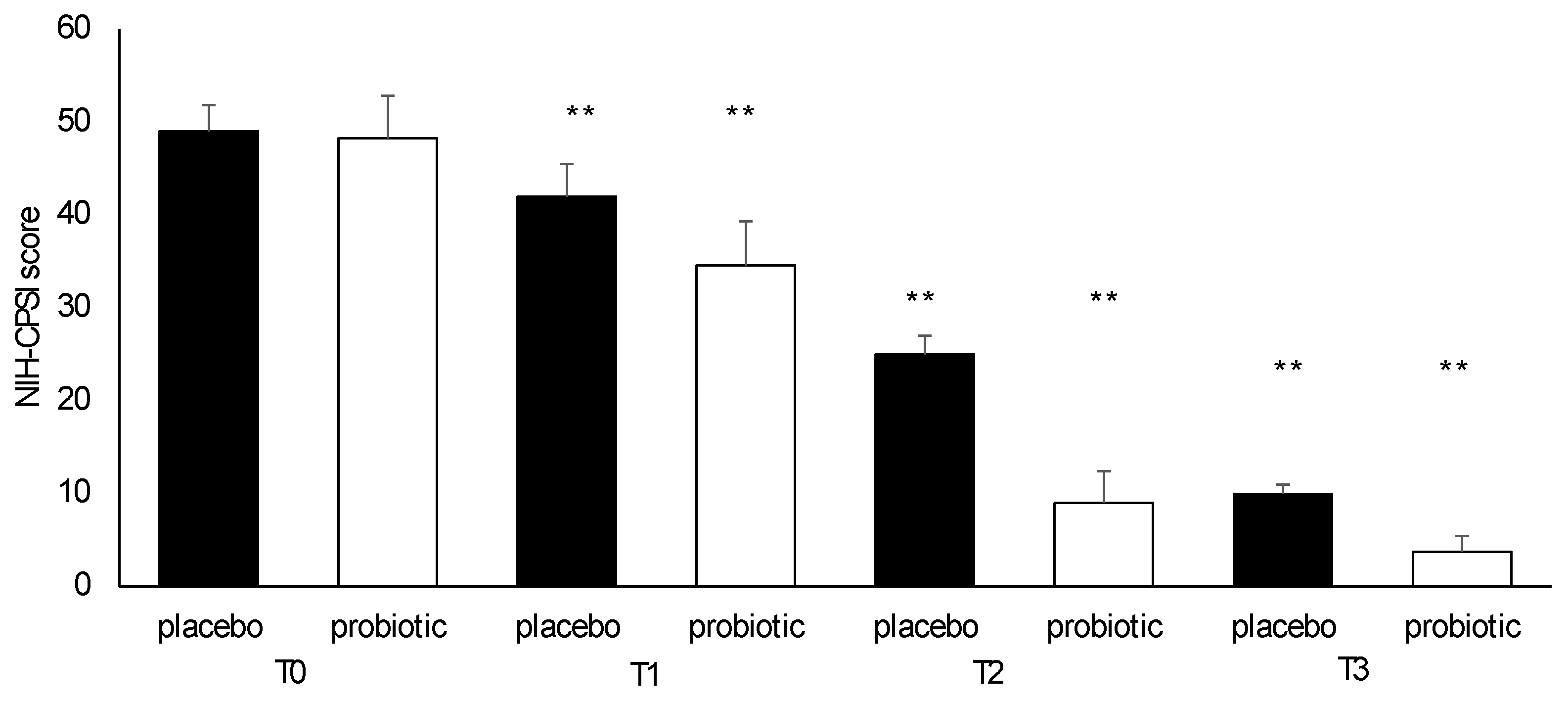
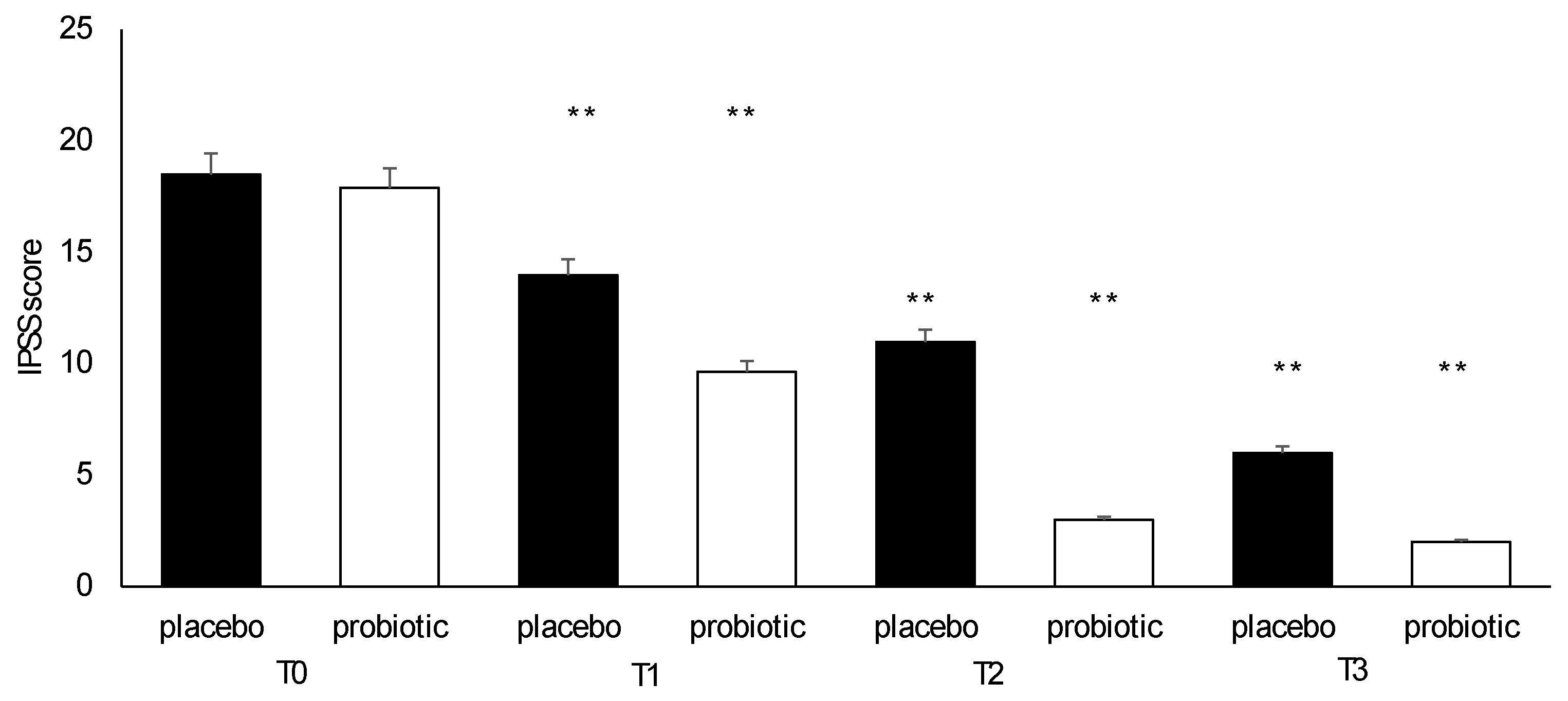
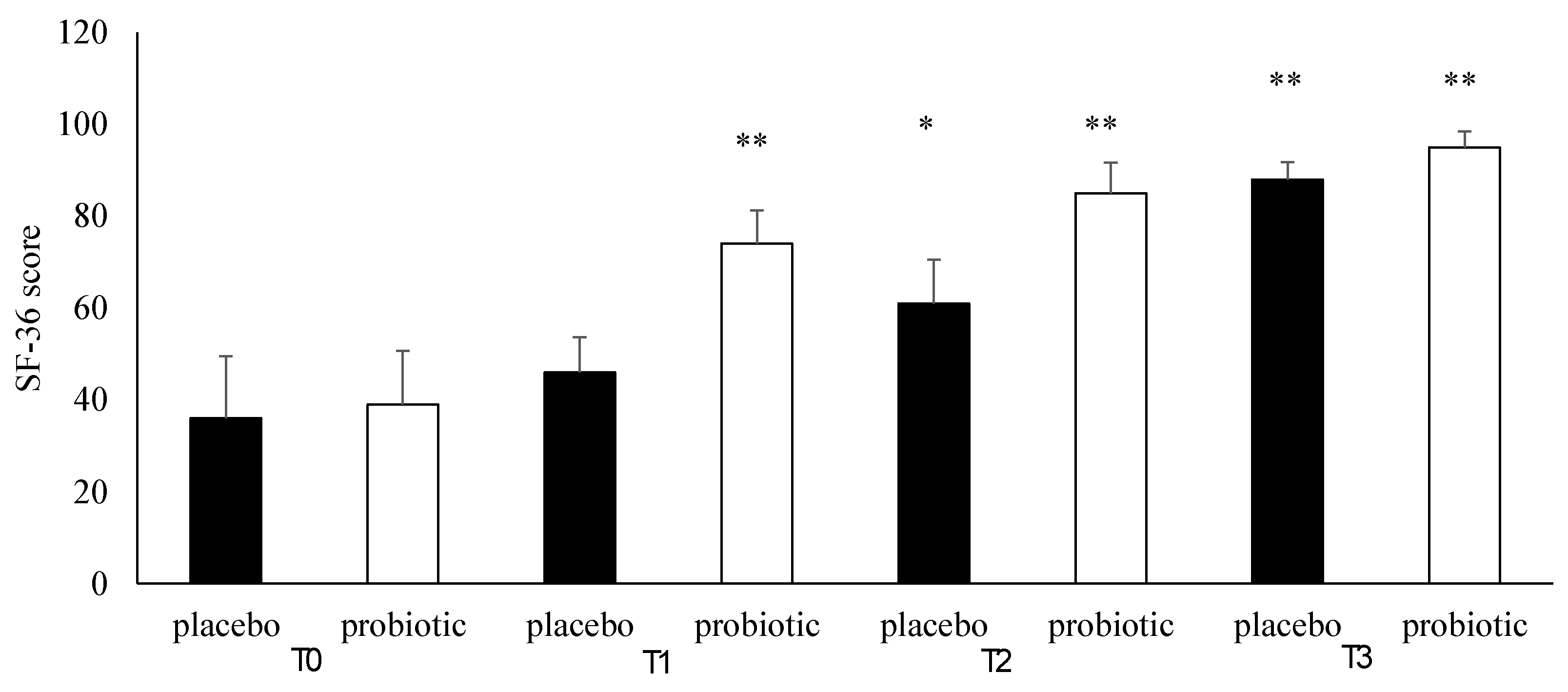
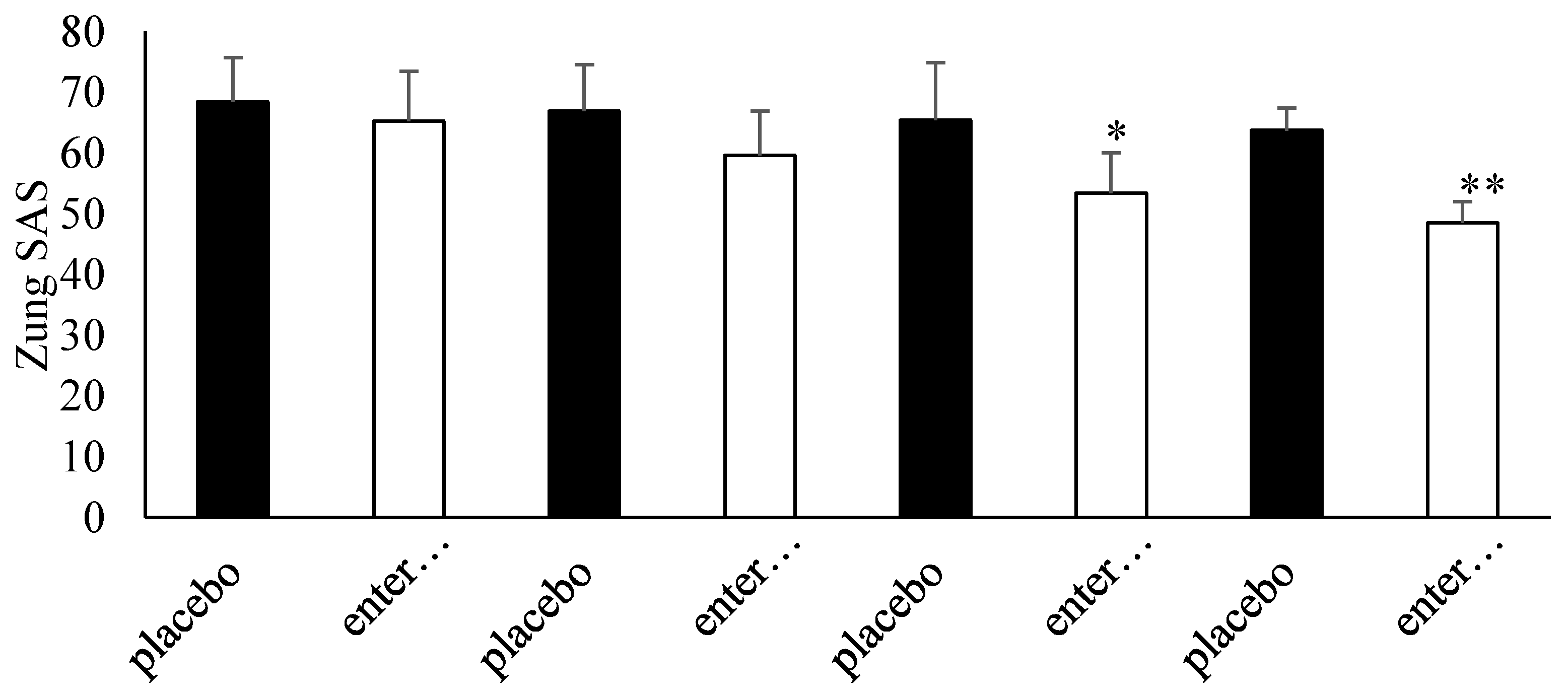
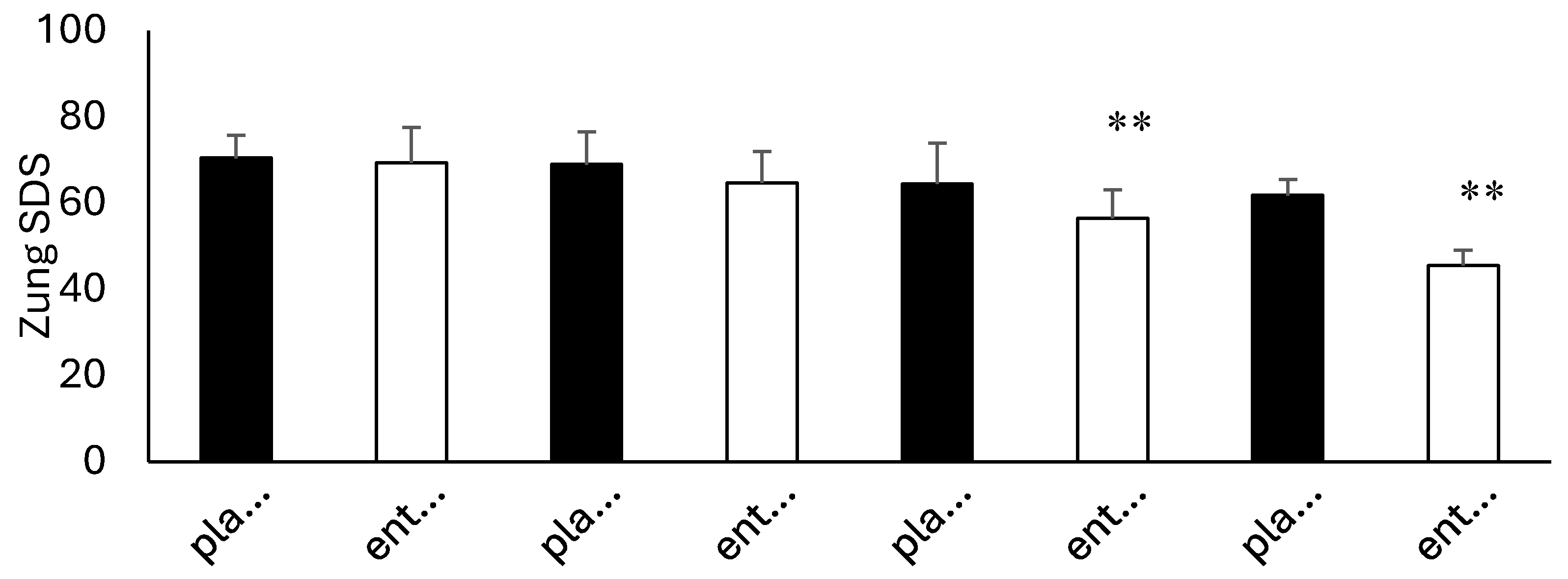
3.4. Questionnaire Analysis
3.5. Clinical Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rees, J.; Abrahams, M.; Doble, A.; Cooper, A. Prostatitis Expert Reference Group (PERG). Diagnosis and treatment of chronic bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome: A consensus guideline. BJU Int 2015, 116, 509–525. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, T.; Alidjanov, J.; Palagin, I.; Medina-Polo, J.; Nickel, J.C.; Wagenlehner, F.M.E. Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): Look to the future. Prostate Cancer Prostatic Dis 2024, 27, 239–241. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Luo, H.; Xu, H.; Qian, B.; Zou, X.; Zhang, G.; Zeng, F.; Zou, J. Preclinical models and evaluation criteria of prostatitis. Front Immunol 2023, 14, 1183895. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kranz, J.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S. , Köves, B.; Schubert, S.; Pilatz, A.; Veeratterapillay, R.; Wagenlehner, F.M.E.; Bausch, K.; Devlies, W.; Horváth, J.; Leitner, L.; Mantica, G.; Mezei, T.; Smith, E.J.; Bonkat, G. European Association of Urology Guidelines on Urological Infections: Summary of the 2024 Guidelines. Eur Urol 2024, 86, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Gallelli, L.; Cione, E.; Perletti, G.; Ciarleglio, F.; Malossini, G.; De Pretis, G.; Palmieri, A.; Mirone, V.; Bartoletti, R.; Johansen, T.E.B. The use of Lactobacillus casei DG® prevents symptomatic episodes and reduces the antibiotic use in patients affected by chronic bacterial prostatitis: Results from a phase IV study. World J Urol 2021, 39, 3433–3440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Badia, X.; Garcia-Losa, M.; Dal-Re, R. Ten-language translation and harmonization of the International Prostate Symptom Score: Developing a methodology for multinational clinical trials. Eur Urol 1997, 31, 129–140. [Google Scholar] [CrossRef]
- Giubilei, G.; Mondaini, N.; Crisci, A.; Raugei, A.; Lombardi, G.; Travaglini, F.; Del Popolo, G.; Bartoletti, R. The Italian version of the National Institutes of Health Chronic Prostatitis Symptom Index. Eur Urol 2005, 47, 805–811. [Google Scholar] [CrossRef]
- Cappelleri, J.C.; Rosen, R.C.; Smith, M.D.; Mishra, A.; Osterloh, I.H. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology 1999, 54, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qu, B.; Lun, S.S.; Guo, Y.; Liu, J. The 36-item short form health survey: Reliability and validity in Chinese medical students. Int J Med Sci 2012, 9, 521–526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laucis, N.C.; Hays, R.D.; Bhattacharyya, T. Scoring the SF-36 in Orthopaedics: A Brief Guide. J Bone Joint Surg Am 2015, 97, 97,1628–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dunstan, D.A.; Scott, N. Norms for Zung's Self-rating Anxiety Scale. BMC Psychiatry 2020, 20, 90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dunstan, D.A.; Scott, N. Clarification of the cut-off score for Zung's self-rating depression scale. BMC Psychiatry 2019, 19, 177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, T.; Tamanini, I.; Odorizzi, K.; Gallelli, L.; Lanzafame, M.; Mazzoli, S.; Lanzafame, P.; Massidda, O.; Palmieri, A.; Wagenlehner, F.M.E.; Bjerklund Johansen, T.E.; De Nunzio, C. The diagnostic yield of the Meares & Stamey test can be significantly improved by symptom-based patient selection and the experience of the test performer. Prostate Cancer Prostatic Dis 2024, 27, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Cossart, Y. Bergey’s Manual of Systematic Bacteriology Volume 2. Pathology 1987, 19, 324; Holt, J.G.; Krieg, N.R.; Sneath, P.H.A. Bergey’s Manual of Determinative Bacterology; The Williams and Wilkins Co.: Baltimore, MD, USA, 1994.
- Krieger, J.N.; Lee, S.W.; Jeon, J.; Cheah, P.Y.; Liong, M.L.; Riley, D.E. Epidemiology of prostatitis. Int J Antimicrob Agents 2008, 31, S85–90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holt, J.D.; Garrett, W.A.; McCurry, T.K.; Teichman, J.M. Common Questions About Chronic Prostatitis. Am Fam Physician 2016, 93, 290–296. [Google Scholar] [PubMed]
- Bartoletti, R.; Cai, T.; Mondaini, N.; Dinelli, N.; Pinzi, N.; Pavone, C.; Gontero, P.; Gavazzi, A.; Giubilei, G.; Prezioso, D.; Mazzoli, S.; Boddi, V.; Naber, K.G.; Italian Prostatitis Study Group. Prevalence, incidence estimation, risk factors and characterization of chronic prostatitis/chronic pelvic pain syndrome in urological hospital outpatients in Italy: Results of a multicenter case-control observational study. J Urol discussion 2415. 2007, 178, 2411–2415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, C.; Shang, X.; Li, H. Chronic Prostatitis/Chronic Pelvic Pain Syndrome: A Disease or Symptom? Current Perspectives on Diagnosis, Treatment, and Prognosis. Am J Mens Health 2020, 14, 1557988320903200. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.F.; Schaeffer, A.J.; Thumbikat, P. Immune mediators of chronic pelvic pain syndrome. Nat Rev Urol 2014, 11, 259–269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hou, D.S.; Long, W.M.; Shen, J.; Zhao, L.P.; Pang, X.Y.; Xu, C. Characterisation of the bacterial community in expressed prostatic secretions from patients with chronic prostatitis/chronic pelvic pain syndrome and infertile men: A preliminary investigation. Asian J Androl 2012, 14, 566–573. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nickel, J.C.; Alexander, R.B.; Schaeffer, A.J.; Landis, J.R.; Knauss, J.S.; Propert, K.J.; Chronic Prostatitis Collaborative Research Network Study Group. Leukocytes and bacteria in men with chronic prostatitis/chronic pelvic pain syndrome compared to asymptomatic controls. J Urol 2003, 170, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Magri, V.; Perletti, G.; Cai, T.; Stamatiou, K.; Trinchieri, A.; Montanari, E. Levofloxacin for NIH Category II Chronic Bacterial Prostatitis: A Real-Life Study. Chemotherapy 2019, 64, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Isolauri, E.; Sütas, Y.; Kankaanpää, P.; Arvilommi, H.; Salminen, S. Probiotics: Effects on immunity. Am J Clin Nutr 2001, 73, 444S–450S. [Google Scholar] [CrossRef] [PubMed]
- Mohamadzadeh, M.; Olson, S.; Kalina, W.V.; Ruthel, G.; Demmin, G.L.; Warfield, K.L.; Bavari, S.; Klaenhammer, T.R. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci U S A 2005, 102, 2880–2885. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Löhr, J.M.; Dominguez-Munoz, E.; Rosendahl, J.; Besselink, M.; Mayerle, J.; Lerch, M.M.; Haas, S.; Akisik, F.; Kartalis, N.; Iglesias-Garcia, J.; Keller, J.; Boermeester, M.; Werner, J.; Dumonceau, J.M.; Fockens, P.; Drewes, A.; Ceyhan, G.; Lindkvist, B.; Drenth, J.; Ewald, N.; Hardt, P.; de Madaria, E.; Witt, H.; Schneider, A.; Manfredi, R.; Brøndum, F.J.; Rudolf, S.; Bollen, T.; Bruno, M.; HaPanEU/UEG Working Group. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterol J 2017, 5, 153–199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aragón, I.M.; Herrera-Imbroda, B.; Queipo-Ortuño, M.I.; Castillo, E.; Del Moral, J.S.; Gómez-Millán, J.; Yucel, G.; Lara, M.F. The Urinary Tract Microbiome in Health and Disease. Eur Urol Focus 2018, 4, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Arora, H.C.; Eng, C.; Shoskes, D.A. Gut microbiome and chronic prostatitis/chronic pelvic pain syndrome. Ann Transl Med 2017, 5, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vicari, E.; La Vignera, S.; Castiglione, R.; Condorelli, R.A.; Vicari, L.O.; Calogero, A.E. Chronic Bacterial Prostatitis and Irritable Bowel Syndrome: Effectiveness of Treatment With Rifaximin Followed by the Probiotic VSL#3. Asian J Androl 2014, 16, 735–739. [Google Scholar]
- Chen, J.; Chen, J.; Fang, Y.; Shen, Q.; Zhao, K.; Liu, C.; Zhang, H. Microbiology and immune mechanisms associated with male infertility. Front Immunol 2023, 14, 1139450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Šutulović, N.; Vesković, M.; Puškaš, N.; Zubelić, A.; Jerotić, D.; Šuvakov, S.; Despotović, S.; Grubač, Ž.; Mladenović, D.; Macut, D.; Rašić-Marković, A.; Simić, T.; Stanojlović, O.; Hrnčić, D. Chronic Prostatitis/Chronic Pelvic Pain Syndrome Induces Depression-Like Behavior and Learning-Memory Impairment: A Possible Link with Decreased Hippocampal Neurogenesis and Astrocyte Activation. Oxid Med Cell Longev 2023, 2023, 3199988. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Characteristics | Data | Probiotic Group | Placebo Group |
|---|---|---|---|
| Age | 50 ± 3.1 | 50.4 ± 2.5 | 49.7 ± 3.4 |
| Body Mass Index (Kg/m2) | 27.7 ± 2.7 | 28.0 ± 2.5 | 27.4 ± 2.7 |
| Occupation Status | |||
| Sedentary | 11 | 4 | 7 |
| Manual | 13 | 8 | 5 |
| Comorbidity | |||
| Copd | 3 | 2 | 1 |
| Diabetes Mellitus Type 2 | 6 | 3 | 3 |
| Hypertension | 8 | 1 | 7 |
| Dyslipidemia | 4 | 4 | 0 |
| Metabolic Syndrome | 3 | 2 | 1 |
| Bacteria | T0 | T1 | T2 | T3 |
|---|---|---|---|---|
| Escherichia coli | 36 | 34 | 38 | 36 |
| C. difficile | 51 | 49 | 52 | 54 |
| Campylobacter spp. (C. jejuni, C. upsaliensis, and C. coli) | 59 | 53 | 54 | 56 |
| Clostridioides difficile tcdA/tcdB | 51 | 52 | 51 | 54 |
| Enteroaggregative E. coli | 56 | 58 | 59 | 59 |
| Enteropathogenic E. coli | 57 | 58 | 59 | 58 |
| Enterotoxigenic E. coli eltA/estA | 61 | 62 | 59 | 62 |
| Shiga toxin–producing E. coli stx1/stx2 | 59 | 62 | 61 | 58 |
| Shiga toxin–producing E. coli stx1/stx2 O157 | 56 | 58 | 59 | 61 |
| enteroinvasive E. coli /Shigella (S. sonnei, S. fexneri, S. boydii, and S. dysenteriae) | 61 | 62 | 64 | 62 |
| Plesiomonas shigelloides | 59 | 60 | 58 | 59 |
| Salmonella spp. | 63 | 64 | 61 | 62 |
| Symptoms | ||||
| IIEF-5 (range: 0–25 points) | Groups at T0 | Groups at T3 | ||
| Probiotic | Placebo | Probiotic | Placebo | |
| Normal (IIEF-5 22–25 points) | 1 | 2 | 4 | 2 |
| Mild (IIEF-5 17–21 points) | 5 | 5 | 7 | 6 |
| Mild to moderate (IIEF 12–16 points) | 3 | 1 | 1 | 2 |
| Moderate to severe (IIEF 8–11 points) | 1 | 2 | 0 | 1 |
| Severe (IIEF-5 0–7 points) | 2 | 2 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





