1. Introduction
Parkinson's disease (PD) is the second most common age-related neurological disorder [
1], and is expected to double in prevalence over the next generation [
2]. The primary motor symptoms of PD include bradykinesia, resting tremor and rigidity [
3]. Pharmacological and surgical therapies are effective ways to manage these symptoms. However, higher dosages of levodopa can cause dyskinesia, akinesia, confusion, hallucination, and psychosis [
4,
5,
6,
7] and deep brain stimulation carries the risks of infection, hardware failure, and an increased incidence of depression and confusion [
8,
9,
10].
High-cadence (forced) cycling has beneficial effects on PD motor symptoms similar to pharmacological and surgical management [
11,
12,
13,
14]. In addition, it has a low risk of falling compared to exercise that requires standing. The beneficial effects of cycling on PD motor symptoms are greater when the pedaling rate (cadence) is higher than self-selected pedaling cadence [
12]. Eight weeks of forced cycling (high-cadence tandem cycling) improved UPDRS motor III score (35%), rigidity (41%), tremor (38%), and bradykinesia (28%). Similarly, high-cadence dynamic cycling (motorized stationary cycling with advanced control of cadence and power) also had significant treatment effects on PD motor symptoms, non-motor symptoms, and functional mobility [
15,
16].
Even though high-cadence cycling offers significant benefits on PD symptoms, it has some limitations. Forced cycling using a tandem bike is not always feasible in clinical settings due to the need for a large space and a relatively fit trainer [
12], and previous studies of dynamic cycling did not adapt setting between or within exercise sessions [
17]. In the current literature, periods of dynamic cycling intervention were short, usually limited to 3 or 6 sessions, which is insufficient to evaluate longer-term benefits [
15,
16]. Most importantly, neither of these paradigms allowed for adaptive exercise for individuals with PD. Every participant in previous studies received uniform exercise prescriptions, regardless of their physical functioning level or degree of PD motor symptoms, which led to some participants experienced greater exercise benefits than others.
To move toward maximizing the effects and minimizing heterogeneity in individual responses, an individually tailored exercise rehabilitation model was developed [
18] using two measured variables for feedback: sample entropy of cadence (SampEn) and effort (percentage of positive power). Previous findings from our lab [
19,
20], indicated significant associations between SampEn of cadence and motor function improvement and between percentage of positive power and motor skill performance following the dynamic cycling intervention. A higher level of SampEn of cadence led to more positive effects on motor symptoms, and a greater percentage of power output during the dynamic cycling session improved motor function skills in individuals with PD. However, these qualitative observations do not translate directly to an individualized strategy that improves motor symptoms and function skills.
These preliminary results suggest that by quantifying the variability of cadence and identifying effort variables, PD motor function improvement can be predicted, and this predictive model can them be used to improve the physiological benefits from high-cadence dynamic cycling for each individual. For this study, we tested an algorithm that uses SampEn of cadence and a measure of effort to determine the resistance settings for high-cadence cycling that is expected to provide benefits to the patient, referred to as patient-specific adaptive dynamic cycling (PSADC) [
21].
In addition, to improve the accuracy of motor function and mobility assessments and enhance the efficacy of the PSADC paradigm, this research incorporates the use of inertial measurement unit (IMU) sensors. IMU sensors offer a highly accurate, reliable, and valid method [
22] for capturing movement patterns and measuring PD motor function and mobility [
23]. Furthermore, they allow for continuous, quantitative monitoring of motor function throughout the intervention period, providing valuable insights into how PD motor symptoms respond to the PSADC paradigm over time. This enables a more precise evaluation of the therapeutic effects of the PSADC paradigm.
This study is a pilot randomized controlled trial (RCT) designed to examine the effects of the PSADC paradigm on PD motor function and functional mobility in comparison to non-adaptive (NA) dynamic cycling. In addition, as a pilot study, our objective is to evaluate the feasibility and efficacy of the PSADC protocol in preparation for a larger, double-blind randomized controlled trial (RCT). Through this process, we aim to gather critical data on the PSADC's practicality, optimize our study design, and estimate the appropriate sample size for future research. Finally, we hypothesized that the PSADC paradigm coupled with the objective data collected through IMU sensors, would improve the therapeutic effects of high-cadence dynamic cycling for each individual as well as reduce heterogeneity in individual changes in PD motor function, and functional mobility measures.
2. Materials and Methods
2.1. Research Design
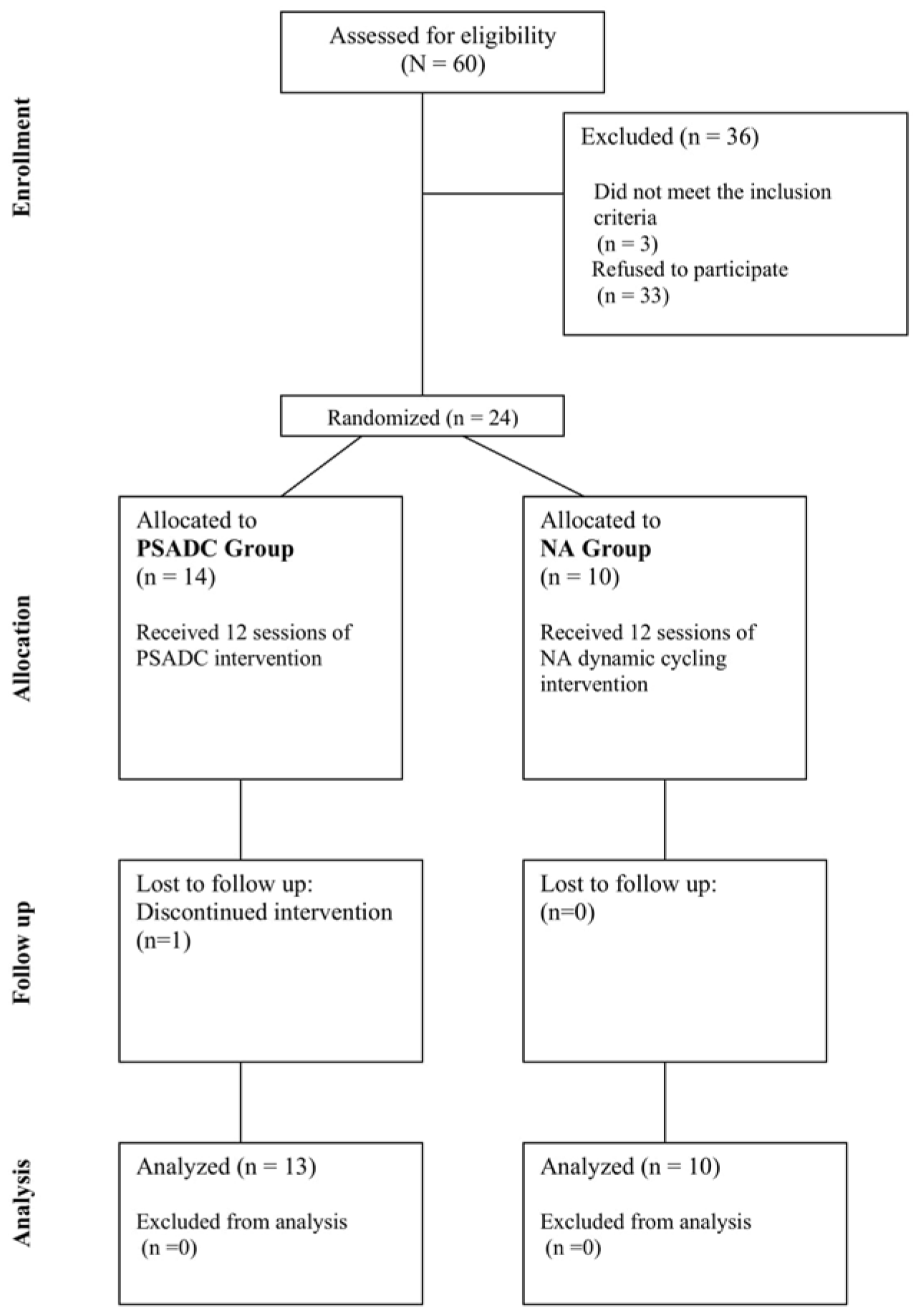
This study was a pilot randomized control trial (RCT) with two arms: (a) PSADC group and (b) NA group. Neither the participants nor the blinded evaluator was aware of the group allocation. It was registered with ClinicalTrials.gov (NCT05361200) and was approved by the Institutional Review Board (IRB) at Kent State University (IRB#87). Written informed consent was provided by the participants.
2.2. Participants
2.2.1. Inclusion and Exclusion Criteria
Inclusion criteria included a diagnosis of idiopathic PD according to the UK Brain Bank Criteria, ability to give written informed consent, 50–79 years old, and on a stable medical regimen of antiparkinsonian medication for at least six months. Exclusion criteria included any sign or symptoms of cardiovascular, metabolic, and/or renal disease without medical clearance from a physician.
2.2.2. Sample Size Calculation
To establish the appropriate sample size for this study, we conducted an a priori power analysis using the G*Power 3.1. This analysis was a paired samples
t-test to assess the difference between two dependent means. The means and standard deviations for the two groups were obtained from prior research [
15]. We set the alpha level at 0.05 and aimed for a power of 0.8. The analysis indicated that a sample size of 20 participants would be necessary. Therefore, we planned to recruit at least 20 participants to ensure sufficient statistical power.
2.3. Group Allocation and Exercise Intervention
Using the REDcap randomization module, we randomly assigned participants to either the PSADC or NA group based on their Hoehn and Yahr stage. Both groups completed 12 dynamic cycling sessions, three times a week for four weeks. Each session included 5 minutes of warm-up at 60 revolutions per minute (RPM), 30 minutes of dynamic cycling at 80 RPM, and 5 minutes of cool-down at 60 RPM. The PSADC group adjusted specific resistance settings on the 3rd, 6th, and 9th sessions based on effort and SampEn of cadence variables from their previous three sessions. If the average SampEn of cadence from the previous 3 sessions was within the interquartile range of healthy adults and effort was over 65%, the resistance level increased for the next 3 sessions. If SampEn was within range but effort was below 65%, or if SampEn was below range but effort was over 65%, the same resistance level was used. When both SampEn and effort were below these thresholds, resistance was reduced for the next 3 sessions. Meanwhile, the NA group maintained the same resistance setting throughout all 12 sessions. The details of the PSADC paradigm are outlined in a study by Kim et al [
21]. Participants were not told which group they were assigned. (
Figure 1).
2.4. Outcome Measurements
2.4.1. PD Motor Functions
The primary outcome measure was Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale Motor III (MDS-UPDRS Motor III). We used this measure at baseline and after the last exercise session. To maintain objectivity, assessments were video-recorded and then evaluated by a blinded assessor who was certified in scoring the MDS-UPDRS. Video-recorded administration of the MDS-UPDRS Motor III not only upholds validity and reliability but is also cost-efficient and practical [
24,
25]. UPDRS Motor III rigidity measures were assessed in person by a trained evaluator. To investigate the effect of PSADC on dopamine-sensitive motor symptoms, the MDS-UPDRS Motor III test was separated into two categories of symptoms: dopamine-sensitive symptoms (such as bradykinesia, resting tremor, and rigidity, which indicate degeneration of dopaminergic neurons) and dopamine less-sensitive symptoms (such as posture, balance, and gait, which reflect the loss of nondopaminergic pathways) [
26].
In addition to the clinical assessment, the Kinesia™ One device (Great Lakes Neuro Technologies, Cleveland, OH) was used to provide an objective and real-time measurement of PD motor function. The Kinesia™ One system incorporates a wireless IMU sensor, which offers detailed data collection by measuring triaxial acceleration and gyroscopic movements along the X, Y, and Z axes. The IMU sensor operates at a sampling rate of 128 Hz, which allows for the capture of 128 data points per second. This is sufficient to detect both slow and rapid motor movements, which are common in individuals with PD. During the tests, the IMU sensor gathers raw data on linear acceleration and angular velocity in all three dimensions (X, Y, and Z), providing a detailed picture of the participant’s movement patterns. This raw data is continuously transmitted to a secure portal where it is processed and analyzed. The IMU sensor data evaluates motor functions like finger tapping, hand movements, and pronation-supination movements of the hands. Movement speed, amplitude, and rhythm data were collected before and after each training session, and baseline and post-intervention data were used for data analysis. The test results are scored on a 0-4 scale, similar to the MDS-UPDRS Motor III [
27]. A decrease in score indicates an improvement in symptom severity, whereas an increase in score indicates a worsening of symptoms.
2.4.2. Mobility
Mobility was assessed with the Timed Up and Go (TUG) test combined with the OPALS system [
28] (ADPM Wearable Technologies, Inc., Portland, OR), an advanced IMU sensor developed for gait analysis. The OPAL IMU sensors feature 3-axis accelerometers, gyroscopes, and magnetometers that enable precise motion detection in all three dimensions (X, Y, and Z axes). The sensors operate at a sampling rate of 128 Hz, providing high-resolution data that enables accurate monitoring of mobility and gait dynamics, such as postural changes or turning dynamics [
29]. The OPAL IMU sensors were attached to the participant’s legs, arms, and trunk to track the total time of the test and to measure postural transitions, including turn duration and turn velocity. TUG was measured at baseline and immediately after the last exercise session.
2.4.3. Exercise Variables
During each cycling session, heartrate (HR) was measured every two minutes using a HR monitor (Mi Band 6, Xaomi, China). Ratings of perceived exertion (RPE) were recorded every four minutes using a 6-20 Borg RPE scale [
30]. Revolutions per minute (RPM, cadence) and power were recorded every second from a micro controller on the dynamic cycle. After each session, these values were averaged across the 30-minute main set. Effort was calculated as the percentage of time a rider produced positive power to maintain the 80 RPM cadence during the session. During cycling, an animation of a balloon over water was provided immediate biofeedback to participants indicating current effort level. Participants were instructed to “keep the balloon over the water” while cycling to encourage positive power and thus high effort.
2.5. Statistical Analysis
All statistical analyses were completed using IBM SPSS version 28 (Armonk, NY: IBM Corp), with an alpha level of 0.05. Independent samples t-tests were used to compare demographic, cycling, and physiological variables, as well as each group's pre- and post-MDS-UPDRS Motor III change scores, to determine any differences between the two groups. For the MDS-UPDRS Motor III, dopamine sensitive and less-sensitive PD symptoms, Kinesia motor function test, and TUG, two-way repeated-measures analysis of variance (ANOVA; 2 groups by 2 time points) was used.
3. Results
23 participants completed the study. Thirteen participants completed 12 sessions of the PDADC protocol, and ten participants completed 12 sessions of the NA protocol. An independent samples
t-test revealed no significant demographic differences between the two groups. No significant difference in the physiological and cycling variables emerged between the two groups except for HR (
t = 2.237,
p = 0.036) and Effort (
t = 2.113,
p = 0.047) (
Table 1).
3.1. MDS-UPDRS Motor III Score Changes
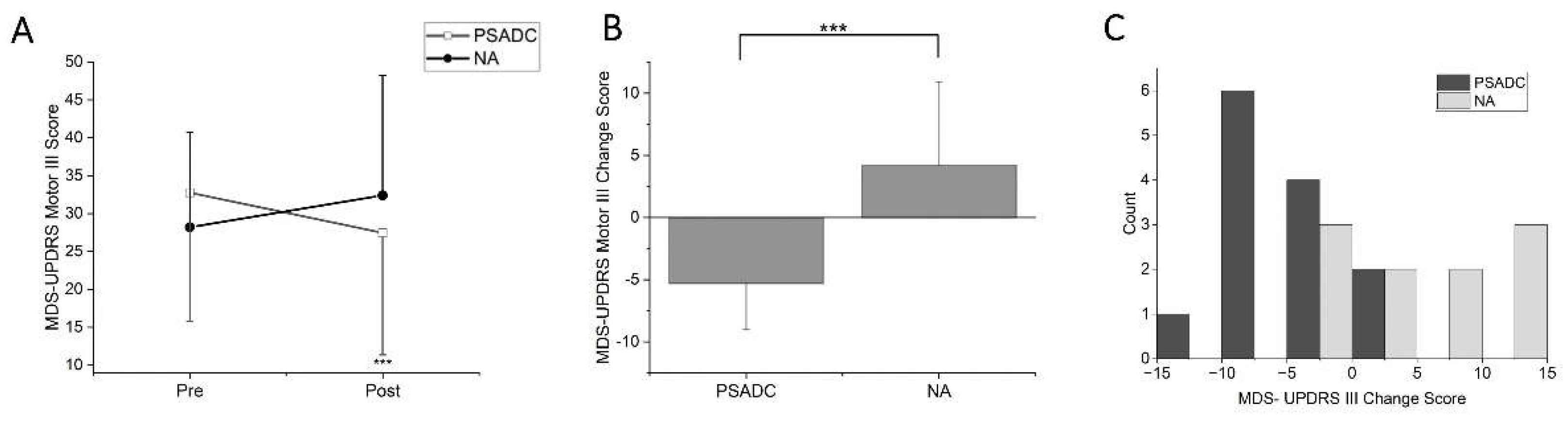
MDS-UPDRS Motor III scores showed a significant group by time interaction (
F = 18.746,
df = 1,
p < 0.001,
ηp2 =0.472) but no significant main effect of time (
F = 254,
df = 1,
p = 0.619,
ηp2 =0.012;
Figure 2A). The PSADC group showed 16.2% improvement (-5.3 ± 3.7 points) while the NA group showed 14.9% decline (4.2 ± 6.7 points). This change was statistically significant (
Figure 2B, t = -4.330,
df = 21,
p < 0.001). Individually, 84.6% (11/13) of the PSADC participants improved their MDS-UPDRS Motor III score, while 30% (3/10) of the NA participants improved (
Figure 2C).
3.2. Dopamine-Sensitive and Less-Sensitive MDS-UPDRS Motor III Score Changes
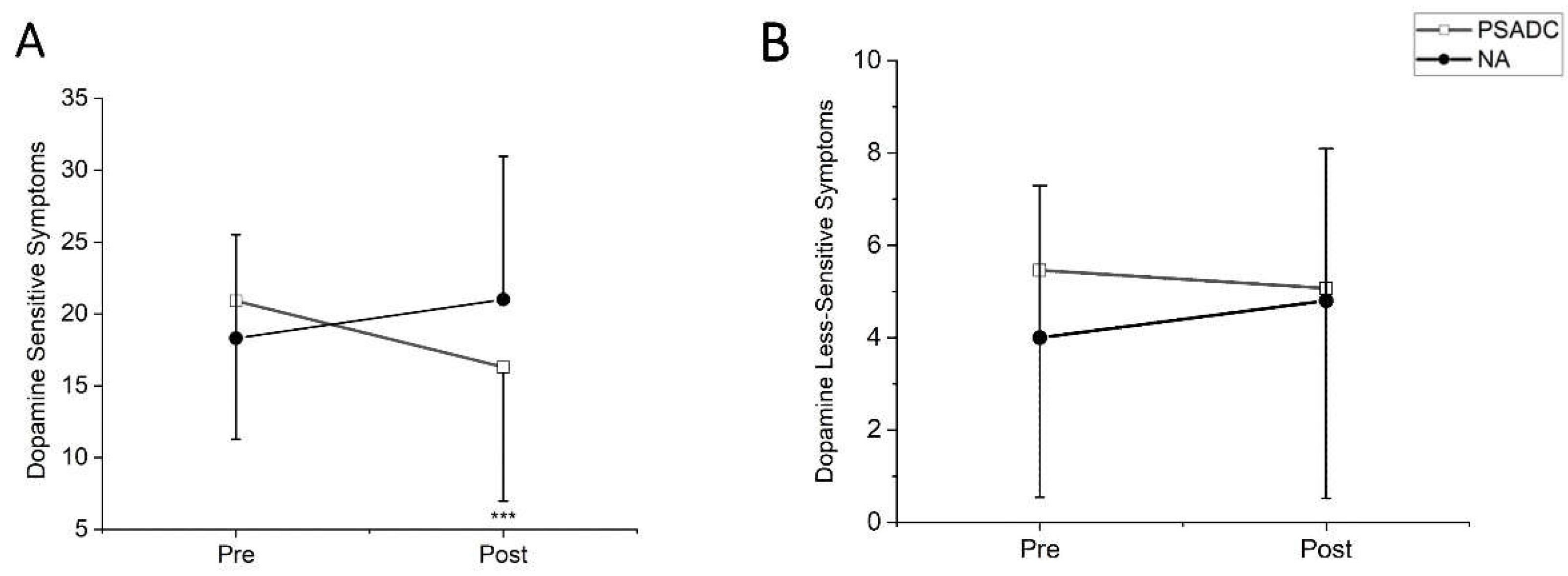
Dopamine-sensitive MDS-UPDRS Motor III symptoms showed a significant group by time interaction (
F = 14.80,
df = 1,
p = 0.001,
ηp2 =0.413) but no significant main effects of time (
F = 1.015,
df = 1,
p = 0.325,
ηp2 =0.046). The PSADC group showed a 22% improvement while the NA group showed a 14.7% decline (
Figure 3A). Dopamine less-sensitive symptoms revealed significant group by time interaction (
F = 5.097,
df = 1,
p = 0.035,
ηp2 =0.195) but no significant main effects of time (
F = 0.627,
df = 1,
p = 0.437,
ηp2 =0.029). The PSADC group showed a 7.1% improvement while the NA group showed a 20% decline (
Figure 3B).
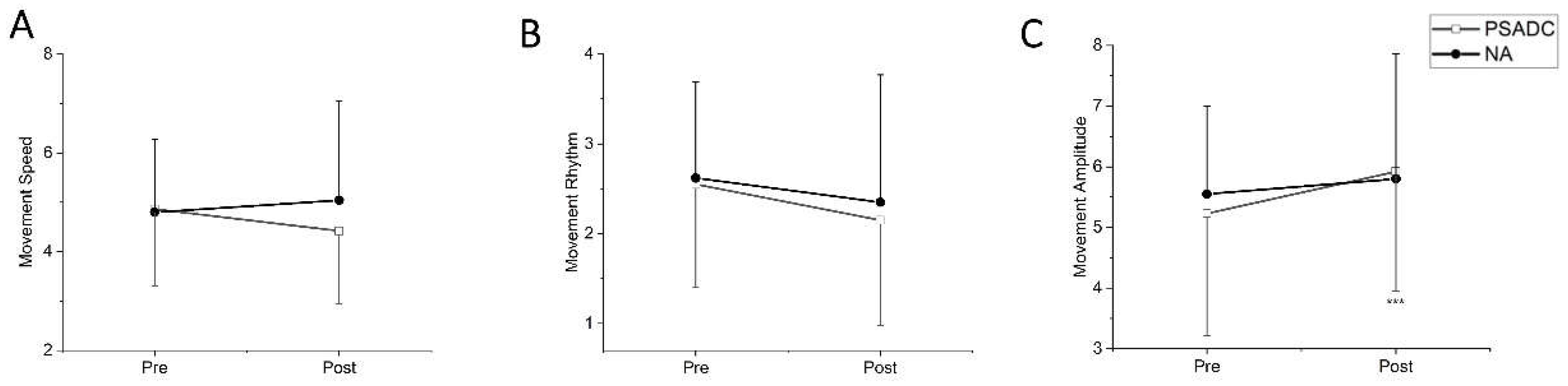
3.3. Kinesia One Motor Function Test Score Changes
The Kinesia One motor function test assessed the speed, amplitude, and rhythm of the movement during finger tapping, hand movement, and pronation-supination movements of hands. One participant in the PSADC group was excluded from data analysis because they had limited upper extremity range of motion and were not able to complete the test. There was no significant group by time interaction (
F = 3.093,
df = 1, p = 0.094,
ηp2 =0.134) or main effect of time (
F = 0.272,
df = 1,
p = 0.607,
ηp2 =0.013) in movement speed between the baseline (pre) and after the intervention (post). However, the PSADC group showed a 9.0% improvement, while the NA group showed a 5.0% worsening (
Figure 4A). For rhythm of movement, there was no significant group by time interaction (
F = 0.127,
df = 1,
p = 0.725,
ηp2 =0.006) nor main effect of time (
F = 3.923,
df = 1,
p = 0.062,
ηp2 =0.164), but the PSADC group showed an improvement of 15.7%, and the NA group showed an improvement of 10.3% (
Figure 4B). Lastly, for movement amplitude, there was a significant main effect of time (
F = 8.452,
df = 1,
p = 0.009,
ηp2 =0.297), but no significant group by time interaction (
F = 1.852,
df = 1,
p = 0.189,
ηp2 =0.085;
Figure 4C).
3.4. Functional Mobility Changes
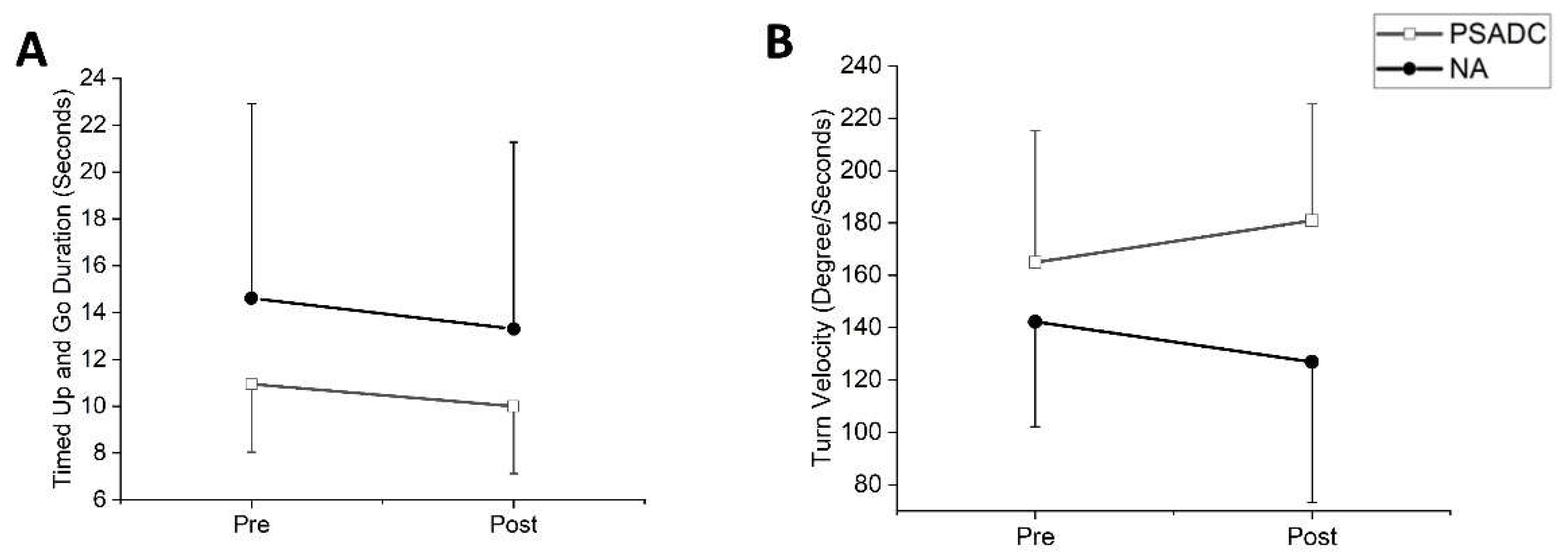
Functional mobility was assessed with Timed up and Go (TUG). One participant in the PSADC group was excluded from data analysis due to an inability to walk without assistance. TUG duration showed a significant main effect of time (
F = 6.00,
df = 1,
p = 0.024,
ηp2 =0.231) but no significant group by time interaction effect (
F = 0.153,
df = 1,
p = 0.700,
ηp2 =0.008). The PSADC group showed an 8.7% improvement, and the NA group showed a 9.0% improvement (
Figure 5A). There was no significant group by time interaction (
F = 0.010,
df = 1,
p = 0.922) or main effect of time (
F = 0.109,
df = 1,
p = 0.745) in turn angle. There was no significant group by time interaction (
F = 0.036,
df = 1,
p = 0.851,
ηp2 =0.002) or main effect of time (
F = 3.068,
df = 1,
p = 0.095,
ηp2 =0.133) for turn duration. The PSADC groups showed a 5.2% improvement, and the NA group showed a 5.4% improvement. Similarly, for turn velocity, there was no significant group by time interaction (
F = 0.2.792,
df = 1,
p = 0.110,
ηp2 =0.123) nor main effect of time (
F = 0.01,
df = 1,
p = 0.974,
ηp2 =0.001). However, the PSADC group showed a 9.7% improvement, and the NA group showed a 10.7% decline (
Figure 5B).
4. Discussion
This study incorporated the use of IMU sensors alongside traditional clinical assessments of motor function and mobility in individuals with PD. This dual approach not only increases the accuracy and depth of motor function and mobility assessment, but also allows for a more accurate understanding of how PD motor function responds to the PSADC paradigm over time, leading to individualized rehabilitation strategies. By combining clinical assessments with objective, quantitative data from IMU sensors, this study achieves a more comprehensive and reliable assessment of motor function in individuals with PD. In assessing mobility, the use of IMU sensors goes beyond traditional stopwatch-based assessments to provide quantitative, objective data that provides more accurate insights into how mobility is affected by PD and how it improves in response to interventions such as PSADC.
In clinical measurement, the PSADC group showed a 5.3-point improvement in MDS-UPDRS Motor III score. This change falls within the range of a moderate clinically important difference (MCID) of 4.5–6.7 points [
31]. While consistent with previous findings after three and six sessions of dynamic cycling [
15,
16], twelve 12 sessions of PSADC over 4 weeks resulted in an improvement that was more than two times greater than previous findings. Moreover, 11 of 13 participants in the PSADC group experienced motor function improvement and nine showed an improvement within MCID. In contrast, only 3 of 10 participants in the NA group showed an improvement in their MDS-UPDRS Motor III score. The exact physiological mechanisms behind the improvements observed with PSADC remain unclear, but several hypotheses have been proposed. PSADC utilizes specific, individualized settings for high-cadence dynamic cycling based on participants' performance, which increases the level of Sample Entropy (SampEn) of cadence. Previous research has demonstrated significant correlations between higher SampEn and motor function improvement [
21]. By providing tailored resistance settings of high-cadence dynamic cycling, participants are exposed to a broader range of movement patterns. These varied movement patterns likely generate more complex and diverse sensory and peripheral afferent input [
32,
33], which is critical for neural plasticity. This increased input may lead to greater activation of the basal ganglia circuits, which play a crucial role in motor control and learning [
34]. Enhanced stimulation of these circuits could facilitate better integration of motor commands and sensory feedback, resulting in improved motor performance in individuals with PD [
35].
The 12 sessions of PSADC paradigm led to 22% improvement dopamine-sensitive PD MDS-UPDRS Motor III symptoms such as tremor, bradykinesia, and rigidity. This suggests that the PSADC paradigm may enhance neuroplasticity and facilitate the upregulation of dopamine production and release like the effects of levodopa therapy but through natural, exercise-induced pathways. This idea is consistent with previous research indicating that exercise has an effect similar to levodopa and could potentially enhance dopaminergic function in the basal ganglia of individuals with PD [
36,
37,
38]. We observed notable improvements in movement speed and rhythm following the PSADC paradigm, consistent with previous research underscoring the beneficial effects of dynamic cycling on bradykinesia and motor timing in the PD population [
15,
16]. In addition, an interesting observation was the decrease in movement amplitude concurrent with the increase in speed. This phenomenon can be interpreted as participants reducing their range of motion as a compensatory mechanism to increase task performance speed. It suggests a potential trade-off between speed and amplitude, reflecting an adaptive strategy by individuals with PD to maintain or improve movement efficiency.
Notably, the NA group demonstrated a slight worsening of symptoms, despite initial motor function improvement through session 6. This finding could be due to insufficient exercise intensity and lack of motivation. The non-adaptive dynamic cycling intervention might not be variable enough to elicit the physiological adaptations that we see with the PSADC paradigm. The resistance setting for the NA group was set at 1 on a scale of 1-6, and this resistance level might have been high enough to provide physiological benefits for the first 6 sessions,[
16] but participants in the NA group might also have acclimated to this low-intensity exercise, limiting the stimuli sufficient to realize the physiological benefits of high-cadence cycling. Moreover, we observed a gradual decrease in effort in some NA group participants during the second half of the intervention. This phenomenon did not emerge in the participants of the PSADC group. As a result, a significant difference in effort emerged between the two groups. We postulate that reduced effort levels and lack of variability in the resistance settings over the intervention may have limited the effect of the dynamic cycling intervention on PD motor symptoms [
20].
Effects of PSADC on Functional Mobility
Both the PSADC and the NA group showed improvement in TUG time to completion. These results align with previous findings [
16] substantiating the positive impact of dynamic cycling on functional mobility. Although the exact physiological and biomechanical mechanisms behind mobility enhancements remain unknown, several hypotheses exist. High cadence cycling, characterized by rapid and repetitive lower body movements, is believed to augment muscle activation, sensory feedback, and motor automaticity [
39,
40]. These enhancements may facilitate smoother transitions in movements required for the TUG test, such as sitting to standing, walking, and turning maneuvers. Furthermore, high cadence dynamic cycling may help alleviate muscle rigidity and joint stiffness, as evidenced by improvements in the MDS-UPDRS Motor III rigidity scores [
15,
16]. This reduction in rigidity can enhance gait and mobility by improving walking efficiency and range of motion, which is particularly important for individuals with PD who are affected by muscle and joint rigidity. These findings are supported by Linder et al.'s study[
41] on aerobic cycling (76 rpm), which demonstrated significant improvements in gait velocity (m/s), cadence (steps/minute), and normalized step length (cm). It is likely that high cadence dynamic cycling may also improve gait characteristics by reducing deviations and promoting more efficient gait patterns, ultimately leading to improved TUG test performance.
Limitations
The current study has several limitations. First, there was a large variation in cycling performance and a wide range of PD symptom severity. The severity of PD symptoms in this sample ranged from individuals with deep brain stimulation (DBS) in wheelchairs to individuals with mild PD symptoms. Although no statistical difference in demographic information emerged between the two groups, there was some variability in levodopa equivalent daily dosage (LEDD) and years with PD in the sample. These two variables often correlate with the severity of PD symptoms, so individuals with more severe motor deficits will likely show greater improvement in motor function [
16]. The other limitation of this study is that all participants were on medication. We wanted to investigate the effects of exercise in real-life conditions [
42], and we strictly controlled their dosage and timing for the baseline measurement, the post-exercise measurements, and the exercise session times to minimize the daily fluctuation of PD medication. The final limitation of this research is the relatively small sample size. Although the study achieved the required statistical power based on the power analysis, the results may not be fully generalizable to the broader PD population due to the relatively small sample size. A larger sample size would enable more definitive conclusions and a more comprehensive representation of the diverse characteristics of individuals with PD. In future research, we plan to address this limitation by recruiting a larger sample size for a double-blinded randomized controlled trial (RCT). This approach will enhance the external validity of our findings and provide more definitive evidence on the effectiveness of the PSADC intervention for improving motor function and functional mobility in individuals with PD.
5. Conclusions
This study is the first pilot randomized controlled study to use SampEn of cadence and effort combined with IMU sensors to evaluate the effectiveness and feasibility of an adaptive cycling program for individuals with PD. The incorporation of IMU sensors allowed for continuous, objective monitoring of motor function and mobility throughout the intervention, providing a more precise and detailed understanding of how participants responded to the PSADC paradigm. Not only did participants show greater improvement in PD motor function and functional mobility, but they also showed less heterogeneity in responses than the NA group. Our findings suggest that a data-driven, individualized exercise rehabilitation plan, supported by IMU sensor data, can improve and potentially maximize the effects of dynamic cycling exercise on PD motor function and mobility. For future research, we plan to conduct a larger double-blind RCT to further validate these finding. This approach will enhance the scientific validity and provide more strong evidence for the PSADC protocol. Additionally, we plan to update the PSADC algorithms using signal processing for feature extraction and machine learning (for feature selection, developing a predictive model, and optimization to maximize individualized benefits) and a larger dataset and examine the application of both within session and session to session-to-session PSADC optimizations to enhance motor function improvement. The continued integration of IMU sensor data into the PSADC paradigm will enable us to provide more accurate, immediate, and patient-specific exercise prescription model for individuals with PD.
6. Patents
Angela Ridgel and Kenneth Loparo are co-inventors on two patents which are related to the device used in this study: “Bike System for Use in Rehabilitation of a Patient,” US 10,058,736.
Author Contributions
Y.K: data collection, statistical analysis, writing- original draft. B.S: data collection, writing -review and editing. L.S: data collection, writing -review and editing. K.L: study design, funding acquisition, writing-review and editing. A.S: study design, participants recruitment, funding acquisition, Writing-review and editing. A.R: Study design, funding acquisition, project administrations and supervision, writing-review and editing.
Funding
This work was funded by the Davis Phinney Foundation (AR/KL), TeCK Fund (AR/KL), NIH R21 (AR), College of Education, Health, and Human Services at Kent State University (YK), and the Brain Health Research Institute (AR) at Kent State University, and U.S. Department of Veteran’s Affairs (AS).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Kent State University (protocol number #87, approved March 2022).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Deidentified data may be available upon request.
Acknowledgments
The authors would like to thank Hassan Abdar (CTI Sensors) for building the dynamic cycle used in this study and for technical support.
Conflicts of Interest
Angela Ridgel and Kenneth Loparo are co-investigators on two U.S patents (US 10,058,736 and US 9,802,081) related to the dynamic cycling that is used in this research. However, dynamic cycling is not commercialized and not available to purchase currently, so there has been no financial benefit.
References
- Willis, A.W.; Roberts, E.; Beck, J.C.; Fiske, B.; Ross, W.; Savica, R.; Van Den Eeden, S.K.; Tanner, C.M.; Marras, C.; Alcalay, R.; et al. Incidence of Parkinson disease in North America. npj Parkinson's Disease 2022, 8, 170. [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson's disease. Lancet Neurol 2021, 20, 385-397. [CrossRef]
- Berardelli, A.; Rothwell, J.C.; Thompson, P.D.; Hallett, M. Pathophysiology of bradykinesia in Parkinson's disease. Brain 2001, 124, 2131-2146. [CrossRef]
- Borovac, J.A. Side effects of a dopamine agonist therapy for Parkinson's disease: A mini-review of clinical pharmacology. Yale J Biol Med 2016, 89, 37-47.
- Patel, A.B.; Jimenez-Shahed, J. Profile of inhaled levodopa and its potential in the treatment of Parkinson's disease: Evidence to date. Neuropsychiatr Dis Treat 2018, 14, 2955-2964. [CrossRef]
- Poewe, W.; Chaudhuri, K.R.; Bergmann, L.; Antonini, A. Levodopa-carbidopa intestinal gel in a subgroup of patients with dyskinesia at baseline from the GLORIA Registry. Neurodegener Dis Manag 2019, 9, 39-46. [CrossRef]
- Trenkwalder, C.; Kuoppamäki, M.; Vahteristo, M.; Müller, T.; Ellmén, J. Increased dose of carbidopa with levodopa and entacapone improves "off" time in a randomized trial. Neurology 2019, 92, e1487-e1496. [CrossRef]
- Jiang, L.; Chen, W.; Guo, Q.; Yang, C.; Gu, J.; Xian, W.; Liu, Y.; Zheng, Y.; Ye, J.; Xu, S.; et al. Eight-year follow-up outcome of subthalamic deep brain stimulation for Parkinson's disease: Maintenance of therapeutic efficacy with a relatively low levodopa dosage and stimulation intensity. CNS Neurosci Ther 2021, 27, 1366-1373. [CrossRef]
- Vergani, F.; Landi, A.; Pirillo, D.; Cilia, R.; Antonini, A.; Sganzerla, E.P. Surgical, medical, and hardware adverse events in a series of 141 patients undergoing subthalamic deep brain stimulation for Parkinson disease. World Neurosurg 2010, 73, 338-344. [CrossRef]
- Zarzycki, M.Z.; Domitrz, I. Stimulation-induced side effects after deep brain stimulation - a systematic review. Acta Neuropsychiatr 2020, 32, 57-64. [CrossRef]
- Alberts, J.L.; Phillips, M.; Lowe, M.J.; Frankemolle, A.; Thota, A.; Beall, E.B.; Feldman, M.; Ahmed, A.; Ridgel, A.L. Cortical and motor responses to acute forced exercise in Parkinson's disease. Parkinsonism Relat Disord 2016, 24, 56-62. [CrossRef]
- Alberts, J.L.; Linder, S.M.; Penko, A.L.; Lowe, M.J.; Phillips, M. It is not about the bike, it is about the pedaling: Forced exercise and Parkinson's disease. Exerc Sport Sci Rev 2011, 39, 177-186. [CrossRef]
- Segura, C.; Eraso, M.; Bonilla, J.; Mendivil, C.O.; Santiago, G.; Useche, N.; Bernal-Pacheco, O.; Monsalve, G.; Sanchez, L.; Hernández, E.; et al. Effect of a High-Intensity Tandem Bicycle Exercise Program on Clinical Severity, Functional Magnetic Resonance Imaging, and Plasma Biomarkers in Parkinson's Disease. Front Neurol 2020, 11, 656. [CrossRef]
- Miner, D.G.; Aron, A.; DiSalvo, E. Therapeutic effects of forced exercise cycling in individuals with Parkinson's disease. J Neurol Sci 2020, 410, 116677. [CrossRef]
- Ridgel; Phillips, R.S.; Walter, B.L.; Discenzo, F.M.; Loparo, K.A. Dynamic High-Cadence Cycling Improves Motor Symptoms in Parkinson's Disease. Front Neurol 2015, 6, 194. [CrossRef]
- Ridgel, A.L.; Ault, D.L. High-Cadence Cycling Promotes Sustained Improvement in Bradykinesia, Rigidity, and Mobility in Individuals with Mild-Moderate Parkinson's Disease. Parkinsons Dis 2019, 2019, 4076862. [CrossRef]
- Mohammadi-Abdar, H.; Ridgel, A.L.; Discenzo, F.M.; Loparo, K.A. Design and Development of a Smart Exercise Bike for Motor Rehabilitation in Individuals with Parkinson's Disease. IEEE ASME Trans Mechatron 2016, 21, 1650-1658. [CrossRef]
- Mohammadi-Abdar, H.; Ridgel, A.L.; Discenzo, F.M.; Phillips, R.S.; Walter, B.L.; Loparo, K.A. Test and Validation of a Smart Exercise Bike for Motor Rehabilitation in Individuals With Parkinson's Disease. IEEE Trans Neural Syst Rehabil Eng 2016, 24, 1254-1264. [CrossRef]
- Ridgel, A.L.; Abdar, H.M.; Alberts, J.L.; Discenzo, F.M.; Loparo, K.A. Variability in cadence during forced cycling predicts motor improvement in individuals with Parkinson's disease. IEEE Trans Neural Syst Rehabil Eng 2013, 21, 481-489. [CrossRef]
- Gates, P.; Ridgel, A.L. Body Mass Index and Exercise Effort Influences Changes in Motor Symptoms After High-Cadence Dynamic Cycling in Parkinson's Disease. Frontiers in Rehabilitation Sciences 2022, 3. [CrossRef]
- Kim, Y.; Smith, B.E.; Shigo, L.M.; Shaikh, A.G.; Loparo, K.A.; Ridgel, A.L. Utilizing Entropy of Cadence to Optimize Cycling Rehabilitation in Individuals With Parkinson’s Disease. Neurorehabilitation and Neural Repair 2024, 0, 15459683241268556. [CrossRef]
- Bo, F.; Yerebakan, M.; Dai, Y.; Wang, W.; Li, J.; Hu, B.; Gao, S. IMU-Based Monitoring for Assistive Diagnosis and Management of IoHT: A Review. Healthcare (Basel) 2022, 10. [CrossRef]
- Sotirakis, C.; Su, Z.; Brzezicki, M.A.; Conway, N.; Tarassenko, L.; FitzGerald, J.J.; Antoniades, C.A. Identification of motor progression in Parkinson's disease using wearable sensors and machine learning. NPJ Parkinsons Dis 2023, 9, 142. [CrossRef]
- Stillerova, T.; Liddle, J.; Gustafsson, L.; Lamont, R.; Silburn, P. Remotely Assessing Symptoms of Parkinson’s Disease Using Videoconferencing: A Feasibility Study. Neurology Research International 2016, 2016, 4802570. [CrossRef]
- Achey, M.; Aldred, J.L.; Aljehani, N.; Bloem, B.R.; Biglan, K.M.; Chan, P.; Cubo, E.; Dorsey, E.R.; Goetz, C.G.; Guttman, M.; et al. The past, present, and future of telemedicine for Parkinson's disease. Mov Disord 2014, 29, 871-883. [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992, 55, 181-184. [CrossRef]
- Heldman, D.A.; Urrea-Mendoza, E.; Lovera, L.C.; Schmerler, D.A.; Garcia, X.; Mohammad, M.E.; McFarlane, M.C.U.; Giuffrida, J.P.; Espay, A.J.; Fernandez, H.H. App-Based Bradykinesia Tasks for Clinic and Home Assessment in Parkinson's Disease: Reliability and Responsiveness. J Parkinsons Dis 2017, 7, 741-747. [CrossRef]
- Morris, R.; S., S.; McBarron, G.; Fino, P.C.; Mancini, M.; Curtze, C. Validity of Mobility Lab (version 2) for gait assessment in young adults, older adults and Parkinson's disease. Physiological measurement 2019, 40. [CrossRef]
- Mancini, M.; Horak, F.B. Potential of APDM mobility lab for the monitoring of the progression of Parkinson's disease. Expert Rev Med Devices 2016, 13, 455-462. [CrossRef]
- Borg, G. Borg's Perceived Exertion and Pain Scales; Human Kinetics: 1998.
- Shulman, L.M.; Gruber-Baldini, A.L.; Anderson, K.E.; Fishman, P.S.; Reich, S.G.; Weiner, W.J. The clinically important difference on the unified Parkinson's disease rating scale. Arch Neurol 2010, 67, 64-70. [CrossRef]
- Perrey, S. Promoting motor function by exercising the brain. Brain Sci 2013, 3, 101-122. [CrossRef]
- Davies, G.; Riemann, B.L.; Manske, R. CURRENT CONCEPTS OF PLYOMETRIC EXERCISE. Int J Sports Phys Ther 2015, 10, 760-786.
- Baladron, J.; Vitay, J.; Fietzek, T.; Hamker, F.H. The contribution of the basal ganglia and cerebellum to motor learning: A neuro-computational approach. PLoS Comput Biol 2023, 19, e1011024. [CrossRef]
- Mazzoni, P.; Shabbott, B.; Cortés, J.C. Motor control abnormalities in Parkinson's disease. Cold Spring Harb Perspect Med 2012, 2, a009282. [CrossRef]
- Sacheli, M.A.; Neva, J.L.; Lakhani, B.; Murray, D.K.; Vafai, N.; Shahinfard, E.; English, C.; McCormick, S.; Dinelle, K.; Neilson, N.; et al. Exercise increases caudate dopamine release and ventral striatal activation in Parkinson's disease. Mov Disord 2019, 34, 1891-1900. [CrossRef]
- Vina, J.; Sanchis-Gomar, F.; Martinez-Bello, V.; Gomez-Cabrera, M.C. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol 2012, 167, 1-12. [CrossRef]
- Ahlskog, J.E. Aerobic Exercise: Evidence for a Direct Brain Effect to Slow Parkinson Disease Progression. Mayo Clin Proc 2018, 93, 360-372. [CrossRef]
- MacIntosh, B.R.; Neptune, R.R.; Horton, J.F. Cadence, power, and muscle activation in cycle ergometry. Medicine & Science in Sports & Exercise 2000, 32.
- Wu, T.; Hallett, M.; Chan, P. Motor automaticity in Parkinson's disease. Neurobiol Dis 2015, 82, 226-234. [CrossRef]
- Linder, S.M.; Baron, E.; Learman, K.; Koop, M.M.; Penko, A.; Espy, D.; Streicher, M.; Alberts, J.L. An 8-week aerobic cycling intervention elicits improved gait velocity and biomechanics in persons with Parkinson's disease. Gait Posture 2022, 98, 313-315. [CrossRef]
- Prodoehl, J.; Rafferty, M.R.; David, F.J.; Poon, C.; Vaillancourt, D.E.; Comella, C.L.; Leurgans, S.E.; Kohrt, W.M.; Corcos, D.M.; Robichaud, J.A. Two-year exercise program improves physical function in Parkinson's disease: The PRET-PD randomized clinical trial. Neurorehabil Neural Repair 2015, 29, 112-122. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).