1. Introduction
Perinatal hypoxic-ischemic encephalopathy (HIE) is a significant cause of perinatal mortality and morbidity in term newborns, leading to severe neurodevelopmental impairments if left untreated [
1,
2,
3]. The extent and nature of the brain injuries caused by HIE depend on the duration and severity of ischemic episodes. Acute cases often impact critical brain areas like the deep perirolandic nuclei and hippocampal regions, while prolonged or partial ischemia tends to affect cerebral intervascular basins [
2,
4]. In moderate or intermittent hypoxia, a mixed injury pattern emerges, impacting both nuclei and cortex along with intervascular regions [
3,
5]. These variations highlight the complexity of HIE and the need for timely, targeted therapeutic interventions [
6].
Therapeutic hypothermia has emerged as a standard intervention for neonates with HIE, showing potential in reducing brain damage by lowering metabolic rates and inhibiting neuronal apoptosis [
7,
8]. It provides neuroprotection, especially by mitigating the effects of reactive oxygen species (ROS) and pro-inflammatory mediators, which play critical roles in HIE’s pathophysiology [
4,
9,
10]. Therapeutic hypothermia in neotanes involves reducing the body temperature to
for whole-body cooling and to
for selective head cooling. Although its specific mechanism of action remains unknown, it is attributed to neuroprotective effects, likely related to reducing cerebral metabolic demand, decreasing oxygen consumption, and other physiological and metabolic effects [
11]. While this therapy requires highly trained personnel and must be carried out in specialized health centers, when successfully implemented in managing hypoxic-ischemic encephalopathy, it has demonstrated benefits in improving clinical outcomes. A review by Ranjan and Gulati [
9], highlights several clinical trials showing reduced mortality and disability attributable to therapeutic hypothermia. Most of these studies have focused on primary outcomes such as mortality and disability. However, no studies have yet been published that demonstrate physiological effects, such as increases in brain structure volumes, which could more directly impact long-term outcomes like neurodevelopment or the preservation of intellectual quotient [
12,
13]. Despite its widespread adoption in high-income settings, access to therapeutic hypothermia remains limited in low- and middle-income countries like Colombia, leading to disparities in clinical outcomes [
3]. Moreover, even with the implementation of therapeutic hypothermia, a substantial proportion of affected neonates still face severe neurodevelopmental deficits or mortality by the age of two [
6,
9].
Advancements in neuroimaging techniques, particularly magnetic resonance imaging (MRI), have enabled a more nuanced understanding of the brain’s response to hypoxic events [
14,
15]. High-resolution imaging, combined with computational analysis methods such as Infant-FreeSurfer, has allowed precise quantification of brain volumes and cortical thickness in neonates [
16]. These tools are crucial for assessing structural changes in the brain following HIE and evaluating the potential benefits of therapeutic interventions such as hypothermia [
17]. Recent developments in machine learning further enhance these evaluations, offering automated segmentation and volumetric assessments, which provide a comprehensive picture of brain recovery [
3]. Additionally, integrating genetic markers into neurodevelopmental evaluations offers insights into the underlying biological processes that might affect patient outcomes, leading to more personalized therapeutic strategies [
2,
9]. In this context, understanding the long-term impact of therapeutic hypothermia on brain volume and development is critical. Previous studies have shown that hypothermia may have region-specific protective effects, particularly on gray and white matter volumes and hippocampal integrity [
18]. However, gaps remain in the literature regarding how these structural changes correlate with neurodevelopmental outcomes and the role of genetic factors in influencing treatment response [
6,
9]. Addressing these gaps requires an integrated approach that combines advanced neuroimaging, genetic analysis, and rigorous clinical assessment to provide a holistic understanding of therapeutic hypothermia’s effects on neonates with perinatal asphyxia [
3,
16]. This study aims to assess the effects of therapeutic hypothermia on brain structure volumes in neonates with perinatal asphyxia and explore potential implications for long-term neurodevelopmental outcomes. Focusing on volumetric changes in key brain regions, this analysis seeks to complement existing evidence on mortality and disability, offering a broader perspective on the potential neuroprotective benefits of therapeutic hypothermia in neonatal care. We assessed structural differences in key brain regions and their associations with neurodevelopmental outcomes using advanced MRI analysis with Infant-FreeSurfer, machine learning algorithms, and genetic evaluations. We compared these findings to a control group of neonates without hypothermia treatment, analyzing differences in gray matter, white matter, and hippocampal volumes. The results provide insights into the region-specific neuroprotective effects of hypothermia, contributing to the optimization of treatment protocols for neonates with HIE. Finally, we aim to enhance our understanding of how therapeutic hypothermia can be effectively integrated into clinical practice to improve the quality of life for affected neonates.
2. Materials and Methods
A prospective cohort study involving term neonates with perinatal asphyxia. Two groups were formed: the exposed group consisted of patients from a specialized healthcare center (HPT) that adhered to clinical practice guidelines for therapeutic hypothermia in neonates with perinatal asphyxia, with the protocol detailed later in the manuscript. The unexposed group consisted of patients from another healthcare facility (CF) where clinical guidelines for therapeutic hypothermia were not implemented, and therefore, they received standard clinical care at the attending physician’s discretion for each individual case. MRI was performed at a time point close to the perinatal insult and again at two years post-event. Clinical data such as complications, birth characteristics, and maternal history were collected and used as control variables in the analysis.
2.1. Participants
Prospective cohort study of 34 asphyxiated newborns, with 12 receiving hypothermia treatment and 22 not receiving it. These newborns were admitted to two neonatal intensive care units in the central region of Colombia from January 2015 to December 2023. The newborns were selected according to the criteria for HIE indicated by the American College of Obstetricians and Gynecologists, such as (a) umbilical cord arterial pH less than 7, (b) Apgar score between 0 to 5 for longer than 5 minutes; (c) neurological manifestations such as seizures, coma, or hypotonia; and (d) multisystem organ dysfunction (e.g., cardiovascular, gastrointestinal, hematological, pulmonary, or renal system). In addition, the severity of its manifestation/neurological damage was evaluated according to the modified SARNAT scale (i.e., clinical staging of HIE), MRI assessment, and Bayley Scale III.
2.2. Therapeutic Hypothermia Procedure in Newborns with HIE
Therapeutic hypothermia is a standard treatment for full-term newborns diagnosed with hypoxic-ischemic encephalopathy (HIE) to reduce the risk of brain injury. This procedure involves lowering the baby’s core body temperature to help decrease the metabolic demands of brain cells and limit the extent of neuronal damage caused by lack of oxygen [
8]. Therapeutic hypothermia can be administered through whole-body cooling or selective head cooling. During whole-body cooling, the baby’s temperature is lowered to
using specialized cooling blankets or devices. With selective head cooling, the temperature is maintained at
using a cooling cap placed over the baby’s head, which allows for targeted temperature control while keeping the rest of the body closer to normal temperature levels [
19].
The cooling process typically starts within six hours of birth to maximize the neuroprotective effects. The target temperature is maintained for 72 hours, followed by a gradual rewarming phase, usually at
per hour until the baby’s temperature reaches
. Close monitoring of vital signs, including heart rate, respiratory function, and electrolyte levels, is essential throughout the procedure to manage potential side effects, such as bradycardia and electrolyte imbalances. Therapeutic hypothermia aims to slow down the cascade of biochemical processes that lead to cell death, including reducing apoptosis and inflammatory responses. It ultimately offers a neuroprotective effect in newborns affected by HIE. While it has shown efficacy in improving survival rates and reducing long-term neurological impairments, its successful application depends on precise temperature control and careful patient monitoring by trained healthcare providers [
20].
2.3. Neurodevelopmental Assessment
Neurodevelopmental outcomes were assessed longitudinally using the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III), which is a standardized tool widely used to evaluate the developmental functioning of infants and toddlers [
21]. The Bayley-III assesses three core domains: cognitive (i.e. problem-solving abilities and understanding of basic concepts), motor (i.e. fine and gross motor skills), and language skills (i.e. receptive and expressive language abilities). Evaluations were conducted at two time points, specifically at 18, and 24 months of age, to monitor the progression of neurodevelopmental milestones and identify any emerging deficits. Trained neuropsychologists conducted all assessments to ensure consistency and reliability of the results. The scores from each domain were analyzed to determine any significant differences between the group of neonates who received hypothermia therapy and those who did not, with adjustments made for potential confounding variables such as gestational age and severity of HIE.
2.4. Genetic Analysis
To determine whether the clinical phenotype of each patient could be associated with genetic alterations potentially acting as a masker for hypoxic-ischemic encephalopathy (HIE), we conducted next-generation sequencing (NGS) on a panel of genes related to neurotransmitters for 34 patients. Additionally, microarray comparative genomic hybridization (CGH) was performed on ten patients exhibiting phenotypic alterations or neurological compromise to identify DNA copy number imbalances, including deletions and duplications, and to conduct whole exome sequencing. Blood samples were collected from all patients between 2017 and 2024 at Comfamiliar Risaralda and processed by GENCEL PHARMA COL in Bogotá. The DNA amplification and bioinformatic analyses were carried out by an external laboratory, ensuring the objectivity and impartiality of the study. The authors were not involved in the sequencing or variant identification processes. Variants identified through these analyses were classified into two categories, providing a clear understanding of the findings: (i) pathogenic variants (PV), and (ii) variants of unknown significance (VUS).
2.5. Brain Volume Analysis
The study used magnetic resonance imaging (MRI) with a 1.5T Siemens Heltenier system to assess brain volume. High-resolution T1-weighted images were obtained using a 3D magnetization-prepared rapid gradient-echo (MPRAGE) protocol. The acquisition parameters were as follows: repetition time = 2,400 ms, echo time = 3.5 ms, inversion time = 1,000 ms, flip angle = 10°, field of view = 256 × 256 mm, acquisition matrix = 320 × 320, 192 slices, and resolution = 1.0 × 1.0 × 1.0 mm. Preprocessing of the images included quality control, motion correction, and spatial normalization to a Montreal Neurological Institute (MNI) template. Cortical parcellation was carried out using the "infant FreeSurfer" framework, designed explicitly for processing infant brain images. This framework allows for precise segmentation and measurement of cortical and subcortical structures [
16].
A senior neuroradiologist labeled all the MRI volumes regarding the brain injury in terms of location and severity. Data analysis was focused on total brain volume, total white matter volume (WMV), gray matter volume (GMV), and specific sub-cortical regions of interest derived from the parcellation process such as basal ganglia and hippocampus. This detailed approach allowed us to gain a comprehensive understanding of the brain’s structure and function.
2.6. Statistical Analysis
An exploratory and descriptive analysis of the data was performed, focusing on the distribution of the calculated brain volumes. From this analysis, a generalized linear model (GLM) with a gamma link function was identified as the most appropriate approach to detect differences between groups, given the non-normal distribution and positively skewed nature of the brain volume variables. This modeling approach enabled the accurate estimation of the relationship between hypothermia exposure and brain volumetric outcomes, while adjusting for potencial confounder like genetic mutations (i.e. GLDC: c.2714T>A; p.Val905Gly, SLC1A4 c.964C>T (p.Arg322*), Del(8)(p11.2) Monosomía 8p11.2, Dup(14)(q11.2) Trisomy 14q11.2, and NSD1: c.3091C>T;p.(Arg1031*)), age at the time of MRI, birth characteristics, and delivery complications. Results are presented as regression coefficients and effect sizes, along with corresponding confidence intervals and p-values. Welch’s t-tests, which do not assume equal variances, were also applied for group comparisons. A Pearson correlation matrix was generated to assess the strength and direction of these relationships. All statistical analyses were performed using R software (version 4.2), with a significance level set at .
3. Results
3.1. Descriptive Results
A total of 34 newborns were included in the study. The sex distribution was similar between both groups, with
females in the hypothermia group and
in the non-hypothermia group (
). The rest of the demographics can be seen in
Table 1.
The use of epidural anesthesia appeared more frequent among those who received hypothermia treatment, with 33% of the mothers in the hypothermia group reporting its use, compared to 14% in the non-hypothermia group (). However, this difference did not reach statistical significance. Furthermore, of mothers in the hypothermia group reported using medication during pregnancy, compared to in the non-hypothermia group ().
These findings suggest that maternal and prenatal characteristics were relatively balanced between the groups, reducing the likelihood that these factors significantly influenced the outcomes of hypothermia treatment.
3.2. Hypotermia Effect
Table 3 presents the results from the adjusted regression models, which assess the volumetric changes in different brain structures in newborns subjected to hypothermia compared to those without hypothermia. The model adjusts for confounding factors such as genetic mutations, age at the time of MRI, birth characteristics, and delivery complications.
Gray matter shows a coefficient of 1.314, indicating that newborns exposed to hypothermia exhibit an average gray matter volume approximately 3.7 times larger than those without hypothermia (p = 0.025). Similarly, white matter volume has a coefficient of 0.798, suggesting that white matter is 2.2 times larger in the hypothermia group (p = 0.025). The WM/GM ratio shows a 3.0-fold increase (p = 0.026). These findings show bigger brain volumes for these structures in the hypothermia group. Additionally, the hippocampus exhibited an increase in volume (3.4-fold, p = 0.032).
However, other regions such as the cerebellum, caudate nucleus, putamen, thalamus, globus pallidus, amygdala, and nucleus accumbens did not show statistically significant differences between the groups (p > 0.05). While some structures demonstrated modest volume increases, these were not statistically robust, indicating a less clear relationship between hypothermia and these brain regions.
These findings indicate that hypothermia may have a potential effect on brain volume in certain regions but does not lead to clinically or statistically significant differences in neurodevelopmental outcomes as measured by the Bayley Scale.
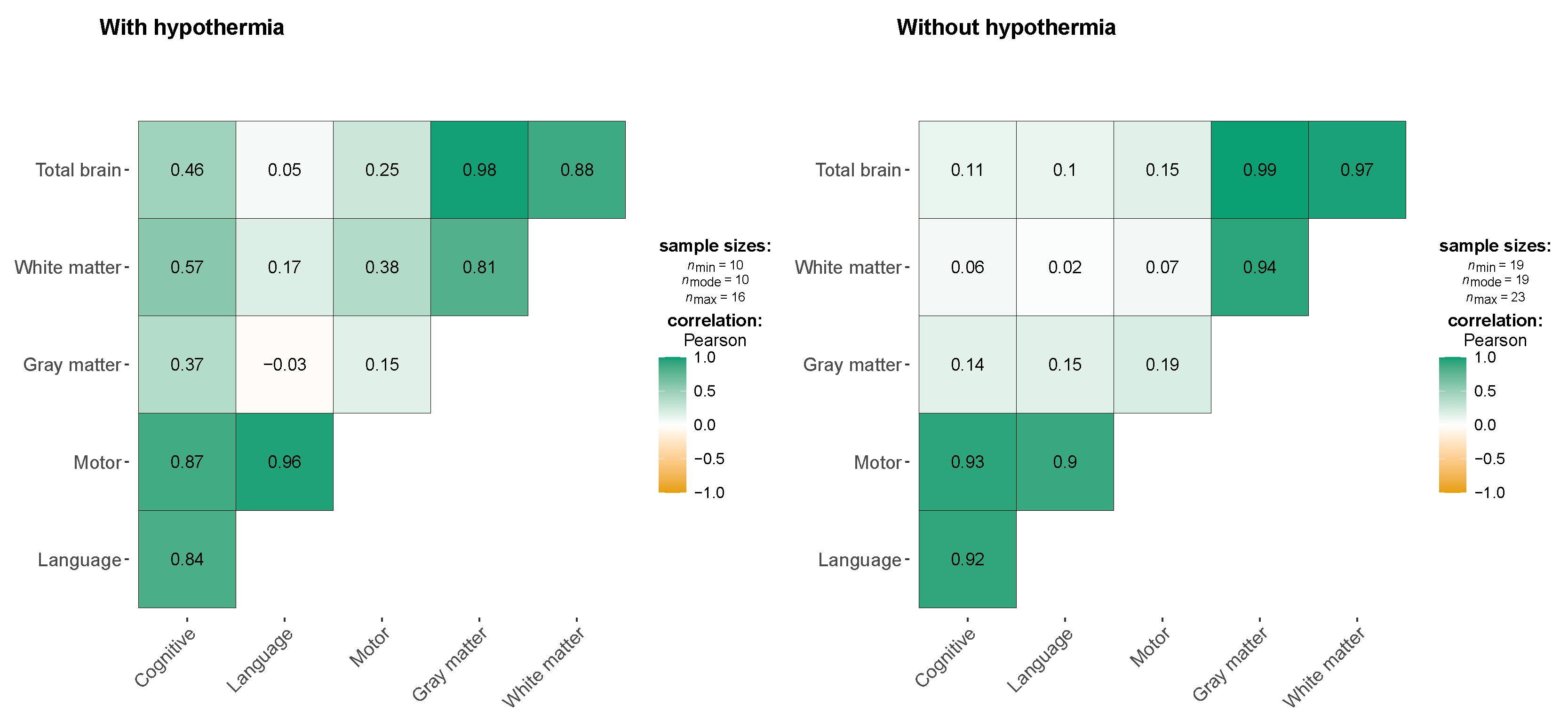
The
Figure 1 shows the correlations between neurodevelopmental outcomes (cognitive, language, and motor functions) and brain volumes (total brain, gray matter, and white matter) in two groups: children treated with hypothermia and those who were not. In the hypothermia group, moderate positive correlations are noted between cognitive function and both white matter volume (r = 0.57) and total brain volume (r = 0.46), though these associations are not statistically significant. This suggests a potential trend where larger brain volumes, particularly in white matter, may support better cognitive outcomes in children who received hypothermia therapy. Additionally, a strong correlation is observed between motor and language functions (r = 0.96), indicating that these neurodevelopmental domains may be closely linked in this group. However, weak correlations between brain volumes and language function (r = -0.03 to 0.17) suggest that brain structure alone may not be a key determinant of language outcomes in these children. In the non-hypothermia group, correlations between neurodevelopmental outcomes and brain volumes are weak. Cognitive function shows minimal correlation with total brain volume (r = 0.11) and white matter volume (r = 0.06), and similar patterns are observed for language and motor functions. This lack of significant associations suggests that brain volume may have less influence on neurodevelopmental outcomes in the absence of hypothermia. These findings highlight the potential protective effect of hypothermia on brain structures like white matter, which may contribute to better cognitive and motor outcomes. However, the absence of statistically significant results across both groups calls for further research with larger sample sizes to better understand these relationships.
3.3. Cortical Thickness and Structural Differences Between Hypothermia and Non-Hypothermia Groups
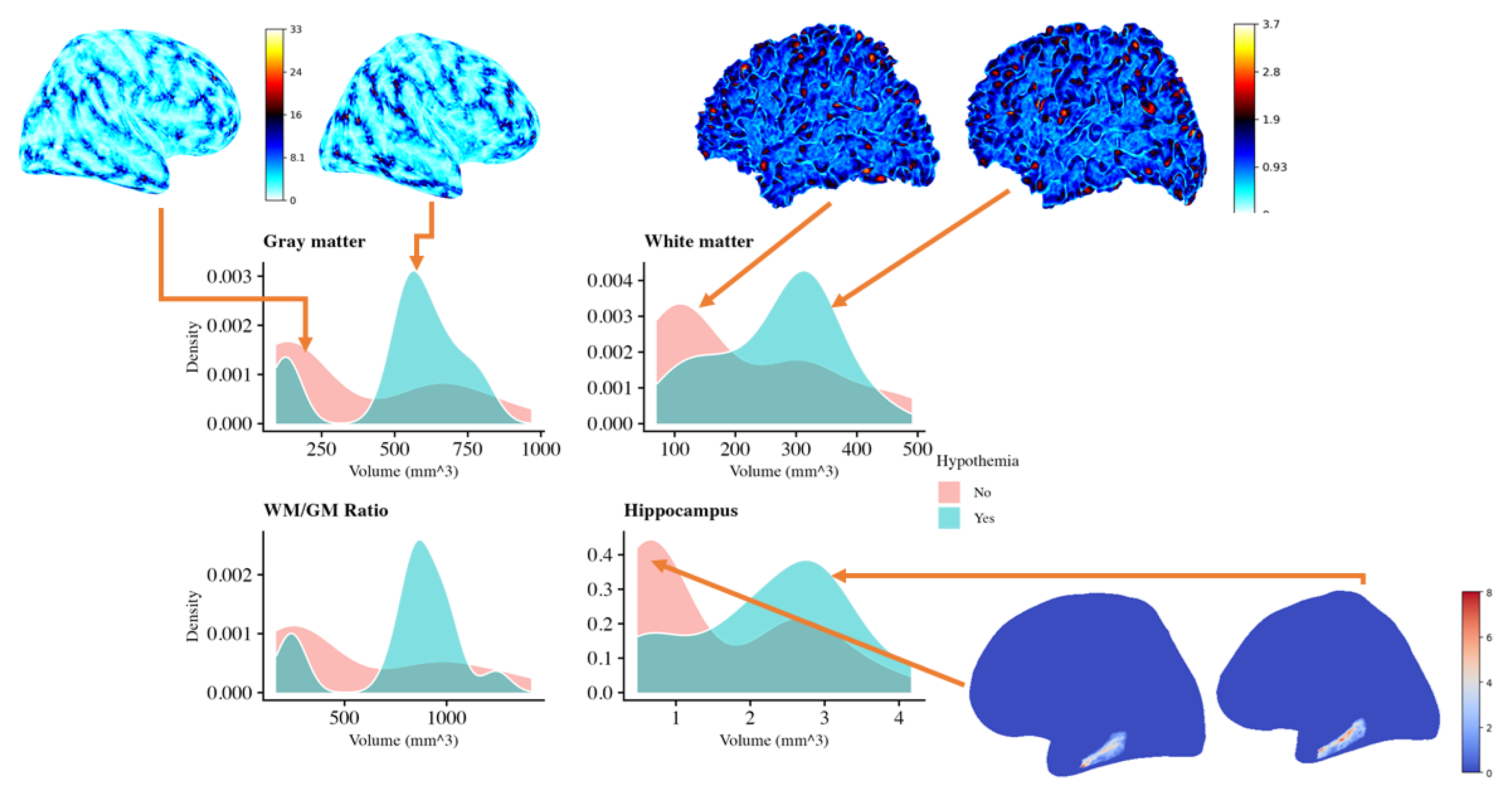
The
Figure 2 compares brain volumes in neonates with and without therapeutic hypothermia, using density plots of gray matter, white matter, hippocampus, and the white matter/gray matter (WM/GM) ratio, along with cortical and subcortical renderings. The plots show that neonates treated with hypothermia (blue) tend to have higher volumes of gray and white matter than untreated neonates (pink), suggesting potential preservation of these tissues. The WM/GM ratio is also higher in the hypothermia group, indicating better overall brain preservation. Notably, the hippocampus, important for memory and cognitive functions, shows a broader range of larger volumes in the hypothermia group, supporting the idea that hypothermia may offer neuroprotective effects. Visualizations of cortical surfaces further emphasize the structural differences, indicating that hypothermia helps maintain cortical integrity. Overall, these results suggest that therapeutic hypothermia may help preserve key brain structures after perinatal asphyxia. This preservation could potentially lead to improved neurodevelopmental outcomes, reinforcing the potential benefits of this treatment. However, further analysis is needed to confirm the significance and clinical impact of these findings.
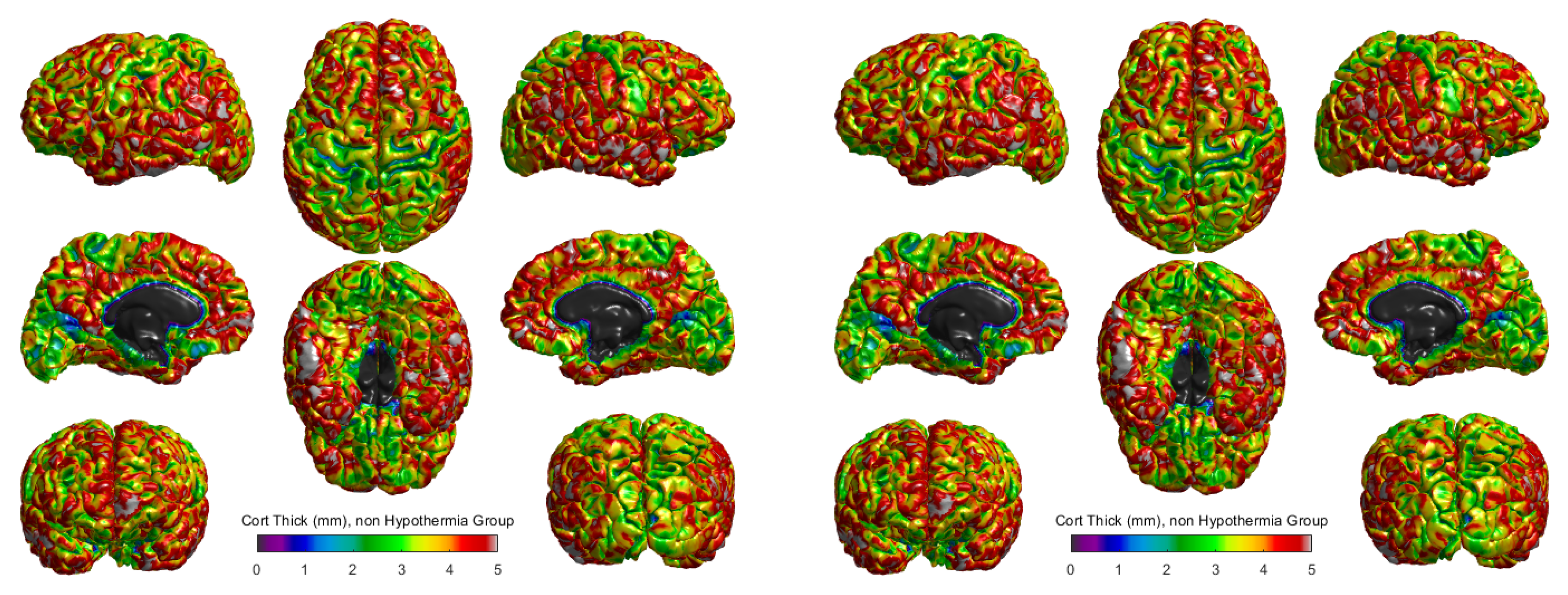
The cortical thickness for the hypothermia-treated group and the non-hypothermia group was compared using 3D surface reconstructions (
Figure 3). In the hypothermia-treated group (
Figure 3 right), a heatmap of cortical thickness revealed a higher degree of preservation, with thicker regions primarily located in the
central sulci and
occipital areas. The cortical thickness ranged from 0 mm to 5 mm, with the highest values represented in red and the lowest in blue. These results suggest that therapeutic hypothermia promotes cortical growth or maintenance in these critical areas.
Conversely, the non-hypothermia group (
Figure 3, left) exhibited more widespread cortical thinning, particularly in the
frontal and
parietal lobes. These regions, responsible for cognitive and motor functions, showed reduced cortical thickness, indicating the potential for developmental delays or impairments in this untreated population. The comparison of these two groups highlights the differential effects of therapeutic hypothermia, suggesting a neuroprotective effect in preserving cortical structure post-injury.
The
Figure 3 shows cortical thickness measurements in millimeters for two groups of term neonates: those who did not receive therapeutic hypothermia (left panel) and those who did (right panel). The scale ranges from purple (indicating thinner cortical areas) to red (indicating thicker cortical areas), with a scale of 0 to 5 mm. Visual comparison between the two groups suggests differences in the distribution and extent of cortical thickness across various brain regions. In the hypothermia-treated group, thicker regions (represented by green to red hues) appear more pronounced and widespread, particularly in motor and sensory processing areas. In contrast, the non-hypothermia group displays a greater prevalence of thinner regions (depicted in shades of blue and purple), suggesting that therapeutic hypothermia may help preserve or promote greater cortical thickness. This preservation of cortical structure might correlate with improved neurodevelopmental outcomes, as larger cortical thickness has been associated with better cognitive and motor functions in previous studies. However, further quantitative analysis would be required to confirm these observations and assess their statistical significance. Additionally, these visual insights highlight the importance of therapeutic interventions in mitigating the effects of hypoxic-ischemic encephalopathy (HIE) on brain development.
4. Discussion
The study included 34 newborns. Among the prenatal characteristics, preeclampsia was observed in of the mothers in the non-hypothermia group. At the same time, no cases were reported in the hypothermia group (), suggesting no significant association between preeclampsia and the application of hypothermia therapy.
Volumetric assessments of the brain, particularly in neonatal populations, provide crucial insights into the potential long-term neurological outcomes following perinatal events such as hypoxic-ischemic encephalopathy (HIE). Our study’s findings on the effects of therapeutic hypothermia on brain volumes in neonates with perinatal asphyxia are of significant importance. Brain volumes, measured through techniques like MRI, allow for the quantification of gray and white matter, which is integral to understanding the effects of HIE and therapeutic interventions like hypothermia. Clinically, larger brain volumes have been associated with better neurodevelopmental outcomes, while reductions in volume often correlate with cognitive and motor impairments [
2].
It is well established that HIE can significantly reduce brain volumes in untreated infants, particularly affecting critical structures like the basal ganglia and hippocampus [
14]. Studies have consistently demonstrated that infants with HIE who do not receive therapeutic intervention show marked reductions in the gray and white matter volumes, with adverse neurodevelopmental consequences [
22]. Our findings align with previous literature, as newborns who underwent therapeutic hypothermia had significantly larger volumes of gray matter, white matter, and the hippocampus than those who did not receive the treatment. Specifically, on average, gray matter volume was 2.47 times more significant, and white matter volume was 2.48 times more significant in the hypothermia group. The hippocampus also showed a notable difference, with an average volume 2.34 times greater in the hypothermia-treated infants. These findings suggest that hypothermia may have a neuroprotective effect on specific brain structures, which could have important clinical implications for the management of HIE. However, other regions, such as the cerebellum, caudate nucleus, putamen, thalamus, and amygdala, did not exhibit significant differences between the groups, indicating that the protective effects of hypothermia may not extend uniformly across all brain structures [
20].
The lack of significant volumetric changes in some brain regions could be attributed to their differential vulnerability to hypoxic damage and varying responses to therapeutic hypothermia. Certain regions, such as the cerebellum and caudate nucleus, may inherently have a higher resistance to hypoxic conditions, possibly due to their specific metabolic profiles or blood supply [
3]. Additionally, these regions may not benefit as much from the neuroprotective mechanisms induced by therapeutic hypothermia, such as the reduction of apoptosis and inflammation [
4]. This selective protection could explain why structures like the hippocampus, which is highly susceptible to ischemic damage, show more pronounced preservation with hypothermia, whereas other regions remain unaffected.
Moreover, the more robust response observed in white matter volume compared to other structures may reflect the higher metabolic activity of white matter during early brain development. White matter is particularly vulnerable to oxidative stress and excitotoxicity during hypoxic events, leading to more severe damage in untreated cases of HIE [
2]. The observed increase in white matter volume in hypothermia-treated infants suggests that therapeutic hypothermia may play a role in reducing white matter injury by mitigating the effects of reactive oxygen species (ROS) and promoting myelin repair processes. These findings align with previous studies that emphasize the critical role of white matter integrity in supporting neurodevelopmental outcomes and highlight the importance of targeted neuroprotective strategies for preserving white matter during therapeutic interventions [
14].
Our study did not find statistically significant correlations between brain volumes and cognitive, language, or motor outcomes. However, moderate correlations were observed between cognitive function and white matter volume (
) and total brain volume (
). The lack of statistical significance may be attributed to the relatively small sample size and incomplete neurodevelopmental assessments for some participants early in the study [
5]. These findings suggest a need for larger cohorts and more extended follow-up periods to validate the potential association between brain volumes and neurodevelopment.
5. Conclusion
Our study contributes to the growing body of evidence supporting the neuroprotective effects of therapeutic hypothermia in neonates with perinatal asphyxia. Specifically, hypothermia-treated infants demonstrated increased volumes in key brain structures such as gray matter, white matter, and the hippocampus, which may translate into improved neurodevelopmental outcomes. However, the region-specific nature of the observed volumetric changes highlights the need for further research to better understand the underlying mechanisms of therapeutic hypothermia and its variable impact on different brain regions.
The findings also underscore the importance of using advanced neuroimaging techniques like cortical parcellation to detect subtle structural changes that conventional methods may miss. Additionally, while our results did not establish significant correlations between brain volumes and neurodevelopmental outcomes, the moderate associations observed in white matter volumes suggest potential avenues for future studies.
In the context of healthcare cost-effectiveness, therapeutic hypothermia may provide long-term economic benefits through reduced disability and improved outcomes, as seen in studies conducted in high-income settings [
23]. Further research is required to determine the applicability of these findings in low- and middle-income countries, including Colombia, where resource constraints and healthcare infrastructure may pose additional challenges. Longitudinal studies are also necessary to evaluate the lasting effects of hypothermia on cognitive and memory functions, especially considering the increased hippocampal volumes in treated neonates. Such insights will be critical for optimizing treatment strategies and improving the quality of life for children affected by HIE.
Author Contributions
Dr. HFG conceptualized the methodology, developed the artificial intelligence methods, and prepared the original draft of the manuscript. Dr. NCR was responsible for data curation, investigation, validation, and contributed to the methodology. Drs. GLPH and FRR curated the data and contributed to the original draft preparation. Drs. JMEA and HFG contributed to the conceptualization, development of artificial intelligence methods, and took part in writing, reviewing, and editing the original draft. Drs. GLPH, CS, FRR and AAOG were involved in the conceptualization, data curation, methods development, and contributed to writing, reviewing, and editing the manuscript. All authors reviewed and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
This research was developed under the project: “SISTEMA DE MONITOREO AUTOMÁTICO PARA LA EVALUACIÓN CLÍNICA DE INFANTES CON ALTERACIONES NEUROLÓGICAS MOTORAS MEDIANTE EL ANÁLISIS DE VOLUMETRÍA CEREBRAL Y PATRÓN DE LA MARCHA” financed by MINCIENCIAS, COLOMBIA with code COL111089784907.
Institutional Review Board Statement
All procedures performed in the study involving human participants were in accordance with the ethical standards of the Colombian institutional and/or national research committee and with the 8430-1993 Declaration and its later amendments or comparable ethical standards. The Ethics committee Review Board approved the study at COMFAMILIAR RISARALDA CLINIC (Approval No. 00049-2019-05-09). The patients legal guardian provided informed consent for publication.
Informed Consent Statement
Written informed consent was obtained from the patient’s parent or guardian for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors want to thank the MINISTRY OF SCIENCES COLOMBIA - MINCIENCIAS and the institutions involved in the present project.
Conflicts of Interest
The authors declare no competing interests.
Abbreviations
The following abbreviations are used in this manuscript:
| HIE |
Hypoxic-Ischemic Encephalopathy |
| TH |
Therapeutic Hypothermia |
| MRI |
Magnetic Resonance Imaging |
| WM |
White Matter |
| GM |
Gray Matter |
Appendix A
Appendix A.1
Table A1 presents the distribution of various characteristics among the newborns. The fetal presentation was predominantly cephalic in both groups, with 92% in the hypothermia group and 91% in the non-hypothermia group (
p > 0.9), indicating no significant difference. The mode of birth, including cesarean and vaginal deliveries, showed a similar distribution across both groups, with cesarean sections occurring in 33% of the hypothermia group and 45% of the non-hypothermia group (
p = 0.7). Instrumental delivery was more frequent in the hypothermia group (33%) compared to the non-hypothermia group (9.1%), although this difference did not reach statistical significance (
p = 0.2). Premature rupture of membranes was reported in 18% of the non-hypothermia group, while no cases were observed in the hypothermia group (
p = 0.3). Meconium-stained amniotic fluid was more common in the non-hypothermia group (36%) compared to the hypothermia group (8.3%) (
p = 0.11). Anthropometric measures, including birth weight, height, and head circumference, showed no significant differences between the two groups, suggesting comparable physical characteristics at birth. These results demonstrate that most maternal and newborn characteristics were similarly distributed between the groups, with no statistically significant differences in perinatal factors.
Table A1.
Characteristics of participants based on hypothermia treatment status.
Table A1.
Characteristics of participants based on hypothermia treatment status.
| Characteristic |
Hypothermia (Yes, N = 12) |
Hypothermia (No, N = 22) |
p-value |
| Fetal presentation |
|
|
>0.9 |
| Cephalic |
11 (92%) |
20 (91%) |
|
| Compound |
0 (0%) |
1 (4.5%) |
|
| Breech |
1 (8.3%) |
1 (4.5%) |
|
| Birth route |
|
|
0.7 |
| Cesarean |
4 (33%) |
10 (45%) |
|
| Vaginal |
8 (67%) |
12 (55%) |
|
| Expulsive |
|
|
0.4 |
| No |
10 (83%) |
15 (68%) |
|
| Yes |
2 (17%) |
7 (32%) |
|
| Prolonged labor |
|
|
>0.9 |
| No |
9 (75%) |
15 (68%) |
|
| Yes |
3 (25%) |
7 (32%) |
|
| Instrumental delivery |
|
|
0.2 |
| No |
8 (67%) |
20 (91%) |
|
| Yes |
4 (33%) |
2 (9.1%) |
|
| Urgent cesarean section |
|
|
0.7 |
| No |
8 (67%) |
13 (59%) |
|
| Yes |
4 (33%) |
9 (41%) |
|
| Emergent cesarean section |
|
|
>0.9 |
| No |
12 (100%) |
21 (95%) |
|
| Yes |
0 (0%) |
1 (4.5%) |
|
| Chorioamnionitis |
|
|
0.4 |
| No |
11 (92%) |
22 (100%) |
|
| Yes |
1 (8.3%) |
0 (0%) |
|
| Premature rupture of membranes |
|
|
0.3 |
| No |
12 (100%) |
18 (82%) |
|
| Yes |
0 (0%) |
4 (18%) |
|
| Oligohydramnios |
|
|
>0.9 |
| No |
11 (92%) |
21 (95%) |
|
| Yes |
1 (8.3%) |
1 (4.5%) |
|
| Meconium |
|
|
0.11 |
| No |
11 (92%) |
14 (64%) |
|
| Yes |
1 (8.3%) |
8 (36%) |
|
| Birth weight (g) |
3,100 (2,921, 3,422) |
3,202 (2,896, 3,404) |
0.8 |
| Height (cm) |
51.00 (49.00, 51.50) |
50.00 (49.00, 52.00) |
>0.9 |
| Head circumference (cm) |
34.25 (33.62, 35.00) |
33.00 (32.00, 36.00) |
0.5 |
References
- Vannucci, R.C.; Perlman, J.M. Interventions for perinatal hypoxic–ischemic encephalopathy. Pediatrics 1997, 100, 1004–1114. [Google Scholar] [CrossRef] [PubMed]
- Douglas-Escobar, M.; Weiss, M.D. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA pediatrics 2015, 169, 397–403. [Google Scholar] [CrossRef] [PubMed]
- She, H.Q.; Sun, Y.F.; Chen, L.; Xiao, Q.X.; Luo, B.Y.; Zhou, H.S.; Zhou, D.; Chang, Q.Y.; Xiong, L.L. Current analysis of hypoxic-ischemic encephalopathy research issues and future treatment modalities. Frontiers in Neuroscience 2023, 17, 1136500. [Google Scholar] [CrossRef] [PubMed]
- Lacerte, M.; Shapshak, A.H.; Mesfin, F.B. Hypoxic brain injury. In StatPearls [Internet]; StatPearls Publishing, 2023.
- Caramelo, I.; Coelho, M.; Rosado, M.; Cardoso, C.M.; Dinis, A.; Duarte, C.B.; Graos, M.; Manadas, B. Biomarkers of hypoxic–ischemic encephalopathy: a systematic review. World Journal of Pediatrics 2023, 19, 505–548. [Google Scholar] [CrossRef] [PubMed]
- Nanyunja, C.; Sadoo, S.; Mambule, I.; Mathieson, S.R.; Nyirenda, M.; Webb, E.L.; Mugalu, J.; Robertson, N.J.; Nabawanuka, A.; Gilbert, G.; others. Protocol for the birth asphyxia in African newborns (baby BRAiN) study: a neonatal encephalopathy feasibility cohort study. Gates open research 2022, 6. [Google Scholar] [CrossRef] [PubMed]
- Sakr, M.; Shah, M.; Balasundaram, P. Neonatal therapeutic hypothermia. In StatPearls [Internet]; StatPearls Publishing, 2024.
- Finder, M.; Boylan, G.B.; Twomey, D.; Ahearne, C.; Murray, D.M.; Hallberg, B. Two-year neurodevelopmental outcomes after mild hypoxic ischemic encephalopathy in the era of therapeutic hypothermia. JAMA pediatrics 2020, 174, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.K.; Gulati, A. Advances in therapies to treat neonatal hypoxic-ischemic encephalopathy. Journal of clinical medicine 2023, 12, 6653. [Google Scholar] [CrossRef] [PubMed]
- Malik, I.; Shah, F.A.; Ali, T.; Tan, Z.; Alattar, A.; Ullah, N.; Khan, A.u.; Alshaman, R.; Li, S. Potent natural antioxidant carveol attenuates MCAO-stress induced oxidative, neurodegeneration by regulating the Nrf-2 pathway. Frontiers in Neuroscience 2020, 14, 659. [Google Scholar] [CrossRef] [PubMed]
- Schock, R.B. Re:“Therapeutic Hypothermia for Hypoxic–Ischemic Brain Injury Is More Effective in Newborn Infants Than in Older Patients: Review and Hypotheses” by Whitelaw and Thoresen. Therapeutic Hypothermia and Temperature Management 2024, 14, 1–1. [Google Scholar] [CrossRef] [PubMed]
- Kurisu, K.; Kim, J.Y.; You, J.; Yenari, M.A. Therapeutic hypothermia and neuroprotection in acute neurological disease. Current Medicinal Chemistry 2019, 26, 5430–5455. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Zhang, Z.Y.; Fan, B.; Li, G.Y. Neuroprotection by therapeutic hypothermia. Frontiers in neuroscience 2019, 13, 586. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, M.; Pennock, J.; Schwieso, J.; Cowan, F.; Dubowitz, L. Hypoxic-ischaemic encephalopathy: early and late magnetic resonance imaging findings in relation to outcome. Archives of Disease in Childhood-Fetal and Neonatal Edition 1996, 75, F145–F151. [Google Scholar] [CrossRef] [PubMed]
- Weeke, L.C.; Groenendaal, F.; Mudigonda, K.; Blennow, M.; Lequin, M.H.; Meiners, L.C.; van Haastert, I.C.; Benders, M.J.; Hallberg, B.; de Vries, L.S. A novel magnetic resonance imaging score predicts neurodevelopmental outcome after perinatal asphyxia and therapeutic hypothermia. The Journal of pediatrics 2018, 192, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zöllei, L.; Iglesias, J.E.; Ou, Y.; Grant, P.E.; Fischl, B. Infant FreeSurfer: An automated segmentation and surface extraction pipeline for T1-weighted neuroimaging data of infants 0–2 years. Neuroimage 2020, 218, 116946. [Google Scholar] [CrossRef] [PubMed]
- Dathe, A.K.; Stein, A.; Bruns, N.; Craciun, E.D.; Tuda, L.; Bialas, J.; Brasseler, M.; Felderhoff-Mueser, U.; Huening, B.M. Early Prediction of Mortality after Birth Asphyxia with the nSOFA. Journal of Clinical Medicine 2023, 12, 4322. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.P.; Lee-Kelland, R.; Brooks, J.C.; Jary, S.; Tonks, J.; Cowan, F.M.; Thoresen, M.; Chakkarapani, E. Brain volumes and functional outcomes in children without cerebral palsy after therapeutic hypothermia for neonatal hypoxic-ischaemic encephalopathy. Developmental Medicine & Child Neurology 2023, 65, 367–375. [Google Scholar]
- Chawla, D. Therapeutic hypothermia for neonatal encephalopathy in developing countries: Current evidence. Clinical Epidemiology and Global Health 2024, p. 101507.
- Thayyil, S.; Pant, S.; Montaldo, P.; Shukla, D.; Oliveira, V.; Ivain, P.; Bassett, P.; Swamy, R.; Mendoza, J.; Moreno-Morales, M.; others. Hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries (HELIX): a randomised controlled trial in India, Sri Lanka, and Bangladesh. The Lancet Global Health 2021, 9, e1273–e1285. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, P.; ID, A. Bayley scales of infant and toddler development.[Updated 2021 Nov 24]. StatPearls [Internet]. StatPearls Publishing 2022.
- Misser, S.K.; Barkovich, A.J.; Lotz, J.W.; Archary, M. A pictorial review of the pathophysiology and classification of the magnetic resonance imaging patterns of perinatal term hypoxic ischemic brain injury–What the radiologist needs to know…. SA Journal of Radiology 2020, 24. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Arias, O.; Eddama, O.; Azzopardi, D.; Edwards, A.D.; Strohm, B.; Campbell, H. Hypothermia for perinatal asphyxia: trial-based resource use and costs at 6–7 years. Archives of Disease in Childhood-Fetal and Neonatal Edition 2019, 104, F285–F292. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).