1. Introduction
Perfluorooctane sulfonate (PFOS) and Perfluorooctanoate (PFOA) are notorious environmental contaminants that fall under the extensive category of per- and polyfluoroalkyl substances (PFAS). Renowned for their multiple industrial applications such as Aqueous Film Forming Foams (AFFFs), fluoropolymer resins, water- and stain-resistant additives, these chemicals have sparked growing alarm due to their extraordinary persistence in the environment, their ability to bioaccumulate in living organisms, and their potential for long term toxicity including cancer [
1]. Of particular concern is the impact of PFOS and PFOA on the nervous system, which is especially susceptible to damage. These compounds can penetrate the blood-brain barrier [
2] and accumulate within the brain, in which emerging research indicates they may interfere with normal neuronal function and contribute to neurotoxicity[
3]. One crucial aspect of neuronal function involves gamma-aminobutyric acid (GABA) receptors, which play a pivotal role in maintaining the excitatory-inhibitory balance in the brain. GABA receptors, particularly the GABA
A subtype, mediate inhibitory synaptic transmission and are essential for various neurological processes, including anxiety modulation, muscle relaxation, and seizure prevention. Disruption of GABA
A receptor-mediated currents can, therefore, have profound implications for brain function and overall health. GABA
A receptors are crucial components of the nervous system, functioning as ligand-gated chloride channels that play a key role in inhibitory neurotransmission. These receptors are composed of five subunits arranged in a pentameric structure, with each subunit drawn from a diverse set of possibilities. In humans, researchers have identified 19 distinct genes that encode the subunits of GABA
A receptors. These include six α (1-6), three β (1-3), three γ (1-3), three ρ (1-3), and one each for δ, ε, π, and θ subunits[
4,
5]. The vast diversity observed in GABA
A receptors stems from the multiple ways these subunits can combine, which is further enhanced by the alternative splicing of several genes. This alternative splicing process allows a single gene to produce multiple different subunit variants, thereby significantly expanding the potential combinations and functional diversity of the receptors. This diversity is critical, as it enables GABA
A receptors to fulfill a wide range of functions in various regions of the brain and to modulate neuronal activity in response to different physiological, and pharmacological stimuli [
6,
7,
8]. The α1β2γ2 subtype is the predominant form in the mammalian nervous system, representing a significant majority. It is estimated that this specific configuration may constitute as much as 60% of all GABA
A receptors [
7], highlighting its crucial role in functioning and regulation of neural activity[
9]. GABA
A receptors are ligand-gated chloride channels, made by pentameric combination of different subunits. GABA
A receptors subunits share a common structure. Each mature subunit consists of approximately 450 amino acid residues and includes several key regions: an N-terminal region, a large hydrophilic extracellular domain (ECD), and four hydrophobic transmembrane domains (TMD: TM1–TM4). The TM2 domain is thought to form the pore of the chloride channel, while an intracellular domain (ICD) located between TM3 and TM4 serves as a critical site for protein interactions and post-translational modifications that regulate receptor activity [
10,
11]. The neurotransmitter GABA, along with psychotropic drugs like benzodiazepines (BZDs), binds to the N-terminal at the α-β and α-γ interfaces, respectively. Additionally, neurosteroids and anesthetics such as barbiturates interact within the TMD of the α and β subunits[
12,
13]. Several GABAergic insecticides, despite their diverse chemical structures, act as noncompetitive antagonists by binding to the same GABA receptor site located in the TM2 domain. Compounds such as β-endosulfan, lindane, and fipronil, along with botanical agents like picrotoxinin, target the chloride channel pore of the GABA receptor, blocking ion flow and leading to neural hyperexcitation, which is critical for the insecticidal action and human neurotoxicity [
14].
This study aims to investigate the impact of PFOS and PFOA on GABA receptor-mediated currents in neuron-like neuroblastoma cells, providing insights into the neurotoxic mechanisms of these pollutants. This research directly reveals how legacy PFAS affect neuron-like cells, representing a major advancement in our understanding. By examining the interactions between PFOS, PFOA, and GABAA receptors, we seek to uncover how these interactions may disrupt neuronal activity. This knowledge will help elucidate the broader health implications of exposure to these widespread environmental contaminants. The study not only deepens our understanding of PFAS-induced neurotoxicity but also highlights the urgent need for stringent regulatory measures to limit exposure and protect public health. The findings are expected to prompt policy changes and interventions aimed at reducing the prevalence of these harmful substances, thereby safeguarding both human health and environmental integrity.
2. Materials and Methods
2.1. Cell Culture
S1 neuroblastoma cells were maintained as reported in[
15]. Briefly, cells were grown in RPMI 1640 medium supplied with 10% FBS, 2 mM L-Glu, 100 μg/ml penicillin-streptomycin, 200 μg/ml geneticin sulfate (G418) and kept at 37ºC in a 5% CO2 incubator. All chemicals were purchased from Merck (Sigma-Aldrich).
2.2. Cytotoxicity Assay
Cells were seeded in 96 multiwell plates (Greiner) at a density of 5×10³ cells per well in complete medium as described in section 2.1. Once settled, the medium was removed and replaced with fresh medium containing varying concentrations of PFOA and PFOS to assess their impact on cell viability. The plates were incubated for 72 hours at 37°C in a 5% CO₂ atmosphere to allow for cell adhesion, proliferation, and PFAS exposure. Following this incubation, resazurin was added to each well at a concentration of 0.1 mg/mL. The plates were further incubated for 2 hours under the same conditions, after which fluorescence was measured using a Tecan Infinite 200 Pro fluorimeter (excitation 535 nm, emission 590 nm) to quantify the reduction of resazurin to resorufin, indicating cell viability.[
17] For statistics, data were evaluated for normality and homoscedasticity and analysed using one-way analysis of variance (ANOVA), followed by Holm-Sidak’s multiple comparisons test using GraphPad Prism™ 9 (GraphPad Software, San Diego, CA, USA).
2.3. Electrophysiological Recordings
S1 neuroblastoma cells were plated in 3.5 cm Petri dishes and submerged in a bath of extracellular solution containing (in mM): 140 NaCl, 5.4 KCl, 2 CaCl₂, 1 MgCl₂, 10 HEPES, and 10 glucose (pH adjusted to 7.4 with NaOH). Observations were performed under an inverted microscope (IMT-2, Olympus Life Science, Italy) using a 40X objective. Whole-cell patch-clamp recordings were conducted at room temperature (22–25 ºC) using an Axopatch 200A amplifier (Molecular Devices, USA). Patch pipettes were fabricated from borosilicate glass capillaries (Hilgenberg, Germany), pulled using a vertical pipette puller (PIP6, HEKA, Germany), and filled with internal solution containing (in mM): 142 KCl, 2 MgCl₂, 2 EGTA, and 10 HEPES (pH adjusted to 7.3 with KOH). Final pipette resistance ranged from 3–5 MΩ.
After establishing a stable whole-cell configuration, cells were clamped at a holding potential (Vh) of -80 mV. Voltage-gated currents were elicited by applying voltage pulses ranging from -100 mV to +100 mV in 20 mV increments, each lasting for 20 seconds. GABA-evoked currents were activated by brief agonist application via a gravity-driven perfusion system localized near the patched cell. Recordings were acquired using a custom program, GePulse (
http://users.ge.ibf.cnr.it/pusch/programs-mik.htm), and whole-cell currents were low-pass filtered at 2 kHz and digitized at 20 kHz.
GABA stock solution (in distilled water), Picrotoxinin (PTX, in DMSO), and PFOS or PFOA (in DMSO or isopropanol, respectively) were freshly prepared and added to the saline solution, ensuring the final concentration of DMSO or isopropanol remained at 0.02% (vol/vol) prior to each experiment. This careful preparation maintained the integrity and consistency of experimental conditions. GABA, PTX, and PFAS were rapidly applied to patched cells using the gravity-driven perfusion system, followed by a 1-minute washout period between applications to allow for receptor recovery and minimize desensitization effects. To prevent cross-contamination, PFOS and PFOA were tested on different S1 cells from separate Petri dishes.
Acquired data were analyzed offline using Clampfit 10.7 (Molecular Devices, USA) and SigmaPlot (Systat Software Inc, USA). Data are expressed as mean ± standard error, and statistical significance (p < 0.05) was determined using a paired t-test
3. Results
3.1. Acute Toxicity of PFAS in S1 Cells
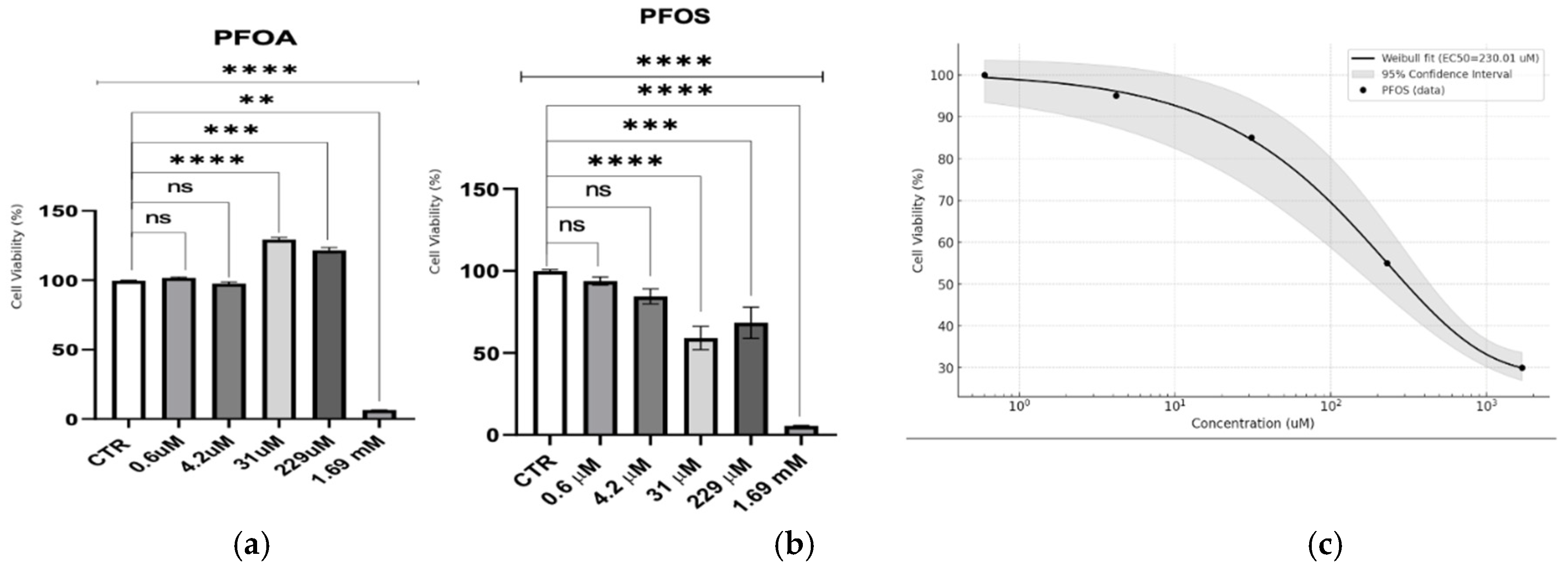
This study assessed the cytotoxic effects of PFOA and PFOS across a range of PFAS concentrations (
Figure 1) in neuron-like S1 neuroblastoma cells. PFOA displayed a threshold response, with minimal toxicity observed until millimolar concentrations. A stimulatory effect was noted at 229 µM, followed by a sharp drop in viability only at 1.69 mM, suggesting that acute toxicity for PFOA is virtually absent at lower doses. In contrast, PFOS exhibited a dose-dependent cytotoxic response. A Weibull regression analysis for PFOS indicated a significant reduction in cell viability across a broader concentration range, with an estimated EC50 of 230.01 ± 26.31 µM, highlighting its higher acute toxicity compared to PFOA.
3.2. Electrophysiological Characterization of S1 Neuron-like Neuroblastoma Cells
After verifying the effect of PFAS on the survival of neuron-like cells, we investigated whether these substances could exert an effect on synaptic transmission on GABA receptors.
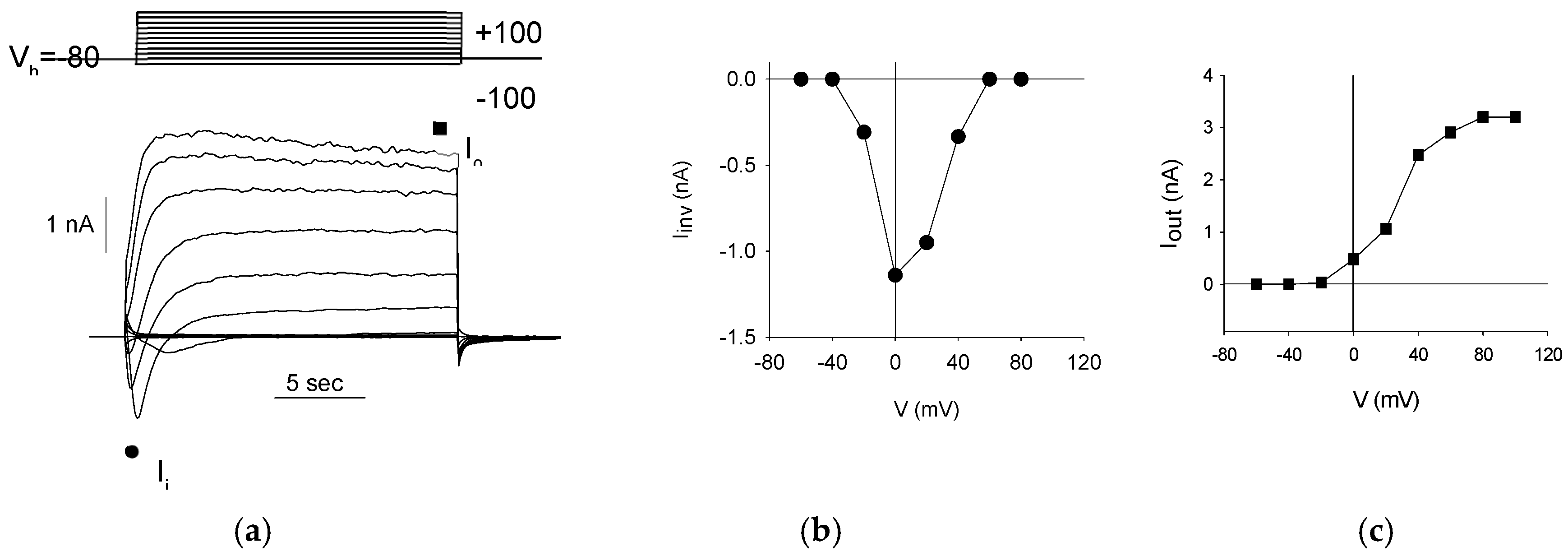
First, to confirm the neuron-like differentiation of S1 neuroblastoma cells, we investigated the presence of voltage-dependent currents. Voltage steps of 20 mV were applied every 20 seconds, ranging from -100 mV to +100 mV, starting from a holding potential (Vh) of -80 mV (
Figure 2a, top). This protocol revealed both inward and outward currents (
Figure 2a, bottom) in all patched cells (330/330, 100%). The inward currents are indicative of Na
+-channel activity, demonstrating a typical voltage-dependent activation and inactivation pattern (
Figure 2b), while the outward currents suggest K
+-channel activity, characterized by a voltage-dependent activation that is sustained over the voltage steps (
Figure 2c).
Since an exhaustive characterization of voltage-gated currents in S1 cells is provided by Gavazzo et al. [
15], further investigation was not pursued. These findings confirm that S1 neuroblastoma cells have differentiated into an excitatory neuron-like phenotype.
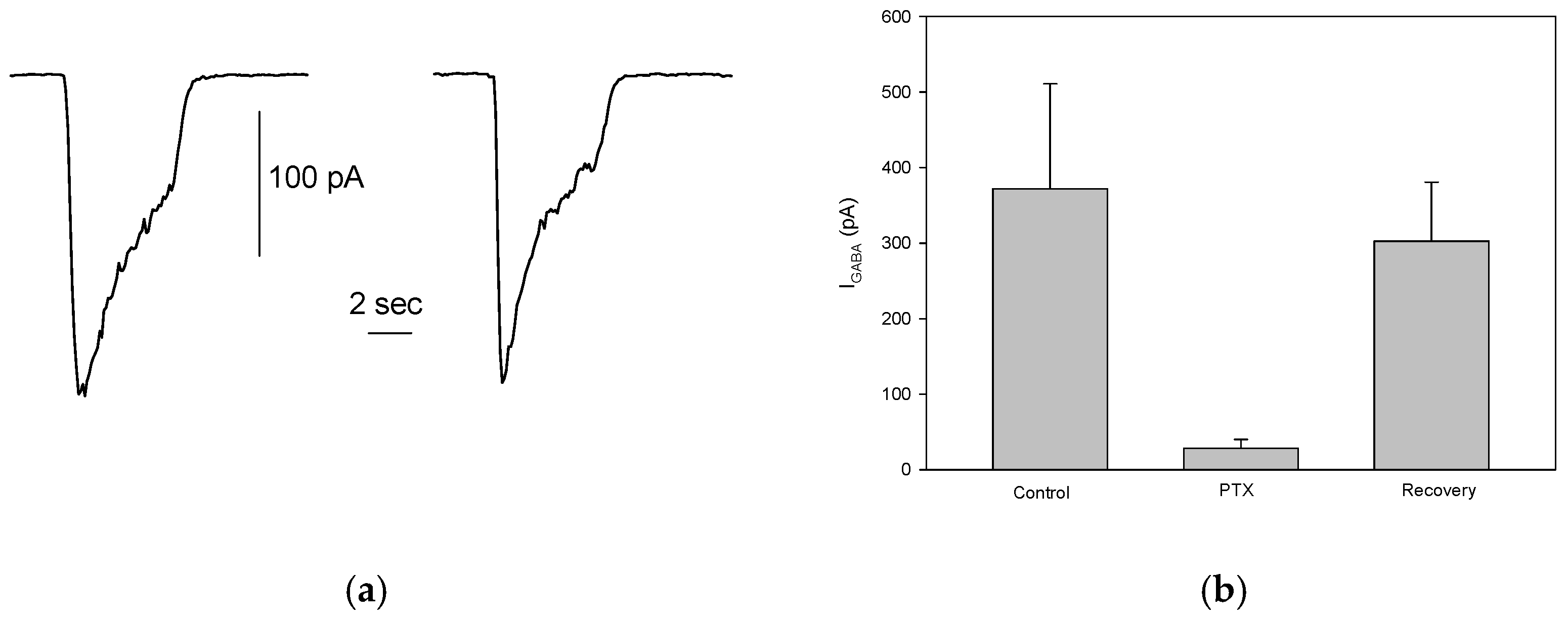
To assess the presence of functional GABAergic receptors in S1 cells, rapid application of 100 μM GABA to patched cells, held at -80 mV, elicited an inward current carried by Cl⁻ ions following the electrochemical gradient. The mean current amplitude was 249 ± 29.5 pA (ranging from 23 to 1314 pA), which aligns well with previous findings [
15]. However, only 27% (89/330) of the cells responded to GABA, suggesting a significant reduction in the proportion of S1 cells expressing functional GABA
A receptors. Despite this, in cells that did respond, the GABA-evoked current was reproducible and stable across multiple stimulations (
Figure 3a). This indicates the reliability of GABA-induced responses in cells expressing functional GABA
A receptors. Additionally, picrotoxinin (PTX, 100 μM), a known GABA
A receptor antagonist, significantly and reversibly inhibited the GABA-evoked currents (control: 372 ± 139 pA; PTX: 28.17 ± 12.1 pA; recovery: 302.5 ± 78.36, n=6,
Figure 3b), confirming that the observed inward currents were indeed mediated by GABA
A receptors, as previously reported [
15].
3.3. Effect of PFOS and PFOA on GABA-Evoked Currents in Neuron-like S1 Neuroblastoma Cells
To determine whether the two PFAS compounds influence baseline current in neuron-like cells, a localized application of sublethal PFOS or PFOA concentrations -10 μM- was administered to patched S1 cells. The results showed that neither compound induced any detectable currents, nor were there any observable effects on the baseline current (data not shown). This lack of response strongly suggests that PFOS and PFOA, when applied alone at this concentration, do not have an immediate or direct impact on the baseline electrical activity of these cells.
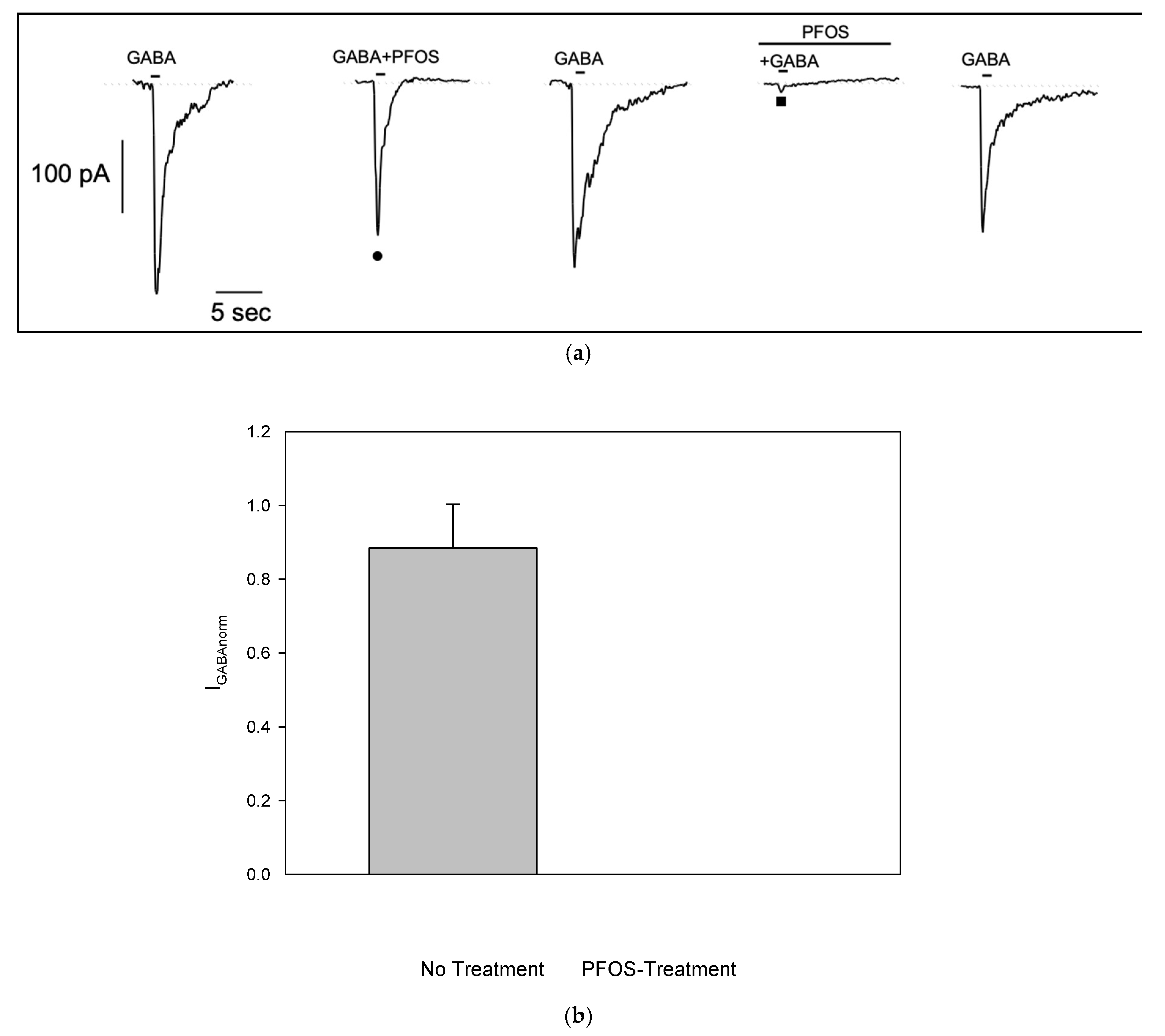
In order to investigate if PFOS is able to modulate GABA-evoked currents, we co-applied GABA with PFOS at 10 μM, a concentration previously shown to be effective in injected oocites[
16]. In GABA-responding S1 cell, brief co-application of PFOS showed no significant effect on GABA-evoked current (
Figure 4a •). However, when S1 cell was pretreated with PFOS for 1 min, current amplitude was almost completely suppressed (
Figure 4a ■). After a 1-minute washout, the current recovered, indicating that PFOS block on GABA-dependent current is reversible. Similar results were observed in n=5 cells. To reduce data variability due to scattering of GABA-evoked current amplitude, responses in the presence of PFOS were normalized to responses recorded without it (no treatment: 0.88±0.12; pre-treatment: 6.8e-5±5.12e-5, see plot on
Figure 4b).
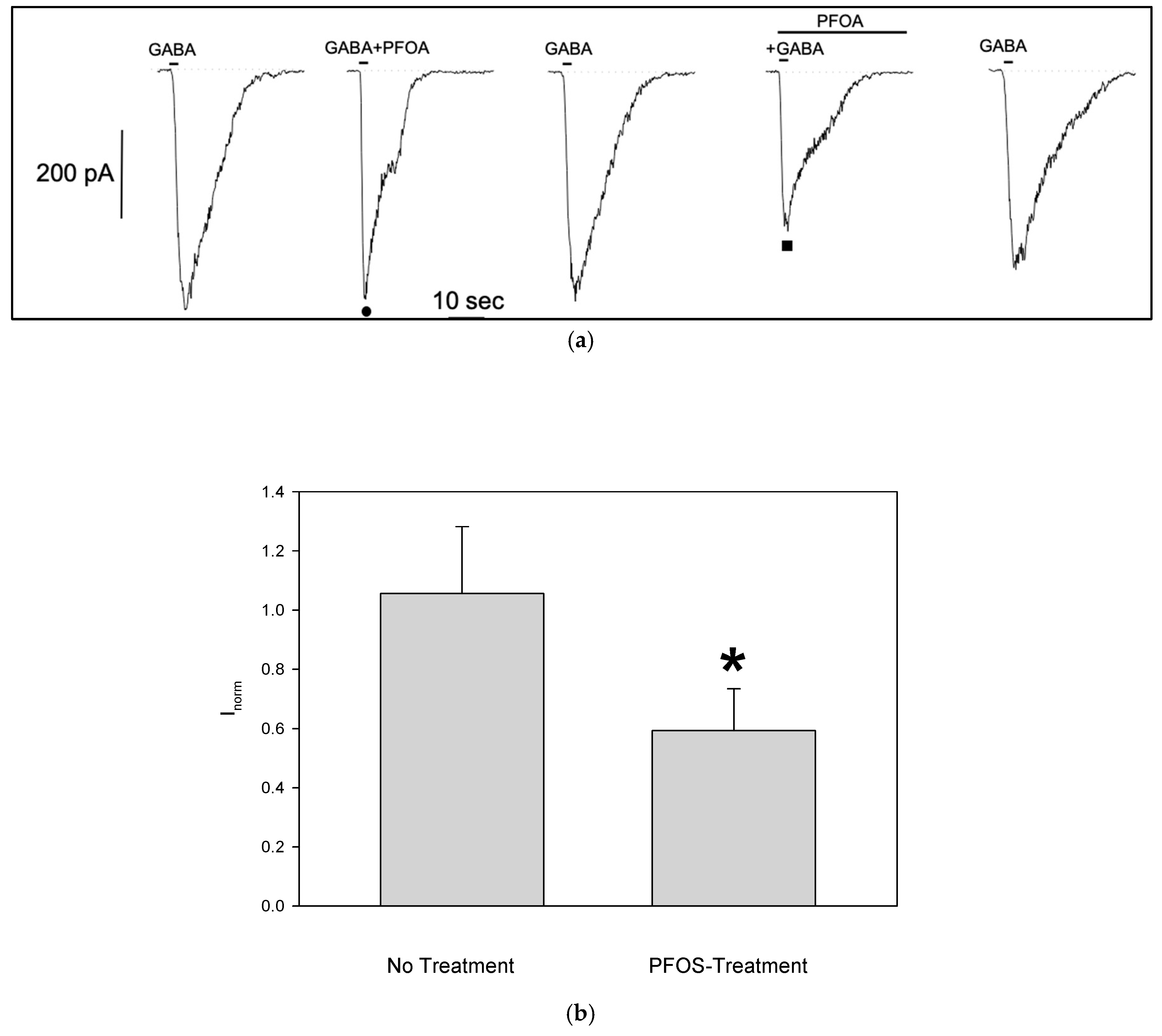
The same experimental protocol was applied to GABA-responsive cells to investigate the effects of PFOA. Like PFOS, PFOA reduced the GABA-evoked current amplitude only when S1 cells were pretreated (
Figure 5a)). Although the block of GABA-dependent current was less pronounced (no treatment normalized current: 1.05±0.23, n=5; pre-treatment: 0.6±0.14, n=6,
Figure 5b), it was still significant, and reversible as seen for the PFOS.
4. Discussion
The results of this study provide compelling evidence that both PFOS and PFOA significantly alter GABAA receptor-mediated currents in neuron-like neuroblastoma S1 cells, revealing important insights into the neurotoxic mechanisms of these persistent pollutants. The reduction in GABA-evoked currents following pre-treatment with PFOS and PFOA highlights a potentially critical pathway by which these compounds exert their neurotoxic effects, specifically through the modulation of inhibitory neurotransmission.
First, it must be pointed out that, due to the GABA amplitude scattering and a limited number of GABA-expressing cells, we used GABA at a very high concentration (100 µM) to increase the probability of finding responsive cells. We are aware that this concentration is saturating, that some other effects of PFAS could be masked, and that it is necessary to conduct experiments with lower doses in a more suitable cellular model to better understand PFAS effects. Nevertheless, our results are in good agreement with Tukker’s data [
16], showing a PFOS/PFOA-dependent reduction of current in GABA-expressing oocytes. Moreover, they claim that both PFAS are non-competitive GABA antagonists. Similarly, our findings indicate that PFOS and PFOA do not directly induce currents or alter baseline electrical activity in the S1 neuroblastoma cells when applied alone. This suggests that their neurotoxic effects may not stem from direct interaction with the GABAA receptor in the absence of the neurotransmitter, supporting the hypothesis of an indirect effect on the receptor. However, the significant reduction in GABA-evoked currents following pre-exposure to these PFAS compounds suggests that PFOS and PFOA may interfere with the receptor’s ability to respond to GABA, potentially by altering receptor conformation, hindering receptor function, or affecting the receptor’s interaction with modulatory binding sites known to be present in the GABAA receptors. There is compelling evidence suggesting that the neurotoxic effects of PFAS may result from their accumulation in cell membranes[
19], as these compounds tend to integrate into cell membranes and other lipid bilayers due to their lipophilic nature[
3,
20,
21,
22]. Moreover, PFAS can alter plasma membrane potential and intracellular pH level[
23]. However, the effects observed in our study are unlikely to be exclusively attributed to these mechanisms. We observed no significant impact on baseline current, and the relative brief 1-minute exposure followed by rapid washout makes membrane accumulation an improbable cause for the observed outcomes. The effect on membrane potentials seems unlikely, as adding just 10 micromolar of negative charge on the extracellular side wouldn’t be sufficient to cause a complete block or even a 60% reduction of GABA-induced current. Furthermore, if the effect were purely charge-dependent, both PFOS and PFOA would produce identical outcomes due to their shared negative charge. Additionally, there was no observed impact on the baseline current. However, the potential impact of PFAS on cell membranes cannot be definitively excluded. While our findings suggest that membrane accumulation may not be the primary mechanism, especially given the brief exposure and rapid washout, it remains a possibility that warrants further investigation. To thoroughly understand the full scope of PFAS effects, including any subtle or delayed interactions with cell membranes, additional experiments are crucial. These studies will be vital in uncovering the precise mechanisms through which PFAS exerts its neurotoxic effects.
An interesting consideration is the potential analogy between PFAS compounds and flumazenil, a well-known antagonist of the benzodiazepine binding site on GABAA receptors. Flumazenil binds to this site and inhibits the positive allosteric modulation induced by benzodiazepines. However, flumazenil does not significantly alter GABA-induced currents on its own but competes with benzodiazepines for the same binding site. In contrast, PFOS and PFOA reduce GABA-evoked currents even when applied alone (after pre-treatment), suggesting a different mechanism of action. Currently, there is no evidence to suggest that PFOS or PFOA interact with the benzodiazepine (flumazenil) binding site on GABAA receptors. Their inhibitory effects are more consistent with non-competitive antagonism or channel blockade rather than competitive antagonism at the benzodiazepine site. Therefore, while both flumazenil and PFOS/PFOA can inhibit GABAA receptor function, they likely do so via different mechanisms and are not analogous in their interaction with the receptor.
Considering other potential mechanisms, PFOS and PFOA might act as non-competitive inhibitors of GABAA receptors, as also shown in Tukker et al. [
16]. Unlike competitive antagonists that bind to the same site as GABA, non-competitive inhibitors bind to allosteric sites, inducing conformational changes that reduce receptor activity. The reversible inhibition observed in our study is reminiscent of channel blockers like picrotoxinin, which obstruct the chloride channel pore of GABAA receptors. However, despite the structural differences between PFOS, PFOA, and known channel blockers, Chen et al. [
14] found that structurally diverse compounds can occupy overlapping sites in the GABAA receptor pore, interacting with critical residues such as A2’, T6’, and L9’ in the TM2 domain. Their findings suggest that non-competitive antagonists, even with distinct structures, can disrupt receptor function by inducing conformational changes in the chloride channel. Given the highly fluorinated nature of PFOS and PFOA, it is plausible that they similarly engage in hydrophobic interactions with these pore-lining residues. This mechanism, akin to that of fluorinated blockers like fipronil, may explain the inhibitory effects of PFOS and PFOA on GABAA receptor activity observed in our study.
In our neuron-like S1 cells, both PFAS effects were reversible within seconds upon washout. However, in GABA-expressing oocytes, PFOS-induced inhibition was poorly reversible, suggesting a stronger and more persistent interaction with the receptor in this model [
16]. This discrepancy could be ascribed to differences in biological systems, as oocytes express recombinant GABAA receptors with controlled subunit composition, which may lack the accessory proteins or native conditions present in neuronal cells [
15]. Additionally, the subunit composition of GABAA receptors can vary significantly between models, potentially affecting PFAS binding affinity and reversibility. Nonetheless, the reversibility of PFAS-dependent inhibition suggests that the interaction between PFOS or PFOA and GABAA receptors is not permanently damaging but rather a temporary interference, particularly when PFAS exposure is brief and localized. This reversible nature also raises concerns about the potential for chronic exposure to cause cumulative neurotoxic effects, where repeated or prolonged exposure might lead to lasting disruptions in inhibitory signaling.
GABAA receptors play a crucial role in maintaining the excitatory-inhibitory balance in the central nervous system. Disruptions to this balance, such as those caused by PFOS and PFOA, can have far-reaching consequences, potentially leading to increased neuronal excitability, impaired neural circuits, and the onset of neurological disorders. The fact that PFOS and PFOA can suppress GABA-evoked currents suggests that their presence could contribute to altered neural excitability and function, particularly under conditions of prolonged exposure where recovery between exposures may be incomplete. These findings are particularly concerning given the widespread and persistent nature of PFOS and PFOA in the environment and their ability to bioaccumulate in human tissues, including the brain. The potential for these compounds to modulate GABAergic signaling may help explain the growing body of epidemiological evidence linking PFAS exposure to cognitive deficits, developmental delays, and other neurological conditions [
3,
24,
25]. The study underscores the broader health implications of exposure to PFOS and PFOA, particularly regarding their potential to disrupt critical neurobiological processes. The observed effects on GABAA receptor-mediated currents provide a mechanistic link between PFAS exposure and neurotoxic outcomes, supporting the hypothesis that these compounds could contribute to the development of neurological disorders.
This research highlights the need for further studies to explore the long-term effects of chronic PFAS exposure on GABAergic neurotransmission and overall brain health. Moreover, the findings call attention to the urgent need for stringent regulatory measures to limit PFAS exposure. The potential for these compounds to interfere with key neurotransmitter systems suggests that current exposure levels, even if deemed safe by existing standards, could pose significant risks to public health, particularly for vulnerable populations such as children and individuals with pre-existing neurological conditions.
5. Perspectives
Looking forward, it is essential to deepen our understanding of the mechanisms underlying PFAS-induced inhibition of GABAA receptor function. Future studies should aim to identify the specific sites of interaction between PFOS/PFOA and the GABAA receptor. Conducting molecular docking studies could provide valuable insights into how these compounds interact at the molecular level with the receptor’s binding sites or transmembrane regions. Such computational approaches can predict potential binding sites and modes, guiding experimental efforts to confirm these interactions.
Additionally, exploring the effects of a broader range of PFAS compounds on GABAA receptor function is crucial. Given the structural diversity within the PFAS family, including congeners and classes that are not yet restricted or banned, testing other PFAS could reveal whether the inhibitory effects observed with PFOS and PFOA are common to other related compounds or are specific legacy ones. This would enhance our understanding of the structure-activity relationships governing PFAS interactions with GABAA receptors and identify potential risks associated with other PFAS substances that are currently in use.
Shifting to more mechanistic models, such as using recombinant systems expressing specific GABAA receptor subunits, could help dissect the precise molecular mechanisms involved. By controlling the subunit composition of the receptors, we can determine whether certain subunits are more susceptible to PFAS-induced inhibition, providing insights into the receptor’s structural features that mediate these effects.
Moreover, biophysical techniques like site-directed mutagenesis and electrophysiological recordings can be employed to identify critical amino acid residues involved in PFAS interactions. This mechanistic approach will contribute to a more detailed understanding of how PFAS compounds affect receptor function at the molecular level.
From a public health perspective, our findings highlight the importance of revisiting safety standards and regulatory policies concerning PFAS exposure. Considering their persistent nature and bioaccumulative properties, even low-level exposure could have significant health implications over time. Collaborative efforts between scientists, policymakers, and industry stakeholders are necessary to develop strategies for reducing PFAS emissions, remediating contaminated environments, and limiting human exposure.
Finally, investigating potential therapeutic interventions to mitigate PFAS-induced neurotoxicity is an important area for future research. Identifying compounds or treatments that can counteract the inhibitory effects of PFAS on GABAA receptors may offer protective strategies for individuals at risk of exposure.
6. Conclusions
This study demonstrates that PFOS and PFOA significantly inhibit GABAA receptor-mediated currents in neuron-like neuroblastoma S1 cells, likely through a non-competitive antagonism. Regardless of PFAS’ mechanisms of action, our findings highlight the potential neurotoxic effects of PFOS and PFOA through the modulation of inhibitory neurotransmission, which may contribute to neurological disorders. Given the widespread presence of these compounds in the environment and their bioaccumulative nature, there is an urgent need to reassess safety standards and limit human exposure to PFAS.
Author Contributions
LL, Conceptualization, Methodology, Investigation, Formal Analysis, Data Curation, Statistical Analysis, Software, Writing—Original Draft, Review, Editing, Visualization. DR, Methodology, Investigation, Formal Analysis, Data Curation, Statistical Analysis, Software, Writing—Original Draft, Review, Editing, Visualization. DG, Formal Analysis, Statistical Analysis, Visualization. AC, Review. CL, Review, Visualization. VM, Conceptualization, Methodology, Investigation, Review, Editing, Visualization. FD, Conceptualization, Methodology, Investigation, Validation, Formal Analysis, Data Curation, Statistical Analysis, Writing—Original Draft, Review, Editing, Visualization, Project administration, Funding acquisition.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 101037509 (SCENARIOS project).
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
S1 neuroblastoma cells were kindly provided to LL by Prof. Aldo Pagano and Dr. Paola Gavazzo. LL also thanks Dr. Michele Fiore for technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zahm, S.; Bonde, J.P.; Chiu, W.A.; Hoppin, J.; Kanno, J.; Abdallah, M.; Blystone, C.R.; Calkins, M.M.; Dong, G.H.; Dorman, D.C.; et al. Schubauer-Berigan, M. K. Carcinogenicity of perfluorooctanoic acid and perfluorooctanesulfonic acid. The Lancet. Oncology 2024, 25, 16–17. [Google Scholar]
- Yu, Y.; Wang, C.; Zhang, X.; Zhu, J.; Wang, L.; Ji, M.; Zhang, Z.; Ji, X.M.; Wang, S.L. Perfluorooctane sulfonate disrupts the blood brain barrier through the crosstalk between endothelial cells and astrocytes in mice. Environ Pollut 2020, 256, 113429. [Google Scholar] [CrossRef] [PubMed]
- Brown-Leung, J.M.; Cannon, J.R. Neurotransmission Targets of Per- and Polyfluoroalkyl Substance Neurotoxicity: Mechanisms and Potential Implications for Adverse Neurological Outcomes. Chem Res Toxicol 2022, 35, 1312–1333. [Google Scholar] [CrossRef]
- Sieghart, W.; Fuchs, K.; Tretter, V.; Ebert, V.; Jechlinger, M.; Hoger, H.; Adamiker, D. Structure and subunit composition of GABA(A) receptors. Neurochem Int 1999, 34, 379–85. [Google Scholar] [CrossRef]
- Steiger, J.L.; Russek, S.J. GABAA receptors: building the bridge between subunit mRNAs, their promoters, and cognate transcription factors. Pharmacol Ther 2004, 101, 259–81. [Google Scholar] [CrossRef]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABA(A) receptors: structure, function, pharmacology, and related disorders. J Genet Eng Biotechnol 2021, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, U.; Knoflach, F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov 2011, 10, 685–97. [Google Scholar] [CrossRef]
- Sigel, E.; Baur, R.; Trube, G.; Mohler, H.; Malherbe, P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron 1990, 5, 703–11. [Google Scholar] [CrossRef]
- Sallard, E.; Letourneur, D.; Legendre, P. Electrophysiology of ionotropic GABA receptors. Cell Mol Life Sci 2021, 78, 5341–5370. [Google Scholar] [CrossRef]
- Chen, Z.W.; Olsen, R.W. GABAA receptor associated proteins: a key factor regulating GABAA receptor function. J Neurochem 2007, 100, 279–94. [Google Scholar] [CrossRef]
- Jacob, T.C.; Moss, S.J.; Jurd, R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci 2008, 9, 331–43. [Google Scholar] [CrossRef] [PubMed]
- Sigel, E.; Luscher, B.P. A closer look at the high affinity benzodiazepine binding site on GABAA receptors. Curr Top Med Chem 2011, 11, 241–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, M. Neurosteroids and GABA-A Receptor Function. Front Endocrinol (Lausanne) 2011, 2, 44. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Durkin, K.A.; Casida, J.E. Structural model for γ-aminobutyric acid receptor noncompetitive antagonist binding: widely diverse structures fit the same site. Proceedings of the National Academy of Sciences, 2006, 103, 5185–5190. [Google Scholar] [CrossRef]
- Gavazzo, P.; Vella, S.; Marchetti, C.; Nizzari, M.; Cancedda, R.; Pagano, A. Acquisition of neuron-like electrophysiological properties in neuroblastoma cells by controlled expression of NDM29 ncRNA. J Neurochem 2011, 119, 989–1001. [Google Scholar] [CrossRef]
- Tukker, A.M.; Bouwman, L.M.S.; van Kleef, R.; Hendriks, H.S.; Legler, J.; Westerink, R.H.S. Perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) acutely affect human alpha(1)beta(2)gamma(2L) GABA(A) receptor and spontaneous neuronal network function in vitro. Sci Rep 2020, 10, 5311. [Google Scholar] [CrossRef]
- Liao, C.Y.; Li, X.Y.; Wu, B.; Duan, S.M.; Jiang, G.B. Acute enhancement of synaptic transmission and chronic inhibition of synaptogenesis induced by perfluorooctane sulfonate through mediation of voltage-dependent calcium channel. Environmental Science & Technology 2008, 42, 5335–5341. [Google Scholar]
- Rampersad, S.N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Teng, M.; Zhao, X.; Li, Y.; Sun, J.; Zhao, W.; Ruan, Y.; Leung, K.M.Y.; Wu, F. Insight into the binding model of per- and polyfluoroalkyl substances to proteins and membranes. Environ Int 2023, 175, 107951. [Google Scholar] [CrossRef]
- Cao, Y.; Ng, C. Absorption, distribution, and toxicity of per- and polyfluoroalkyl substances (PFAS) in the brain: a review. Environ Sci Process Impacts 2021, 23, 1623–1640. [Google Scholar] [CrossRef]
- Harada, K.H.; Ishii, T.M.; Takatsuka, K.; Koizumi, A.; Ohmori, H. Effects of perfluorooctane sulfonate on action potentials and currents in cultured rat cerebellar Purkinje cells. Biochemical and Biophysical Research Communications 2006, 351, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Naumann, A.; Alesio, J.; Poonia, M.; Bothun, G.D. PFAS fluidize synthetic and bacterial lipid monolayers based on hydrophobicity and lipid charge. J Environ Chem Eng 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Kleszczynski, K.; Skladanowski, A.C. Mechanism of cytotoxic action of perfluorinated acids. I. alteration in plasma membrane potential and intracellular pH level. Toxicol Appl Pharmacol 2009, 234, 300–5. [Google Scholar] [CrossRef]
- Panieri, E.; Baralic, K.; Djukic-Cosic, D.; Buha Djordjevic, A.; Saso, L. PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Reardon, A.J.F.; Hajihosseini, M.; Dinu, I.; Field, C.J.; Kinniburgh, D.W.; MacDonald, A.M.; Dewey, D.; England-Mason, G.; Martin, J.W. ; APrON Study Maternal co-exposure to mercury and perfluoroalkyl acid isomers and their associations with child neurodevelopment in a Canadian birth cohort. Environ Int. 2023, 178, 108087. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).