Submitted:
17 October 2024
Posted:
18 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction to Cannabis and the Endocannabinoid System
1.1. The Endocannabinoid System
1.2. Cannabinoid Receptors
1.3. Endocannabinoids
1.3.2. Anandamide (AEA)
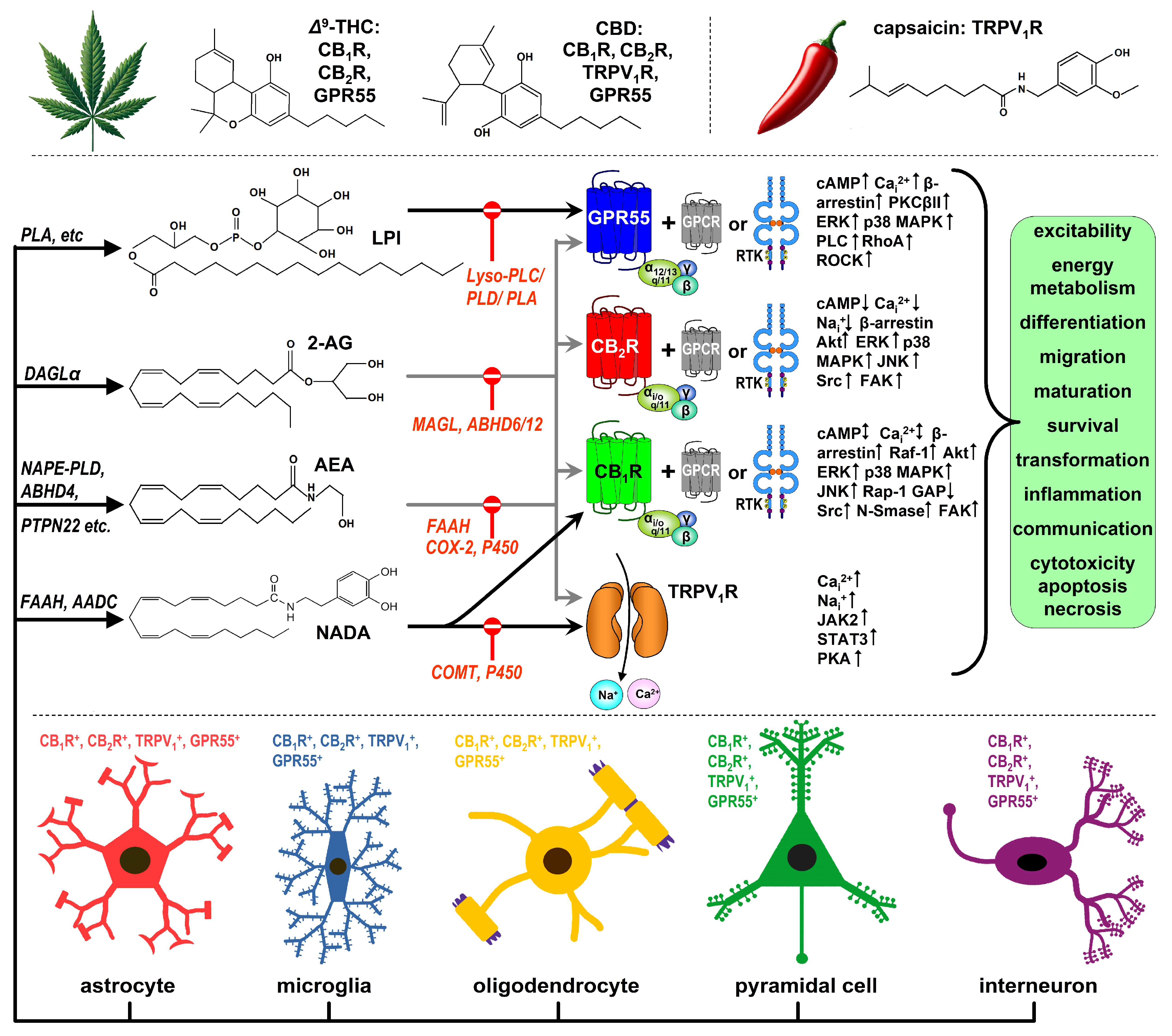
2. Cannabinoid Receptors and Brain Development
2.1. Cannabinoid Receptors and the Development of Brain Cytoarchitecture
2.2. Cannabinoid Receptors and the Development of Brain Circuitry
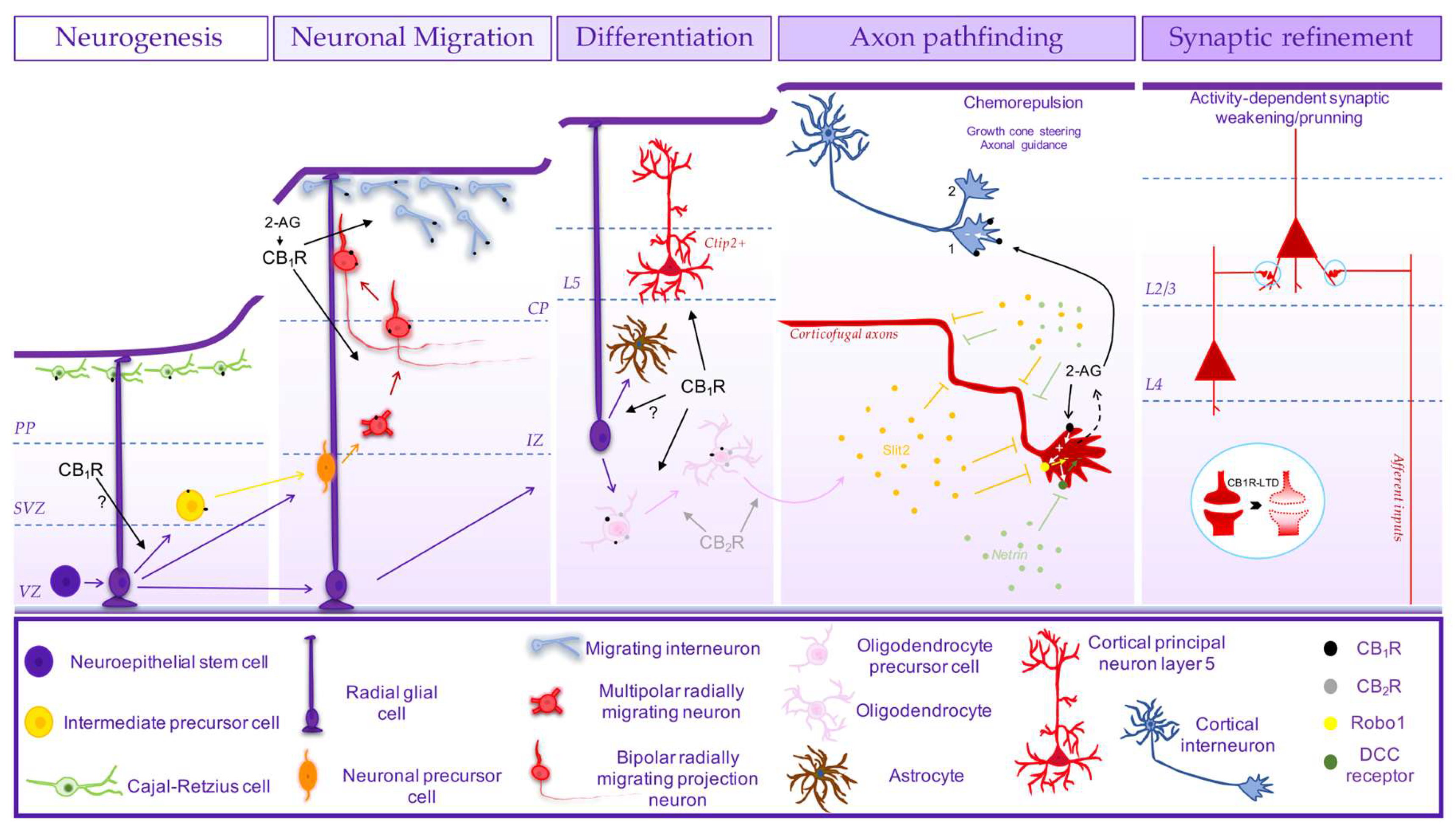
3. Cannabinoids and the Adolescent Brain
3.1. Animal Studies on the Role of the Endocannabinoid System in the Adolescent Brain
3.2. The Maturating Human Brain Is Vulnerable to Cannabinoids
- Positive Symptoms: CBD has been shown to ameliorate hyperlocomotion and stereotypies, which are proxies for positive symptoms like psychomotor agitation and hallucinations in schizophrenia. CBD may exert antipsychotic effects by normalizing dopamine signalling and counteracting Δ9-THC’s psychotomimetic effects.
- Negative Symptoms: There is evidence that CBD can improve social interaction deficits and reduce immobility in animal models of schizophrenia, suggesting it could treat negative symptoms such as social withdrawal, anhedonia, and lack of motivation.
- Cognitive Symptoms: CBD has shown promise in reversing cognitive deficits in preclinical models, particularly in memory and attention tasks. It has been shown to restore object recognition memory and working memory, likely by modulating PFC and hippocampal circuits.
4. Conclusions
| ReceptorEnzyme Ligand |
Function in the Adolescent Brain | Effects of External Cannabinoids | Consequences if Perturbed | Sex-Dependent Effects | References |
|---|---|---|---|---|---|
| CB1R | Regulates excitatory/inhibitory neurotransmission, synaptic pruning, and maturation of corticolimbic circuits (e.g., PFC, hippocampus). Peaks during adolescence, declines in adulthood. | Δ9-THC acts as a CB1R agonist. Chronic exposure downregulates CB1R, desensitizes receptors, impairs synaptic plasticity, and reduces dendritic complexity. | Persistent changes in PFC and hippocampal structure. Increased risk of psychiatric disorders (e.g., anxiety, schizophrenia). Impaired executive function, memory, and emotional regulation. | Greater CB1R density in males, more efficient CB1R coupling in females. More pronounced cognitive and emotional impairments in female rodents. Males show delayed onset of CB1R-mediated synaptic plasticity. | [206,214,254] |
| CB2R | Involved in immune regulation and neuroinflammation. Low neuronal expression in the brain but increases in microglia with neuroinflammation. | Chronic Δ9-THC exposure reduces CB2R density in adolescent brains. Selective acute CB2R activation (e.g., by AM1241) can reduce neuroinflammation, prevent anxiety-like behaviors during adolescence. | Chronic Δ9-THC exposure unequivocally downregulates CB2R expression, which may exacerbate anxiety and neuroinflammation caused by substance abuse or stress. | Two-fold greater CB2R expression in adolescent but not adult females. | [214,215,216] |
| TRPV1R | Involved in modulating stress and anxiety responses during adolescence. Opposes CB1R effects on anxiety regulation. | TRPV1R activation by CBD or stress can exacerbate anxiety responses. TRPV1R-dependent LTP in hippocampus may be linked to cognitive deficits caused by alcohol exposure. | Increased anxiety and cognitive deficits when activated by cannabinoids or stress. TRPV1 blockade may provide therapeutic potential for treating anxiety disorders. | Females show earlier onset of TRPV1-mediated synaptic plasticity. Male rodents show stronger anxiety-related responses to TRPV1 activation. | [214,218,219] |
| Δ9-THC | Partial agonist of the CB1R and the CB2R. Interferes with the maturation of corticolimbic circuits, synaptic pruning, and neuroplasticity during adolescence. | May lead to downregulation and desensitization of its receptors with chronic use. Disrupts synaptic plasticity, reduces dendritic complexity, and impairs signaling in the PFC and hippocampus. Triggers hypoGABAergic and hyperdopaminergic state. | Affects cortical thickness and wiring. Long-lasting cognitive impairments (e.g., memory, decision-making) and emotional dysregulation. Increases the risk of psychiatric disorders like anxiety, depression, and schizophrenia. | Females are more susceptible to Δ9-THC-induced emotional and cognitive impairments, showing greater downregulation of CB1R. Males tend to exhibit delayed onset of Δ9-THC-induced synaptic plasticity changes. | [208,229,230,243,244,254] |
| CBD | Negative allosteric modulator of CB1R and CB2R. Activates TRPV1R and inhibits eCB reuptake. Potential neuroprotective role during brain development. | Long-term CBD exposure can affect glutamatergic synapses and synaptic plasticity. May have neuroprotective effects but can also exacerbate disruptions in brain network connectivity when co-administered with Δ9-THC. | Reduction in GluA1 AMPA subunit and increased PSD95. Alters brain connectivity, especially when combined with Δ9-THC. No adverse effects on cognitive or motor functions in healthy adolescents. | Males may experience greater cognitive protection from CBD. Females show increased susceptibility to CBD’s effects on synaptic plasticity when combined with Δ9-THC. | [234,238,245] |
| FAAH | Breaks down anandamide. Controls anandamide levels and regulates emotional responses, stress, and cognitive functions. | Polymorphism FAAH C385A reduces enzyme activity, leading to elevated anandamide levels and altered stress responses. Chronic Δ9-Δ9-THC exposure interferes with FAAH activity, but reports are conflicting. | Reduced FAAH activity is associated with heightened emotional regulation and impulsivity, but can increase susceptibility to substance abuse and psychiatric disorders. | Females generally have lower FAAH expression during adolescence, leading to prolonged anandamide signaling. Males with FAAH C385A polymorphism show stronger reward-related acivity, impulsivity and risk-taking behaviors. | [214,215,240,241,242,255,256,257] |
| MAGL | Breaks down 2-AG. Regulates synaptic plasticity, excitatory/inhibitory balance, and emotional regulation. | Decrease in function during adolescence. Chronic Δ9-THC exposure reduces microglial but increases overall MAGL expression, leading to altered synaptic transmission. | Dysregulation of synaptic connections and plasticity in corticolimbic circuits. Long-term emotional and cognitive deficits. | Inhibiting MAGL uncover LTD in juvenile males. | [214,215,249,257] |
| DAGLα | Synthesizes 2-AG, essential for synaptic plasticity and connectivity in brain maturation. Peaks during adolescence. | Its expression peaks during adolescence. Δ9-THC can alter DAGL activity, affecting 2-AG synthesis and overall cannabinoid signaling during brain development. | Disruption in the production of 2-AG, leading to impaired synaptic connectivity, memory, and emotional regulation. | Females show earlier DAGL maturation and heightened synaptic plasticity, whereas males exhibit more delayed effects on synaptic development. | [201,249,257] |
| NAPE-PLD | Synthesizes anandamide, plays a role in regulating emotional and cognitive functions. | Gain of function during adolescence. Chronic Δ9-THC exposure can reduce NAPE-PLD expression in microglia, leading to altered anandamide production. | Impaired emotional regulation and stress response. Increased risk of psychiatric disorders due to disrupted anandamide signaling. | Females show higher baseline NAPE-PLD activity, contributing to sex differences in emotional regulation under stress or cannabinoid exposure. | [214,215,249] |
| ABHD6 | Degrades 2-AG, plays a role in regulating synaptic plasticity and emotional responses. | Chronic Δ9-THC exposure can increase ABHD6 expression in the placenta but not in the brain. ABHD6 expression is increased in the PFC of schizophrenic adolescents. | Dysregulated synaptic transmission and plasticity in corticolimbic circuits, leading to cognitive and emotional deficits. | Males show higher ABHD6 levels during adolescence, leading to stronger inhibition of 2-AG signaling compared to females. | [214,258,259] |
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Mechoulam, R.; Hanuš, L.O.; Pertwee, R.; Howlett, A.C. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat. Rev. Neurosci. 2014, 15(11), 757–764. [Google Scholar] [CrossRef] [PubMed]
- Solymosi, K.; Köfalvi, A. Cannabis: A treasure trove or Pandora’s box? Mini Rev. Med. Chem. 2017, 17(13), 1223–1291. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A.; Borrelli, F.; Capasso, R.; Di Marzo, V.; Mechoulam, R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009, 30(10), 515–527. [Google Scholar] [CrossRef]
- Sideris, A.; Doan, L.V. An Overview of Cannabidiol. Anesth. Analg. 2024, 138(1), 54–68. [Google Scholar] [CrossRef]
- Di Marzo, V.; Fontana, A.; Cadas, H.; Schinelli, S.; Cimino, G.; Schwartz, J.C.; Piomelli, D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 1994, 372(6507), 686–691. [Google Scholar] [CrossRef]
- Katona, I.; Freund, T.F. Multiple functions of endocannabinoid signaling in the brain. Annu. Rev. Neurosci. 2012, 35, 529–558. [Google Scholar] [CrossRef] [PubMed]
- Araque, A.; Castillo, P.E.; Manzoni, O.J.; Tonini, R. Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology 2017, 124, 13–24. [Google Scholar] [CrossRef]
- Bacci, A.; Huguenard, J.R.; Prince, D.A. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature 2004, 431(7006), 312–316. [Google Scholar] [CrossRef]
- Marinelli, S.; Pacioni, S.; Cannich, A.; Marsicano, G.; Bacci, A. Self-modulation of neocortical pyramidal neurons by endocannabinoids. Nat. Neurosci. 2009, 12(12), 1488–1490. [Google Scholar] [CrossRef]
- Den Boon, F.S.; Chameau, P.; Schaafsma-Zhao, Q.; van Aken, W.; Bari, M.; Oddi, S.; Kruse, C.G.; Maccarrone, M.; Wadman, W.J.; Werkman, T.R. Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc. Natl. Acad. Sci. USA 2012, 109(9), 3534–3539. [Google Scholar] [CrossRef]
- Stempel, A.V.; Stumpf, A.; Zhang, H.Y.; Özdoğan, T.; Pannasch, U.; Theis, A.K.; Otte, D.M.; Wojtalla, A.; Rácz, I.; Ponomarenko, A.; Xi, Z.X.; Zimmer, A.; Schmitz, D. Cannabinoid type 2 receptors mediate a cell type-specific plasticity in the hippocampus. Neuron 2016, 90(4), 795–809. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, A.; Parthier, D.; Sammons, R.P.; Stempel, A.V.; Breustedt, J.; Rost, B.R.; Schmitz, D. Cannabinoid type 2 receptors mediate a cell type-specific self-inhibition in cortical neurons. Neuropharmacology 2018, 139, 217–225. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19(3), 833. [Google Scholar] [CrossRef]
- Alhouayek, M.; Masquelier, J.; Muccioli, G.G. Lysophosphatidylinositols, from cell membrane constituents to GPR55 ligands. Trends Pharmacol. Sci. 2018, 39(6), 586–604. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.A.; Whalley, B.J. The proposed mechanisms of action of CBD in epilepsy. Epileptic Disord 2020, 22(S1), 10–15. [Google Scholar] [CrossRef]
- Katona, I.; Sperlágh, B.; Sík, A.; Köfalvi, A.; Vizi, E.S.; Mackie, K.; Freund, T.F. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J. Neurosci. 1999, 19(11), 4544–4558. [Google Scholar] [CrossRef]
- Katona, I.; Sperlágh, B.; Maglóczky, Z.; Sántha, E.; Köfalvi, A.; Czirják, S.; Mackie, K.; Vizi, E.S.; Freund, T.F. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience 2000, 100(4), 797–804. [Google Scholar] [CrossRef]
- Lutz, B. Neurobiology of cannabinoid receptor signaling. Dialogues Clin. Neurosci. 2020, 22(3), 207–222. [Google Scholar] [CrossRef]
- Callén, L.; Moreno, E.; Barroso-Chinea, P.; Moreno-Delgado, D.; Cortés, A.; Mallol, J.; Casadó, V.; Lanciego, J.L.; Franco, R.; Lluis, C.; Canela, E.I.; McCormick, P.J. Cannabinoid receptors CB1 and CB2 form functional heteromers in brain. J. Biol. Chem. 2012, 287(25), 20851–20865. [Google Scholar] [CrossRef]
- Ishiguro, H.; Kibret, B.G.; Horiuchi, Y.; Onaivi, E.S. Potential role of cannabinoid type 2 receptors in neuropsychiatric and neurodegenerative disorders. Front. Psychiatry 2022, 13, 828895. [Google Scholar] [CrossRef]
- Grabon, W.; Rheims, S.; Smith, J.; Bodennec, J.; Belmeguenai, A.; Bezin, L. CB2 receptor in the CNS: From immune and neuronal modulation to behavior. Neurosci. Biobehav. Rev. 2023, 150, 105226. [Google Scholar] [CrossRef] [PubMed]
- Harkany, T.; Guzmán, M.; Galve-Roperh, I.; Berghuis, P.; Devi, L.A.; Mackie, K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol. Sci. 2007, 28(2), 83–92. [Google Scholar] [CrossRef] [PubMed]
- Dalton, G.D.; Carney, S.T.; Marshburn, J.D.; Norford, D.C.; Howlett, A.C. CB1 Cannabinoid receptors stimulate Gβγ-GRK2-mediated FAK phosphorylation at tyrosine 925 to regulate ERK activation involving neuronal focal adhesions. Front. Cell. Neurosci. 2020, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Oyagawa, C.R.M.; Grimsey, N.L. Cannabinoid receptor CB1 and CB2 interacting proteins: Techniques, progress and perspectives. Methods Cell Biol. 2021, 166, 83–132. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Reggio, P.H. An update on non-CB1, non-CB2 cannabinoid related G-protein-coupled receptors. Cannabis Cannabinoid Res. 2017, 2(1), 265–273. [Google Scholar] [CrossRef]
- Navarro, G.; Varani, K.; Lillo, A.; Vincenzi, F.; Rivas-Santisteban, R.; Raïch, I.; Reyes-Resina, I.; Ferreiro-Vera, C.; Borea, P.A.; Sánchez de Medina, V.; Nadal, X.; Franco, R. Pharmacological data of cannabidiol- and cannabigerol-type phytocannabinoids acting on cannabinoid CB1, CB2 and CB1/CB2 heteromer receptors. Pharmacol. Res. 2020, 159, 104940. [Google Scholar] [CrossRef]
- Dalton, G.D.; Howlett, A.C. Cannabinoid CB1 receptors transactivate multiple receptor tyrosine kinases and regulate serine/threonine kinases to activate ERK in neuronal cells. Br. J. Pharmacol. 2012, 165(8), 2497–2511. [Google Scholar] [CrossRef]
- Berghuis, P.; Dobszay, M.B.; Wang, X.; Spano, S.; Ledda, F.; Sousa, K.M.; Schulte, G.; Ernfors, P.; Mackie, K.; Paratcha, G.; Hurd, Y.L.; Harkany, T. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc. Natl. Acad. Sci. USA 2005, 102(52), 19115–19120. [Google Scholar] [CrossRef]
- Baker, D.; Pryce, G.; Davies, W.L.; Hiley, C.R. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol. Sci. 2006, 27(1), 1–4. [Google Scholar] [CrossRef]
- Kapur, A.; Zhao, P.; Sharir, H.; Bai, Y.; Caron, M.G.; Barak, L.S.; Abood, M.E. Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands. J. Biol. Chem. 2009, 284(43), 29817–29827. [Google Scholar] [CrossRef]
- Henstridge, C.M.; Balenga, N.A.; Schröder, R.; Kargl, J.K.; Platzer, W.; Martini, L.; Arthur, S.; Penman, J.; Whistler, J.L.; Kostenis, E.; Waldhoer, M.; Irving, A.J. GPR55 ligands promote receptor coupling to multiple signalling pathways. Br. J. Pharmacol. 2010, 160(3), 604–614. [Google Scholar] [CrossRef]
- Ross, R.A. L-α-lysophosphatidylinositol meets GPR55: A deadly relationship. Trends Pharmacol. Sci. 2011, 32(5), 265–269. [Google Scholar] [CrossRef] [PubMed]
- Calvillo-Robledo, A.; Cervantes-Villagrana, R.D.; Morales, P.; Marichal-Cancino, B.A. The oncogenic lysophosphatidylinositol (LPI)/GPR55 signaling. Life Sci. 2022, 301, 120596. [Google Scholar] [CrossRef]
- He, Y.; Shen, H.; Bi, G.H.; Zhang, H.Y.; Soler-Cedeño, O.; Alton, H.; Yang, Y.; Xi, Z.X. GPR55 is expressed in glutamate neurons and functionally modulates drug taking and seeking in rats and mice. Transl. Psychiatry 2024, 14(1), 101. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Szállási, Á. Transient receptor potential channels as drug targets: From the science of basic research to the art of medicine. Pharmacol. Rev. 2014, 66(3), 676–814. [Google Scholar] [CrossRef]
- Muller, C.; Reggio, P.H. An analysis of the putative CBD binding site in the ionotropic cannabinoid receptors. Front. Cell. Neurosci. 2020, 14, 615811. [Google Scholar] [CrossRef]
- Nagy, I.; White, J.P.M.; Paule, C.C.; Maze, M.; Urban, L. Functional molecular biology of the TRPV1 ion channel. In Cannabinoids and the Brain; Köfalvi, A., Ed.; Springer: New York, USA, 2007; pp. 101–130. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Eilers, H. TRPV1 splice variants: Structure and function. Front. Biosci. 2010, 15(3), 872–882. [Google Scholar] [CrossRef]
- Vos, M.H.; Neelands, T.R.; McDonald, H.A.; Choi, W.; Kroeger, P.E.; Puttfarcken, P.S.; Faltynek, C.R.; Moreland, R.B.; Han, P. TRPV1b overexpression negatively regulates TRPV1 responsiveness to capsaicin, heat and low pH in HEK293 cells. J. Neurochem. 2006, 99(4), 1088–1102. [Google Scholar] [CrossRef] [PubMed]
- Köles, L.; Garção, P.; Zádori, Z.S.; Ferreira, S.G.; Pinheiro, B.S.; da Silva-Santos, C.S.; Ledent, C.; Köfalvi, A. Presynaptic TRPV1 vanilloid receptor function is age- but not CB1 cannabinoid receptor-dependent in the rodent forebrain. Brain Res. Bull. 2013, 97, 126–135. [Google Scholar] [CrossRef]
- Alger, B.E. Endocannabinoids at the synapse a decade after the dies mirabilis (29 March 2001): What we still do not know. J. Physiol. 2012, 590(10), 2203–2212. [Google Scholar] [CrossRef]
- Murataeva, N.; Straiker, A.; Mackie, K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br. J. Pharmacol. 2014, 171(6), 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Fukaya, M.; Uchigashima, M.; Miura, E.; Kamiya, H.; Kano, M.; Watanabe, M. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J. Neurosci. 2006, 26(18), 4740–4751. [Google Scholar] [CrossRef] [PubMed]
- Mulder, J.; Zilberter, M.; Pasquaré, S.J.; Alpár, A.; Schulte, G.; Ferreira, S.G.; Köfalvi, A.; Martín-Moreno, A.M.; Keimpema, E.; Tanila, H.; Watanabe, M.; Mackie, K.; Hortobágyi, T.; de Ceballos, M.L.; Harkany, T. Molecular reorganization of endocannabinoid signalling in Alzheimer’s disease. Brain 2011, 134(4), 1041–1060. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 2012, 70-81, 70–81. [Google Scholar] [CrossRef]
- Savinainen, J.R.; Saario, S.M.; Laitinen, J.T. The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol. (Oxf.) 2012, 204(2), 267–276. [Google Scholar] [CrossRef]
- Maccarrone, M.; Di Marzo, V.; Gertsch, J.; Grether, U.; Howlett, A.C.; Hua, T.; Makriyannis, A.; Piomelli, D.; Ueda, N.; van der Stelt, M. Goods and bads of the endocannabinoid system as a therapeutic target: Lessons learned after 30 years. Pharmacol. Rev. 2023, 75(5), 885–958. [Google Scholar] [CrossRef]
- Blankman, J.L.; Cravatt, B.F. Chemical probes of endocannabinoid metabolism. Pharmacol. Rev. 2013, 65(2), 849–871. [Google Scholar] [CrossRef]
- Simon, G.M.; Cravatt, B.F. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J. Biol. Chem. 2008, 283(14), 9341–9349. [Google Scholar] [CrossRef]
- Maccarrone, M. Metabolism of the endocannabinoid anandamide: Open questions after 25 years. Front. Mol. Neurosci. 2017, 10, 166. [Google Scholar] [CrossRef]
- Wei, B.Q.; Mikkelsen, T.S.; McKinney, M.K.; Lander, E.S.; Cravatt, B.F. A second fatty acid amide hydrolase with variable distribution among placental mammals. J. Biol. Chem. 2006, 281(48), 36569–36578. [Google Scholar] [CrossRef]
- Moreno-Luna, R.; Esteban, P.F.; Paniagua-Torija, B.; Arevalo-Martin, A.; Garcia-Ovejero, D.; Molina-Holgado, E. Heterogeneity of the endocannabinoid system between cerebral cortex and spinal cord oligodendrocytes. Mol. Neurobiol. 2021, 58(2), 689–702. [Google Scholar] [CrossRef] [PubMed]
- Grabiec, U.; Dehghani, F. N-Arachidonoyl dopamine: A novel endocannabinoid and endovanilloid with widespread physiological and pharmacological activities. Cannabis Cannabinoid Res. 2017, 2(1), 183–196. [Google Scholar] [CrossRef] [PubMed]
- Paria, B.C.; Wangm, H.; Dey, S.K. Endocannabinoid signaling in synchronizing embryo development and uterine receptivity for implantation. Chem. Phys. Lipids 2002, 121, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dey, S.K. Lipid signaling in embryo implantation. Prostaglandins Other Lipid. Mediat. 2004, 77, 84–102. [Google Scholar] [CrossRef]
- Sun, X. & Dey, K. Aspects of encocannabinoid signaling in periimplantation biology. Mol. Cell. Endocrinol. 2008, 286S, S3–S11. [Google Scholar] [CrossRef]
- Macarrone, M. CB2 receptors in reproduction. Br. J. Pharmacol. 2008, 153, 189–198. [Google Scholar] [CrossRef]
- Correa, F.; Wolfson, M.L.; Valchi, P.; Aisemberg, J.; Franchi, A.M. Endocannabinoid system and pregnancy. Reprod. 2016, 152, R191–R200. [Google Scholar] [CrossRef]
- Innocenzi, E.; De Domenico, E.; Ciccarone, F.; Zampieri, M.; Rossi, G.; Cicconi, R.; Bernardini, R.; Mattei, M.; Grimaldi, P. Paternal activation of CB2 cannabinoid receptor impairs placental and embryonic growth via an epigenetic mechanism. Sci. Rep. 2019, 9, 17034. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Paria, B.C.; Dey, S.K.; Armant, D.R. Stage-specific excitation of cannabinoid receptor exhibits differential effects on mouse embryonic development. Biol. Reprod. 1999, 60, 839–844. [Google Scholar] [CrossRef]
- Taylor, A.H.; Ang, C.; Bell, S.C.; Konje, J.C. The role of the endocannabinoid system in gametogenesis implantation and early pregnancy. Hum. Reprod. Update 2007, 13(5), 501–513. [Google Scholar] [CrossRef]
- Habayeb, O.M.H.; Taylor, A.H.; Bell, S.C.; Taylor, D.J.; Konje, J.C. Expression of the endocannabinoid system in human first trimester placenta and its role in trophoblast proliferation. Endocrinol. 2008, 149(10), 5052–5060. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xie, H.; Yang, J.; Wang, H.; Bradshaw, H.B.; Dey, S.K. Endocannabinoid signaling directs differentiation of trophoblast cell lineages and placentation. Proc. Natl. Acad. Sci. U S A 2010, 107(39), 16887–16892. [Google Scholar] [CrossRef]
- Jiang, S.; Fu, Y.; Williams, J.; Wood, J.; Pandarinathan, L.; Avraham, S.; Makriyannis, A.; Avraham, S.; Avraham, H.K. Expression and function of cannabinoid receptors CB1 and CB2 and their cognate cannabinoid ligands in murine embryonic stem cells. PLoS ONE 2007, 2(7), e641. [Google Scholar] [CrossRef]
- Sharov, A.; Piao, Y.; Matoba, R.; Dudekula, D.B.; Qian, Y.; VanBuren, V.; Falco, G.; Martin, P.R.; Stagg, C.A.; Bassey, U. C.; Wang, Y.; Carter, M. G.; Hamatani, T.; Aiba, K.; Akutsu, H.; Sharova, L.; Tanaka, T. S.; Kimber, W. L.; Yoshikawa, T.; Jaradat, S. A.; Pantano, S.; Nagaraja, R.; Boheler, K. R.; Taub, D.; Hodes, R. J.; Longo, D. L.; Schlessinger, D.; Keller, J.; Klotz, E.; Kelsoe, G.; Umezawa, A.; Vescovi, A. L.; Rossant, J.; Kunath, T.; Hogan, B.L.M.; Curci, A.; D’Urso, M.; Kelso, J.; Hide, W.; Ko, M.S.H. Transcriptome analysis of mouse stem cells and early embryos. PLoS Biol. 2003, 1(3), e74. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.; Tedesco, M.; Battista, N.; Pasquariello, N.; Pucci, M; Gasperi, V.; Scaldaferri, M.L.; Farini, D.; De Felici, M.; Maccarrone, M. Characterization of the endocannabinoid system in mouse embryonic stem cells. Stem Cells Dev 2011, 20, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Nones, J.; Spohr, T.C.; Furtado, D.R.; Sartore, R.C.; Paulsen, B.S.; Guimarães, M.Z.; Rehen, S.K. Cannabinoids modulate cell survival in embryoid bodies. Cell Biol. Int. 2010, 34(4), 399–408. [Google Scholar] [CrossRef]
- Oh, H.A.; Kwon, S.; Choi, S.; Shin, H.; Yoon, K.H.; Kim, W.J.; Lim, H.J. Uncovering a role for endocannabinoid signaling in autophagy in preimplantation mouse embryos. Mol. Hum. Reprod. 2013, 19(2), 93–101. [Google Scholar] [CrossRef]
- Galve-Roperh, I.; Chiurchiù, V.; Díaz-Alonso, J.; Bari, M.; Guzmán, M.; Maccarrone, M. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog. Lip. Res. 2013, 52, 633–650. [Google Scholar] [CrossRef]
- Psychoyos, D.; Hungund, B.; Cooper, T.; Finneli, R. A cannabinoid analogue of Δ9-tetrahydrocannabinol disrupts neural development in chick. Birth Defects Res. 2008, 83, 477–488. [Google Scholar] [CrossRef]
- Ahmed, K.T.; Amin, M.R.; Shah, P.; Ali, D.W. Motor neuron development in zebrafish is altered by brief (5-hr) exposures to THC (∆9-tetrahydrocannabinol) or CBD (cannabidiol) during gastrulation. Sci. Rep. 2018, 8(1), 10518. [Google Scholar] [CrossRef]
- Amin, M.R.; Ahmed, K.T.; Ali, D.W. Early exposure to THC alters M-cell development in zebrafish embryos. Biomedicines 2020, 8(1), 5. [Google Scholar] [CrossRef] [PubMed]
- Kanyo, R.; Amin, M.R.; Locskai, L.F.; Bouvier, D.; Olthuis, A.M.; Allison, W.T.; Ali, D.W. Medium-throughput zebrafish optogenetic platform identifies deficits in subsequent neural activity following brief early exposure to cannabidiol and Δ9-tetrahydrocannabinol. Sci. Rep. 2021, 11, 11515. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.R.; Ahmed, K.T.; Ali, D.W. Cannabinoid receptor 2(Cb2r) mediates cannabinol (CBN) induced developmental defects in zebrafish. Sci. Rep. 2022, 12, 20251. [Google Scholar] [CrossRef] [PubMed]
- Khaliullina, H.; Bilgin, M.; Sampaio, J.L.; Shevchenko, A.; Eaton, S. Endocannabinoids are conserved inhibitors of the Hedgehog pathway. Proc. Natl. Acad. Sci. U S A 2015, 112(11), 3415–3420. [Google Scholar] [CrossRef] [PubMed]
- Boa-Amponsem, O.; Zhang, C.; Burton, D.; Williams, K.P.; Cole, G.J. Ethanol and cannabinoids regulate zebrafish GABAergic neuron development and behavior in a Sonic Hedgehog and Fibroblast Growth Factor-dependent mechanism. Alcohol Clin. Exp. Res. 2020, 44(7), 1366–1377. [Google Scholar] [CrossRef]
- Begbie, J.; Doherty, P.; Graham, A. Cannabinoid receptor, CB1, expression follows neuronal differentiation in the early chick embryo. J. Anat. 2004, 205(3), 213–218. [Google Scholar] [CrossRef]
- Psychoyos, D.; Vinod, K.Y.; Cao, J.; Xie, S.; Hyson, R.L.; Wlodarczyk, B.; He, W.; Cooper, T.B.; Hungund, B.L.; Finnell, R.H. Cannabinoid receptor 1 signaling in embryo neurodevelopment. Birth Defects Res. 2012, 95(2), 137–50. [Google Scholar] [CrossRef]
- Migliarini, B.; Carnevali, O. A novel role for the endocannabinoid system during zebrafish development. Mol. Cell Endocrinol. 2009, 299(2), 172–177. [Google Scholar] [CrossRef]
- Son, H.W.; Ali, D.W. Endocannabinoid receptor expression in early zebrafish development. Dev. Neurosci. 2022, 44(3), 142–152. [Google Scholar] [CrossRef]
- Buckley, N.E.; Hansson, S.; Harta, G.; Mezey, E. Expression of the CB1 and CB2 receptor messenger RNAs during embryonic development in the rat. Neuroscience 1998, 82(4), 1131–49. [Google Scholar] [CrossRef]
- Mulder, J.; Aguado, T.; Keimpema, E.; Barabás, K.; Ballester-Rosado, C.J.; Nguyen, L.; Monory, K.; Marsicano, G.; Di Marzo, V.; Hurd, Y.L.; Guillemot, F.; Mackie, K.; Lutz, B.; Guzmán, M.; Lu, H.C.; Galve-Roperh, I.; Harkany, T. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc. Natl. Acad. Sci. U S A 2008, 105(25), 8760–8765. [Google Scholar] [CrossRef] [PubMed]
- Vitalis, T.; Lainé, J.; Simon, A.; Roland, A.; Leterrier, C.; Lenkei, Z. The type 1 cannabinoid receptor is highly expressed in embryonic cortical projection neurons and negatively regulates neurite growth in vitro. Eur. J. Neurosci. 2008, 28(9), 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Alonso, J.; Aguado, T.; Wu, CS.; Palazuelos, J.; Hofmann, C.; Garcez, P.; Guillemot, F.; Lu, H.C.; Lutz, B.; Guzmán, M.; Galve-Roperh, I. The CB(1) cannabinoid receptor drives corticospinal motor neuron differentiation through the Ctip2/Satb2 transcriptional regulation axis. J. Neurosci. 2012, 32(47), 16651–16665. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.; Chambers, D.; Hobbs, C.; Doherty, P.; Graham, A. The endocannabinoid receptor, CB1, is required for normal axonal growth and fasciculation. Mol. Cell. Neurosci. 2008, 38(1), 89–97. [Google Scholar] [CrossRef]
- Berrendero, F.; Sepe, N.; Ramos, J.A.; Di Marzo, V.; Fernández-Ruiz, J.J. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse 1999, 33(3), 181–191. [Google Scholar] [CrossRef]
- Aguado, T.; Monory, K.; Palazuelos, J.; Stella, N.; Cravatt, B.; Lutz, B.; Marsicano, G.; Kokaia, Z.; Guzmán, M.; Galve-Roperh, I. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005, 19(12), 1704–1706. [Google Scholar] [CrossRef]
- Palazuelos, J.; Ortega, Z.; Díaz-Alonso, J.; Guzmán, M.; Galve-Roperh, I. CB2 cannabinoid receptors promote neural progenitor cell proliferation via mTORC1 signaling. J. Biol. Chem. 2012, 287(2), 1198–209. [Google Scholar] [CrossRef]
- Compagnucci, C.; Di Siena, S.; Bustamante, M.B.; Di Giacomo, D.; Di Tommaso, M.; Maccarrone, M.; Grimaldi, P.; Sette, C. Type-1 (CB1) cannabinoid receptor promotes neuronal differentiation and maturation of neural stem cells. PLoS ONE 2013, 8(1), e54271. [Google Scholar] [CrossRef]
- Morozov, Y.M.; Torii, M.; Rakic, P. Origin, early commitment, migratory routes, and destination of cannabinoid type 1 receptor-containing interneurons. Cereb. Cortex 2009, 19 (Suppl 1), i78–89. [Google Scholar] [CrossRef]
- Díaz-Alonso, J.; Aguado, T.; de Salas-Quiroga, A.; Ortega, Z.; Guzmán, M.; Galve-Roperh, I. CB1 cannabinoid receptor-dependent activation of mTORC1/Pax6 signaling drives Tbr2 expression and basal progenitor expansion in the developing mouse cortex. Cereb. Cortex 2015, 25(9), 2395–2408. [Google Scholar] [CrossRef]
- Morozov, Y.M.; Mackie, K.; Rakic, P. Cannabinoid type 1 receptor is undetectable in rodent and primate cerebral neural stem cells but participates in radial neuronal migration. Int. J. Mol. Sci. 2020, 21(22), 8657. [Google Scholar] [CrossRef] [PubMed]
- Keimpema, E.; Barabas, K.; Morozov, Y.M.; Tortoriello, G.; Torii, M.; Cameron, G.; Yanagawa, Y.; Watanabe, M.; Mackie, K.; Harkany, T. Differential subcellular recruitment of monoacylglycerol lipase generates spatial specificity of 2-arachidonoyl glycerol signaling during axonal pathfinding. J. Neurosci. 2010, 30(42), 13992–134007. [Google Scholar] [CrossRef] [PubMed]
- Saez, T.M.; Aronne, M.P.; Caltana, L.; Brusco, A.H. Prenatal exposure to the CB1 and CB2 cannabinoid receptor agonist WIN 55,212-2 alters migration of early-born glutamatergic neurons and GABAergic interneurons in the rat cerebral cortex. J Neurochem. 2014, 129(4), 637–648. [Google Scholar] [CrossRef] [PubMed]
- Zurolo, E.; Iyer, A.M.; Spliet, W.G.; Van Rijen, P.C.; Troost, D.; Gorter, J.A.; Aronica, E. CB1 and CB2 cannabinoid receptor expression during development and in epileptogenic developmental pathologies. Neuroscience 2010, 170(1), 28–41. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Alonso, J.; de Salas-Quiroga, A.; Paraíso-Luna, J.; García-Rincón, D.; Garcez, P.P.; Parsons, M.; Andradas, C.; Sánchez, C.; Guillemot, F.; Guzmán, M.; Galve-Roperh, I. Loss of cannabinoid CB1 receptors induces cortical migration malformations and increases seizure susceptibility. Cereb. Cortex 2017, 27(11), 5303–5317. [Google Scholar] [CrossRef]
- Morozov, Y.M.; Freund, T.F. Post-natal development of type 1 cannabinoid receptor immunoreactivity in the rat hippocampus. Eur. J. Neurosci. 2003, 18(5), 1213–1222. [Google Scholar] [CrossRef]
- Berghuis, P.; Rajnicek, A.M.; Morozov, Y.M.; Ross, R.A.; Mulder, J.; Urbán, G.M.; Monory, K.; Marsicano, G.; Matteoli, M.; Canty, A.; Irving, A.J.; Katona, I.; Yanagawa, Y.; Rakic, P.; Lutz, B.; Mackie, K.; Harkany, T. Hardwiring the brain: Endocannabinoids shape neuronal connectivity. Science 2007, 316(5828), 1212–1216. [Google Scholar] [CrossRef]
- Paraíso-Luna, J.; Aguareles, J.; Martín, R.; Ayo-Martín, A.C.; Simón-Sánchez, S.; García-Rincón, D.; Costas-Insua, C.; García-Taboada, E.; de Salas-Quiroga, A.; Díaz-Alonso, J.; Liste, I.; Sánchez-Prieto, J.; Cappello, S.; Guzmán, M.; Galve-Roperh, I. Endocannabinoid signalling in stem cells and cerebral organoids drives differentiation to deep layer projection neurons via CB1 receptors. Development 2020, 147(24), dev192161. [Google Scholar] [CrossRef]
- Wu, C.S.; Zhu, J.; Wager-Miller, J.; Wang, S.; O’Leary, D.; Monory, K.; Lutz, B.; Mackie, K.; Lu, H.C. Requirement of cannabinoid CB(1) receptors in cortical pyramidal neurons for appropriate development of corticothalamic and thalamocortical projections. Eur. J. Neurosci. 2010, 32(5), 693–706. [Google Scholar] [CrossRef]
- Barnes, J.L.; Mohr, C.; Ritchey, C.R.; Erikson, C.M.; Shiina, H.; Rossi, D.J. Developmentally transient CB1Rs on cerebellar afferents suppress afferent input, dowstream synaptic excitation, and signaling to migrating neurons. J. Neurosci. 2020, 40(32), 6133–6145. [Google Scholar] [CrossRef]
- Martinez, L.R.; Black, K.C.; Webb, B.T.; Bell, A.; Baygani, S.K.; Mier, T.J.; Dominguez, L.; Mackie, K.; Kalinovsky, A. Components of endocannabinoid signaling system are expressed in the perinatal mouse cerebellum and required for its normal development. eNeuro 2020, 7(2), 0471–0519. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.; Hernández, M.L.; Pazos, M.R.; Tolón, R.M.; Romero, J.; Fernández-Ruiz, J. Colocalization of CB1 receptors with L1 and GAP-43 in forebrain white matter regions during fetal rat brain development: Evidence for a role of these receptors in axonal growth and guidance. Neuroscience 2008, 153(3), 687–699. [Google Scholar] [CrossRef]
- Biegon, A.; Kerman, I.A. Autoradiographic study of pre- and postnatal distribution of cannabinoid receptors in human brain. Neuroimage 2001, 14(6), 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Mato, S.; Del Olmo, E.; Pazos, A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur. J. Neurosci. 2003, 17(9), 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dow-Edwards, D.; Keller, E.; Hurd, Y.L. Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience 2003, 118(3), 681–694. [Google Scholar] [CrossRef] [PubMed]
- Keimpema, E.; Tortoriello, G.; Alpár, A.; Capsoni, S.; Arisi, I.; Calvigioni, D.; Hu, S.S.; Cattaneo, A.; Doherty, P.; Mackie, K.; Harkany, T. Nerve growth factor scales endocannabinoid signaling by regulating monoacylglycerol lipase turnover in developing cholinergic neurons. Proc. Natl. Acad. Sci. USA 2013, 110(5), 1935–1940. [Google Scholar] [CrossRef] [PubMed]
- Berrendero, F.; Sepe, N.; Ramos, J.A.; Di Marzo, V.; Fernández-Ruiz, J.J. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse 1999, 33(3), 181–191. [Google Scholar] [CrossRef]
- Paria, B.C.; Song, H.; Wang, X.; Schmid, P.C.; Krebsbach, R.J.; Schmid, H.H.; Bonner, T.I.; Zimmer, A.; Dey, S.K. Dysregulated cannabinoid signaling disrupts uterine receptivity for embryo implantation. J. Biol. Chem. 2001, 276(23), 20523–20528. [Google Scholar] [CrossRef]
- Bisogno, T.; Howell, F.; Williams, G.; Minassi, A.; Cascio, M.G.; Ligresti, A.; Matias, I.; Schiano-Moriello, A.; Paul, P.; Williams, E.J.; Gangadharan, U.; Hobbs, C.; Di Marzo, V.; Doherty, P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell. Biol. 2003, 163(3), 463–468. [Google Scholar] [CrossRef]
- Keimpema, E.; Alpár, A.; Howell, F.; Malenczyk, K.; Hobbs, C.; Hurd, Y.L.; Watanabe, M.; Sakimura, K.; Kano, M.; Doherty, P.; Harkany, T. Diacylglycerol lipase α manipulation reveals developmental roles for intercellular endocannabinoid signaling. Sci. Rep. 2013, 3, 2093. [Google Scholar] [CrossRef]
- Palazuelos, J.; Aguado, T.; Egia, A.; Mechoulam, R.; Guzmán, M.; Galve-Roperh, I. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006, 20(13), 2405–2407. [Google Scholar] [CrossRef] [PubMed]
- Molina-Holgado, F.; Rubio-Araiz, A.; García-Ovejero, D.; Williams, R.J.; Moore, J.D.; Arévalo-Martín, A.; Gómez-Torres, O.; Molina-Holgado, E. CB2 cannabinoid receptors promote mouse neural stem cell proliferation. Eur. J. Neurosci. 2007, 25(3), 629–634. [Google Scholar] [CrossRef]
- Duff, G.; Argaw, A.; Cecyre, B.; Cherif, H.; Tea, N.; Zabouri, N.; Casanova, C.; Ptito, M.; Bouchard, J.F. Cannabinoid receptor CB2 modulates axon guidance. PLoS ONE 2013, 8(8), e70849. [Google Scholar] [CrossRef] [PubMed]
- Alpár, A.; Tortoriello, G.; Calvigioni, D.; Niphakis, M.J.; Milenkovic, I.; Bakker, J.; Cameron, G.A.; Hanics, J.; Morris, C.V.; Fuzik, J.; Kovacs, G.G.; Cravatt, B.F.; Parnavelas, J.G.; Andrews, W.D.; Hurd, Y.L; Keimpema, E.; Harkany, T. Endocannabinoids modulate cortical development by configuring Slit2/Robo1 signalling. Nat. Commun. 2014, 5, 4421. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, D.J.; Chesler, A.T.; Jackson, A.C.; Sigal, Y.M.; Yamanaka, H.; Grant, R.; O’Donnell, D.; Nicoll, R.A.; Shah, N.M.; Julius, D.; Basbaum, A.I. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J. Neurosci. 2011, 31(13), 5067–5077. [Google Scholar] [CrossRef]
- Perchuk, A.; Bierbower, S.M.; Canseco-Alba, A.; Mora, Z.; Tyrell, L.; Joshi, N.; Schanz, N.; Gould, G.G.; Onaivi, E.S. Developmental and behavioral effects in neonatal and adult mice following prenatal activation of endocannabinoid receptors by capsaicin. Acta Pharmacol. Sin. 2019, 40(3), 418–424. [Google Scholar] [CrossRef]
- Cherif, H.; Argaw, A.; Cécyre, B.; Bouchard, A.; Gagnon, J.; Javadi, P.; Desgent, S.; Mackie, K.; Bouchard, J.F. Role of GPR55 during axon growth and target innervation. eNeuro 2015, 2, ENEURO.0011-15.2015. [Google Scholar] [CrossRef]
- de Salas-Quiroga, A.; Díaz-Alonso, J.; García-Rincón, D.; Remmers, F.; Vega, D.; Gómez-Cañas, M.; Lutz, B.; Guzmán, M.; Galve-Roperh, I. Prenatal exposure to cannabinoids evokes long-lasting functional alterations by targeting CB1 receptors on developing cortical neurons. Proc. Natl. Acad. Sci. U S A 2015, 112(44), 13693–13698. [Google Scholar] [CrossRef]
- Rogers, S.A.; Kempen, T.A.; Pickel, V.M.; Milner, T.A. Enkephalin levels and the number of neuropeptide Y-containing interneurons in the hippocampus are decreased in female cannabinoid-receptor 1 knock-out mice. Neurosci. Lett. 2016, 620, 97–103. [Google Scholar] [CrossRef]
- de Salas-Quiroga, A.; García-Rincón, D.; Gómez-Domínguez, D.; Valero, M.; Simón-Sánchez, S.; Paraíso-Luna, J.; Aguareles, J.; Pujadas, M.; Muguruza, C.; Callado, L.F.; Lutz, B.; Guzmán, M.; de la Prida, L.M.; Galve-Roperh, I. Long-term hippocampal interneuronopathy drives sex-dimorphic spatial memory impairment induced by prenatal THC exposure. Neuropsychopharmacology 2020, 45(5), 877–886. [Google Scholar] [CrossRef]
- Rubio-Araiz, A.; Arévalo-Martín, A.; Gómez-Torres, O.; Navarro-Galve, B.; García-Ovejero, D.; Suetterlin, P.; Sánchez-Heras, E.; Molina-Holgado, E.; Molina-Holgado, F. The endocannabinoid system modulates a transient TNF pathway that induces neural stem cell proliferation. Mol. Cell Neurosci. 2008, 38, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Trazzi, S.; Steger, M.; Mitrugno, V.M.; Bartesaghi, R.; Ciani, E. CB1 cannabinoid receptors increase neuronal precursor proliferation through AKT/glycogen synthase kinase-3beta/beta-catenin signaling. J. Biol. Chem. 2010, 285(13), 10098–10109. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Xie, L.; Kim, S.H.; Parmentier-Batteur, S.; Sun, Y.; Mao, X.O.; Childs, J.; Greenberg, D.A. Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol. Pharmacol. 2004, 66(2), 204–208. [Google Scholar] [CrossRef] [PubMed]
- Aguado, T.; Palazuelos, J.; Monory, K.; Stella, N.; Cravatt, B.; Lutz, B.; Marsicano, G.; Kokaia, Z.; Guzmán, M.; Galve-Roperh, I. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J. Neurosci. 2006, 26(5), 1551–1561. [Google Scholar] [CrossRef]
- Rueda, D.; Navarro, B.; Martinez-Serrano, A.; Guzman, M.; Galve-Roperh, I. The endocannabinoid anandamide inhibits neuronal progenitor cell differentiation through attenuation of the Rap1/B-Raf/ERK pathway. J. Biol. Chem. 2002, 277(48), 46645–46650. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, Y.; Xiao, L.; Van Cleemput, J.; Ji, S.P.; Bai, G.; Zhang, X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J. Clin. Invest. 2005, 115, 3104–3116. [Google Scholar] [CrossRef]
- Soltys, J.; Yushak, M.; Mao-Draayer, Y. Regulation of neural progenitor cell fate by anandamide. Biochem. Biophys. Res. Commun. 2010, 400(1), 21–26. [Google Scholar] [CrossRef]
- Hill, J.D.; Zuluaga-Ramirez, V.; Gajghate, S.; Winfield, M.; Persidsky, Y. Activation of GPR55 increases neural stem cell proliferation and promotes early adult hippocampal neurogenesis. Br. J. Pharmacol. 2018, 175(16), 3407–3421. [Google Scholar] [CrossRef]
- Abbas-Farishta, R.; Robert, C.; Turcot, O.; Thomas, S.; Vanni, M.P.; Bouchard, J.F.; Casanova, C. Impact of CB1 receptor deletion on visual responses and organization of primary visual cortex in adult mice. Invest. Ophthalmol. Vis. Sci. 2015, 56(13), 7697–7707. [Google Scholar] [CrossRef]
- Hedrich, J.; Angamo, E.A.; Conrad, A.; Lutz, B.; Luhmann, H.J. Cell type specific impact of cannabinoid receptor signaling in somatosensory barrel map formation in mice. J. Comp. Neurol. 2020, 528(1), 3–13. [Google Scholar] [CrossRef]
- Tortoriello, G.; Morris, C.V.; Alpar, A.; Fuzik, J.; Shirran, S.L.; Calvigioni, D.; Keimpema, E.; Botting, C.H.; Reinecke, K.; Herdegen, T.; Courtney, M.; Hurd, Y.L.; Harkany, T. Miswiring the brain: Δ9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J. 2014, 33(7), 668–685. [Google Scholar] [CrossRef] [PubMed]
- Turunen, P.M.; Louhivuori, L.M.; Louhivuori, V.; Kukkonen, J.P.; Åkerman, K.E. Endocannabinoid signaling in embryonic neuronal motility and cell-cell contact - Role of mGluR5 and TRPC3 Channels. Neuroscience 2018, 375, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, Y.; Hisanaga, S.; Heizmann, C.W.; Murakami, F. Distinct migratory behavior of early- and late-born neurons derived from the cortical ventricular zone. J. Comp. Neurol. 2004, 479(1), 1–14. [Google Scholar] [CrossRef]
- Calderon de Anda, F.; Gärtner, A.; Tsai, L.H.; Dotti, C.G. Pyramidal neuron polarity axis is defined at the bipolar stage. J. Cell Sci. 2008, 121(Pt 2) Pt 2, 178–185. [Google Scholar] [CrossRef]
- Jossin, Y.; Cooper, J.A. Reelin, Rap1 and N-cadherin orient the migration of multipolar neurons in the developing neocortex. Nat. Neurosci. 2011, 14(6), 697–703. [Google Scholar] [CrossRef]
- Nadarajah, B.; Brunstrom, J.E.; Grutzendler, J.; Wong, R.O.; Pearlman, A.L. Two modes of radial migration in early development of the cerebral cortex. Nat. Neurosci. 2001, 4(2), 143–50. [Google Scholar] [CrossRef] [PubMed]
- Noctor, S.C.; Martínez-Cerdeño, V.; Ivic, L.; Kriegstein, A.R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004, 7(2), 136–44. [Google Scholar] [CrossRef]
- Franco, S.J.; Martinez-Garay, I.; Gil-Sanz, C.; Harkins-Perry, S.R.; Müller, U. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron. 2011, 69(3), 482–97. [Google Scholar] [CrossRef]
- Gil-Sanz, C.; Franco, S.J.; Martinez-Garay, I.; Espinosa, A.; Harkins-Perry, S.; Müller, U. Cajal-Retzius cells instruct neuronal migration by coincidence signaling between secreted and contact-dependent guidance cues. Neuron. 2013, 79, 461–477. [Google Scholar] [CrossRef]
- Polleux, F.; Whitford, K.L.; Dijkhuizen, P.A.; Vitalis, T.; Ghosh, A. Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development. 2002, 129, 3147–3160. [Google Scholar] [CrossRef]
- Fukumitsu, H.; Ohtsuka, M.; Murai, R.; Nakamura, H.; Itoh, K.; Furukawa, S. Brain-derived neurotrophic factor participates in determination of neuronal laminar fate in the developing mouse cerebral cortex. J. Neurosci. 2006, 26(51), 13218–30. [Google Scholar] [CrossRef] [PubMed]
- Flames, N.; Long, J.E.; Garratt, A.N.; Fischer, T.M.; Gassmann, M.; Birchmeier, C.; Lai, C.; Rubenstein, J.L.; Marín, O. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004, 44(2), 251–61. [Google Scholar] [CrossRef]
- Du, H.; Kwon, I.K.; Kim, J. Neuregulin-1 impairs the long-term depression of hippocampal inhibitory synapses by facilitating the degradation of endocannabinoid 2-AG. J. Neurosci. 2013, 33(38), 15022–31. [Google Scholar] [CrossRef] [PubMed]
- Gomez, O.; Arevalo-Martin, A.; Garcia-Ovejero, D.; Ortega-Gutierrez, S.; Cisneros, J.A.; Almazan, G.; Sánchez-Rodriguez, M.A.; Molina-Holgado, F.; Molina-Holgado, E. The constitutive production of the endocannabinoid 2-arachidonoylglycerol participates in oligodendrocyte differentiation. Glia 2010, 58, 1913–1927. [Google Scholar] [CrossRef]
- Molina-Holgado, E.; Vela, J.M.; Arévalo-Martín, A.; Almazán, G.; Molina-Holgado, F.; Borrell, J.; Guaza, C. Cannabinoids promote oligodendrocyte progenitor survival: Involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J. Neurosci. 2002, 22(22), 9742–53. [Google Scholar] [CrossRef]
- Gomez, O.; Sanchez-Rodriguez, M.A.; Ortega-Gutierrez, S.; Vazquez-Villa, H.; Guaza, C.; Molina-Holgado, F.; Molina-Holgado, E. A basal tone of 2-arachidonoylglycerol contributes to early oligodendrocyte progenitor proliferation by activating phosphatidylinositol 3-kinase (PI3K)/akt and the mammalian target of rapamycin (mTOR) pathways. J. Neuroimmune Pharmacol. 2015, 10(2), 309–317. [Google Scholar] [CrossRef] [PubMed]
- Gomez, O.; Sanchez-Rodriguez, A.; Le, M.; Sanchez-Caro, C.; Molina-Holgado, F.; Molina-Holgado, E. Cannabinoid receptor agonists modulate oligodendrocyte differentiation by activating PI3K/Akt and the mammalian target of rapamycin (mTOR) pathways. Br. J. Pharmacol. 2011, 163(7), 1520–32. [Google Scholar] [CrossRef]
- Arévalo-Martín, A.; García-Ovejero, D.; Rubio-Araiz, A.; Gómez, O.; Molina-Holgado, F.; Molina-Holgado, E. Cannabinoids modulate Olig2 and polysialylated neural cell adhesion molecule expression in the subventricular zone of post-natal rats through cannabinoid receptor 1 and cannabinoid receptor 2. Eur. J. Neurosci. 2007, 26(6), 1548–59. [Google Scholar] [CrossRef]
- Huerga-Gómez A, Aguado T, Sánchez-de la Torre A, Bernal-Chico A, Matute C, Mato S, Guzmán M, Galve-Roperh I, Palazuelos J. Δ9-Tetrahydrocannabinol promotes oligodendrocyte development and CNS myelination in vivo. Glia. 2021, 69, 532–545. [CrossRef]
- Leterrier, C. ; Lainé J, Darmon, M.; Boudin, H.; Rossier, J.; Lenkei, Z. Constitutive activation drives compartment-selective endocytosis and axonal targeting of type 1 cannabinoid receptors. J. Neurosci. 2006, 26, 3143–3153. [Google Scholar] [CrossRef]
- Argaw, A.; Duff, G.; Zabouri, N.; Cécyre, B.; Chainé, N.; Cherif, H.; Tea, N.; Lutz, B.; Ptito, M.; Bouchard, J.F. Concerted action of CB1 cannabinoid receptor and deleted in colorectal cancer in axon guidance. J. Neurosci. 2011, 31(4), 1489–99. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.J.; Walsh, F.S.; Doherty, P. The FGF receptor uses the endocannabinoid signaling system to couple to an axonal growth response. J. Cell Biol. 2003, 160(4), 481–6. [Google Scholar] [CrossRef]
- Zuccarini, G.; D’Atri, I.; Cottone, E.; Mackie, K.; Shainer, I.; Gothilf, Y.; Provero, P.; Bovolin, P.; Merlo, G.R. Interference with the Cannabinoid Receptor CB1R Results in Miswiring of GnRH3 and AgRP1 Axons in Zebrafish Embryos. Int. J. Mol Sci. 2019, 21(1), 168. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, J.; Berrendero, F.; Hernández, M.L.; Ramos, J.A. The endogenous cannabinoid system and brain development. Trends Neurosci. 2000, 23(1), 14–20. [Google Scholar] [CrossRef]
- Itami, C.; Huang, J.Y.; Yamasaki, M.; Watanabe, M.; Lu, H.C.; Kimura, F. Developmental switch in spike timing-dependent plasticity and cannabinoid-dependent reorganization of the thalamocortical projection in the barrel cortex. J. Neurosci. 2016, 36(26), 7039–54. [Google Scholar] [CrossRef]
- Walker, D.J.; Suetterlin, P.; Reisenberg, M.; Williams, G.; Doherty, P. Down-regulation of diacylglycerol lipase-alpha during neural stem cell differentiation: Identification of elements that regulate transcription. J. Neurosci. Res. 2010, 88(4), 735–745. [Google Scholar] [CrossRef]
- Crittenden, J.R.; Yoshida, T.; Venu, S.; Mahar, A.; Graybiel, A.M. Cannabinoid receptor 1 is required for neurodevelopment of striosome-dendron bouquets. eNeuro. 2022, 9, ENEURO.0318-21.2022. [Google Scholar] [CrossRef] [PubMed]
- Black, B.; Vishwakarma, V.; Dhakal, K.; Bhattarai, S.; Pradhan, P.; Jain, A.; Kim, Y.T.; Mohanty, S. Spatial temperature gradients guide axonal outgrowth. Sci. Rep. 2016, 6, 29876. [Google Scholar] [CrossRef]
- Roland, A.B.; Ricobaraza, A.; Carrel, D.; Jordan, B.M.; Rico, F.; Simon, A.; Humbert-Claude, M.; Ferrier, J.; McFadden, M.H.; Scheuring, S.; Lenkei, Z. Cannabinoid-induced actomyosin contractility shapes neuronal morphology and growth. Elife. 2014, 3, e03159. [Google Scholar] [CrossRef]
- Njoo, C.; Agarwal, N.; Lutz, B.; Kuner, R. The Cannabinoid receptor CB1 interacts with the WAVE1 complex and plays a role in actin dynamics and structural plasticity in neurons. PLoS Biol. 2015, 13(10), e1002286. [Google Scholar] [CrossRef]
- Moore, S.W.; Correia, J.P.; Lai Wing Sun, K.; Pool, M.; Fournier, A.E.; Kennedy, T.E. Rho inhibition recruits DCC to the neuronal plasma membrane and enhances axon chemoattraction to netrin 1. Development. 2008, 135(17), 2855–64. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bashaw, G.J. Son of sevenless directly links the Robo receptor to rac activation to control axon repulsion at the midline. Neuron. 2006, 52(4), 595–607. [Google Scholar] [CrossRef] [PubMed]
- Manna, T.; Grenningloh, G.; Miller, H.P.; Wilson, L. Stathmin family protein SCG10 differentially regulates the plus and minus end dynamics of microtubules at steady state in vitro: Implications for its role in neurite outgrowth. Biochemistry. 2007, 46(11), 3543–52. [Google Scholar] [CrossRef] [PubMed]
- Saez, T.M.M.; Fernandez-Bessone, I.; Rodriguez, M.S.; Alloatti, M.; Otero, M.G.; Cromberg, L.E.; Pozo-Devoto, V.M.; Oubiña, G.; Sosa, L.; Buffone, M.G.; Gelman, D.M.; Falzone, T.L. Kinesin-1-mediated axonal transport of CB1 receptors is required for cannabinoid-dependent axonal growth and guidance. Development 2020, 147(8), dev184069. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Thayer, S.A. Cannabinoids inhibit the formation of new synapses between hippocampal neurons in culture. J. Neurosci. 2001, 21(10), RC146. [Google Scholar] [CrossRef]
- Papariello, A.; Taylor, D.; Soderstrom, K.; Litwa, K. CB1 antagonism increases excitatory synaptogenesis in a cortical spheroid model of fetal brain development. Sci. Rep. 2021, 11(1), 9356. [Google Scholar] [CrossRef]
- Hu, H.Y.; Kruijssen, D.L.H.; Frias, C.P.; Rózsa, B.; Hoogenraad, C.C.; Wierenga, C.J. Endocannabinoid Signaling Mediates Local Dendritic Coordination between Excitatory and Inhibitory Synapses. Cell Rep. 2019, 27, 666–675.e5. [Google Scholar] [CrossRef]
- Liang, J.; Kruijssen, D.L.H.; Verschuuren, A.C.J.; Voesenek, B.J.B.; Benavides, F.F.W.; Sáez Gonzalez, M.; Ruiter, M.; Wierenga, C.J. Axonal CB1 Receptors Mediate Inhibitory Bouton Formation via cAMP Increase and PKA. J. Neurosci. 2021, 41(40), 8279–8296. [Google Scholar] [CrossRef]
- Deshmukh, S.; Onozuka, K.; Bender, K.J.; Bender, V.A.; Lutz, B.; Mackie, K.; Feldman, D.E. Postnatal development of cannabinoid receptor type 1 expression in rodent somatosensory cortex. Neuroscience. 2007, 145(1), 279–87. [Google Scholar] [CrossRef]
- Liu, C.H.; Heynen, A.J.; Shuler, M.G.; Bear, M.F. Cannabinoid receptor blockade reveals parallel plasticity mechanisms in different layers of mouse visual cortex. Neuron. 2008, 58(3), 340–5. [Google Scholar] [CrossRef]
- Li, L.; Bender, K.J.; Drew, P.J.; Jadhav, S.P.; Sylwestrak, E.; Feldman, D.E. Endocannabinoid signaling is required for development and critical period plasticity of the whisker map in somatosensory cortex. Neuron. 2009, 64(4), 537–49. [Google Scholar] [CrossRef] [PubMed]
- Bender, K.J.; Allen, C.B.; Bender, V.A.; Feldman, D.E. Synaptic basis for whisker deprivation-induced synaptic depression in rat somatosensory cortex. J. Neurosci. 2006, 26(16), 4155–65. [Google Scholar] [CrossRef] [PubMed]
- Nevian, T.; Sakmann, B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J. Neurosci. 2006, 26(43), 11001–13. [Google Scholar] [CrossRef] [PubMed]
- Rubino, T.; Prini, P.; Piscitelli, F.; Zamberletti, E.; Trusel, M.; Melis, M.; Sagheddu, C.; Ligresti, A.; Tonini, R.; Di Marzo, V.; Parolaro, D. Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol. Dis. 2015, 73, 60–9. [Google Scholar] [CrossRef]
- Miller, M.L.; Chadwick, B.; Dickstein, D.L.; Purushothaman, I.; Egervari, G.; Rahman, T.; Tessereau, C.; Hof, P.R.; Roussos, P.; Shen, L.; Baxter, M.G.; Hurd, Y.L. Adolescent exposure to Δ9-tetrahydrocannabinol alters the transcriptional trajectory and dendritic architecture of prefrontal pyramidal neurons. Mol. Psychiatry. 2019, 24(4), 588–600. [Google Scholar] [CrossRef]
- Filbey, F.M.; McQueeny, T.; DeWitt, S.J.; Mishra, V. Preliminary findings demonstrating latent effects of early adolescent marijuana use onset on cortical architecture. Dev. Cogn. Neurosci. 2015, 16, 16–22. [Google Scholar] [CrossRef]
- Huang, Y.; Yasuda, H.; Sarihi, A.; Tsumoto, T. Roles of endocannabinoids in heterosynaptic long-term depression of excitatory synaptic transmission in visual cortex of young mice. J Neurosci. 2008, 28(28), 7074–83. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Huang, Y.; Tsumoto, T. Regulation of excitability and plasticity by endocannabinoids and PKA in developing hippocampus. Proc. Natl. Acad. Sci. U S A. 2008, 105(8), 3106–11. [Google Scholar] [CrossRef]
- Scheyer, A.F.; Borsoi, M.; Wager-Miller, J.; Pelissier-Alicot, A.L.; Murphy, M.N.; Mackie, K.; Manzoni, O.J.J. Cannabinoid exposure via lactation in rats disrupts perinatal programming of the gamma-aminobutyric acid trajectory and select early-life behaviors. Biol. Psychiatry. 2020, 87(7), 666–677. [Google Scholar] [CrossRef]
- García-Gil, L.; De Miguel, R.; Muñoz, R.M.; Cebeira, M.; Villanua, M.A.; Ramos, J.A.; Fernández-Ruiz, J.J. Perinatal delta(9)-tetrahydrocannabinol exposure alters the responsiveness of hypothalamic dopaminergic neurons to dopamine-acting drugs in adult rats. Neurotoxicol. Teratol. 1997, 19(6), 477–87. [Google Scholar] [CrossRef]
- Wang, X.; Dow-Edwards, D.; Anderson, V.; Minkoff, H.; Hurd, Y.L. In utero marijuana exposure associated with abnormal amygdala dopamine D2 gene expression in the human fetus. Biol. Psychiatry. 2004, 56(12), 909–15. [Google Scholar] [CrossRef] [PubMed]
- DiNieri, J.A.; Wang, X.; Szutorisz, H.; Spano, S.M.; Kaur, J.; Casaccia, P.; Dow-Edwards, D.; Hurd, Y.L. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol. Psychiatry. 2011, 70(8), 763–769. [Google Scholar] [CrossRef] [PubMed]
- Molina-Holgado, F.; Amaro, A.; González, M.I.; Alvarez, F.J.; Leret, M.L. Effect of maternal delta 9-tetrahydrocannabinol on developing serotonergic system. Eur. J. Pharmacol. 1996, 316(1), 39–42. [Google Scholar] [CrossRef]
- Molina-Holgado, F.; Alvarez, F.J.; Gonzalez, I.; Antonio, M.T.; Leret, M.L. Maternal exposure to delta 9-tetrahydrocannabinol (Δ9-THC) alters indolamine levels and turnover in adult male and female rat brain regions. Brain Res. Bull. 1997, 43(2), 173–8. [Google Scholar] [CrossRef]
- Giedd, J.N.; Blumenthal, J.; Jeffries, N.O.; Castellanos, F.X.; Liu, H.; Zijdenbos, A.; Paus, T.; Evans, A.C.; Rapoport, J.L. Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 1999, 2(10), 861–863. [Google Scholar] [CrossRef]
- Sicher, A.R.; Duerr, A.; Starnes, W.D.; Crowley, N.A. Adolescent alcohol and stress exposure rewires key cortical neurocircuitry. Front. Neurosci. 2022, 16, 896880. [Google Scholar] [CrossRef]
- Huttenlocher, P.R.; Dabholkar, A.S. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997, 387(2), 167–178. [Google Scholar] [CrossRef]
- Sturman, D.A.; Moghaddam, B. The neurobiology of adolescence: Changes in brain architecture, functional dynamics, and behavioral tendencies. Neurosci. Biobehav. Rev. 2011, 35(8), 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Paus, T.; Keshavan, M.; Giedd, J.N. Why do many psychiatric disorders emerge during adolescence? Rev. Neurosci. 2008, 9(12), 947–957. [Google Scholar] [CrossRef]
- Gogtay, N.; Giedd, J.N.; Lusk, L.; Hayashi, K.M.; Greenstein, D.; Vaituzis, A.C.; Nugent, T.F. 3rd; Herman, D.H.; Clasen, L.S.; Toga, A.W.; Rapoport, J.L.; Thompson, P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. USA 2004, 101(21), 8174–8179. [Google Scholar] [CrossRef]
- Sowell, E.R.; Peterson, B.S.; Thompson, P.M.; Welcome, S.E.; Henkenius, A.L.; Toga, A.W. Mapping cortical change across the human life span. Nat. Neurosci. 2003, 6(3), 309–315. [Google Scholar] [CrossRef] [PubMed]
- Paus, T.; Zijdenbos, A.; Worsley, K.; Collins, D.L.; Blumenthal, J.; Giedd, J.N.; Rapoport, J.L.; Evans, A.C. Structural maturation of neural pathways in children and adolescents: In vivo study. Science 1999, 283(5409), 1908–1911. [Google Scholar] [CrossRef] [PubMed]
- Paus, T.; Collins, D.L.; Evans, A.C.; Leonard, G.; Pike, B.; Zijdenbos, A. Maturation of white matter in the human brain: A review of magnetic resonance studies. Brain Res. Bull. 2001, 54(3), 255–266. [Google Scholar] [CrossRef]
- Hwang, K.; Velanova, K.; Luna, B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: A functional magnetic resonance imaging effective connectivity study. J. Neurosci. 2010, 30(46), 15535–15545. [Google Scholar] [CrossRef]
- Luna, B.; Padmanabhan, A.; O’Hearn, K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010, 72(1), 101–113. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, L.; Albert, D.; Cauffman, E.; Banich, M.; Graham, S.; Woolard, J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Dev. Psychol. 2008, 44(6), 1764–1778. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Baler, R.D.; Compton, W.M.; Weiss, S.R. Adverse health effects of marijuana use. N. Engl. J. Med. 2014, 370(23), 2219–2227. [Google Scholar] [CrossRef]
- Lubman, D.I.; Cheetham, A.; Yücel, M. Cannabis and adolescent brain development. Pharmacol. Ther. 2015, 148, 1–16. [Google Scholar] [CrossRef]
- Peters, K.Z.; Naneix, F. The role of dopamine and endocannabinoid systems in prefrontal cortex development: Adolescence as a critical period. Front. Neural Circuits 2022, 16, 939235. [Google Scholar] [CrossRef]
- Ellgren, M.; Artmann, A.; Tkalych, O.; Gupta, A.; Hansen, H.S.; Hansen, S.H.; Devi, L.A.; Hurd, Y.L. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur. Neuropsychopharmacol. 2008, 18(11), 826–834. [Google Scholar] [CrossRef]
- Marco, E.M.; Echeverry-Alzate, V.; López-Moreno, J.A.; Giné, E.; Peñasco, S.; Viveros, M.P. Consequences of early life stress on the expression of endocannabinoid-related genes in the rat brain. Behav. Pharmacol. 2014, 25, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Li, C.; Jaffe, A.E.; Shin, J.H.; Deep-Soboslay, A.; Yamin, R.; Weinberger, D.R.; Hyde, T.M.; Kleinman, J.E. Cannabinoid receptor CNR1 expression and DNA methylation in human prefrontal cortex, hippocampus and caudate in brain development and schizophrenia. Transl. Psychiatry 2020, 10(1), 158. [Google Scholar] [CrossRef]
- Dallabrida, K.G.; de Oliveira Bender, J.M.; Chade, E.S.; Rodrigues, N.; Sampaio, T.B. Endocannabinoid system changes throughout life: Implications and therapeutic potential for autism, ADHD, and Alzheimer’s disease. Brain Sci. 2024, 14(6), 592. [Google Scholar] [CrossRef] [PubMed]
- Renard, J.; Krebs, M.O.; Jay, T.M.; Le Pen, G. Long-term cognitive impairments induced by chronic cannabinoid exposure during adolescence in rats: A strain comparison. Psychopharmacology (Berl.) 2013, 225(4), 781–790. [Google Scholar] [CrossRef] [PubMed]
- Renard, J.; Vitalis, T.; Rame, M.; Krebs, M.O.; Lenkei, Z.; Le Pen, G.; Jay, T.M. Chronic cannabinoid exposure during adolescence leads to long-term structural and functional changes in the prefrontal cortex. Eur. Neuropsychopharmacol. 2016, 26(1), 55–64. [Google Scholar] [CrossRef]
- Schneider, M. Adolescence as a vulnerable period to alter rodent behavior. Cell Tissue Res. 2013, 354(1), 99–106. [Google Scholar] [CrossRef]
- Rubino, T.; Parolaro, D. The impact of exposure to cannabinoids in adolescence: Insights from animal models. Biol. Psychiatry 2016, 79(7), 578–585. [Google Scholar] [CrossRef]
- Katona, I.; Freund, T.F. Multiple functions of endocannabinoid signaling in the brain. Annu. Rev. Neurosci. 2012, 35, 529–558. [Google Scholar] [CrossRef]
- Rodríguez de Fonseca, F.; Ramos, J.A.; Bonnin, A.; Fernández-Ruiz, J.J. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport 1993, 4(2), 135–138. [Google Scholar] [CrossRef]
- Molla, H.M.; Miguelez Fernández, A.M.M.; Tseng, K.Y. Late-adolescent onset of prefrontal endocannabinoid control of hippocampal and amygdalar inputs and its impact on trace-fear conditioning behavior. Neuropsychopharmacology 2024, 49(9), 1417–1424. [Google Scholar] [CrossRef]
- Meyer, H.C.; Lee, F.S.; Gee, D.G. The role of the endocannabinoid system and genetic variation in adolescent brain development. Neuropsychopharmacology 2018, 43(1), 21–33. [Google Scholar] [CrossRef] [PubMed]
- De Felice, M.; Renard, J.; Hudson, R.; Szkudlarek, H.J.; Pereira, B.J.; Schmid, S.; Rushlow, W.J.; Laviolette, S.R. l-Theanine prevents long-term affective and cognitive side effects of adolescent Δ-9-tetrahydrocannabinol exposure and blocks associated molecular and neuronal abnormalities in the mesocorticolimbic circuitry. J. Neurosci. 2021, 41(4), 739–750. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, A.; Bara, A.; Murphy Green, M.N.; Manduca, A.; Wager-Miller, J.; Borsoi, M.; Lassalle, O.; Pelissier-Alicot, A.L.; Chavis, P.; Mackie, K.; Manzoni, O.J.J. Sexually dimorphic adolescent trajectories of prefrontal endocannabinoid synaptic plasticity equalize in adulthood, reflected by endocannabinoid system gene expression. Cannabis Cannabinoid Res. 2023, 8(5), 749–767. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Jung, K.M.; Fotio, Y.; Squire, E.; Palese, F.; Lin, L.; Torrens, A.; Ahmed, F.; Mabou Tagne, A.; Ramirez, J.; Su, S.; Wong, C.R.; Jung, D.H.; Scarfone, V.M.; Nguyen, P.U.; Wood, M.; Green, K.; Piomelli, D. Frequent low-dose Δ9-tetrahydrocannabinol in adolescence disrupts microglia homeostasis and disables responses to microbial infection and social stress in young adulthood. Biol. Psychiatry 2022, 92(11), 845–860. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, H.; Liu, D.; Li, X.; He, L.; Pan, J.; Shen, Q.; Peng, Y. CB2R activation ameliorates late adolescent chronic alcohol exposure-induced anxiety-like behaviors during withdrawal by preventing morphological changes and suppressing NLRP3 inflammasome activation in prefrontal cortex microglia in mice. Brain Behav. Immun. 2023, 110, 60–79. [Google Scholar] [CrossRef]
- Romero-Torres, B.M.; Alvarado-Ramírez, Y.A.; Duran-Alonzo, S.R.; Ruiz-Contreras, A.E.; Herrera-Solis, A.; Amancio-Belmont, O.; Prospéro-García, O.E.; Méndez-Díaz, M. A potential role of hippocampus on impulsivity and alcohol consumption through CB1R. Pharmacol. Biochem. Behav. 2023, 225, 173558. [Google Scholar] [CrossRef]
- Rico-Barrio, I.; Peñasco, S.; Lekunberri, L.; Serrano, M.; Egaña-Huguet, J.; Mimenza, A.; Soria-Gómez, E.; Ramos, A.; Buceta, I.; Gerrikagoitia, I.; Mendizabal-Zubiaga, J.; Elezgarai, I.; Puente, N.; Grandes, P. Environmental enrichment rescues endocannabinoid-dependent synaptic plasticity lost in young adult male mice after ethanol exposure during adolescence. Biomedicines 2021, 9(7), 825. [Google Scholar] [CrossRef]
- Micale, V.; Cristino, L.; Tamburella, A.; Petrosino, S.; Leggio, G.M.; Drago, F.; Di Marzo, V. Anxiolytic effects in mice of a dual blocker of fatty acid amide hydrolase and transient receptor potential vanilloid type-1 channels. Neuropsychopharmacology 2009, 34(3), 593–606. [Google Scholar] [CrossRef]
- Renard, J.; Krebs, M.O.; Le Pen, G.; Jay, T.M. Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Front. Neurosci. 2014, 8, 361. [Google Scholar] [CrossRef]
- Saravia, R.; Ten-Blanco, M.; Julià-Hernández, M.; Gagliano, H.; Andero, R.; Armario, A.; Maldonado, R.; Berrendero, F. Concomitant THC and stress adolescent exposure induces impaired fear extinction and related neurobiological changes in adulthood. Neuropharmacology 2019, 144, 345–357. [Google Scholar] [CrossRef]
- Akirav, I. The role of cannabinoids in modulating emotional and non-emotional memory processes in the hippocampus. Front. Behav. Neurosci. 2011, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Rubino, T.; Viganò, D.; Realini, N.; Guidali, C.; Braida, D.; Capurro, V.; Castiglioni, C.; Cherubino, F.; Romualdi, P.; Candeletti, S.; Sala, M.; Parolaro, D. Chronic delta-9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: Behavioral and biochemical correlates. Neuropsychopharmacology 2008, 33(11), 2760–2771. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.T.; Hill, M.N.; Lee, F.S. Developmental regulation of fear learning and anxiety behavior by endocannabinoids. Genes Brain Behav. 2016, 15(1), 108–124. [Google Scholar] [CrossRef] [PubMed]
- Abboussi, O.; Andaloussi, Z.L.; Chris, A.D.; Taghzouti, K. Chronic exposure to WIN55,212-2 during adolescence alters prefrontal dopamine turnover and induces sensorimotor deficits in adult rats. Neurotox. Res. 2020, 38(3), 682–690. [Google Scholar] [CrossRef]
- Demaili, A.; Portugalov, A.; Dudai, M.; Maroun, M.; Akirav, I.; Braun, K.; Bock, J. Epigenetic (re)programming of gene expression changes of CB1R and FAAH in the medial prefrontal cortex in response to early life and adolescence stress exposure. Front. Cell. Neurosci. 2023, 17, 1129946. [Google Scholar] [CrossRef]
- Renard, J.; Rosen, L.G.; Loureiro, M.; De Oliveira, C.; Schmid, S.; Rushlow, W.J.; Laviolette, S.R. Adolescent cannabinoid exposure induces a persistent sub-cortical hyper-dopaminergic state and associated molecular adaptations in the prefrontal cortex. Cereb. Cortex 27(2), 1297–1310. [CrossRef]
- Renard, J.; Szkudlarek, H.J.; Kramar, C.P.; Jobson, C.E.L.; Moura, K.; Rushlow, W.J.; Laviolette, S.R. Adolescent THC exposure causes enduring prefrontal cortical disruption of GABAergic inhibition and dysregulation of sub-cortical dopamine function. Sci. Rep. 7(1), 11420. [CrossRef]
- Renard, J.; Rushlow, W.J.; Laviolette, S.R. Effects of adolescent THC exposure on the prefrontal GABAergic system: Implications for schizophrenia-related psychopathology. Front. Psychiatry 2018, 9, 281. [Google Scholar] [CrossRef]
- Köfalvi, A.; Fritzsche, M. The endocannabinoid system is a major player in schizophrenia. In Köfalvi, A., Ed.; Cannabinoids and the Brain; Springer: New York, USA, 2008; pp. 485–528. [Google Scholar] [CrossRef]
- Bossong, M.G.; Niesink, R.J. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog. Neurobiol. 2010, 92(3), 370–385. [Google Scholar] [CrossRef]
- Vitale, R.M.; Iannotti, F.A.; Amodeo, P. The (poly)pharmacology of cannabidiol in neurological and neuropsychiatric disorders: Molecular mechanisms and targets. Int. J. Mol. Sci. 2021, 22(9), 4876. [Google Scholar] [CrossRef]
- Castillo-Arellano, J.; Canseco-Alba, A.; Cutler, S.J.; León, F. The polypharmacological effects of cannabidiol. Molecules 2023, 28(7), 3271. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.F.L.; Campos, R.M.P.; Isaac, A.R.; Paes-Colli, Y.; Carvalho, V.M.; Sampaio, L.S.; de Melo Reis, R.A. Long-term treatment with cannabidiol-enriched cannabis extract induces synaptic changes in the adolescent rat hippocampus. Int. J. Mol. Sci. 2023, 24(14), 11775. [Google Scholar] [CrossRef] [PubMed]
- Poleg, S.; Golubchik, P.; Offen, D.; Weizman, A. Cannabidiol as a suggested candidate for treatment of autism spectrum disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 90–96. [Google Scholar] [CrossRef]
- Dow-Edwards, D.; Silva, L. Endocannabinoids in brain plasticity: Cortical maturation, HPA axis function and behavior. Brain Res. 2017, 1654(Pt B), 157–164. [Google Scholar] [CrossRef]
- Stark, T.; Di Martino, S.; Drago, F.; Wotjak, C.T.; Micale, V. Phytocannabinoids and schizophrenia: Focus on adolescence as a critical window of enhanced vulnerability and opportunity for treatment. Pharmacol. Res. 2021, 174, 105938. [Google Scholar] [CrossRef]
- Ertl, N.; Freeman, T.P.; Mokrysz, C.; Ofori, S.; Borissova, A.; Petrilli, K.; Curran, H.V.; Lawn, W.; Wall, M.B. Acute effects of different types of cannabis on young adult and adolescent resting-state brain networks. Neuropsychopharmacology 2024, 49(10), 1640–1651. [Google Scholar] [CrossRef] [PubMed]
- Medina, K.L.; Nagel, B.J.; Park, A.; McQueeny, T.; Tapert, S.F. Depressive symptoms in adolescents: Associations with white matter volume and marijuana use. J. Child Psychol. Psychiatry 2007, 48(6), 592–600. [Google Scholar] [CrossRef]
- Desai, S.; Zundel, C.G.; Evanski, J.M.; Gowatch, L.C.; Bhogal, A.; Ely, S.; Carpenter, C.; Shampine, M.; O’Mara, E.; Rabinak, C.A.; Marusak, H.A. Genetic variation in endocannabinoid signaling: Anxiety, depression, and threat- and reward-related brain functioning during the transition into adolescence. Behav. Brain Res. 2024, 463, 114925. [Google Scholar] [CrossRef]
- Hariri, A.R.; Gorka, A.; Hyde, L.W.; Kimak, M.; Halder, I.; Ducci, F.; Ferrell, R.E.; Goldman, D.; Manuck, S.B. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol. Psychiatry 2009, 66(1), 9–16. [Google Scholar] [CrossRef]
- Kovner, R.; Oler, J.A.; Kalin, N.H. Cortico-limbic interactions mediate adaptive and maladaptive responses relevant to psychopathology. Am. J. Psychiatry 2019, 176(12), 987–999. [Google Scholar] [CrossRef]
- Di Forti, M.; Marconi, A.; Carra, E.; Fraietta, S.; Trotta, A.; Bonomo, M.; Bianconi, F.; Gardner-Sood, P.; O’Connor, J.; Russo, M.; Stilo, S.A.; Marques, T.R.; Mondelli, V.; Dazzan, P.; Pariante, C.; David, A.S.; Gaughran, F.; Atakan, Z.; Iyegbe, C.; Powell, J.; Morgan, C.; Lynskey, M.; Murray, R.M. Proportion of patients in south London with first-episode psychosis attributable to use of high-potency cannabis: A case-control study. Lancet Psychiatry 2015, 2(3), 233–238. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.H.; Caspi, A.; Ambler, A.; Harrington, H.; Houts, R.; Keefe, R.S.; McDonald, K.; Ward, A.; Poulton, R.; Moffitt, T.E. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc. Natl. Acad. Sci. USA 2012, 109(40), E2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Hermann, D.; Schneider, M. Potential protective effects of cannabidiol on neuroanatomical alterations in cannabis users and psychosis: A critical review. Curr. Pharm. Des. 2012, 18(32), 4897–4905. [Google Scholar] [CrossRef]
- Albaugh, M.D.; Ottino-Gonzalez, J.; Sidwell, A.; Lepage, C.; Juliano, A.; Owens, M.M.; Chaarani, B.; Spechler, P.; Fontaine, N.; Rioux, P.; Lewis, L.; Jeon, S.; Evans, A.; D’Souza, D.; Radhakrishnan, R.; Banaschewski, T.; Bokde, A.L.W.; Quinlan, E.B.; Conrod, P.; Desrivières, S.; Flor, H.; Grigis, A.; Gowland, P.; Heinz, A.; Ittermann, B.; Martinot, J.L.; Paillère Martinot, M.L.; Nees, F.; Papadopoulos Orfanos, D.; Paus, T.; Poustka, L.; Millenet, S.; Fröhner, J.H.; Smolka, M.N.; Walter, H.; Whelan, R.; Schumann, G.; Potter, A.; Garavan, H.; IMAGEN Consortium. Association of cannabis use during adolescence with neurodevelopment. Association of cannabis use during adolescence with neurodevelopment. JAMA Psychiatry 2021, 78(9), 1–11. [Google Scholar] [CrossRef]
- Allick, A.; Park, G.; Kim, K.; Vintimilla, M.; Rathod, K.; Lebo, R.; Nanavati, J.; Hammond, C.J. Age- and sex-related cortical gray matter volume differences in adolescent cannabis users: A systematic review and meta-analysis of voxel-based morphometry studies. Front. Psychiatry 2021, 12, 745193. [Google Scholar] [CrossRef]
- Canas, P.M.; Duarte, J.M.; Rodrigues, R.J.; Köfalvi, A.; Cunha, R.A. Modification upon aging of the density of presynaptic modulation systems in the hippocampus. Neurobiol. Aging 2009, 30(11), 1877–1884. [Google Scholar] [CrossRef]
- Long, L.E.; Lind, J.; Webster, M.; Weickert, C.S. Developmental trajectory of the endocannabinoid system in human dorsolateral prefrontal cortex. BMC Neurosci. 2012, 13, 87. [Google Scholar] [CrossRef]
- Kinon, B.J.; Leucht, S.; Tamminga, C.; Breier, A.; Marcus, R.; Paul, S.M. Rationale for adjunctive treatment targeting multiple mechanisms in schizophrenia. J. Clin. Psychiatry 2024, 85(3), 23nr15240. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.C.; Sewell, R.A.; Ranganathan, M. Cannabis and psychosis/schizophrenia: Human studies. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 259(7), 413–431. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Crippa, J.A.; Hallak, J.E.; Bhattacharyya, S.; Atakan, Z.; Martin-Santos, R.; McGuire, P.K.; Guimarães, F.S. A critical review of the antipsychotic effects of cannabidiol: 30 years of a translational investigation. Curr. Pharm. Des. 2012, 18(32), 5131–5140. [Google Scholar] [CrossRef]
- Gorbenko, A.A.; Heuberger, J.A.A.C.; Klumpers, L.E.; de Kam, M.L.; Strugala, P.K.; de Visser, S.J.; Groeneveld, G.J. Cannabidiol increases psychotropic effects and plasma concentrations of Δ9-tetrahydrocannabinol without improving its analgesic properties. Clin. Pharmacol. Ther. 2024. [CrossRef] [PubMed]
- Rubino, T.; Parolaro, D. Sexually dimorphic effects of cannabinoid compounds on emotion and cognition. Front. Behav. Neurosci. 2011, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Elmes, M.W.; Kaczocha, M.; Berger, W.T.; Leung, K.; Ralph, B.P.; Wang, L.; Sweeney, J.M.; Miyauchi, J.T.; Tsirka, S.E.; Ojima, I.; Deutsch, D.G. Fatty acid-binding proteins (FABPs) are intracellular carriers for Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J. Biol. Chem. 2015, 290(14), 8711–8721. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.R.; Watts, J.J.; Da Silva, T.; Tyndale, R.F.; Rusjan, P.M.; Houle, S.; Wilson, A.A.; Ross, R.A.; Boileau, I.; Mizrahi, R. Fatty acid amide hydrolase is lower in young cannabis users. Addict. Biol. 2021, 26(1), e12872. [Google Scholar] [CrossRef]
- Ostlund, I.; Von Gunten, M.; Smith, C.; Edwards, J.G. Chronic Δ9-tetrahydrocannabinol impact on plasticity, and differential activation requirement for CB1-dependent long-term depression in ventral tegmental area GABA neurons in adult versus young mice. Front. Neurosci. 2023, 16, 1067493. [Google Scholar] [CrossRef] [PubMed]
- Volk, D.W.; Siegel, B.I.; Verrico, C.D.; Lewis, D.A. Endocannabinoid metabolism in the prefrontal cortex in schizophrenia. Schizophr. Res. 2013, 147(1), 53–57. [Google Scholar] [CrossRef]
- Maia, J.; Fonseca, B.M.; Cunha, S.C.; Braga, J.; Gonçalves, D.; Teixeira, N.; Correia-da-Silva, G. Impact of tetrahydrocannabinol on the endocannabinoid 2-arachidonoylglycerol metabolism: ABHD6 and ABHD12 as novel players in human placenta. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865(12), 158807. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





