Submitted:
18 October 2024
Posted:
18 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Risk
3. Influence
4. Prevention
5. Management
6. Conclusions
Funding
Conflicts of Interest
References
- Ninomiya, I.; Osugi, H.; Fujimura, T.; Fushida, S.; Okamoto, K.; Maruzen, S.; Oyama, K.; Kinoshita, J.; Tsukada, T.; Kitagawa, H.; Takamura, H.; Nakagawara, H.; Tajima, H.; Hayashi, H.; Makino, I.; Ohta, T. Thoracoscopic esophagectomy with extended lymph node dissection in the left lateral position: technical feasibility and oncologic outcomes. 2014 Dis Esophagus. 2014, 27,159-167.
- Jang, H.J.; Lee, H.S.; Kim, M.S.; Lee, J.M.; Zo, J.I. Patterns of lymph node metastasis and survival for upper esophageal squamous cell carcinoma. Ann Thorac Surg. 2011, 92, 1091-1097.
- Kanemura, T.; Makino, T.; Miyazaki, Y.; Takahashi, T.; Kurokawa, Y.; Yamasaki, M.; Nakajima, K.; Takiguchi, S.; Mori, M.; Doki, Y. Distribution patterns of metastases in recurrent laryngeal nerve lymph nodes in patients with squamous cell esophageal cancer. Dis Esophagus. 2017, 30, 1-7.
- Taniyama, Y.; Nakamura, T.; Mitamura, A.; Teshima, J.; Katsura, K.; Abe, S.; Nakano, T.; Kamei, T.; Miyata, G.; Ouchi, N. A strategy for supraclavicular lymph node dissection using recurrent laryngeal nerve lymph node status in thoracic esophageal squamous cell carcinoma. Ann Thorac Surg. 2013, 95, 1930-1937.
- Wu, J.; Chen, Q.X.; Zhou, X.M.; Mao, W.M.; Krasna, M.J. Does recurrent laryngeal nerve lymph node metastasis really affect the prognosis in node-positive patients with squamous cell carcinoma of the middle thoracic esophagus? BMC Surg. 2014, 12, 14:43.
- Hong, T.H.; Kim, H.K.; Lee, G.; Shin, S.; Cho, J.H.; Choi, Y.S.; Zo, J.I.; Shim, Y.M. Role of recurrent laryngeal nerve lymph node dissection in surgery of early-stage esophageal squamous cell carcinoma. Ann Surg Oncol. 2022, 29, 627-639.
- Xu, S.; Chen, D.; Liu, Z.; Song, P.; Zheng, Y.; Xue, X.; Sang, Y.; Li, Z.; Chen, Y. Impact of the extent of recurrent laryngeal nerve lymphadenectomy on thoracic esophageal squamous cell carcinoma: a real-world multicentre study. Eur J Cardiothorac Surg. 2023, 63. ezad168.
- Liu, F.; Yang, W.; Yang, W.; Xu, R.; Chen, L.; He, Y.; Liu, Z.; Zhou, F.; Hou, B.; Zhang, L.; Zhang, L.; Zhang, F.; Cai, F.; Xu, H.; Lin, M.; Liu, M.; Pan, Y.; Liu, Y.; Hu, Z.; Chen, H.; He, Z.; Ke, Y. Minimally invasive or open esophagectomy for treatment of resectable esophageal squamous cell carcinoma? Answer from a real-world multicenter study. Ann Surg. 2023, 277, e777-e784.
- Mao, Y.; Gao, S.; Li, Y.; Chen, C.; Hao, A.; Wang, Q.; Tan, L.; Ma, J.; Xiao, G.; Fu, X.; Fang, W.; Li, Z.; Han, Y.; Chen, K.; Zhang, R.; Li, X.; Rong, T.; Fu, J.; Liu, Y.; Mao, W.; Xu, M.; Liu, S.; Yu, Z.; Zhang, Z.; Fang, Y.; Fu, D.; Wei, X.; Yuan, L.; Muhammad, S.; He, J. Minimally invasive versus open esophagectomy for resectable thoracic esophageal cancer (NST 1502): a multicenter prospective cohort study. J Natl Cancer Cent. 2023, 3, 106-114.
- Na, K.J.; Kang, C.H.; Park, S.; Park, I.K.; Kim, Y.T. Robotic esophagectomy versus open esophagectomy in esophageal squamous cell carcinoma: a propensity-score matched analysis. J Robot Surg. 2022, 16, 841-848.
- Takeuchi, H.; Miyata, H.; Ozawa, S.; Udagawa, H.; Osugi, H.; Matsubara, H.; Konno, H.; Seto, Y.; Kitagawa, Y. Comparison of short-term outcomes between open and minimally invasive esophagectomy for esophageal cancer using a nationwide database in Japan. Ann Surg Oncol. 2017, 24, 1821-1827.
- Taniyama, Y.; Miyata, G.; Kamei, T.; Nakano, T.; Abe, S.; Katsura, K.; Sakurai, T.; Teshima, J.; Hikage, M.; Ohuchi, N. Complications following recurrent laryngeal nerve lymph node dissection in esophageal cancer surgery. Interact Cardiovasc Thorac Surg. 2015, 20, 41-46.
- Li, Z.G.; Zhang, X.B.; Wen, Y.W.; Liu, Y.H.; Chao, Y.K. Incidence and predictors of unsuspected recurrent laryngeal nerve lymph node metastases after neoadjuvant chemoradiotherapy in patients with esophageal squamous cell carcinoma. World J Surg. 2018, 42, 2485-2492.
- Lee, J.O.; Yun, J.K.; Jeong, Y.H.; Lee, Y.S.; Kim, Y.H. Management for recurrent laryngeal nerve paralysis following oesophagectomy for oesophageal cancer: thoracic surgeon perspective. J Thorac Dis. 2024, 16, 3805-3817.
- Jeon, Y.J.; Cho, J.H.; Lee, H.K.; Kim, H.K.; Choi, Y.S.; Zo, J.I.; Shim, Y.M. Management of patients with bilateral recurrent laryngeal nerve paralysis following esophagectomy. Thorac Cancer. 2021, 12, 1851-1856.
- Van Workum, F.; Berkelmans, G.H.; Klarenbeek, B.R.; Nieuwenhuijzen, G.A.P.; Luyer, M.D.P.; Rosman, C. McKeown or Ivor Lewis totally minimally invasive esophagectomy for cancer of the esophagus and gastroesophageal junction: systematic review and meta-analysis. J Thorac Dis. 2017, 9, S826-S833.
- Wang, J.; Hu, J.; Zhu, D.; Wang, K.; Gao, C.; Shan, T.; Yang, Y. McKeown or Ivor Lewis minimally invasive esophagectomy: a systematic review and meta-analysis. Transl Cancer Res. 2020, 9, 1518-1527.
- Saito, Y.; Takeuchi, H.; Fukuda, K.; Suda, K.; Nakamura, R.; Wada, N.; Kawakubo, H.; Kitagawa, Y. Size of recurrent laryngeal nerve as a new risk factor for postoperative vocal cord paralysis. Dis Esophagus. 2018, 31, 1-8.
- Ohi, M.; Toiyama, Y.; Yasuda, H.; Ichikawa, T.; Imaoka, H.; Okugawa, Y.; Fujikawa, H.; Okita, Y.; Yokoe, T.; Hiro, J.; Kusunoki, M. Preoperative computed tomography predicts the risk of recurrent laryngeal nerve paralysis in patients with esophageal cancer undergoing thoracoscopic esophagectomy in the prone position. Esophagus. 2021, 18, 228-238.
- Liu, N.; Chen, B.; Li, L.; Zeng, Q.; Sheng, L.; Zhang, B.; Liang, W.; Lv, B. Mechanisms of recurrent laryngeal nerve injury near the nerve entry point during thyroid surgery: a retrospective cohort study. Int J Surg. 2020, 83, 125-130.
- Hayami, M.; Watanabe, M.; Mine, S.; Imamura, Y.; Okamura, A.; Yuda, M.; Yamashita, K.; Shoji, Y.; Toihata, T.; Kozuki, R.; Ishizuka, N. Steam induced by the activation of energy devices under a wet condition may cause thermal injury. Surg Endosc. 2020, 34, 2295-2302.
- Sulica, L. The natural history of idiopathic unilateral vocal fold paralysis: evidence and problems. Laryngoscope. 2008, 118, 1303-1307.
- Lee, D.H.; Lee, S.Y.; Lee, M.; Seok, J.; Park, S.J.; Jin, Y.J.; Lee, D.Y.; Kwon, T.K. Natural course of unilateral vocal fold paralysis and optimal timing of permanent treatment. JAMA Otolaryngol Head Neck Surg. 2020, 146, 30-35.
- Kuo, C.T.; Chiu, C.H.; Fang, T.J.; Chao, Y.K. Prognostic factors for recovery from left recurrent laryngeal nerve palsy after minimally invasive McKeown esophagectomy: a retrospective study. Ann Surg Oncol. 2024, 31, 1546-1552.
- Shimizu, H.; Shiozaki, A.; Fujiwara, H.; Konishi, H.; Kosuga, T.; Komatsu, S.; Ichikawa, D.; Okamoto, K.; Otsuji, E. Short- and long-term progress of recurrent laryngeal nerve paralysis after subtotal esophagectomy. Anticancer Res. 2017, 37, 2019-2023.
- Takatsu, J.; Higaki, E.; Abe, T.; Fujieda, H.; Yoshida, M.; Yamamoto, M.; Shimizu, Y. Critical swallowing functions contributing to dysphagia in patients with recurrent laryngeal nerve paralysis after esophagectomy. Esophagus. 2024, 21, 111-119.
- Baba, M.; Aikou, T.; Natsugoe, S.; Kusano, C.; Shimada, M.; Nakano, S.; Fukumoto, T.; Yoshinaka, H. Quality of life following esophagectomy with three-field lymphadenectomy for carcinoma, focusing on its relationship to vocal cord palsy. Dis Esophagus. 1998, 11, 28-34.
- Scholtemeijer, M.G.; Seesing, M.F.J.; Brenkman, H.J.F.; Janssen, L.M.; van Hillegersberg, R.; Ruurda, J.P. Recurrent laryngeal nerve injury after esophagectomy for esophageal cancer: incidence, management, and impact on short- and long-term outcomes. J Thorac Dis. 2017, 9, S868-S878.
- Oshikiri, T.; Takiguchi, G.; Hasegawa, H.; Yamamoto, M.; Kanaji, S.; Yamashita, K.; Matsuda, T.; Nakamura, T.; Suzuki, S.; Kakeji, Y. Postoperative recurrent laryngeal nerve palsy is associated with pneumonia in minimally invasive esophagectomy for esophageal cancer. Surg Endosc. 2021, 35, 837-844.
- Yang, Y.; Li, B.; Xu, X.; Liu, Z.; Jiang, C.; Wu, X.; Yang, Y.; Li, Z. Short-term and long-term effects of recurrent laryngeal nerve injury after robotic esophagectomy. Eur J Surg Oncol. 2023, 49, 107009.
- Booka, E.; Takeuchi, H.; Nishi, T.; Matsuda, S.; Kaburagi, T.; Fukuda, K.; Nakamura, R.; Takahashi, T.; Wada, N.; Kawakubo, H.; Omori, T.; Kitagawa, Y. The impact of postoperative complications on survival after esophagectomy for esophageal cancer. Medicine (Baltimore). 2015, 94, e1369.
- Qu, R.; Tu, D.; Ping, W.; Fu, X. The impact of recurrent laryngeal nerve injury on prognosis after McKeown esophagectomy for ESCC. Cancer Manag Res. 2021, 13, 1861-1868.
- Tsunoda, S.; Shinohara, H.; Kanaya, S.; Okabe, H.; Tanaka, E.; Obama, K.; Hosogi, H.; Hisamori, S.; Sakai, Y. Mesenteric excision of upper esophagus: a concept for rational anatomical lymphadenectomy of the recurrent laryngeal nodes in thoracoscopic esophagectomy. Surg Endosc. 2020, 34, 133-141.
- Otsuka, K.; Murakami, M.; Goto, S.; Ariyoshi, T.; Yamashita, T.; Saito, A.; Kohmoto, M.; Kato, R.; Lefor, A.K.; Aoki, T. Minimally invasive esophagectomy and radical lymph node dissection without recurrent laryngeal nerve paralysis. Surg Endosc. 2020, 34, 2749-2757.
- Saeki, H.; Nakashima, Y.; Hirose, K.; Sasaki, S.; Jogo, T.; Taniguchi, D.; Edahiro, K.; Korehisa, S.; Kudou, K.; Nakanishi, R.; Kubo, N.; Ando, K.; Kabashima, A.; Oki, E.; Maehara, Y. “Energy-less technique” with mini-clips for recurrent laryngeal nerve lymph node dissection in prone thoracoscopic esophagectomy for esophageal cancer. Am J Surg. 2018, 216, 1212-1214.
- Fu, J.; Li, Y.; Wang, Z.; Cheng, Y.; Chen, N.; Sun, X.; Zhang, B.; Peng, Z.; Chen, W.; Qian, R.; Shi, A.; Yan, X.; Wang, H.; Ma, F.; Lv, Y.; Zhang, Y. The role of magnetic anchoring and traction technique in thoracoscopic lymphadenectomy along the left recurrent laryngeal nerve. Surg Endosc. 2022, 36, 3653-3662.
- Dongming, G.; Yuequan, J.; Qi, Z.; Huajie, X.; Zhiqiang, W. A novel technique for lymphadenectomy along the left recurrent laryngeal nerve during minimally invasive esophagectomy: a retrospective cohort study. BMC Surg. 2023, 23, 355.
- Hikage, M.; Kamei, T.; Nakano, T.; Abe, S.; Katsura, K.; Taniyama, Y.; Sakurai, T.; Teshima, J.; Ito, S.; Niizuma, N.; Okamoto, H.; Fukutomi, T.; Yamada, M.; Maruyama, S.; Ohuchi, N. Impact of routine recurrent laryngeal nerve monitoring in prone esophagectomy with mediastinal lymph node dissection. Surg Endosc. 2017, 31, 2986-2996.
- Kanemura, T.; Miyata, H.; Yamasaki, M.; Makino, T.; Miyazaki, Y.; Takahashi, T.; Kurokawa, Y.; Takiguchi, S.; Mori, M.; Doki, Y. Usefulness of intraoperative nerve monitoring in esophageal cancer surgery in predicting recurrent laryngeal nerve palsy and its severity. Gen Thorac Cardiovasc Surg. 2019, 67, 1075-1080.
- Huang, C.L.; Chen, C.M.; Hung, W.H.; Cheng, Y.F.; Hong, R.P.; Wang, B.Y.; Cheng, C.Y. Clinical outcome of intraoperative recurrent laryngeal nerve monitoring during thoracoscopic esophagectomy and mediastinal lymph node dissection for esophageal cancer. J Clin Med. 2022, 11, 4949.
- Chen, B.; Yang, T.; Wang, W.; Tang, W.; Xie, J.; Kang, M. Application of intraoperative neuromonitoring (IONM) of the recurrent laryngeal nerve during esophagectomy: a systematic review and meta-analysis. J Clin Med. 2023, 12, 565.
- Wong, I.; Tong, D.K.H.; Tsang, R.K.Y.; Wong, C.L.Y.; Chan, D.K.K.; Chan, F.S.Y.; Law, S. Continuous intraoperative vagus nerve stimulation for monitoring of recurrent laryngeal nerve during minimally invasive esophagectomy. J Vis Surg. 2017, 3, 9.
- Komatsu, S.; Konishi, T.; Matsubara, D.; Soga, K.; Shimomura, K.; Ikeda, J.; Taniguchi, F.; Fujiwara, H.; Shiozaki, A.; Otsuji, E. Continuous recurrent laryngeal nerve monitoring during single-port mediastinoscopic radical esophagectomy for esophageal cancer. J Gastrointest Surg. 2022, 26, 2444-2450.
- Oshikiri, T.; Goto, H.; Horikawa, M.; Urakawa, N.; Hasegawa, H.; Kanaji, S.; Yamashita, K.; Matsuda, T.; Nakamura, T.; Kakeji, Y. Incidence of recurrent laryngeal nerve palsy in robot-assisted versus conventional minimally invasive McKeown esophagectomy in prone position: a propensity score-matched study. Ann Surg Oncol. 2021, 28, 7249-7257.
- Chao, Y.K.; Li, Z.; Jiang, H.; Wen, Y.W.; Chiu, C.H.; Li, B.; Shang, X.; Zhou, D.; Li, Y.; Yan, X.; Wu, H. Comparative study of surgical outcomes between robot-assisted and video-assisted thoracoscopic esophagectomy for esophageal cancer: a meta-analysis. Surg Endosc. 2023, 37, 3443-3455.
- Watanabe M, Kuriyama K, Terayama M, Okamura A, Kanamori J, Imamura Y. Robotic- Assisted Esophagectomy: Current Situation and Future Perspectives. Ann Thorac Cardiovasc Surg. 2023, 29, 168-176.
- Yang, Y.; Li, B.; Yi, J.; Hua, R.; Chen, H.; Tan, L.; Li, H.; He, Y.; Guo, X.; Sun, Y.; Yu, B.; Li, Z. Robot-assisted versus conventional minimally invasive esophagectomy for resectable esophageal squamous cell carcinoma: early results of a multicenter randomized controlled trial: the RAMIE trial. Ann Surg. 2022, 275, 646-653.
- Sato, K.; Fujita, T.; Matsuzaki, H.; Takeshita, N.; Fujiwara, H.; Mitsunaga, S.; Kojima, T.; Mori, K.; Daiko, H. Real-time detection of the recurrent laryngeal nerve in thoracoscopic esophagectomy using artificial intelligence. Surg Endosc. 2022, 36, 5531-5539.
- Natsugoe, S.; Okumura, H.; Matsumoto, M.; Ishigami, S.; Owaki, T.; Nakano, S.; Aikou, T. Reconstruction of recurrent laryngeal nerve with involvement by metastatic node in esophageal cancer. Ann Thorac Surg. 2005, 79, 1886-1889.
- Koyanagi, K.; Igaki, H.; Iwabu, J.; Ochiai, H.; Tachimori, Y. Recurrent laryngeal nerve paralysis after esophagectomy: respiratory complications and role of nerve reconstruction. Tohoku J Exp Med. 2015, 237, 1-8.
- Filauro, M.; Vallin, A.; Marcenaro, E.; Missale, F.; Fragale, M.; Mora, F.; Marrosu, V.; Sampieri, C.; Carta, F.; Puxeddu, R.; Peretti, G. Quality of life after transoral CO2 laser posterior cordotomy with or without partial arytenoidectomy for bilateral adductor vocal cord paralysis. Eur Arch Otorhinolaryngol. 2021, 278, 4391-4401.
- Tarihci Cakmak E, Sen EI, Doruk C, Sen C, Sezikli S, Yaliman A. The Effects of Neuromuscular Electrical Stimulation on Swallowing Functions in Post-stroke Dysphagia: A Randomized Controlled Trial. Dysphagia. 2023, 38, 874-885.
- Wang Z, Xiao Z, Shen Q, Zhao N, Zhang W. Neuromuscular Electrical Stimulation for Post-Stroke Dysphagia Treatment: A Systemic Evaluation and Meta-Analysis of Randomized Controlled Trials. Dysphagia. 2024, 39, 424-432.
- Mattioli, F.; Menichetti, M.; Bergamini, G.; Molteni, G.; Alberici, M.P.; Luppi, M.P.; Nizzoli, F.; Presutti, L. Results of early versus intermediate or delayed voice therapy in patients with unilateral vocal fold paralysis: our experience in 171 patients. J Voice. 2015, 29, 455-458.
- Walton, C.; Carding, P.; Flanagan, K. Perspectives on voice treatment for unilateral vocal fold paralysis. Curr Opin Otolaryngol Head Neck Surg. 2018, 26, 157-161.
- Kojima, K.; Fukushima, T.; Kurita, D.; Matsuoka, A.; Ishiyama, K.; Oguma, J.; Daiko, H. Perioperative decrease in tongue pressure is an intervenable predictor of aspiration after esophagectomy. Dysphagia. 2023, 38, 1147-1155.
- Yu, Y.; Li, Y.; Lu, Y.; Hua, X.; Ma, H.; Li, H.; Wei, X.; Zhang, J.; Chen, X.; Liu, Q.; Zhu, Z.; Xu, L.; Zhang, R.; Sun, H.; Wang, Z. Chin-down-plus-larynx-tightening maneuver improves choking cough after esophageal cancer surgery. Ann Transl Med. 2019, 7, 376.
- Chen, X.; Wan, P.; Yu, Y.; Li, M.; Xu, Y.; Huang, P.; Huang, Z. Types and timing of therapy for vocal fold paresis/paralysis after thyroidectomy: a systematic review and meta-analysis. J Voice. 2014, 28, 799-808.
- Hasukawa, A.; Mochizuki, R.; Sakamoto, H.; Shibano, A.; Kitahara, T. Type I thyroplasty or fat injection laryngoplasty versus arytenoid adduction: effects of surgery on voice recovery in patients with unilateral vocal fold paralysis. Ear Nose Throat J. 2023, 19, 1455613231176153.
- Jeong, G.E.; Lee, D.H.; Lee, Y.S.; Ahn, D.S.; Lee, D.K.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Treatment efficacy of voice therapy following injection laryngoplasty for unilateral vocal fold paralysis. J Voice. 2022, 36, 242-248.
- Haddad, R.; Ismail, S.; Khalaf, M.G.; Matar, N. Lipoinjection for unilateral vocal fold paralysis treatment: a systematic review and meta-analysis. Laryngoscope. 2022, 132, 1630-1640.
- Watanabe, K.; Hirano, A.; Kobayashi, Y.; Sato, T.; Honkura, Y.; Katori, Y. Long-term voice evaluation after arytenoid adduction surgery in patients with unilateral vocal fold paralysis. Eur Arch Otorhinolaryngol. 2023, 280, 5011-5017.
- Lindeman, R.C. Diverting the paralyzed larynx: a reversible procedure for intractable aspiration. Laryngoscope. 1975, 85, 157-180.
- Ninomiya, H.; Yasuoka, Y.; Inoue, Y.; Toyoda, M.; Takahashi, K.; Miyashita, M.; Furuya, N. Simple and new surgical procedure for laryngotracheal separation in pediatrics. Laryngoscope. 2008, 118, 958-961.
- Shino, M.; Yasuoka, Y.; Murata, T.; Ninomiya, H.; Takayasu, Y.; Takahashi, K.; Chikamatsu, K. Improvement of tracheal flap method for laryngotracheal separation. Laryngoscope. 2013, 123, 440-445.
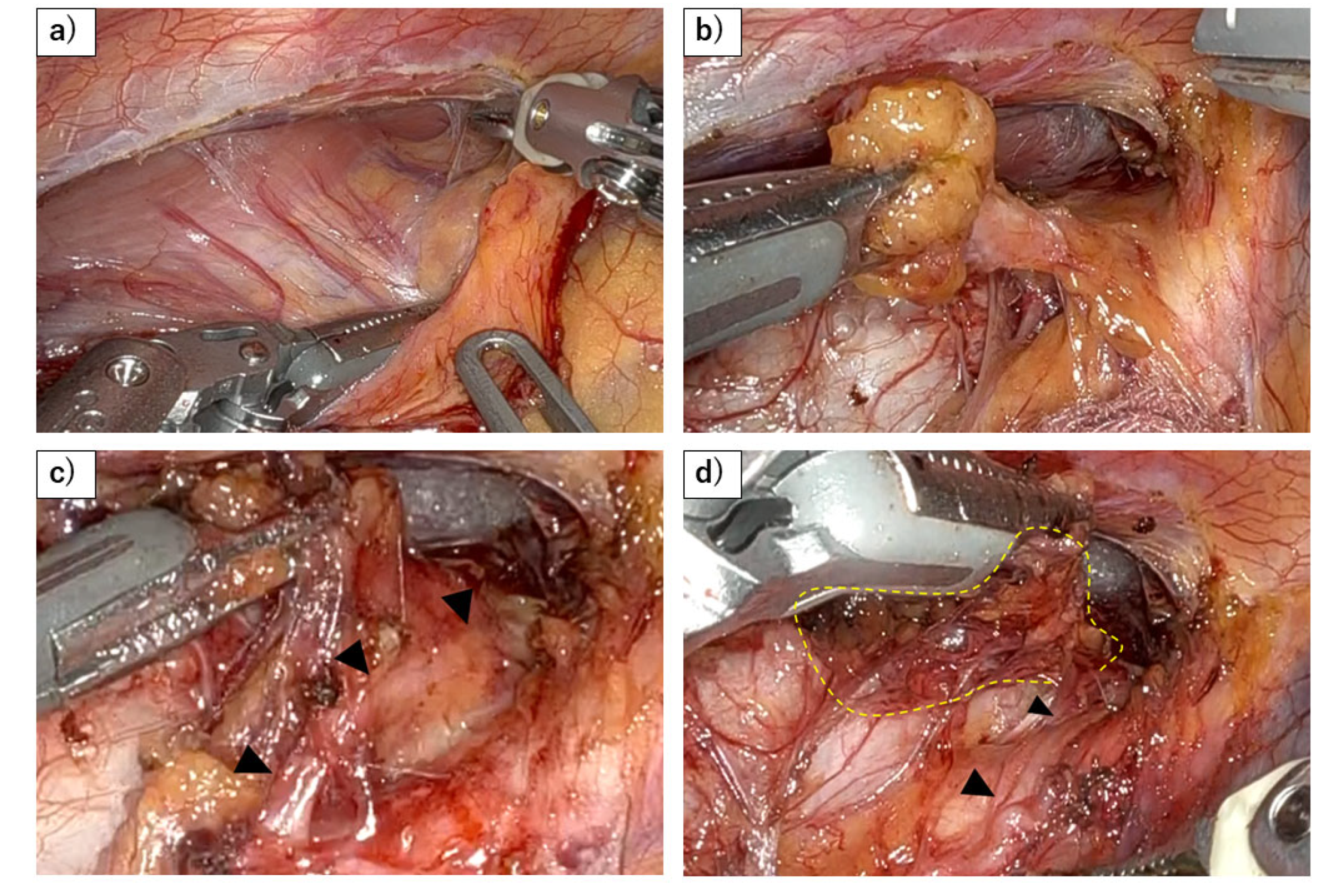
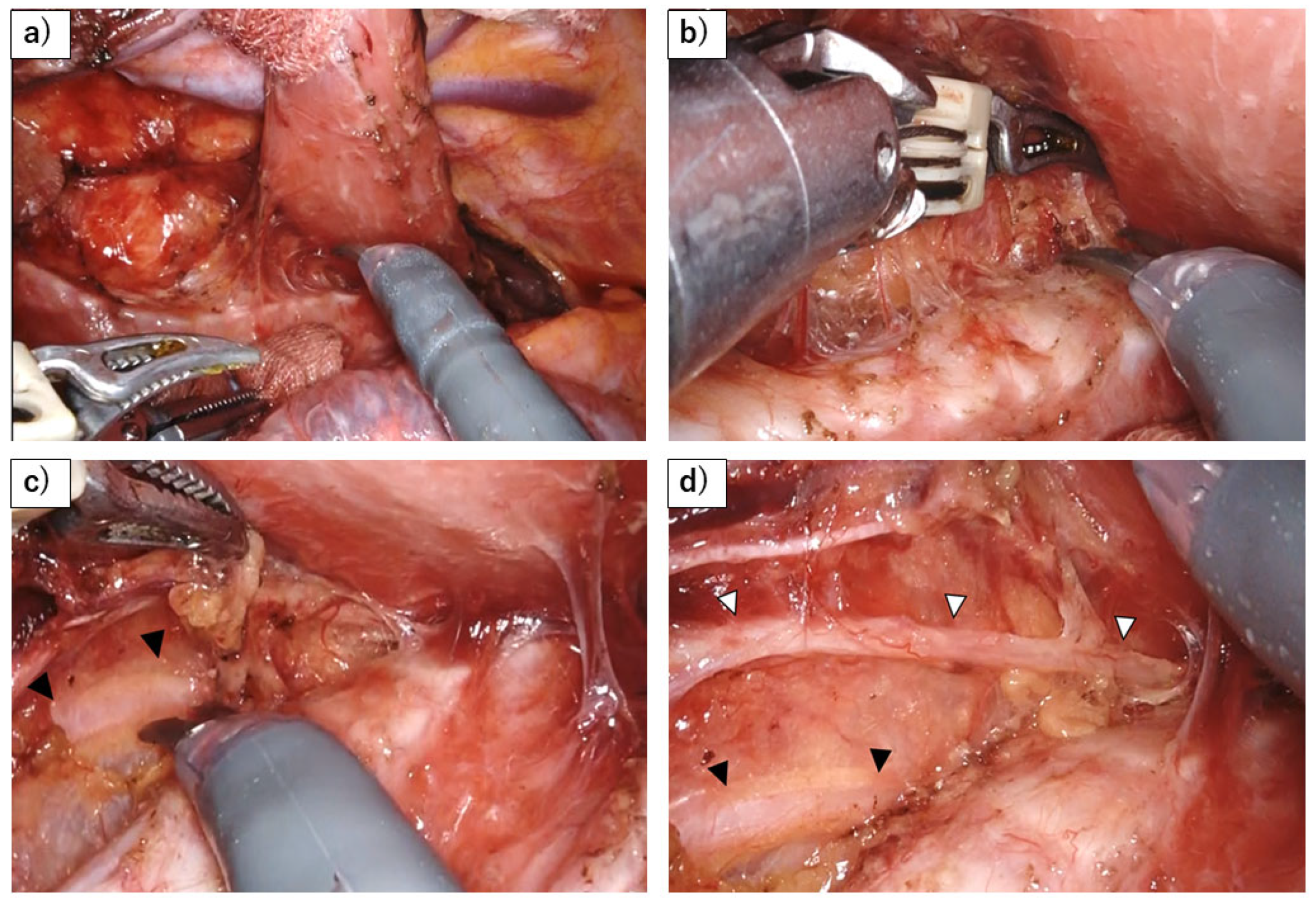
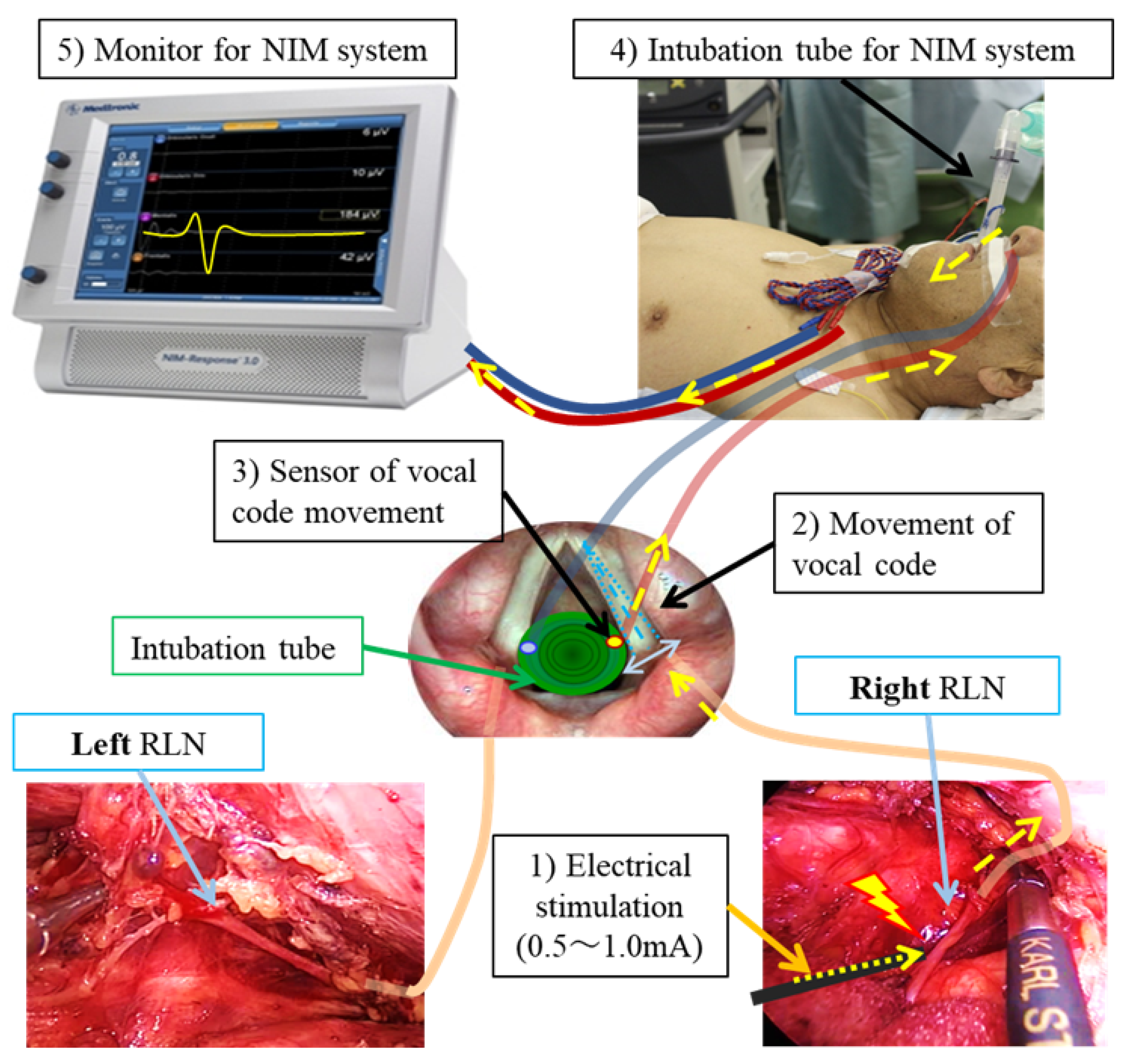
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




