1. Introduction
The rise of wearable sensors has pointed out the way towards athlete monitoring, a cornerstone of sports performance optimisation and injury prevention 1,2. Based on human locomotion, these sensors mainly apply to endurance and team sports, allowing coaches and athletes to understand the exercise demand objectively. However, the use of micro-technology and sensors in resistance training (RT) stands low compared to endurance and field sports. Without such support, the capture of an objective exercise demand for athlete monitoring purposes remains challenging for physical trainers and coaches 3. Popular among amateurs and athletes aiming for fitness, performance enhancement 4, injury prevention 5 and health 6, RT induces a wide range of adaptations at the physiological 4,7, hormonal 8,9, neuromuscular 10 and cardiovascular 11 levels. In this context, one may consider a simple dose-response model in which the training dose (i.e. a quantitative representation of the mechanical work performed) induces adaptations (i.e. the response, illustrated by the adaptations mentioned above). Monitoring RT is therefore a prerequisite for optimising training programs. Inappropriate training doses may indeed lead to performance impairments and injuries 12.
To date, athlete monitoring in RT relies on training load (TL) indexes. According to Wallace et al. (2009) 13 and Impellizzeri et al. (2005) 14, TL usually refers to (i) an external load defined by the mechanical work completed by the athlete, independent of their internal characteristics, and (ii) an internal load, corresponding to the psycho-physiological stresses imposed on the athlete in response to the external load. TLs are derivatives of volume and intensity parameters. The latter is quantified either in an objective way or by subjective estimates 3. Accordingly, the method chosen to quantify TL has specific advantages and drawbacks 3,15,16. In RT, the objective quantification of volume usually refers to the total work performed within a session, while intensity relies on the average intensity of the lifting session 16. A variety of quantification methods may be employed, including those based on relative intensities, normalisation to body mass, or consideration of the load displacement in the calculation. From an athlete monitoring perspective, it is safe to say that internal TL indexes should reflect the body’s adaptations to exercise (i.e. external TL). Ultimately, both should be used to elucidate the athlete’s progression.
The physiological relevance of objective TL indexes in RT has been scarcely studied 17,18. Generally, authors have found limited relevance of the simplest formulation of TL indexes (e.g. the so-called volume load, VL) 16 in terms of metabolic and hormonal responses to resistance exercise 17,18. These results give credit to subjective methods, such as those based on ratings of perceived exertion (RPE), which correlate better with acute physiological responses 17. However, pairwise correlations between common TL indexes (objective and subjective) remain weak or, at best, moderate 18,19. Training load quantification methods used in RT have several limitations. First, the basic formulation of VL, which is based on the product of the number of repetitions and the intensity of the weight lifted, has a reciprocal implication. In terms of training responses, it is theoretically incorrect due to the various effects of resistance exercise intensity on physiological (e.g. fibre types I and II hypertrophic responses 7), hormonal (e.g. growth hormone and cortisol responses 20, chronic changes in insulin-like growth factor-1, β-Endorphins and fluid regulatory hormones changes 8) and metabolic changes (e.g. blood lactate concentrations) 21. Second, the movement of the load should be considered either as a weighting factor of VL or by using a mechanical work calculation to differentiate resistance exercises. Otherwise, one may encounter a rough depiction of the overall TL 15,16,18. Third, sessional intensity is affected by the design of the training bout, such as the inter-set recovery time, which impacts training outcomes in several ways 22. For instance, energetic metabolism benefits from more extended rest periods by recovering the adenosine triphosphate and phosphocreatine energy sources 23, while blood lactate and hormonal concentrations are also influenced 8,24. Thus, inter-set recovery time should be considered in any TL estimates 18. Finally, none of the above TL calculation methods (i.e. VL and derivatives, mechanical work) consider the time a muscle is held under tension (TUT) or the exercise velocity. Yet, it is known that TUT stands for a key factor of the exercise response, influencing muscle contractile properties and leading to chronic neuromuscular adaptations 25,26.
Given these limitations, the common TL quantification methods used in RT lack the requisite physiological evidence. In the context of long-term athlete monitoring, the use of approximated TL may result in practitioners failing to identify meaningful adaptations to exercise, potentially leading to flawed training prescriptions. In line with the current state of the art, and by analogy with the training impulse method of Banister & Hamilton (1985) applied to endurance exercises, we hypothesise that exponential weighting resistance exercise intensity within a TL quantification would improve its physiological relevance. This approach would not necessitate any specific measurement systems for athlete monitoring purposes, but it could also support their use, if any.
The objective of this study was to evaluate the accuracy of the most prevalent methods for quantifying training load (TL), namely, the rating of perceived exertion (RPE), mechanical work, and their primary variants. These methods were evaluated in relation to a set of acute physiological responses specifically designed to assess isolated resistance exercises at sub-maximal intensity. The investigation was conducted within a controlled experimental design, with the aim of obtaining comprehensive and accurate physiological responses to exercise at the muscle level. Following a primary exploratory analysis, two alternative approaches for quantifying relevant TL estimates for resistance exercises were proposed: i) A TL quantification method based on individual physiological responses to exercise; ii) A compressed representation of TL quantification methods and training-related parameters.
2. Materials and Methods
2.1. Experimental Approach to the Problem
To assess the validity of TL quantification methods regarding physiological responses under individually controlled conditions, resistance exercises were performed on an isokinetic dynamometer using concentric contractions only. The experiment was composed of a first testing session for individual-based protocol calibration and three testing sessions that involved low, moderate, and high-intensity resistance exercise modalities (LI, MI and HI, respectively). These three sessions were theoretically volume-equated according to the VL method 16 and in line with previous studies 15,28–31.
2.2. Participants
Fifteen participants were voluntary engaged in the study (eleven males, age: 27 ± 3.3 years, height: 178.36 ± 3.95 cm, body mass (BM): 77.48 ± 7.74 kg, fat mass: 11.11 ± 3.53 % BM; and four healthy females, age: 21.7 ± 1.5 years, height: 169.25 ± 5.03 cm, body mass: 60.62 ± 3.91 kg, fat mass: 21.1 ± 5.28 % BM). To be eligible, participants had to satisfy three conditions: they had to be (i) currently engaged in resistance training with at least six months of experience prior to the start of the study, (ii) familiar with resistance exercises performed at maximal intensities, and (iii) to have no current recurrent lower limbs injury or functional limitations regarding to a knee extension task performed at maximal intensity. The study was conducted in accordance with the standards set by the declaration of Helsinki (2013) involving human subjects. Following an explanation of all procedures, risks and benefits associated with the experimental protocol, each participant gave his/her written informed consent prior to the experimentation. The protocol was reviewed and approved by the local research Ethics Committee (IRB-EM 2001-B, EuroMov, France).
2.3. Experimental Design
Torque - Velocity Profile Modelling
The first testing session allowed for modelling individual torque - velocity profiles (T-V) of the quadriceps group of the dominating leg during an isokinetic leg extension task. Prior to testing, participants completed a four-minute global cycling warm-up at 50 W and a cadence of 50 to 60 revolutions per minute on an ergocycle (Ergoselect, ergoline GmbH, Germany).
Then, the participant was seated on an isokinetic dynamometer (Biodex system 3, Biodex Medical Systems, USA). The shaft was aligned with the axis of rotation of the knee joint to be tested. The torso, waist, pelvis and working leg were secured with straps. Handles were disposed on either side of the chair for open hand placement during the exercise. A shin pad attached to the distal extremity of the mechanical arm was firmly secured to the working leg about 5 cm above the medial malleolus. Once the participant was poised, lever arm amplitudes were recorded in internal to external positions (i.e. from naturally bent knee to fully extended knee, approximately zero degree). The working leg was weighed in an external position and considered in isokinetic measurements.
A specific warm-up followed the setting step. Participants were asked for performing four repetitions of concentric extension at with a progressive increase in intensity. Then, participants performed two repetitions of concentric extension at maximal intensity. Since the knee extension was the only movement of interest, the knee flexion was assisted by returning to the initial position at a velocity of . After a passive rest period of four minutes, the participants performed seven series of concentric extensions, 3 minutes apart at the following velocities in a quasi-randomised order: , , , , , , . To limit the fatiguing effect of the lowest velocities, only two repetitions were performed at and , against three repetitions at other velocities. These velocities were performed before the sixth of the seven series. A one-second break was set between two consecutive contractions to avoid any possible influence of the stretch-shortening cycle. The use of seven points enabled to model a valid and reproducible T-V profile 32.
2.4. Resistance Exercise Protocols
To assign an equated volume between LI, MI, and HI testing sessions (named C1, C2, and C3, respectively), the equivalent relative intensity was obtained from individual T-V profiles. The repetition maximum (RM) and their corresponding relative intensities were then estimated from a nonlinear equation from Reynolds et al. (2006)
33, such as:
Here,
denotes the percentage of relative intensity (% maximal torque) and
denotes the number of expected RM. Hence, the three conditions were performed at 58 %, 77 % and 93 % of the theoretical maximal torque value for which the velocity is null (MVC), corresponding to 24, 9 and 3 theoretical RM. An overview of the testing protocols is given in Appendix 1,
Table 1.
In order to match recordings on a single time frame, mechanical (position, velocity, torque), cardiovascular and neuromuscular measurement systems were coupled using analog signals (Trigno Analog Input Adapter, Delsys, MA, USA).
2.5. Systemic Measurements
Cardiac Measurements
Participants wore two ECG sensors (Trigno EKG Biofeedback, Delsys, MA, USA) for a continuous measure of heart rate (HR) activity. Prior to starting the experiment, the quality of the HR activity recording was visually checked over Q-, R- and S-waves displayed in real time on EMGworks software (Delsys, MA, USA). Heart rate was further extracted from the R-R intervals. The continuous signal was then averaged using a 10-second bin moving average filter. The rate decay of HR during recovery was estimated using a mono-exponential function
with
being a gain constant,
denotes an intercept and
a negative constant for exponential decay.
2.6. Pulmonary Gas Exchange Measurements
Breath-by-breath gas exchanges were analysed through a portable metabolic cart (k4b2, Cosmed, Italy), previously validated by several independent authors in locomotor activities 34. Before each session, the portable system was powered on to warm up for 10 min. Calibration of the oxygen () and carbon dioxide () analysers was performed before every test using two-point calibration with two precision-analysed gas mixtures (room air and a high-precision certified calibration tank gas containing O2 16 %, CO2 5 % and balance nitrogen). Turbine flow calibration was determined using a high-precision 3-L calibration syringe in an eight-pump series. For subsequent numerical analysis, the recorded breath-by-breath gas exchange measurements were linearly interpolated on a second-by-second basis. A moving average filter was applied to the raw data to get an exploitable signal. From the net pulmonary oxygen uptake () and considering a major contribution of glycolytic pathways during exercise, we estimated the net energy expenditure (EE) according to an energy equivalent of per millilitre of 35.
During exercise, the rate of was computed from the linear relationship between and time. At rest, the rate of recovery was given by the generic mono-exponential function defined in Equation 1.
2.7. Metabolic and Hormonal Measurements
Blood lactate concentrations in were collected four times during each testing session using a finger prick and a valid hand-held lactate analyser (Lactate Pro, KDK Corporation, Arkray, Japan) 36. The first sample was collected after the participant being fully equipped and prior to any exercise. A second sample was taken at the onset of the testing (both global and specific warm-ups being completed). Changes in were evaluated at 1 min and 3 min post-exercise in order to cover several possible kinetics of responses following exercise.
In addition, 10 ml of blood was taken at the fingertips for plasma cortisol concentration ) analysis. Immediately after collection, samples were centrifuged for 10 min at 2000 . Then, plasma was collected from the centrifuged sample and stored at . Plasma cortisol analysis was performed using enzyme-linked immunosorbent assay kits (Cortisol ELISA, MN, USA).
2.8. Local Measurements
Mechanical Measurements
For any exercises, torque (Nm), angular velocity () and position () were recorded at a 148 Hz sampling frequency. From the torque production over time, we extracted rate of force development (RFD) values from the onset of exercise to 100 ms, peak RFD and mechanical impulse over the entire repetition ( and in , IMP in , respectively).
2.9. Skeletal Muscle Oxygenation and Oxidative Function Measurements
Locally, skeletal muscle oxidative capacity of the vastus lateralis (VLat) was evaluated by in vivo near-infrared spectroscopy (NIRS). The use of NIRS, which has gained popularity in sports application since the early 2000's 37 is considered as a valid method for evaluating skeletal muscle oxygenation and oxidative metabolism 38,39. The portable NIRS device (PortaLite, Artinis Medical Systems BV, The Netherlands) used in this study was a continuous dual-wavelength system that simultaneously uses the modified Beer-Lambert and spatially resolved spectroscopy (SRS) methods. Changes in myoglobin were assumed to be small compared to haemoglobin 40. Changes in tissue oxyhemoglobin, deoxyhemoglobin and total hemoglobin concentration (, , and , respectively) were measured using the difference in absorption characteristics of light at 750 and 850 nm. The tissue saturation index (TSI) was calculated using the SRS method. Skinfold measurement at the NIRS optodes location was done prior to the first session to ensure valid measurements regarding the adipose tissue thickness. This allowed us to determine an oxygenation index () for subsequent analysis.
From measurements, we estimated the muscle oxygen consumption through the rate decay of during the most representative of the first repetitions per series, in which the ischemia arterial occlusion remains unchanged 41.
2.10. Neuromuscular Measurements
Activity of the VLat, vastus medialis (VMed) and rectus femoris (RFem) were assessed through surface electromyography (EMG) using three sensors (Trigno Avanti, Delsys, MA, USA) located in respect of the SENIAM recommendations
42. Electrode sites were properly shaved and cleaned with alcohol before electrode placement. The sampling frequency of the EMG signals was set at 2048 Hz, recorded through the EMGworks software and exported using the Delsys file utility application (Delsys, MA, USA). Activity of quadriceps muscles were analysed in both time and frequency domains. In time-domain analysis, the integrated signals amplitude was calculated from VLat, VMed and RFem for each knee extensions using a root mean square (
) function (see Equation 2), following a signal rectification and filtering using a second-order low-pass Butterworth filter with a cut-off frequency of 10 Hz. Then, normalisation to the mean signal computed from the first repetition and a time-normalisation were processed, ensuring unbiased within-session and within-participant analysis
43.
In frequency domain analysis, and because testing exercises involved dynamic contractions, short term Fourier transform (STFT) was processed on 125 ms overlapping samples of length
. Then, a power spectral density (PSD) representation allowed for the extraction of median frequencies (MDF) to detect impairment in EMG signals due to muscle fatigue
44. It is defined in the sequel:
where
is the EMG power spectrum at a frequency bin
, and
is the length of frequency bin
.
2.11. Statistical Analysis
First, normality and variance homogeneity of the residual errors were checked by a Shapiro-wilk and a Levene tests, respectively. The distributions of the physiological responses across the three testing sessions were then compared through ANOVAs followed by Tukey’s post-hoc analysis. The marginal mean differences were reported for comparisons. Effect size from ANOVAs was reported as within 95 % confidence intervals (CI). Linear mixed models (LMM) were computed to assess the contribution of variables related to each resistance exercise protocol, with training-related parameters as fixed effects, and participant as a random effect. Standardised coefficients , together with their 95 % CI, were reported as a measure of effect size. In the case of a single fixed effect or continuous variable, unstandardised coefficients were provided. The threshold of significance was set at . Lastly, a principal component analysis (PCA) was performed to build a linear combination of the initial variables that maximizes the variance onto orthogonal axes. A compressed representation of the data was either determined by the first principal component (PC), or a combination of the most contributing PCs using a meta-regression model.
3. Results
In this section, we sequentially present the physiological responses to exercise accounting for in resistance exercise parameters and individual T-V profiles.
3.1. Neuromuscular Responses
Neuromechanics
A first analysis of the mechanical measurement distributions showed significant differences in terms of mechanical work, normalised averaged torque, and mechanical impulse between the three testing conditions (see subfigures in
Figure 1). As expected, exercises performed at higher relative intensities -associated with a lower exercise velocity and hence, a greater TUT- induced the greatest values. Positive correlations were thus found between total mechanical work on the one hand and averaged torque and mechanical impulse on the other (
and
for averaged torque and mechanical impulse, respectively).
Intra-session analysis showed that the torque produced significantly decreased with the accumulation of repetitions (
). Yet, this is not consistent across the testing conditions. An interaction between the protocol and the accumulation of repetitions over the individual torque response suggests that higher relative intensities (
i.e. C2 and C3) induce smaller changes. Details about the contribution of the protocol and the accumulation of repetitions over the torque produced are given in Appendix,
Table 2.
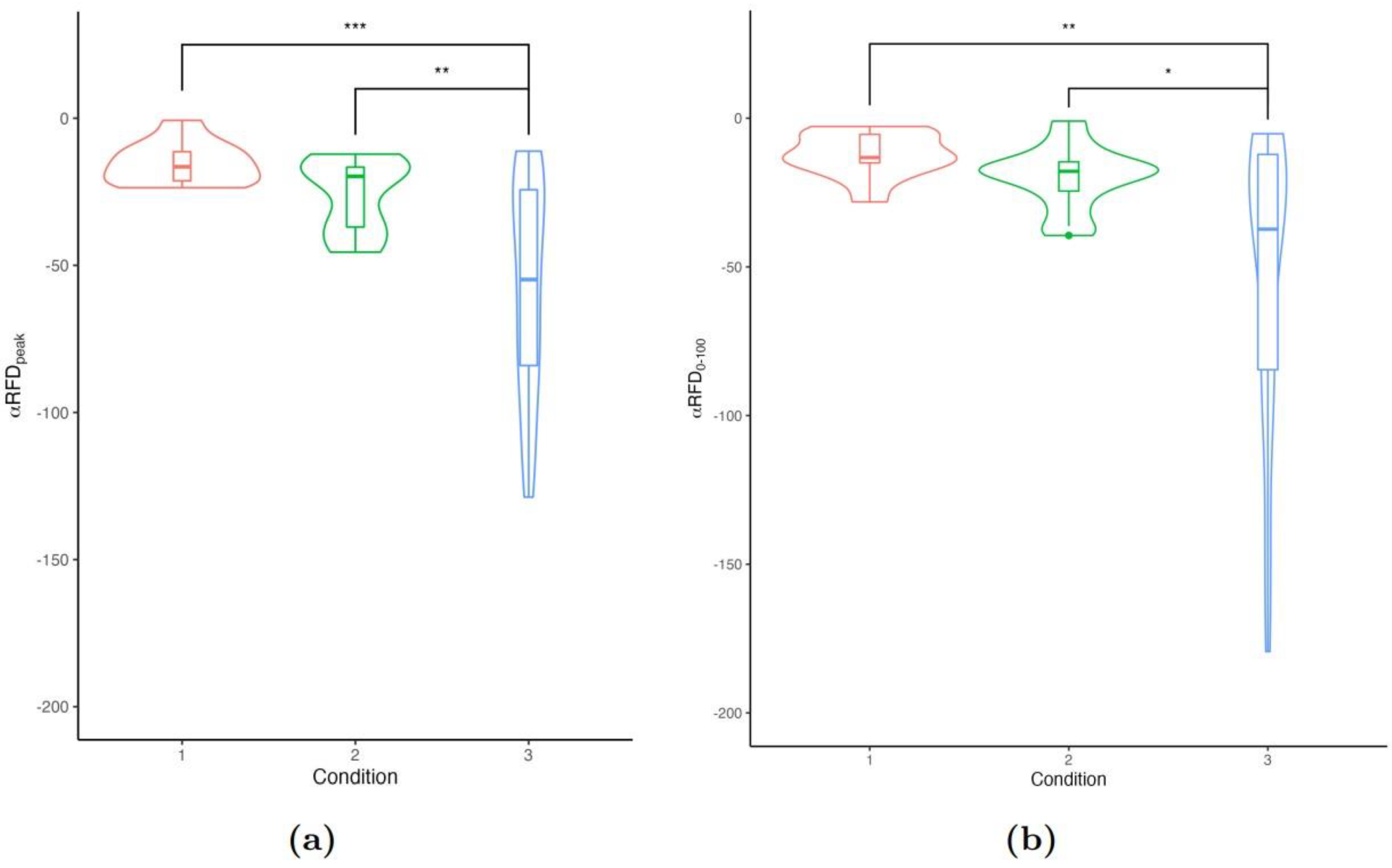
An overiew of averaged RFD over testing sessions indicated that
and
significantly increased between C1 and C2 (LI and MI protocols, p < 0.001). However, changes remain not significant between C2 and C3 sessions despite a large increase of exercise intensity (see subfigures in
Figure 1). Within-session analysis showed that performing numerous repetitions within or across series lowered both
and
(
and
, respectively).
Besides, a significant interaction between the testing condition (i.e. the relative intensity) and the accumulation of repetitions showed that C2 had a greater sustained RFD than C3 and C1.
Having a decreasing effect of the repetitions’ accumulation over
and
suggests that exercise induces progressive impairment of neuromuscular function. However, changes in IMP did not evoke any significant decay across repetitions. Details about model estimates are given in
Table 1.
On this basis, it is possible to estimate the rate of muscle fatigue occurrence from the individual regression slopes. The low intensity condition C1 showed a homogeneous distribution of RFD rate decays, suggesting a relatively consistent apparition of muscle fatigue across participants.
In contrast, C2 and C3 showed a greater variability in
and
rate decays across participants, supporting the singularity in response to exercise at theoretical MI and HI modalities. We note that, according to the averaged population studied, the distributions of RFD slopes are not significantly different between C1 and C2 (
, see Appendix,
Figure 1).
3.2. Electromyographic Activity
In time domain, amplitude of EMG signals from knee extensors was given by the linear combination between values computed from averaged VLat, VMed and RFem signals. Assuming that resistance exercises might induce muscle fatigue, we first investigated the contribution of performing multiple sets (in C2 and C3) to a potential muscular fatigue apparition.
Changes in EMG amplitude distributions over repetitions showed a small increase in values over repetitions () and a slight decrease in normalised averaged torque, as expected and seen in subsection “Neuromechanics” ().
Considering only multiple set testing conditions (C2 and C3), we found through LMM that the averaged RMS computed across sets significantly decreased during C2 (
). As with exercise intensity, absolute exercise velocity had a significant positive effect on changes in leg extensors RMS slope (
). However, the heaviest session, C3, did not show a significant effect on RMS slopes, for which their relationship with the exercise modality remains homogeneous (
). Hence, the results indicate that performing slower repetitions induces greater impairments in RMS, at least for exercises performed at MI. Details about estimated coefficients are provided in Appendix,
Table 3.
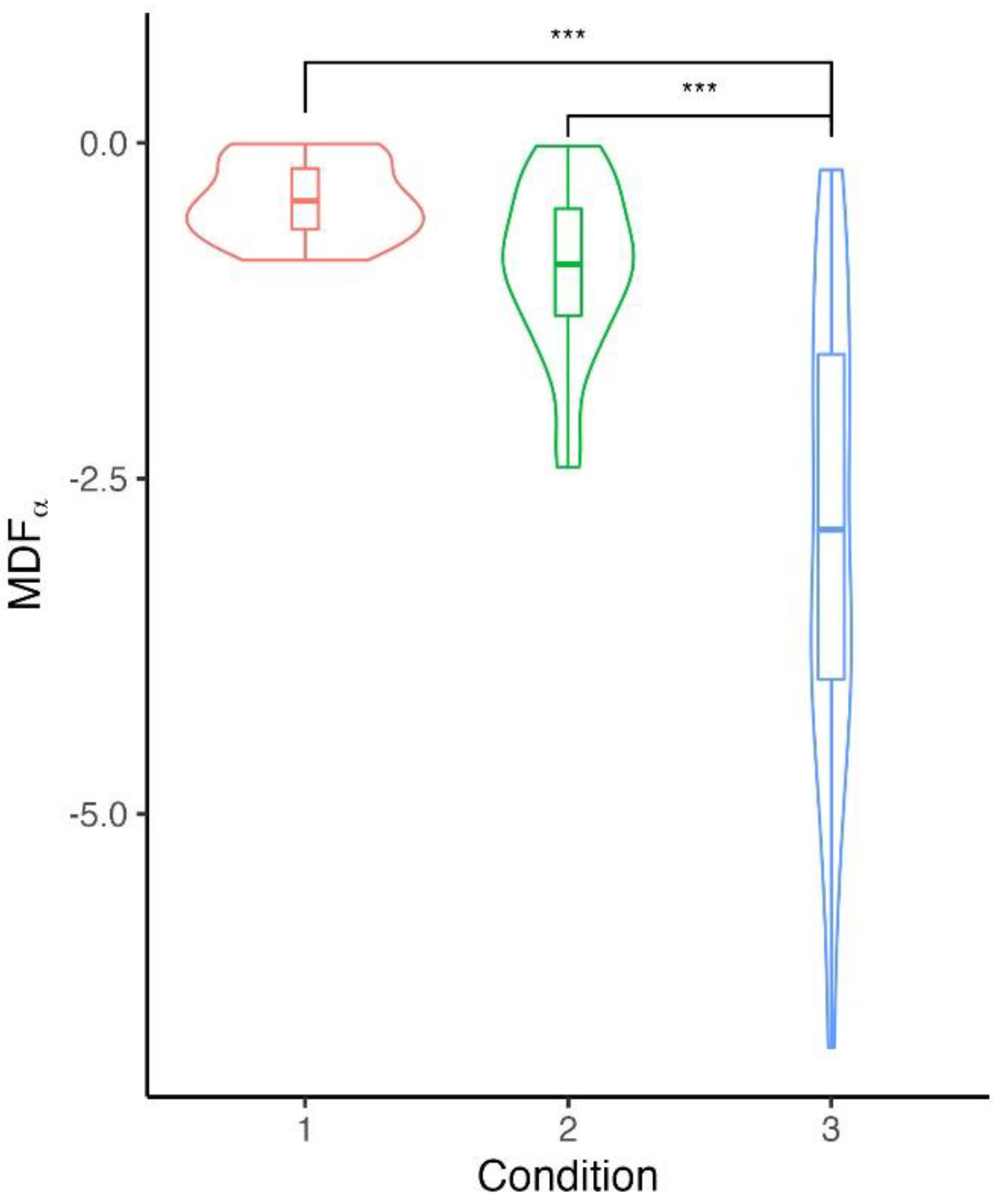
In the frequency domain, we observed a slight downward shift of averaged MDF values over VMed, VLat, and RFem muscles with the accumulation of repetitions (
). Such a decrease was heterogeneous through testing conditions, with a greater decrease for the protocol performed at the highest intensities (
), see
Table 2).
From STFT samples, slopes of MDF averaged over muscles (
) showed a greater magnitude of muscle function impairments at high intensities (see Appendix,
Figure 2) which is supported by the mixed effect regression (
, see
Table 2)
3.3. Metabolic and Hormonal Responses
3.3.1. Blood Lactate Concentrations
Metabolic responses to exercise showed that changes in
were mostly influenced by the individually fitted protocol. The session performed at low intensity and associated with a single set - high volume induced the greatest changes in
regarding baseline values. At the opposite, the higher the exercise intensity, the lower the variation in
after exercise completion (
compared to C1). In addition, a significant interaction between the testing condition and the exercise velocity indicated smaller changes in
in response to high intensity -low velocity- exercises. Full details are provided in Appendix,
Table 4.
3.3.2. Plasma Cortisol Concentrations
Distributions of did not show any significant differences between the three testing conditions (). Considering the experimental design, neither C1, nor C2 and C3 significantly induced a noticeable hormonal stress state when was measured at five minutes post exercise.
3.4. Cardiac and Pulmonary Gas Exchange Kinetics
Heart Rate
One-way repeated measures ANOVAs indicated that distributions of post-exercise HR slopes computed from Equation 1 were significantly lower within C3 than C1 (). In addition, distributions of recovery amplitudes were significantly different only between C3 and C2 ().
3.5. Oxygen Uptake Measurements
At exercise, distributions of the average rate of were not significantly different between testing conditions (), despite substantial differences in terms of exercise intensity and repetitions.
A similar observation was made post-exercise, where
slopes averaged over the session were not significantly different between testing conditions. However, amplitudes of
averaged over each session showed greater amplitudes at recovery for C1, which were associated with higher
values at the onset of recovery phase (
, see
Figure 2).
In analogy with the total mechanical work, we observed a significant increase of total energy expenditure from
measurements across testing conditions (see subfigures in
Figure 2). Naturally, such metabolic measurements are mostly impacted by the magnitude of
as an index of exercise intensity and the exercise duration ruled by the total number of repetitions and TUT.
3.6. Muscle Tissue Oxygenation
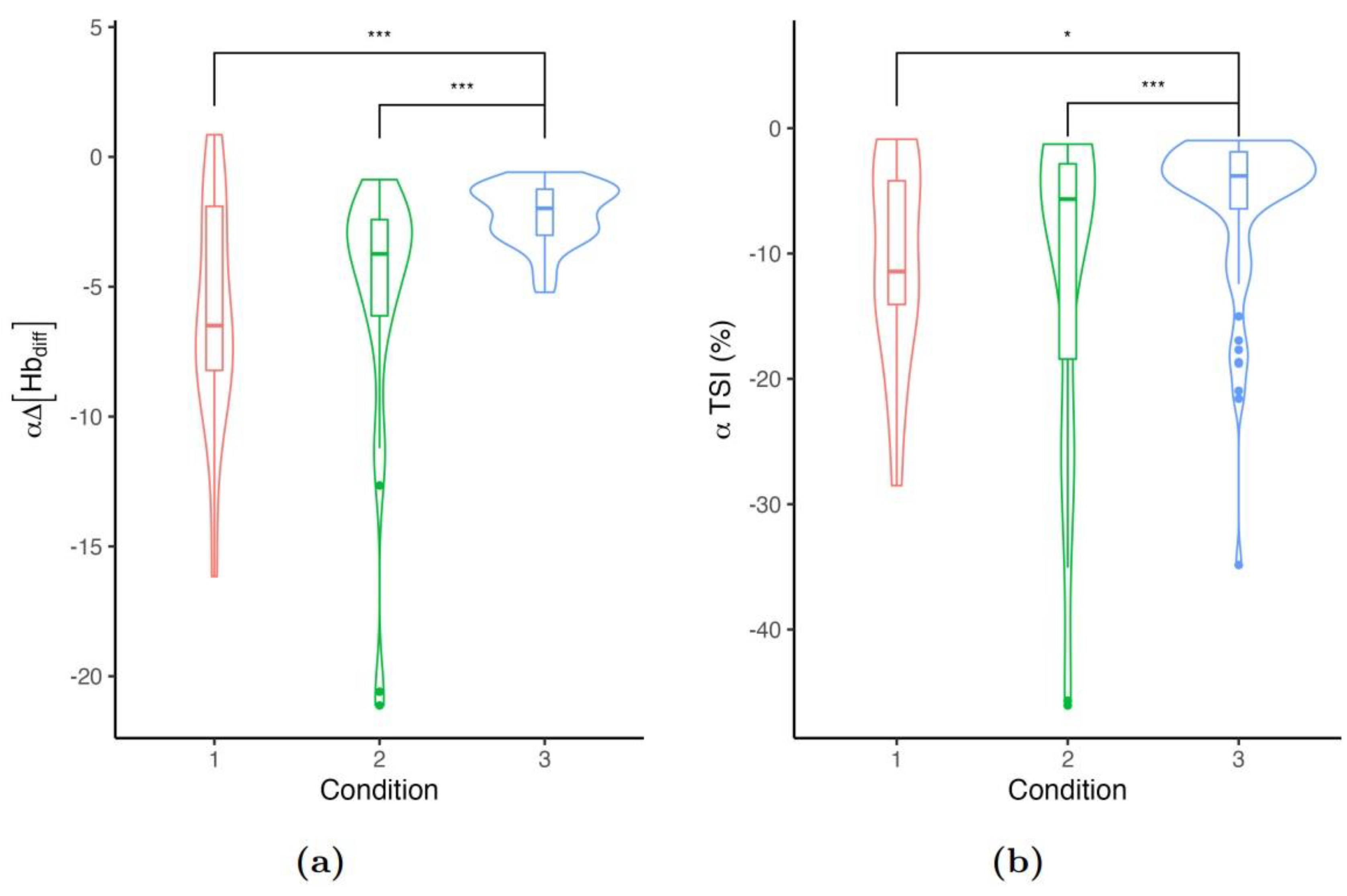
Locally and during exercise, the distributions of
rate decays showed greater shifts within LI and MI testing protocols (
i.e. C1 and C2, respectively, see Appendix,
Figure 3. Likely, TSI measurements reported a similar pattern. For both, we found a significant and negative relationship between the respective physiological measurement and the exercise velocity (
and
for
and TSI, respectively). However, the amplitudes of
and TSI were not significantly different across testing protocols (
).
Figure 3.
Distribution of (a) rate decay of and (b) rate decay of TSI at exercise.
Figure 3.
Distribution of (a) rate decay of and (b) rate decay of TSI at exercise.
3.7. Relationships between Training Load Indexes and Physiological Responses
Using an estimation of muscle fatigue as a weighting factor of objective training load indexes
In analogy with the training impulses of Banister & Hamilton (1985), we defined a new model based on neuromuscular impairments measured at different exercise intensities of resistance exercise. In this context, RFD appears to be (i) a relevant indicator of fatigue apparition and neuromechanics impairments according to the results presented in subsection “Neuromuscular responses” and supported by the literature 10,45, and (ii) a practical, raising and non-invasive parameter that benefits from the recent technological improvements in measurement systems (e.g. linear position transducers and inertial measurement units).
From the averaged rate decays of
observed during exercise, we modelled the non-linear relationship between
and exercise intensity (see
Figure 3) according to a mono-exponential function (see Equation 1)
This relationship allowed for considering a neuromuscular function that is exponentially impaired by exercise intensity. Hence, we defined three formulations of a
-based model of TL quantification in the sequel:
Where
is the amount of repetitions performed,
denotes the relative intensity (% MVC) and
the rate decay such as
. From Equation 3, we write its density correspondence
with
R being the total inter-set recovery duration.
Finally, and like RFD, the IMP measure is becoming increasingly accessible. It could be used as a surrogate for the product of volume and number of repetitions. Hence,
becomes
with
being the number of repetitions,
S denotes the duration of each repetition and
T is the torque produced.
3.8. A Linear Combination of Quantification Methods and Exercise Related Variables
From the results presented so far, we have processed a PCA based on training-related features, the usual TL indexes and the three -based models presented in subsection “Using an estimation of muscle fatigue as a weighting factor of objective training load indexes”.
The first two dimensions express 81.5 % of the total data set inertia (58.3 % and 23.2 % explained by the first and second dimensions, respectively). Graphically, the circle of correlation in Figure 4 shows the correlated features along the first and second axes.
Of the physiological responses observed above, only changes in were likely to be represented on the second dimension. In contrast, MDF, RFD-related ones and EE were mainly represented on the first dimension.
Hence, RPE-related variables seemed to be key indicators of responses, whereas the other variables were likely better suitable to explain neuromuscular and cardio-respiratory responses. In addition, TL methods represented through their density (i.e and ) were likely correlated and anti-correlated with other objective TL indexes and training-related parameters (see Figure 4).
From the projection of the individuals, we identified each cluster that maps with the three testing protocols (see Figure 4). Each of the clusters was well represented on the first dimension, while the second dimension likely depicted the dispersion of the individuals' projection for each protocol.
Similar to the physiological responses, Figure 4 showed that the RPE-related indexes mostly contributed to explaining the individuals projected on the second dimension. On the contrary, the other variables were more likely to represent individuals projected on the first dimension and explained a more significant part of the total variance.
3.9. Relationship between Training Load Quantification Methods and Physiological Responses
Linear relationships between each TL quantification method and the main physiological responses to resistance exercise are provided in
Table 3. A mixed model analysis showed that aggregating the first two PC using a meta-model provided the greatest variance explained by linear relationships for most physiological responses. One notch below, coordinates of the first PC,
and
(see Equations 3 and 5) provided satisfying
values regarding the subjective TL indexes and the VL-based indexes. The latter showed a significant lack in its ability to explain neuromuscular and cardio-respiratory responses.
4. Discussion
This section will first discuss the physiological responses during and after resistance exercises. Then, we will review the relevance of all TL quantification methods investigated in the study to the key physiological responses.
4.1. Physiological Responses to Various Resistance Exercise Protocols
First, differences in terms of mechanical measurement across testing sessions (i.e. averaged torque, total , RFD and impulse, RFD, etc.) were expected, since higher exercise intensity results in a greater force production corresponding to higher levels of muscle activation 46.
Besides, we observed a significant decrease in the torque produced during exercise in the three protocols (C1, C2, and C3), suggesting an accumulation of fatigue through repetitions. Based on a significant interaction effect between the number of repetitions and the testing condition, a reasonable explanation for such heterogeneity in torque decrease might come from a longer recovery time between sets and a shorter set of exercises performed at high intensity.
Similarly, the protocol design might partly explain the heterogeneity of RFD responses to exercise among the testing sessions. Beyond the relative intensity, the interset recovery time and the total number of repetitions might contribute to the sustainability of RFD across the series of exercises. Such results highlight that the neuromuscular responses are singular, protocol design dependent and therefore multifactorial (Borresen & Lambert, 2009; Ratamess et al., 2014; Rodriguez-Rosell et al., 2018) 47,48.
The downward shift of and over the accumulation of repetitions suggests impairment of the neuromuscular function with an increase in exercise repetitions. This is in line with the literature, as force generation (including the rate of RFD) and inorganic phosphate release are closely related 49. Indeed, under muscle fatigue, ions H+ and inorganic phosphate concentrations increase in the myoplasm, impairing the strong bindings in the actomyosin complex and inhibiting the release of calcium in the sarcoplasmic reticulum. These chemo-mechanical changes therefore result in a decrease in force production 50,51. On this basis, RFD has been considered a key indicator of neuromuscular fatigue 45,48. Regarding mechanical impulse, our results support that it does not reflect neuromuscular responses such as the muscular fatigue highlighted by the early phase RFD. However, it is evidence of mechanical work production.
Concurrent decrease of torque output and increase of averaged EMG signals over repetitions indicates neuromuscular adaptations to strenuous exercise 52. Among the potential causes of such neuromuscular changes, some authors have reported a high correlation between the decline of peak torque and the percentage of type II fibers 53,54 and an increase in muscle lactate 54.
We note that adaptation mainly concerns the first testing session (i.e. C1) based on a single set of twenty-four repetitions performed at an intensity close to 60 % of the theoretical MVC. The results support the fact that the relationship between EMG amplitude according to RMS and the torque produced is non-linear (or at least quadratic) 55–57. This might be related to (i) the fusion of individual motor units (MU), and subsequent tetanus phenomena that occur between 60 % and 80 % of MVC 56 and (ii) the fact that the number and amplitude of recruited MUs are not directly related to changes in isokinetic exercise velocities 55. Despite the normalisation of EMG signals used in our study would not allow for inter-session and inter-participant comparisons, the large and significant effect of exercise velocity over EMG-RMS responses remains in agreement with the literature 55.
Carried out at the highest relative intensity, performing shorter sets of repetitions with longer interset recovery time (i.e. C3, HI condition) tends to inhibit such neuromuscular adaptations. This was expected since four minutes of passive rest between three theoretical RM exercises would allow a substantial recovery of neuromuscular function 24. However, EMG analysis in the frequency domain underlines that HI sessions induced the most significant magnitude of muscle function alteration. Specifically, the mechanisms behind the decline of MDF can be attributed to (i) a fall in conduction velocity throughout repetitions and (ii) the muscle phenotype and particularly its fiber type distribution 58. Furthermore, such neuromuscular adaptation could be an essential factor in exercise realisation, optimising force and ensuring economical activation of fatigued muscle by the central nervous system 59.
Regarding the metabolic responses to exercise, changes in also agree with the literature since the lactate response is a function of exercise intensity, volume (according to the accumulation of exercise repetitions), TUT and inter-set recovery time 21,60. As expected, exercises performed at moderate to high intensities, with a moderate to a large number of repetitions and short recovery time, induced the greatest changes in 18.
With respect to , none of the three testing conditions elicited significant modifications. Our results do not corroborate previous findings in which was substantially impacted by resistance exercise performed at moderate intensity and associated with a high volume and low resting periods 17, even in isokinetic conditions 61. In our study, participants performed only a small number of repetitions (equal to or less than 24 repetitions) in a concentric mode only. Therefore, we can safely suppose that our protocols were not strenuous enough to elicit noticeable hormonal stress as measured by .
Cardio-pulmonary results indicate that HR kinetics slightly differ between the three testing sessions. Shorter time courses of HR kinetics are observed at high exercise intensities, whether HR is measured during exercise or recovery. However, the magnitude of HR differences between the protocols remains marginal and points out the negligible impact of localised resistance exercise on cardiac function.
In terms of oxygen uptake, the slight elevation of reported in our study is consistent with the literature 62 and highlights the weak contribution of an isolated muscle group on cardio-pulmonary function 63. Assuming that higher intensity sessions do not induce substantial changes in, our results also corroborate the changes in (a proxy of the anaerobic glycolysis contribution to energy supply), for which the changes were significantly greater for C1 and C2 than C3 (i.e. LI, MI, and HI sessions, respectively). This suggests that performing fewer repetitions at higher intensities does not induce an elevation of during exercise and, therefore, no significant changes in anaerobic metabolism contribution to the task completion, which also supports previous findings 64. However, observed should be interpreted with caution since breath irregularities and apnea times occurred during exercises performed at low velocities.
Locally, a proxy of was estimated through by the rate of , which is a more suitable measurement of oxygen consumption at the level of a muscle group 41,65 than . A correlation is naturally expected between local and systemic measurements. However, apnoea times that occurred at the lowest exercise velocities impaired measurements and their relationship with .
4.2. Training Load Indexes and Their Relationship with Physiological Responses
From a new space of dimensions, PCA reveals that (i) objective TL indexes and mechanical training-related parameters (e.g. exercise velocity and intensity, recovery time, impulse) can represent an average response for a given testing condition, and (ii) subjective TL indexes (i.e. RPE-based features) are likely to discriminate an inter-individual dispersion (see Figure 4).
This is in line with the literature since subjective measures of TL provide extra individual information as a psychophysiological integrator 66, able to differentiate training responses between individuals for a given external TL.
Upon closer examination of the explanatory power of the TL methods with respect to a set of key physiological responses to resistance exercise (see
Table 3), it becomes evident that the common VL method
16 is severely lacking in its ability to describe the physiological responses. That was indeed expected, since testing sessions were theoretically volume-equated using the VL method, whereas the participants showed heterogeneous T-V profiles and different exercise velocities. This questions the physiological relevance of the VL index for training programming purposes. However, accounting for interset recovery time through a weighted representation of VL (
) likely improved its explanatory power regarding the set of physiological responses, as suggested by Marston et al. (2017)
18.
Compared to VL indexes, TL indexes based on dimension reduction (
i.e. the first dimension of PCA
Dim.1, represented by the first PC, and a stacked representation of the first two PCs) come with a broader consideration of exercise and naturally better explain all physiological responses. In practice, performing a PCA over a set of TL methods and training-related parameters is relatively accessible and could be implemented in software for coaches (
e.g. athlete management systems). Using the coordinates of the first PC could thus be a more suitable way of quantifying TL than using a single raw index (see
Dim.1 in
Table 3).
However, making the most of PCs through a stacked representation
67 implies building a model on top of these PCs (see
Stacked.Dim in
Table 3), and hence measuring physiological parameters to calibrate the model correctly. Even though measurement systems have become increasingly democratised in resistance training, this could be a limitation for daily use in an ecological situation.
In addition, weighting objective TL indexes by a generic neuromuscular impairment (
) as shown in
Figure 3 and Equation 3, has also substantially improved the explanation of the main physiological responses to resistance exercise. Yet, dividing
by the interset recovery duration impaired the neuromuscular representation of the proposed model and should be discarded accordingly. By analogy with the training impulses
27, these TL indexes may provide a more relevant estimate of the demand of a resistance training session. Hence, they could be directly integrated into athlete monitoring processes.
Among the indexes compared so far, the Borg RPE, the category ratio subjective scales (CR10) 68 and session RPE (sRPE) could stand valuable as a unique index of TL. That is in line with the literature since their robustness and relevance in athlete monitoring purposes have been proven 3. However, sRPE failed to explain neuromuscular responses to exercise. It suggests that sRPE may not be the most appropriate RPE-based feature to illustrate a neuromuscular response to exercise. A possible explanation could be an interaction effect between RPE and the number of repetitions that could be blurred by the overall session duration, suggesting that physiological outcomes and RPE relationships differ between testing sessions. In addition, the pertinence of considering the time for estimating an RPE-based index (i.e. sRPE) has been questioned by authors 69.
This study has limitations, nevertheless. First, our results apply to localised exercises performed in highly controlled conditions where participants achieved concentric contractions of knee extensors in an open kinetic chain setting. While this experimental setting ensures a comprehensive analysis of physiological acute responses at the muscle, it might only represent a part of the overall responses underpinning resistance exercises, which could be performed in ecological conditions with polyarticular and conventional movements. Even if we succeeded in measuring local (i.e. at the muscle level) responses, systemic responses would be probably underestimated.
In addition, heterogeneous T-V profiles were observed among participants (ranging from hyperbolic and double-hyperbolic to likely linear profiles). Although our profile modelling methodology is recognised as valid 32, we could expect mainly quasi-linear profile shapes if modelled from multi-joint exercises 70. Accordingly, differences in physiological responses to exercise between participants may be somewhat lowered. Yet, it does not discredit the relevance of force-velocity (or T-V) profiling for training programming. Further investigations remain necessary to determine the relationships between TL quantification methods and physiological responses underpinning resistance exercises in ecological conditions.
As a final note, the relevance of TL indexes is essential for athlete monitoring applied to performance improvement and injury prevention 71. In this study, we provided objective TL indexes based on neuromuscular impairments following resistance exercise. Then, we compared them to former TL indexes and showed how they could be integrated into a multidimensional approach to human adaptations to RT. Using a broader set of information -through objective and subjective TL estimations, scheduling, environment, and other training-related factors- would ensure, or at least allow for a thorough understanding of individual responses to exercise for training programming and decision support. In this multidimensional perspective, providing critical insights regarding athletic performance and injuries through key performance indexes and influencing factors is essential. Therefore, dealing with different sources of information requires an appropriate modelling methodology (e.g. dimension reduction methods for high dimensionality and multicollinearity issues) to investigate relationships and causal pathways between a phenomenon and a set of explanatory features with consistency 72. That usually implies a multidisciplinary and close collaboration between sports scientists and data scientists, mainly when the phenomenon of interest is highly complex (e.g. the injury occurrence)73 and where its relationship with training indicators is not straightforward 71. Linking TL estimations to athletic injuries in a unidimensional or restrictive framework may result in the identification of spurious correlations rather than the delineation of the actual causal pathways of training effects and injury occurrence 67,71. It thus emphasizes the importance of a multidimensional and systemic approach to understanding an athlete's response to exercise.
5. Conclusions
In the present study, we measured a set of physiological responses to isolated resistance exercises to provide a more relevant and objective index of TL. Individual muscular properties were considered in the testing through individual torque-velocity profiles. Our results mainly show that at the muscle level, the current objective TL indexes suffer from a simplistic representation of exercises, whereas a more comprehensive approach better describes physiological outcomes. Accordingly, a generic equation of TL based on objective quantification methods and neuromuscular impairment contributes to a greater understanding of the physiological responses to resistance exercise. However, our prime results should be supported by further investigations involving polyarticular resistance exercises in ecological condition.
In conclusion, a condensed representation of the various TL indexes and training-related parameters consistently reflected individual responses to exercise. In order to achieve an accurate differentiation of human responses to exercise, it is essential to consider the complex and multidimensional nature of human adaptations, as well as the concurrent objective and subjective estimates of TL.
6. Practical Applications
Force-velocity profiles strongly impact physiological responses to isolated lower limb resistance exercises and should constitute the basis for individual training programming.
Conventional and objective methods of training load quantification are limited to explaining physiological responses. Considering the muscle fatigue onset using a generic exponential function contributes to a more relevant expression of objective training load indexes and is supported by observed physiological processes. It would apply to any resistance training sessions with or without biomechanical measurement systems, using the generic function where is the amount of repetitions performed, denotes the relative intensity and the rate decay such as .
However, as responses to resistance exercises are heterogeneous and complex, it is not sufficient to consider training load indexes in isolation; they should be considered in conjunction with other methods and training parameters. As an alternative approach, dimension reduction methods, such as principal component analysis, are a valuable tool for compressing information into a single or a few features that can serve as a surrogate for traditional training load indexes. This observational study paves the way for further investigations into ecological conditions. However, the proposed objective methods and training load indexes are applicable in real-world settings and can contribute to a deeper understanding of the athletic response to training for monitoring purposes.
Supplemental Data: Not applicable.
Author Contributions
Conceptualisation, F.I., R.C, S.P.; methodology and investigation, F.I., R.C, T.B., S.P.; recruitment, F.I.; formal analysis and data curation F.I., T.B., S.P.; resources, R.C., S.P.; writing original draft preparation, F.I.; writing—review and editing, F.I., R.C, S.P.; visualisation, F.I.; supervision, R.C., S.P.; project administration, R.C., S.P.; funding acquisition, F.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Association Nationale de la Recherche et de la Technologie (ANRT) grant number 2018/0653.
Data Availability Statement
The datasets analyzed for this study can be available on demand.
Acknowledgments
We would like to acknowledge Simon Pla, Manon Faedy and Chloé Dorey for their technical assistance during the experiment.
Conflicts of Interest Statement
F.I. was employed by the company Seenovate, Montpellier, France. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
|
plasma cortisol concentration; |
|
blood lactate concentration; |
|
muscle oxygenation index; |
|
carbon dioxide; |
| EE |
net energy expenditure; |
| EMG |
electromyography; |
| IMP |
mechanical impulse; |
| LI |
low-intensity; |
| LMM |
linear mixed models; |
| MI |
moderate intensity; |
|
muscle oxygen consumption; |
| HI |
high-intensity; |
| HR |
heart rate; |
| MDF |
median frequency; |
| MVC |
maximum voluntary contraction; |
| NIRS |
near-infrared spectroscopy; |
| PC |
principal component; |
| PCA |
principal component analysis; |
| RFD |
rate of force development; |
|
rate of force development over the first 100 ms of exercise; |
|
peak rate of force development reached over a repetition; |
| RFem |
rectus femoris; |
| RM |
repetition maximum; |
| RMS |
root mean square; |
| RPE |
rate of perceived exertion; |
| RT |
resistance training; |
| SRS |
spatially resolved spectroscopy; |
| STFT |
short term Fourier transform; |
| TL |
training load; |
| TSI |
tissue saturation index; |
| TUT |
time under tension; |
| T-V |
torque-velocity; |
| VMed |
vastus medialis; |
| VL |
volume-load; |
| VLat |
vastus lateralis; |
|
net oxygen uptake. |
References
- Foster C, Daines E, Hector L, Snyder AC, Welsh R. Athletic performance in relation to training load. Wis Med J. 1996;95(6):370-374.
- Halson SL. Monitoring training load to understand fatigue in athletes. Sports Med. 2014;44(2):139-147.
- Scott BR, Duthie GM, Thornton HR, Dascombe BJ. Training monitoring for resistance exercise: theory and applications. Sports Med. 2016;46(5):687-698. [CrossRef]
- Deschenes MR, Kraemer WJ. Performance and physiologic adaptations to resistance training. Am J Phys Med Rehabil. 2002;81(11):S3–S16. [CrossRef]
- Faigenbaum AD, Myer GD. Resistance training among young athletes: safety, efficacy and injury prevention effects. Br J Sports Med. 2010;44(1):56-63. [CrossRef]
- Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116(5):572-584.
- Fry AC. The role of resistance exercise intensity on muscle fibre adaptations. Sports Med. 2004;34(10):663-679. [CrossRef]
- Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35(4):339-361. [CrossRef]
- Walker S, Häkkinen K, Newton RU, et al. Acute responses of comprehensive gonadosteroids and corticosteroids to resistance exercise before and after 10 weeks of supervised strength training. Exp Physiol. 2020;105(3):438-448. [CrossRef]
- Häkkinen K, Alen M, Kraemer WJ, et al. Neuromuscular adaptations during concurrent strength and endurance training versus strength training. Eur J Appl Physiol. 2003;89(1):42-52.
- Fleck S. Cardiovascular adaptations to resistance training. Med Sci Sports Exerc. 1988;20(5). [CrossRef]
- Kibler WB, Chandler TJ, Stracener ES. Musculoskeletal adaptations and injuries due to overtraining. Exerc Sport Sci Rev. 1992;20:99-126.
- Wallace LK, Slattery KM, Coutts AJ. The ecological validity and application of the session-RPE method for quantifying training loads in swimming. J Strength Cond Res. 2009;23(1):33-38. [CrossRef]
- Impellizzeri FM, Rampinini E, Marcora SM. Physiological assessment of aerobic training in soccer. J Sports Sci. 2005;23(6):583-592. [CrossRef]
- McBride JM, McCaulley GO, Cormie P, Nuzzo JL, Cavill MJ, Triplett NT. Comparison of methods to quantify volume during resistance exercise. J Strength Cond Res. 2009;23(1):106-110.
- Haff GG. Quantifying workloads in resistance training: a brief review. Strength Cond J. 2010;10:31-40.
- Genner KM, Weston M. A comparison of workload quantification methods in relation to physiological responses to resistance exercise. J Strength Cond Res. 2014;28(9):2621-2627.
- Marston KJ, Peiffer JJ, Newton MJ, Scott BR. A comparison of traditional and novel metrics to quantify resistance training. Sci Rep. 2017;7(1):1-8. [CrossRef]
- Martorelli AS, De Lima FD, Vieira A, et al. The interplay between internal and external load parameters during different strength training sessions in resistance-trained men. Eur J Sport Sci. 2021;21(1):16-25.
- Ahtiainen JP, Pakarinen A, Kraemer WJ, Häkkinen K. Acute hormonal and neuromuscular responses and recovery to forced vs. maximum repetitions multiple resistance exercises. Int J Sports Med. 2003;24(06):410-418. [CrossRef]
- Lagally KM, Robertson RJ, Gallagher KI, et al. Perceived exertion, electromyography, and blood lactate during acute bouts of resistance exercise. Med Sci Sports Exerc. 2002;34(3):552-559.
- Bird SP, Tarpenning KM, Marino FE. Designing resistance training programmes to enhance muscular fitness. Sports Med. 2005;35(10):841-851. [CrossRef]
- Harris RC, Edwards RHT, Hultman E, Nordesjö LO, Nylind B, Sahlin K. The time course of phosphorylcreatine resynthesis during recovery of the quadriceps muscle in man. Pflugers Arch. 1976;367(2):137-142. [CrossRef]
- Abdessemed D, Duche P, Hautier C, Poumarat G, Bedu M. Effect of recovery duration on muscular power and blood lactate during the bench press exercise. Int J Sports Med. 1999;20(06):368-373.
- Tran QT, Docherty D, Behm D. The effects of varying time under tension and volume load on acute neuromuscular responses. Eur J Appl Physiol. 2006;98(4):402-410. [CrossRef]
- Burd NA, Andrews RJ, West DWD, et al. Muscle time under tension during resistance exercise stimulates differential muscle protein sub-fractional synthetic responses in men. J Physiol. 2012;590(2):351-362. [CrossRef]
- Banister EW, Hamilton CL. Variations in iron status with fatigue modelled from training in female distance runners. Eur J Appl Physiol Occup Physiol. 1985;54(1):16-23. [CrossRef]
- Thornton MK, Potteiger JA. Effects of resistance exercise bouts of different intensities but equal work on EPOC. Med Sci Sports Exerc. 2002;34(4):715-722.
- Lasevicius T, Ugrinowitsch C, Schoenfeld BJ, et al. Effects of different intensities of resistance training with equated volume load on muscle strength and hypertrophy. Eur J Sport Sci. 2018;18(6):772-780.
- Longo AR, Silva-Batista C, Pedroso K, et al. Volume load rather than resting interval influences muscle hypertrophy during high-intensity resistance training. J Strength Cond Res. 2022;36(6), 1554-1559. [CrossRef]
- Pearson J, Wadhi T, Barakat C, et al. Does Varying Repetition Tempo in a Single-Joint Lower Body Exercise Augment Muscle Size and Strength in Resistance-Trained Men? J Strength Cond Res. 2021;36(8), 2162-2168.
- Lemaire A, Ripamonti M, Ritz M, Rahmani A. Agreement of three vs. eight isokinetic preset velocities to determine knee extensor torque-and power-velocity relationships. Isokin Exerc Sci. 2014;22(1):1-7.
- Reynolds JM, Gordon TJ, Robergs RA. Prediction of one repetition maximum strength from multiple repetition maximum testing and anthropometry. J Strength Cond Res. 2006;20(3):584-592.
- Duffield R, Dawson B, Pinnington HC, Wong P. Accuracy and reliability of a Cosmed K4b2 portable gas analysis system. J Sci Med Sport. 2004;7(1):11-22.
- Candau R, Belli A, Millet GY, Georges D, Barbier B, Rouillon JD. Energy cost and running mechanics during a treadmill run to voluntary exhaustion in humans. Eur J Appl Physiol Occup Physiol. 1998;77(6):479-485. [CrossRef]
- Baldari C, Bonavolontà V, Emerenziani G Pietro, Gallotta MC, Silva AJ, Guidetti L. Accuracy, reliability, linearity of Accutrend and Lactate Pro versus EBIO plus analyzer. Eur J Appl Physiol. 2009;107(1):105-111.
- Perrey S, Quaresima V, Ferrari M. Muscle Oximetry in Sports Science: An Updated Systematic Review. Sports Medicine. 2024;54(4):975-996. doi:10.1007/s40279-023-01987-x.
- Barstow TJ. Understanding near infrared spectroscopy and its application to skeletal muscle research. J Appl Physiol. 2019;126(5):1360-1376. [CrossRef]
- Hamaoka T, McCully KK. Review of early development of near-infrared spectroscopy and recent advancement of studies on muscle oxygenation and oxidative metabolism. J Physiol Sci. 2019;69(6):799-811.
- Spires J, Lai N, Zhou H, Saidel GM. Hemoglobin and myoglobin contributions to skeletal muscle oxygenation in response to exercise. In: Oxygen Transport to Tissue XXXII. Springer; 2011:347-352.
- Ferrari M, Muthalib M, Quaresima V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments. Philos Trans A Math Phys Eng Sci. 2011;369(1955):4577-4590.
- Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10(5):361-374.
- Halaki M, Ginn K. Normalization of EMG signals: to normalize or not to normalize and what to normalize to. In: Computational Intelligence in Electromyography Analysis-a Perspective on Current Applications and Future Challenges. InTech Rijeka; 2012:175-194.
- Thongpanja S, Phinyomark A, Phukpattaranont P, Limsakul C. Mean and median frequency of EMG signal to determine muscle force based on time-dependent power spectrum. Elektron Elektrotech. 2013;19(3):51-56.
- D’Emanuele S, Maffiuletti NA, Tarperi C, Rainoldi A, Schena F, Boccia G. Rate of Force Development as an Indicator of Neuromuscular Fatigue: A Scoping Review. Front Hum Neurosci. 2021;15:387.
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28(3):560-580. [CrossRef]
- Borresen J, Lambert MI. The quantification of training load, the training response and the effect on performance. Sports Med. 2009;39(9):779-795.
- Rodriguez-Rosell D, Pareja-Blanco F, Aagaard P, González-Badillo JJ. Physiological and methodological aspects of rate of force development assessment in human skeletal muscle. Clin Physiol Funct Imaging. 2018;38(5):743-762.
- Tesi C, Colomo F, Nencini S, Piroddi N, Poggesi C. The effect of inorganic phosphate on force generation in single myofibrils from rabbit skeletal muscle. Biophys J. 2000;78(6):3081-3092.
- Allen DG, Westerblad H. Role of phosphate and calcium stores in muscle fatigue. J Physiol. 2001;536(3):657-665.
- Tesi C, Colomo F, Piroddi N, Poggesi C. Characterization of the cross-bridge force-generating step using inorganic phosphate and BDM in myofibrils from rabbit skeletal muscles. J Physiol. 2002;541(1):187-199.
- Ebersole KT, O’Connor KM, Wier AP. Mechanomyographic and electromyographic responses to repeated concentric muscle actions of the quadriceps femoris. J Electromyogr Kinesiol. 2006;16(2):149-157.
- Nilsson J, Tesch P, Thorstensson A. Fatigue and EMG of repeated fast voluntary contractions in man. Acta Physiol Scand. 1977;101(2):194-198.
- Horita T, Ishiko T. Relationships between muscle lactate accumulation and surface EMG activities during isokinetic contractions in man. Eur J Appl Physiol Occup Physiol. 1987;56(1):18-23.
- Smith DB, Housh TJ, Johnson GO, Evetovich TK, Ebersole KT, Perry SR. Mechanomyographic and electromyographic responses to eccentric and concentric isokinetic muscle actions of the biceps brachii. Muscle Nerve. 1998;21(11):1438-1444.
- Madeleine P, Bajaj P, Søgaard K, Arendt-Nielsen L. Mechanomyography and electromyography force relationships during concentric, isometric and eccentric contractions. J Electromyogr Kinesiol. 2001;11(2):113-121.
- Kuriki HU, Mello EM, De Azevedo FM, Takahashi LSO, Alves N, de Faria Negrão Filho R. The Relationship between Electromyography and Muscle Force. INTECH Open Access Publisher; 2012.
- Häkkinen K, Komi P V. Electromyographic and mechanical characteristics of human skeletal muscle during fatigue under voluntary and reflex conditions. Electroencephalogr Clin Neurophysiol. 1983;55(4):436-444.
- Enoka RM, Stuart DG. Neurobiology of muscle fatigue. J Appl Physiol. 1992;72(5):1631-1648.
- Lacerda LT, Martins-Costa HC, Diniz RCR, et al. Variations in repetition duration and repetition numbers influence muscular activation and blood lactate response in protocols equalized by time under tension. J Strength Cond Res. 2016;30(1):251-258. [CrossRef]
- Paccotti P, Minetto M, Terzolo M, et al. Effects of high-intensity isokinetic exercise on salivary cortisol in athletes with different training schedules: relationships to serum cortisol and lactate. Int J Sports Med. 2005;26(09):747-755. [CrossRef]
- Ratamess NA, Rosenberg JG, Kang J, et al. Acute oxygen uptake and resistance exercise performance using different rest interval lengths: The influence of maximal aerobic capacity and exercise sequence. J Strength Cond Res. 2014;28(7):1875-1888.
- Muramatsu S, Katao S, Homma I. Cardiorespiratory Responses and Mechanical Efficiency During Repeated Isokinetic Extension-Flexion Exercises of the Upper and Lower Limbs. Showa Uni J Med Sci. 1995;7(2):163-172.
- Kang J, Hoffman JR, Im J, et al. Evaluation of physiological responses during recovery following three resistance exercise programs. J Strength Cond Res. 2005;19(2):305-309.
- Perrey S, Ferrari M. Muscle oximetry in sports science: a systematic review. Sports Med. 2018;48(3):597-616.
- Eston R. Use of ratings of perceived exertion in sports. Int J Sports Physiol Perform. 2012;7(2):175-182.
- Imbach F, Sutton-Charani N, Montmain J, Candau R, Perrey S. The Use of Fitness-Fatigue Models for Sport Performance Modelling: Conceptual Issues and Contributions from Machine-Learning. Sports Med Open. 2022;8(1):1-6.
- Borg G. Borg’s Perceived Exertion and Pain Scales. Human kinetics; 1998.
- Agostinho MF, Philippe AG, Marcolino GS, et al. Perceived training intensity and performance changes quantification in judo. J Strength Cond Res. 2015;29(6):1570-1577.
- Rivière JR, Morin JB, Bowen M, Cross MR, Messonnier LA, Samozino P. Exploring the Low Force-High Velocity Domain of the Force–Velocity Relationship in Acyclic Lower-Limb Extensions. Sports Med Open. 2023;9(1). doi:10.1186/s40798-023-00598-0.
- Kalkhoven JT, Watsford ML, Coutts AJ, Edwards WB, Impellizzeri FM. Training load and injury: causal pathways and future directions. Sports Med. 2021;51(6):1137-1150.
- Imbach F, Perrey S, Chailan R, Meline T, Candau R. Training load responses modelling and model generalisation in elite sports. Sci Rep. 2022;12(1):1-14.
- Vallance E, Sutton-Charani N, Imoussaten A, Montmain J, Perrey S. Combining internal- and external-training-loads to predict non-contact injuries in soccer. Applied Sciences (Switzerland). 2020;10(15). doi:10.3390/APP10155261.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).