Submitted:
17 October 2024
Posted:
21 October 2024
You are already at the latest version
Abstract
Keywords:
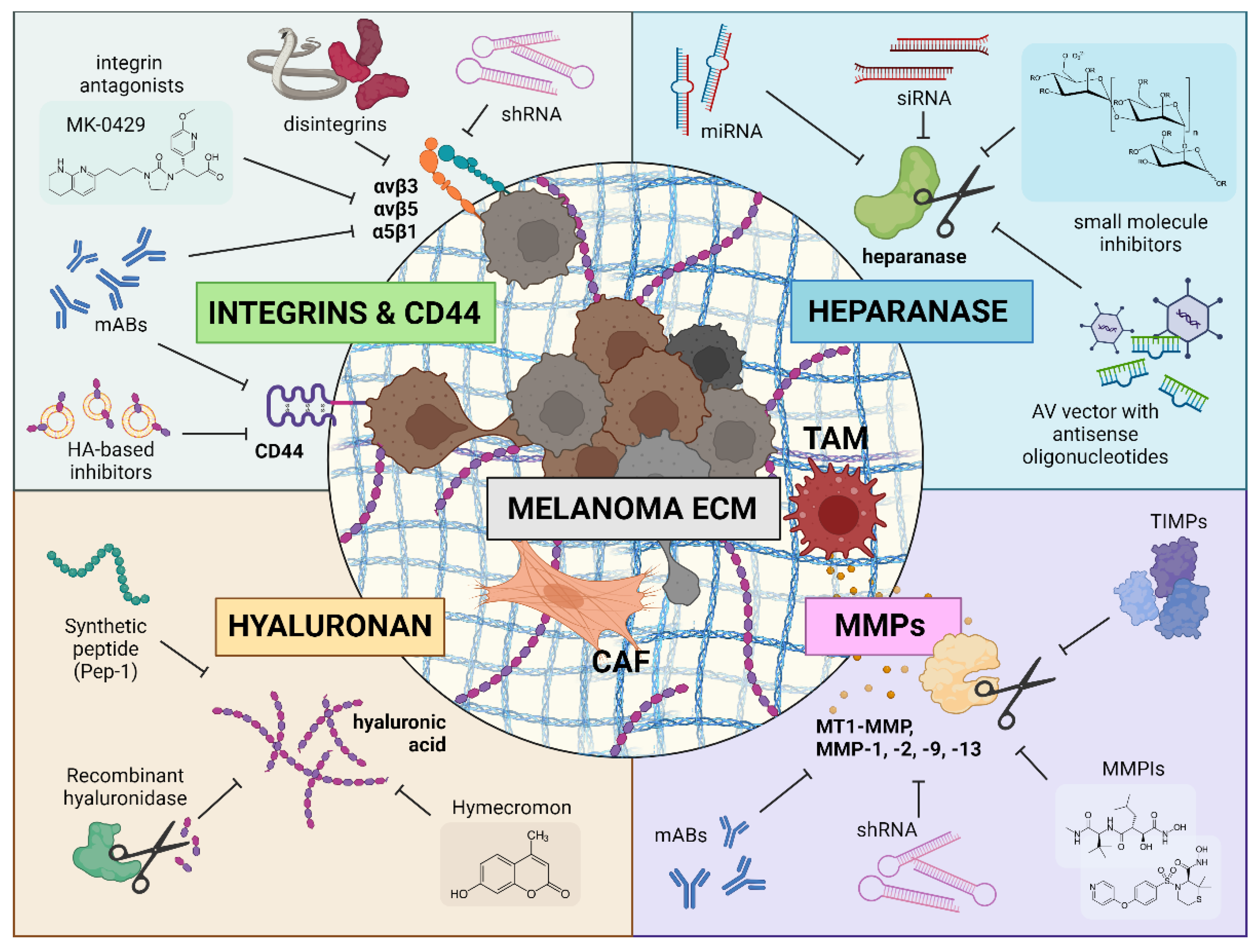
1. Introduction
2. Extracellular Matrix in Melanoma
3. Heparanase Targeting
3.1. PI-88
3.2. PG545
4. MMP Targeting
4.1. MMP Inhibitors
4.1.1. First Generation MMPI
4.1.2. Second Generation MMPI
4.1.3. Alternative Approaches to MMP Inhibition
4.2. Tissue Inhibitors of Metalloproteinases
4.2.1. Recombinant TIMPs
4.2.2. Genetic Vectors Encoding TIMPs
5. Hyaluronic Acid Targeting
5.1. Low-Molecular Weight Inhibitors
5.2. Hyaluronidases
6. Integrins Targeting
6.1. Disintegrins
6.2. Non-Disintegrins Inhibitors
7. Non-Integrin Receptors Targeting
7.1. CD44 Inhibitors
7.1.1. CD44 Hyaluronic Acid-Based Inhibitors
7.1.2. CD44 Monoclonal Antibody Inhibitors
7.1.3. CD44 Alternative Inhibitors
7.2. DDR1/2 Inhibitors
8. Conclusion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Strashilov, S.; Yordanov, A. Aetiology and Pathogenesis of Cutaneous Melanoma: Current Concepts and Advances. International Journal of Molecular Sciences 2021, 22, 6395. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fang, Y.; Chen, H.; Zhang, T.; Yin, X.; Man, J.; Yang, X.; Lu, M. Spatiotemporal Trends of the Global Burden of Melanoma in 204 Countries and Territories from 1990 to 2019: Results from the 2019 Global Burden of Disease Study. Neoplasia 2022, 24, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, G. Histopathological Diagnosis of Malignant Melanoma at the Dawn of 2023: Knowledge Gained and New Challenges. Dermatopathology 2023, 10, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol 2022, 158, 495–503. [Google Scholar] [CrossRef]
- Park, S.L.; Le Marchand, L.; Wilkens, L.R.; Kolonel, L.N.; Henderson, B.E.; Zhang, Z.-F.; Setiawan, V.W. Risk Factors for Malignant Melanoma in White and Non-White/Non-African American Populations: The Multiethnic Cohort. Cancer Prev Res (Phila) 2012, 5, 423–434. [Google Scholar] [CrossRef]
- Mattia, G.; Puglisi, R.; Ascione, B.; Malorni, W.; Carè, A.; Matarrese, P. Cell Death-Based Treatments of Melanoma:Conventional Treatments and New Therapeutic Strategies. Cell Death Dis 2018, 9, 112. [Google Scholar] [CrossRef]
- Zhou, A.Y.; Johnson, D.B. Combinatorial Therapies in Melanoma: MAPK Inhibitors and Beyond. Am J Clin Dermatol 2018, 19, 181–193. [Google Scholar] [CrossRef]
- Sun, J.; Carr, M.J.; Khushalani, N.I. Principles of Targeted Therapy for Melanoma. Surg Clin North Am 2020, 100, 175–188. [Google Scholar] [CrossRef]
- Broman, K.K.; Dossett, L.A.; Sun, J.; Eroglu, Z.; Zager, J.S. Update on BRAF and MEK Inhibition for Treatment of Melanoma in Metastatic, Unresectable, and Adjuvant Settings. Expert Opin Drug Saf 2019, 18, 381–392. [Google Scholar] [CrossRef]
- Falcone, I.; Conciatori, F.; Bazzichetto, C.; Ferretti, G.; Cognetti, F.; Ciuffreda, L.; Milella, M. Tumor Microenvironment: Implications in Melanoma Resistance to Targeted Therapy and Immunotherapy. Cancers 2020, 12, 2870. [Google Scholar] [CrossRef]
- Kharouf, N.; Flanagan, T.W.; Hassan, S.-Y.; Shalaby, H.; Khabaz, M.; Hassan, S.-L.; Megahed, M.; Haikel, Y.; Santourlidis, S.; Hassan, M. Tumor Microenvironment as a Therapeutic Target in Melanoma Treatment. Cancers 2023, 15, 3147. [Google Scholar] [CrossRef] [PubMed]
- Giavina-Bianchi, M.H.; Giavina-Bianchi, P.F.; Festa, C. Melanoma: Tumor Microenvironment and New Treatments*. An. Bras. Dermatol. 2017, 92, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Blansfield, J.A.; Caragacianu, D.; Alexander, H.R.; Tangrea, M.A.; Morita, S.Y.; Lorang, D.; Schafer, P.; Muller, G.; Stirling, D.; Royal, R.E.; et al. Combining Agents That Target the Tumor Microenvironment Improves the Efficacy of Anticancer Therapy. Clin Cancer Res 2008, 14, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef]
- Swartz, M.A.; Iida, N.; Roberts, E.W.; Sangaletti, S.; Wong, M.H.; Yull, F.E.; Coussens, L.M.; DeClerck, Y.A. Tumor Microenvironment Complexity: Emerging Roles in Cancer Therapy. Cancer Res 2012, 72, 2473–2480. [Google Scholar] [CrossRef]
- Mhaidly, R.; Mechta-Grigoriou, F. Fibroblast Heterogeneity in Tumor Micro-Environment: Role in Immunosuppression and New Therapies. Semin Immunol 2020, 48, 101417. [Google Scholar] [CrossRef]
- Monteran, L.; Erez, N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front Immunol 2019, 10, 1835. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers (Basel) 2014, 6, 1670–1690. [Google Scholar] [CrossRef]
- Gao, J.; Liang, Y.; Wang, L. Shaping Polarization Of Tumor-Associated Macrophages In Cancer Immunotherapy. Front Immunol 2022, 13, 888713. [Google Scholar] [CrossRef]
- Scott, E.N.; Gocher, A.M.; Workman, C.J.; Vignali, D.A.A. Regulatory T Cells: Barriers of Immune Infiltration Into the Tumor Microenvironment. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Chaudhary, B.; Elkord, E. Regulatory T Cells in the Tumor Microenvironment and Cancer Progression: Role and Therapeutic Targeting. Vaccines 2016, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Ostrand-Rosenberg, S.; Sinha, P.; Beury, D.W.; Clements, V.K. Cross-Talk between Myeloid-Derived Suppressor Cells (MDSC), Macrophages, and Dendritic Cells Enhances Tumor-Induced Immune Suppression. Semin Cancer Biol 2012, 22, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Fleming, V.; Hu, X.; Weber, R.; Nagibin, V.; Groth, C.; Altevogt, P.; Utikal, J.; Umansky, V. Targeting Myeloid-Derived Suppressor Cells to Bypass Tumor-Induced Immunosuppression. Front Immunol 2018, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, A.A.; Watkins, S.K. Immune Suppression in the Tumor Microenvironment: A Role for Dendritic Cell-Mediated Tolerization of T Cells. Cancer Immunol Immunother 2012, 61, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Shaul, M.E.; Fridlender, Z.G. Neutrophils as Active Regulators of the Immune System in the Tumor Microenvironment. J Leukoc Biol 2017, 102, 343–349. [Google Scholar] [CrossRef]
- Duong, M.N.; Geneste, A.; Fallone, F.; Li, X.; Dumontet, C.; Muller, C. The fat and the bad: Mature adipocytes, key actors in tumor progression and resistance. Oncotarget 2017, 8, 57622–57641. [Google Scholar] [CrossRef]
- Varol, C. Tumorigenic Interplay Between Macrophages and Collagenous Matrix in the Tumor Microenvironment. Methods Mol Biol 2019, 1944, 203–220. [Google Scholar] [CrossRef]
- Lu, P.; Weaver, V.M.; Werb, Z. The Extracellular Matrix: A Dynamic Niche in Cancer Progression. Journal of Cell Biology 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Vannucci, L. Stroma as an Active Player in the Development of the Tumor Microenvironment. Cancer Microenviron 2015, 8, 159–166. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular Matrix (ECM) Stiffness and Degradation as Cancer Drivers. J Cell Biochem 2019, 120, 2782–2790. [Google Scholar] [CrossRef]
- Brassart-Pasco, S.; Brézillon, S.; Brassart, B.; Ramont, L.; Oudart, J.-B.; Monboisse, J.C. Tumor Microenvironment: Extracellular Matrix Alterations Influence Tumor Progression. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Pampena, R.; Kyrgidis, A.; Lallas, A.; Moscarella, E.; Argenziano, G.; Longo, C. A Meta-Analysis of Nevus-Associated Melanoma: Prevalence and Practical Implications. J Am Acad Dermatol 2017, 77, 938–945e4. [Google Scholar] [CrossRef] [PubMed]
- Martín-Gorgojo, A.; Nagore, E. Melanoma Arising in a Melanocytic Nevus. Actas Dermosifiliogr (Engl Ed) 2018, 109, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Botti, G.; Cerrone, M.; Scognamiglio, G.; Anniciello, A.; Ascierto, P.A.; Cantile, M. Microenvironment and Tumor Progression of Melanoma: New Therapeutic Prospectives. J Immunotoxicol 2013, 10, 235–252. [Google Scholar] [CrossRef]
- Van Duinen, C.M.; Fleuren, G.J.; Bruijn, J.A. The Extracellular Matrix in Pigmented Skin Lesions: An Immunohistochemical Study. Histopathology 1994, 24, 33–40. [Google Scholar] [CrossRef]
- McGary, E.C.; Lev, D.C.; Bar-Eli, M. Cellular Adhesion Pathways and Metastatic Potential of Human Melanoma. Cancer Biol Ther 2002, 1, 459–465. [Google Scholar] [CrossRef]
- Smolle, J.; Fiebiger, M.; Hofmann-Wellenhof, R.; Kerl, H. Quantitative Morphology of Collagen Fibers in Cutaneous Malignant Melanoma and Melanocytic Nevus. Am J Dermatopathol 1996, 18, 358–363. [Google Scholar] [CrossRef]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The Extracellular Matrix Modulates the Hallmarks of Cancer. EMBO Rep 2014, 15, 1243–1253. [Google Scholar] [CrossRef]
- Kavuturu, A.; Alves Constantino, M.; Sassano, A.; Merlino, G. Abstract 4148: Extracellular Matrix Biophysical Properties Change during Nevus to Melanoma Progression. Cancer Research 2024, 84, 4148–4148. [Google Scholar] [CrossRef]
- Kumar, S.M.; Yu, H.; Edwards, R.; Chen, L.; Kazianis, S.; Brafford, P.; Acs, G.; Herlyn, M.; Xu, X. Mutant V600E BRAF Increases Hypoxia Inducible Factor-1α Expression in Melanoma. Cancer Research 2007, 67, 3177–3184. [Google Scholar] [CrossRef]
- O’Connell, M.P.; Marchbank, K.; Webster, M.R.; Valiga, A.A.; Kaur, A.; Vultur, A.; Li, L.; Herlyn, M.; Villanueva, J.; Liu, Q.; et al. Hypoxia Induces Phenotypic Plasticity and Therapy Resistance in Melanoma via the Tyrosine Kinase Receptors ROR1 and ROR2. Cancer Discov 2013, 3, 1378–1393. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, M.; Zhang, L.; Shen, D.; Xu, X.; Yi, Q.; Tang, L. SNF5, a Core Subunit of SWI/SNF Complex, Regulates Melanoma Cancer Cell Growth, Metastasis, and Immune Escape in Response to Matrix Stiffness. Transl Oncol 2022, 17, 101335. [Google Scholar] [CrossRef]
- Brás, M.M.; Radmacher, M.; Sousa, S.R.; Granja, P.L. Melanoma in the Eyes of Mechanobiology. Front Cell Dev Biol 2020, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.M.; Yeyeodu, S.T.; McLaughlin, A.; Schuman, D.; Taylor, D.K. In Situ Drug Delivery to Breast Cancer-Associated Extracellular Matrix. ACS Chem. Biol. 2018, 13, 2825–2840. [Google Scholar] [CrossRef] [PubMed]
- Jalil, S.M.A.; Henry, J.C.; Cameron, A.J.M. Targets in the Tumour Matrisome to Promote Cancer Therapy Response. Cancers 2024, 16, 1847. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.-Q.; Liu, H.; Navarro, E.; Kussie, P.; Zhu, Z. Development of Heparanase Inhibitors for Anti-Cancer Therapy. Curr Med Chem 2006, 13, 2101–2111. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, Y.; Zhou, H.; Quan, J.; Liu, C.; Wang, Y.; Zhang, Y.; Yu, X. Heparanase in Cancer Progression: Structure, Substrate Recognition and Therapeutic Potential. Front Chem 2022, 10, 926353. [Google Scholar] [CrossRef]
- Jayatilleke, K.M.; Hulett, M.D. Heparanase and the Hallmarks of Cancer. Journal of Translational Medicine 2020, 18, 453. [Google Scholar] [CrossRef]
- Vornicova, O.; Boyango, I.; Feld, S.; Naroditsky, I.; Kazarin, O.; Zohar, Y.; Tiram, Y.; Ilan, N.; Ben-Izhak, O.; Vlodavsky, I.; et al. The prognostic significance of heparanase expression in metastatic melanoma. Oncotarget 2016, 7, 74678–74685. [Google Scholar] [CrossRef]
- Murry, B.P.; Greiter-Wilke, A.; Paulsen, D.P.; Hiatt, K.M.; Beltrami, C.A.; Marchetti, D. Selective Heparanase Localization in Malignant Melanoma. Int J Oncol 2005, 26, 345–352. [Google Scholar] [CrossRef]
- Nakajima, M.; DeChavigny, A.; Johnson, C.E.; Hamada, J.; Stein, C.A.; Nicolson, G.L. Suramin. A Potent Inhibitor of Melanoma Heparanase and Invasion. J Biol Chem 1991, 266, 9661–9666. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Miao, H.-Q.; Xu, Y.-J.; Navarro, E.C.; Tonra, J.R.; Corcoran, E.; Lahiji, A.; Kussie, P.; Kiselyov, A.S.; Wong, W.C.; et al. 1-[4-(1H-Benzoimidazol-2-Yl)-Phenyl]-3-[4-(1H-Benzoimidazol-2-Yl)-Phenyl]-Urea Derivatives as Small Molecule Heparanase Inhibitors. Bioorg Med Chem Lett 2006, 16, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Irimura, T.; Nakajima, M.; Nicolson, G.L. Chemically Modified Heparins as Inhibitors of Heparan Sulfate Specific Endo-Beta-Glucuronidase (Heparanase) of Metastatic Melanoma Cells. Biochemistry 1986, 25, 5322–5328. [Google Scholar] [CrossRef] [PubMed]
- Vlodavsky, I.; Mohsen, M.; Lider, O.; Cm, S.; Hp, E.; Vigoda, M.; Ishai-Michaeli, R.; Peretz, T. Inhibition of Tumor Metastasis by Heparanase Inhibiting Species of Heparin. Invasion & metastasis 1994.
- Bitan, M.; Mohsen, M.; Levi, E.; Wygoda, M.R.; Miao, H.Q.; Lider, O.; Svahn, C.M.; Ekre, H.P.; Ishai-Michaeli, R.; Bar-Shavit, R. Structural Requirements for Inhibition of Melanoma Lung Colonization by Heparanase Inhibiting Species of Heparin. Isr J Med Sci 1995, 31, 106–118. [Google Scholar]
- Kaur, R.; Deb, P.K.; Diwan, V.; Saini, B. Heparanase Inhibitors in Cancer Progression: Recent Advances. Current Pharmaceutical Design 27, 43–68. [CrossRef]
- Mohan, C.D.; Hari, S.; Preetham, H.D.; Rangappa, S.; Barash, U.; Ilan, N.; Nayak, S.C.; Gupta, V.K.; Basappa, null; Vlodavsky, I.; et al. Targeting Heparanase in Cancer: Inhibition by Synthetic, Chemically Modified, and Natural Compounds. iScience 2019, 15, 360–390. [CrossRef]
- Chhabra, M.; Ferro, V. PI-88 and Related Heparan Sulfate Mimetics. Adv Exp Med Biol 2020, 1221, 473–491. [Google Scholar] [CrossRef]
- Chow, L.Q.M.; Gustafson, D.L.; O’Bryant, C.L.; Gore, L.; Basche, M.; Holden, S.N.; Morrow, M.C.; Grolnic, S.; Creese, B.R.; Roberts, K.L.; et al. A Phase I Pharmacological and Biological Study of PI-88 and Docetaxel in Patients with Advanced Malignancies. Cancer Chemother Pharmacol 2008, 63, 65. [Google Scholar] [CrossRef]
- Holden, S.; Basche, M.; O’Bryant, C.; Morrow, M.; Grolnic, S.; Persky, M.; Deem, C.; Roberts, K.; Ribbons, K.; Eckhardt, S. A Phase I Study of the Heparanase Inhibitor PI-88 given Subcutaneously (Sq) in Patients (Pts) with Advanced Solid Malignancies. European Journal of Cancer 2002, 38, S74–S75. [Google Scholar] [CrossRef]
- Rosenthal, M.A.; Rischin, D.; McArthur, G.; Ribbons, K.; Chong, B.; Fareed, J.; Toner, G.; Green, M.D.; Basser, R.L. Treatment with the Novel Anti-Angiogenic Agent PI-88 Is Associated with Immune-Mediated Thrombocytopenia. Ann Oncol 2002, 13, 770–776. [Google Scholar] [CrossRef]
- Millward, M.; Hamilton, A.; Thomson, D.; Gautam, A.; Wilson, E. Final Results of a Phase I Study of Daily PI-88 as a Single Agent and in Combination with Dacarbazine (D) in Patients with Metastatic Melanoma. JCO 2007, 25, 8532–8532. [Google Scholar] [CrossRef]
- Basche, M.; Gustafson, D.L.; Holden, S.N.; O’Bryant, C.L.; Gore, L.; Witta, S.; Schultz, M.K.; Morrow, M.; Levin, A.; Creese, B.R.; et al. A Phase I Biological and Pharmacologic Study of the Heparanase Inhibitor PI-88 in Patients with Advanced Solid Tumors. Clin Cancer Res 2006, 12, 5471–5480. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.D.; Robinson, W.A.; Millward, M.J.; Powell, A.; Price, T.J.; Thomson, D.B.; Walpole, E.T.; Haydon, A.M.; Creese, B.R.; Roberts, K.L.; et al. A Phase II Study of the Heparanase Inhibitor PI-88 in Patients with Advanced Melanoma. Invest New Drugs 2008, 26, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Lanzi, C.; Cassinelli, G. Heparan Sulfate Mimetics in Cancer Therapy: The Challenge to Define Structural Determinants and the Relevance of Targets for Optimal Activity. Molecules 2018, 23, 2915. [Google Scholar] [CrossRef] [PubMed]
- Dredge, K.; Hammond, E.; Davis, K.; Li, C.P.; Liu, L.; Johnstone, K.; Handley, P.; Wimmer, N.; Gonda, T.J.; Gautam, A.; et al. The PG500 Series: Novel Heparan Sulfate Mimetics as Potent Angiogenesis and Heparanase Inhibitors for Cancer Therapy. Invest New Drugs 2010, 28, 276–283. [Google Scholar] [CrossRef]
- Mohamed, S.; Coombe, D.R. Heparin Mimetics: Their Therapeutic Potential. Pharmaceuticals 2017, 10, 78. [Google Scholar] [CrossRef]
- Johnstone, K.D.; Karoli, T.; Liu, L.; Dredge, K.; Copeman, E.; Li, C.P.; Davis, K.; Hammond, E.; Bytheway, I.; Kostewicz, E.; et al. Synthesis and Biological Evaluation of Polysulfated Oligosaccharide Glycosides as Inhibitors of Angiogenesis and Tumor Growth. J. Med. Chem. 2010, 53, 1686–1699. [Google Scholar] [CrossRef]
- Winterhoff, B.; Freyer, L.; Hammond, E.; Giri, S.; Mondal, S.; Roy, D.; Teoman, A.; Mullany, S.A.; Hoffmann, R.; von Bismarck, A.; et al. PG545 Enhances Anti-Cancer Activity of Chemotherapy in Ovarian Models and Increases Surrogate Biomarkers Such as VEGF in Preclinical and Clinical Plasma Samples. Eur J Cancer 2015, 51, 879–892. [Google Scholar] [CrossRef]
- Lemech, C.; Dredge, K.; Bampton, D.; Hammond, E.; Clouston, A.; Waterhouse, N.J.; Stanley, A.C.; Mouttie, L.L.-E.; Chojnowski, G.M.; Haydon, A.; et al. Phase Ib Open-Label, Multicenter Study of Pixatimod, an Activator of TLR9, in Combination with Nivolumab in Subjects with Microsatellite-Stable Metastatic Colorectal Cancer, Metastatic Pancreatic Ductal Adenocarcinoma and Other Solid Tumors. J Immunother Cancer 2023, 11, e006136. [Google Scholar] [CrossRef]
- Roy, M.; Reiland, J.; Murry, B.P.; Chouljenko, V.; Kousoulas, K.G.; Marchetti, D. Antisense-Mediated Suppression of Heparanase Gene Inhibits Melanoma Cell Invasion. Neoplasia 2005, 7, 253–262. [Google Scholar] [CrossRef]
- Liu, X.; Fang, H.; Chen, H.; Jiang, X.; Fang, D.; Wang, Y.; Zhu, D. An Artificial miRNA against HPSE Suppresses Melanoma Invasion Properties, Correlating with a down-Regulation of Chemokines and MAPK Phosphorylation. PLoS One 2012, 7, e38659. [Google Scholar] [CrossRef]
- Edovitsky, E.; Elkin, M.; Zcharia, E.; Peretz, T.; Vlodavsky, I. Heparanase Gene Silencing, Tumor Invasiveness, Angiogenesis, and Metastasis. J Natl Cancer Inst 2004, 96, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Reunanen, N.; Kähäri, V. Matrix Metalloproteinases in Cancer Cell Invasion. In Madame Curie Bioscience Database [Internet]; Landes Bioscience, 2013.
- Hidalgo, M.; Eckhardt, S.G. Development of Matrix Metalloproteinase Inhibitors in Cancer Therapy. JNCI: Journal of the National Cancer Institute 2001, 93, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures into Future Successes. Mol Cancer Ther 2018, 17, 1147–1155. [Google Scholar] [CrossRef]
- Hofmann, U.B.; Westphal, J.R.; Van Muijen, G.N.; Ruiter, D.J. Matrix Metalloproteinases in Human Melanoma. J Invest Dermatol 2000, 115, 337–344. [Google Scholar] [CrossRef]
- Skiles, J.W.; Gonnella, N.C.; Jeng, A.Y. The Design, Structure, and Clinical Update of Small Molecular Weight Matrix Metalloproteinase Inhibitors. Curr Med Chem 2004, 11, 2911–2977. [Google Scholar] [CrossRef]
- Lazar, A.M.; Costea, D.O.; Popp, C.G.; Mastalier, B. Skin Malignant Melanoma and Matrix Metalloproteinases: Promising Links to Efficient Therapies. Int J Mol Sci 2024, 25, 7804. [Google Scholar] [CrossRef]
- Alves, R.; Pires, A.; Jorge, J.; Balça-Silva, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B. Batimastat Induces Cytotoxic and Cytostatic Effects in In vitro Models of Hematological Tumors. Int J Mol Sci 2024, 25, 4554. [Google Scholar] [CrossRef]
- Rasmussen, H.S.; McCann, P.P. Matrix Metalloproteinase Inhibition as a Novel Anticancer Strategy: A Review with Special Focus on Batimastat and Marimastat. Pharmacol Ther 1997, 75, 69–75. [Google Scholar] [CrossRef]
- Chirivi, R.G.; Garofalo, A.; Crimmin, M.J.; Bawden, L.J.; Stoppacciaro, A.; Brown, P.D.; Giavazzi, R. Inhibition of the Metastatic Spread and Growth of B16-BL6 Murine Melanoma by a Synthetic Matrix Metalloproteinase Inhibitor. Int J Cancer 1994, 58, 460–464. [Google Scholar] [CrossRef]
- Wylie, S.; MacDonald, I.C.; Varghese, H.J.; Schmidt, E.E.; Morris, V.L.; Groom, A.C.; Chambers, A.F. The Matrix Metalloproteinase Inhibitor Batimastat Inhibits Angiogenesis in Liver Metastases of B16F1 Melanoma Cells. Clin Exp Metastasis 1999, 17, 111–117. [Google Scholar] [CrossRef]
- Macaulay, V.M.; O’Byrne, K.J.; Saunders, M.P.; Braybrooke, J.P.; Long, L.; Gleeson, F.; Mason, C.S.; Harris, A.L.; Brown, P.; Talbot, D.C. Phase I Study of Intrapleural Batimastat (BB-94), a Matrix Metalloproteinase Inhibitor, in the Treatment of Malignant Pleural Effusions1. Clinical Cancer Research 1999, 5, 513–520. [Google Scholar] [PubMed]
- Steward, W.P. Marimastat (BB2516): Current Status of Development. Cancer Chemother Pharmacol 1999, 43 Suppl, S56–60. [Google Scholar] [CrossRef]
- Bodurtha, A.; Eisenhauer, E.; Steward, W.; Rusthoven, J.; Quirt, I.; Lohmann, R.; Wainman, N.; Rugg, T. Phase I-II Study of Marimastat (BB2516) in Patients with Metastatic Melanoma. In Proceedings of the Proc Am Soc Clin Oncol; 1997; Vol. 16, p. 493a.
- Toppmeyer, D.L.; Gounder, M.; Much, J.; Musanti, R.; Vyas, V.; Medina, M.; Orlando, T.; Pennick, M.; Lin, Y.; Shih, W.; et al. A Phase I and Pharmacologic Study of the Combination of Marimastat and Paclitaxel in Patients with Advanced Malignancy. Med Sci Monit 2003, 9, PI99–104. [Google Scholar]
- Quirt, I.; Bodurth, A.; Lohmann, R.; Rusthoven, J.; Belanger, K.; Young, V.; Wainman, N.; Stewar, W.; Eisenhauer, E. ; National Cancer Institute of Canada Clinical Trials Group Phase II Study of Marimastat (BB-2516) in Malignant Melanoma: A Clinical and Tumor Biopsy Study of the National Cancer Institute of Canada Clinical Trials Group. Invest New Drugs 2002, 20, 431–437. [Google Scholar] [CrossRef]
- Ferrario, A.; Chantrain, C.F.; von Tiehl, K.; Buckley, S.; Rucker, N.; Shalinsky, D.R.; Shimada, H.; DeClerck, Y.A.; Gomer, C.J. The Matrix Metalloproteinase Inhibitor Prinomastat Enhances Photodynamic Therapy Responsiveness in a Mouse Tumor Model. Cancer Res 2004, 64, 2328–2332. [Google Scholar] [CrossRef]
- Ozerdem, U.; Mach-Hofacre, B.; Varki, N.; Folberg, R.; Mueller, A.J.; Ochabski, R.; Pham, T.; Appelt, K.; Freeman, W.R. The Effect of Prinomastat (AG3340), a Synthetic Inhibitor of Matrix Metalloproteinases, on Uveal Melanoma Rabbit Model. Current Eye Research 2002, 24, 86–91. [Google Scholar] [CrossRef]
- Shalinsky, D.R.; Brekken, J.; Zou, H.; McDermott, C.D.; Forsyth, P.; Edwards, D.; Margosiak, S.; Bender, S.; Truitt, G.; Wood, A.; et al. Broad Antitumor and Antiangiogenic Activities of AG3340, a Potent and Selective MMP Inhibitor Undergoing Advanced Oncology Clinical Trials. Ann N Y Acad Sci 1999, 878, 236–270. [Google Scholar] [CrossRef]
- Hande, K.R.; Collier, M.; Paradiso, L.; Stuart-Smith, J.; Dixon, M.; Clendeninn, N.; Yeun, G.; Alberti, D.; Binger, K.; Wilding, G. Phase I and Pharmacokinetic Study of Prinomastat, a Matrix Metalloprotease Inhibitor. Clinical Cancer Research 2004, 10, 909–915. [Google Scholar] [CrossRef]
- Lokeshwar, B.L.; Escatel, E.; Zhu, B. Cytotoxic Activity and Inhibition of Tumor Cell Invasion by Derivatives of a Chemically Modified Tetracycline CMT-3 (COL-3). Curr Med Chem 2001, 8, 271–279. [Google Scholar] [CrossRef]
- Rudek, M.A.; Figg, W.D.; Dyer, V.; Dahut, W.; Turner, M.L.; Steinberg, S.M.; Liewehr, D.J.; Kohler, D.R.; Pluda, J.M.; Reed, E. Phase I Clinical Trial of Oral COL-3, a Matrix Metalloproteinase Inhibitor, in Patients With Refractory Metastatic Cancer. Journal of Clinical Oncology 2016. [CrossRef]
- Kasaoka, T.; Nishiyama, H.; Okada, M.; Nakajima, M. Matrix Metalloproteinase Inhibitor, MMI270 (CGS27023A) Inhibited Hematogenic Metastasis of B16 Melanoma Cells in Both Experimental and Spontaneous Metastasis Models. Clin Exp Metastasis 2008, 25, 827–834. [Google Scholar] [CrossRef]
- Nakamura, E.S.; Koizumi, K.; Yamaura, T.; Saiki, I. Anti-Tumor Angiogenic Effect of a Matrix Metalloproteinase Inhibitor MMI270. Anticancer Res 2003, 23, 411–417. [Google Scholar] [PubMed]
- Naglich, J.G.; Jure-Kunkel, M.; Gupta, E.; Fargnoli, J.; Henderson, A.J.; Lewin, A.C.; Talbott, R.; Baxter, A.; Bird, J.; Savopoulos, R.; et al. Inhibition of Angiogenesis and Metastasis in Two Murine Models by the Matrix Metalloproteinase Inhibitor, BMS-275291. Cancer Research 2001, 61, 8480–8485. [Google Scholar] [PubMed]
- Romanchikova, N.; Trapencieris, P.; Zemītis, J.; Turks, M. A Novel Matrix Metalloproteinase-2 Inhibitor Triazolylmethyl Aziridine Reduces Melanoma Cell Invasion, Angiogenesis and Targets ERK1/2 Phosphorylation. Journal of Enzyme Inhibition and Medicinal Chemistry 2014, 29, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Kuang, X.; Xie, Z.; Liang, L.; Zhang, Z.; Zhang, Y.; Ma, F.; Gao, Q.; Chang, R.; Lee, H.-H.; et al. Small-Molecule MMP2/MMP9 Inhibitor SB-3CT Modulates Tumor Immune Surveillance by Regulating PD-L1. Genome Medicine 2020, 12, 83. [Google Scholar] [CrossRef]
- Tao, P.; Fisher, J.F.; Mobashery, S.; Bernhard Schlegel, H. DFT Studies of the Ring Opening Mechanism of SB-3CT, a Potent Inhibitor of Matrix Metalloproteinase 2. Org Lett 2009, 11, 2559. [Google Scholar] [CrossRef]
- Krüger, A.; Arlt, M.J.E.; Gerg, M.; Kopitz, C.; Bernardo, M.M.; Chang, M.; Mobashery, S.; Fridman, R. Antimetastatic Activity of a Novel Mechanism-Based Gelatinase Inhibitor. Cancer Research 2005, 65, 3523–3526. [Google Scholar] [CrossRef]
- Marusak, C.; Bayles, I.; Ma, J.; Gooyit, M.; Gao, M.; Chang, M.; Bedogni, B. The Thiirane-Based Selective MT1-MMP/MMP2 Inhibitor ND-322 Reduces Melanoma Tumor Growth and Delays Metastatic Dissemination. Pharmacol Res 2016, 113, 515–520. [Google Scholar] [CrossRef]
- Reich, R.; Katz, Y.; Hadar, R.; Breuer, E. Carbamoylphosphonate Matrix Metalloproteinase Inhibitors 3: In vivo Evaluation of Cyclopentylcarbamoylphosphonic Acid in Experimental Metastasis and Angiogenesis. Clin Cancer Res 2005, 11, 3925–3929. [Google Scholar] [CrossRef]
- Devy, L.; Arulanandam, T.; Buckler, D.; Finnern, R.; Naa, L.; Rank, D.; Ladner, R.C.; Rabbani, S.; Dransfield, D.T.; Henderikx, P. 203 POSTER Antitumor Efficacy of DX-2400, a Potent and Selective Human Antibody MMP-14 Inhibitor Discovered Using Phage Display Technology. European Journal of Cancer Supplements 2006, 4, 63–64. [Google Scholar] [CrossRef]
- Devy, L.; Huang, L.; Naa, L.; Yanamandra, N.; Pieters, H.; Frans, N.; Chang, E.; Tao, Q.; Vanhove, M.; Lejeune, A.; et al. Selective Inhibition of Matrix Metalloproteinase-14 Blocks Tumor Growth, Invasion, and Angiogenesis. Cancer Research 2009, 69, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.; Efremov, E.; Niedzwiecki, A.; Rath, M. Matrix Metalloproteinases-9 as a Promising Target for Anti-Cancer Vaccine: Inhibition of Melanoma Tumor Growth in Mice Immunized with Syngeneic MMP-9 Peptides. World Cancer Research Journal 2019, 6. [Google Scholar] [CrossRef]
- Roomi, M.W.; Niedzwiecki, A.; Rath, A. Peptide Vaccines Directed against Human Metalloproteinases (MMPs) with Anti-Tumor Efficacy in vitro and in vivo. J Cell Med Nat Health 2018, 1–12. [Google Scholar]
- Blackburn, J.S.; Rhodes, C.H.; Coon, C.I.; Brinckerhoff, C.E. RNA Interference Inhibition of Matrix Metalloproteinase-1 Prevents Melanoma Metastasis by Reducing Tumor Collagenase Activity and Angiogenesis. Cancer Res 2007, 67, 10849–10858. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Saji, S.; Sato, F.; Noda, M.; Toi, M. Potential Clinical Applications of Matrix Metalloproteinase Inhibitors and Their Future Prospects. Int J Biol Markers 2013, 28, 117–130. [Google Scholar] [CrossRef]
- Brand, K. Cancer Gene Therapy with Tissue Inhibitors of Metalloproteinases (TIMPs). Curr Gene Ther 2002, 2, 255–271. [Google Scholar] [CrossRef]
- Gomis-Rüth, F.X.; Maskos, K.; Betz, M.; Bergner, A.; Huber, R.; Suzuki, K.; Yoshida, N.; Nagase, H.; Brew, K.; Bourenkov, G.P.; et al. Mechanism of Inhibition of the Human Matrix Metalloproteinase Stromelysin-1 by TIMP-1. Nature 1997, 389, 77–81. [Google Scholar] [CrossRef]
- Schultz, R.M.; Silberman, S.; Persky, B.; Bajkowski, A.S.; Carmichael, D.F. Inhibition by Human Recombinant Tissue Inhibitor of Metalloproteinases of Human Amnion Invasion and Lung Colonization by Murine B16-F10 Melanoma Cells. Cancer Res 1988, 48, 5539–5545. [Google Scholar]
- Djafarzadeh, R.; Milani, V.; Rieth, N.; von Luettichau, I.; Skrablin, P.S.; Hofstetter, M.; Noessner, E.; Nelson, P.J. TIMP-1-GPI in Combination with Hyperthermic Treatment of Melanoma Increases Sensitivity to FAS-Mediated Apoptosis. Cancer Immunol Immunother 2009, 58, 361–371. [Google Scholar] [CrossRef]
- Oku, T.; Ata, N.; Yonezawa, K.; Tokai, H.; Fujii, H.; Shinagawa, A.; Ohuchi, E.; Saiki, I. Antimetastatic and Antitumor Effect of a Recombinant Human Tissue Inhibitor of Metalloproteinases-2 in Murine Melanoma Models. Biol Pharm Bull 1997, 20, 843–849. [Google Scholar] [CrossRef]
- Kang, W.K.; Park, E.-K.; Lee, H.S.; Park, B.-Y.; Chang, J.-Y.; Kim, M.-Y.; Kang, H.A.; Kim, J.-Y. A Biologically Active Angiogenesis Inhibitor, Human Serum Albumin-TIMP-2 Fusion Protein, Secreted from Saccharomyces Cerevisiae. Protein Expr Purif 2007, 53, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Bourboulia, D.; Jensen-Taubman, S.; Rittler, M.R.; Han, H.Y.; Chatterjee, T.; Wei, B.; Stetler-Stevenson, W.G. Endogenous Angiogenesis Inhibitor Blocks Tumor Growth via Direct and Indirect Effects on Tumor Microenvironment. Am J Pathol 2011, 179, 2589–2600. [Google Scholar] [CrossRef]
- Shi, Y.; Parhar, R.S.; Zou, M.; Al-Mohanna, F.A.; Paterson, M.C. Gene Therapy of Melanoma Pulmonary Metastasis by Intramuscular Injection of Plasmid DNA Encoding Tissue Inhibitor of Metalloproteinases-1. Cancer Gene Ther 2002, 9, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, A.M.; Mueller, B.M.; Reisfeld, R.A.; Taylor, S.M.; DeClerck, Y.A. Effect of Tissue Inhibitor of the Matrix Metalloproteinases-2 Expression on the Growth and Spontaneous Metastasis of a Human Melanoma Cell Line. Cancer Res 1994, 54, 5467–5473. [Google Scholar]
- Ahonen, M.; Baker, A.H.; Kähäri, V.M. Adenovirus-Mediated Gene Delivery of Tissue Inhibitor of Metalloproteinases-3 Inhibits Invasion and Induces Apoptosis in Melanoma Cells. Cancer Res 1998, 58, 2310–2315. [Google Scholar]
- Ahonen, M.; Ala-Aho, R.; Baker, A.H.; George, S.J.; Grénman, R.; Saarialho-Kere, U.; Kähäri, V.-M. Antitumor Activity and Bystander Effect of Adenovirally Delivered Tissue Inhibitor of Metalloproteinases-3. Mol Ther 2002, 5, 705–715. [Google Scholar] [CrossRef]
- Takabe, P.; Siiskonen, H.; Rönkä, A.; Kainulainen, K.; Pasonen-Seppänen, S. The Impact of Hyaluronan on Tumor Progression in Cutaneous Melanoma. Front Oncol 2022, 11, 811434. [Google Scholar] [CrossRef]
- Gagneja, S.; Capalash, N.; Sharma, P. Hyaluronic Acid as a Tumor Progression Agent and a Potential Chemotherapeutic Biomolecule against Cancer: A Review on Its Dual Role. Int J Biol Macromol 2024, 275, 133744. [Google Scholar] [CrossRef]
- Turley, E.A.; Wood, D.K.; McCarthy, J.B. Carcinoma Cell Hyaluronan as a “Portable” Cancerized Prometastatic Microenvironment. Cancer Res 2016, 76, 2507–2512. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan-CD44 Interactions in Cancer: Paradoxes and Possibilities. Clin Cancer Res 2009, 15, 7462–7468. [Google Scholar] [CrossRef]
- Xu, Y.; Benedikt, J.; Ye, L. Hyaluronic Acid Interacting Molecules Mediated Crosstalk between Cancer Cells and Microenvironment from Primary Tumour to Distant Metastasis. Cancers 2024, 16, 1907. [Google Scholar] [CrossRef] [PubMed]
- Sapudom, J.; Nguyen, K.-T.; Martin, S.; Wippold, T.; Möller, S.; Schnabelrauch, M.; Anderegg, U.; Pompe, T. Biomimetic Tissue Models Reveal the Role of Hyaluronan in Melanoma Proliferation and Invasion. Biomater Sci 2020, 8, 1405–1417. [Google Scholar] [CrossRef]
- Edward, M.; Gillan, C.; Micha, D.; Tammi, R.H. Tumour Regulation of Fibroblast Hyaluronan Expression: A Mechanism to Facilitate Tumour Growth and Invasion. Carcinogenesis 2005, 26, 1215–1223. [Google Scholar] [CrossRef]
- Voelcker, V.; Gebhardt, C.; Averbeck, M.; Saalbach, A.; Wolf, V.; Weih, F.; Sleeman, J.; Anderegg, U.; Simon, J. Hyaluronan Fragments Induce Cytokine and Metalloprotease Upregulation in Human Melanoma Cells in Part by Signalling via TLR4. Exp Dermatol 2008, 17, 100–107. [Google Scholar] [CrossRef]
- Ahrens, T.; Assmann, V.; Fieber, C.; Termeer, C.; Herrlich, P.; Hofmann, M.; Simon, J.C. CD44 Is the Principal Mediator of Hyaluronic-Acid-Induced Melanoma Cell Proliferation. J Invest Dermatol 2001, 116, 93–101. [Google Scholar] [CrossRef]
- Ichikawa, T.; Itano, N.; Sawai, T.; Kimata, K.; Koganehira, Y.; Saida, T.; Taniguchi, S. Increased Synthesis of Hyaluronate Enhances Motility of Human Melanoma Cells. J Invest Dermatol 1999, 113, 935–939. [Google Scholar] [CrossRef]
- Mummert, M.; Mummert, D.I.; Ellinger, L.; Takashima, A. Functional Roles of Hyaluronan in B16-F10 Melanoma Growth and Experimental Metastasis in Mice. Molecular cancer therapeutics 2003.
- Kultti, A.; Pasonen-Seppänen, S.; Jauhiainen, M.; Rilla, K.J.; Kärnä, R.; Pyöriä, E.; Tammi, R.H.; Tammi, M.I. 4-Methylumbelliferone Inhibits Hyaluronan Synthesis by Depletion of Cellular UDP-Glucuronic Acid and Downregulation of Hyaluronan Synthase 2 and 3. Exp Cell Res 2009, 315, 1914–1923. [Google Scholar] [CrossRef]
- Yoshihara, S.; Kon, A.; Kudo, D.; Nakazawa, H.; Kakizaki, I.; Sasaki, M.; Endo, M.; Takagaki, K. A Hyaluronan Synthase Suppressor, 4-Methylumbelliferone, Inhibits Liver Metastasis of Melanoma Cells. FEBS Lett 2005, 579, 2722–2726. [Google Scholar] [CrossRef]
- Kudo, D.; Kon, A.; Yoshihara, S.; Kakizaki, I.; Sasaki, M.; Endo, M.; Takagaki, K. Effect of a Hyaluronan Synthase Suppressor, 4-Methylumbelliferone, on B16F-10 Melanoma Cell Adhesion and Locomotion. Biochem Biophys Res Commun 2004, 321, 783–787. [Google Scholar] [CrossRef]
- Edward, M.; Quinn, J.A.; Pasonen-Seppänen, S.M.; McCann, B.A.; Tammi, R.H. 4-Methylumbelliferone Inhibits Tumour Cell Growth and the Activation of Stromal Hyaluronan Synthesis by Melanoma Cell-Derived Factors. Br J Dermatol 2010, 162, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Mummert, M.E.; Mohamadzadeh, M.; Mummert, D.I.; Mizumoto, N.; Takashima, A. Development of a Peptide Inhibitor of Hyaluronan-Mediated Leukocyte Trafficking. J Exp Med 2000, 192, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Spruss, T.; Bernhardt, G.; Schönenberger, H.; Schiess, W. Hyaluronidase Significantly Enhances the Efficacy of Regional Vinblastine Chemotherapy of Malignant Melanoma. J Cancer Res Clin Oncol 1995, 121, 193–202. [Google Scholar] [CrossRef]
- McGuire, J.; Taguchi, T.; Tombline, G.; Paige, V.; Janelsins, M.; Gilmore, N.; Seluanov, A.; Gorbunova, V. Hyaluronidase Inhibitor Delphinidin Inhibits Cancer Metastasis. Sci Rep 2024, 14, 14958. [Google Scholar] [CrossRef]
- Locke, K.W.; Maneval, D.C.; LaBarre, M.J. ENHANZE® Drug Delivery Technology: A Novel Approach to Subcutaneous Administration Using Recombinant Human Hyaluronidase PH20. Drug Deliv 2019, 26, 98–106. [Google Scholar] [CrossRef]
- Frost, G.I. Recombinant Human Hyaluronidase (rHuPH20): An Enabling Platform for Subcutaneous Drug and Fluid Administration. Expert Opin Drug Deliv 2007, 4, 427–440. [Google Scholar] [CrossRef]
- Styles, I.K.; Feeney, O.M.; Nguyen, T.-H.; Brundel, D.H.S.; Kang, D.W.; Clift, R.; McIntosh, M.P.; Porter, C.J.H. Removal of Interstitial Hyaluronan with Recombinant Human Hyaluronidase Improves the Systemic and Lymphatic Uptake of Cetuximab in Rats. J Control Release 2019, 315, 85–96. [Google Scholar] [CrossRef]
- Lonardi, S.; Lugowska, I.; Jackson, C.G.C.A.; O’Donnell, A.; Bahleda, R.; Garrido, M.; Latten-Jansen, L.; Chacon, M.; Yimer, H.A.; Camacho, T.; et al. CheckMate 8KX: Phase 1/2 Multitumor Preliminary Analyses of a Subcutaneous Formulation of Nivolumab (± rHuPH20). JCO 2021, 39, 2575–2575. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Mohr, P.; Dronca, R.; Harris, S.; Wilson, M.; Gurm, B.; Howansky, M.; Ng, W.-T.; Ravimohan, S.; Vezina, H.; et al. 882TiP Subcutaneous vs Intravenous Nivolumab in Patients with Melanoma Following Complete Resection. Annals of Oncology 2022, 33, S951–S952. [Google Scholar] [CrossRef]
- Huang, R.; Rofstad, E.K. Integrins as Therapeutic Targets in the Organ-Specific Metastasis of Human Malignant Melanoma. J Exp Clin Cancer Res 2018, 37, 92. [Google Scholar] [CrossRef]
- Pickarski, M.; Gleason, A.; Bednar, B.; Duong, L.T. Orally Active Avβ3 Integrin Inhibitor MK-0429 Reduces Melanoma Metastasis. Oncol Rep 2015, 33, 2737–2745. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, F.; Graziani, G.; Levati, L.; Tentori, L.; D’Atri, S.; Lacal, P.M. Cilengitide Downmodulates Invasiveness and Vasculogenic Mimicry of Neuropilin 1 Expressing Melanoma Cells through the Inhibition of Avβ5 Integrin. Int J Cancer 2015, 136, E545–558. [Google Scholar] [CrossRef] [PubMed]
- Mortarini, R.; Gismondi, A.; Santoni, A.; Parmiani, G.; Anichini, A. Role of the A5β1 Integrin Receptor in the Proliferative Response of Quiescent Human Melanoma Cells to Fibronectin. Cancer Research 1992.
- Arias-Mejias, S.M.; Warda, K.Y.; Quattrocchi, E.; Alonso-Quinones, H.; Sominidi-Damodaran, S.; Meves, A. The Role of Integrins in Melanoma: A Review. Int J Dermatol 2020, 59, 525–534. [Google Scholar] [CrossRef]
- Cheresh, D.A. Structure, Function and Biological Properties of Integrin Alpha v Beta 3 on Human Melanoma Cells. Cancer Metastasis Rev 1991, 10, 3–10. [Google Scholar] [CrossRef]
- Lu, X.; Lu, D.; Scully, M.F.; Kakkar, V.V. Modulation of Integrin-Binding Selectivity by Mutation within the RGD-Loop of Snake Venom Proteins: A Novel Drug Development Approach. Curr Med Chem Cardiovasc Hematol Agents 2003, 1, 189–196. [Google Scholar] [CrossRef]
- Lu, X.; Lu, D.; Scully, M.F.; Kakkar, V.V. Integrins in Drug Targeting-RGD Templates in Toxins. Curr Pharm Des 2006, 12, 2749–2769. [Google Scholar] [CrossRef]
- Almeida, G. de O.; de Oliveira, I.S.; Arantes, E.C.; Sampaio, S.V. Snake Venom Disintegrins Update: Insights about New Findings. J Venom Anim Toxins Incl Trop Dis 29, e20230039. [CrossRef]
- Shih, C.-H.; Chiang, T.-B.; Wang, W.-J. Inhibition of Integrins Av/A5-Dependent Functions in Melanoma Cells by an ECD-Disintegrin Acurhagin-C. Matrix Biol 2013, 32, 152–159. [Google Scholar] [CrossRef]
- Saviola, A.J.; Burns, P.D.; Mukherjee, A.K.; Mackessy, S.P. The Disintegrin Tzabcanin Inhibits Adhesion and Migration in Melanoma and Lung Cancer Cells. Int J Biol Macromol 2016, 88, 457–464. [Google Scholar] [CrossRef]
- Trikha, M.; De Clerck, Y.A.; Markland, F.S. Contortrostatin, a Snake Venom Disintegrin, Inhibits Beta 1 Integrin-Mediated Human Metastatic Melanoma Cell Adhesion and Blocks Experimental Metastasis. Cancer Res 1994, 54, 4993–4998. [Google Scholar]
- Kang, I.C.; Kim, D.S.; Jang, Y.; Chung, K.H. Suppressive Mechanism of Salmosin, a Novel Disintegrin in B16 Melanoma Cell Metastasis. Biochem Biophys Res Commun 2000, 275, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.E.; Rodríguez-Acosta, A.; Palomar, R.; Lucena, S.E.; Bashir, S.; Soto, J.G.; Pérez, J.C. Colombistatin: A Disintegrin Isolated from the Venom of the South American Snake (Bothrops Colombiensis) That Effectively Inhibits Platelet Aggregation and SK-Mel-28 Cell Adhesion. Arch Toxicol 2009, 83, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Lucena, S.; Sanchez, E.E.; Perez, J.C. Anti-Metastatic Activity of the Recombinant Disintegrin, r-Mojastin 1, from the Mohave Rattlesnake. Toxicon 2011, 57, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Lucena, S.E.; Jia, Y.; Soto, J.G.; Parral, J.; Cantu, E.; Brannon, J.; Lardner, K.; Ramos, C.J.; Seoane, A.I.; Sánchez, E.E. Anti-Invasive and Anti-Adhesive Activities of a Recombinant Disintegrin, r-Viridistatin 2, Derived from the Prairie Rattlesnake (Crotalus Viridis Viridis). Toxicon 2012, 60, 31–39. [Google Scholar] [CrossRef]
- Carey, C.M.; Bueno, R.; Gutierrez, D.A.; Petro, C.; Lucena, S.E.; Sanchez, E.E.; Soto, J.G. Recombinant Rubistatin (r-Rub), an MVD Disintegrin, Inhibits Cell Migration and Proliferation, and Is a Strong Apoptotic Inducer of the Human Melanoma Cell Line SK-Mel-28. Toxicon 2012, 59, 241–248. [Google Scholar] [CrossRef]
- Arruda Macêdo, J.K.; Fox, J.W.; de Souza Castro, M. Disintegrins from Snake Venoms and Their Applications in Cancer Research and Therapy. Curr Protein Pept Sci 2015, 16, 532–548. [Google Scholar] [CrossRef]
- Mizejewski, G. Disintegrin-Like Peptides Derived from Naturally-Occurring Proteins: A Proposed Adjunct Treatment for Cancer Therapy: A Commentary. International Journal of Cancer Research and Molecular Mechanisms 2020, 6. [Google Scholar] [CrossRef]
- Pisano, M.; Paola, I. de; Nieddu, V.; Sassu, I.; Cossu, S.; Galleri, G.; Gatto, A.D.; Budroni, M.; Cossu, A.; Saviano, M.; et al. In vitro Activity of the Avβ3 Integrin Antagonist RGDechi-hCit on Malignant Melanoma Cells. Anticancer research 2013.
- Nasulewicz-Goldeman, A.; Uszczyńska, B.; Szczaurska-Nowak, K.; Wietrzyk, J. siRNA-Mediated Silencing of Integrin Β3 Expression Inhibits the Metastatic Potential of B16 Melanoma Cells. Oncol Rep 2012, 28, 1567–1573. [Google Scholar] [CrossRef]
- Tong, H.; Jiang, G.; Qi, D.; Bi, J.; Tian, D.; Guan, X.; Zheng, S.; Sun, X. Bupleurum Chinense Polysaccharide Inhibit Adhesion of Human Melanoma Cells via Blocking Β1 Integrin Function. Carbohydr Polym 2017, 156, 244–252. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, X.; Sun, G.; Bao, Y. Codonopsis Lanceolata Polysaccharide CLPS Inhibits Melanoma Metastasis via Regulating Integrin Signaling. Int J Biol Macromol 2017, 103, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Kothari, M.; Wanjari, A.; Acharya, S.; Karwa, V.; Chavhan, R.; Kumar, S.; Kadu, A.; Patil, R. A Comprehensive Review of Monoclonal Antibodies in Modern Medicine: Tracing the Evolution of a Revolutionary Therapeutic Approach. Cureus 16, e61983. [CrossRef]
- Mitjans, F.; Sander, D.; Adán, J.; Sutter, A.; Martinez, J.M.; Jäggle, C.S.; Moyano, J.M.; Kreysch, H.G.; Piulats, J.; Goodman, S.L. An Anti-Alpha v-Integrin Antibody That Blocks Integrin Function Inhibits the Development of a Human Melanoma in Nude Mice. J Cell Sci 1995, 108 ( Pt 8), 2825–2838. [Google Scholar] [CrossRef]
- Montgomery, A.M.; Reisfeld, R.A.; Cheresh, D.A. Integrin Alpha v Beta 3 Rescues Melanoma Cells from Apoptosis in Three-Dimensional Dermal Collagen. Proc Natl Acad Sci U S A 1994, 91, 8856–8860. [Google Scholar] [CrossRef] [PubMed]
- Borst, A.J.; James, Z.M.; Zagotta, W.N.; Ginsberg, M.; Rey, F.A.; DiMaio, F.; Backovic, M.; Veesler, D. The Therapeutic Antibody LM609 Selectively Inhibits Ligand Binding to Human αVβ3 Integrin via Steric Hindrance. Structure 2017, 25, 1732–1739e5. [Google Scholar] [CrossRef] [PubMed]
- McNeel, D.G.; Eickhoff, J.; Lee, F.T.; King, D.M.; Alberti, D.; Thomas, J.P.; Friedl, A.; Kolesar, J.; Marnocha, R.; Volkman, J.; et al. Phase I Trial of a Monoclonal Antibody Specific for Avβ3 Integrin (MEDI-522) in Patients with Advanced Malignancies, Including an Assessment of Effect on Tumor Perfusion. Clin Cancer Res 2005, 11, 7851–7860. [Google Scholar] [CrossRef]
- Patel, S.R.; Jenkins R.N., J.; Papadopolous, N.; Burgess, M.A.; Plager, C.; Gutterman, J.; Benjamin, R.S. Pilot Study of Vitaxin—an Angiogenesis Inhibitor—in Patients with Advanced Leiomyosarcomas. Cancer 2001, 92, 1347–1348. [Google Scholar] [CrossRef]
- Gutheil, J.C.; Campbell, T.N.; Pierce, P.R.; Watkins, J.D.; Huse, W.D.; Bodkin, D.J.; Cheresh, D.A. Targeted Antiangiogenic Therapy for Cancer Using Vitaxin: A Humanized Monoclonal Antibody to the Integrin Alphavbeta3. Clin Cancer Res 2000, 6, 3056–3061. [Google Scholar]
- Posey, J.A.; Khazaeli, M.B.; DelGrosso, A.; Saleh, M.N.; Lin, C.Y.; Huse, W.; LoBuglio, A.F. A Pilot Trial of Vitaxin, a Humanized Anti-Vitronectin Receptor (Anti Alpha v Beta 3) Antibody in Patients with Metastatic Cancer. Cancer Biother Radiopharm 2001, 16, 125–132. [Google Scholar] [CrossRef]
- Moschos, S.J.; Sander, C.A.; Wang, W.; Reppert, S.L.; Drogowski, L.M.; Jukic, D.M.; Rao, U.N.M.; Athanassiou, C.; Buzoianu, M.; Mandic, M.; et al. Pharmacodynamic (Phase 0) Study Using Etaracizumab in Advanced Melanoma. J Immunother 2010, 33, 316–325. [Google Scholar] [CrossRef]
- Delbaldo, C.; Raymond, E.; Vera, K.; Hammershaimb, L.; Kaucic, K.; Lozahic, S.; Marty, M.; Faivre, S. Phase I and Pharmacokinetic Study of Etaracizumab (AbegrinTM), a Humanized Monoclonal Antibody against Avβ3 Integrin Receptor, in Patients with Advanced Solid Tumors. Invest New Drugs 2008, 26, 35–43. [Google Scholar] [CrossRef]
- Hersey, P.; Sosman, J.; O’Day, S.; Richards, J.; Bedikian, A.; Gonzalez, R.; Sharfman, W.; Weber, R.; Logan, T.; Buzoianu, M.; et al. A Randomized Phase 2 Study of Etaracizumab, a Monoclonal Antibody against Integrin Alpha(v)Beta(3), + or - Dacarbazine in Patients with Stage IV Metastatic Melanoma. Cancer 2010, 116, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Sainio, A.; Järveläinen, H. Extracellular Matrix-Cell Interactions: Focus on Therapeutic Applications. Cell Signal 2020, 66, 109487. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, S.; Chen, S.; Ma, F. The Prognostic Value and Immunological Role of CD44 in Pan-Cancer Study. Sci Rep 2023, 13, 7011. [Google Scholar] [CrossRef]
- Wagner, D.L.; Klotzsch, E. Barring the Gates to the Battleground: DDR1 Promotes Immune Exclusion in Solid Tumors. Sig Transduct Target Ther 2022, 7, 1–2. [Google Scholar] [CrossRef]
- Harris, E.N.; Baker, E. Role of the Hyaluronan Receptor, Stabilin-2/HARE, in Health and Disease. International Journal of Molecular Sciences 2020, 21, 3504. [Google Scholar] [CrossRef]
- Karinen, S.; Juurikka, K.; Hujanen, R.; Wahbi, W.; Hadler-Olsen, E.; Svineng, G.; Eklund, K.K.; Salo, T.; Åström, P.; Salem, A. Tumour Cells Express Functional Lymphatic Endothelium-Specific Hyaluronan Receptor in vitro and in vivo: Lymphatic Mimicry Promotes Oral Oncogenesis? Oncogenesis 2021, 10, 1–11. [Google Scholar] [CrossRef]
- Hinneh, J.A.; Gillis, J.L.; Moore, N.L.; Butler, L.M.; Centenera, M.M. The Role of RHAMM in Cancer: Exposing Novel Therapeutic Vulnerabilities. Front Oncol 2022, 12, 982231. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A Guide to the Composition and Functions of the Extracellular Matrix. The FEBS Journal 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front Cell Dev Biol 2017, 5, 18. [Google Scholar] [CrossRef]
- Goodison, S.; Urquidi, V.; Tarin, D. CD44 Cell Adhesion Molecules. Mol Pathol 1999, 52, 189–196. [Google Scholar] [CrossRef]
- Naor, D.; Nedvetzki, S.; Golan, I.; Melnik, L.; Faitelson, Y. CD44 in Cancer. Crit Rev Clin Lab Sci 2002, 39, 527–579. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhou, M.; Li, X.; Hu, M.; Li, C.; Li, M.; Sheng, F.; Li, Z.; Wu, G.; Luo, M.; et al. Synergistic Active Targeting of Dually Integrin Avβ3/CD44-Targeted Nanoparticles to B16F10 Tumors Located at Different Sites of Mouse Bodies. J Control Release 2016, 235, 1–13. [Google Scholar] [CrossRef]
- Coradini, D.; Zorzet, S.; Rossin, R.; Scarlata, I.; Pellizzaro, C.; Turrin, C.; Bello, M.; Cantoni, S.; Speranza, A.; Sava, G.; et al. Inhibition of Hepatocellular Carcinomas in vitro and Hepatic Metastases in vivo in Mice by the Histone Deacetylase Inhibitor HA-But. Clin Cancer Res 2004, 10, 4822–4830. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Kim, H.R.; Saravanakumar, G.; Heo, R.; Chae, S.Y.; Um, W.; Kim, K.; Kwon, I.C.; Lee, J.Y.; Lee, D.S.; et al. Bioreducible Hyaluronic Acid Conjugates as siRNA Carrier for Tumor Targeting. J Control Release 2013, 172, 653–661. [Google Scholar] [CrossRef]
- Peer, D.; Margalit, R. Tumor-Targeted Hyaluronan Nanoliposomes Increase the Antitumor Activity of Liposomal Doxorubicin in Syngeneic and Human Xenograft Mouse Tumor Models. Neoplasia 2004, 6, 343–353. [Google Scholar] [CrossRef]
- Peer, D.; Margalit, R. Loading Mitomycin C inside Long Circulating Hyaluronan Targeted Nano-Liposomes Increases Its Antitumor Activity in Three Mice Tumor Models. Int J Cancer 2004, 108, 780–789. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, J.; Wang, J.; Che, X.; Narula, J.; Bigby, M.; Wu, M.; Sy, M.S. Inhibition of Human Melanoma Growth and Metastasis in vivo by Anti-CD44 Monoclonal Antibody. Cancer Res 1994, 54, 1561–1565. [Google Scholar]
- Menke-van der Houven van Oordt, C.W.; Gomez-Roca, C.; van Herpen, C.; Coveler, A.L.; Mahalingam, D.; Verheul, H.M.W.; van der Graaf, W.T.A.; Christen, R.; Rüttinger, D.; Weigand, S.; et al. First-in-Human Phase I Clinical Trial of RG7356, an Anti-CD44 Humanized Antibody, in Patients with Advanced, CD44-Expressing Solid Tumors. Oncotarget 2016, 7, 80046–80058. [Google Scholar] [CrossRef]
- Fänder, J.; Kielstein, H.; Büttner, M.; Koelblinger, P.; Dummer, R.; Bauer, M.; Handke, D.; Wickenhauser, C.; Seliger, B.; Jasinski-Bergner, S. Characterizing CD44 Regulatory microRNAs as Putative Therapeutic Agents in Human Melanoma. Oncotarget 2019, 10, 6509–6525. [Google Scholar] [CrossRef]
- Ahrens, T.; Sleeman, J.P.; Schempp, C.M.; Howells, N.; Hofmann, M.; Ponta, H.; Herrlich, P.; Simon, J.C. Soluble CD44 Inhibits Melanoma Tumor Growth by Blocking Cell Surface CD44 Binding to Hyaluronic Acid. Oncogene 2001, 20, 3399–3408. [Google Scholar] [CrossRef]
- Rezler, E.M.; Khan, D.R.; Lauer-Fields, J.; Cudic, M.; Baronas-Lowell, D.; Fields, G.B. Targeted Drug Delivery Utilizing Protein-like Molecular Architecture. J Am Chem Soc 2007, 129, 4961–4972. [Google Scholar] [CrossRef] [PubMed]
- Leitinger, B. Discoidin Domain Receptor Functions in Physiological and Pathological Conditions. Int Rev Cell Mol Biol 2014, 310, 39–87. [Google Scholar] [CrossRef]
- Cario, M. DDR1 and DDR2 in Skin. Cell Adhesion & Migration 2018, 12, 386–393. [Google Scholar] [CrossRef]
- Reger de Moura, C.; Battistella, M.; Sohail, A.; Caudron, A.; Feugeas, J.P.; Podgorniak, M.-P.; Pages, C.; Mazouz Dorval, S.; Marco, O.; Menashi, S.; et al. Discoidin Domain Receptors: A Promising Target in Melanoma. Pigment Cell Melanoma Res 2019, 32, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Poudel, B.; Lee, Y.-M.; Kim, D.-K. DDR2 Inhibition Reduces Migration and Invasion of Murine Metastatic Melanoma Cells by Suppressing MMP2/9 Expression through ERK/NF-κB Pathway. Acta Biochim Biophys Sin (Shanghai) 2015, 47, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Badiola, I.; Villacé, P.; Basaldua, I.; Olaso, E. Downregulation of Discoidin Domain Receptor 2 in A375 Human Melanoma Cells Reduces Its Experimental Liver Metastasis Ability. Oncol Rep 2011, 26, 971–978. [Google Scholar] [CrossRef]
- Berestjuk, I.; Lecacheur, M.; Carminati, A.; Diazzi, S.; Rovera, C.; Prod’homme, V.; Ohanna, M.; Popovic, A.; Mallavialle, A.; Larbret, F.; et al. Targeting Discoidin Domain Receptors DDR1 and DDR2 Overcomes Matrix-mediated Tumor Cell Adaptation and Tolerance to BRAF-targeted Therapy in Melanoma. EMBO Mol Med 2022, 14, e11814. [Google Scholar] [CrossRef]
| Target | Type of drug | Additional Terms | Research object | Results | Reference |
|---|---|---|---|---|---|
| Heparanase | Suramin (polysulfonated naphthylurea) | _ | allograft B16-F10 in mice in vivo | Strong inhibitory effect on heparanase activity in melanoma cells; demonstration of reduced invasiveness in reconstructed basal membranes | [51] |
| 1,3-bis-[4-(1H-benzoimidazol-2-yl)-phenyl]-urea | _ | B16-BL6 cell line in vitro; syngeneic B16 in C57 mice in vivo | Inhibitory effect observed on the proliferative activity of melanoma cells in vitro (less than 50%); reduction in metastatic potential of these cells in mouse models (about 50% reduction) | [52] | |
| Chemically modified heparins | _ | syngeneic B16-BL6 in C57BL/6 mice in vivo | Significant reductions in the numbers of experimental melanoma lung metastases occurred | [53] | |
| Modified species of heparin and size-homogeneous oligosaccharides derived from depolymerized heparins | _ | syngeneic B16-BL6 in C57BL/6 mice in vivo | Effective inhibition of heparanase-mediated degradation of heparan sulfate in the ECM and reduction of lung colonization by melanoma cells | [54] | |
| Adenoviral vector carrying a cDNA with an antisense sequence of the heparanase gene HSPE-1 | _ | B16-B15b and 70W in nude mouse models in vivo | Significant reduction of HPSE-1 content in melanoma cells after adenoviral vector infection; significant decrease in melanoma invasiveness | [71] | |
| Artificial microRNA (miRNA) | _ | A375 cell line in vitro | Effective inhibition of HPSE protein expression and mRNA synthesis; reduction of invasive properties of melanoma cells in vitro and in vivo | [72] | |
| Plasmid vector carrying a small interfering RNA (siRNA) construct | _ | syngeneic B16-BL6 in C57BL/6 mice in vivo | Less vascularization of tumors and formation of fewer metastases; longer lifespan of mice injected with modified cells compared to mice injected with control cells without the genetic constructs | [73] | |
| MMPs | Prinomastat (AG3340) | Carboplatin; Taxol | syngeneic B16-F10 in C57BL/6 mice in vivo | Reduction of tumor growth, angiogenesis, and proliferation with increased necrosis and apoptosis; enhanced efficacy of carboplatin and taxol; decreased metastasis in melanoma; improved therapeutic index over cytotoxic drugs | [91] |
| MMI270 (CGS27023A) | _ | syngeneic B16-F10 and B16-BL6 in BDF1 mice in vivo | Significant reduction of the metastatic colonies in the lungs; no effect on colony size | [95] | |
| _ | syngeneic B16-BL6 in mice in vivo | Reduction of vessels leading to the primary tumor | [96] | ||
| Rebimastat (BMS-275291) | _ | syngeneic B16-BL6 in C57BL/6 mice in vivo | Dose-dependent inhibition of tumor metastasis to the lungs; dose-dependent anti-angiogenic effect | [97] | |
| JaZ-30 (C(2)-monosubstituted aziridine - aryl-1,2,3-triazole conjugate) | _ | B16 4A5 cell line in vitro | Reduction of VEGF secretion and ERK1/2 phosphorylation; inhibition of invasion through Matrigel and angiogenesis reduction in HUVEC cells; moderate decreasion of cell viability | [98] | |
| Small-molecule MMP2/MMP9 inhibitor SB-3CT | ICB (anti-PD-1/anti-CTLA-4) | A375 and SK-Mel-28 cell lines in vitro; syngeneic B16-F10 in C57/BL6 mice in vivo | Significant reduction in mRNA and protein levels of PD-L1 in melanoma cell lines; suppression of lung metastases when combined with ICB therapy | [99] | |
| ND-322 | _ | xenograft WM266-4 in mice in vivo | Effective inhibition of MT1-MMP and MMP2 activity resulting in reduction of melanoma cells growth, migration and invasion in vitro | [102] | |
| CPCPA (cyclopentylcarbamoylphosphonic acid) | _ | syngeneic B16-F10 in C57BL mice in vivo | Effective inhibition of tumor cell invasion through Matrigel without affecting cell proliferation; reduction of metastasis, inhibition of MMP expression and angiogenesis in mice | [103] | |
| Anti-MT1-MMP antibody DX-2400 | _ | allograft B16-F10 in mice in vivo | Blockade of proMMP-2 activation, reduction of MMP-9 expression, reduction of endothelial cell invasion, inhibition of tumor progression, reduction of metastasis rate and angiogenesis | [105] | |
| Peptide vaccines based on synthetic immunogenic oligopeptides with MMP sequences | Human MMP-2 and MMP-9 | syngeneic B16-F0 in C57BL/6 mice in vivo | Up to 88% reduction in tumor volume with human MMP-2 oligopeptide; 80% reduction with one of the human MMP-9 oligopeptides; no pronounced side effects | [106] | |
| MMP-9 of mice and rats | syngeneic B16-F0 in C57BL/6J mice in vivo | Reduction in tumor size (55 to 77% depending on the oligopeptide); no differences in clinical serum analyses, haematological parameters and histopathology of major organs compared to controls | [107] | ||
| MMP-1 inhibitory shRNA | _ | VMM12 cell line in vitro; xenograft VMM12 in immunodeficient nu/nu mice in vivo | Suppression of MMP-1 expression in vitro; reduction of metastatic activity in the lungs; reduction of collagenase activity and mediated suppression of invasion and angiogenesis | [108] | |
| Recombinant human TIMP | _ | B16-F10 cell line with amniotic membrane in vitro; syngeneic B16-F10 in C57BL/6 mice in vivo | Inhibition of metastasis; no effect on tumor growth | [112] | |
| Recombinant TIMP-1 conjugated to glycosylphosphatidylinositol | Sub-lethal hyperthermic treatment | 624.38-MEL, 93.04A12MEL, SK-MEL23, WM115, WM266-4 cell lines in vitro | Inhibition of proMMP-2 and proMMP-9 release from melanoma cells; significant increase in sensitivity to FAS-induced apoptosis | [113] | |
| Recombinant human TIMP-2 (r-hTIMP-2) | _ | syngeneic B16-BL6 in C57BL/6 mice in vivo | Inhibition of metastatic foci formation and limited inhibitory effect on tumor cell growth under in vitro and in vivo | [114] | |
| Recombinant human TIMP-2 fused to human serum albumin | Fluorouracil | syngeneic B16-BL6 in C57BL/6 mice in vivo | Inhibition of tumor growth | [115] | |
| Plasmid vector encoding TIMP-1 cDNA | Intraperitoneal injection of IL-2 | syngeneic B16-F10 in C57BL/6 mice in vivo | Significant reduction of lung metastasis; Further reduction of pulmonary metastases and increased survival were achieved by IL-2 administration combined with TIMP-1 treatment | [117] | |
| cDNA encoding human TIMP-2 | _ | xenograft M24 net in immunodeficient mice in vivo | Suppression of melanoma cell growth due to TIMP-2-mediated occlusion of interstitial collagen; no effect on metastatic activity | [118] | |
| Recombinant adenoviruses encoding TIMP-3 | _ | SK-Mel-5 and A2058 cell lines in Matrigel in vitro | Inhibition of invasion through the basal membrane; reduction of cell attachment to collagen types I and IV and fibronectin; induction of apoptosis | [119] | |
| Recombinant adenovirus encoding TIMP-3 | _ | xenograft A2058 in SCID/SCID mice in vivo | Inhibition of gelatinase activity and xenograft growth; Induction of apoptosis | [120] | |
| Integrins | 4-Methylumbelliferone (4-MU) | _ | syngeneic B16-F10 in C57BL/6 mice in vivo | Enhancement of melanoma cell adhesion and motility due to the presence of HA; inhibition of HA formation on the cell surface by 4-MU; decrease in the number of metastatic nodules by 32% in liver tissue | [133] |
| _ | B16-F10 cell line in vitro | Promotion of melanoma cell adhesion and locomotion by HA; dose-dependent reduction of cell adhesion (up to 49%) and locomotion (up to 37%) by 4-MU | [134] | ||
| _ | C8161 and MV3 cell lines in vitro | Reduction of hyaluronan levels in the matrix by 4-MU; inhibition of both growth and invasion in collagen lattices of melanoma cells; reversible growth suppression without induction of apoptosis | [135] | ||
| Synthetic peptide Pep-1 | _ | B16-F10 cell line in vitro; allograft B16-F10 in mice in vivo | Blocking of CD44-mediated adhesion to HA by Pep-1; no reduction in melanoma cell proliferation in vitro or growth in vivo; significant reduction in lung metastasis incidence and increased survival observed following a single intravenous injection of Pep-1 | [136] | |
| Hyaluronidase | Vinblastin | xenograft SK-Mel-2, -3, -5, -24 in nu/nu mice in vivo | Pronounced antitumor effect of combination therapy; ineffectiveness of individual drugs; prevention of inflammatory reactions with prior hyaluronidase; disappearance of tumor cells after 18 weeks, with no lymph node metastases | [137] | |
| Delphinidin | _ | syngeneic B16-F10 in C57BL/6 mice in vivo | Inhibition of cell proliferation, migration, and invasion; reduction of melanoma cell growth by 50% and over 90%; decrease in migration by approximately 45%; reduction of metastasis to sentinel lymph nodes from 80% in control mice to 25% | [138] | |
| Integrin inhibitor MK-0429 | Cyclophosphamide | allograft B16-F10 in B6D2F1 mice in vivo | Reduction of metastatic tumor colonies by 64%, decrease in tumor area by 60%, inhibition of tumor progression, and a 40% reduction in lung tumor burden | [145] | |
| Acurhagin-C | Methotrexate | B16-F10 and SK-Mel-1 cell lines in vitro | Reducing cell adhesion and transendothelial migration; induction of apoptosis via caspase-8 and -9 activation; enhancement of methotrexate's anti-proliferative effects in melanoma cells, sparing human epidermal melanocytes | [153] | |
| Tzabcanin | _ | A375 cell line in vitro | Reduction of melanoma cell adhesion to vitronectin with an IC50 of 747 nM; inhibition of melanoma cell migration by approximately 45% | [154] | |
| Contortrostatin | _ | M24 met cell line in vitro; xenograft M24 met in SCID mice in vivo | Reduction of adhesion to type I collagen (IC50 = 20 nM), vitronectin (IC50 = 75 nM), and fibronectin (IC50 = 220 nM); reduction of lung tumor foci by 51% at 20 µg and by 73% at 100 µg in vivo. | [155] | |
| Recombinant Salmosin | _ | B16-F10 cell line in vitro; syngeneic B16-F10 in C57BL/6 mice in vivo | Reduction of adhesion and invasion in vitro by blocking αvβ3 integrin; inhibition of cell proliferation on collagen I-coated plates; inhibition of lung colonization by melanoma cells in vivo | [156] | |
| Recombinant Colombistatin | _ | SK-Mel-28 cell line in vitro | Inhibition of adhesion of melanoma cells to fibronectin; reduction of migration activity | [157] | |
| Recombinant Mujastin 1 | _ | SK-Mel-28 and B16-F10 cell lines in vitro | Inhibition of SK-Mel-28 cell adhesion to fibronectin; reduction of lung tumor colonization in mouse models | [158] | |
| Recombinant Viridistatin 2 | _ | xenograft SK-Mel-28 and syngeneic B16-F10 in C57BL/6 and BALB/c mice in vivo | Inhibition of SK-Mel-28 cell adhesion, migration, and invasion; reduction of SK-Mel-28 migration by 96% and invasion of various cell lines by up to 85%; significant reduction of lung colonization of murine melanoma cells by 71% in vivo | [159] | |
| Recombinant Rubistatin | _ | SK-Mel-28 cell line in vitro | Inhibition of cell migration, proliferation, and adhesion to fibronectin | [160] | |
| Selective antagonist of αvβ3 RGDechi-hCit | Cisplatinum; Etoposide | A375, WM266-4, SK-Mel-28, Sbcl2, LB24Dagi, PR-Mel и PNP-Mel cell lines in vitro | Partial inhibition of adhesion and migration was observed, particularly in WM266 cells with the highest αvβ3 levels; no direct correlation between inhibition and αvβ3 expression | [163] | |
| siRNA against β3 integrin | _ | B16 cell line in matrigel in vitro; syngeneic B16 in C57BL/6/IiW mice in vivo | Over 90% reduction in β3 expression; significant impairment of fibronectin binding and migration through Matrigel; lung metastases decreation | [164] | |
| Bupleurum chinense Polysaccharides | _ | A375 cell line in vitro | Reduction of F-actin stress fibers by 54% to 28% compared to control; reduction of melanoma adhesion to fibronectin by 35% to 64%; reduction of phosphorylation of FAK by 50% to 65% and paxillin by 55% to 70% at various concentrations; reduction of focal adhesions per cell by 36% | [165] | |
| Codonopsis lanceolata Polysaccharides | _ | B16-F10 cell line in vitro; syngeneic B16-F10 in C57BL/6 mice in vivo | Inhibition of cell proliferation and pulmonary metastasis; disruption of integrin β1-mediated cell migration under in vitro conditions | [166] | |
| Anti-αv-integrin 17E6 antibody | _ | xenograft M21 cell line in Balb/c nu/nu mice in vivo | Inhibition of tumor growth and metastasis mediated by integrins; lack of inhibitory effects on melanoma cells themselves or antibody-mediated cellular cytotoxicity | [168] | |
| Anti-αvβ3 integrin monoclonal antibody LM609 | _ | xenograft M21 in Balb/c nu/nu mice in vivo | Elimination of the survival advantage from αvβ3 ligation in melanoma cells; significant reduction of melanoma cell viability in collagen matrices; no significant impact on cell adhesion or migration in cells with low αvβ3 expression | [169] | |
| CD44 | Hyaluronan (HA) + tetraiodothyroacetic acid (tetrac) conjugate (TeHA-SLN) | Docetaxel (DTX) | syngeneic B16-F10 in mice in vivo; melanoma metastasis in situ | Tumor growth inhibition was significant due to the action of TeHA-SLN/DTX; efficacy of TeHA-SLN as a bidirectional drug delivery system was demonstrated | [188] |
| Hyaluronan esterified with butyric acid residues | _ | syngeneic B16-F10 in C57BL/6 mice in vivo | Complete suppression of metastases in animals and significantly prolonged life expectancy compared to control groups | [189] | |
| HPD-siRNA complexes | _ | syngeneic B16-F10 in Balb/c nude mice in vivo | Selective accumulation of siRNA-HPD complexes at the tumor site after systemic administration to mice resulted in effective suppression of target gene expression; significant impact on tumor growth and progression was observed | [190] | |
| Nano-sized hyaluronan-liposomes (tHA-LIP) | Doxorubicin (DXR); Doxil | syngeneic B16-F10.9 in C57BL/6 mice in vivo | Selective accumulation of DXR in tumors; enhanced therapeutic effects observed; reduced tumor progression and metastatic burden; improved survival rates in syngeneic models compared to control | [191] | |
| Mitomycin C (MMC) | Increased potency of MMC-loaded tHA-LIP in receptor-overexpressing cells; prolonged circulation and enhanced accumulation in tumor-bearing lungs; improved delivery of MMC; significant improvements in tumor progression, metastasis, and survival outcomes | [192] | |||
| Anti-CD44 monoclonal antibody GKW.A2 | _ | xenograft SMMU-1 and SMMU-2 in mice in vivo | Local tumor development was not suppressed one week after subcutaneous injection in mice; however, the formation of metastatic tumors was inhibited, and animal survival was prolonged | [193] | |
| miR-143-3p | _ | BLM cell line in vitro | Decrease in melanoma cell proliferation; reduction in cell migration; increase in apoptosis of melanoma cells | [195] | |
| cDNA encoding the soluble form of CD44 | _ | 1F6 cell lines in vitro; xenograft 1F6 in MF1 nu/nu mice in vivo | Ihibition of cell growth by competitively blocking cell surface binding of CD44 to hyaluronic acid | [196] | |
| A peptide mimetic of collagen triple helix peptide (α1(IV)1263-1277 PA) | Liposomes loaded with rhodamine | М14#5 cell line in vitro | PA-associated improvement in targeting specificity; promotion of greater accumulation of therapeutic agents in tumor cells within melanoma models compared to non-targeting liposomes | [197] | |
| DDR | siRNA against DDR2 | _ | B16-BL6 cell line in vitro | Suppression of migration, invasion, and survival in human melanoma cell lines | [201] |
| DDR tyrosine kinase inhibitor (DDR1-IN-1) | siRNA against DDR1 | M10 cell line in vitro; xenograft C8161 and SK-Mel-5 in nude/c mice in vivo | Significant inhibition of melanoma cell proliferation in vitro and in vivo | [200] | |
| Imatinib | BRAF inhibitors | SK-Mel-5 and MM099 cell lines in vitro; xenograft 1205Lu in nude mice in vivo | Increase in the efficacy of BRAF inhibitors; counteraction of collagen remodeling; delay in melanoma recurrence | [203] | |
| siRNA against DDR2 | _ | A375 cell line in vitro | Reduction in gelatinase activity and JNK phosphorylation in melanoma cells; decrease in proliferation and migration rates compared to mock-transfected cells | [202] |
| Target | Type of drug | Additional Terms | Clinical Trials ID | Phase | Disease | Status/Results | Reference |
|---|---|---|---|---|---|---|---|
| Heparanase | PI-88 (metformin) | Docetaxel | _ | I | Advanced Malignancies (including melanoma) | Completed. No PR or CR was observed during the study period. However, at least 2 of 5 melanoma patients (40%) evaluable for response had SD at the end of ≥2 cycles of therapy | [59] |
| Dexamethasone | _ | I | Advanced Solid Malignancies (including melanoma) | Completed. Despite no PR or CR, 3/15 (20%) evaluable patients showed SD at 2, 4, and 10 years. One patient with melanoma (6.7%) refractory to biochemotherapy showed PR accompanied by a reduction in the size and number of pulmonary metastase | [60] | ||
| _ | _ | I | Advanced Malignancies (including melanoma) | Completed. 14 patients with advanced malignancies, including melanoma, were included in the study, where only one patient (7.1%) with metastatic melanoma achieved SD, but after four cycles of therapy (12 weeks), he was diagnosed with PD, as were the other melanoma patients in the study | [61] | ||
| Dacarbazin | _ | I | Unresectable Metastatic Melanoma | Completed. No CR or PR were observed with PI-88 monotherapy, but one patient showed radiologic SD at 4 months. However, PR was observed in 2/5 patients (40%) initially receiving monotherapy but who later had dacarbazine added to PI-88. 3/9 patients (33%) initially receiving combination therapy had radiologic PR | [62] | ||
| NCT00130442 | II | Metastatic Melanoma | Completed. Twenty-four of 65 patients (36.9%) showed SD with a median duration of 117 days. However, in the combination therapy option, more subjects (30.77% vs. 19.70%) experienced serious adverse effects including neutropenia (30.77%) and thrombocytopenia (27.27%) | _ | |||
| _ | NCT00073892 | II | Progressive Melanoma | Completed. One (2.4%) patient achieved PR, six (14.6%) patients showed SD as the best response, and the remaining 30 participants (73.2%) showed PD. At the end of six cycles of treatment, 3 of the 41 patients studied had no disease progression | [64] | ||
| PG545 (pixatimod) | _ | NCT01252095 | I | Melanoma | Terminated (Unexpected injection site reactions). The results are unpublished, but there is additional clinical data (summarised below). As a result, no RECIST responses were recorded and all patients had PD. Plasma levels of VEGF and FGF-2 increased 3.5-fold and 1.5-fold, respectively, after 22 days of treatment with PG545 | [69] | |
| Nivolumab | NCT05061017 | Ib | Solid Tumors (not including melanoma) | Completed. Of the 58 participants, three people (5.2%) with metastatic colorectal cancer had confirmed PR and eight (13.8%) had SD for at least 9 weeks | [70] | ||
| Nivolumab; Cyclophosphamide | IIA | Refractory Metastatic Melanoma | Completed. Results not published | _ | |||
| MMPs | BB-94 (Batimastat) | _ | _ | I | Malignant Pleural Effusion (including melanoma) | Completed. The melanoma patient treated with an intrapleural dose of 60 mg/m² showed a PR with a reduced need for pleural aspirations and some improvement in dyspnoea scores one month after treatment. Although BB-94 did not induce systemic tumor regression, the patient experienced symptomatic relief | [84] |
| BB2516 (Marimastat) | Paclitaxel | _ | I | Advanced Malignancies (including melanoma) | Completed. Two melanoma patients were included in the study. While no CR or PR was observed, seven patients achieved SD. One melanoma patient showed symptomatic relief, but the disease progressed to PD. The combination was well tolerated with no effect on the pharmacokinetics of paclitaxel, suggesting safe co-administration at single agent doses | [87] | |
| _ | _ | II | Malignant Melanoma | Completed. No CR were observed among the 28 eligible patients. Two patients (7.1%) achieved confirmed PR, lasting approximately 3 months. Five patients (17.9%) experienced SD for a median duration of 1.8 months, while 16 patients showed PD | [88] | ||
| AG3340 (Prinomastat) | _ | _ | I | Advanced Cancer (including melanoma) | Completed. No confirmed tumor responses to therapy. The primary toxicities identified were joint and muscle pain, generally reversible with rest and/or dose reduction | [92] | |
| COL-3 (Incyclinide) | _ | NCT00001683 | I | Refractory Metastatic Cancer (including melanoma) | Completed. Demonstrated limited efficacy in the form of SD in eight patients (22.9%) with tumors of non-epithelial origin over two months | [94] | |
| Hyaluronic acid | Recombinant human hyaluronidase PH20 (rHuPH20) | Nivolumab | NCT03656718 | I/II | Unresectable Melanoma; Metastatic Melanoma | Completed. The SC Nivolumab + rHuPH20 dose-related exposures were well tolerated | [142] |
| NCT05297565 | III | Stage III A/B/C/D or Stage IV Melanoma | Completed. Results not published | [143] | |||
| Rituximab | NCT03719131 | II | Stage III A/B/C/D or Stage IV Cutaneous melanoma; Unresectable Melanoma | Active, not recruiting. Results not published | _ | ||
| Relatlimab; Nivolumab | NCT05625399 | III | Stage III or Stage IV Melanoma | Recruiting. Results not published | _ | ||
| Relatlimumab; Nivolumab | NCT06101134 | II | Melanoma | Recruiting. Results not published | _ | ||
| Nivolumab | NCT05496192 | II | Stage III A/B/C/D or Stage IV Melanoma | Withdrawn (Replaced it with another clinical trial) | _ | ||
| Hyaluronidase | Pembrolizumab | NCT06099782 | II | Stage II B/C or Stage III Melanoma | Recruiting. Results not published | _ | |
| Integrins | MEDI-523 (Vitaxin) | _ | _ | Metastatic Cancer (including melanoma) | Completed. One melanoma patient received two maximum doses of the drug, but continued to have PD, leading to withdrawal from the study. Notably, in this patient, the labelled Vitaxin successfully visualised and localised the tumor, likely due to the high expression of the αvβ3 integrins | [174] | |
| MEDI-522 (Etaracizumab) | _ | _ | I | Advanced Malignancies (including melanoma) | Completed. No CR or PR was observed in patients with advanced malignancies; however, long-term SD (34 weeks, >1 year, >2 years) was reported in patients with renal cell cancer. Two patients with melanoma and one patient with ocular melanoma showed PD after 6-8 weeks of therapy | [171] | |
| _ | _ | 0 | Advanced Melanoma | Completed. Pharmacodynamics in patients with advanced melanoma showed that the drug effectively saturates tumor cells at a dose of 8 mg/kg. Demonstrated an acceptable safety profile with no serious toxic effects and although no clear antitumor effects were observed, some patients may still benefit from inhibition of αvβ3 integrin-related signalling pathways | [175] | ||
| _ | _ | I | Advanced Solid Tumors (including melanoma) | Completed. All patients showed absence of PR and CR, but the melanoma patient showed SD for more than 4 months | [176] | ||
| Dacarbazin | NCT00066196 | II | Stage IV Melanoma | Completed. Responses were seen in the etoracizumab plus dacarbazine group, with 7 of 55 patients (12.7%) achieving a PR. There were no responses in the monotherapy group. SD was observed in 26 of 57 (45.6%) patients receiving etoracizumab alone and 22 of 55 (40%) in the combination group. PD was observed in 47.4% and 40%, respectively | [177] | ||
| _ | NCT00111696 | I | Stage IV Melanoma; Recurrent Malignant Melanoma | Completed. Results not published | _ | ||
| _ | NCT00263783 | I | Melanoma | Completed. Results not published | _ | ||
| _ | NCT00111696 | I | Advanced Malignant Melanoma | Completed. Results not published | _ | ||
| CD44 | Anti-CD44 Antibody RG7356 | _ | NCT01358903 | I | Melanoma | Completed. Has shown low clinical efficacy for patients with a variety of solid tumors, including melanoma. Only 13 out of 61 patients (21%) experienced SD lasting an average of 12 weeks. Labelled antibody showed efficacy in tumor tracing | [194] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





