Submitted:
18 October 2024
Posted:
21 October 2024
You are already at the latest version
Abstract
Quorum Sensing (QS) and Machine Learning (ML) hybrid systems represent a groundbreaking innovation in synthetic biology, offering unprecedented control and adaptability in microbial gene regulation and metabolic processes. QS, a microbial communication mechanism, is crucial for coordinating gene expression in response to population density, impacting behaviors such as biofilm formation, virulence, and resource optimization. However, traditional QS systems are constrained by their reliance on static, pre-programmed feedback loops, limiting their flexibility in dynamic, complex environments. This review highlights how integrating advanced ML algorithms—such as reinforcement learning and deep learning—into QS systems can overcome these limitations by enabling real-time data processing, predictive modeling, and dynamic feedback control. Through these innovations, QS-ML systems can autonomously adjust gene expression and metabolic outputs, making them more efficient and scalable in applications ranging from pathogen control to precision medicine and industrial biomanufacturing. Key case studies illustrate the successful deployment of QS-ML systems to combat antimicrobial resistance, optimize bio-production, and enhance therapeutic precision in cancer and immune modulation. Despite the clear advantages, challenges remain in data integration, system robustness, and regulatory oversight. Addressing these hurdles through interdisciplinary collaboration and developing scalable, multi-omics data platforms will be critical for advancing QS-ML systems from experimental settings to real-world applications. This review underscores the transformative potential of QS-ML systems in revolutionizing synthetic biology, with profound implications for personalized medicine, sustainable biomanufacturing, and environmental health.Quorum Sensing (QS) and Machine Learning (ML) hybrid systems represent a groundbreaking innovation in synthetic biology, offering unprecedented control and adaptability in microbial gene regulation and metabolic processes. QS, a microbial communication mechanism, is crucial for coordinating gene expression in response to population density, impacting behaviors such as biofilm formation, virulence, and resource optimization. However, traditional QS systems are constrained by their reliance on static, pre-programmed feedback loops, limiting their flexibility in dynamic, complex environments. This review highlights how integrating advanced ML algorithms—such as reinforcement learning and deep learning—into QS systems can overcome these limitations by enabling real-time data processing, predictive modeling, and dynamic feedback control. Through these innovations, QS-ML systems can autonomously adjust gene expression and metabolic outputs, making them more efficient and scalable in applications ranging from pathogen control to precision medicine and industrial biomanufacturing. Key case studies illustrate the successful deployment of QS-ML systems to combat antimicrobial resistance, optimize bio-production, and enhance therapeutic precision in cancer and immune modulation. Despite the clear advantages, challenges remain in data integration, system robustness, and regulatory oversight. Addressing these hurdles through interdisciplinary collaboration and developing scalable, multi-omics data platforms will be critical for advancing QS-ML systems from experimental settings to real-world applications. This review underscores the transformative potential of QS-ML systems in revolutionizing synthetic biology, with profound implications for personalized medicine, sustainable biomanufacturing, and environmental health.
Keywords:
1. Introduction
1.1. Context: The Role of Quorum Sensing in Synthetic Biology
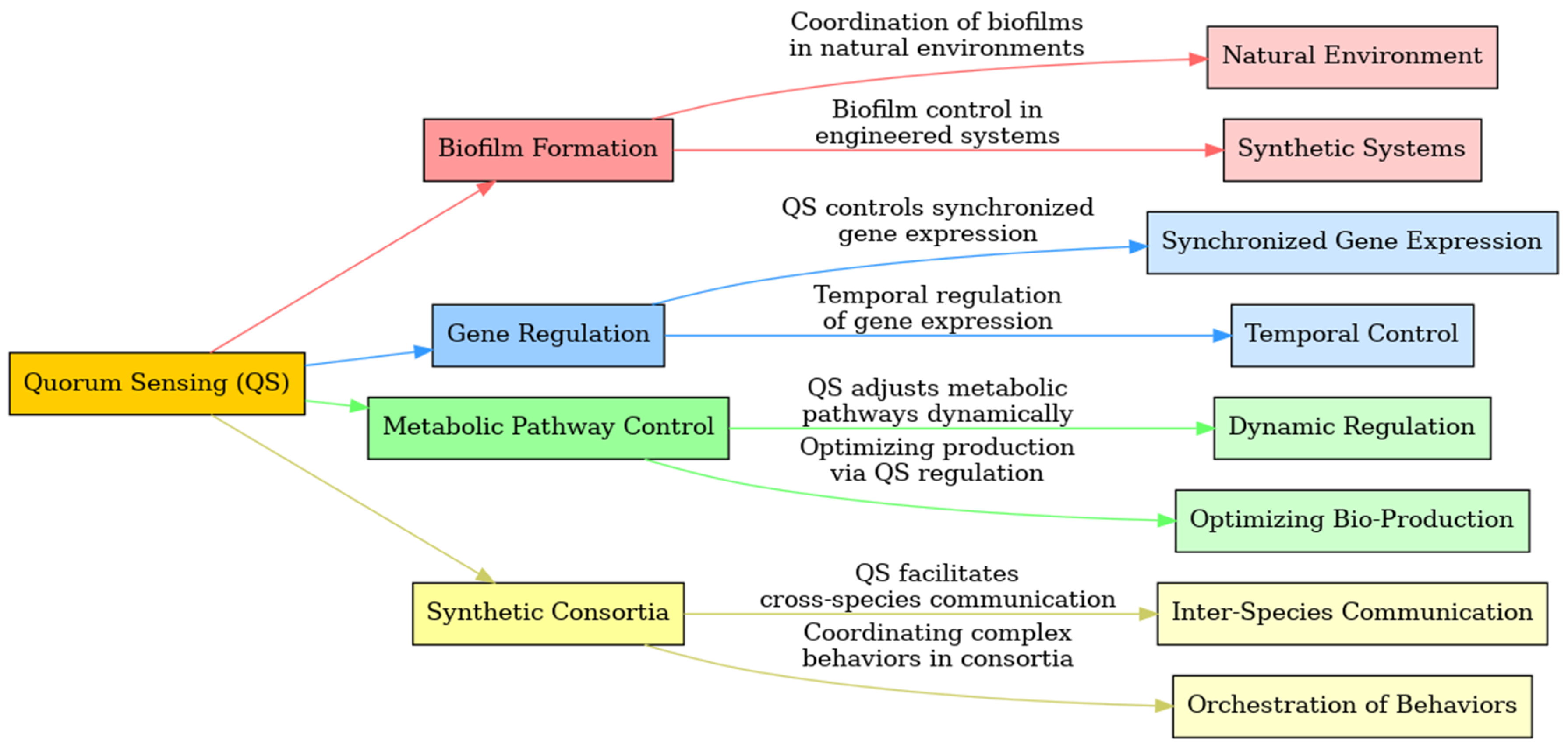
1.1.1. Biofilm Formation
1.1.2. Gene Regulation
1.1.3. Metabolic Pathway Control
1.1.4. Synthetic Consortia
1.2. The Potential of Machine Learning in Optimizing Quorum Sensing Systems
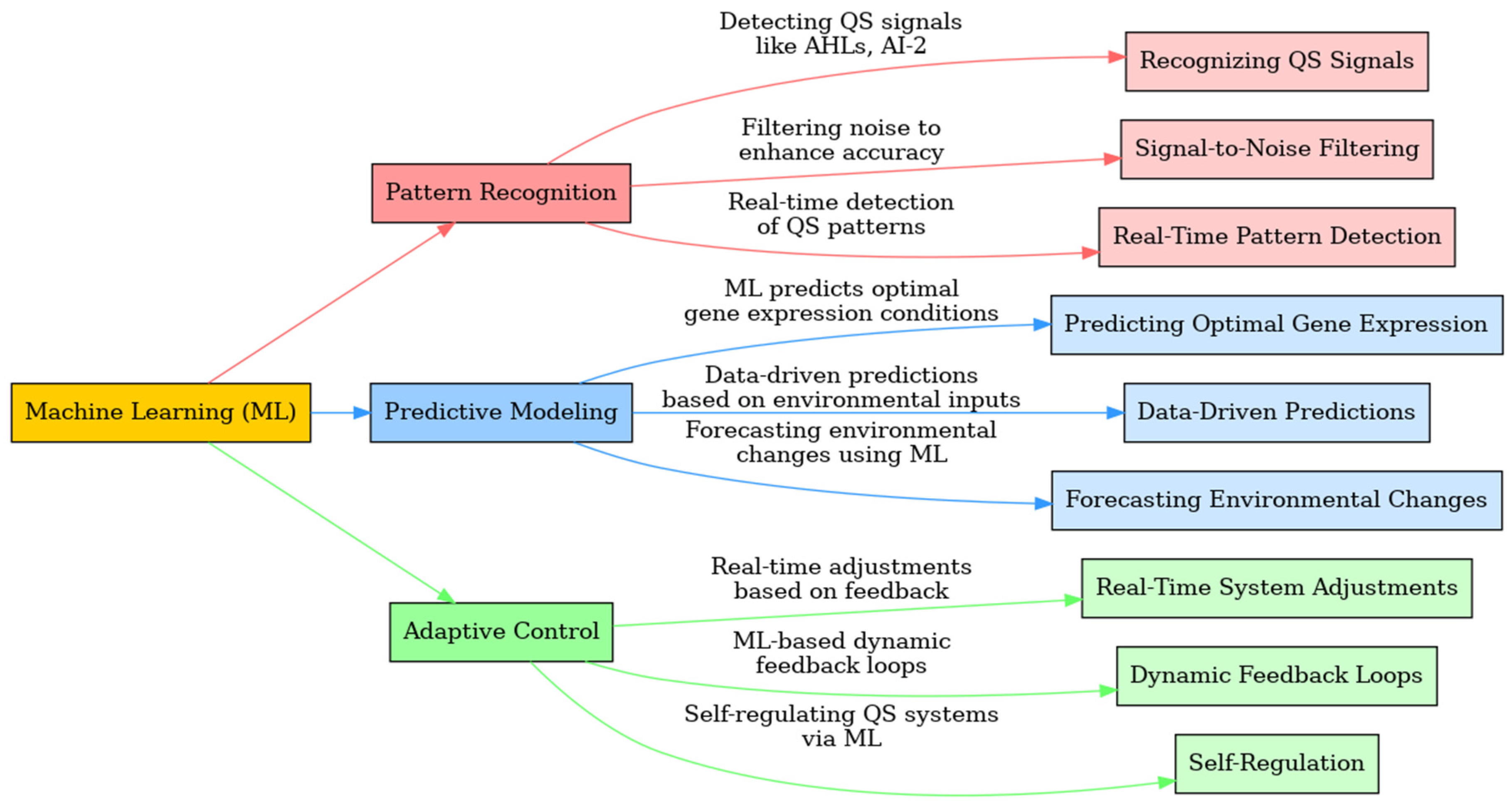
- Recognizing QS Signals: ML algorithms can detect subtle variations in autoinducer signals within microbial populations, allowing for more accurate regulation of behaviors like biofilm formation or metabolic control. For example, in Pseudomonas aeruginosa, ML can identify changes in AHL concentration, enabling dynamic control of biofilm growth. [31,118,119]
- Signal-to-Noise Filtering: In multi-species microbial consortia, where overlapping QS signals can cause interference, ML algorithms are capable of distinguishing relevant signals from background noise. This improves the reliability of QS circuits in mixed microbial environments, such as those used in environmental remediation or industrial production. [66,82,120,121,122,123]
- Real-Time Pattern Detection: ML allows QS systems to detect and respond to real-time changes in the microbial environment. For instance, in bio-reactors, ML algorithms can continuously monitor QS signals and make dynamic adjustments to gene regulation or enzyme production as cell density changes. [124,125,126,127,128,129,130,131]
- Predicting Optimal Gene Expression: ML models can forecast the best moments to trigger gene expression in QS systems based on past data and environmental conditions. This ensures that key genes are activated when the population density is optimal for enzyme production or biofuel synthesis, increasing overall efficiency. [139,140,141,142,143,144,145]
- Data-Driven Predictions: By integrating data from various biological layers—such as genomics, proteomics, and metabolomics—ML can provide holistic predictions of how microbial populations will behave in response to environmental changes. This is particularly valuable in bio-manufacturing, where ML-driven models can predict how cells will respond to nutrient fluctuations or temperature shifts, allowing for real-time adjustments to QS circuits. [146,147,148,149,150,151,152]
- Forecasting Environmental Changes: ML systems can also forecast environmental changes, such as shifts in pH or temperature, and adjust QS-regulated pathways in advance. For example, ML algorithms can predict an upcoming temperature fluctuation in a bio-reactor and modify metabolic activity in microbes to maintain stability and optimize production. [153,154,155,156,157,158,159]
- Real-Time System Adjustments: ML-driven QS systems can make real-time adjustments to gene expression, metabolic activity, or biofilm formation based on environmental feedback. In bio-reactors, for instance, ML models can continuously optimize enzyme production based on real-time data, ensuring that production remains efficient even as conditions change. [49,106,164,165,166]
- Dynamic Feedback Loops: ML enables the creation of dynamic feedback loops where changes in QS signals trigger modifications in gene expression, which are then monitored and further refined by the ML system. This ensures that QS-regulated behaviors, such as metabolic pathway control, remain efficient under varying conditions. [78,167,168,169,170,171,172]
- Self-Regulation: One of the most promising aspects of integrating ML with QS systems is the potential for self-regulation. ML-driven synthetic organisms can autonomously adjust their metabolic pathways in response to environmental fluctuations, optimizing processes like biofuel production without the need for human intervention. This is particularly valuable in large-scale industrial applications where maintaining optimal performance requires continuous monitoring and adjustment. [46,60,106,173,174,175]
2. Mechanisms of Quorum Sensing in Synthetic Biology
2.1. Biological Basis of Quorum Sensing
| QS System | Microbial Species | Autoinducer Molecule | Controlled Processes | Synthetic Applications |
|---|---|---|---|---|
| LuxI/LuxR | Vibrio fischeri | N-acyl homoserine lactone (AHL) | Bioluminescence, Virulence | Bioluminescence signaling, synchronized gene expression in engineered systems |
| LasI/LasR | Pseudomonas aeruginosa | N-3-oxo-dodecanoyl homoserine lactone | Biofilm formation, Toxin production, Motility | Anti-biofilm strategies, biofilm engineering, coordination in microbial consortia |
| AgrC/AgrA | Staphylococcus aureus | Autoinducing peptide (AIP) | Toxin production, Virulence, Biofilm development | QS-based control of virulence in pathogens, disruption of biofilms in medical devices |
| ComX/ComP | Bacillus subtilis | Competence-stimulating peptide (CSP) | Sporulation, Competence development | Synchronized sporulation in microbial factories, nutrient regulation in engineered systems |
| AI-2 | Multi-species (e.g., Escherichia coli) | Furanosyl borate diester | Inter-species communication, Virulence regulation | Cross-species synthetic consortia, inter-species gene circuits for microbial factories |
2.2. Challenges in Traditional Quorum Sensing Systems
| Challenge | Description | Impact on Synthetic Systems | Potential Solutions |
|---|---|---|---|
| Environmental Variability | Traditional QS systems cannot adapt to rapid or unpredictable environmental changes | Leads to loss of control over gene expression and process inefficiency | Integration of real-time environmental sensors with QS systems using ML |
| Static Feedback Loops | QS circuits often rely on pre-programmed responses that don’t adjust dynamically | Limits flexibility, causing suboptimal responses in fluctuating conditions | Dynamic feedback loops with machine learning algorithms |
| Signal Crosstalk | Overlap of QS signals in mixed microbial populations leading to interference | Unintended activation of gene circuits, reducing system reliability | Design of orthogonal QS systems to prevent signal interference |
| Scalability | Difficulty scaling QS systems in large, diverse microbial consortia | Impairs ability to regulate gene expression in complex environments | ML-driven modeling to manage scalability in diverse consortia |
| Lack of Predictive Capacity | QS systems respond reactively rather than preemptively | Inefficiency in changing environments, delayed system response | Predictive modeling with machine learning for anticipatory adjustments |
3. Machine Learning in Synthetic Biology: A New Paradigm for Optimizing QS Systems
3.1. Overview of Machine Learning for Biological Optimization
| Algorithm | Key Features | Applications in QS-ML Systems | Examples of Use |
|---|---|---|---|
| Supervised Learning | Learns from labeled data to make predictions | Analyzing QS signaling data to predict optimal gene expression states | Predicting gene regulation patterns in microbial consortia |
| Reinforcement Learning | Trial-and-error approach to learning optimal actions | Continuous optimization of metabolic processes based on feedback from QS signals | Real-time adjustment of metabolic pathways in response to environmental changes |
| Unsupervised Learning | Identifies patterns in unlabeled data | Clustering QS signaling data to detect emerging patterns or novel behaviors | Detecting novel QS pathways in synthetic microbial systems |
| Deep Learning | Uses neural networks for complex pattern recognition | Predicting complex relationships between QS signals and environmental conditions | Analyzing high-dimensional QS data for metabolic regulation in real-time |
| Transfer Learning | Applies knowledge from one domain to another | Applying models trained on one set of microbial species to another | Generalizing QS systems across different microbial species and environments |
| Evolutionary Algorithms | Optimization techniques inspired by natural evolution | Optimizing synthetic gene circuits to evolve more efficient QS responses | Designing more efficient QS circuits through iterative simulation |
3.2. Integrating ML and QS for Hybrid Adaptive Systems
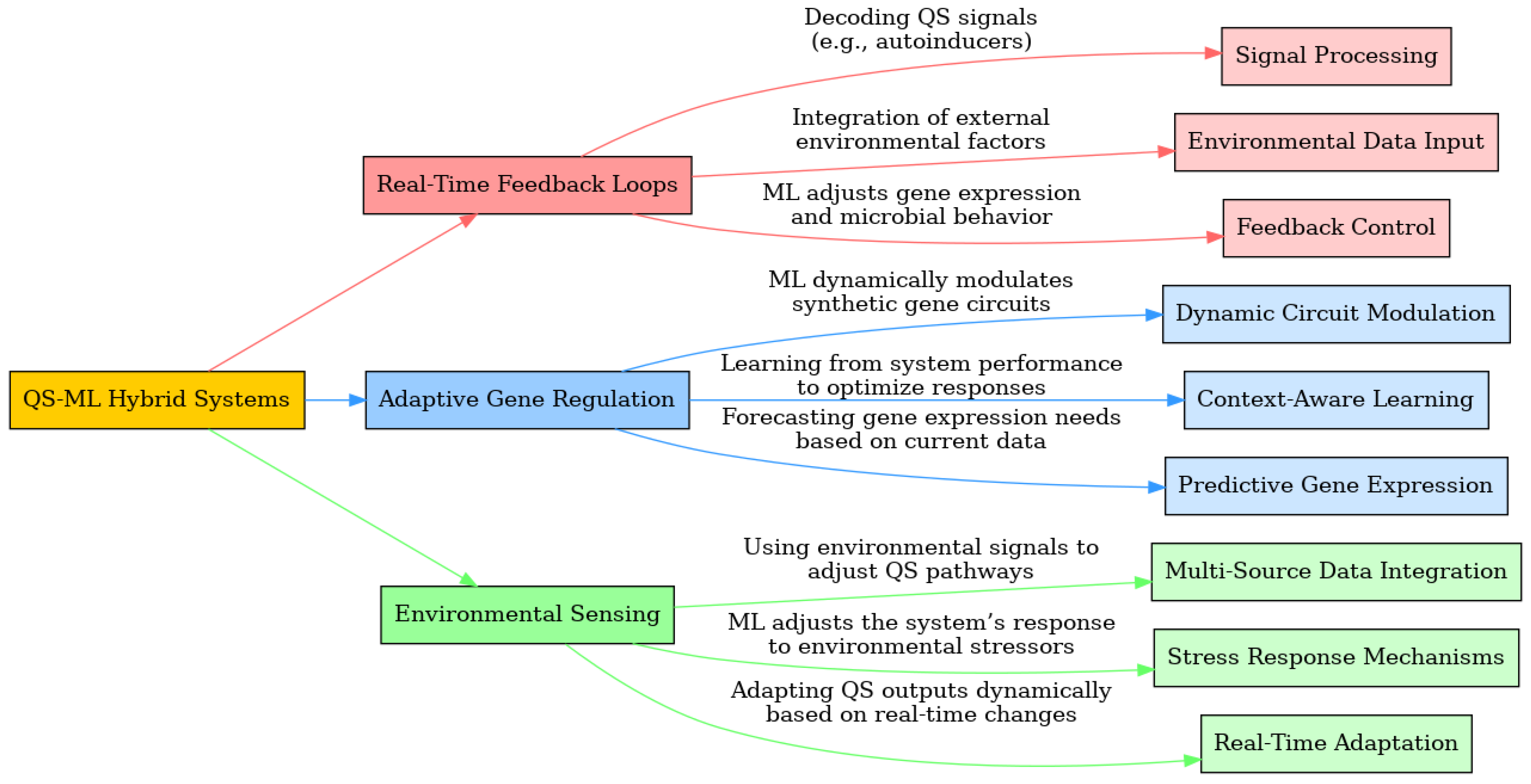
- Signal Processing: ML models decode QS signals from microbial communities in real-time, identifying the concentration and timing of autoinducer molecules such as AHL, AI-2, and peptides. This signal processing allows the system to predict changes in population density and adjust gene expression accordingly. For example, an ML-driven system processes AHL levels to predict upcoming increases in population density, allowing for preemptive adjustments in gene expression to optimize biofilm formation or suppression based on the environmental context. [64,66,67,179,336,337]
- Environmental Data Input: ML continuously integrates data from environmental sensors, monitoring factors such as temperature, pH, and nutrient availability. This allows the QS system to consider external factors that may affect microbial behavior. For example, a reinforcement learning model predicts the impact of temperature changes on microbial communication and adjusts QS responses accordingly, ensuring that gene circuits remain active only under optimal conditions. [334,338,339,340,341]
- Feedback Control: ML algorithms provide feedback control by predicting the optimal state of gene expression based on QS inputs and environmental data, dynamically adjusting microbial behavior. For example, ML adjusts the production of biofilm-forming genes in microbial populations based on real-time assessments of nutrient availability, ensuring that resources are efficiently used to maintain or suppress biofilm growth. The combination of signal processing, environmental data input, and feedback control ensures that QS-ML systems are capable of making real-time adjustments that optimize both microbial behavior and system outputs (Figure 3). [237,252,342,343,344,345]
- Dynamic Circuit Modulation: ML algorithms dynamically modulate synthetic gene circuits in response to QS inputs, allowing for rapid adjustments in gene expression. This dynamic modulation ensures that gene circuits can adapt to changing metabolic demands, optimizing the system for bio-production or other industrial applications. For example, in a bio-production system, ML dynamically adjusts gene expression levels of synthetic pathways responsible for producing antibiotics or enzymes, ensuring that the metabolic output matches current resource availability and system demands. [8,47,55,349,350,351]
- Context-Aware Learning: ML models learn from past system performance to improve efficiency over time, adapting gene regulation strategies based on historical data. This context-aware learning enables the system to refine its responses based on previous metabolic cycles, making future gene expression more efficient. For example, a neural network model learns from the outcomes of past metabolic cycles to adjust the expression of key genes, increasing the yield of a desired product (e.g., enzymes or biofuels) in subsequent cycles by optimizing resource allocation and metabolic pathway activation. [352,353,354,355,356,357,358]
- Predictive Gene Expression: ML models predict future gene expression needs based on real-time environmental signals and resource availability. By forecasting future metabolic demands, the system can adjust gene expression proactively rather than reactively, ensuring a more efficient and stable microbial system. For example, ML predicts when microbial populations in synthetic consortia will need to upregulate genes responsible for producing virulence factors or enzymes, allowing the system to optimize output based on the anticipated demand. [111,139,141,145,174,359,360]
- Multi-Source Data Integration: ML integrates data from multiple environmental sensors (e.g., temperature, pH, osmotic pressure) to make comprehensive decisions about gene regulation and metabolic activity. This multi-source data integration enables the system to react holistically to changing environmental conditions. For example, by integrating data from both pH and temperature sensors, an ML-driven QS system can predict the optimal moment for QS-mediated gene activation in an industrial-scale bioprocess, ensuring that metabolic processes occur under ideal conditions. [152,367,372,373,374,375,376,377,378]
- Stress Response Mechanisms: ML enhances QS systems’ ability to respond to environmental stressors by regulating protective genes that help microbial populations resist stress. This capability is essential for maintaining microbial health and productivity in challenging environments. For example, when nutrient levels suddenly drop, ML modulates QS pathways to initiate stress response mechanisms, upregulating protective genes that help the microbial population survive until conditions improve. [379,380,381,382,383,384,385,386,387]
- Real-Time Adaptation: ML enables real-time adaptation of QS systems to sudden changes in environmental conditions, ensuring that microbial populations remain stable and productive even in the face of unexpected stressors. By dynamically adjusting gene expression and metabolic pathways, the system can maintain balance and optimize its outputs under variable conditions. For example, ML detects real-time changes in nutrient availability or toxin levels and dynamically adjusts microbial activity, shifting from metabolic production to stress response when necessary to ensure long-term system stability. [348,388,389,390,391,392,393]
4. Case Studies: Applications of QS-ML Hybrid Systems in Disease Control
4.1. Combatting Pathogenic Bacteria with QS-ML Systems
- Public Health Application: In epidemic-prone areas, QS-ML hybrid systems could be deployed to monitor environmental and population-level QS signals, triggering preemptive responses that help mitigate the spread of cholera and reduce the need for large-scale medical interventions (Table 4). [338,442,445]
| Pathogen | QS System | ML Intervention | Outcome | Clinical/Practical Application |
|---|---|---|---|---|
| Pseudomonas aeruginosa | LasI/LasR | Machine learning to predict and disrupt biofilm formation | Reduced biofilm density, improved antimicrobial efficacy | Enhanced treatment of cystic fibrosis and wound infections |
| Staphylococcus aureus | AgrC/AgrA | ML-driven suppression of QS signals regulating toxin production | Reduced virulence factor expression, improved patient outcomes | Lower virulence in hospital-acquired infections (e.g., MRSA) |
| Escherichia coli(EHEC) | AI-2 | Real-time QS monitoring using ML for early detection of virulence activation | Preemptive mitigation of toxin production | Food safety: preventing foodborne illness outbreaks |
| Vibrio cholerae | LuxO | ML-enhanced QS disruption to prevent cholera toxin production | Significant reduction in cholera toxin levels | Cholera control strategies in epidemic-prone areas |
4.2. Precision Therapies and Gene Regulation
- Example Application: In the context of inherited genetic diseases, such as Duchenne muscular dystrophy (DMD), QS-ML systems have been used to regulate the expression of corrective genes, ensuring that gene delivery is synchronized with the patient’s metabolic cycles for better therapeutic outcomes (Table 5). [480,481,482,483,484,485,486,487]
- ML Optimization: By analyzing tumor microenvironment data—such as oxygen levels, tumor-associated antigens, and immune checkpoint signals—ML models can optimize immune cell activation in QS-controlled systems, adjusting the production of cytokines or other immunomodulatory molecules in response to real-time feedback. [491,492,493,494,495,496,497]
- Example Application: In trials involving adaptive immune system modulation, QS-ML systems have been shown to improve the efficacy of T-cell-based immunotherapies, allowing engineered immune cells to dynamically adjust their activity based on tumor conditions, reducing the risk of excessive immune responses or off-target effects (Table 5). [504,505,506,507,508]
- Example Application: In chemotherapy, QS-ML hybrid systems have been applied to deliver drugs directly to tumor cells, using ML to predict when the tumor is most vulnerable and adjust the release of chemotherapy agents accordingly, improving treatment outcomes and reducing side effects (Table 5). [425,525,526,527]
- Example Application: In regenerative therapies for cartilage repair or neural tissue regeneration, QS-ML systems have been used to guide stem cell differentiation, ensuring that cells develop into the appropriate tissue type and integrate seamlessly with surrounding tissues (Table 5). [541,542,543,544,545,546]
- Example Application: In clinical trials, QS-controlled synthetic probiotics have been used to modulate the gut environment in patients with irritable bowel syndrome (IBS) or Crohn's disease, improving symptoms by dynamically adjusting their activity in response to ML predictions about the gut's changing conditions (Table 5). [556,559,560]
| Therapy Type | QS Component | ML Optimization | Outcome | Example Application |
|---|---|---|---|---|
| Gene Therapy | QS-regulated gene circuits | Real-time gene expression adjustment based on patient data | Improved efficacy of gene delivery, reduced side effects | Personalized gene therapy for inherited genetic diseases |
| Cancer Immunotherapy | QS-controlled immune cell activation | ML to optimize timing and intensity of immune response | Enhanced tumor targeting, minimized damage to healthy tissues | Adaptive immune system modulation in cancer treatment |
| Drug Delivery Systems | QS-triggered release mechanisms | ML-driven prediction of drug release timing and dosage | Precise targeting of diseased cells, reduced systemic toxicity | Controlled release systems for chemotherapy drugs |
| Stem Cell Therapy | QS-guided differentiation | ML to predict optimal differentiation pathways | Improved stem cell integration and tissue regeneration | Regenerative medicine: cartilage or neural tissue repair |
| Synthetic Probiotic Therapies | QS-controlled synthetic probiotics | ML to optimize probiotic behavior in response to gut environment | Enhanced gut health, reduced inflammation | Treatment of gastrointestinal disorders, microbiome modulation |
5. Discussion
5.1. Technical Challenges in Hybrid QS-ML Systems
| Challenge | Description | Impact | Potential Solutions |
|---|---|---|---|
| Data Integration | Difficulty in merging biological, genomic, and QS signaling data | Leads to incomplete or inconsistent models | Development of unified data integration platforms |
| Computational Complexity | High computational costs of real-time processing of QS-ML systems | Slows down system performance, limits scalability | Use of cloud computing and edge computing for real-time processing |
| Training Data Availability | Lack of large, labeled datasets for training ML models | Hinders model accuracy and generalization | Generating synthetic datasets and using transfer learning |
| Scalability | Difficulty in scaling systems for large, complex microbial consortia | Reduces effectiveness in multi-species synthetic biology setups | Designing modular, hierarchical QS-ML systems |
| System Robustness | Vulnerability to environmental disturbances or unexpected inputs | Causes system breakdowns or unintended outcomes | Building robust safety mechanisms and fail-safe designs |
- Potential Solutions: To address this issue, there is a need for the development of unified data integration platforms that can seamlessly merge genomic, proteomic, and QS signaling data into a consistent format for ML models. This would require innovations in bioinformatics tools and data standardization protocols that ensure high data fidelity across different sources (Table 6). [578,582,586,587]
- Potential Solutions: One potential solution is to leverage cloud computing or edge computing platforms, which can provide the necessary computational resources to handle large-scale real-time data processing. Edge computing is especially useful for decentralized processing, allowing real-time adjustments to be made closer to where the data is generated, thus reducing latency and improving system responsiveness (Table 6). [598,599,600,601]
- Impact: Without sufficient training data, ML models may lack the accuracy needed to reliably predict gene expression outcomes or metabolic responses in novel environments, reducing the system's overall effectiveness in practical applications.
- Potential Solutions: To mitigate this challenge, researchers can generate synthetic datasets using in silico simulations of microbial behaviors, which can help train models even in the absence of real-world data. Additionally, transfer learning—where models trained on one dataset are adapted for use in a different but related context—can be employed to improve model generalization in environments with limited training data (Table 6).
- Impact: The difficulty in scaling these systems leads to reduced effectiveness in multi-species synthetic biology setups, limiting the potential for applying QS-ML systems to large industrial processes or complex therapeutic applications, such as those involving human microbiomes. [603,604,605,607,608,609]
- Potential Solutions: One promising solution is to design modular, hierarchical QS-ML systems that can function independently at smaller scales but are capable of interacting in a coordinated manner when combined. These modular systems allow for better management of complexity, as each unit can be optimized for a specific task or environment, while the overall system remains adaptable to larger setups (Table 6). [603,604,610,611,612]
- Potential Solutions: To enhance system robustness, multi-layered safety mechanisms should be integrated into QS-ML systems. These include fail-safe designs that automatically revert the system to a safe state if anomalies are detected, and robust feedback loops that allow the system to dynamically adjust its behavior in response to environmental disturbances. Additionally, redundancy in key circuits can provide backup functions in case of failure, ensuring that critical operations continue even when parts of the system malfunction (Table 6). [620,621,622,623]
5.2. Balancing Flexibility with Control
- Control Mechanisms: One solution to this challenge is to implement failsafes and feedback loops that can intervene if the system begins to deviate from its intended path. These mechanisms could involve manual overrides or automated safety checks that halt problematic system behaviors before they cause harm. Furthermore, robust safety designs—discussed in section 5.1—must be integrated to detect system anomalies and course-correct dynamically. [634,637]
- Proposed Solutions: Multi-layered security mechanisms can help prevent these unintended consequences. For example, integrating kill-switches within the system design ensures that the QS-ML circuit can be shut down immediately if it behaves erratically. Additionally, predictive monitoring tools can analyze the system’s performance in real time, allowing researchers to preemptively intervene if the system shows signs of failure. These fail-safe designs, combined with robust feedback loops, can ensure that the QS-ML system maintains both flexibility and control, even under fluctuating conditions. [650,651,652,653]
5.3. Ethical and Safety Considerations
- Ethical Concerns: This raises questions about liability and regulation—who is responsible if an autonomous system causes harm? The unpredictability inherent in QS-ML systems, especially in unpredictable environments like the human body, makes it difficult to establish clear regulatory oversight. As these systems make real-time, data-driven decisions, there is a need for ethical frameworks that address how these systems should be monitored and who is accountable for their behavior. [671,676,677,678,679,680]
- Proposed Solutions: To address these concerns, regulatory bodies must establish guidelines that balance the system's autonomy with human oversight. As discussed in section 5.2, integrating failsafe mechanisms into QS-ML systems can ensure that medical professionals can override the system if it deviates from its intended course. Furthermore, predictive tools should be incorporated into the system design to anticipate potential issues before they arise, enhancing patient safety and predictability. [574,674,681,682,683]
- Ethical Considerations: The potential for ecological disruption raises concerns about the long-term effects of releasing synthetic organisms into the environment. If QS-ML systems inadvertently alter microbial interactions or introduce imbalances into the food chain, the consequences could be far-reaching and difficult to reverse. [666,690]
- Proposed Solutions: To mitigate these risks, it is critical to implement stringent biocontainment protocols and biosafety mechanisms that prevent the unintended spread of QS-ML systems in natural environments. One approach is to design non-replicating organisms or gene-editing kill-switches that allow for system deactivation if the system deviates from its intended function. Additionally, multi-layered security checks, as discussed in section 5.2, should be used to monitor the system's behavior and ensure it operates within ethical and ecological boundaries. [691,692,693]
5.4. Opportunities for Future Research and Development
- Genomics Integration: Incorporating genomic data into QS-ML systems can help improve gene regulation by allowing the system to predict gene expression changes in response to environmental signals. By integrating genomic sequencing data, ML models can forecast how different QS pathways will affect microbial behavior at a molecular level. For example, predicting gene expression changes in microbial consortia based on genomic data can improve the control of synthetic biological systems, such as those used in bio-production. [147,710]
- Proteomics Integration: Proteomics data—which maps the interactions between proteins—can enhance the ability of QS-ML systems to adjust cellular behaviors more precisely. By integrating protein expression profiles, ML can optimize metabolic pathways based on real-time analysis of protein networks. For example, ML-driven QS systems could analyze protein interaction networks to adjust metabolic pathways in real time, improving the efficiency of biosensor applications or other synthetic biology processes. [711,712]
- Metabolomics Integration: Metabolomics data tracks metabolic activities, providing insight into cellular energy use, nutrient uptake, and metabolic fluxes. This data is critical for bio-production applications where the timely adjustment of gene expression is necessary to optimize production yields and efficiency. For example, an ML-driven QS system that adjusts gene expression based on real-time metabolomic data could enhance the productivity of bio-reactors or optimize microbial behavior in biosensors. [709,710]
- Cross-Omics Correlation: Future advancements will involve building models that correlate data across genomics, proteomics, and metabolomics layers to fine-tune predictions and improve the accuracy of cellular responses. This cross-omics approach will help QS-ML systems become more adaptive and predictive in diverse biological environments. For example, developing ML models that integrate cross-omics data to optimize microbial bio-production processes, ensuring that the system reacts dynamically to environmental changes and improves output predictability (Figure 4). [713,714]
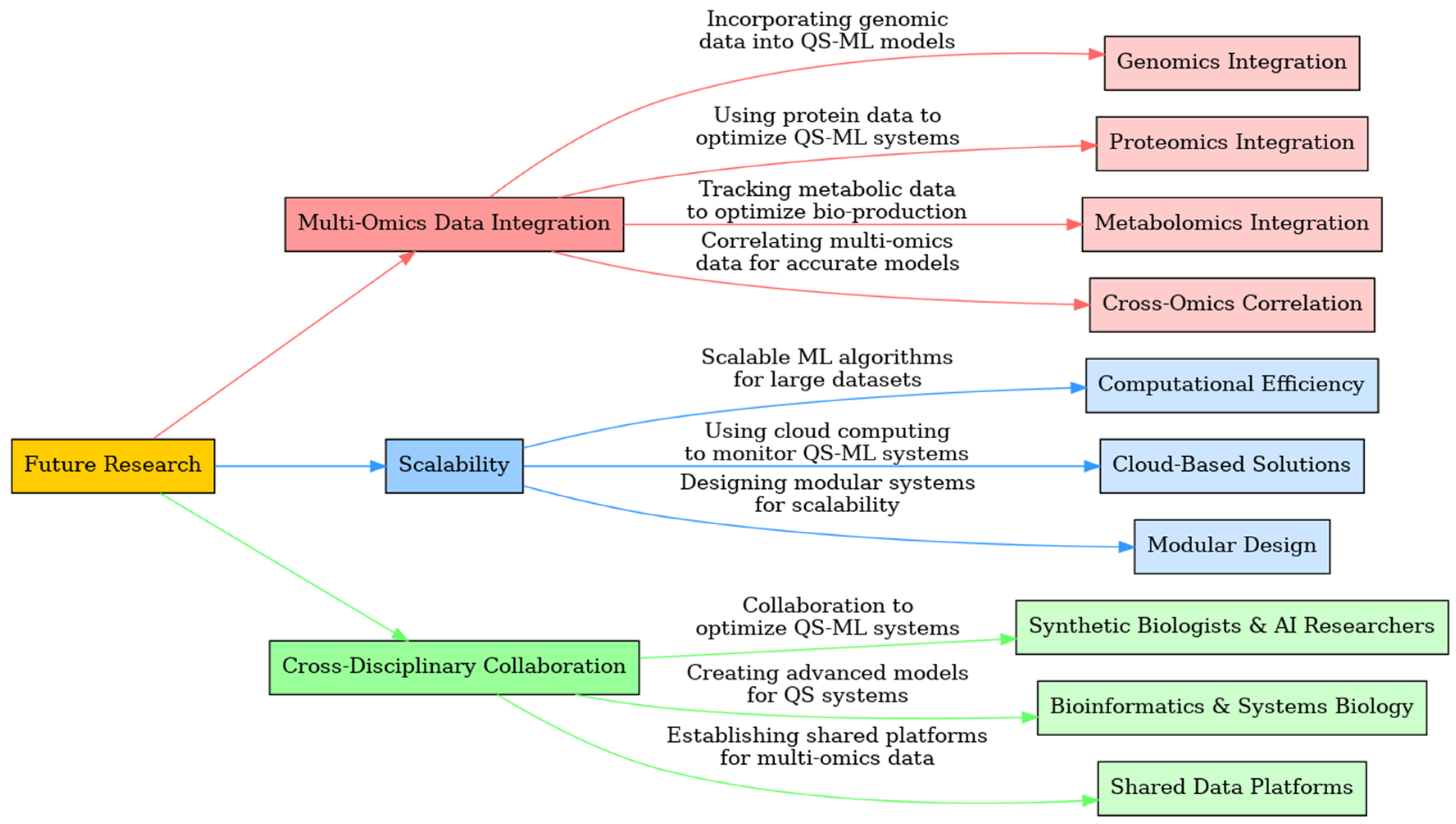
- Computational Efficiency: Future QS-ML systems must be capable of processing large datasets in real time without overloading computational resources. The development of scalable ML algorithms that can handle real-time data from large microbial populations will be essential for expanding the scope of QS-ML applications. For example, scalable ML models that process data in multi-species bioreactors could allow for real-time monitoring and adjustments of microbial behavior in large-scale industrial processes, improving efficiency and reducing waste. [716,717]
- Cloud-Based Solutions: Leveraging cloud computing can help overcome the limitations of local processing power by enabling remote monitoring and control of QS-ML systems. Cloud-based platforms would allow for continuous monitoring of biological processes in real-time, with the ability to make adjustments based on the analysis of large, complex datasets. For example, cloud-based monitoring of bioreactors in industrial applications could enable QS-ML systems to make real-time adjustments to microbial behavior from remote locations, enhancing the scalability of bio-production processes (Figure 4). [715,718]
- Modular Design for QS-ML Systems: Modular designs would allow for the flexible scaling of QS-ML systems, from lab-scale experiments to large industrial bioprocesses. By creating self-contained modules that can function independently or as part of a larger system, QS-ML systems could be easily adapted to different applications. For example, modular systems that control different microbial populations in large bioreactors could allow for independent regulation of each population, improving system performance in complex industrial setups (Figure 4). [719,720,721]
- Synthetic Biologists & AI Researchers: Collaboration between synthetic biologists and AI researchers will be crucial for optimizing the design and control of QS-ML systems. AI researchers can develop novel ML algorithms tailored specifically to biological datasets, while synthetic biologists provide the biological context necessary for these systems to function effectively. For example, AI researchers developing new ML algorithms optimized for biological data could work with synthetic biologists to refine QS-ML systems that manage microbial behavior in real-time bioprocesses. [707,725]
- Bioinformatics & Systems Biology: Collaboration between bioinformatics and systems biology experts will help create more advanced models for predicting biological behaviors. By leveraging bioinformatics tools to generate detailed datasets, systems biologists can test and validate these models in experimental settings, ensuring that the predictions hold up in real-world applications. For example, bioinformatics researchers providing detailed datasets for ML models that optimize QS interactions, with systems biologists conducting experiments to validate predictions in real-time applications (Figure 4). [146,723]
- Shared Data Platforms: Establishing shared data platforms will be critical for fostering collaboration between biological and AI researchers. Open-source databases that contain multi-omics data could accelerate the development of QS-ML systems by providing a common resource for researchers to train ML models and optimize synthetic biology applications. For example, open-source databases that house genomic, proteomic, and metabolomic data could be used to train ML models for more accurate control of QS systems in industrial or medical applications, allowing for faster innovation and wider adoption (Figure 4). [146,707]
6. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konda, M. , et al., Quorum Sensing: A New Target for Anti-infective Drug Therapy. 2023.
- Li, Y. , et al., An Engineered Escherichia coli Community for Studying Quorum Sensing. SynBio 2023, 1, 144–157. [Google Scholar] [CrossRef]
- Snehashis Koley, A.B. , Mandira Mukherjee, Quorum Sensing in Gram-Negative Bacteria, in Natural Products. 2023, CRC Press. p. 37.
- Swetha, B.M., M. Saravanan, and P. Piruthivraj, Emerging trends in the inhibition of bacterial molecular communication: An overview. Microbial Pathogenesis, 2023: p. 106495.
- Taylor-Robinson, Z.S.a.A.W. , Quorum Sensing in Biofilm, in Recent Advances in Bacterial Biofilm Studies - Formation, Regulation, and Eradication in Human Infections. 2023, IntechOpen.
- Ge, C. , et al., Redesigning regulatory components of quorum-sensing system for diverse metabolic control. Nature communications 2022, 13, 2182. [Google Scholar] [CrossRef] [PubMed]
- Gu, F. , et al., A synthetic population-level oscillator in non-microfluidic environments. Communications Biology 2023, 6, 515. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. , et al., Dynamic cell programming with quorum sensing-controlled CRISPRi circuit. ACS Synthetic Biology 2020, 9, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.Y. , et al., Designing microbial cell factories for programmable control of cellular metabolism. Current Opinion in Systems Biology, 2023: p. 100493.
- Saha, R. , et al., Microbial quorum sensing systems: new and emerging trends of biotechnology in bioremediation. Microbes and Microbial Biotechnology for Green Remediation, 2022: p. 795-811.
- Soma, Y. , et al., Design of synthetic quorum sensing achieving induction timing-independent signal stabilization for dynamic metabolic engineering of E. coli. ACS Synthetic Biology 2021, 10, 1384–1393. [Google Scholar] [CrossRef]
- Wan, X. , et al., Programming living sensors for environment, health and biomanufacturing. Microbial biotechnology 2021, 14, 2334–2342. [Google Scholar] [CrossRef]
- =, *!!! REPLACE !!!*. Al-Tayawi, T.S., E.M. Adel, and F.H. Omer, =An Overview of Biofilm as a Virulence Factor for Bacteria to Survive in the Harsh Environment. =International Journal of Medical Science and Clinical Research Studies 2023, 3, 1188–1197. [Google Scholar]
- Aransiola, S.A., B. Selvaraj, and N.R. Maddela, Bacterial biofilm formation and anti-biofilm strategies. Research in Microbiology 2024, 175, 104172.
- Bello, O.O. , et al., Occurrence and Role of Bacterial Biofilms in Different Systems. Acta microbiologica Bulgarica, 2023.
- Gaurav G Khandalkar, P.K.U. , Rahul G Ingle, Natural Products Could be a Promising Remedy against Biofilm-Forming Bacteria. International Journal of Pharmaceutical Quality Assurance 2024, 15, 1035–1039. [Google Scholar] [CrossRef]
- Handa, V. , et al., Quorum Sensing Mechanisms, Biofilm Growth, and Microbial Corrosion Effects of Bacterial Species, in Machine Learning in 2D Materials Science. 2023, CRC Press. p. 133-146.
- Harpke, M. and E. Kothe, Biofilm formation in Gram-positives as an answer to combined salt and metal stress. Journal of Basic Microbiology 2023, 63, 888–896. [Google Scholar] [CrossRef]
- Sivakumar Krishnan, S.A.P., Y. V. Nancharaiah, Environmental microbial biofilms: formation, characteristics, and biotechnological applications, in Material-Microbes Interactions. 2023: Academic Press. p. 3-45.
- Xu, F. , et al., Protective effects of antibiotic resistant bacteria on susceptibles in biofilm: Influential factors, mechanism, and modeling. Science of The Total Environment 2024, 930, 172668. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I., T.M. Usman, and S. Varjani, Exploring the role of microbial biofilm for industrial effluents treatment. Bioengineered 2022, 13, 6420–6440. [CrossRef] [PubMed]
- Jaiswal, S. and P. Shukla, Alternative strategies for microbial remediation of pollutants via synthetic biology. Frontiers in microbiology 2020, 11, 808. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.Q. , Synthetic biology engineering of biofilms as nanomaterials factories. Biochemical Society Transactions 2017, 45, 585–597. [Google Scholar] [CrossRef]
- Sahreen, S. , et al., Exploring the function of quorum sensing regulated biofilms in biological wastewater treatment: A review. International Journal of Molecular Sciences 2022, 23, 9751. [Google Scholar] [CrossRef]
- Wang, S. , et al., Advances in the Application of Quorum Sensing to Regulate Electrode Biofilms in Bioelectrochemical Systems. Fermentation 2023, 9, 625. [Google Scholar] [CrossRef]
- Xue, Y.-M. , et al., Engineering a Pseudomonas putida as living quorum quencher for biofilm formation inhibition, benzenes degradation, and environmental risk evaluation. Water Research 2023, 246, 120690. [Google Scholar] [CrossRef]
- Yaashikaa, P., M. K. Devi, and P.S. Kumar, Engineering microbes for enhancing the degradation of environmental pollutants: A detailed review on synthetic biology. Environmental Research 2022, 214, 113868. [Google Scholar] [CrossRef]
- Zhou, L. , et al., Regulatory mechanisms and promising applications of quorum sensing-inhibiting agents in control of bacterial biofilm formation. Frontiers in microbiology 2020, 11, 589640. [Google Scholar] [CrossRef]
- Kraikivski, P. , Synchronization of Oscillatory Gene Networks. Case Studies in Systems Biology, 2021: p. 123-136.
- Martinelli, V., et al., Multicellular PI control for gene regulation in microbial consortia. IEEE Control Systems Letters 2022, 6, 3373–3378. [CrossRef]
- Rattray, J.B. , et al., Bacterial quorum sensing allows graded and bimodal cellular responses to variations in population density. MBio 2022, 13, e00745–22. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.K. , et al., Mathematical modeling of RNA-based architectures for closed loop control of gene expression. ACS synthetic biology 2018, 7, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Cannarsa, M.C. , et al., Light-driven synchronization of optogenetic clocks. bioRxiv, 2023: p. 2023.10. 24.563722.
- Chang, M., et al., Gene expression modulation tools for bacterial synthetic biology. Biotechnology for Sustainable Materials 2024, 1, 6.
- Glasscock, C.J. , et al., Dynamic control of gene expression with riboregulated switchable feedback promoters. ACS synthetic biology 2021, 10, 1199–1213. [Google Scholar] [CrossRef]
- Jang, S. , et al., Synthetic regulatory tools to engineer microbial cell factories for chemical production, in Current Developments in Biotechnology and Bioengineering. 2019, Elsevier. p. 115-141.
- Liu, H. , et al., Synthetic gene circuits enable Escherichia coli to use endogenous H2S as a signaling molecule for quorum sensing. ACS Synthetic Biology 2019, 8, 2113–2120. [Google Scholar] [CrossRef]
- Moore, J.C., I. Ramos, and S. Van Dien, Practical genetic control strategies for industrial bioprocesses. Journal of Industrial Microbiology and Biotechnology 2022, 49, kuab088.
- Simmons, T.R. , et al., Rewiring native post-transcriptional carbon regulators to build multi-layered genetic circuits and optimize engineered microbes for bioproduction. bioRxiv, 2023: p. 2023.10. 04.560922.
- Tang, C. , et al., On-demand biomanufacturing through synthetic biology approach. Materials Today Bio 2023, 18, 100518. [Google Scholar] [CrossRef]
- Bramhachari, P.V. and G. Mohana Sheela, Vibrio fischeri symbiotically synchronizes bioluminescence in marine animals via quorum sensing mechanism. Implication of Quorum Sensing System in Biofilm Formation and Virulence, 2018: p. 207-219.
- Miyashiro, T. and E.G. Ruby, Shedding light on bioluminescence regulation in Vibrio fischeri. Molecular microbiology 2012, 84, 795–806. [CrossRef]
- Venturi, V. and B.M. Ahmer, LuxR solos are becoming major players in cell–cell communication in bacteria. 2015, Frontiers Media SA. p. 89.
- Cao, Z., Z. Liu, and X. Mao, Application of quorum sensing in metabolic engineering. Journal of Agricultural and Food Chemistry 2023, 71, 5062–5074. [CrossRef]
- Min, B.E., et al., Optimization of industrial microorganisms: recent advances in synthetic dynamic regulators. Journal of Industrial Microbiology and Biotechnology 2017, 44, 89–98. [CrossRef]
- Song, J. , et al., Self-regulated efficient production of L-threonine via an artificial quorum sensing system in engineered Escherichia coli. Microbiological Research 2024, 284, 127720. [Google Scholar] [CrossRef]
- Wu, J. , et al., Developing a pathway-independent and full-autonomous global resource allocation strategy to dynamically switching phenotypic states. Nature Communications 2020, 11, 5521. [Google Scholar] [CrossRef] [PubMed]
- Bäcker, L.E. , et al., Tuning and functionalization of logic gates for time resolved population programming. 2024.
- Li, X., Q. Qi, and Q. Liang, Construction of cascade circuits for dynamic temporal regulation and its application to PHB production. Biotechnology for Biofuels and Bioproducts 2023, 16, 158. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z. , et al., Programming dissipation systems by DNA timer for temporally regulating enzyme catalysis and nanostructure assembly. ACS nano 2022, 16, 14274–14283. [Google Scholar] [CrossRef] [PubMed]
- Siebert, D., J. Altenbuchner, and B. Blombach, A timed off-switch for dynamic control of gene expression in Corynebacterium glutamicum. Frontiers in Bioengineering and Biotechnology 2021, 9, 704681.
- Yu, W. , et al., A pathway independent multi-modular ordered control system based on thermosensors and CRISPRi improves bioproduction in Bacillus subtilis. Nucleic Acids Research 2022, 50, 6587–6600. [Google Scholar] [CrossRef]
- Cui, S. , et al., Multilayer genetic circuits for dynamic regulation of metabolic pathways. ACS Synthetic Biology 2021, 10, 1587–1597. [Google Scholar] [CrossRef]
- Dinh, C.V. and K.L. Prather, Development of an autonomous and bifunctional quorum-sensing circuit for metabolic flux control in engineered Escherichia coli. Proceedings of the National Academy of Sciences 2019, 116, 25562–25568. [Google Scholar] [CrossRef]
- Tian, J. , et al., Developing an endogenous quorum-sensing based CRISPRi circuit for autonomous and tunable dynamic regulation of multiple targets in Streptomyces. Nucleic Acids Research 2020, 48, 8188–8202. [Google Scholar] [CrossRef]
- Wu, Y. , et al., Design of a programmable biosensor-CRISPRi genetic circuits for dynamic and autonomous dual-control of metabolic flux in Bacillus subtilis. Nucleic acids research 2020, 48, 996–1009. [Google Scholar] [CrossRef]
- Lopez-Garcia, C.L. , Synthetic biology approaches for the construction of improved microbial cell factories. 2021, Iowa State University.
- Lv, X., et al., New synthetic biology tools for metabolic control. Current Opinion in Biotechnology 2022, 76, 102724.
- Sarath, R. , et al., Metabolic Engineering Approaches for Bioenergy Production, in Applied Biotechnology for Emerging Pollutants Remediation and Energy Conversion. 2023, Springer. p. 305-332.
- Zou, Y. , et al., A self-regulated network for dynamically balancing multiple precursors in complex biosynthetic pathways. Metabolic Engineering 2024, 82, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Leitão, A.L. and F.J. Enguita, Synthetic biology approaches for secondary metabolism engineering, in Microbial Cell Factories Engineering for Production of Biomolecules. 2021, Elsevier. p. 51-64.
- Czárán, T. , et al., Cue-driven microbial cooperation and communication: evolving quorum sensing with honest signaling. BMC biology 2024, 22, 73. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q. , et al., Structure and signal regulation mechanism of interspecies and interkingdom quorum sensing system receptors. Journal of agricultural and food chemistry 2022, 70, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y. , et al., Constructions of quorum sensing signaling network for activated sludge microbial community. ISME communications 2024, 4, ycae018. [Google Scholar] [CrossRef] [PubMed]
- Marken, J.P. and R.M. Murray, Addressable and adaptable intercellular communication via DNA messaging. Nature Communications 2023, 14, 2358. [CrossRef]
- Wu, S. , et al., Deciphering and Constructing the Quorum Sensing Language “Interpreter” Ecosystem for Microbial Community. 2024.
- Wu, S. , et al., Design and analysis of quorum sensing language “Interpreter” ecosystem for microbial community. Chemical Engineering Journal 2024, 496, 153148. [Google Scholar] [CrossRef]
- Wu, X. , et al., Communication mediated interaction between bacteria and microalgae advances photogranulation. Science of The Total Environment 2024, 914, 169975. [Google Scholar] [CrossRef]
- Gorochowski, T.E., et al., Toward engineering biosystems with emergent collective functions. Frontiers in bioengineering and biotechnology 2020, 8, 705.
- Karkaria, B.D., et al., From microbial communities to distributed computing systems. Frontiers in Bioengineering and Biotechnology 2020, 8, 834.
- Kylilis, N. , et al., Tools for engineering coordinated system behaviour in synthetic microbial consortia. Nature communications 2018, 9, 2677. [Google Scholar] [CrossRef]
- Li, S., et al., Synthetic microbial consortia with programmable ecological interactions. Methods in Ecology and Evolution 2022, 13, 1608–1621. [CrossRef]
- Rodríguez Amor, D. and M. Dal Bello, Bottom-up approaches to synthetic cooperation in microbial communities. Life 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J., et al., Engineering microbial consortia through synthetic biology approach. Sheng wu Gong Cheng xue bao= Chinese Journal of Biotechnology 2023, 39, 2517–2545.
- Bonidia, R.P. and A.C.P. de Leon Ferreira. BioAutoML: Democratizing Machine Learning in Life Sciences. in Simpósio Brasileiro de Computação Aplicada à Saúde (SBCAS). 2024. SBC.
- Ding, N. , et al., AI-Assisted Rational Design and Activity Prediction of Biological Elements for Optimizing Transcription-Factor-Based Biosensors. Molecules, 2024. 29(15).
- Goshisht, M.K. , Machine learning and deep learning in synthetic biology: Key architectures, applications, and challenges. ACS omega 2024, 9, 9921–9945. [Google Scholar] [CrossRef] [PubMed]
- Merzbacher, C. and D.A. Oyarzún, Applications of artificial intelligence and machine learning in dynamic pathway engineering. Biochemical Society Transactions 2023, 51, 1871–1879. [CrossRef]
- Murala, P. and K. Sivaraman. Data science Analysis Using Deep Learning Techniques in Mining Biological Data. in 2024 International Conference on Knowledge Engineering and Communication Systems (ICKECS). 2024. IEEE.
- Soni, M. , et al., A comprehensive analysis of the convergence between deep learning technologies and bioinformatics, catalyzing groundbreaking innovations in biological data interpretation, in Explainable Artificial Intelligence for Biomedical and Healthcare Applications. 2024, CRC Press. p. 211-229.
- Valeri, J.A. , et al., BioAutoMATED: An end-to-end automated machine learning tool for explanation and design of biological sequences. Cell Systems 2023, 14, 525–542. [Google Scholar] [CrossRef]
- Li, X. , et al., Synthetic neural-like computing in microbial consortia for pattern recognition. Nature communications 2021, 12, 3139. [Google Scholar] [CrossRef]
- Mahmud, M., et al., Deep learning in mining biological data. Cognitive computation 2021, 13, 1–33. [CrossRef]
- Min, S., B. Lee, and S. Yoon, Deep learning in bioinformatics. Briefings in bioinformatics 2017, 18, 851–869.
- Sameer, A.A.S.a.R. , Artificial Intelligence Techniques in Bioinformatics: Unravelling Complex Biological Systems. International Journal of Advanced Research in Science, Communication and Technology 2023. 3(1): p. 269-275.
- Tawseef Ahmed Teli, F.S.M. , Zubair Masoodi, Application of ML and DL on Biological Data, in Applications of Machine Learning and Deep Learning on Biological Data. 2023, Auerbach Publications eBooks. p. 159-180.
- Vakilipoor, F., et al. Hybrid deep learning-based feature-augmented detection for molecular communication systems. in Proceedings of the 9th ACM International Conference on Nanoscale Computing and Communication. 2022.
- Weiss, R. , et al., Applications of neural networks in biomedical data analysis. Biomedicines 2022, 10, 1469. [Google Scholar] [CrossRef]
- Bartoli, V., M. di Bernardo, and T.E. Gorochowski, Self-adaptive biosystems through tunable genetic parts and circuits. Current Opinion in Systems Biology 2020, 24, 78–85. [CrossRef]
- Bhattacharya, P., K. Raman, and A.K. Tangirala, A generic systems-theoretic approach to identify biological networks capable of adaptation. bioRxiv, 2021: p. 2021.05. 27.445914.
- Bhattacharya, P., K. Raman, and A.K. Tangirala, On biological networks capable of robust adaptation in the presence of uncertainties: A linear systems-theoretic approach. Mathematical Biosciences 2023, 358, 108984. [Google Scholar] [CrossRef] [PubMed]
- Dasauni, K., D. Singh, and T.K. Nailwal, Understanding the molecular and genetics determinants of microbial adaptation in changing environment, in Microbiome Under Changing Climate. 2022, Elsevier. p. 333-352.
- Prakash, S. , et al., Engineered sensor bacteria evolve master-level gameplay through accelerated adaptation. bioRxiv, 2022: p. 2022.04. 22.489191.
- Rattray, J.B. , et al., The dynamic response of quorum-sensing to density is robust to signal supplementation and signal synthase knockouts. bioRxiv, 2022: p. 2022.09. 12.507654.
- Preiser, R. , Complex adaptive systems, in Elgar Encyclopedia of Interdisciplinarity and Transdisciplinarity, F. Darbellay, Editor. 2024. p. 86–90.
- Alamnie, G. and B. Andualem, Biosafety Issues of Unintended Horizontal Transfer of Recombinant DNA, in Genetic Transformation in Crops. 2020, IntechOpen.
- Cimolato, C. , et al., Exploring alternative quorum sensing model structures and quorum quenching strategies. bioRxiv, 2023: p. 2023.07. 07.548074.
- Haudiquet, M. , et al., Selfish, promiscuous and sometimes useful: how mobile genetic elements drive horizontal gene transfer in microbial populations. Philosophical Transactions of the Royal Society B 2022, 377, 20210234. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y., A. Manna, and T.S. Moon, Advances in engineering genetic circuits for microbial biocontainment. Current Opinion in Systems Biology, 2023: p. 100483.
- Qiu, X. , et al., Regulation of quorum sensing for the manipulation of conjugative transfer of antibiotic resistance genes in wastewater treatment system. Water Research 2024, 253, 121222. [Google Scholar] [CrossRef] [PubMed]
- Rycroft, T. , et al., A quantitative risk assessment method for synthetic biology products in the environment. Science of the Total Environment 2019, 696, 133940. [Google Scholar] [CrossRef] [PubMed]
- Sarangi, R., et al., Horizontal gene transfer: Implications in human health and diseases. Journal of Integrative Medicine and Research 2023, 1, 13–18. [CrossRef]
- Vidiella, B. and R. Solé, Ecological firewalls for synthetic biology. Iscience, 2022. 25(7).
- Zhu, X. , et al., Innovative microbial disease biocontrol strategies mediated by quorum quenching and their multifaceted applications: A review. Frontiers in Plant Science 2023, 13, 1063393. [Google Scholar] [CrossRef]
- Ding, Q. and L. Liu, Reprogramming cellular metabolism to increase the efficiency of microbial cell factories. Critical Reviews in Biotechnology 2024, 44, 892–909. [Google Scholar] [CrossRef]
- Kugler, A. and K. Stensjö, Machine learning predicts system-wide metabolic flux control in cyanobacteria. Metabolic engineering 2024, 82, 171–182. [Google Scholar] [CrossRef]
- Lu, H. , et al., Cell factory design with advanced metabolic modelling empowered by artificial intelligence. Metabolic Engineering, 2024.
- Parker, B. , How can we design robust manufacturing metabolisms and reconsider the bioreactor? Research Directions: Biotechnology Design 2023, 1, e3. [Google Scholar] [CrossRef]
- Phaneuf, P. , et al., Data-Driven Strain Design Towards Mitigating Biomanufacturing Stresses. bioRxiv, 2023: p. 2023.09. 17.558093.
- Ryu, G., et al., Deep learning for metabolic pathway design. Metabolic Engineering 2023, 80, 130–141. [CrossRef] [PubMed]
- Srikanth, M. and M. Bhanurangarao, Deep learning approaches for predictive modeling and optimization of metabolic fluxes in engineered microorganism. International Journal of Research in Science &Amp, 2023: p. 1-11.
- Adugna, T., A. Ramu, and A. Haldorai, A review of pattern recognition and machine learning. J. Machine Comput 2024, 4, 210–220.
- de Ridder, D., J. De Ridder, and M.J. Reinders, Pattern recognition in bioinformatics. Briefings in bioinformatics 2013, 14, 633–647. [CrossRef] [PubMed]
- Hense, B., C. Kuttler, and J. Müller, Functionality of Autoinducer Systems in Complex Environments, in The Physical Basis of Bacterial Quorum Communication. 2014, Springer. p. 83-103.
- Li, X., et al. Application Methods of Artificial Neural Network in Pattern Recognition. in 2020 13th International Conference on Intelligent Computation Technology and Automation (ICICTA). 2020. IEEE.
- Singh, C. , Machine Learning in Pattern Recognition. European Journal of Engineering and Technology Research, 2023. 8(2).
- Nidhi Pateriya, N.T. , Gulafsha Anjum, A Review Paper on Machine Learning in Pattern Recognition. International Journal of Innovative Research in Computer and Communication Engineering 2024, 12, 86–90. [Google Scholar] [CrossRef]
- Wu, S. , et al., Quorum sensing for population-level control of bacteria and potential therapeutic applications. Cellular and Molecular Life Sciences 2020, 77, 1319–1343. [Google Scholar] [CrossRef]
- Xiong, F. , et al., Enhanced AHL-mediated quorum sensing accelerates the start-up of biofilm reactors by elevating the fitness of fast-growing bacteria in sludge and biofilm communities. Water Research 2024, 257, 121697. [Google Scholar] [CrossRef]
- Ford, G., et al. Unknown signal detection in interference and noise using hidden Markov models. in 2021 IEEE Statistical Signal Processing Workshop (SSP). 2021. IEEE.
- Silva, K.P.T. and J.Q. Boedicker, A neural network model predicts community-level signaling states in a diverse microbial community. PLoS Computational Biology 2019, 15, e1007166. [Google Scholar] [CrossRef]
- Wang, M. and Q. Tu, Effective data filtering is prerequisite for robust microbial association network construction. Frontiers in Microbiology 2022, 13, 1016947. [Google Scholar]
- Ankita, D. Jain, C.M.H., Noise reduction using machine learning, B. Corporation, Editor. 2019.
- Bravo-Frank, N. , et al., Realtime bacteria detection and analysis in sterile liquid products using deep learning holographic imaging. npj Biosensing 2024, 1, 8. [Google Scholar] [CrossRef]
- Butcher, R.J. and J.J. Tabor, Real-time detection of response regulator phosphorylation dynamics in live bacteria. Proceedings of the National Academy of Sciences 2022, 119, e2201204119. [CrossRef]
- Chen, N. , et al., Real-Time Monitoring of Dynamic Chemical Processes in Microbial Metabolism with Optical Sensors. Chinese Journal of Chemistry 2023, 41, 1836–1840. [Google Scholar] [CrossRef]
- Jiang, W. and J. Shan. Design of Real-Time Detection System of Bacteria Concentration Changes in Biological Fermentation. in Advanced Hybrid Information Processing: Third EAI International Conference, ADHIP 2019, Nanjing, China, September 21–22, 2019, Proceedings, Part II. 2019. Springer.
- Mukherjee, S. and B.L. Bassler, Bacterial quorum sensing in complex and dynamically changing environments. Nature Reviews Microbiology 2019, 17, 371–382. [CrossRef] [PubMed]
- Remya, S. and T. Anjali, An intelligent and optimal deep learning approach in sensor based networks for detecting microbes. IEEE Sensors Journal, 2023.
- Sharma, S. and L. Tharani, Optical sensing for real-time detection of food-borne pathogens in fresh produce using machine learning. Science Progress 2024, 107, 00368504231223029. [Google Scholar] [CrossRef] [PubMed]
- Sun Li, C.L. , Real-time signal sequence detection method based on deep learning. 2020.
- Abdul Subhahan Shaik, N.S., Dr. C. Krishna Priya, Predictive Modeling in Remote Sensing Using Machine Learning Algorithms. International Journal of Current Science Research and Review 2024, 7, 4116–4123.
- Baron, M. , et al., Use of a systems-biology informed machine learning model to predict drug response using clinically available NGS data. 2023, American Society of Clinical Oncology.
- Chango, D.A.C., A.D.P. Anchatipán, and F.R.R. Bedón, Análisis del Uso de Machine Learning para Sistema de control predictivo a nivel industrial. Polo del Conocimiento 2024, 9, 1023–1040. [CrossRef]
- Karanam, V.S.S.L. and B. Ramamurthy. Improving Transfer Time Prediction of ML Models via Auto-correcting Dynamical Systems Modeling. in 2024 IEEE 10th International Conference on Network Softwarization (NetSoft). 2024. IEEE.
- King, R. , A machine learning approach to predict gene expression levels based on stochastic simulation. 2024.
- Toma, M. and O.C. Wei, Predictive modeling in medicine. Encyclopedia 2023, 3, 590–601. [CrossRef]
- Zaidi, A.R. , et al., Developing MLP based prediction system for anticancer drug response using hybrid features of genomics and cheminformatics. 2024.
- Al Taweraqi, N. and R.D. King, Improved prediction of gene expression through integrating cell signalling models with machine learning. BMC bioinformatics 2022, 23, 323. [Google Scholar] [CrossRef]
- Huiqing Wang, C.L. , Chunlin Dong, Gene expression prediction method based on LSTM loop neural network. 2018.
- Kamal, I.M., N.A. Wahid, and H. Bae. Gene expression prediction using stacked temporal convolutional network. in 2020 IEEE International Conference on Big Data and Smart Computing (BigComp). 2020. IEEE.
- Kumar, N.P. , et al., Design of optimal Elman Recurrent Neural Network based prediction approach for biofuel production. Scientific Reports 2023, 13, 8565. [Google Scholar]
- Lugagne, J.-B., C.M. Blassick, and M.J. Dunlop, Deep model predictive control of gene expression in thousands of single cells. Nature Communications 2024, 15, 2148. [CrossRef]
- Mateescu, C., et al. Artificial intelligence approach in predicting biomass-to-biofuels conversion performances. in 2022 23rd International Carpathian Control Conference (ICCC). 2022. IEEE.
- Wang, H., et al., A new LSTM-based gene expression prediction model: L-GEPM. Journal of Bioinformatics and Computational Biology 2019, 17, 1950022.
- Agarwal, N. , et al., Artificial Intelligence and Machine Learning for Analysis of Multi-omics, in Multi-Omics Analysis of the Human Microbiome: From Technology to Clinical Applications. 2024, Springer. p. 339-354.
- Firdous, A. , et al., Machine learning approaches for multiomics data analysis, in Biological Insights of Multi-Omics Technologies in Human Diseases. 2024, Elsevier. p. 311-338.
- McElhinney, J.M. , et al., Interfacing machine learning and microbial omics: a promising means to address environmental challenges. Frontiers in Microbiology 2022, 13, 851450. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.S., et al. A Novel Machine Learning Approach of Multi-omics Data Prediction. in 2022 International Conference on Innovative Computing, Intelligent Communication and Smart Electrical Systems (ICSES). 2022. IEEE.
- Shi, Z. , et al., Data-driven synthetic cell factories development for industrial biomanufacturing. BioDesign Research, 2022. 2022.
- Wu, L. , et al., Data-driven prediction of colonization outcomes for complex microbial communities. Nature Communications 2024, 15, 2406. [Google Scholar] [CrossRef] [PubMed]
- Zitnik, M. , et al., Machine learning for integrating data in biology and medicine: Principles, practice, and opportunities. Information Fusion 2019, 50, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Aboagyea, E.A. , et al., Machine Learning Methods for the Forecasting of Environmental Impacts in Early-stage Process Design. 2024.
- Amato, F. , et al., A novel framework for spatio-temporal prediction of environmental data using deep learning. Scientific reports 2020, 10, 22243. [Google Scholar] [CrossRef]
- Deb, S. , et al., Machine learning methods trained on simple models can predict critical transitions in complex natural systems. Royal Society Open Science 2022, 9, 211475. [Google Scholar] [CrossRef]
- Kolesnikova, K., et al. Use the neural networks in prediction of environmental processes. in 2024 IEEE 4th International Conference on Smart Information Systems and Technologies (SIST). 2024. IEEE.
- Mitchell, A. , et al., Adaptive prediction of environmental changes by microorganisms. Nature 2009, 460, 220–224. [Google Scholar] [CrossRef]
- Volkova, E., S. Muratchaev, and A. Volkov. Development of a Automated Environmental Monitoring System with Forecasting. in Futuristic Trends in Network and Communication Technologies: Third International Conference, FTNCT 2020, Taganrog, Russia, October 14–16, 2020, Revised Selected Papers, Part I 3. 2021. Springer.
- Yang, J., et al. Prediction of temperature change with multi-dimensional environmental characteristic based on CNN-LSTM-ATTENTION model. in 2022 IEEE 10th Joint International Information Technology and Artificial Intelligence Conference (ITAIC). 2022. IEEE.
- Caldas, R.D., et al. A hybrid approach combining control theory and AI for engineering self-adaptive systems. in Proceedings of the IEEE/ACM 15th International Symposium on Software Engineering for Adaptive and Self-Managing Systems. 2020.
- Harris, A.W., et al., Designing genetic feedback controllers. IEEE Transactions on Biomedical Circuits and Systems 2015, 9, 475–484. [CrossRef]
- Zile, M. , Intelligent and Adaptive Control, in Microgrid Architectures, Control and Protection Methods. 2020, Springer, Cham. p. 423-446.
- Zhou, Z. , Design and analysis of adaptive control systems: Adaptive control algorithms using machine learning. Applied and Computational Engineering 2024, 39, 51–56. [Google Scholar] [CrossRef]
- de Avila Ferreira, T., et al. Real-time optimization of uncertain process systems via modifier adaptation and gaussian processes. in 2018 European Control Conference (ECC). 2018. IEEE.
- Morais, M.S. and A. Araujo, Real-time optimization strategy of the alcoholic fermentation process using metamodeling. 2023.
- Williams, T. , et al., Machine learning and metabolic modelling assisted implementation of a novel process analytical technology in cell and gene therapy manufacturing. Scientific Reports 2023, 13, 834. [Google Scholar] [CrossRef]
- Bauer, K. , et al., Feedback loops in machine learning: A study on the interplay of continuous updating and human discrimination. Journal of the Association for Information Systems 2024, 25, 804–866. [Google Scholar] [CrossRef]
- Dietrich, J. , A Methodology for Vertical Translation Between Molecular and Organismal Level in Biological Feedback Loops. bioRxiv, 2021: p. 2021.09. 20.461028.
- Gesmundo, A., A continual development methodology for large-scale multitask dynamic ML systems. arXiv preprint arXiv:2209.07326, 2022.
- Pithan, L. , et al., Closing the loop: autonomous experiments enabled by machine-learning-based online data analysis in synchrotron beamline environments. Journal of synchrotron radiation, 2023. 30(6).
- Wang, T. , et al., ML-NPI: predicting interactions between noncoding RNA and protein based on meta-learning in a large-scale dynamic graph. Journal of Chemical Information and Modeling 2023, 64, 2912–2920. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J., R. Ramanathan, and W.-F. Wong, Synthesis of the Dynamical Properties of Feedback Loops in Bio-Pathways. IEEE/ACM Transactions on Computational Biology and Bioinformatics 2019, 18, 1217–1226.
- Borri, A., M. d'Angelo, and P. Palumbo, Self-regulation in a stochastic model of chemical self-replication. International Journal of Robust and Nonlinear Control 2023, 33, 4908–4922. [CrossRef]
- Espinel-Ríos, S., et al., Machine learning-supported cybergenetic modeling, optimization and control for synthetic microbial communities, in Computer Aided Chemical Engineering. 2023, Elsevier. p. 2601-2606.
- Ji, Y., T. Chakraborty, and S.V. Wegner, Self-regulated and bidirectional communication in synthetic cell communities. ACS nano 2023, 17, 8992–9002. [CrossRef]
- Archisman Bhunia, K.N. , Abhilasha Singh, Asmeeta Sircar, Nivedita Chatterjee, Quorum Sensing, in Omics for Environmental Engineering and Microbiology Systems. 2022, CRC Press.
- Escobar-Muciño, E., M.M. Arenas-Hernández, and M.L. Luna-Guevara, Mechanisms of Inhibition of Quorum Sensing as an Alternative for the Control of E. coli and Salmonella. Microorganisms 2022, 10, 884. [CrossRef]
- Huang, S. , et al., Insights into adaptive mechanisms of extreme acidophiles based on quorum sensing/quenching-related proteins. Msystems 2022, 7, e01491–21. [Google Scholar] [CrossRef]
- Moreno-Gámez, S., M.E. Hochberg, and G. Van Doorn, Quorum sensing as a mechanism to harness the wisdom of the crowds. Nature communications 2023, 14, 3415. [CrossRef]
- Shen, Y. , et al., New antibacterial targets: Regulation of quorum sensing and secretory systems in zoonotic bacteria. Microbiological Research 2023, 274, 127436. [Google Scholar] [CrossRef]
- Ampomah-Wireko, M. , et al., Chemical probe of AHL modulators on quorum sensing in Gram-Negative Bacteria and as antiproliferative agents: A review. European Journal of Medicinal Chemistry 2021, 226, 113864. [Google Scholar] [CrossRef]
- Bazhenov, S. , et al., Influence of the luxR regulatory gene dosage and expression level on the sensitivity of the whole-cell biosensor to acyl-homoserine lactone. Biosensors 2021, 11, 166. [Google Scholar] [CrossRef]
- Dominelli, N. , et al., Interkingdom Signaling of the Insect Pathogen Photorhabdus luminescens with Plants Via the LuxR solo SdiA. Microorganisms 2023, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M. , et al., AHL-Based Quorum Sensing Regulates the Biosynthesis of a Variety of Bioactive Molecules in Bacteria. Journal of Natural Products 2024, 87, 1268–1284. [Google Scholar] [CrossRef] [PubMed]
- Li, S. , et al., Characterization of differentiated autoregulation of LuxI/LuxR-type quorum sensing system in Pseudoalteromonas. Biochemical and Biophysical Research Communications 2022, 590, 177–183. [Google Scholar] [CrossRef]
- Liu, L. , et al., AHL-mediated quorum sensing to regulate bacterial substance and energy metabolism: A review. Microbiological Research 2022, 262, 127102. [Google Scholar] [CrossRef]
- Zeng, M. , et al., Synthetic Homoserine Lactone Sensors for Gram-Positive Bacillus subtilis Using LuxR-Type Regulators. ACS Synthetic Biology 2023, 13, 282–299. [Google Scholar] [CrossRef] [PubMed]
- Ábrahám, Á. , et al., Single-cell level LasR-mediated quorum sensing response of Pseudomonas aeruginosa to pulses of signal molecules. Scientific Reports 2024, 14, 16181. [Google Scholar] [CrossRef] [PubMed]
- Ballante, F. , et al., Modified N-acyl-L-homoserine lactone compounds abrogate Las-dependent quorum-sensing response in human pathogen Pseudomonas aeruginosa. Frontiers in Molecular Biosciences 2023, 10, 1264773. [Google Scholar] [CrossRef]
- de Oliveira Pereira, T., M. -C. Groleau, and E. Déziel, Surface growth of Pseudomonas aeruginosa reveals a regulatory effect of 3-oxo-C12-homoserine lactone in the absence of its cognate receptor, LasR. Mbio 2023, 14, e00922–23. [Google Scholar] [CrossRef]
- Manson, D.E. , et al., Abiotic small molecule inhibitors and activators of the LasR quorum sensing receptor in Pseudomonas aeruginosa with potencies comparable or surpassing N-acyl homoserine lactones. ACS infectious diseases 2024, 10, 1212–1221. [Google Scholar] [CrossRef]
- Mohan, M.S. , et al., Attenuation of Las/Rhl quorum sensing regulated virulence and biofilm formation in Pseudomonas aeruginosa PAO1 by Artocarpesin. Microbial Pathogenesis 2024, 189, 106609. [Google Scholar] [CrossRef]
- Rex, D.A.B. , et al., Pleotropic potential of quorum sensing mediated N-acyl homoserine lactones (AHLs) at the LasR and RhlR receptors of Pseudomonas aeruginosa. Structural Chemistry 2023, 34, 1327–1339. [Google Scholar] [CrossRef]
- Singothu, S. and V. Bhandari, Computational assessment of marine natural products as LasR inhibitors for attenuating quorum sensing in Pseudomonas aeruginosa. Journal of Biomolecular Structure and Dynamics, 2024: p. 1-15.
- Eisenbraun, E.L. , et al., Synthetic Peptides Capable of Potent Multigroup Staphylococcal Quorum Sensing Activation and Inhibition in Both Cultures and Biofilm Communities. Journal of the American Chemical Society, 2024.
- Fang, L., C. Cosgriff, and F. Alonzo III, Determinants of maturation of the Staphylococcus aureus autoinducing peptide. Journal of Bacteriology 2024, 206, e00195–24.
- Inagaki, R. , et al., Eliminating extracellular autoinducing peptide signals inhibits the Staphylococcus aureus quorum sensing agr system. Biochemical and Biophysical Research Communications 2024, 711, 149912. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. , et al., AgrA directly binds to the promoter of vraSR and downregulates its expression in Staphylococcus aureus. Antimicrobial Agents and Chemotherapy 2024, 68, e00893–23. [Google Scholar] [CrossRef] [PubMed]
- Milly, T.A. and Y. Tal-Gan, Targeting peptide-based quorum sensing systems for the treatment of gram-positive bacterial infections. Peptide Science 2023, 115, e24298. [Google Scholar] [CrossRef]
- Stock, M.R. , et al., Characterization of MroQ-dependent maturation and export of the Staphylococcus aureus accessory gene regulatory system autoinducing peptide. Infection and Immunity 2022, 90, e00263–22. [Google Scholar] [CrossRef]
- Williams, P. , et al., Quorum-sensing, intra-and inter-species competition in the staphylococci. Microbiology 2023, 169, 001381. [Google Scholar] [CrossRef]
- Wittekind, M.A. , et al., The Small Protein ScrA Influences Staphylococcus aureus Virulence-Related Processes via the SaeRS System. Microbiology Spectrum 2023, 11, e05255–22. [Google Scholar] [CrossRef]
- Zhou, J. , et al., The Functional Study of Response Regulator ArlR Mutants in Staphylococcus Aureus. Applied Biochemistry and Biotechnology, 2024: p. 1-16.
- Anju, S. , et al., Novel insights on the bacillus quorum sensing mechanism: Its role in competence, virulence, sporulation and biofilm formation. Implication of quorum sensing system in biofilm formation and virulence, 2018: p. 313-327.
- Auchtung, J.M. and A.D. Grossman, Extracellular Peptide Signaling and Quorum Responses in Development, Self-Recognition, and Horizontal Gene Transfer in Bacillus subtilis. Chemical communication among bacteria, 2008: p. 13-30.
- Dandach, S.H. and M. Khammash. Stochastic strategies for survival: bacterial competence in Bacillus Subtilis. in Proceedings of the 2011 American Control Conference. 2011. IEEE.
- Gupta, R. , et al., Competence and Sporulation in Bacillus subtilis. Fundamentals of Bacterial Physiology and Metabolism, 2021: p. 653-670.
- Kumar, A. and T.R. Singh, A quantitative study of gene regulatory pathways in Bacillus subtilis for virulence and competence phenotype by quorum sensing. Systems and synthetic biology 2013, 7, 33–39. [Google Scholar] [CrossRef]
- Muers, M. , Racing to decide. Nature Reviews Microbiology 2012, 10, 84–84. [Google Scholar] [CrossRef]
- Rai, N., R. Rai, and K. Venkatesh, Quorum sensing in competence and sporulation, in Quorum sensing vs quorum quenching: a battle with no end in sight. 2014, Springer. p. 61-64.
- Schultz, D., et al., Deciding fate in adverse times: sporulation and competence in Bacillus subtilis. Proceedings of the National Academy of Sciences 2009, 106, 21027–21034. [CrossRef] [PubMed]
- Xi, H., L. Duan, and M. Turcotte, Point-cycle bistability and stochasticity in a regulatory circuit for Bacillus subtilis competence. Mathematical Biosciences 2013, 244, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Combarnous, Y. and T.M.D. Nguyen, Cell communications among microorganisms, plants, and animals: origin, evolution, and interplays. International Journal of Molecular Sciences 2020, 21, 8052. [CrossRef] [PubMed]
- Gamby, S. , et al., Altering the communication networks of multispecies microbial systems using a diverse toolbox of AI-2 analogues. ACS chemical biology 2012, 7, 1023–1030. [Google Scholar] [CrossRef]
- Guo, L., X. He, and W. Shi, Intercellular communications in multispecies oral microbial communities. Frontiers in microbiology 2014, 5, 328.
- Mohana Sheela, G. , et al., Intra and inter-species communication in microbes: living with complex and sociable neighbors. Implication of quorum sensing system in biofilm formation and virulence, 2018: p. 7-16.
- Pereira, C.S., J.A. Thompson, and K.B. Xavier, AI-2-mediated signalling in bacteria. FEMS microbiology reviews 2013, 37, 156–181. [CrossRef]
- Pierson III, L.S., R. M. Maier, and I.L. Pepper, Microbial communication: bacteria/bacteria and bacteria/host, in Environmental Microbiology. 2015, Elsevier. p. 461-481.
- Rodrigues, M.V. , et al., Synthesis and Potential of Autoinducer-2 and Analogs to Manipulate Inter-Species Quorum Sensing. Israel Journal of Chemistry, 2023. 63(5-6): p. e202200091.
- Scott, S.R. and J. Hasty, Quorum sensing communication modules for microbial consortia. ACS synthetic biology 2016, 5, 969–977. [CrossRef]
- Sedlmayer, F. , et al., Designer cells programming quorum-sensing interference with microbes. Nature communications 2018, 9, 1822. [Google Scholar] [CrossRef]
- Li, X. , et al., Quantifying the optimal strategy of population control of quorum sensing network in Escherichia coli. Npj Systems Biology and Applications 2021, 7, 35. [Google Scholar] [CrossRef]
- Walton, S.J., S. E. Clamons, and R.M. Murray, Analysis of Circuits for Dosage Control in Microbial Populations. bioRxiv, 2020: p. 2020.12. 18.423556.
- XU, M. XU, M., et al., 合成生物学应用于微生物群体感应的研究进展. SCIENTIA SINICA Vitae 2023, 53, 64–81. [Google Scholar] [CrossRef]
- Duncker, K.E., Z.A. Holmes, and L. You, Engineered microbial consortia: strategies and applications. Microbial Cell Factories 2021, 20, 1–13.
- Liu, Y. , et al., Rational construction of synthetic consortia: Key considerations and model-based methods for guiding the development of a novel biosynthesis platform. Biotechnology Advances, 2024: p. 108348.
- Matuszyńska, A. , et al., A new era of Synthetic Biology-microbial community design. Synthetic Biology, 2024: p. ysae011.
- Mittermeier, F., et al., Artificial microbial consortia for bioproduction processes. Engineering in life sciences 2023, 23, e2100152.
- Stephens, K. , et al., Bacterial co-culture with cell signaling translator and growth controller modules for autonomously regulated culture composition. Nature communications 2019, 10, 4129. [Google Scholar] [CrossRef] [PubMed]
- Wang, L., et al., Engineering consortia by polymeric microbial swarmbots. Nature Communications 2022, 13, 3879. [CrossRef] [PubMed]
- Blöbaum, L. , et al., Quantifying microbial robustness in dynamic environments using microfluidic single-cell cultivation. Microbial Cell Factories 2024, 23, 44. [Google Scholar] [CrossRef]
- Kashyap, A., W. Wang, and B.A. Camley, Trade-offs in concentration sensing in dynamic environments. Biophysical Journal 2024, 123, 1184–1194. [CrossRef]
- Lynch, M.R. , Environmental Sensing, in Evolutionary Cell Biology: The Origins of Cellular Architecture. 2024, Oxford University Press. p. 570–600.
- Mahdavi, S. , et al., Flexibility and sensitivity in gene regulation out of equilibrium. bioRxiv, 2023: p. 2023.04. 11.536490.
- Cavraro, G. , Advocating Feedback Control for Human-Earth System Applications. IEEE Control Systems Letters, 2024.
- Hu, G. and F. You, AI-enabled cyber-physical-biological systems for smart energy management and sustainable food production in a plant factory. Applied Energy 2024, 356, 122334. [Google Scholar] [CrossRef]
- M. Zand, A. M. Zand, A., et al., Multi-Layer Autocatalytic Feedback Enables Integral Control Amidst Resource Competition and Across Scales. bioRxiv, 2024: p. 2024.08. 22.609155.
- Rino, T., F. Giannino, and D. Fiore. Feedback control of plant-soil autotoxicity via pulse-width modulation. in 2024 European Control Conference (ECC). 2024. IEEE.
- Wang, H. , et al., A feedback control method for plant factory environment based on photosynthetic rate prediction model. Computers and Electronics in Agriculture 2023, 211, 108007. [Google Scholar] [CrossRef]
- Wang, W., et al., Online Feedback Optimization over Networks: A Distributed Model-free Approach. arXiv preprint arXiv:2403.19834, 2024.
- Babaniyi, G.G., U. J. Akor, and B.R. Babaniyi, Anti-quorum Sensing Therapies: Issues and Limitations. 2023.
- Banerjee, A. , et al., Quorum Sensing and Environmental Sustainability. Microbial Biotechnology: Role in Ecological Sustainability and Research, 2022: p. 75-88.
- Dubern, J.-F. , et al., Growth rate and nutrient limitation as key drivers of extracellular quorum sensing signal molecule accumulation in Pseudomonas aeruginosa. Microbiology 2023, 169, 001316. [Google Scholar] [CrossRef]
- Tripathi, S. , et al., Quorum sensing-a promising tool for degradation of industrial waste containing persistent organic pollutants. Environmental Pollution 2022, 292, 118342. [Google Scholar] [CrossRef]
- Ceroni, F., et al., Burden-driven feedback control of gene expression. Nature methods 2018, 15, 387–393. [CrossRef] [PubMed]
- Nikita, S., et al., Advances in bioreactor control for production of biotherapeutic products. Biotechnology and Bioengineering 2023, 120, 1189–1214. [CrossRef] [PubMed]
- Pang, W.L. , Quantitative analysis of genetic expression responses to dynamic microenvironmental perturbation. 2007, UC San Diego.
- Boo, A. , et al., Host-aware RNA-based control of synthetic microbial consortia. bioRxiv, 2023: p. 2023.05. 15.540816.
- Daniel, R., V. Kravchik, and N. Geva-Zatorsky, Utilizing Incoherent Feedforward Loops for Enhanced Response Stability and Coordination of Microbial Consortia-Based Biosensors. 2023.
- Kang, C.W. , et al., Circuit-guided population acclimation of a synthetic microbial consortium for improved biochemical production. Nature communications 2022, 13, 6506. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.A. , et al., Cybernetic control of a natural microbial co-culture. bioRxiv, 2024: p. 2024.07. 04.602068.
- Martinelli, V., et al., Multicellular pd control in microbial consortia. IEEE Control Systems Letters 2023, 7, 2641–2646. [CrossRef]
- VanArsdale, E. , et al., Electrogenetic signaling and information propagation for controlling microbial consortia via programmed lysis. Biotechnology and Bioengineering 2023, 120, 1366–1381. [Google Scholar] [CrossRef]
- Hoekstra, N.D. , Characterization of a novel quorum quenching enzyme and determination of autoinducer signal receptor specificity. 2021, University of Minnesota.
- Müller, I.E., et al., Gene networks that compensate for crosstalk with crosstalk. Nature Communications 2019, 10, 4028. [CrossRef]
- Munir, S. , et al., Quorum sensing interfering strategies and their implications in the management of biofilm-associated bacterial infections. Brazilian Archives of Biology and Technology 2020, 63, e20190555. [Google Scholar] [CrossRef]
- Sanders, J. , et al., Jump-start and push-start: Mutual activation through crosstalk in coupled quorum sensing pathways. bioRxiv, 2023: p. 2023.05. 08.539885.
- Sanders, J.G. , et al., Crosstalk enables mutual activation of coupled quorum sensing pathways through “jump-start” and “push-start” mechanisms. Scientific Reports 2023, 13, 19230. [Google Scholar] [CrossRef]
- Wang, S., G.F. Payne, and W.E. Bentley, Quorum sensing communication: molecularly connecting cells, their neighbors, and even devices. Annual review of chemical and biomolecular engineering 2020, 11, 447–468. [CrossRef]
- Wellington, S. and E.P. Greenberg, Quorum sensing signal selectivity and the potential for interspecies cross talk. MBio 2019, 10, 10–1128.
- Liu, B. , et al., A portable regulatory RNA array design enables tunable and complex regulation across diverse bacteria. Nature Communications 2023, 14, 5268. [Google Scholar] [CrossRef] [PubMed]
- Borchert, A.J., et al., Machine learning analysis of RB-TnSeq fitness data predicts functional gene modules in Pseudomonas putida KT2440. Msystems 2024, 9, e00942–23.
- Merzbacher, C., O. Mac Aodha, and D.A. Oyarzun, Modelling dynamic host-pathway interactions at the genome scale. bioRxiv, 2024: p. 2024.04. 09.588720.
- R. Moimenta, A. R. Moimenta, A., et al., A continuous dynamic genome-scale model explains batch fermentations led by species of the Saccharomyces genus. bioRxiv, 2024: p. 2024.05. 03.592398.
- Santos, A.P.A. , Exploration of non-coding RNA in complex microbial communities with machine learning. 2024, Universidade de São Paulo.
- Wu, S. , et al., Potential of orthogonal and cross-talk quorum sensing for dynamic regulation in cocultivation. Chemical Engineering Journal 2022, 445, 136720. [Google Scholar] [CrossRef]
- Alsiyabi, A., S. A. Shahid, and A. Al-Harrasi, An Automated Machine Learning Framework for Antimicrobial Resistance Prediction Through Transcriptomics. bioRxiv, 2024: p. 2024.06. 22.600223.
- Koblitz, J. , et al., Predicting bacterial phenotypic traits through improved machine learning using high-quality, curated datasets. bioRxiv, 2024: p. 2024.08. 12.607695.
- Naga, N.G. , et al., It is the time for quorum sensing inhibition as alternative strategy of antimicrobial therapy. Cell Communication and Signaling 2023, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Panjla, A. , et al., Applying Machine Learning for Antibiotic Development and Prediction of Microbial Resistance. Chemistry–An Asian Journal, 2024: p. e202400102.
- Thompson, J.C., V. M. Zavala, and O.S. Venturelli, Integrating a tailored recurrent neural network with Bayesian experimental design to optimize microbial community functions. PLOS Computational Biology 2023, 19, e1011436. [Google Scholar] [CrossRef]
- Zhang, H. , et al., Machine learning and genetic algorithm-guided directed evolution for the development of antimicrobial peptides. Journal of Advanced Research, 2024.
- Fang, Y., et al., Molecular Communication for Quorum Sensing Inspired Cooperative Drug Delivery. IEEE Transactions on Molecular, Biological and Multi-Scale Communications 2023, 9, 100–105. [CrossRef]
- Ilyas, H. , et al., Commands of Synthetic Biology to Modernize and Re-design the Biological Systems. Advancements in Life Sciences 2024, 11, 549–557. [Google Scholar] [CrossRef]
- Wu, S. , et al., Machine learning aided construction of the quorum sensing communication network for human gut microbiota. Nature Communications 2022, 13, 3079. [Google Scholar] [CrossRef]
- Kohyama, S. , et al., Machine learning-aided design and screening of an emergent protein function in synthetic cells. Nature Communications 2024, 15, 2010. [Google Scholar] [CrossRef]
- Mafat, I.H. , et al., Exploring Machine Learning Applications in Chemical Production through Valorization of Biomass, Plastics, and Petroleum Resources: A Comprehensive Review. Journal of Analytical and Applied Pyrolysis, 2024: p. 106512.
- Vaishali Jain, S.K.T. , Overview: machine learning, in Machine Learning: An Art of Computer Thinking. 2024: IIP Series. p. 130-144.
- Munde, A. , The Machine Learning Pipeline, in Deep Learning Concepts in Operations Research. 2024, Auerbach Publications. p. 18.
- Anders Hansson, M.A. , Supervised Learning, in Optimization for Learning and Control. 2023.
- Emilian, R. Vankov, K.G., Supervised Learning, in Statistical Methods in Epilepsy. 2024, Chapman and Hall/CRC. p. 30.
- Li, H. , Summary of Supervised Learning Methods, in Machine Learning Methods. 2024, Springer Nature Singapore: Singapore. p. 273-280.
- Nagaraj, A. , Supervised Learning Algorithms, in COVID 19 – Monitoring with IoT Devices. 2023. p. 23-75.
- Najjar, E. and A.M. Breesam, Supervised machine learning a brief survey of approaches. Al-Iraqia Journal for Scientific Engineering Research 2023, 2, 71–82.
- Narula, P. , Analysis of Common Supervised Learning Algorithms Through Application. Advanced Computational Intelligence: An International Journa, 2023. 10(1/2/3): p. 29-48.
- Takahashi, K. and L. Takahashi, Supervised Machine Learning, in Materials Informatics and Catalysts Informatics: An Introduction. 2024, Springer Nature Singapore: Singapore. p. 191-226.
- Amin, S. , An Introduction to Reinforcement Learning and Its Application in Various Domains, in Deep Learning, Reinforcement Learning, and the Rise of Intelligent Systems. 2024, IGI Global. p. 1-25.
- Han, I., et al., Monte Carlo and Temporal Difference Methods in Reinforcement Learning [AI-eXplained]. IEEE Computational Intelligence Magazine 2023, 18, 64–65. [CrossRef]
- ISMAIL, A.S., et al., REINFORCEMENT LEARNING MODEL FOR FINDING OPTIMAL PATH. Journal of Duhok University 2023, 26, 489–501. [CrossRef]
- Krichen, M. Deep Reinforcement Learning. in 2023 14th International Conference on Computing Communication and Networking Technologies (ICCCNT). 2023.
- Moussaoui, H.E.a., Nabil; Benslimane, Mohamed, Reinforcement Learning: A review. University of Bahrain Scientific Journals, 2023. 13(1).
- Shaik, N. , et al., 5 Reinforcement learning, in Toward Artificial General Intelligence, C.d.A. Victor Hugo, R. Pethuru, and Y. Satya Prakash, Editors. 2024, De Gruyter: Berlin, Boston. p. 109-124.
- Sumedha Sharma, T.J., Sanidhya Chaturvedi, Swapnil Saraswat, and R. Sharma, Advancements in Reinforcement Learning - An Overview. Journal of Analysis and Computation (JAC) 2023, 17, 263–274.
- Sonal, A.R.G., Dr. Ashima Mehta, Advancements in Reinforcement Learning. International Journal of Advanced Research in Science, Communication and Technology (IJARSCT) 2024, 4, 127–132. [Google Scholar] [CrossRef]
- AL-Gburi, A.F.J. , et al., Multi-Objective Unsupervised Feature Selection and Cluster Based on Symbiotic Organism Search. Algorithms 2024, 17, 355. [Google Scholar] [CrossRef]
- Anders Hansson, M.A. , Unsupervised Learning, in Optimization for Learning and Control. 2023: John Wiley & Sons, Inc.
- Dowrick, J.M. , et al., Unsupervised machine learning highlights the challenges of subtyping disorders of gut-brain interaction. Neurogastroenterology & Motility, 2024: p. e14898.
- Li, H. , Introduction to Unsupervised Learning, in Machine Learning Methods. 2024, Springer Nature Singapore: Singapore. p. 281-292.
- Takahashi, K. and L. Takahashi, Unsupervised Machine Learning and Beyond Machine Learning, in Materials Informatics and Catalysts Informatics: An Introduction. 2023, Springer. p. 227-244.
- Vinci, G. , Unsupervised Learning, in Statistical Methods in Epilepsy. 2024: Chapman and Hall/CRC. p. 21.
- Watson, D.S. , On the philosophy of unsupervised learning. Philosophy & Technology 2023, 36, 28. [Google Scholar]
- Khurana, D.C.a.R. , Unsupervised Learning, in Introduction to Machine Learning with Python. 2023. p. 103-112.
- Hsieh, W.W. , 10 - Unsupervised Learning, in Introduction to Environmental Data Science. 2023. p. 330 - 371.
- Das, P. , et al., Deep Learning Techniques Revolutionizing Biomedical Applications: Arrhythmia Detection, Cardiac Sensed Signals, and Cell-Free Synthetic Biology, in Applications of Synthetic Biology in Health, Energy, and Environment. 2023, IGI Global. p. 68-91.
- Kumar, S., et al., Deep learning in computational biology: Advancements, challenges, and future outlook. arXiv preprint arXiv:2310.03086, 2023.
- Mohanta, S.K. , et al., Deep Learning is a State-of-the-Art Approach to Artificial Intelligence, in Deep Learning Concepts in Operations Research. 2024, Auerbach Publications. p. 27-43.
- Ravanmehr, R. and R. Mohamadrezaei, Deep Learning Overview, in Session-Based Recommender Systems Using Deep Learning. 2024, Springer Nature Switzerland: Cham. p. 27-72.
- Shafik, W. , Deep Learning Impacts in the Field of Artificial Intelligence, in Deep Learning Concepts in Operations Research. 2024, Auerbach Publications. p. 9-26.
- Choe, D. , et al., Advancing the scale of synthetic biology via cross-species transfer of cellular functions enabled by iModulon engraftment. Nature Communications 2024, 15, 2356. [Google Scholar] [CrossRef]
- Latyshev, P. , et al., Unsupervised domain adaptation methods for cross-species transfer of regulatory code signals. Frontiers in Big Data 2023, 6, 1140663. [Google Scholar] [CrossRef]
- Shamreen Ahamed, B. , Dharani, V., Poonkodi, M., Sangeetha, G., & Sanaa Fathima, B, Transfer learning and domain adaptation in deep networks. 2023, Jupiter Publications Consortium.
- Xia, Y. , et al., Species-specific design of artificial promoters by transfer-learning based generative deep-learning model. Nucleic Acids Research, 2024: p. gkae429.
- Xu, T. , et al., A locally weighted, correlated subdomain adaptive network employed to facilitate transfer learning. Image and Vision Computing 2024, 141, 104887. [Google Scholar] [CrossRef]
- Kala, R. , Chapter 15 - An introduction to evolutionary computation, in Autonomous Mobile Robots, R. Kala, Editor. 2024, Academic Press. p. 715-759.
- Kruse, R. , et al., Introduction to Evolutionary Algorithms, in Computational Intelligence: A Methodological Introduction. 2022, Springer International Publishing: Cham. p. 225-254.
- Liu, L., et al. A Survey of Evolutionary Algorithms. in 2023 4th International Conference on Big Data, Artificial Intelligence and Internet of Things Engineering (ICBAIE). 2023.
- Luo, F., G. Ranzi, and Z.Y. Dong, Chapter 7 - Evolutionary optimization, in Building Energy Management Systems and Techniques, F. Luo, G. Ranzi, and Z.Y. Dong, Editors. 2024, Elsevier. p. 111-138.
- Plump, C., B.J. Berger, and R. Drechsler. Improving evolutionary algorithms by enhancing an approximative fitness function through prediction intervals. in 2021 IEEE Congress on Evolutionary Computation (CEC). 2021. IEEE.
- Pon Bharathi, A. , et al., A Study on Evolutionary Algorithms and Its Applications. Electrical and Automation Engineering, 2022. 1(1).
- Tamilselvi, S. , Introduction to Evolutionary Algorithms, in Genetic Algorithms, V. Sebastián, L. José María, and M. José María, Editors. 2022, IntechOpen: Rijeka. p. Ch. 1.
- Yoshida, H., A. M. Adams, and O. Witkowski, Evolutionary Computation: Perspectives on Past and Future, in Frontiers in Genetics Algorithm Theory and Applications. 2024, Springer. p. 3-13.
- Zhang, X., X. Zhang, and W. Wang, Evolutionary Computing, in Intelligent Information Processing with Matlab. 2023, Springer Nature Singapore: Singapore. p. 173-219.
- Yan-Fu Li, E.Z. , Evolutionary Algorithms (EAs), in Reliability Assessment and Optimization: Methods and Applications. 2022: John Wiley & Sons Ltd.
- Bansal, H. and K. Sharma, A review study on various algorithms of machine learning. Journal of emerging technologies and innovative research, 2020.
- Nesbeth, D.N., et al., Synthetic biology routes to bio-artificial intelligence. Essays in biochemistry 2016, 60, 381–391. [CrossRef] [PubMed]
- Tan, F. and J.-J. Slotine. A quorum sensing inspired algorithm for dynamic clustering. in 52nd IEEE Conference on Decision and Control. 2013. IEEE.
- Wei, L. , et al., Comparative analysis and prediction of quorum-sensing peptides using feature representation learning and machine learning algorithms. Briefings in Bioinformatics 2020, 21, 106–119. [Google Scholar] [PubMed]
- A., V., Kychkin., Oleg, V., Gorshkov., Mikhail, Kukarkin, Predictive models integration with an environmental monitoring IoT platform. Прикладная инфoрматика 2022, 17, 5–16.
- Bontorin, G., et al. A real-time closed-loop setup for hybrid neural networks. in 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2007. IEEE.
- DeBenedictis, E.A. , et al., A high-throughput platform for feedback-controlled directed evolution. BioRxiv, 2020: p. 2020.04. 01.021022.
- Elias, J.R., et al., Real-Time Streaming and Event-driven Control of Scientific Experiments. arXiv preprint arXiv:2205.01476, 2022.
- Kulkarni Sheetal Vijay., S.S., Mrunali Bajare, Rahul Deshmukh, Real Time Monitoring of Temperature And Humidity Using LabVIEW and ML. International Journal for Research in Applied Science & Engineering Technology (IJRA) 2024, 12, 4701–4710.
- Sahay, M.R., et al., Environmental monitoring system using IoT and cloud service at real-time. EasyChair Preprint 2019, 968, 2.
- Schmidt, J.Q. and B. Kerkez, Machine learning-assisted, process-based quality control for detecting compromised environmental sensors. Environmental Science & Technology 2023, 57, 18058–18066. [Google Scholar]
- Tao, W. , Real-time data feedback artificial intelligence control system and control method thereof. 2020.
- Jaiswal, S., et al., Using Signal Processing in Tandem With Adapted Mixture Models for Classifying Genomic Signals. arXiv preprint arXiv:2211.01603, 2022.
- Sampara, P., et al., Advancing environmental biotechnology with microbial community modeling rooted in functional ‘omics. Current Opinion in Biotechnology 2024, 88, 103165.
- Ebulue, C.C., et al., Environmental data in epidemic forecasting: Insights from predictive analytics. Computer Science & IT Research Journal 2024, 5, 1113–1125.
- Konecny, J. and M. Prauzek, Environmental Monitoring Stations Data Transmission Using Reinforcement Learning Wavelet Compression Method. IFAC-PapersOnLine 2022, 55, 127–132. [Google Scholar] [CrossRef]
- Malakar, N. , Environmental Intelligence using Machine Learning Applications. Journal of Nepal Physical Society 2022, 8, 42–47. [Google Scholar] [CrossRef]
- Singh, G. , ENVIRONMENTAL MONITORING WITH MACHINE LEARNING. EPRA International Journal of Multidisciplinary Research (IJMR), 9 (5), 208, 2023. 212.
- Diveev, A. and E. Shmalko, Machine Learning Feedback Control Approach Based on Symbolic Regression for Robotic Systems. Mathematics 2022, 10, 4100. [Google Scholar] [CrossRef]
- Kuk, E., S. Bobek, and G.J. Nalepa. ML-based proactive control of industrial processes. in International Conference on Computational Science.
- Sveen, T.R., S. Hannula, and M. Bahram, Microbial regulation of feedbacks to ecosystem change. Trends in Microbiology, 2024.
- Yang, J. , et al., A Model Predictive Control Algorithm Based on Biological Regulatory Mechanism and Operational Research. IEEE/CAA Journal of Automatica Sinica 2023, 10, 2174–2176. [Google Scholar] [CrossRef]
- Boada, Y. , et al., Modeling and optimization of a molecular biocontroller for the regulation of complex metabolic pathways. Frontiers in Molecular Biosciences 2022, 9, 801032. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M. and K. Kaneko, Entangled gene regulatory networks with cooperative expression endow robust adaptive responses to unforeseen environmental changes. Physical Review Research 2021, 3, 033183. [Google Scholar] [CrossRef]
- López-Maury, L., S. Marguerat, and J. Bähler, Tuning gene expression to changing environments: from rapid responses to evolutionary adaptation. Nature Reviews Genetics 2008, 9, 583–593. [Google Scholar] [CrossRef]
- Benzinger, D., S. Ovinnikov, and M. Khammash, Synthetic gene networks recapitulate dynamic signal decoding and differential gene expression. Cell Systems 2022, 13, 353–364. [Google Scholar] [CrossRef]
- Jiang, T. , et al., Establishing Tunable Genetic Logic Gates with Versatile Dynamic Performance by Varying Regulatory Parameters. ACS Synthetic Biology 2023, 12, 3730–3742. [Google Scholar] [CrossRef]
- Velazquez Sanchez, A.K., et al., Tailored synthetic sRNAs dynamically tune multilayer genetic circuits. ACS synthetic biology 2023, 12, 2524–2535. [CrossRef]
- Kaenampornpan, M. and E. O Neill, Integrating history and activity theory in context aware system design. COGNITIVE SCIENCE RESEARCH PAPER-UNIVERSITY OF SUSSEX CSRP 2005, 577, 87.
- Lee, S.H. , et al., Metabolic flux optimization of iterative pathways through orthogonal gene expression control: Application to the β-oxidation reversal. Metabolic Engineering 2024, 82, 262–273. [Google Scholar] [CrossRef]
- Lengerich, B., et al., Contextualized machine learning. arXiv preprint arXiv:2310.11340, 2023.
- Li, C.-T. , et al., Optimization of nutrient utilization efficiency and productivity for algal cultures under light and dark cycles using genome-scale model process control. npj Systems Biology and Applications 2023, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Piga, D., F. Pura, and M. Forgione, On the adaptation of in-context learners for system identification. IFAC-PapersOnLine 2024, 58, 277–282. [CrossRef]
- Shakiba, N. , et al., Context-aware synthetic biology by controller design: Engineering the mammalian cell. Cell systems 2021, 12, 561–592. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G., et al., Continual learning of context-dependent processing in neural networks. Nature Machine Intelligence 2019, 1, 364–372. [CrossRef]
- Gerngross, D., N. Beerenwinkel, and S. Panke, Systematic investigation of synthetic operon designs enables prediction and control of expression levels of multiple proteins. bioRxiv, 2022: p. 2022.06. 10.495604.
- Yamamoto, Y. , et al., Construction of a machine-learning model to predict the optimal gene expression level for efficient production of D-lactic acid in yeast. World Journal of Microbiology and Biotechnology 2023, 39, 69. [Google Scholar] [CrossRef]
- Cobongela, S.Z.Z. , Bioremediation of Pharmaceutical Waste, in Bioremediation for Environmental Pollutants. 2023, Bentham Science Publisher. p. 303-328.
- Khuat, T.T. , et al., Applications of machine learning in biopharmaceutical process development and manufacturing: Current trends, challenges, and opportunities. arXiv preprint arXiv:, arXiv:2310.09991, 2023.
- Narayanan, H. , et al., A new generation of predictive models: The added value of hybrid models for manufacturing processes of therapeutic proteins. Biotechnology and bioengineering 2019, 116, 2540–2549. [Google Scholar] [CrossRef]
- Singh, M.V.P. and K.R. Shankar, Next-generation hybrid technologies for the treatment of pharmaceutical industry effluents. Journal of Environmental Management 2024, 353, 120197. [CrossRef]
- Sudhir, S.K. , et al., Treatment of pharmaceutical pollutants from industrial wastewater, in Current Developments in Biotechnology and Bioengineering. 2023, Elsevier. p. 1-16.
- Walsh, I. , et al., Harnessing the potential of machine learning for advancing “Quality by Design” in biomanufacturing. mAbs, 14 (1), 2013593. 2022.
- Divya Singhal, U.C. , Ayushi Singh, Environmental monitoring in the digital age: embracing iot solutions, in Futuristic Trends in IoT. 2024: IIP Series. p. 207-217.
- Popescu, S.M. , et al., Artificial intelligence and IoT driven technologies for environmental pollution monitoring and management. Frontiers in Environmental Science 2024, 12, 1336088. [Google Scholar] [CrossRef]
- Sachdev, A. , et al. Innovative 2D Nanomaterials-Based Electrochemical Microfluidic Sensors for Food Safety and Environmental Ecology. in Electrochemical Society Meeting Abstracts.
- Zhou, A.Y. , et al., A portable bioelectronic sensing system (BESSY) for environmental deployment incorporating differential microbial sensing in miniaturized reactors. PloS one 2017, 12, e0184994. [Google Scholar] [CrossRef]
- Deepak Sharma, A.P. , Deepika Sharma, Sampan Attri, Abhishek Chaudhary, Microbial Sensors, in Microbial Approaches for Sustainable Green Technologies. 2024, CRC Press. p. 20.
- B. Jansi, S.J., Dr. B. Jansi, S.J., Dr. V. Sumalatha, Exploiting synergies for improved applications through the fusion of deep learning and machine learning, in Futuristic Trends in Information Technology. 2024, IIP Series. p. 1-16.
- Fouché, A. and A. Zinovyev, Omics data integration in computational biology viewed through the prism of machine learning paradigms. Frontiers in Bioinformatics 2023, 3, 1191961. [Google Scholar] [CrossRef]
- Kumaran, S., et al. An Intelligent Framework for Wireless Sensor Networks in Environmental Monitoring. in 2023 2nd International Conference on Automation, Computing and Renewable Systems (ICACRS). 2023. IEEE.
- Nandhini, B. Sensor Fusion Techniques for Enhanced Environmental Monitoring in IoT. in 2023 International Conference on Sustainable Communication Networks and Application (ICSCNA). 2023. IEEE.
- Pokkuluri, K.S. and A. Khang, Integration of Machine Learning Augmented With Biosensors for Enhanced Water Quality Monitoring, in Agriculture and Aquaculture Applications of Biosensors and Bioelectronics. 2024, IGI Global. p. 181-192.
- Sen, P. and M. Orešič, Integrating Omics Data in Genome-Scale Metabolic Modeling: A Methodological Perspective for Precision Medicine. Metabolites 2023, 13, 855. [Google Scholar] [CrossRef] [PubMed]
- Wu, D. , et al., Towards a hybrid model-driven platform based on flux balance analysis and a machine learning pipeline for biosystem design. Synthetic and Systems Biotechnology 2024, 9, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Baral, R. , et al., A General Mechanism for the General Stress Response in Bacteria. bioRxiv, 2024: p. 2024.02. 16.580724.
- Chan, E., M. Au, and R. Chan, Functional profiling and strategic antimicrobial manipulation of a universal nutrition-sensing network to regulate microbial virulence, antibiotic tolerance, and stress protection. Hong Kong Med J, 2017. 23(4 Supplement 5).
- García-Contreras, R. , et al., Quorum sensing enhancement of the stress response promotes resistance to quorum quenching and prevents social cheating. The ISME journal 2015, 9, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Laslo, É., É. György, and M.-R. Szentpáli, Stress response in bacteria originated from dairy products. Acta Universitatis Sapientiae, Alimentaria 2023, 16, 90–102. [CrossRef]
- Mills, S. , et al., Enhancing the stress responses of probiotics for a lifestyle from gut to product and back again. Microbial cell factories 2011, 10, 1–15. [Google Scholar] [CrossRef]
- Society, A.C. , Microbial Stress Response: Mechanisms and Data Science. 2023: ACS Publications.
- Tang, M.-X., S. Dyrma, and T. Dong, Stress responses modulate bacterial competitive fitness in polymicrobial communities, in Stress: Immunology and Inflammation. 2024, Elsevier. p. 161-171.
- Yuan, B. , et al., Regulatory mechanisms underlying yeast chemical stress response and development of robust strains for bioproduction. Current Opinion in Biotechnology 2024, 86, 103072. [Google Scholar] [CrossRef]
- Zoheir, A.E. , et al., A three-colour stress biosensor reveals multimodal response in single cells and spatiotemporal dynamics of biofilms. npj Biofilms and Microbiomes 2023, 9, 57. [Google Scholar] [CrossRef]
- Haouari, B., R. Mzid, and O. Mosbahi, A reinforcement learning-based approach for online optimal control of self-adaptive real-time systems. Neural Computing and Applications 2023, 35, 20375–20401. [CrossRef]
- Kulkarni, S., A. Marda, and K. Vaidhyanathan. Towards self-adaptive machine learning-enabled systems through QoS-aware model switching. in 2023 38th IEEE/ACM International Conference on Automated Software Engineering (ASE). 2023. IEEE.
- Rothman, D.L., P.B. Moore, and R.G. Shulman, The impact of metabolism on the adaptation of organisms to environmental change. Frontiers in Cell and Developmental Biology 2023, 11, 1197226.
- Töpfer, M., et al. Online ML Self-adaptation in Face of Traps. in 2023 IEEE International Conference on Autonomic Computing and Self-Organizing Systems (ACSOS). 2023. IEEE.
- Xavier, J.B. , Machine learning of cellular metabolic rewiring. Biology Methods and Protocols 2024, 9, bpae048. [Google Scholar] [CrossRef]
- Kulkarni Sheetal Vijay., S.S. Kulkarni Sheetal Vijay., S.S., Mrunali Bajare, Rahul Deshmukh, Real Time Monitoring of Temperature And Humidity Using LabVIEW and ML. International Journal for Research in Applied Science & Engineering Technology (IJRA), 2024. 12(5).
- MOE, N.P.S. , Machine-learning-assisted environment-adaptive thermal metamaterials. Cornell University, 2023.
- Paterova, T., M. Prauzek, and J. Konecny. Robustness Analysis of Data-Driven Self-Learning Controllers for IoT Environmental Monitoring Nodes based on Q-learning Approaches. in 2022 IEEE Symposium Series on Computational Intelligence (SSCI). 2022. IEEE.
- Saleh, S. and B. Battseren, AI-driven Solutions for Sustainable Environment Monitoring. Embedded Selforganising Systems 2023, 10, 1–2. [CrossRef]
- Ankit Mahule, K.R. , Ankush D. Sawarkar, Sagar Lachure, Enhancing Environmental Resilience, in Artificial Intelligence for Air Quality Monitoring and Prediction. 2024, CRC Press.
- Aida, H. , et al., Machine learning-assisted discovery of growth decision elements by relating bacterial population dynamics to environmental diversity. Elife 2022, 11, e76846. [Google Scholar] [CrossRef] [PubMed]
- Somathilaka, S., et al., Inferring Gene Regulatory Neural Networks for Bacterial Decision Making in Biofilms. arXiv preprint arXiv:2301.04225, 2023.
- Somathilaka, S.S. , et al., Revealing gene regulation-based neural network computing in bacteria. Biophysical Reports, 2023. 3(3).
- Alam, M. and S. Ghosh. Deepqmlp: A scalable quantum-classical hybrid deep neural network architecture for classification. in 2022 35th International Conference on VLSI Design and 2022 21st International Conference on Embedded Systems (VLSID). 2022. IEEE.
- Dai, C. , et al., QSP: An open sequence database for quorum sensing related gene analysis with an automatic annotation pipeline. Water research 2023, 235, 119814. [Google Scholar] [CrossRef] [PubMed]
- Faure, L. , et al., A neural-mechanistic hybrid approach improving the predictive power of genome-scale metabolic models. Nature Communications 2023, 14, 4669. [Google Scholar] [CrossRef]
- Wu, S. , et al., Combinational quorum sensing devices for dynamic control in cross-feeding cocultivation. Metabolic Engineering 2021, 67, 186–197. [Google Scholar] [CrossRef]
- Gotti, C. , et al., LC-SRM combined with machine learning enables fast identification and quantification of bacterial pathogens in urinary tract infections. bioRxiv, 2024: p. 2024.05. 31.596829.
- Mishra, A. , et al., Artificial Intelligence-Driven Analysis of Antimicrobial-Resistant and Biofilm-Forming Pathogens on Biotic and Abiotic Surfaces. Antibiotics 2024, 13, 788. [Google Scholar] [CrossRef]
- Rahman, M.H.-U. , et al., Machine learning-assisted raman spectroscopy and SERS for bacterial pathogen detection: clinical, food safety, and environmental applications. Chemosensors 2024, 12, 140. [Google Scholar] [CrossRef]
- Zhang, X. , et al., Artificial intelligence applications in the diagnosis and treatment of bacterial infections. Frontiers in Microbiology 2024, 15, 1449844. [Google Scholar]
- Fakhar, M., M. Ahmed, and A.N. Sabri, Computational and experimental strategies for combating MBL P. aeruginosa (MBLPA) biofilms using phytochemicals: Targeting the quorum sensing network. Saudi Journal of Biological Sciences 2024, 31, 104001. [Google Scholar] [CrossRef]
- Galvez-Llompart, M. , et al., Targeting bacterial growth in biofilm conditions: rational design of novel inhibitors to mitigate clinical and food contamination using QSAR. Journal of Enzyme Inhibition and Medicinal Chemistry 2024, 39, 2330907. [Google Scholar] [CrossRef]
- Munagala, S. , et al., Integrating QQ with Nano-techniques–A Potent Antibacterial Therapy. 2023.
- Song, K. , et al., Whole-transcriptome analysis reveals mechanisms underlying antibacterial activity and biofilm inhibition by a malic acid combination (MAC) in Pseudomonas aeruginosa. PeerJ 2023, 11, e16476. [Google Scholar] [CrossRef] [PubMed]
- Tang, D. , et al., Effect of L-HSL on biofilm and motility of Pseudomonas aeruginosa and its mechanism. Applied Microbiology and Biotechnology 2024, 108, 418. [Google Scholar] [CrossRef] [PubMed]
- Basu, B. , et al., The Future of Cystic Fibrosis Care: Exploring AI's Impact on Detection and Therapy. Current Respiratory Medicine Reviews 2024, 20, 302–321. [Google Scholar] [CrossRef]
- Graña-Miraglia, L. , et al., Predictive modeling of antibiotic eradication therapy success for new-onset Pseudomonas aeruginosa pulmonary infections in children with cystic fibrosis. PLOS Computational Biology 2023, 19, e1011424. [Google Scholar] [CrossRef]
- Nafee, N. , et al., Enzyme-Linked Lipid Nanocarriers for Coping Pseudomonal Pulmonary Infection. Would Nanocarriers Complement Biofilm Disruption or Pave Its Road? International Journal of Nanomedicine, 2024: p. 3861-3890.
- A. Yu. Voronkova, E.K.Z., V. A. Yu. Voronkova, E.K.Z., V. D. Sherman, E. L. Amelina, A. V. Orlov, O. V. Usacheva, V. V. Tarakanova, L. V. Smirnova, E. V. Pasnova, N. D. Odinaeva, E. I. Kondratyeva, Therapy of gram-negative infection in the complex treatment of cystic fibrosis. Russian Pulmonology Journal, 2023. 33(4).
- Chung, J. , et al., Targeting Matrix Exopolysaccharides to Disrupt Pseudomonas aeruginosa Biofilms in Cystic Fibrosis. 2023.
- Kenneth, N. , Cystic fibrosis and pulmonary biofilms. The Southwest Respiratory and Critical Care Chronicles, 2023. 11(49).
- Rappo, U. , et al., Late Breaking Abstract-A novel treatment for chronic P. aeruginosa pulmonary infection in CF subjects-A phase 1b/2a randomized, double-blind, placebo-controlled, multicenter study to evaluate phage therapy. 2023, Eur Respiratory Soc.
- Fernandes, P.O. , et al., Machine Learning-Based Virtual Screening of Antibacterial Agents against Methicillin-Susceptible and Resistant Staphylococcus aureus. Journal of Chemical Information and Modeling 2024, 64, 1932–1944. [Google Scholar] [CrossRef]
- Nigo, M. , et al., Deep learning model for personalized prediction of positive MRSA culture using time-series electronic health records. Nature Communications 2024, 15, 2036. [Google Scholar] [CrossRef]
- Rusic, D. , et al., Tackling the Antimicrobial Resistance “Pandemic” with Machine Learning Tools: A Summary of Available Evidence. Microorganisms 2024, 12, 842. [Google Scholar] [CrossRef]
- Shehadeh, F. , et al., Machine learning-assisted high-throughput screening for Anti-MRSA compounds. IEEE/ACM Transactions on Computational Biology and Bioinformatics, 2024.
- Zhang, L., et al., Quantum Long Short-Term Memory for Drug Discovery. arXiv preprint arXiv:2407.19852, 2024.
- Ho, L.-C. , et al., Impact of the implementation of the Intelligent Antimicrobial System (iAMS) on clinical outcomes among patients with bacteraemia caused by methicillin-resistant Staphylococcus aureus. International Journal of Antimicrobial Agents 2024, 63, 107142. [Google Scholar] [CrossRef]
- Max, S. , AI Tools in Food Quality Monitoring. 2023.
- Mayer, C. , et al., Quorum sensing architecture network in Escherichia coli virulence and pathogenesis. FEMS Microbiology Reviews 2023, 47, fuad031. [Google Scholar] [CrossRef]
- O’Shea, N., D. Greene, and M.A. Fenelon, Artificial Intelligence in Food Safety, in Encyclopedia of Food Safety (Second Edition), G.W. Smithers, Editor. 2024, Academic Press: Oxford. p. 178-184.
- Wang, Z. , et al., Establishment of real-time fluorescence and visual LAMP for rapid detection of Escherichia coli O157: H7 and kits construction. Letters in Applied Microbiology 2023, 76, ovad122. [Google Scholar] [CrossRef]
- Yadav, P.K. , et al. Detection of E. coli concentration levels using CSI-D+ handheld with UV-C fluorescence imaging and deep learning on leaf surfaces. in Sensing for Agriculture and Food Quality and Safety XVI.
- Yi, J. , et al., AI-enabled biosensing for rapid pathogen detection: From liquid food to agricultural water. Water Research 2023, 242, 120258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C., et al., Screening of E. coli O157: H7 AI-2 QS inhibitors and their inhibitory effect on biofilm formation in combination with disinfectants. Food Bioscience 2024, 58, 103821.
- Yin, B. , et al., Chip-based automated equipment for dual-mode point-of-care testing foodborne pathogens. Biosensors and Bioelectronics 2024, 257, 116338. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S., V. Kadam, and S. Bhatlawande. Real Time Food Monitoring and Quality Alert System Using IoT and Streamlit. in International Conference on Information Science and Applications. 2023. Springer.
- Radhakrishnan, A., et al. An Automated Alert System for Monitoring the Hygiene in Restaurants Using Machine Vision. in 2024 1st International Conference on Trends in Engineering Systems and Technologies (ICTEST). 2024. IEEE.
- Li, Y. , et al., A novel quorum sensing regulator LuxT contributes to the virulence of Vibrio cholerae. Virulence 2023, 14, 2274640. [Google Scholar] [CrossRef] [PubMed]
- Mahima M, K.g.K., P. Vasanthakumari, G. Jayalakshmi, Kothandapani Sundar, Quorum Sensing Based Drug Screening Against Vibrio Cholerae. Journal of Microbes and Research, 2022. 1(1).
- Sajeevan, A., T. Ramamurthy, and A.P. Solomon, Vibrio cholerae virulence and its suppression through the quorum-sensing system. Critical Reviews in Microbiology, 2024: p. 1-22.
- Walker, L.M. , et al., A simple mechanism for integration of quorum sensing and cAMP signalling in V. cholerae. BioRxiv, 2023.
- El Bushra, H. , A Tool to Monitor Multi-Sectoral Response Activities During Outbreaks of Cholera in Sudan. African Journal of Health and Medical Sciences (AFJHMS) 2024, 9, 20–28. [Google Scholar] [CrossRef]
- El Morr, C., et al., AI-based epidemic and pandemic early warning systems: A systematic scoping review. Health Informatics Journal 2024, 30, 14604582241275844. [CrossRef]
- Fieldhouse, J., et al., How feasible or useful are timeliness metrics as a tool to optimise One Health outbreak responses? BMJ Global Health 2024, 9, e013615. [CrossRef]
- Nwankwo, E.I., et al., Artificial Intelligence in predictive analytics for epidemic outbreaks in rural populations. International Journal of Life Science Research Archive 2024, 7, 078–094. [CrossRef]
- Isiaka, A. , et al., Harnessing Artificial Intelligence for Early Detection and Management of Infectious Disease Outbreaks. International Journal of Innovative Research and Development, 2024.
- Kuik, C. , et al., Matrix-assisted laser desorption/ionization mass spectrometry imaging for quorum sensing. AMB Express 2024, 14, 45. [Google Scholar] [CrossRef]
- Roy, M., M. Adhikari, and B. Tiwary, Phytocompound-Mediated Inhibition of Quorum-Sensing Cascade in Pathogenic Bacteria, in Natural Products. 2024, CRC Press. p. 79-101.
- Charlebois, D.A. , Quantitative systems-based prediction of antimicrobial resistance evolution. NPJ Systems Biology and Applications 2023, 9, 40. [Google Scholar] [CrossRef]
- Kim, J.I. , et al., Machine learning for antimicrobial resistance prediction: current practice, limitations, and clinical perspective. Clinical microbiology reviews 2022, 35, e00179–21. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, V., et al., Use of Predictive Analytics in Antimicrobial Resistance: A Review. Vinoth Kumar Kolluru et al., Cognizance Journal of Multidisciplinary Studies. 2024, 4, 404–414.
- Nsubuga, M. , et al., Generalizability of machine learning in predicting antimicrobial resistance in E. coli: a multi-country case study in Africa. BMC genomics 2024, 25, 287. [Google Scholar] [CrossRef]
- Roche-Lima, A. , et al., ML-SyPred–Machine Learning Synergy Predictor: A Tool to Predict Drug Combinations. 2022.
- Weis, C. , et al., Direct Antimicrobial Resistance Prediction from MALDI-TOF mass spectra profile in clinical isolates through Machine Learning. bioRxiv 2020, 1, 1–35. [Google Scholar]
- Yang, J. , et al., Interpretable machine learning-based decision support for prediction of antibiotic resistance for complicated urinary tract infections. npj Antimicrobials and Resistance 2023, 1, 14. [Google Scholar] [CrossRef]
- Yun, B. , et al., Machine learning-enabled prediction of antimicrobial resistance in foodborne pathogens. CyTA-Journal of Food 2024, 22, 2324024. [Google Scholar] [CrossRef]
- Ashreetha, B., et al. Precision Medicine in Oncology-Leveraging Machine Learning for Accurate Neoplasm Diagnosis. in 2024 5th International Conference for Emerging Technology (INCET). 2024. IEEE.
- Bhuvaneswari, R. , et al., Advancing Precision Medicine: Integrating AI and Machine Learning for Personalized Healthcare Solutions, in Artificial Intelligence Transformations for Healthcare Applications: Medical Diagnosis, Treatment, and Patient Care. 2024, IGI Global. p. 344-361.
- Deliu, N. and B. Chakraborty, Artificial Intelligence-based Decision Support Systems for Precision and Digital Health. arXiv preprint arXiv:2407.16062, 2024.
- Folguera-Blasco, N. , et al., Coupling quantitative systems pharmacology modelling to machine learning and artificial intelligence for drug development: its pAIns and gAIns. Frontiers in Systems Biology 2024, 4, 1380685. [Google Scholar] [CrossRef]
- Ganga, S., et al. The use of ML Algo Techniques in Translational Way of HC System (Medicine). in 2024 4th International Conference on Advance Computing and Innovative Technologies in Engineering (ICACITE). 2024. IEEE.
- Pfeil, J. , et al., tauX: A Gene Expression Ratio Strategy to Improve Machine Learning Applications in Precision Medicine. bioRxiv, 2024: p. 2024.07. 01.601595.
- Rehan, H. , Advancing Cancer Treatment with AI-Driven Personalized Medicine and Cloud-Based Data Integration. Journal of Machine Learning in Pharmaceutical Research 2024, 4, 1–40. [Google Scholar]
- Viduedo, P.H.Z., et al., Harnessing the Power of Ai and Machine Learning for Next-Generation Sequencing Data Analysis: A Comprehensive Review of Applications, Challenges, and Future Directions in Precision Oncology. Revista Ibero-Americana de Humanidades, Ciências e Educação. 2024, 10, 2898–2904.
- Reena Lokare, J.W. , Sunita Patil, Ganesh Wadmare, Darshan Patil, Transparent precision: Explainable AI empowered breast cancer recommendations for personalized treatment. IAES International Journal of Artificial Intelligence 2024, 13, 2694–2694. [Google Scholar]
- Bansal, A. , et al., Gene Therapy and Its Applications. Journal of Medical Evidence, 2023. 4(1).
- Carneiro, D.C., et al., Therapeutic applications of synthetic gene/genetic circuits: a patent review. Frontiers in Bioengineering and Biotechnology 2024, 12, 1425529.
- Deyhimfar, R. , et al., The clinical impact of mRNA therapeutics in the treatment of cancers, infections, genetic disorders, and autoimmune diseases. Heliyon, 2024.
- He, Y., A. P. Johnston, and C.W. Pouton, Therapeutic applications of cell engineering using mRNA technology. Trends in Biotechnology, 2024.
- Li, Y. mRNA therapeutics in diseases: Light inspect into a new horizon. in Theoretical and Natural Science. 2024.
- Mohandoss, K. , A gene therapy approach to the design of personalized medicine, in Futuristic Trends in Pharmacy & Nursing. 2024: IIP Series. p. 167-181.
- Datta, B.N. and B. Sahoo, Machine learning, regression and optimization. Data Science and SDGs: Challenges, Opportunities and Realities, 2021: p. 177-197.
- Hanzely, S., Adaptive Optimization Algorithms for Machine Learning. arXiv preprint arXiv:2311.10203, 2023.
- Kumar, D., et al., Machine learning's role in personalized medicine & treatment optimization. World Journal of Advanced Research and Reviews 2024, 21, 1675–1686.
- Zhang, B., Y.J. Ong, and T. Nakamura. Simpo: Simultaneous prediction and optimization. in 2022 IEEE International Conference on Services Computing (SCC). 2022. IEEE.
- Pradeep Verma, N.M.a.V.S. , Machine learning for personalized medicine: Tailoring treatment strategies through data analysis. The Pharma Innovation Journal 2019, 8, 11–14. [Google Scholar] [CrossRef]
- Behdani, A.M., et al., Optimizing pharmacogenomic decision-making by data science. PLOS Digital Health 2024, 3, e0000451.
- Li, P. , et al., Utility based approach in individualized optimal dose selection using machine learning methods. Statistics in medicine 2022, 41, 2957–2977. [Google Scholar] [CrossRef]
- Mhatre, S. , et al., AI and ML for development of cell and gene therapy for personalized treatment. Bioinformatics Tools for Pharmaceutical Drug Product Development, 2023: p. 371-400.
- Szűcs, T.D., et al. Model predictive fuzzy control in chemotherapy optimization. in 2023 IEEE 17th International Symposium on Applied Computational Intelligence and Informatics (SACI). 2023. IEEE.
- Elangkovan, N. and G. Dickson, Gene therapy for Duchenne muscular dystrophy. Journal of neuromuscular diseases, 2021. 8(s2): p. S303-S316.
- Happi Mbakam, C. and J.P. Tremblay, Gene therapy for Duchenne muscular dystrophy: an update on the latest clinical developments. Expert Review of Neurotherapeutics 2023, 23, 905–920. [CrossRef]
- Markati, T., et al., Emerging therapies for Duchenne muscular dystrophy. The Lancet Neurology 2022, 21, 814–829. [CrossRef]
- Olson, E.N. , Toward the correction of muscular dystrophy by gene editing. Proceedings of the National Academy of Sciences 2021, 118, e2004840117. [Google Scholar] [CrossRef]
- Podkalicka, P. , et al., Molecular mechanisms of Duchenne muscular dystrophy and new therapeutic strategies. Postepy Biochemii 2022, 68, 109–122. [Google Scholar]
- Richard, I. , Basic notions about gene therapy from the nucleic acid perspective and applications in a pediatric disease: Duchenne muscular dystrophy. Archives de Pédiatrie.
- Saad, F.A., et al., Duchenne muscular dystrophy gene therapy. Current Gene Therapy 2024, 24, 17–28. [CrossRef] [PubMed]
- Superson, M., et al., Applications of gene modification technologies in the treatment of inherited diseases. Journal of Education, Health and Sport 2024, 66, 50073–50073. [CrossRef]
- Damane, B.P. , et al., Applying artificial intelligence prediction tools for advancing precision oncology in immunotherapy: future perspectives in personalized care, in Artificial intelligence and precision oncology: bridging cancer research and clinical decision support. 2023, Springer. p. 239-258.
- Leek, L.V. , et al., Integrative multi-omic machine learning model predicts neoadjuvant immunotherapy response using molecular data and deep learning-derived features from digital pathology. Cancer Research, 2024. 84(6_Supplement): p. 4951-4951.
- Li, Y. , et al., Informing immunotherapy with multi-omics driven machine learning. npj Digital Medicine 2024, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Ying, X. and B. Li, Machine-learning Modeling for Personalized Immunotherapy-An Evaluation Module. Biomedical journal of scientific & technical research 2022, 47, 38211.
- Arulraj, T., et al., Leveraging multi-omics data to empower quantitative systems pharmacology in immuno-oncology. Briefings in bioinformatics 2024, 25, bbae131.
- Finotello, F. and F. Eduati, Multi-omics profiling of the tumor microenvironment: paving the way to precision immuno-oncology. Frontiers in Oncology 2018, 8, 430. [CrossRef]
- Merlano, M., et al., Knowing the tumour microenvironment to optimise immunotherapy. Acta Otorhinolaryngologica Italica 2019, 39, 2. [CrossRef]
- Pomponio, R.J. , et al., Classification of the Tumor Immune Microenvironment Using Machine-Learning-Based CD8 Immunophenotyping As a Potential Biomarker for Immunotherapy and TGF-β Blockade in Nonsmall Cell Lung Cancer. AI in Precision Oncology 2024, 1, 106–118. [Google Scholar] [CrossRef]
- Popel, A.S. , Immunoactivating the tumor microenvironment enhances immunotherapy as predicted by integrative computational model. Proceedings of the National Academy of Sciences 2020, 117, 4447–4449. [Google Scholar] [CrossRef]
- Sun, J. , et al., Neural-network-based immune optimization regulation using adaptive dynamic programming. IEEE Transactions on Cybernetics 2022, 53, 1944–1953. [Google Scholar] [CrossRef]
- Ahmad, A. , et al., Precision cancer nanotherapy: evolving role of multifunctional nanoparticles for cancer active targeting. Journal of medicinal chemistry 2019, 62, 10475–10496. [Google Scholar] [CrossRef] [PubMed]
- Broadwater, D. , et al., Counterion tuning of near-infrared organic salts dictates phototoxicity to inhibit tumor growth. ACS Applied Materials & Interfaces 2022, 14, 53511–53522. [Google Scholar]
- Chen, J. , et al., Precise fibrin decomposition and tumor mechanics modulation with hydroxyethyl starch-based smart nanomedicine for enhanced antitumor efficacy. Journal of Materials Chemistry B 2022, 10, 8193–8210. [Google Scholar] [CrossRef] [PubMed]
- Haze, H. , et al., Modulation of the Tumor Stroma and Associated Novel Nanoparticle Strategies to Enhance Tumor Targeting. Surgeries 2024, 5, 49–62. [Google Scholar] [CrossRef]
- Manzari, M.T., et al., Targeted drug delivery strategies for precision medicines. Nature Reviews Materials 2021, 6, 351–370. [CrossRef]
- Vishwakarma, M. , et al., Cationic nanocarriers: A potential approach for targeting negatively charged cancer cell. Advances in Colloid and Interface Science, 2024: p. 103160.
- Fedorov, V.D., M. Sadelain, and C.C. Kloss, Novel approaches to enhance the specificity and safety of engineered T cells. The Cancer Journal 2014, 20, 160–165. [CrossRef]
- Gamboa, L., A.H. Zamat, and G.A. Kwong, Synthetic immunity by remote control. Theranostics 2020, 10, 3652. [CrossRef]
- Hickey, J.W., A.K. Kosmides, and J.P. Schneck, Engineering platforms for t cell modulation. International Review of Cell and Molecular Biology 2018, 341, 277–362.
- McBride, D.A., et al., Applications of molecular engineering in T-cell-based immunotherapies. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 2019, 11, e1557.
- Ti, D., et al., Adaptive T cell immunotherapy in cancer. Science China Life Sciences 2021, 64, 363–371. [CrossRef]
- Bhandari, A. , et al., Image-based predictive modelling frameworks for personalised drug delivery in cancer therapy. Journal of Controlled Release 2024, 370, 721–746. [Google Scholar] [CrossRef]
- Das, K.P. , Nanoparticles and convergence of artificial intelligence for targeted drug delivery for cancer therapy: Current progress and challenges. Frontiers in Medical Technology 2023, 4, 1067144. [Google Scholar] [CrossRef] [PubMed]
- Haval, A.M., K. Shwetabh, and S.S. Dash, Machine learning-based enhanced drug delivery system and its applications–a systematic review. J Angiother 2023, 642, 123098. [Google Scholar]
- Hristova-Panusheva, K. , et al., Nanoparticle-Mediated Drug Delivery Systems for Precision Targeting in Oncology. Pharmaceuticals 2024, 17, 677. [Google Scholar] [CrossRef] [PubMed]
- Husseini, G.A. , et al., Deep Learning for the Accurate Prediction of Triggered Drug Delivery. IEEE Transactions on NanoBioscience, 2024.
- Kallepalli, B. , et al., Intelligent Drug Delivery: Pioneering Stimuli-Responsive Systems to Revolutionize Disease Management-An In-depth Exploration. Current Drug Delivery, 2024.
- R, D. , Guided therapeutics: the art and science of drug targeting, in Futuristic Trends in Pharmacy & Nursing Volume. 2024: IIP Series. p. 16-30.
- Bräutigam, K. , Optimization of chemotherapy regimens using mathematical programming. Computers & Industrial Engineering 2024, 191, 110078. [Google Scholar]
- Floares, A. , et al., Optimal drug dosage regimens in cancer chemotherapy with neural networks. Journal of Clinical Oncology, 2004. 22(14_suppl): p. 2134-2134.
- Hakami, M.A. , Harnessing machine learning potential for personalised drug design and overcoming drug resistance. Journal of Drug Targeting 2024, 32, 918–930. [Google Scholar] [CrossRef]
- Hosseinpour, Z., A.H. Nikoofard, and E. Nejabat. Model Predictive Control for Optimal Drug Administration of Cancer Chemotherapy. in 2023 31st International Conference on Electrical Engineering (ICEE). 2023. IEEE.
- Pistikopoulos, E.N., I. Nascu, and E.G. Velliou, Modelling optimization and control of biomedical systems. 2018: John Wiley & Sons.
- Chaudhari, D.D., S. D. Mali, and V. Chaudhari, A Short Review on Recent Approches in Targeted Drug Dilvairy System (TDDS). 2023.
- Ezike, T.C. , et al., Advances in drug delivery systems, challenges and future directions. Heliyon, 2023. 9(6).
- Mirić, A. and N. Milivojević. Intelligent Drug Delivery Systems. 2023. Cham: Springer International Publishing.
- Tărăbuță, P.A. , et al., Drug Delivery Systems for Tissue Engineering, in Biomaterials and Tissue Engineering. 2023, Springer. p. 205-238.
- Oishi, M. , et al., Practical QSP application from the preclinical phase to enhance the probability of clinical success: Insights from case studies in oncology. Drug Metabolism and Pharmacokinetics, 2024: p. 101020.
- Sagingalieva, A. , et al., Hybrid quantum neural network for drug response prediction. Cancers 2023, 15, 2705. [Google Scholar] [CrossRef]
- Dhiwin Samrich J, D.S.J.N.R. , Quantum-Inspired Machine Learning: Transformative Applications and Implications for Industry Disruption. International Journal of Research Publication and Reviews 2024, 5, 13006–13012. [Google Scholar]
- Alsberg, E., H.A. von Recum, and M.J. Mahoney, Environmental cues to guide stem cell fate decision for tissue engineering applications. Expert opinion on biological therapy 2006, 6, 847–866. [CrossRef]
- Kong, Y. , et al., Evaluating Differentiation Status of Mesenchymal Stem Cells by Label-Free Microscopy System and Machine Learning. Cells 2023, 12, 1524. [Google Scholar] [CrossRef]
- Li, C., Y. Fan, and L. Zheng, Differentiation of stem cells regulated by biophysical cues. Sheng wu yi xue Gong Cheng xue za zhi= Journal of Biomedical Engineering= Shengwu Yixue Gongchengxue Zazhi 2023, 40, 609–616.
- Park, D.B. , et al., Tissue specific stem cell therapy for airway regeneration. Cell Proliferation, 2024: p. e13662.
- Song, E. , et al., P157 Differentiation of Tonsil-derived mesenchymal stem cells to intestinal stem cells-like cells for cell therapy of inflammatory bowel disease. Journal of Crohn's and Colitis, 2024. 18(Supplement_1): p. i462-i462.
- Yang, X. , et al., A live-cell image-based machine learning strategy for reducing variability in PSC differentiation systems. Cell Discovery 2023, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Adil, A. , et al., Stem cell therapy in the era of machine learning, in Computational Biology for Stem Cell Research. 2024, Elsevier. p. 77-84.
- Ashraf, M., M. Khalilitousi, and Z. Laksman, Applying machine learning to stem cell culture and differentiation. Current Protocols 2021, 1, e261. [CrossRef] [PubMed]
- Guo, J.L., M. Januszyk, and M.T. Longaker, Machine learning in tissue engineering. Tissue Engineering Part A, 2023. 29(1-2): p. 2-19.
- Yadav, M.K. , et al., Application of machine learning–based approaches in stem cell research, in Computational biology for stem cell research. 2024, Elsevier. p. 65-76.
- Zaman, W.S.W.K. , et al., Machine learning in stem cells research: application for biosafety and bioefficacy assessment. IEEE Access 2021, 9, 25926–25945. [Google Scholar] [CrossRef]
- Goyal, P. and R. Malviya, Developments in Stem Cell Therapy by Utilizing Artificial Intelligence. Current Pharmaceutical Design 2023, 29, 2223–2228. [CrossRef]
- Richard Webb, A.M. , Alwyn D'Souza, Regenerative medicine, in Medical Innovation. 2023: CRC Press. p. 13.
- Chen, M. , et al., Advancements in tissue engineering for articular cartilage regeneration. Heliyon, 2024.
- Gharravi, A.M. , et al., Stem Cell for Cartilage Repair, in Handbook of Stem Cell Therapy, K.H. Haider, Editor. 2022, Springer Nature Singapore: Singapore. p. 1-35.
- Gupta, A. and S. Singh, Potential role of growth factors controlled release in achieving enhanced neuronal trans-differentiation from mesenchymal stem cells for neural tissue repair and regeneration. Molecular Neurobiology, 2022: p. 1-19.
- Liu, Y.Y.F. , et al., Machine learning to predict mesenchymal stem cell efficacy for cartilage repair. PLoS Computational Biology 2020, 16, e1008275. [Google Scholar] [CrossRef]
- Velikic, G. , et al., Harnessing the stem cell niche in regenerative medicine: innovative avenue to combat neurodegenerative diseases. International journal of molecular sciences 2024, 25, 993. [Google Scholar] [CrossRef]
- Wang, P. , et al., Treatment and application of stem cells from different sources for cartilage injury: a literature review. Annals of Translational Medicine, 2022. 10(10).
- Baranwal, M. , et al., Deep learning enables design of multifunctional synthetic human gut microbiome dynamics. bioRxiv, 2021: p. 2021.09. 27.461983.
- Lebovich, M., M. Zeng, and L.B. Andrews, Algorithmic programming of sequential logic and genetic circuits for recording biochemical concentration in a probiotic bacterium. ACS synthetic biology 2023, 12, 2632–2649. [Google Scholar] [CrossRef]
- Liu, Y., Z. Zhu, and L. Jiang, Programming therapeutic probiotics by self-tunable sense-and-respond genetic circuits. Trends in Microbiology, 2023.
- Xia, J.Y., et al., Engineered calprotectin-sensing probiotics for IBD surveillance in humans. Proceedings of the National Academy of Sciences 2023, 120, e2221121120.
- Zhou, J. , et al., Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nature Communications 2022, 13, 3432. [Google Scholar] [CrossRef]
- Baranwal, M. , et al., Recurrent neural networks enable design of multifunctional synthetic human gut microbiome dynamics. Elife 2022, 11, e73870. [Google Scholar] [CrossRef] [PubMed]
- Barra, M., T. Danino, and D. Garrido, Engineered probiotics for detection and treatment of inflammatory intestinal diseases. Frontiers in Bioengineering and Biotechnology 2020, 8, 265.
- Fonseca, D.C. , et al., Evaluation of gut microbiota predictive potential associated with phenotypic characteristics to identify multifactorial diseases. Gut Microbes 2024, 16, 2297815. [Google Scholar] [CrossRef]
- Li, M. , et al., Engineered probiotics with sustained release of interleukin-2 for the treatment of inflammatory bowel disease after oral delivery. Biomaterials 2024, 309, 122584. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S. , et al., Optimization of probiotic therapeutics using machine learning in an artificial human gastrointestinal tract. Scientific reports 2021, 11, 1067. [Google Scholar] [CrossRef]
- Wu, S. , et al., metaProbiotics: a tool for mining probiotic from metagenomic binning data based on a language model. Briefings in Bioinformatics 2024, 25, bbae085. [Google Scholar] [CrossRef]
- Scott, B.M. , et al., Self-tunable engineered yeast probiotics for the treatment of inflammatory bowel disease. Nature medicine 2021, 27, 1212–1222. [Google Scholar] [CrossRef]
- Ermolenko, E. , et al., Evaluation of the effectiveness of personalised therapy for the patients with irritable bowel syndrome. Beneficial Microbes 2023, 14, 119–129. [Google Scholar] [CrossRef]
- McKay, R. , et al., A platform of genetically engineered bacteria as vehicles for localized delivery of therapeutics: Toward applications for Crohn's disease. Bioengineering & translational medicine 2018, 3, 209–221. [Google Scholar]
- Joy, Z.H. , et al., INTEGRATING MACHINE LEARNING AND BIG DATA ANALYTICS FOR REAL-TIME DISEASE DETECTION IN SMART HEALTHCARE SYSTEMS. International Journal of Health and Medical 2024, 1, 16–27. [Google Scholar]
- Yazzourh, S. , et al., Medical Knowledge Integration into Reinforcement Learning Algorithms for Dynamic Treatment Regimes. arXiv preprint arXiv:, arXiv:2407.00364, 2024.
- Zlobina, K. , et al., The role of machine learning in advancing precision medicine with feedback control. Cell Reports Physical Science, 2022. 3(11).
- Mahmood, A.A.R., A. M. Jha, and K. Manivannan, Precision Medicine: Personalizing The Fight Against Cancer. International Journal of Trends in OncoScience, 2024: p. 10-18.
- Prabhakar, P.K. , Advancements in Precision Medicine: Tailoring Healthcare to Individual Needs, in Biomedical Research Developments for Improved Healthcare, P.K. Prabhakar, Editor. 2024, IGI Global: Hershey, PA, USA. p. 310-326.
- Seyhan, A.A. and C. Carini, Precision medicine: success stories and challenges from science to implementation, in The New Era of Precision Medicine. 2024, Elsevier. p. 83-113.
- Tom, A. , Knowledge, Attitude and Ethical Perception Towards Precision Medicine Among Healthcare Professionals-a Questionnaire Survey. Journal of pharmaceutical research and development, 2024.
- Altmann, P., et al., Challenges for reinforcement learning in quantum computing. arXiv preprint arXiv:2312.11337, 2023.
- De Domenico, M., et al., Challenges and opportunities for digital twins in precision medicine: a complex systems perspective. arXiv preprint arXiv:2405.09649, 2024.
- Li, M., et al. Hybrid Quantum Classical Machine Learning with Knowledge Distillation. in ICC 2024-IEEE International Conference on Communications. 2024. IEEE.
- Lin, Z. , Progress and challenges in the symbiosis of AI with science and medicine. European Journal of Clinical Investigation, 2024: p. e14222-e14222.
- Mahanty, B. , Hybrid modeling in bioprocess dynamics: Structural variabilities, implementation strategies, and practical challenges. Biotechnology and Bioengineering 2023, 120, 2072–2091. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R., C. Rajaramesh, and U. Ghugar, Challenges in Building Predictive Models. Intelligent Techniques for Predictive Data Analytics, 2024: p. 25.
- Ong, J.C.L., et al., Ethical and regulatory challenges of large language models in medicine. The Lancet Digital Health 2024, 6, e428–e432. [CrossRef] [PubMed]
- Ullah, E. , et al., Challenges and barriers of using large language models (LLM) such as ChatGPT for diagnostic medicine with a focus on digital pathology–a recent scoping review. Diagnostic pathology 2024, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Farina, L. , AI will not give us precision medicine. Ann Ist Super Sanità 2024, 60, 8–13. [Google Scholar]
- Flores, J.E. , et al., Missing data in multi-omics integration: Recent advances through artificial intelligence. Frontiers in Artificial Intelligence 2023, 6, 1098308. [Google Scholar] [CrossRef]
- Li, Z. , et al., Amalur: Data integration meets machine learning. IEEE Transactions on Knowledge and Data Engineering, 2024.
- Martínez-García, M. and E. Hernández-Lemus, Data integration challenges for machine learning in precision medicine. Frontiers in medicine 2022, 8, 784455. [CrossRef]
- Merieme, E.A., et al. A survey on the challenges of data integration. in 2022 9th International Conference on Wireless Networks and Mobile Communications (WINCOM). 2022.
- Rapin, A., M.B. Sleiman, and J. Auwerx, Data integration in systems genetics and aging research. arXiv preprint arXiv:2207.03540, 2022.
- Vidhya, G., D. Nirmala, and T. Manju, Quality challenges in deep learning data collection in perspective of artificial intelligence. Journal of Information Technology and Computing 2023, 4, 46–58. [CrossRef]
- Dube, L. and T. Verster, Assessing the performance of machine learning models for default prediction under missing data and class imbalance: A simulation study. ORiON 2024, 40, 1–24. [Google Scholar]
- Ferri, P. , et al., Extremely missing numerical data in Electronic Health Records for machine learning can be managed through simple imputation methods considering informative missingness: A comparative of solutions in a COVID-19 mortality case study. Computer Methods and Programs in Biomedicine 2023, 242, 107803. [Google Scholar] [CrossRef]
- Shi, J. , et al., Implicit bias in Critical Care Data: Factors affecting sampling frequencies and missingness patterns of clinical and biological variables in ICU Patients. medRxiv, 2024: p. 2024.06. 09.24308661.
- Emili, A. , State of the art in data integration and network biology. Authorea Preprints, 2024.
- Gonesh Chandra Saha, M.B. , Muhammad Jahanzeb Khan, Hasi Saha,Dinesh Kumar, Avinash, Integrative Analysis of Multi-Omics Data with Deep Learning: Challenges and Opportunities in Bioinformatics. Tuijin Jishu/Journal of Propulsion Technology 2023, 44, 1384–1392. [Google Scholar]
- Adams, A.K. , et al., Qmatey: an automated pipeline for fast exact matching-based alignment and strain-level taxonomic binning and profiling of metagenomes. Briefings in Bioinformatics 2023, 24, bbad351. [Google Scholar] [CrossRef]
- Carrieri, A.P., et al. A fast machine learning workflow for rapid phenotype prediction from whole shotgun metagenomes. in Proceedings of the AAAI Conference on Artificial Intelligence. 2019.
- Dghais, W., M. Alam, and Y. Chen, Real Time Modelling and Processing, in Real-Time Modelling and Processing for Communication Systems: Applications and Practices, M. Alam, W. Dghais, and Y. Chen, Editors. 2018, Springer International Publishing: Cham. p. 1-13.
- Durge, A.R. and D.D. Shrimankar. MRQPMS: Design of a Map Reduce Bioinspired Model for Solving Quorum Planted Motif Search for High-Speed Deployments. in BIOINFORMATICS. 2023.
- Khodabakhsh, A. , et al., Predicting decision-making time for diagnosis over ngs cycles: An interpretable machine learning approach. bioRxiv, 2023: p. 2023.03. 07.530760.
- Nussinov, R. and J.A. Papin, How can computation advance microbiome research? 2017, Public Library of Science San Francisco, CA USA. p. e1005547.
- Karuvally, A. and J.E.B. Moss, Model Complexity of Program Phases. arXiv preprint arXiv:2310.03865, 2023.
- Prybutok, A.N. , et al., Fighting fire with fire: deploying complexity in computational modeling to effectively characterize complex biological systems. Current Opinion in Biotechnology 2022, 75, 102704. [Google Scholar] [CrossRef]
- Raajaraam, L. and K. Raman, Modeling Microbial Communities: Perspective and Challenges. ACS Synthetic Biology 2024, 13, 2260–2270. [CrossRef]
- V. Yu. Zadiraka, O.M.K. V. Yu. Zadiraka, O.M.K., Inna V. Shvidchenko, Models of Computer Calculations. Cybernetics and Computer Technologies, 2022(2): p. 38-51.
- Gupta, I. , et al., A Multiple Controlled Toffoli Driven Adaptive Quantum Neural Network Model for Dynamic Workload Prediction in Cloud Environments. IEEE Transactions on Pattern Analysis and Machine Intelligence, 2024.
- Sokolov, A., A. Larionov, and A. Mukhtarov. Distributed System for Scientific and Engineering Computations with Problem Containerization and Prioritization. in International Conference on Distributed Computer and Communication Networks. 2023. Springer.
- Tiwari, R.K., A.B.B.A. Hamid, and T.E. Nyamasvisva. Potential in Quantum Machine Learning for Real-World Problems. in 2024 5th International Conference on Recent Trends in Computer Science and Technology (ICRTCST). 2024. IEEE.
- Narotam Dass, A.K. , Mohit Sungroya, Optimizing real-time systems with parallel computing: techniques and challenges, in Futuristic Trends in Information Technology. 2024: IIP Series. p. 246-258.
- Alzubaidi, L. , et al., A survey on deep learning tools dealing with data scarcity: definitions, challenges, solutions, tips, and applications. Journal of Big Data 2023, 10, 46. [Google Scholar] [CrossRef]
- Chai, C., et al. Mitigating Data Scarcity in Supervised Machine Learning Through Reinforcement Learning Guided Data Generation. in 2024 IEEE 40th International Conference on Data Engineering (ICDE). 2024. IEEE.
- Dens, C., et al., Machine learning strategies to tackle data challenges in mass spectrometry-based proteomics. Journal of the American Society for Mass Spectrometry 2024, 35, 2143–2155. [CrossRef]
- Li, Y., et al., StableQ: Enhancing Data-Scarce Quantization with Text-to-Image Data. arXiv preprint arXiv:2312.05272, 2023.
- Niel, O., A novel algorithm can generate data to train machine learning models in conditions of extreme scarcity of real world data. arXiv preprint arXiv:2305.00987, 2023.
- Hakami, A. , Strategies for overcoming data scarcity, imbalance, and feature selection challenges in machine learning models for predictive maintenance. Scientific Reports 2024, 14, 9645. [Google Scholar] [CrossRef]
- Xu, M. Machine Unlearning: Challenges in Data Quality and Access. in Proceedings of the Thirty-Third International Joint Conference on Artificial Intelligence (IJCAI-24). 2024.
- Xu, M. , et al., Embracing limited and imperfect training datasets: opportunities and challenges in plant disease recognition using deep learning. Frontiers in Plant Science 2023, 14, 1225409. [Google Scholar] [CrossRef]
- Chen, J., et al., Unveiling the Flaws: Exploring Imperfections in Synthetic Data and Mitigation Strategies for Large Language Models. arXiv preprint arXiv:2406.12397, 2024.
- Gandhi, S., et al., Better Synthetic Data by Retrieving and Transforming Existing Datasets. arXiv preprint arXiv:2404.14361, 2024.
- Miletic, M. and M. Sariyar, Challenges of Using Synthetic Data Generation Methods for Tabular Microdata. Applied Sciences 2024, 14, 5975. [Google Scholar] [CrossRef]
- Braiek, H.B. and F. Khomh, Machine Learning Robustness: A Primer. arXiv preprint arXiv:2404.00897, 2024.
- Guillaume, F. , Genetic and environmental robustness are distinct yet correlated evolvable traits in a gene network. Peer Community In Evolutionary Biology, 2022: p. 100138.
- Olsson, L., et al., Robustness: linking strain design to viable bioprocesses. Trends in Biotechnology 2022, 40, 918–931. [CrossRef] [PubMed]
- Zhang, C., D. Garlan, and E. Kang. A behavioral notion of robustness for software systems. in Proceedings of the 28th ACM Joint Meeting on European Software Engineering Conference and Symposium on the Foundations of Software Engineering. 2020.
- Herken, B.W. , et al., Environmental challenge rewires functional connections among human genes. bioRxiv, 2023.
- Kochanowski, K. , et al., Dissecting the impact of metabolic environment on three common cancer cell phenotypes. bioRxiv, 2020: p. 2020.06. 23.167437.
- Libiseller-Egger, J., et al., Environmental flexibility does not explain metabolic robustness. NPJ Systems Biology and Applications 2020, 6, 39.
- Hanif, M.A. , et al., Robust computing for machine learning-based systems. Dependable Embedded Systems, 2021: p. 479-503.
- Wang, S., Y. Jin, and M. Cai, Enhancing the robustness of networks against multiple damage models using a multifactorial evolutionary algorithm. IEEE Transactions on Systems, Man, and Cybernetics: Systems 2023, 53, 4176–4188. [CrossRef]
- Yan, Y. , Improve robustness of machine learning via efficient optimization and conformal prediction. AI Magazine, 2024.
- Zhou, X., M. Kouzel, and H. Alemzadeh. Robustness testing of data and knowledge driven anomaly detection in cyber-physical systems. in 2022 52nd Annual IEEE/IFIP International Conference on Dependable Systems and Networks Workshops (DSN-W). 2022. IEEE.
- Habibullah, K.M., et al. Scoping of non-functional requirements for machine learning systems. in 2024 IEEE 32nd International Requirements Engineering Conference (RE). 2024. IEEE.
- Liu, J. and S. Liu, Applications of Large Language Models in Clinical Practice: Path, Challenges, and Future Perspectives. OSF PREPRINTS, 2024.
- Farazin, A.Z.A. , Advancements and Challenges in the Application of Machine Learning for Biomedical Diagnostics and Disease Prediction. SciBase Journals, 2024. 2(3).
- Hamann, A. and S. Wölk, Performance analysis of a hybrid agent for quantum-accessible reinforcement learning. New Journal of Physics 2022, 24, 033044. [Google Scholar] [CrossRef]
- Joshua, R. Bertram, B.R.W. and R.A. Mclean Angus L, Safe and secure practical autonomy, R. Collins, Editor. 2020.
- Menta, S., et al., Learning Q-function approximations for hybrid control problems. IEEE Control Systems Letters 2021, 6, 1364–1369.
- Rohde, W., M. Perepechaenko, and R. Kuang. Quantum-Secure Autonomous Factories: Hybrid TLS 1.3 for Inter-and Intra-plant Communication. in International Conference on Communication and Network Technology. 2022. Springer.
- Wang, H., L. Stobaugh, and W. Stobaugh, Hybrid Quanvolutional Regression Neural Network: Quantum Advantage for Near-term Autonomous Machine. Authorea Preprints, 2024.
- Zhang, H., et al., Controlling Sequential Hybrid Evolutionary Algorithm by Q-Learning [Research Frontier][Research Frontier]. IEEE Computational Intelligence Magazine 2023, 18, 84–103. [CrossRef]
- Ziegler, J., et al., Toward robust autotuning of noisy quantum dot devices. Physical Review Applied 2022, 17, 024069. [CrossRef]
- Bakirtzis, G., M. Chiou, and A. Theodorou, Negotiating Control: Neurosymbolic Variable Autonomy. arXiv preprint arXiv:2407.16254, 2024.
- Da Poian, V., et al. Science Autonomy and Planetary Missions: ML and Data Science Applied to the ExoMars Mission. in 2023 IEEE Aerospace Conference. 2023. IEEE.
- Fard, N.E., R. R. Selmic, and K. Khorasani, Public policy challenges, regulations, oversight, technical, and ethical considerations for autonomous systems: a survey. IEEE Technology and Society Magazine 2023, 42, 45–53. [Google Scholar] [CrossRef]
- Hamad, M. and S. Steinhorst, Security Challenges in Autonomous Systems Design. arXiv preprint arXiv:2312.00018, 2023.
- Saisubramanian, S., E. Kamar, and S. Zilberstein, Avoiding negative side effects of autonomous systems in the open world. Journal of Artificial Intelligence Research 2022, 74, 143–177. [CrossRef]
- Samin, H., N. Bencomo, and P. Sawyer, Decision-making under uncertainty: be aware of your priorities. Software and Systems Modeling 2022, 21, 2213–2242. [CrossRef]
- Straube, S. , et al., The Challenge of Autonomy: What We Can Learn from Research on Robots Designed for Harsh Environments, in Robots in Care and Everyday Life: Future, Ethics, Social Acceptance. 2022, Springer International Publishing Cham. p. 57-80.
- Bruggeman, F.J., B. Teusink, and R. Steuer, Trade-offs between the instantaneous growth rate and long-term fitness: consequences for microbial physiology and predictive computational models. Bioessays 2023, 45, 2300015. [Google Scholar] [CrossRef] [PubMed]
- Kapasiawala, M. and R.M. Murray, Metabolic perturbations to an E. coli-based cell-free system reveal a trade-off between transcription and translation. bioRxiv, 2024: p. 2023.03.22.533877.
- Pittman, J.M., A Measure for Level of Autonomy Based on Observable System Behavior. arXiv preprint arXiv:2407.14975, 2024.
- Sparks, E.E. and A. Rasmussen, Trade-offs in plant responses to the environment. 2023, Wiley Online Library. p. 2943-2945.
- Abdelkader, H., et al. Robustness Attributes to Safeguard Machine Learning Models in Production. in 2023 IEEE Engineering Informatics. 2023. IEEE.
- Pang, R.Y. and K. Reinecke, Anticipating Unintended Consequences of Technology Using Insights from Creativity Support Tools. arXiv preprint arXiv:2304.05687, 2023.
- Shen, J., Z. Han, and W. Wang. Robustness of Modified Control Barrier Functions subject to External Disturbances. in 2023 IEEE 18th Conference on Industrial Electronics and Applications (ICIEA). 2023. IEEE.
- Praveen Kumar, S.B. , Robustness Testing for AI/ML Models: Strategies for Identifying and Mitigating Vulnerabilities. International Journal of Science and Research (IJSR) 2024, 13, 923–930. [Google Scholar] [CrossRef]
- Clay-Williams, R. , Complex systems and unintended consequences, in Implementation Science. 2022: Routledge.
- Ferreira, R.S. Towards safety monitoring of ML-based perception tasks of autonomous systems. in 2020 IEEE International Symposium on Software Reliability Engineering Workshops (ISSREW). 2020. IEEE.
- Uddin, J., M. Ahad, and A.H. Kafi. Wireless event-based kill-switch for safe and autonomous UAS operation. in 2023 International Conference on Electronics, Information, and Communication (ICEIC). 2023. IEEE.
- Weiss, M. and P. Tonella. Fail-safe execution of deep learning based systems through uncertainty monitoring. in 2021 14th IEEE conference on software testing, verification and validation (ICST). 2021. IEEE.
- Dinesh Reddy Chittibala, S.R.J. , Security in Machine Learning (ML) Workflows. International Journal of Computing and Engineering 2024, 5, 52–63. [Google Scholar] [CrossRef]
- Causevic, A., A.V. Papadopoulos, and M. Sirjani. Towards a framework for safe and secure adaptive collaborative systems. in 2019 IEEE 43rd Annual Computer Software and Applications Conference (COMPSAC). 2019. IEEE.
- Chaudhary, S. On Adaptivity and Safety in Sequential Decision Making. in IJCAI. 2023.
- Cho, M. and C. Sun. Constrained Meta-Reinforcement Learning for Adaptable Safety Guarantee with Differentiable Convex Programming. in Proceedings of the AAAI Conference on Artificial Intelligence. 2024.
- Dubslaff, C., et al., Breaking the limits of redundancy systems analysis. arXiv preprint arXiv:1912.05364, 2019.
- Henkel, E., et al. Scalable Redundancy Detection for Real-Time Requirements. in 2024 IEEE 32nd International Requirements Engineering Conference (RE). 2024. IEEE.
- Kajmakovic, A., et al. Predictive fail-safe improving the safety of industrial environments through model-based analytics on hidden data sources. in 2018 IEEE 13th International Symposium on Industrial Embedded Systems (SIES). 2018. IEEE.
- Ozer, E. , Safety-based prediction apparatus, system and method. 2022, Google Patents.
- Zhu, Q., et al. Safety-assured design and adaptation of learning-enabled autonomous systems. in Proceedings of the 26th Asia and South Pacific Design Automation Conference. 2021.
- Abdelkhalak El Hami, M.E. , Modeling of Systems with Redundancy, in Reliability-based Modeling of System Performance. 2023: Wiley Online Library.
- Federico, C.A. and A.A. Trotsyuk, Biomedical Data Science, Artificial Intelligence, and Ethics: Navigating Challenges in the Face of Explosive Growth. Annual Review of Biomedical Data Science, 2024. 7.
- Johnson, S.G., G. Simon, and C. Aliferis, Regulatory Aspects and Ethical Legal Societal Implications (ELSI). Artificial Intelligence and Machine Learning in Health Care and Medical Sciences: Best Practices and Pitfalls, 2024: p. 659-692.
- Mestre, R., et al., Ethics and responsibility in biohybrid robotics research. Proceedings of the National Academy of Sciences 2024, 121, e2310458121.
- Ou, Y. and S. Guo, Safety risks and ethical governance of biomedical applications of synthetic biology. Frontiers in Bioengineering and Biotechnology 2023, 11, 1292029.
- Sharma, A.K. and R. Sharma, Navigating the Ethical Landscape: Implementing Machine Learning in Smart Healthcare Informatics. Indian Journal of Community Health 2024, 36, 149–152. [Google Scholar] [CrossRef]
- Undheim, T.A. , The whack-a-mole governance challenge for AI-enabled synthetic biology: literature review and emerging frameworks. Frontiers in Bioengineering and Biotechnology 2024, 12, 1359768. [Google Scholar] [CrossRef] [PubMed]
- Kochetova, J.Y. , Ethical risks of artificial intelligence and prospects for joint decision-making in medicine. Čelovek 2024, 35, 96–106. [Google Scholar] [CrossRef]
- Love, C.S. , “Just the Facts Ma’am”: Moral and Ethical Considerations for Artificial Intelligence in Medicine and its Potential to Impact Patient Autonomy and Hope. The Linacre Quarterly 2023, 90, 375–394. [Google Scholar] [CrossRef] [PubMed]
- Saenz, A.D. , et al., Autonomous AI systems in the face of liability, regulations and costs. NPJ digital medicine 2023, 6, 185. [Google Scholar] [CrossRef]
- Savulescu, J., et al., Ethics of artificial intelligence in medicine. Singapore Medical Journal 2024, 65, 150–158. [CrossRef] [PubMed]
- Smith, D.H., et al. Ethics of Trust/worthiness in Autonomous Systems: a scoping review. in Proceedings of the First International Symposium on Trustworthy Autonomous Systems. 2023.
- Uygun İlikhan, S., et al., How to mitigate the risks of deployment of artificial intelligence in medicine? Turkish Journal of Medical Sciences 2024, 54, 483–492. [CrossRef]
- Youssef, A., M. Abramoff, and D. Char, Is the algorithm good in a bad world, or has it learned to be bad? The ethical challenges of “locked” versus “continuously learning” and “autonomous” versus “assistive” ai tools in healthcare. The American Journal of Bioethics 2023, 23, 43–45. [Google Scholar] [CrossRef]
- Ansarullah, S.I. , et al., Ethical issues around artificial intelligence, in A Biologist s Guide to Artificial Intelligence. 2024, Elsevier. p. 301-314.
- Grabb, D., M. Lamparth, and N. Vasan, Risks from Language Models for Automated Mental Healthcare: Ethics and Structure for Implementation. medRxiv, 2024: p. 2024.04. 07.24305462.
- Kläes, M., et al. Handling uncertainties of data-driven models in compliance with safety constraints for autonomous behaviour. in 2021 17th European Dependable Computing Conference (EDCC). 2021. IEEE.
- Lupo, G., B. Q. Vo, and N. Locke, Continuous automation approach for autonomous ethics-based audit of AI systems, in Trolley Crash. 2024, Elsevier. p. 141-162.
- Prunkl, C. , Human Autonomy at Risk? An Analysis of the Challenges from AI. Minds and Machines 2024, 34, 26. [Google Scholar] [CrossRef]
- Ong, J.C.L. , et al., Medical Ethics of Large Language Models in Medicine. NEJM AI, 2024: p. AIra2400038.
- Sambare, G. , et al., Autonomous Healthcare Systems: Deep Learning-Based IoT Solutions for Continuous Monitoring and Adaptive Treatment. Journal of Electrical Systems, 2024. 20(1s): p. 393-407.
- Sparrow, R. , et al., Should the use of adaptive machine learning systems in medicine be classified as research? The American Journal of Bioethics, 2024: p. 1-12.
- Bakar, N.F.A. , et al., Environmental impact of quantum dots, in Graphene, Nanotubes and Quantum Dots-Based Nanotechnology. 2022, Elsevier. p. 837-867.
- Dolezel, M. , et al., Challenges for the Post-Market Environmental Monitoring in the European Union Imposed by Novel Applications of Genetically Modified and Genome-Edited Organisms. BioTech 2024, 13, 14. [Google Scholar] [CrossRef]
- James, C.A. , et al., The screening and prioritization of contaminants of emerging concern in the marine environment based on multiple biological response measures. Science of The Total Environment 2023, 886, 163712. [Google Scholar] [CrossRef]
- Koren, K. and C.M. McGraw, Let’s Talk about Slime; or Why Biofouling Needs More Attention in Sensor Science. ACS sensors 2023, 8, 2432–2439. [Google Scholar] [CrossRef] [PubMed]
- Lensch, A. , et al., Safety aspects of microorganisms deliberately released into the environment. EFB Bioeconomy Journal, 2023: p. 100061.
- Tran, T.-K. , et al., Review on fate, transport, toxicity and health risk of nanoparticles in natural ecosystems: Emerging challenges in the modern age and solutions toward a sustainable environment. Science of The Total Environment, 2023: p. 169331.
- Zeng, X., et al., Regulation and management of the biosecurity for synthetic biology. Synthetic and Systems Biotechnology 2022, 7, 784–790. [CrossRef] [PubMed]
- Godwin, S. , et al., Environmental Health and Safety Offers a Biosafety Risk Assessment for a Theoretical Model of a Gene Therapy Process Transfer from Research and Development to Large-Scale Manufacturing. Applied Biosafety 2023, 28, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Niegowska, M. and M. Wögerbauer, Improving the risk assessment of antimicrobial resistance (AMR) along the food/feed chain and from environmental reservoirs using qMRA and probabilistic modelling. EFSA Journal 2022, 20, e200407. [Google Scholar] [CrossRef]
- Saarimäki, L.A., G. Del Giudice, and D. Greco, Expanding adverse outcome pathways towards one health models for nanosafety. Frontiers in Toxicology 2023, 5, 1176745. [CrossRef]
- Chen, J., V. Storchan, and E. Kurshan, Beyond fairness metrics: Roadblocks and challenges for ethical ai in practice. arXiv preprint arXiv:2108.06217, 2021.
- Di Cara, N. , et al., Using Data Hazards to support safe and ethical digital footprint research. International Journal of Population Data Science, 2023. 8(3).
- Janarthanan, V. , et al., Legal and Ethical Issues Associated With Challenges in the Implementation of the Electronic Medical Record System and Its Current Laws in India. Cureus, 2024. 16(3).
- Mandeep Singh, S.B. , Kanhaiya Sharma, Ethical and Privacy Considerations in Medical Big Data: Balancing Innovation and Safeguarding Patient. International Journal of Research Publication and Reviews, 2024. 5(5).
- Suresh, N. , et al., Ethical Considerations in AI Implementation for Patient Data Security and Privacy, in AI Healthcare Applications and Security, Ethical, and Legal Considerations. 2024, IGI Global. p. 139-147.
- Al-kfairy, M., et al. Ethical Challenges and Solutions of Generative AI: An Interdisciplinary Perspective. in Informatics. 2024. MDPI.
- Shivagouda M Patil, P.T., Mr. Hrishikesh Mogare, Regulatory Frameworks for Ethical AI Development in Coding. International Journal for Research in AppliedScience & Engineering Technology (IJRASET 2024, 12, 2018–2024. [Google Scholar]
- Baena-Miret, S., F. Reverter, and E. Vegas, A framework for block-wise missing data in multi-omics. Plos one 2024, 19, e0307482.
- Ivanisevic, T. and R.N. Sewduth, Multi-omics integration for the design of novel therapies and the identification of novel biomarkers. Proteomes 2023, 11, 34. [Google Scholar] [CrossRef]
- Lac, L., C. K. Leung, and P. Hu, Computational frameworks integrating deep learning and statistical models in mining multimodal omics data. Journal of Biomedical Informatics, 2024: p. 104629.
- Mohr, A.E. , et al., Navigating challenges and opportunities in multi-omics integration for personalized healthcare. Biomedicines 2024, 12, 1496. [Google Scholar] [CrossRef]
- Nam, Y. , et al., Harnessing Artificial Intelligence in Multimodal Omics Data Integration: Paving the Path for the Next Frontier in Precision Medicine. Annual Review of Biomedical Data Science, 2024. 7.
- Yang, G.-H. , et al., A hybrid forecasting framework based on MCS and machine learning for higher dimensional and unbalanced systems. Physica A: Statistical Mechanics and its Applications 2024, 637, 129612. [Google Scholar] [CrossRef]
- Zhou, Y. , et al., Utilizing AI-Enhanced Multi-Omics Integration for Predictive Modeling of Disease Susceptibility in Functional Phenotypes. Journal of Theory and Practice of Engineering Science 2024, 4, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Chetty, A. and R. Blekhman, Multi-omic approaches for host-microbiome data integration. Gut Microbes 2024, 16, 2297860.
- Dang, T. , et al., An integrative framework of stochastic variational variable selection for joint analysis of multi-omics microbiome data. bioRxiv, 2023: p. 2023.08. 18.553796.
- Gonçalves, D.M., R. Henriques, and R.S. Costa, Predicting metabolic fluxes from omics data via machine learning: Moving from knowledge-driven towards data-driven approaches. Computational and Structural Biotechnology Journal 2023, 21, 4960–4973. [CrossRef] [PubMed]
- Can, H. , et al., Integration of Meta-Multi-Omics Data Using Probabilistic Graphs and External Knowledge. Cells 2023, 12, 1998. [Google Scholar] [CrossRef]
- Gonesh Chandra Saha, M.B. , Muhammad Jahanzeb Khan, Hasi Saha,Dinesh Kumar, Avinash, Integrative Analysis of Multi-Omics Data with Deep Learning: Challenges and Opportunities in Bioinformatics. Tuijin Jishu/Journal of Propulsion Technology, 2023. 44(3).
- Avesani, S., et al. MODIMO: Workshop on Multi-Omics Data Integration for Modelling Biological Systems. in Proceedings of the 32nd ACM International Conference on Information and Knowledge Management. 2023.
- Muller, E., I. Shiryan, and E. Borenstein, Multi-omic integration of microbiome data for identifying disease-associated modules. Nature Communications 2024, 15, 2621.
- Jindal, M. , Advancements in Cloud-Based Machine Learning: Navigating Deployment and Scalability. International Journal For Multidisciplinary Research, 2023. 5(6).
- Padmanaban, H. , Machine Learning Algorithms Scaling on Large-Scale Data Infrastructure. Journal of Artificial Intelligence General science (JAIGS) ISSN: 3006-4023 2024, 3, 171–196. [Google Scholar]
- Tarzi, C. , et al., Emerging methods for genome-scale metabolic modeling of microbial communities. Trends in Endocrinology & Metabolism, 2024.
- Zimelewicz, E., et al. ML-Enabled Systems Model Deployment and Monitoring: Status Quo and Problems. in International Conference on Software Quality. 2024. Springer.
- Chen, M. and J. Xia, Optimization parameters for efficient scale-up of fermentation process, in Scale-up and Chemical Process for Microbial Production of Plant-Derived Bioactive Compounds. 2024, Elsevier. p. 29-42.
- Xu, B. , et al., Quorum Quenching in Membrane Bioreactors for Fouling Retardation: Complexity Provides Opportunities. Environmental Science & Technology 2024, 58, 13171–13193. [Google Scholar]
- Xu, J. , et al., Service Function Chain Deployment Using Deep Q Learning and Tidal Mechanism. IEEE Internet of Things Journal, 2023.
- Li, J. and Q. Yu, Scientists’ disciplinary characteristics and collaboration behaviour under the convergence paradigm: A multilevel network perspective. Journal of Informetrics 2024, 18, 101491. [Google Scholar] [CrossRef]
- Harle, S.M. , Integrative Approaches to Tackle Multidisciplinary Challenges: A Review of Multi-science Problem Analysis. Current materials science (Bentham Science), 2024. 17.
- Boon, M. , Interdisciplinarity through modelling, in The Routledge Handbook of Philosophy of Scientific Modeling. 2024: Routledge. p. 395-411.
- Busquim, G. , et al., Towards Effective Collaboration between Software Engineers and Data Scientists developing Machine Learning-Enabled Systems. arXiv preprint arXiv:, arXiv:2407.15821, 2024.
- Pastor, Ó., et al. Towards an AI-based Genomic Medicine of Precision that Integrates Predictive and Explainable Knowledge Dimensions. in Congresso Ibero-Americano em Engenharia de Software (CIbSE). 2024. SBC.
- Turanli, B. , et al., Genome-scale metabolic models in translational medicine: the current status and potential of machine learning in improving the effectiveness of the models. Molecular Omics 2024, 20, 234–247. [Google Scholar] [CrossRef]
- Wu, Y. and L. Xie, AI-driven multi-omics integration for multi-scale predictive modeling of causal genotype-environment-phenotype relationships. arXiv preprint arXiv:2407.06405, 2024.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





