Introduction
Lithium was discovered over two hundred years ago in 1817, But it was not until the seminal work of the Australian physician, John Cade, some 7
5 years ago, that lithium was recognized by modern psychiatry as an effective treatment for manic-depressive illness (MDI) [
1]. Initially, Cade’s research did not focus on lithium itself; rather, he utilized it to enhance the water solubility of uric acid. His investigation aimed to assess the toxicity of urea obtained from MDI patients compared to healthy subjects when injected into guinea pigs (i.p.). Soon, he noticed that animals injected with lithium carbonate exhibited increased calmness and decreased responsiveness for a couple of hours, between 3 to 5 hours post-injection. Consequently, he decided to try lithium salts in the treatment of two disorders, mania and epilepsy, and by 1948 he reported 10 successful cases of lithium treatment for MDI patients.
For many years since, lithium’s mechanism of action remained poorly understood despite extensive research, which focused first on its effects on electrolytes in serum,
CSF, brain and other tissues, in these studies a consistent finding continuously emerged: chronic lithium administration resulted in elevated serum magnesium levels [
2]. Subsequent developments in molecular biology tools allowed the study of lithium’s effects on second messenger pathways and neurotransmitters, one of which was the inositol second messenger signaling pathway. Lithium was found to deplete inositol levels by inhibiting inositol monophosphatase (IMPase) [
3], an enzyme that require magnesium for the reaction, Notably, some of lithium’s effects observed in these studies could be reversed by magnesium supplementation. In the last two decades, attention has shifted towards understanding lithium’s effects on gene expression [
4], particularly in light of its recently reported neuroprotective activity. These studies were grounded in the premise that lithium’s effectivity necessitated long-term (chronic) treatment, reducing the likelihood of significant contributions from short-term changes to its therapeutic mechanism.
From initial ignorance of lithium’s mechanism of action to our current understanding, significant progress has been made. However, despite ongoing discoveries of new targets for lithium action, the key mechanisms underlying its therapeutic effects remain elusive.
Subject Matter
Over the past two decades, many reported effects of lithium have been associated with its capacity to primarily enhance cell survival, particularly in neurons, both in vivo and in cell culture [
5]. The remarkable preference for promoting cell survival in neurons is astonishing. While lithium is not a selective drug and is far from being so, these two predominant characteristics shared by the majority of its reported effects are hard to ignore: it consistently induces pathways promoting cell survival
and demonstrates a preference for exerting its effects on neurons, that lately, there are growing call from several researchers for using lithium as a treatment or preventive medication for neurological disorders [
6] . It’s true that the majority of studies have focused on evaluating lithium’s protective effects in neurons. However, this emphasis on brain tissue isn’t merely coincidental; rather, it suggests that lithium’s efficacy is particularly pronounced in the brain.
Battle of the Ions: Lithium vs. Magnesium
One of the key mechanisms through which lithium exerts its effects is by competing with magnesium for protein binding sites [
7,
8,
9]. Given its comparable ionic radius (Li:Mg 0.76Å vs. 0.72Å) but distinct electric charge (electronegativity, Li:Mg 0.98:1.31 Pauling scale), lithium competes effectively with magnesium for binding sites, leading to enzymatic reactions disturbance. But to accomplish this, lithium needs first to reach cellular concentrations that would enable it to efficiently compete with magnesium, the extent of these concentrations is dependent on local free magnesium levels.
Magnesium chemical characteristics favor its binding to many species, such as proteins, phospholipids or nucleic acids. Among all intracellular species, ATP is the one that displays the highest affinity for magnesium; therefore it is considered the major intracellular chelator or ‘buffer’ of intracellular magnesium . Consequently, free cytosolic [Mg
2+] Measured in the human brain is about half of that assessed in the human skeletal muscle, this is likely related to the continuous higher ATP concentration in brain tissue. On the other hand, Lithium concentrations are found to be equivalent in skeletal muscle and brain, generally at less than half the serum concentrations [
10]. These variations, serving as the first factor we present here elucidating why lithium’s preferentially target cells in the central nervous system (CNS) where lower non-toxic lithium concentrations are still sufficient to compete with magnesium over binding sites in the CNS, unlike in other cell types where intracellular magnesium levels are higher due to the lower ATP content.
GSK3β and Lithium: A Brainy Bromance
Glycogen synthase kinase 3 beta (GSK3β) is a crucial enzyme involved in regulating various cellular processes, including cell proliferation, differentiation, apoptosis, and metabolism. Its importance lies in its central role within signaling pathways that control these fundamental cellular functions. Lithium inhibits this enzyme both directly[
11] by competing with magnesium and indirectly by affecting post-translational modifications [
12].
GSK3β regulates the delicate balance between cell survival and apoptosis by phosphorylating key substrates involved in apoptotic pathways. Inhibition of GSK3β generally leads to increased cell survival, while its activation tends to promote apoptosis. Interestingly, recent insights suggest that GSK3β may exhibit a more nuanced response, promoting cell death triggered by internal factors and resisting apoptosis induced by external signals. Dysregulation of GSK3β has been implicated in various neurological disorders, including Alzheimer’s disease, where it contributes to the formation of neurofibrillary tangles, a hallmark pathology of the disease [
13], bipolar disorder [
14], Parkinson’s disease [
15] and schizophrenia [
16]. Additionally, GSK3β plays a significant role in cancer development and progression by influencing cell proliferation, migration, and invasion, making it a potential target for cancer therapy due to its involvement in oncogenic signaling pathways [
17].
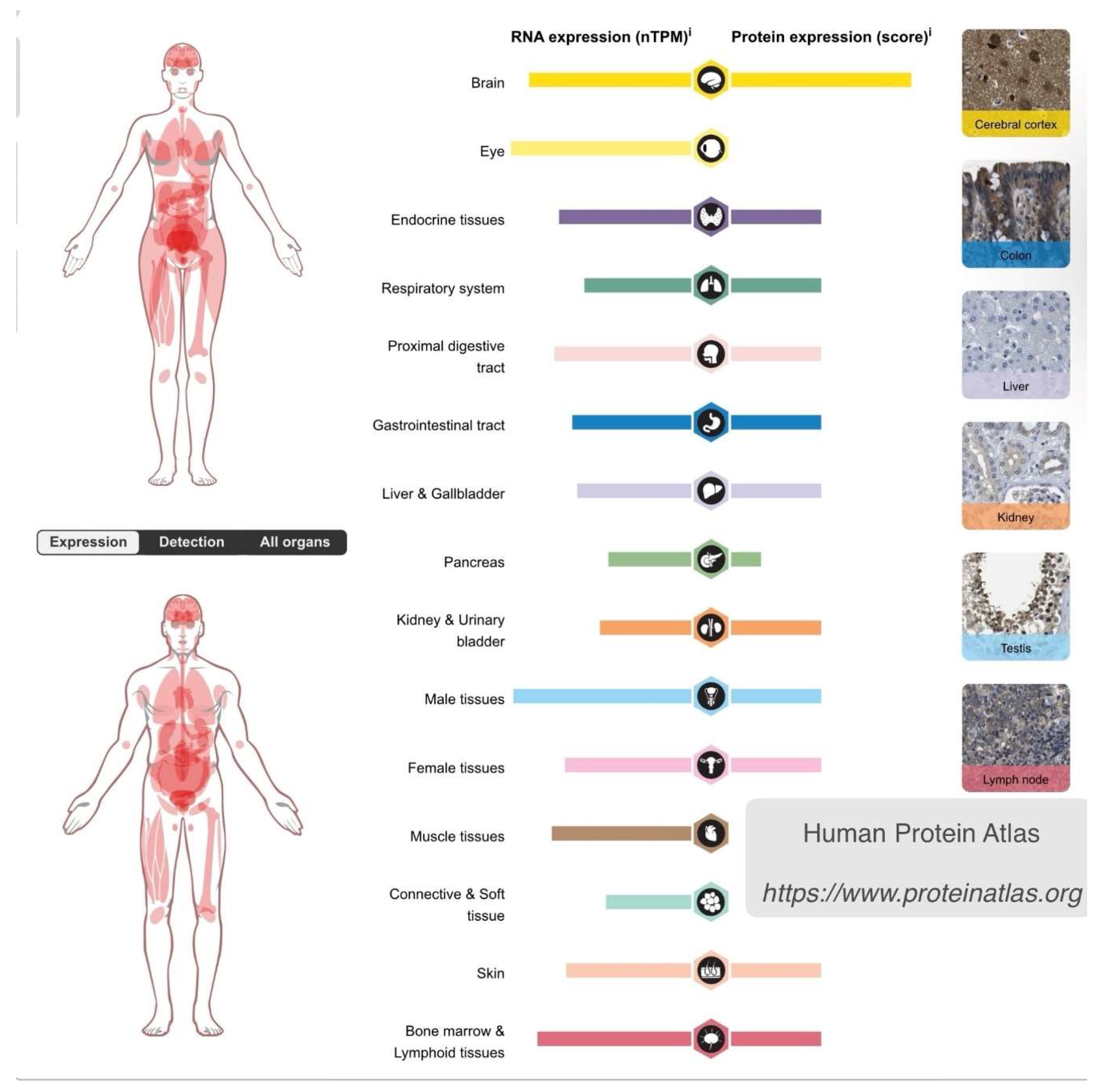
The importance of this kinase in regulating cell fate cannot be overstated, as it occupies a central position in major pathways that influence numerous cellular processes across all body tissues. However, the expression of GSK3β varies among different body organs. In the brain, both GSK3β RNA and protein levels are higher compared to all other body organs [
18] (
Figure 1). This differential expression serves as the second factor explaining why lithium’s effects preferentially impact cells in the central nervous system (CNS) and why it favors cell survival pathways over others. In this regard, lithium is already utilized in medicine to stimulate cell proliferation, particularly in bone marrow, a tissue abundant in GSK3β. It is administered to mitigate the drop in circulating blood cells during chemotherapy [
19].
This underscores why any agent that modulates this enzyme, such as lithium, would exert a greater impact on tissues rich in GSK3β like the brain, further elucidating lithium’s preference for affecting brain cells. It’s crucial to note also, unlike most other kinases, GSK3β remains continuously active and is primarily regulated through inhibition, ensuring a profound influence on its activity by its modulators.
In this paper, we propose that GSK3β inhibition in the brain is likely to be more robust than in other tissues due to the lower levels of intracellular free magnesium, as well as the higher expression of GSK3β. As a result, it’s anticipated that the effects of lithium, mediated by GSK3β inhibition, will be significantly amplified in the brain compared to peripheral tissues. This perspective may provide insights into the conflicting observations regarding GSK3β inhibition in peripheral tissues [
20].
Conclusion
In conclusion, lithium’s mechanism of action primarily involves competing with magnesium for protein binding sites. Given the lower levels of free magnesium in the brain and neurons compared to other tissues, lithium exerts a more pronounced effect on brain cells. Additionally, the key enzyme affected by lithium, GSK3β, is highly expressed in the brain. When inactivated, GSK3β promotes cell survival. Importantly, GSK3β activity is dependent on magnesium, which levels are lower in the brain as discussed above, further enhancing lithium’s impact on brain cells. This, coupled with its significant presence in brain tissue, explains why lithium predominantly promotes cell survival in neurons.
Conflict of Interest
The author have no conflicts of interest to declare and there is no financial interest to report.
References
- Cade, J. F. Lithium salts in the treatment of psychotic excitement. 1949. Bull World Health Organ 78, 349–52 (1949).
- Coppen, A. & Shaw, D. M. The distribution of electrolytes and water in patients after taking lithium carbonate. Lancet 2, 805–6 (1967). [CrossRef]
- Belmaker, R. H. et al. Role of inositol 1-phosphatase inhibition in the mechanism of action of lithium. Pharmacological Toxicology 66, 76–83 (1990). [CrossRef]
- Farah, R., Khamisy-Farah, R., Amit, T., Youdim, M. B. H. & Arraf, Z. Lithium’s gene expression profile, relevance to neuroprotection A cDNA microarray study. Cell Mol Neurobiol 33, 411–420 (2013).
- Chuang, D. M. et al. Neuroprotective effects of lithium in cultured cells and animal models of diseases. Bipolar Disord 4, 129–136 (2002).
- Chiu, C. T., Wang, Z., Hunsberger, J. G. & Chuang, D. M. Therapeutic potential of mood stabilizers lithium and valproic acid: Beyond bipolar disorder. Pharmacological Reviews vol. 65 105–142. Preprint at https://doi.org/10.1124/pr.111.005512 (2013). [CrossRef]
- Dudev, T. & Lim, C. Competition between Li+ and Mg2+ in metalloproteins. Implications for lithium therapy. J Am Chem Soc 133, 9506–9515 (2011).
- Layden, B., Diven, C., Minadeo, N., Bryant, F. B. & Mota de Freitas, D. Li+/Mg2+ competition at therapeutic intracellular Li+ levels in human neuroblastoma SH-SY5Y cells. Bipolar Disord 2, 200–4 (2000).
- De Freitas, D. M., Castro, M. M. C. A. & Geraldes, C. F. G. C. Is competition between Li + and Mg 2+ the underlying theme in the proposed mechanisms for the pharmacological action of lithium salts in bipolar disorder? Acc Chem Res 39, 283–291 (2006).
- El Balkhi, S., Megarbane, B., Poupon, J., Baud, F. J. & Galliot-Guilley, M. Lithium poisoning: Is determination of the red blood cell lithium concentration useful? Clin Toxicol 47, 8–13 (2009).
- Ryves, W. J. & Harwood, A. J. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun 280, 720–725 (2001).
- Jope, R. S. Lithium and GSK-3: One inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol Sci 24, 441–443 (2003).
- Hanger, D. P., Hughes, K., Woodgett, J. R., Brion, J. P. & Anderton, B. H. Glycogen synthase kinase-3 induces Alzheimer’s disease-like phosphorylation of tau: Generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci Lett 147, 58–62 (1992).
- Valvezan, A. J. & Klein, P. S. GSK-3 and Wnt signaling in neurogenesis and bipolar disorder. Frontiers in Molecular Neuroscience Preprint at https://doi.org/10.3389/fnmol.2012.00001 (2012). [CrossRef]
- Duka, T., Duka, V., Joyce, J. N. & Sidhu, A. α-Synuclein contributes to GSK-3β-catalyzed Tau phosphorylation in Parkinson’s disease models. The FASEB Journal 23, 2820–2830 (2009).
- Lovestone, S., Killick, R., Di Forti, M. & Murray, R. Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci 30, 142–149 (2007).
- Mccubrey, J. A. et al. GSK-3 as Potential Target for Therapeutic Intervention in Cancer. Oncotarget vol. 5 www.impactjournals.com/oncotarget/.
- Uhlén, M. et al. Tissue-based map of the human proteome. Science (1979) 347, (2015).
- Zhu, Z. et al. Lithium stimulates human bone marrow derived mesenchymal stem cell proliferation through GSK-3β-dependent β-catenin/Wnt pathway activation. FEBS Journal 281, 5371–5389 (2014).
- Azab, A. N., Vainer, E., Agam, G. & Bersudsky, Y. Lymphocyte phospho-ser-9-GSK-3β/total GSK-3β protein levels ratio is not affected by chronic lithium or valproate treatment in euthymic patients with bipolar disorder. J Clin Psychopharmacol 37, 226–230 (2017).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).