Submitted:
20 October 2024
Posted:
21 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction

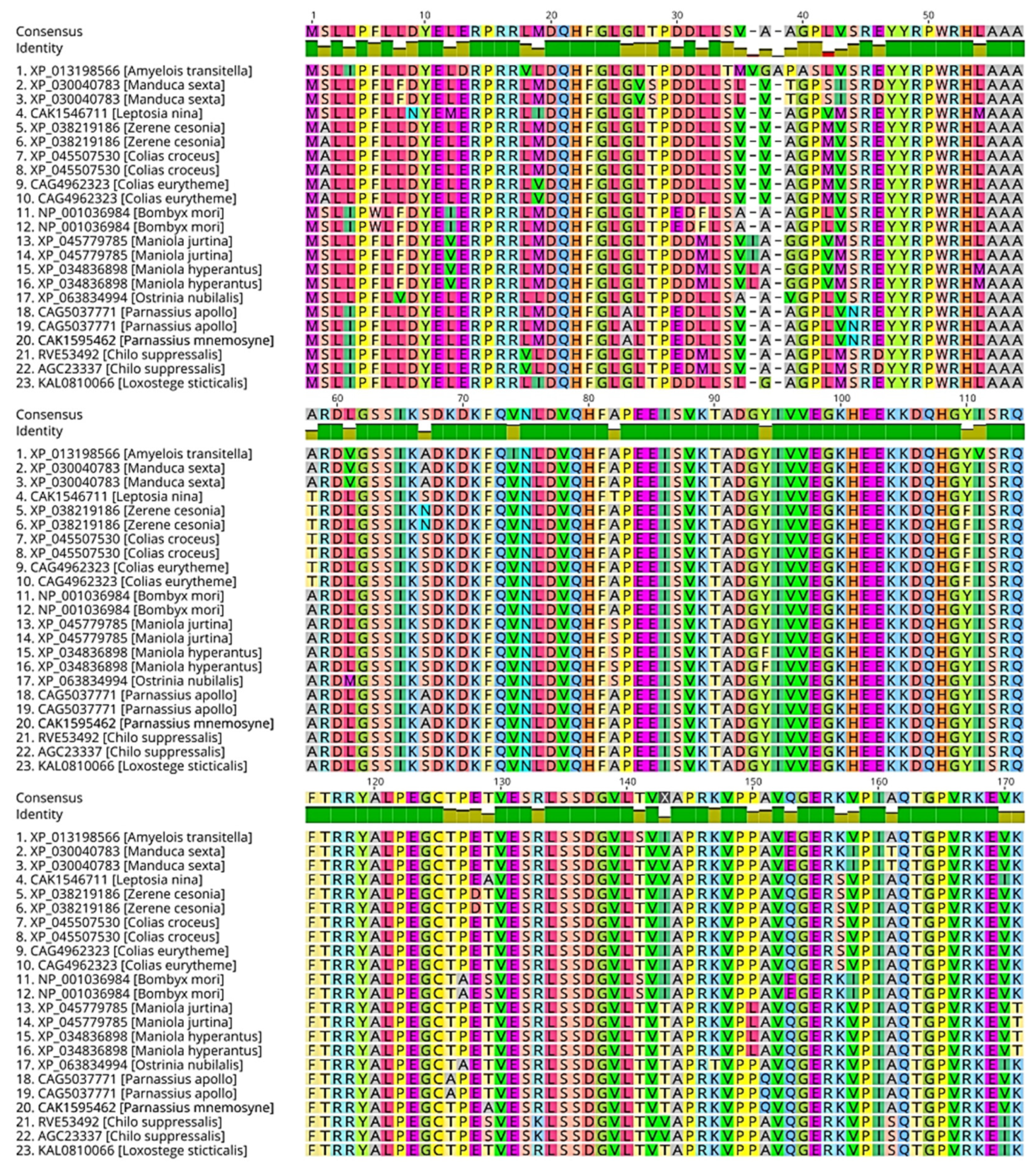
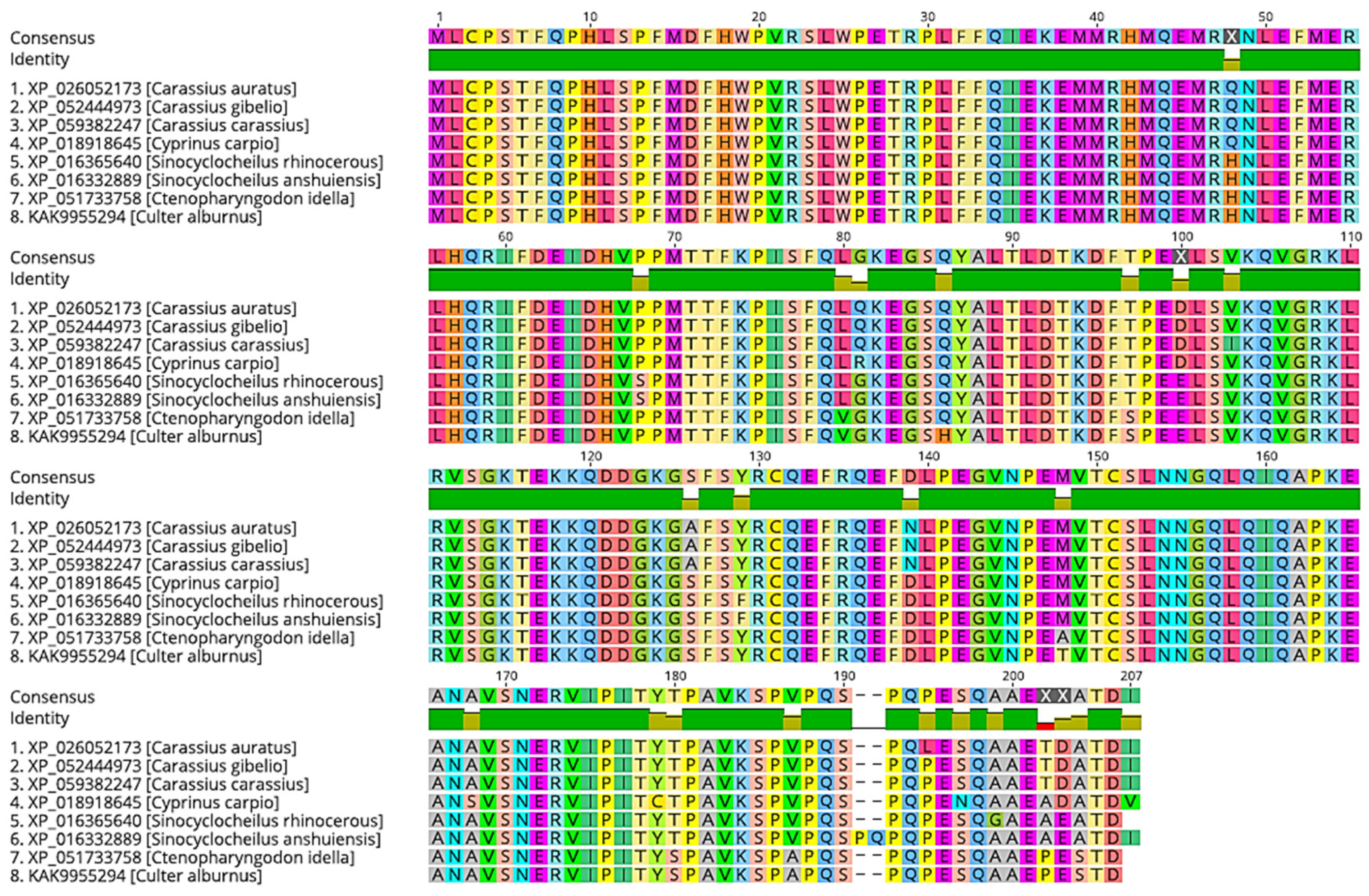
2. Small Heat Shock Proteins in Insects
2.1. Temperature Extremes Stressor
2.2. Desiccation Stressor
2.3. Pesticides and Heavy Metals Stressor
2.4. Hypoxia/Anoxia Stressor
2.5. Crowding and Starvation Stressors
2.6. Parasitism Stressor
2.7. UV Radiation Stressor
2.8. Energy Cost of sHSps Response
| Insect species | Small heat shock protein/gene | Expression pattern | Reference |
|---|---|---|---|
| TEMPERATURE stressor | |||
| Corn stalk borer (Sesamia nonagrioides larvae) | SnoHsp19.5 and SnoHsp20.8 | Both genes were upregulated (within 15 min) by heat shock at 40 °C and when larvae recovered after cold shock. |
Gkouvitsas et al. [21] |
| Leaf beetle (Gastrophysa atrocyanea) | sHsps 21 and sHsps 23 | RNAi knockdown of both genes decreased viability and lowered heat resistance. | Atungulu et al. [47] |
| Liriomyza sativae pupae |
ls-hsp19.5, ls-hsp20.8 and ls-hsp21.7 |
All significantly induced by cold treatment with ls-hsp20.8 displayed the greatest sensitivity. This suggests that different sHsps may be responsive to various stressor intensities. |
Huang et al. [98] |
| Chaperone proteins aid winter survival of freeze-tolerant gall fly larvae Eurosta solidaginis. | α- crystallins and β-crystallins | The sHSPs αB-crystallin increased in levels just prior to and during cold weather (i.e., in late fall and winter). Both αA and αB crystallin were highly induced in response to freeze/thaw conditions. |
Zhang et al. [44] |
| Silkworm (Bombyx mori) | shsp19.9, shsp20.1, shsp20.4, shsp20.8, shsp21.4, shsp23.7 and shsp21.4. | All genes were upregulated by heat stress except shsp21.4, which was downregulated. |
Sakano et al. [99] Li et al. [100] |
| Red flour beetle (Tribolium castaneum) | Tchsp18.3 | Gene was upregulated in response to heat stress but not to cold stress. | Xie et al. [81] |
| Drosophila melanogaster | Hsp22 and Hsp23 | The removal of the genes mRNA by RNAi interrupted recovery (time to recover and mobility following recovery) from chill injury thus showing that upregulation of the genes is required for recovery, but not during the cold stress itself. | Colinet et al. [101] |
| flesh fly (Sarcophaga cras-sipalpis) | Hsp23 | Deletion of genes’ mRNA reduced cold hardiness. | Rinehart et al. [102] |
| Western flower thrip (Frankliniella occidentalis) | FoHSP11.6 and FoHSP28.0 | Both generes were induced by both low and high temperature with maximum expression levels attained after 0.5 – 1 h of temperature stress exposure. Also, thermotolerance reduced when both genes were silenced by RNAi. | Yuan et al. [103] |
| Chilo suppressalis (Walker) | Cshsp19.0 | Gene was upregulated as a response to heat and cold stress exposure for 2 h. | Dong et al. [104] |
| Spodoptera frugiperda | SfsHsp21.3, SfsHsp20, SfsHsp20.1, SfsHsp19.3, and SfsHsp29. | All genes were significantly upregulated at both temperature extremes (42°C and 4°C) with the exception of two genes (SfsHsp20.1 and SfsHsp19.3) in the adult males that did not respond to the 4°C treatment. | Yang et al. [89] |
| PESTICIDE and heavy metal stressor | |||
| Apis cerana cerana | AccsHSP21.7 | A knockdown of the sHSP gene decreased the insect’s resistance to a commercial herbicide glyphosate, resulting in significant mortality. |
Huang et al. [65] |
| Fall armyworm (FAW) (Spodoptera frugiperda) | sHsp19.07, sHsp20.7 and sHsp19.74. | All genes were upregulated following exposure to the Chlorantraniliprole pesticide. Though sHsp19.74 reached maximum mRNA expression levels faster (8 h after exposure) than the rest (12h), its levels plummeted at 12 h after exposure, suggesting a momentary responsiveness of sHSPS to pesticide treatment. |
Samanta et al. [105] |
| Diamondback moth (Plutella xylostella L) | Fourteen sHSPs (sHSP27.5, sHSP28.9, sHSP21.6, sHSP18.8, sHSP19.22, sHSP21.8, sHSP21.9, sHSP22.1, sHSP23.4, sHSP19.5, sHSP20.06, sHSP20.09, sHSP19.23, sHSP20.1) | Fourth instar larvae were exposed to various pesticides and heavy metals for 24 hr. sHSPs responses were as below. Beta-cypermethrin pesticide significantly upregulated all except sHSP20.09, whereas chlorfenapyr pesticide downregulated all except sHSP28.9. Expression responses to Indoxacarb and Cantharidin were irregular. Exposure to H2O2 for 24 h downregulated five sHSPs (sHSP19.22, sHSP19.23, sHSP21.6, sHSP22.1, and sHSP23.4) Copper (Cu2+) downregulated three sHSPs (sHSP20.1 sHSP22.1, sHSP28.9) and upregulated seven sHSPs (sHSP19.22, sHSP19.23, sHSP20.06, sHSP20.09, sHSP21.8, sHSP21.9, sHSP27.5). Manganese (Mn2+) upregulated four sHSPs (sHSP20.1, sHSP21.6, sHSP22.1, sHSP28.9) and upregulated all the rest. Nickel (Ni2+) upregulated (sHSP19.22, sHSP19.5, sHSP20.06, sHSP20.09), not induced (sHSP20.1, sHSP21.8, sHSP21.9), and the rest were downregulated. Gene expression response to Lead (Pb2+) was irregular. |
Chen & Zhang [33] |
| Daphnia magna | eleven sHSP genes (termed DmsHSP1 - DmsHSP11) | Insect exposure to heavy metals (Cd2+, Cu2+, and Zn2+) upregulated DmsHSP1 and DmsHSP5. RNAi knockdown of genes DmsHSP1–21, except DmsHSP11–12.8, increased susceptibility to heavy metal stress exposure. |
Li et al. [56] |
| Acquatic larvae of Chironomus riparius. |
hsp17, hsp21, hsp22, hsp23, hsp24, hsp27, and hsp34) | Following acute exposure to Cadmium (Cd), hsp23, hsp24, hsp27, and hsp34 were upregulated, whereas levels of hsp17 and hsp21 remained unaltered. This indicates that sHSPs have diverse roles during response to Cd. | Martín-Folgar & Martínez-Guitarte [71] |
| Chinese rice grasshopper (Oxya chinensis) | OcGrp78, OcHsp70, OcHsp90, and OcHsp40 | Following exposure to Cadmium (Cd), mRNA expression levels of all genes increased, reaching a maximum within a short period (6 h), albeit decreasing significantly after 12 h. |
Zhang et al. [106] |
| HYPOXIA OR ANOXIA stressor | |||
| Flesh fly (Sarcophaga crassipalpis) | hsp25, hsp23, and hsp18 | hsp25, hsp23, and hsp18 were upregulated by at least 10-fold within two days of hypoxia (3 % oxygen) treatment application. Upregulation was maintained for the whole treatment period (10 days) and during recovery - 2 h post-treatment – after which expression levels declined. | Michaud et al. [75] |
| Gall fly larvae (Eurosta solidaginis) | αA and αB crystallin | Both sHSPs increased in response to anoxia (exposure period of 24 h under N2 gas at 15 °C) |
Zhang et al. [44] |
| CROWDING stressor | |||
| Migratory locusts (Locusta migratoria L) | Hsp20.5, Hsp20.6, and Hsp20.7 | mRNAs of all sHPS were more expressed in gregarious phases (representing high population density) compared to solitary phases (representing low population density) | Wang et al. [77] |
| fifth-instar nymphs of the Australian plague locust (Chortoicetes terminifera) | Hsp20.5 and Hsp20.7 | Crowding (during the gregarious phase) resulted in a 2 – 3-fold significant upregulation of both genes. | Chapuis et al. [107] |
| STARVATION stressor | |||
| Mulberry pyralid caterpillar (Glyphodes pyloalis) | GpHSP19.5, 20, 20.2, and 21.6 GpHSP21.8 and GpHSP21.4 |
Genes were upregulated time-dependently, reaching maximum levels on the sixth day of food deprivation. On the contrary, expression levels of two GpsHSPs (GpHSP21.8 and GpHSP21.4) demonstrated intermittent downregulation in comparison to the control at 2 or 4 days following the starvation period. | Chu et al. [80] |
| Fruitfly (Drosophila melanogaster) |
Hsp27 | sHPS was knocked out, and flies showed a significant decrease in resistance to starvation. | Hao et al. [82] |
| 4-day-old larvae of Housefly (Musca domestica) | MdomHSP27 MdomHSP10, MdomHSP27.1, and MdomHSP27.2 |
the expression of MdomHSP27 was significantly downregulated after a 6h starvation period, whereas the other 3 MdomHSPs (MdomHSP10, MdomHSP27.1 and MdomHSP27.2) were not significantly affected |
Tian et al. [108] |
| Red flour beetle (Tribolium castaneum) | Tchsp18.3 | When sHPS was knocked down, the lifespan of adult beetles was reduced by 15.8% (they died within 18 days after starvation) compared to the control group. |
Xie et al. [81] |
| Parasitoid wasp (Pteromalus puparum Linnaeus) | PpHSP20 | Gene expression increased significantly after 6 h of starvation but declined after 24 h |
Wang et al. [109] |
| Diamondback moth (Plutella xylostella L.) | sHSP20.1, sHSP21.6, sHSP22.1, and sHSP28.9, | Expression levels of all sHSPs were significantly downregulated following food starvation for 21 h. | Chen & Zhang [33] |
3. Small Heat Shock Proteins in Nematodes
3.1. Heat Stressor
3.2. Cold Stressor
3.3. Desiccation Stressor
3.4. Anhydrobiosis and Hypoxia Stressors
3.5. Chemical Stressor
4. Small Heat Shock Proteins in Aquatic Animals
4.1. Heavy Metal, Temperature, and Salinity Stress
4.3. Pathogenic Infection
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. Anthropogenic Temperature Fluctuations and Their Effect on Aquaculture: A Comprehensive Review. Aquac Fish 2022, 7, 223–243. [Google Scholar] [CrossRef]
- Viitasalo, M.; Bonsdorff, E. Global Climate Change and the Baltic Sea Ecosystem: Direct and Indirect Effects on Species, Communities and Ecosystem Functioning. Earth System Dynamics 2022, 13, 711–747. [Google Scholar] [CrossRef]
- Häder, D.-P.; Banaszak, A.T.; Villafañe, V.E.; Narvarte, M.A.; González, R.A.; Helbling, E.W. Anthropogenic Pollution of Aquatic Ecosystems: Emerging Problems with Global Implications. Science of The Total Environment 2020, 713, 136586. [Google Scholar] [CrossRef] [PubMed]
- Basha, E.; O’Neill, H.; Vierling, E. Small Heat Shock Proteins and α-Crystallins: Dynamic Proteins with Flexible Functions. Trends Biochem Sci 2012, 37, 106–117. [Google Scholar] [CrossRef]
- Walsh, B.S.; Parratt, S.R.; Mannion, N.L.M.; Snook, R.R.; Bretman, A.; Price, T.A.R. Plastic Responses of Survival and Fertility Following Heat Stress in Pupal and Adult Drosophila Virilis. Ecol Evol 2021, 11, 18238–18247. [Google Scholar] [CrossRef]
- Guillén, L.; Pascacio-Villafán, C.; Osorio-Paz, I.; Ortega-Casas, R.; Enciso-Ortíz, E.; Altúzar-Molina, A.; Velázquez, O.; Aluja, M. Coping with Global Warming: Adult Thermal Thresholds in Four Pestiferous Anastrepha Species Determined under Experimental Laboratory Conditions and Development/Survival Times of Immatures and Adults under Natural Field Conditions. Front Physiol 2022, 13. [Google Scholar] [CrossRef]
- Nooten, S.S.; Korten, H.; Schmitt, T.; Kárpáti, Z. The Heat Is on: Reduced Detection of Floral Scents after Heatwaves in Bumblebees. Proceedings of the Royal Society B: Biological Sciences 2024, 291, 20240352. [Google Scholar] [CrossRef]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in Fluctuating Thermal Environments. Annu Rev Entomol 2015, 60, 123–140. [Google Scholar] [CrossRef]
- King, A.M.; MacRae, T.H. Insect Heat Shock Proteins During Stress and Diapause. Annu Rev Entomol 2015, 60, 59–75. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Kristensen, T.N.; Loeschcke, V. The Evolutionary and Ecological Role of Heat Shock Proteins. Ecol Lett 2003, 6, 1025–1037. [Google Scholar] [CrossRef]
- González-Tokman, D.; Córdoba-Aguilar, A.; Dáttilo, W.; Lira-Noriega, A.; Sánchez-Guillén, R.A.; Villalobos, F. Insect Responses to Heat: Physiological Mechanisms, Evolution and Ecological Implications in a Warming World. Biological Reviews 2020, 95, 802–821. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.E.; Hofmann, G.E. Heat-Shock Proteins, Molecular Chaperones, and the Stress Response: Evolutionary and Ecological Physiology. Annu Rev Physiol 1999, 61, 243–282. [Google Scholar] [CrossRef] [PubMed]
- Gething, M.-J.; Sambrook, J. Protein Folding in the Cell. Nature 1992, 355, 33–45. [Google Scholar] [CrossRef]
- Colinet, H.; Lee, S.F.; Hoffmann, A. Temporal Expression of Heat Shock Genes during Cold Stress and Recovery from Chill Coma in Adult Drosophila Melanogaster. FEBS J 2010, 277, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.M.; Aquilina, J.A. Evidence for Specific Subunit Distribution and Interactions in the Quaternary Structure of A-crystallin. Proteins: Structure, Function, and Bioinformatics 2010, 78, 2546–2553. [Google Scholar] [CrossRef]
- Sun, W.; Montagu, M. Van; Verbruggen, N. Small Heat Shock Proteins and Stress Toler.Pdf. Plant Physiology and Biochemistry 2002, 1577, 1–9. [Google Scholar]
- Miernyk, J.A. Protein Folding in the Plant Cell. Plant Physiol 1999, 121, 695–703. [Google Scholar] [CrossRef]
- Sun, Y.; MacRae, T.H. Small Heat Shock Proteins: Molecular Structure and Chaperone Function. Cellular and Molecular Life Sciences 2005, 62, 2460–2476. [Google Scholar] [CrossRef]
- Eisenhardt, B.D. Small Heat Shock Proteins: Recent Developments. Biomol Concepts 2013, 4, 583–595. [Google Scholar] [CrossRef]
- Tsvetkova, N.M.; Horváth, I.; Török, Z.; Wolkers, W.F.; Balogi, Z.; Shigapova, N.; Crowe, L.M.; Tablin, F.; Vierling, E.; Crowe, J.H.; et al. Small Heat-Shock Proteins Regulate Membrane Lipid Polymorphism. Proc Natl Acad Sci U S A 2002, 99, 13504–13509. [Google Scholar] [CrossRef]
- Gkouvitsas, T.; Kontogiannatos, D.; Kourti, A. Differential Expression of Two Small Hsps during Diapause in the Corn Stalk Borer Sesamia Nonagrioides (Lef. ). J Insect Physiol 2008, 54, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Morrow, G.; Battistini, S.; Zhang, P.; Tanguay, R.M. Decreased Lifespan in the Absence of Expression of the Mitochondrial Small Heat Shock Protein Hsp22 in Drosophila. J Biol Chem 2004, 279, 43382–43385. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Bossier, P.; Wang, X.; Bojikova-Fournier, S.; MacRae, T.H. Diversity, Structure, and Expression of the Gene for P26, a Small Heat Shock Protein from Artemia. Genomics 2006, 88, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Willsie, J.K.; Clegg, J.S. Nuclear P26, a Small Heat Shock/α-Crystallin Protein, and Its Relationship to Stress Resistance in Artemia Franciscana Embryos. Journal of Experimental Biology 2001, 204, 2339–2350. [Google Scholar] [CrossRef]
- Willsie, J.K.; Clegg, J.S. Small Heat Shock Protein P26 Associates with Nuclear Lamins and HSP70 in Nuclei and Nuclear Matrix Fractions from Stressed Cells. J Cell Biochem 2002, 84, 601–614. [Google Scholar] [CrossRef]
- Hibshman, J.D.; Carra, S.; Goldstein, B. Tardigrade Small Heat Shock Proteins Can Limit Desiccation-Induced Protein Aggregation. Commun Biol 2023, 6, 121. [Google Scholar] [CrossRef]
- Villeneuve, T.S.; Ma, X.; Sun, Y.; Oulton, M.M.; Oliver, A.E.; MacRae, T.H. Inhibition of Apoptosis by P26: Implications for Small Heat Shock Protein Function during Artemia Development. Cell Stress Chaperones 2006, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol Biol Evol 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.-I.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform.
- Tissières, A.; Mitchell, H.K.; Tracy, U.M. Protein Synthesis in Salivary Glands of Drosophila Melanogaster: Relation to Chromosome Puffs. J Mol Biol 1974, 84, 389–398. [Google Scholar] [CrossRef]
- Russnak, R.H.; Peter, E.; Candido, M. Nucleic Acids Research Aoning and Analysis of CDNA Sequences Coding for Two 16 Kilodalton Heat Shock Proteins (Hsps) in Caenorhabditis Elegans: Homology with the Small Hsps of Drosophila; 1983; Vol. 1;
- Liang, P.; MacRae, T.H. The Synthesis of a Small Heat Shock/α-Crystallin Protein in Artemia and Its Relationship to Stress Tolerance during Development. Dev Biol 1999, 207, 445–456. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y. Identification of Multiple Small Heat-Shock Protein Genes in Plutella Xylostella (L.) and Their Expression Profiles in Response to Abiotic Stresses. Cell Stress Chaperones 2015, 20, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Flis, Ł.; Malewski, T.; Dobosz, R. Temperature Effects on Expression Levels of Hsp Genes in Eggs and Second-Stage Juveniles of Meloidogyne Hapla Chitwood, 1949. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, M.; Luo, X.; Zhang, Z.; Hu, X. Molecular Characterization of Heat Shock Protein 20 (Hsp20) in Goldfish (Carassius Auratus) and Expression Analysis in Response to Environmental Stresses. Aquac Rep 2022, 24, 101106. [Google Scholar] [CrossRef]
- Denlinger, D.L.; Rinehart, J.P.; Yocum, G.D. Stress Proteins. In Insect Timing: Circadian Rhythmicity to Seasonality; Elsevier, 2001; pp. 155–171 ISBN 978-0-444-50608-5.
- Wang, F.; Li, D.; Chen, Q.; Ma, L. Genome-Wide Survey and Characterization of the Small Heat Shock Protein Gene Family in Bursaphelenchus Xylophilus. Gene 2016, 579, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in Global Climate Change Research: Direct Effects of Rising Temperature on Insect Herbivores. Glob Chang Biol 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Abdullah, M. BEHAVIOURAL EFFECTS OF TEMPERATURE ON INSECTS.; 1961.
- Gilbert, N.; Raworth, D.A. FORUM: INSECTS AND TEMPERATURE—A GENERAL THEORY. Can Entomol 1996, 128, 1–13. [Google Scholar] [CrossRef]
- Li, M.; Wei, X.M.; Li, J.; Wei, S.M.; Zhang, J.L.; Chen, G.H.; Zhang, X.M. Effect of Short-Term Exposure to High Temperatures on the Reproductive Behavior and Physiological Enzyme Activities in the Fruit Fly Zeugodacus Tau (Walker). Front Physiol 2023, 14, 1036397. [Google Scholar] [CrossRef]
- Huang, L.-H.; Chen, B.; Kang, L. Impact of Mild Temperature Hardening on Thermotolerance, Fecundity, and Hsp Gene Expression in Liriomyza Huidobrensis. J Insect Physiol 2007, 53, 1199–1205. [Google Scholar] [CrossRef]
- Dahlgaard, J.; Loeschcke, V.; Michalak, P.; Justesen, J. Induced Thermotolerance and Associated Expression of the Heat-shock Protein Hsp70 in Adult Drosophila Melanogaster. Funct Ecol 1998, 12, 786–793. [Google Scholar] [CrossRef]
- Zhang, G.; Storey, J.M.; Storey, K.B. Chaperone Proteins and Winter Survival by a Freeze Tolerant Insect. J Insect Physiol 2011, 57, 1115–1122. [Google Scholar] [CrossRef]
- Chown, S.L.; Terblanche, J.S. Physiological Diversity in Insects: Ecological and Evolutionary Contexts. Adv In Insect Phys 2006, 33, 50–152. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.E. A Primer on Insect Cold-Tolerance. In Low Temperature Biology of Insects; Denlinger, D.L., Lee Richard, E., J., *!!! REPLACE !!!*, Eds.; Cambridge University Press: Cambridge, 2010; ISBN 9780521886352. [Google Scholar]
- Atungulu, E.; Tanaka, H.; Fujita, K.; Yamamoto, K.; Sakata, M.; Sato, E.; Hara, M.; Yamashita, T.; Suzuki, K. A Double Chaperone Function of the SHsp Genes against Heat-Based Environmental Adversity in the Soil-Dwelling Leaf Beetles. J Insect Biotechnol Sericology 2006, 75, 15–22. [Google Scholar]
- Hadley, N.F. Water Relations of Terrestrial Arthropods.; 1994.
- Hansen, J.M.; Go, Y.-M.; Jones, D.P. Nuclear and Mitochondrial Compartmentation of Oxidative Stress and Redox Signaling. Annu Rev Pharmacol Toxicol 2006, 46, 215–234. [Google Scholar] [CrossRef]
- Lopez-Martinez, G.; Elnitsky, M.A.; Benoit, J.B.; Lee, R.E.; Denlinger, D.L. High Resistance to Oxidative Damage in the Antarctic Midge Belgica Antarctica, and Developmentally Linked Expression of Genes Encoding Superoxide Dismutase, Catalase and Heat Shock Proteins. Insect Biochem Mol Biol 2008, 38, 796–804. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide Decline of the Entomofauna: A Review of Its Drivers. Biol Conserv 2019, 232, 8–27. [Google Scholar] [CrossRef]
- van Klink, R.; Bowler, D.E.; Gongalsky, K.B.; Swengel, A.B.; Gentile, A.; Chase, J.M. Meta-Analysis Reveals Declines in Terrestrial but Increases in Freshwater Insect Abundances. Science 2020, 368, 417–420. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S. Oxidative Stress and Apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.; Zhongyuan, Z.; Wang, J.; Wong, C.-O.; Karagas, N.E.; Roessner, U.; Rupasinghe, T.; Venkatachalam, K.; Perry, T.; Bellen, H.J.; et al. Low Doses of the Neonicotinoid Insecticide Imidacloprid Induce ROS Triggering Neurological and Metabolic Impairments in Drosophila. Proc Natl Acad Sci U S A 2020, 117, 25840–25850. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, D.; Voth, W.; Jakob, U. Maintaining a Healthy Proteome during Oxidative Stress. Mol Cell 2018, 69, 203–213. [Google Scholar] [CrossRef]
- Li, M.; Tang, T.; Yuan, F.; Zhang, Y.; Li, F.; Liu, F. Protective Effects of Small Heat Shock Proteins in Daphnia Magna against Heavy Metal Exposure. Science of The Total Environment 2022, 848, 157565. [Google Scholar] [CrossRef]
- Cheng, S.; Lin, R.; Wang, L.; Qiu, Q.; Qu, M.; Ren, X.; Zong, F.; Jiang, H.; Yu, C. Comparative Susceptibility of Thirteen Selected Pesticides to Three Different Insect Egg Parasitoid Trichogramma Species. Ecotoxicol Environ Saf 2018, 166, 86–91. [Google Scholar] [CrossRef] [PubMed]
- D’Ávila, V.A.; Barbosa, W.F.; Guedes, R.N.C.; Cutler, G.C. Effects of Spinosad, Imidacloprid, and Lambda-Cyhalothrin on Survival, Parasitism, and Reproduction of the Aphid Parasitoid Aphidius Colemani. J Econ Entomol 2018, 111, 1096–1103. [Google Scholar] [CrossRef]
- Baglan, H.; Lazzari, C.R.; Guerrieri, F.J. Glyphosate Impairs Learning in Aedes Aegypti Mosquito Larvae at Field-Realistic Doses. J Exp Biol 2018, 221, jeb187518. [Google Scholar] [CrossRef]
- Rajak, P.; Roy, S. Heat Shock Proteins and Pesticide Stress. In Regulation of Heat Shock Protein Responses; Asea, A.A.A., Kaur, P., Eds.; Springer International Publishing: Cham, 2018; ISBN 978-3-319-74714-9. [Google Scholar]
- Otaka, M.; Odashima, M.; Watanabe, S. Role of Heat Shock Proteins (Molecular Chaperones) in Intestinal Mucosal Protection. Biochem Biophys Res Commun 2006, 348, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Somensi, N.; Brum, P.O.; de Miranda Ramos, V.; Gasparotto, J.; Zanotto-Filho, A.; Rostirolla, D.C.; da Silva Morrone, M.; Moreira, J.C.F.; Pens Gelain, D. Extracellular HSP70 Activates ERK1/2, NF-KB and Pro-Inflammatory Gene Transcription Through Binding with RAGE in A549 Human Lung Cancer Cells. Cell Physiol Biochem 2017, 42, 2507–2522. [Google Scholar] [CrossRef]
- Yu, L.-M.; Zhang, W.-H.; Han, X.-X.; Li, Y.-Y.; Lu, Y.; Pan, J.; Mao, J.-Q.; Zhu, L.-Y.; Deng, J.-J.; Huang, W.; et al. Hypoxia-Induced ROS Contribute to Myoblast Pyroptosis during Obstructive Sleep Apnea via the NF- κ B/HIF-1 α Signaling Pathway. Oxid Med Cell Longev 2019, 2019, 1–19. [Google Scholar] [CrossRef]
- Shan, Y.; Yan, S.; Hong, X.; Zha, J.; Qin, J. Effect of Imidacloprid on the Behavior, Antioxidant System, Multixenobiotic Resistance, and Histopathology of Asian Freshwater Clams (Corbicula Fluminea). Aquatic Toxicology 2020, 218, 105333. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, Y.; Niu, X.; Sun, Y.; Wang, H.; Guo, X.; Xu, B.; Wang, C. AccsHSP21. 7 Enhances the Antioxidant Capacity of Apis Cerana Cerana. J Sci Food Agric 2023, 103, 5401–5411. [Google Scholar] [CrossRef]
- Wang, X.X.; Geng, S.L.; Zhang, X.S.; Xu, W.H. P-S6K Is Associated with Insect Diapause via the ROS/AKT/ S6K/CREB/HIF-1 Pathway in the Cotton Bollworm, Helicoverpa Armigera. Insect Biochem Mol Biol 2020, 120. [Google Scholar] [CrossRef]
- Brunquell, J.; Morris, S.; Lu, Y.; Cheng, F.; Westerheide, S.D. The Genome-Wide Role of HSF-1 in the Regulation of Gene Expression in Caenorhabditis Elegans. BMC Genomics 2016, 17, 559. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip Toxicol 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in Metal-Induced Oxidative Stress and Human Disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Hartwig, A.; Asmuss, M.; Blessing, H.; Hoffmann, S.; Jahnke, G.; Khandelwal, S.; Pelzer, A.; Bürkle, A. Interference by Toxic Metal Ions with Zinc-Dependent Proteins Involved in Maintaining Genomic Stability. Food Chem Toxicol 2002, 40, 1179–1184. [Google Scholar] [CrossRef]
- Martín-Folgar, R.; Martínez-Guitarte, J.-L. Cadmium Alters the Expression of Small Heat Shock Protein Genes in the Aquatic Midge Chironomus Riparius. Chemosphere 2017, 169, 485–492. [Google Scholar] [CrossRef]
- Morales, M.; Planelló, R.; Martínez-Paz, P.; Herrero, O.; Cortés, E.; Martínez-Guitarte, J.L.; Morcillo, G. Characterization of Hsp70 Gene in Chironomus Riparius: Expression in Response to Endocrine Disrupting Pollutants as a Marker of Ecotoxicological Stress. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2011, 153, 150–158. [Google Scholar] [CrossRef]
- Hoback, W.W.; Stanley, D.W. Insects in Hypoxia. J Insect Physiol 2001, 47, 533–542. [Google Scholar] [CrossRef]
- Liu, G.; Roy, J.; Johnson, E.A. Identification and Function of Hypoxia-Response Genes in Drosophila Melanogaster. Physiol Genomics 2006, 25, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.R.; Teets, N.M.; Peyton, J.T.; Blobner, B.M.; Denlinger, D.L. Heat Shock Response to Hypoxia and Its Attenuation during Recovery in the Flesh Fly, Sarcophaga Crassipalpis. J Insect Physiol 2011, 57, 203–210. [Google Scholar] [CrossRef]
- Basson, C.H.; Terblanche, J.S. Metabolic Responses of Glossina Pallidipes (Diptera: Glossinidae) Puparia Exposed to Oxygen and Temperature Variation: Implications for Population Dynamics and Subterranean Life. J Insect Physiol 2010, 56, 1789–1797. [Google Scholar] [CrossRef]
- Wang, H. -S.; Wang, X. -H.; Zhou, C. -S.; Huang, L. -H.; Zhang, S. -F.; Guo, W.; Kang, L. CDNA Cloning of Heat Shock Proteins and Their Expression in the Two Phases of the Migratory Locust. Insect Mol Biol 2007, 16, 207–219. [Google Scholar] [CrossRef]
- ALBRECHT, F.O.; VERDIER, M.; BLACKITH, R.E. Maternal Control of Ovariole Number in the Progeny of the Migratory Locust. Nature 1959, 184, 103–104. [Google Scholar] [CrossRef]
- Parsell, D.A.; Lindquist, S. The Function of Heat-Shock Proteins in Stress Tolerance: Degradation and Reactivation of Damaged Proteins. Annu Rev Genet 1993, 27, 437–496. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Jiang, D.; Yan, M.; Li, Y.; Wang, J.; Wu, F.; Sheng, S. Identifications, Characteristics, and Expression Patterns of Small Heat Shock Protein Genes in a Major Mulberry Pest, Glyphodes Pyloalis (Lepidoptera: Pyralidae). Journal of Insect Science 2020, 20. [Google Scholar] [CrossRef]
- Xie, J.; Hu, X.-X.; Zhai, M.-F.; Yu, X.-J.; Song, X.-W.; Gao, S.-S.; Wu, W.; Li, B. Characterization and Functional Analysis of Hsp18.3 Gene in the Red Flour Beetle, Tribolium Castaneum. Insect Sci 2019, 26, 263–273. [Google Scholar] [CrossRef]
- Hao, X.; Zhang, S.; Timakov, B.; Zhang, P. The Hsp27 Gene Is Not Required for Drosophila Development but Its Activity Is Associated with Starvation Resistance. Cell Stress Chaperones 2007, 12, 364. [Google Scholar] [CrossRef]
- Rinehart, J.P.; Denlinger, D.L.; Rivers, D.B. Upregulation of Transcripts Encoding Select Heat Shock Proteins in the Flesh Fly Sarcophaga Crassipalpis in Response to Venom from the Ectoparasitoid Wasp Nasonia Vitripennis. J Invertebr Pathol 2002, 79, 62–63. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; Wu, G.-X.; Ye, G.-Y.; Hu, C. Heat Shock Protein Genes (Hsp20, Hsp75 and Hsp90) from Pieris Rapae: Molecular Cloning and Transcription in Response to Parasitization by Pteromalus Puparum. Insect Sci 2013, 20, 183–193. [Google Scholar] [CrossRef]
- Shim, J.-K.; Ha, D.-M.; Nho, S.-K.; Song, K.-S.; Lee, K.-Y. Upregulation of Heat Shock Protein Genes by Envenomation of Ectoparasitoid Bracon Hebetor in Larval Host of Indian Meal Moth Plodia Interpunctella. J Invertebr Pathol 2008, 97, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Sage, E.; Douki, T. Ultraviolet Radiation-Mediated Damage to Cellular DNA. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2005, 571, 3–17. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Kashiwagi, T.; Eguchi, E. Selective Photoreceptor Damage in Four Species of Insects Induced by Experimental Exposures to UV-Irradiation. Micron 2002, 33, 23–31. [Google Scholar] [CrossRef]
- Meng, J.-Y.; Zhang, C.-Y.; Zhu, F.; Wang, X.-P.; Lei, C.-L. Ultraviolet Light-Induced Oxidative Stress: Effects on Antioxidant Response of Helicoverpa Armigera Adults. J Insect Physiol 2009, 55, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-L.; Meng, J.-Y.; Zhou, L.; Yao, M.-S.; Zhang, C.-Y. Identification of Five Small Heat Shock Protein Genes in Spodoptera Frugiperda and Expression Analysis in Response to Different Environmental Stressors. Cell Stress Chaperones 2021, 26, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Guo, X.; Li, Y.; Gao, H.; Guo, X.; Xu, B. SHsp22.6, an Intronless Small Heat Shock Protein Gene, Is Involved in Stress Defence and Development in Apis Cerana Cerana. Insect Biochem Mol Biol 2014, 53, 1–12. [Google Scholar] [CrossRef]
- Liu, Z.; Xi, D.; Kang, M.; Guo, X.; Xu, B. Molecular Cloning and Characterization of Hsp27.6: The First Reported Small Heat Shock Protein from Apis Cerana Cerana. Cell Stress Chaperones 2012, 17, 539–551. [Google Scholar] [CrossRef]
- Xie, J.; Xiong, W.; Hu, X.; Gu, S.; Zhang, S.; Gao, S.; Song, X.; Bi, J.; Li, B. Characterization and Functional Analysis of Hsp21.8b: An Orthologous Small Heat Shock Protein Gene in Tribolium Castaneum. Journal of Applied Entomology 2018, 142, 654–666. [Google Scholar] [CrossRef]
- Sang, W.; Ma, W.-H.; Qiu, L.; Zhu, Z.-H.; Lei, C.-L. The Involvement of Heat Shock Protein and Cytochrome P450 Genes in Response to UV-A Exposure in the Beetle Tribolium Castaneum. J Insect Physiol 2012, 58, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.E.; Cartaño, N. V; Milos, L.; Krebs, R.A.; Lindquist, S.L. Effect of Engineering Hsp70 Copy Number on Hsp70 Expression and Tolerance of Ecologically Relevant Heat Shock in Larvae and Pupae of Drosophila Melanogaster. J Exp Biol 1996, 199, 1837–1844. [Google Scholar] [CrossRef]
- Feder, J.H.; Rossi, J.M.; Solomon, J.; Solomon, N.; Lindquist, S. The Consequences of Expressing Hsp70 in Drosophila Cells at Normal Temperatures. Genes Dev 1992, 6, 1402–1413. [Google Scholar] [CrossRef]
- Pener, M.P. Locust Phase Polymorphism and Its Endocrine Relations. In; 1991; pp. 1–79.
- Wang, H.; Dong, S.-Z.; Li, K.; Hu, C.; Ye, G. A Heat Shock Cognate 70 Gene in the Endoparasitoid, Pteromalus Puparum, and Its Expression in Relation to Thermal Stress. BMB Rep 2008, 41, 388–393. [Google Scholar] [CrossRef]
- Huang, L.-H.; Wang, C.-Z.; Kang, L. Cloning and Expression of Five Heat Shock Protein Genes in Relation to Cold Hardening and Development in the Leafminer, Liriomyza Sativa. J Insect Physiol 2009, 55, 279–285. [Google Scholar] [CrossRef]
- Sakano, D.; Li, B.; Xia, Q.; Yamamoto, K.; Banno, Y.; Fujii, H.; Aso, Y. Genes Encoding Small Heat Shock Proteins of the Silkworm, Bombyx Mori. Biosci Biotechnol Biochem 2006, 70, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Moghaddam, S.H.H.; Du, X.; Zhong, B.-X.; Chen, Y.-Y. Comparative Analysis on the Expression of Inducible HSPs in the Silkworm, Bombyx Mori. Mol Biol Rep 2012, 39, 3915–3923. [Google Scholar] [CrossRef] [PubMed]
- Colinet, H.; Lee, S.F.; Hoffmann, A. Knocking down Expression of Hsp22 and Hsp23 by RNA Interference Affects Recovery from Chill Coma in Drosophila Melanogaster. Journal of Experimental Biology 2010, 213, 4146–4150. [Google Scholar] [CrossRef]
- Rinehart, J.P.; Li, A.; Yocum, G.D.; Robich, R.M.; Hayward, S.A.L.; Denlinger, D.L. Up-Regulation of Heat Shock Proteins Is Essential for Cold Survival during Insect Diapause. Proceedings of the National Academy of Sciences 2007, 104, 11130–11137. [Google Scholar] [CrossRef]
- Yuan, J.-W.; Song, H.-X.; Chang, Y.-W.; Yang, F.; Xie, H.-F.; Gong, W.-R.; Du, Y.-Z. Identification, Expression Analysis and Functional Verification of Two Genes Encoding Small Heat Shock Proteins in the Western Flower Thrips, Frankliniella Occidentalis (Pergande). Int J Biol Macromol 2022, 211, 74–84. [Google Scholar] [CrossRef]
- Dong, C.-L.; Zhu, F.; Lu, M.-X.; Du, Y.-Z. Characterization and Functional Analysis of Cshsp19. 0 Encoding a Small Heat Shock Protein in Chilo Suppressalis (Walker). Int J Biol Macromol 2021, 188, 924–931. [Google Scholar] [CrossRef]
- Samanta, S.; Barman, M.; Chakraborty, S.; Banerjee, A.; Tarafdar, J. Involvement of Small Heat Shock Proteins (SHsps) in Developmental Stages of Fall Armyworm, Spodoptera Frugiperda and Its Expression Pattern under Abiotic Stress Condition. Heliyon 2021, 7, e06906. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhang, J.; Guo, Y.; Ma, E. Molecular Cloning and MRNA Expression of Heat Shock Protein Genes and Their Response to Cadmium Stress in the Grasshopper Oxya Chinensis. PLoS One 2015, 10, e0131244. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, M.-P.; Simpson, S.J.; Blondin, L.; Sword, G.A. Taxa-Specific Heat Shock Proteins Are over-Expressed with Crowding in the Australian Plague Locust. J Insect Physiol 2011, 57, 1562–1567. [Google Scholar] [CrossRef]
- Tian, L.; Wang, X.; Wang, X.; Lei, C.; Zhu, F. Starvation-, Thermal- and Heavy Metal- Associated Expression of Four Small Heat Shock Protein Genes in Musca Domestica. Gene 2018, 642, 268–276. [Google Scholar] [CrossRef]
- Wang, H.; Li, K.; Zhu, J.-Y.; Fang, Q.; Ye, G.-Y.; Wang, H.; Li, K.; Zhu, J.-Y. Cloning and Expression Pattern of Heat Shock Protein Genes from the Endoparasitoid Wasp, Pteromalus Puparum in Response to Environmental Stresses. Arch Insect Biochem Physiol 2012, 79, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Kayastha, P.; Wieczorkiewicz, F.; Pujol, M.; Robinson, A.; Michalak, M.; Kaczmarek, Ł.; Poprawa, I. Elevated External Temperature Affects Cell Ultrastructure and Heat Shock Proteins (HSPs) in Paramacrobiotus Experimentalis Kaczmarek, Mioduchowska, Poprawa, & Roszkowska, 2020. Sci Rep 2024, 14. [Google Scholar] [CrossRef]
- Kyriakou, E.; Taouktsi, E.; Syntichaki, P. The Thermal Stress Coping Network of the Nematode Caenorhabditis Elegans. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Zevian, S.C.; Yanowitz, J.L. Methodological Considerations for Heat Shock of the Nematode Caenorhabditis Elegans. Methods 2014, 68, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Strayer, A.; Wu, Z.; Christen, Y.; Link, C.D.; Luo, Y. Expression of the Small Heat-shock Protein Hsp-16-2 in Caenorhabditis Elegans Is Suppressed by Ginkgo Biloba Extract EGb 761. The FASEB Journal 2003, 17, 2305–2307. [Google Scholar] [CrossRef]
- Jecock, R.M.; Devaney, E. Expression of Small Heat Shock Proteins by the Third-Stage Larva of Brugia Pahangi; 1992; Vol. 56;
- Younis, A.E.; Geisinger, F.; Ajonina-Ekoti, I.; Soblik, H.; Steen, H.; Mitreva, M.; Erttmann, K.D.; Perbandt, M.; Liebau, E.; Brattig, N.W. Stage-Specific Excretory-Secretory Small Heat Shock Proteins from the Parasitic Nematode Strongyloides Ratti - Putative Links to Host’s Intestinal Mucosal Defense System. FEBS Journal 2011, 278, 3319–3336. [Google Scholar] [CrossRef]
- Fonseca, F.; Pénicaud, C.; Tymczyszyn, E.E.; Gómez-Zavaglia, A.; Passot, S. Factors Influencing the Membrane Fluidity and the Impact on Production of Lactic Acid Bacteria Starters. Appl Microbiol Biotechnol 2019, 103, 6867–6883. [Google Scholar] [CrossRef]
- Sadura, I.; Janeczko, A. Are Heat Shock Proteins Important in Low-Temperature-Stressed Plants? A Minireview. Agronomy 2024, 14. [Google Scholar] [CrossRef]
- Peter, E.; Candido, M. Caenorhabditis Elegans: Structure, Regulation and Biology.
- Horikawa, M.; Fukuyama, M.; Antebi, A.; Mizunuma, M. Regulatory Mechanism of Cold-Inducible Diapause in Caenorhabditis Elegans. Nat Commun 2024, 15. [Google Scholar] [CrossRef]
- Thorne, M.A.S.; Kagoshima, H.; Clark, M.S.; Marshall, C.J.; Wharton, D.A. Molecular Analysis of the Cold Tolerant Antarctic Nematode, Panagrolaimus Davidi. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- Adhikari, B.N.; Wall, D.H.; Adams, B.J. Effect of Slow Desiccation and Freezing on Gene Transcription and Stress Survival of an Antarctic Nematode. Journal of Experimental Biology 2010, 213, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Erkut, C.; Vasilj, A.; Boland, S.; Habermann, B.; Shevchenko, A.; Kurzchalia, T. V Molecular Strategies of the Caenorhabditis Elegans Dauer Larva to Survive Extreme Desiccation. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Tyson, T.; O’Mahony Zamora, G.; Wong, S.; Skelton, M.; Daly, B.; Jones, J.T.; Mulvihill, E.D.; Elsworth, B.; Phillips, M.; Blaxter, M.; et al. A Molecular Analysis of Desiccation Tolerance Mechanisms in the Anhydrobiotic Nematode Panagrolaimus Superbus Using Expressed Sequenced Tags. BMC Res Notes 2012, 5. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J. Hypoxia-Inducible Factor-1 (HIF-1)-Independent Hypoxia Response of the Small Heat Shock Protein Hsp-16.1 Gene Regulated by Chromatin-Remodeling Factors in the Nematode Caenorhabditis Elegans. Journal of Biological Chemistry 2013, 288, 1582–1589. [Google Scholar] [CrossRef]
- Balakumaran, M.; Chidambaranathan, P.; Tej Kumar, J.P.J.P.; Sirohi, A.; Kumar Jain, P.; Gaikwad, K.; Iyyappan, Y.; Rao, A.R.; Sahu, S.; Dahuja, A.; et al. Deciphering the Mechanism of Anhydrobiosis in the Entomopathogenic Nematode Heterorhabditis Indica through Comparative Transcriptomics. PLoS One 2022, 17. [Google Scholar] [CrossRef]
- Hong, M.; Kwon, J.Y.; Shim, J.; Lee, J. Differential Hypoxia Response of Hsp-16 Genes in the Nematode. J Mol Biol 2004, 344, 369–381. [Google Scholar] [CrossRef]
- Kim, B.M.; Rhee, J.S.; Jeong, C.B.; Seo, J.S.; Park, G.S.; Lee, Y.M.; Lee, J.S. Heavy Metals Induce Oxidative Stress and Trigger Oxidative Stress-Mediated Heat Shock Protein (Hsp) Modulation in the Intertidal Copepod Tigriopus Japonicus. Comparative Biochemistry and Physiology Part - C: Toxicology and Pharmacology 2014, 166, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch Toxicol 2023, 97, 2499–2574. [Google Scholar]
- Sampayo, J.N.; Olsen, A.; Lithgow, G.J. Oxidative Stress in Caenorhabditis Elegans: Protective Effects of Superoxide Dismutase/Catalase Mimetics. Aging Cell 2003, 2, 319–326. [Google Scholar] [CrossRef]
- Ezemaduka, A.N.; Wang, Y.; Li, X. Expression of CeHSP17 Protein in Response to Heat Shock and Heavy Metal Ions; 2017; Vol. 49;
- Shashikumar, S.; Rajini, P.S. Cypermethrin Elicited Responses in Heat Shock Protein and Feeding in Caenorhabditis Elegans. Ecotoxicol Environ Saf 2010, 73, 1057–1062. [Google Scholar] [CrossRef]
- Sonone, S.S.; Jadhav, S.; Sankhla, M.S.; Kumar, R. Water Contamination by Heavy Metals and Their Toxic Effect on Aquaculture and Human Health through Food Chain. Letters in Applied NanoBioScience 2021, 10, 2148–2166. [Google Scholar] [CrossRef]
- Gaur, V.K.; Sharma, P.; Sirohi, R.; Awasthi, M.K.; Dussap, C.G.; Pandey, A. Assessing the Impact of Industrial Waste on Environment and Mitigation Strategies: A Comprehensive Review. J Hazard Mater 2020, 398, 123019. [Google Scholar] [CrossRef]
- Khan, Z.; Elahi, A.; Bukhari, D.A.; Rehman, A. Cadmium Sources, Toxicity, Resistance and Removal by Microorganisms-A Potential Strategy for Cadmium Eradication. Journal of Saudi Chemical Society 2022, 26, 101569. [Google Scholar] [CrossRef]
- Moiseenko, T.I.; Gashkina, N.A. Distribution and Bioaccumulation of Heavy Metals (Hg, Cd and Pb) in Fish: Influence of the Aquatic Environment and Climate. Environmental Research Letters 2020, 15. [Google Scholar] [CrossRef]
- Das, S.; Kar, I.; Patra, A.K. Cadmium Induced Bioaccumulation, Histopathology, Gene Regulation in Fish and Its Amelioration – A Review. Journal of Trace Elements in Medicine and Biology 2023, 79, 127202. [Google Scholar] [CrossRef]
- Tamás, L.; Mistrík, I.; Zelinová, V. Heavy Metal-Induced Reactive Oxygen Species and Cell Death in Barley Root Tip. Environ Exp Bot 2017, 140, 34–40. [Google Scholar] [CrossRef]
- Georgiadou, E.C.; Kowalska, E.; Patla, K.; Kulbat, K.; Smolińska, B.; Leszczyńska, J.; Fotopoulos, V. Influence of Heavy Metals (Ni, Cu, and Zn) on Nitro-Oxidative Stress Responses, Proteome Regulation and Allergen Production in Basil (Ocimum Basilicum L.) Plants. Front Plant Sci 2018, 9. [Google Scholar] [CrossRef]
- Tamás, M.J.; Sharma, S.K.; Ibstedt, S.; Jacobson, T.; Christen, P. Heavy Metals and Metalloids as a Cause for Protein Misfolding and Aggregation. Biomolecules 2014, 4, 252–267. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front Pharmacol 2021, 12, 1–19. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, Z.; Tang, T.; Xie, S.; Liu, F. A 28.6-KD Small Heat Shock Protein (MnHSP28.6) Protects Macrobrachium Nipponense against Heavy Metal Toxicity and Oxidative Stress by Virtue of Its Anti-Aggregation Activity. Fish Shellfish Immunol 2019, 95, 635–643. [Google Scholar] [CrossRef]
- Zhang, A.; Lu, Y.; Li, C.; Zhang, P.; Su, X.; Li, Y.; Wang, C.; Li, T. A Small Heat Shock Protein (SHSP) from Sinonovacula Constricta against Heavy Metals Stresses. Fish Shellfish Immunol 2013, 34, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.-L.; Yao, C.-L.; Wang, Z.-Y. Acute Temperature and Cadmium Stress Response Characterization of Small Heat Shock Protein 27 in Large Yellow Croaker, Larimichthys Crocea. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2012, 155, 190–197. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Ning, X.; Chen, A.; Zhang, L.; Qin, S.; Wu, H.; Zhao, J. Identification of Two Small Heat Shock Proteins with Different Response Profile to Cadmium and Pathogen Stresses in Venerupis Philippinarum. Cell Stress Chaperones 2010, 15, 897–904. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; He, C.; Gao, X.; Yuan, X. Identification of a Small HSP Gene from Hard Clam Meretrix Meretrix and Its Potential as an Environmental Stress Biomarker. Aquat Biol 2013, 18, 243–252. [Google Scholar] [CrossRef]
- Bildik, A.E.; Ekren, G.S.A.; Akdeniz, G.; Kiral, F.K. Effect of Enviromental Temperature on Heat Shock Proteins (HSP30, HSP70, HSP90) and IGF-I MRNA Expression in Sparus Aurata. Iran J Fish Sci 2019, 18, 1014–1024. [Google Scholar]
- Currie, S. The Effects of Heat Shock and Acclimation Temperature on Hsp70 and Hsp30 MRNA Expression in Rainbow Trout: In Vivo and in Vitro Comparisons. J Fish Biol 2000, 56, 398–408. [Google Scholar] [CrossRef]
- Liu, X.; Shi, H.; Liu, Z.; Kang, Y.; Wang, J.; Huang, J. Effect of Heat Stress on Heat Shock Protein 30 (Hsp 30) MRNA Expression in Rainbow Trout (Oncorhynchus Mykiss).; 2019.
- Shekhar, M.S.; Kiruthika, J.; Ponniah, A.G. Identification and Expression Analysis of Differentially Expressed Genes from Shrimp (Penaeus Monodon) in Response to Low Salinity Stress. Fish Shellfish Immunol 2013, 35, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Zarei, S.; Ghafouri, H.; Vahdatiraad, L.; Heidari, B. The Influence of HSP Inducers on Salinity Stress in Sterlet Sturgeon (Acipenser Ruthenus): In Vitro Study on HSP Expression, Immune Responses, and Antioxidant Capacity. Cell Stress Chaperones 2024, 29, 552–566. [Google Scholar] [CrossRef]
- Muthusamy, S.K.; Dalal, M.; Chinnusamy, V.; Bansal, K.C. Genome-Wide Identification and Analysis of Biotic and Abiotic Stress Regulation of Small Heat Shock Protein (HSP20) Family Genes in Bread Wheat. J Plant Physiol 2017, 211, 100–113. [Google Scholar] [CrossRef]
- Arockiaraj, J.; Vanaraja, P.; Easwvaran, S.; Singh, A.; Othman, R.Y.; Bhassu, S. Gene Expression and Functional Studies of Small Heat Shock Protein 37 (MrHSP37) from Macrobrachium Rosenbergii Challenged with Infectious Hypodermal and Hematopoietic Necrosis Virus (IHHNV). Mol Biol Rep 2012, 39, 6671–6682. [Google Scholar] [CrossRef]
- Huang, P.; Kang, S.; Chen, W.; Hsu, T.; Lo, C.; Liu, K.; Chen, L. Identification of the Small Heat Shock Protein, HSP21, of Shrimp Penaeus Monodon and the Gene Expression of HSP21 Is Inactivated after White Spot Syndrome Virus (WSSV) Infection. Fish Shellfish Immunol 2008, 25, 250–257. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





