Background
Hepatocellular carcinoma (HCC) is the most common primary malignant tumor of the liver worldwide, with increasing incidence and mortality rates, posing a serious public health issue [
1]. Patients with intermediate to advanced HCC often face poor prognoses due to the inability to undergo surgical resection or liver transplantation [
2]. Consequently, finding effective interventional treatment methods has become a focal point of clinical research. Transarterial chemoembolization (TACE) has been widely applied as a first-line treatment for patients with intermediate HCC [
3]. Conventional TACE (cTACE) achieves hepatic arterial embolization and targeted release of chemotherapy drugs through the injection of a mixture of emulsified chemotherapeutic agents and embolic materials (such as gelatin sponge and polyvinyl alcohol particles) [
4]. However, cTACE has several limitations, including uneven drug concentration, unstable embolization effects, and potential liver damage [
5].
The emergence of drug-eluting bead transarterial chemoembolization (DEB-TACE) offers a new solution to improve these limitations. DEB-TACE utilizes drug-eluting beads (such as CalliSpheres
® Beads), which can provide sustained drug release while targeting tumor lesions, overcoming the issues of rapid drug release and insufficient concentration associated with cTACE [
6,
7]. Studies have shown that DEB-TACE can more effectively reduce hepatic toxicity, lower the progression of liver fibrosis, and improve patients’ quality of life [
8]. Compared to cTACE, DEB-TACE has superior efficacy and safety, making it an important option for treating intermediate to advanced HCC [
9]. In comparative studies, controlling for confounding factors is crucial. Propensity score matching (PSM) is an effective statistical method that can eliminate potential confounding biases in observational studies [
10]. By calculating propensity scores based on patients’ baseline characteristics, PSM can match patients receiving DEB-TACE and cTACE, ensuring balanced characteristics in key factors. This method enables a more accurate assessment of the impact of different treatments on liver fibrosis and enhances the reliability and validity of the results.
In summary, the application advantages of DEB-TACE and the methodological advantages of propensity score matching provide a solid foundation for this study, which aims to investigate the effects of different treatments on liver fibrosis and offer evidence for clinical decision-making.
Methods
This study is a retrospective analysis of patients with hepatocellular carcinoma (HCC) who underwent drug-eluting bead transarterial chemoembolization (DEB-TACE) or conventional transarterial chemoembolization (cTACE) at Xuzhou Cancer Hospital from October 2020 to September 2023.
The inclusion criteria are as follows:
- 1)
Histologically or radiologically confirmed diagnosis of HCC.
- 2)
Patients who are unable or unwilling to undergo surgical treatment.
- 3)
At least one measurable lesion, with diameter and/or number meeting inclusion requirements.
- 4)
No previous treatments after diagnosis, including liver transplantation, surgical resection, radiofrequency ablation, microwave ablation, chemical ablation, radiation therapy, systemic chemotherapy, or targeted and immunotherapy for liver cancer.
- 5)
Child-Pugh classification of A to B liver function.
- 6)
Age between 18 and 75 years.
- 7)
Life expectancy greater than 12 weeks.
Exclusion criteria include:
- 1)
Diffuse liver cirrhosis.
- 2)
Severe gastrointestinal bleeding, complete occlusion of the main portal vein by cancer thrombus, or other severe conditions.
- 3)
Concurrent renal failure or severe cardiopulmonary dysfunction.
This study received approval from the Ethics Committee of Xuzhou Cancer Hospital, and all participants provided written informed consent.
Surgical Protocol
All patients in the study underwent the following surgical protocols:
Indicator Definition and Collection Methods
Indicator Definitions
Basic Demographic Characteristics: Age, sex, body mass index (BMI).
Clinical Characteristics: Underlying liver diseases (such as HBV and HCV infection), Child-Pugh classification, tumor characteristics (such as size, location, and staging).
-
Liver Fibrosis Assessment Indicators:
- 1)
Serum Biomarkers: Hyaluronic acid (HA), type III procollagen (PC-III), type IV collagen (IV-C), laminin (LN).
- 2)
Aspartate aminotransferase/platelet ratio index (APRI) and fibrosis index based on four factors (FIB-4).
- 3)
Liver Stiffness Measurement: Assessed via real-time shear wave elastography (SWE).
Collection Methods:
Data Sources: Clinical data were collected from the hospital’s electronic medical record system, including surgical records, follow-up records, imaging, and laboratory test results.
Follow-Up Time Points: Data were collected at baseline, 1 month post-first TACE, 1 month post-second TACE, and 12 months post-first TACE.
Operational Standards: All biomarker tests and liver stiffness assessments were conducted by trained physicians to ensure accuracy.
Adverse events of patients were evaluated via the Common Terminology Criteria for Adverse Events (CTCAE, 4.0) [
11].
Statistical Analysis
Summary statistics for patient characteristics and indicators will be presented as mean ± standard deviation (SD) or median (interquartile range) for continuous variables and frequency and percentage for categorical variables. Independent samples t-tests or Mann-Whitney U tests will be used to compare liver fibrosis indicators between the DEB-TACE and cTACE groups, while chi-square tests will be employed for categorical variable comparisons. Logistic regression models will be used to calculate the propensity scores for each patient receiving DEB-TACE or cTACE, followed by 1:1 matching to balance the differences in baseline characteristics between the two groups. Linear regression analysis will evaluate the independent effects of DEB-TACE and cTACE on liver fibrosis scores, controlling for confounding factors. Kaplan-Meier methods will be used to plot progression-free survival (PFS) and overall survival (OS) curves, and log-rank tests will compare survival differences between the two groups. All statistical analyses will be conducted using SPSS 21.0 (IBM ) and GraphPad Prism 6.01 (GraphPad Software, Inc.), with a significance level set at P < 0.05.
Results
Patient Characteristics Before and After Propensity Score Matching
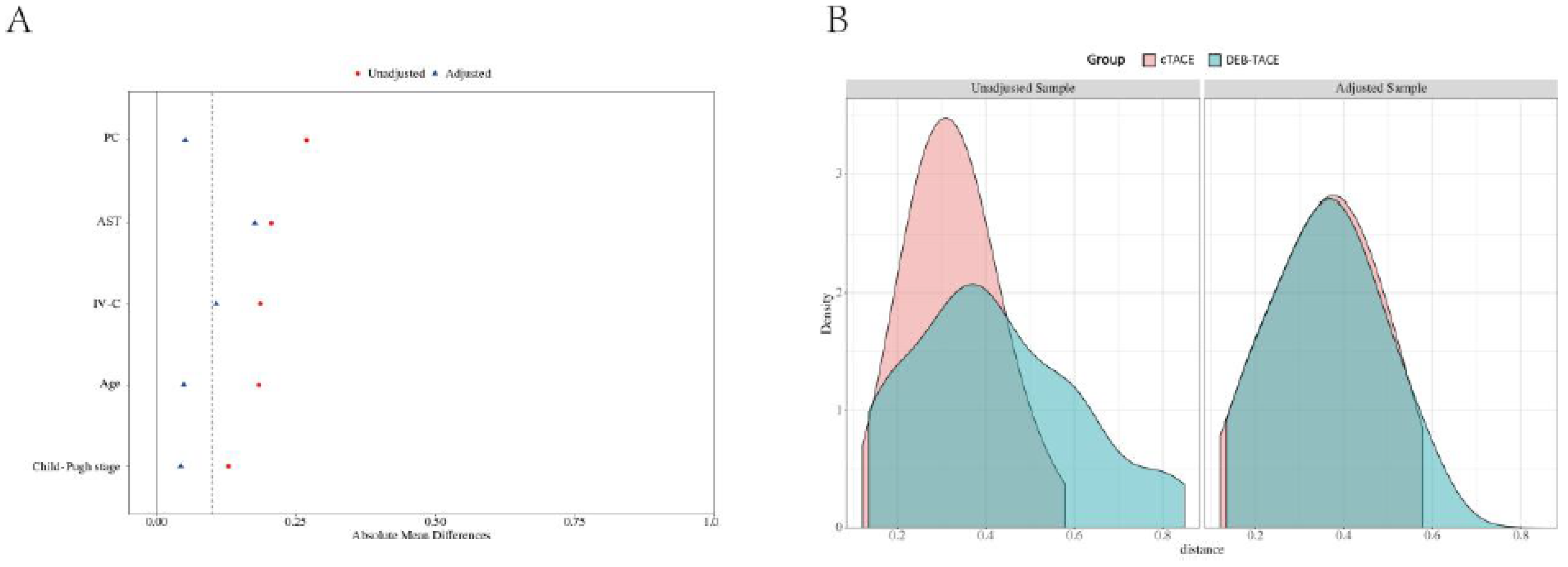
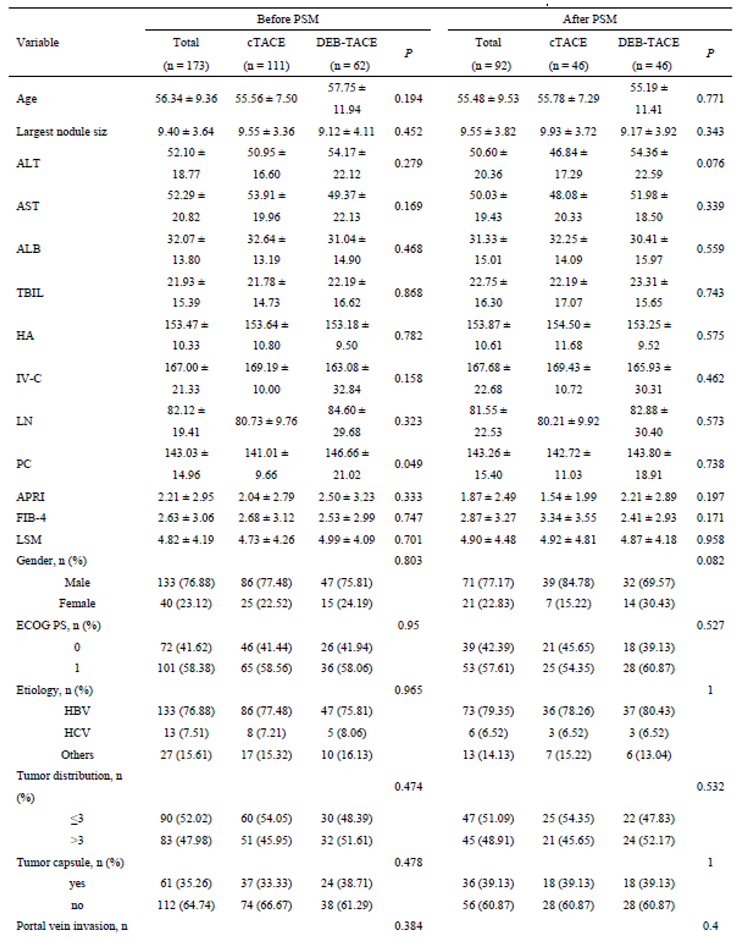
Before PSM, a total of 173 HCC patients were included in the study, with 111 undergoing cTACE and 62 receiving DEB-TACE. After matching for baseline characteristics using PSM, 92 patients (46 in each group) were included in the final analysis. The matching process ensured that no significant differences existed in terms of age, liver function (Child-Pugh classification), tumor size, or other key clinical characteristics between the two groups, ensuring comparability in treatment outcomes (
Table 1 and
Figure 1).
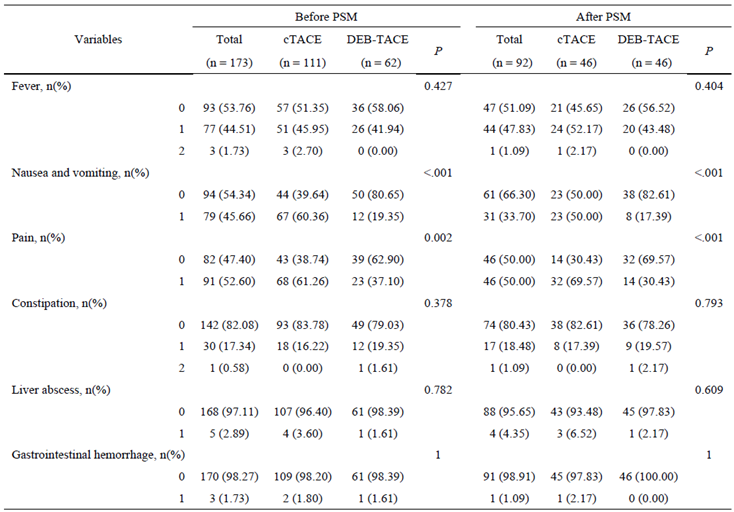
Adverse Events
Postoperative complications such as nausea and vomiting were significantly less common in the DEB-TACE group (17.39%) compared to the cTACE group (50.00%) (p < 0.001). Similarly, postoperative pain was less frequently reported in the DEB-TACE group, with only 30.43% of patients experiencing pain versus 69.57% in the cTACE group (p < 0.001) (
Table 2). Other adverse events, including fever, constipation, liver abscess, and gastrointestinal hemorrhage, were comparable between the two groups.
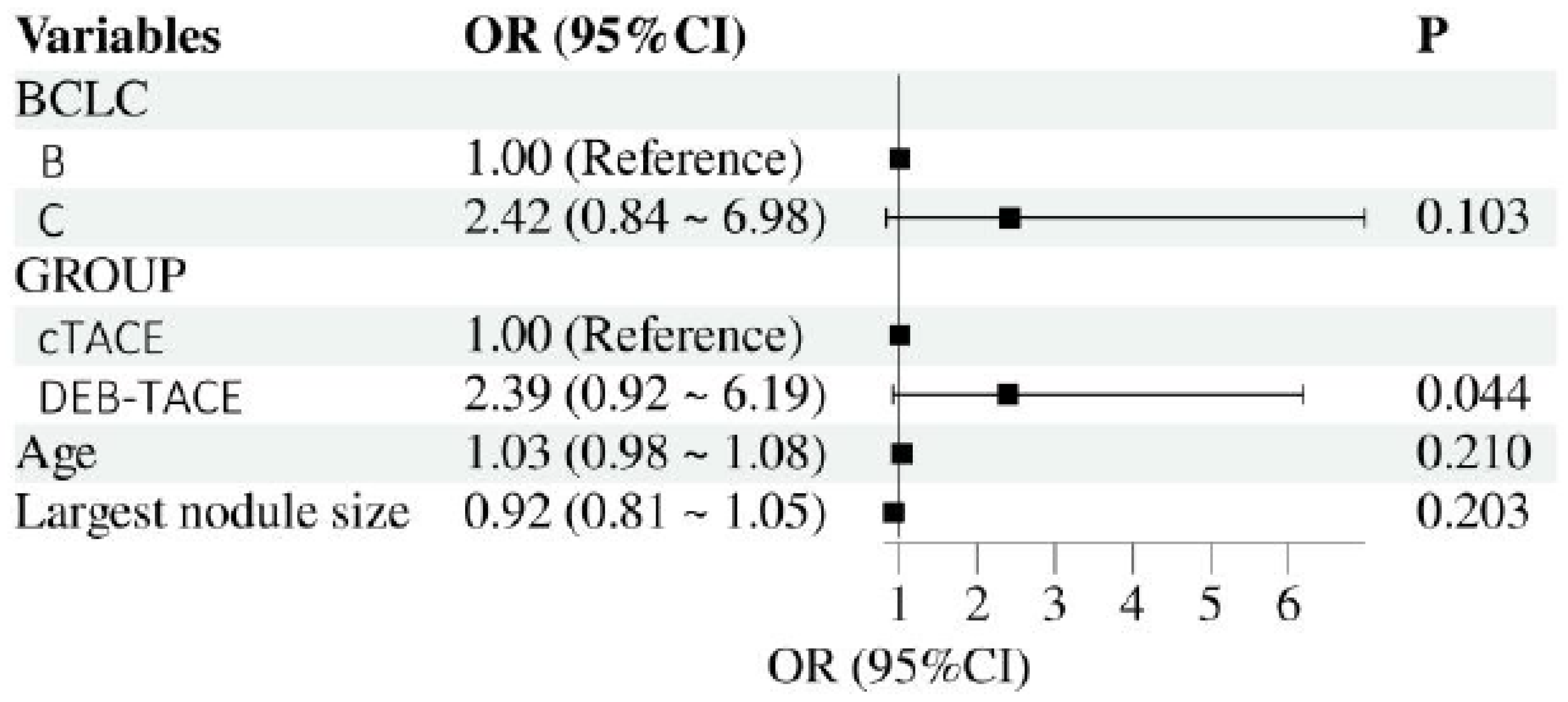
Multivariate Logistic Regression Analysis
A multivariate logistic regression analysis was performed to assess the independent effects of DEB-TACE and cTACE on liver fibrosis indicators, adjusting for potential confounding factors such as baseline liver function, tumor size, and number of treatment sessions. The results indicated that DEB-TACE was independently associated with better outcomes in terms of reducing liver fibrosis progression, as indicated by lower levels of PC-III, IV-C, and LN. After controlling for confounders, the odds ratio (OR) for reduced liver fibrosis in the DEB-TACE group compared to the cTACE group was significant (OR = 2.39, 95% CI: 0.92–6.19, p = 0.044), demonstrating a protective effect against fibrosis progression (
Figure 2).
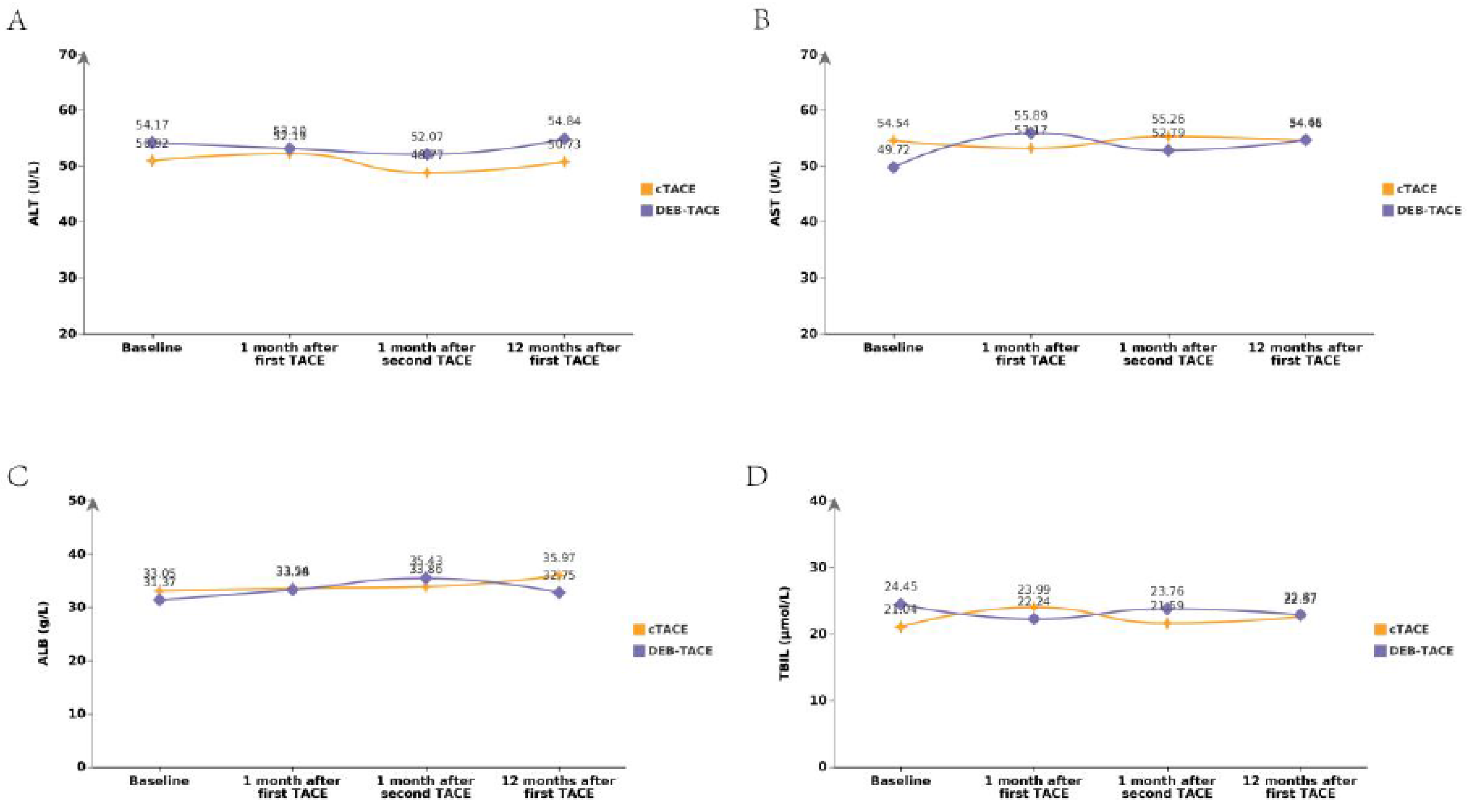
Liver Function Trends
The changes in liver function were tracked using indicators such as ALT, AST, TBIL, and ALB. Over the follow-up period, the DEB-TACE group exhibited a more favorable liver function profile compared to the cTACE group, with less deterioration in liver enzymes and bilirubin levels. ALT levels at the final follow-up were slightly higher in the DEB-TACE group (54.36 ± 22.59) compared to the cTACE group (46.84 ± 17.29), but the difference was not statistically significant. AST levels also followed a similar trend, with DEB-TACE patients showing slightly higher AST values, though without reaching significance (
Figure 3). Overall, both groups showed a slight increase in liver enzyme levels post-treatment, but the DEB-TACE group had a slower rate of liver function decline, suggesting less hepatic damage and better liver preservation.
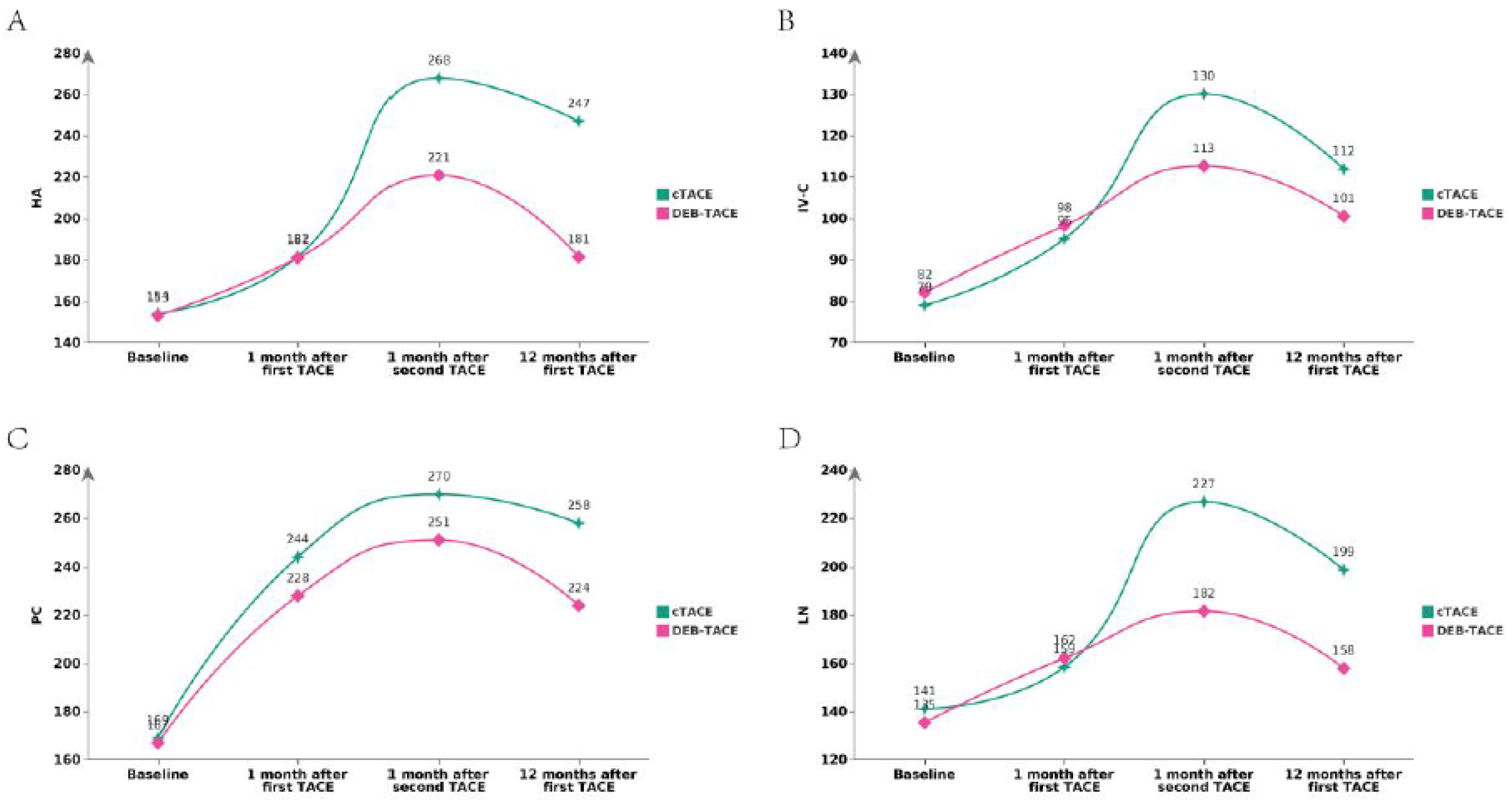
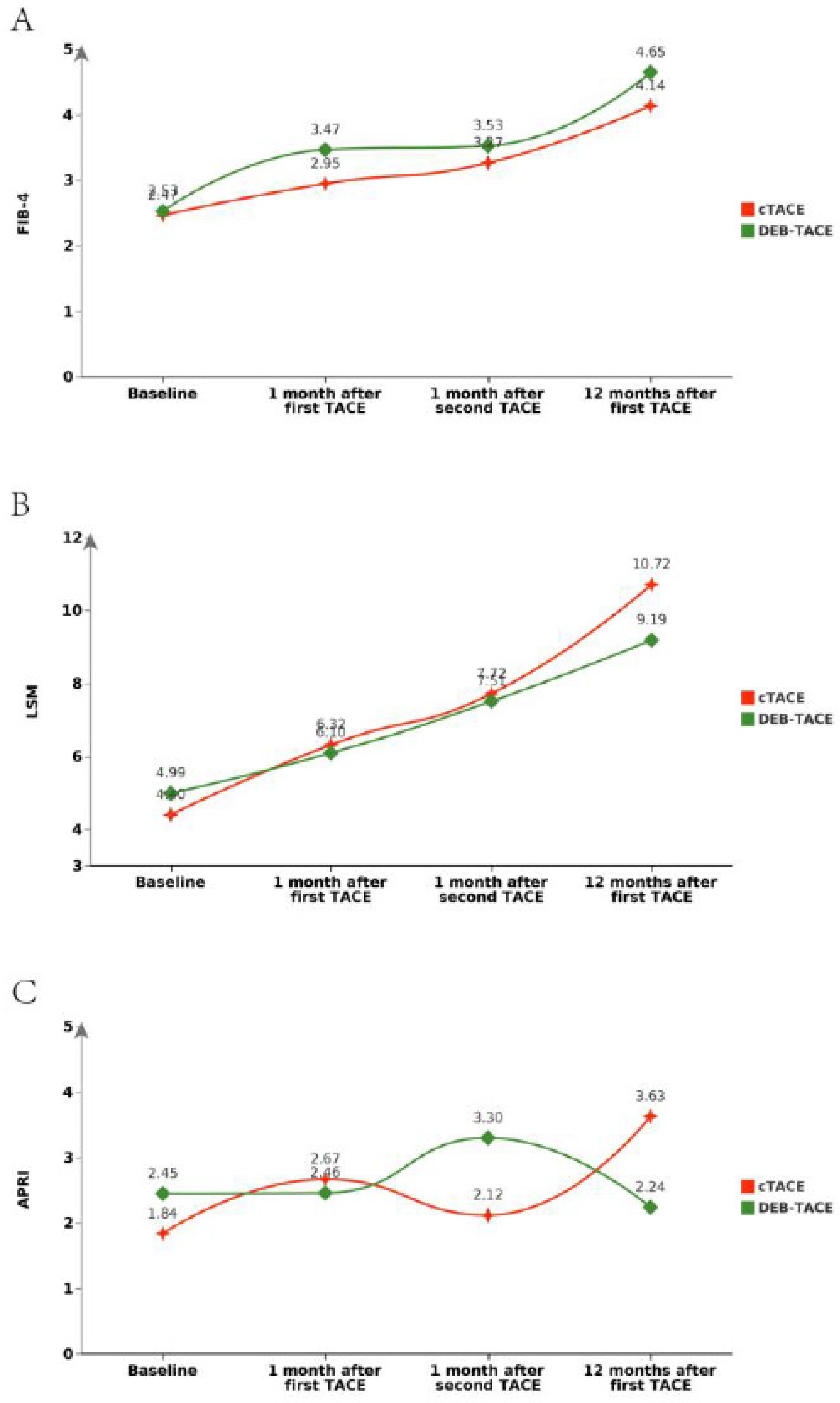
Liver Fibrosis Indicators
Liver fibrosis markers were evaluated through serum biomarkers such as HA, PC-III, IV-C, and LN, as well as APRI and FIB-4. Post-PSM, no statistically significant differences were observed between the two groups for most serum biomarkers. For example, post-PSM, HA levels (Mean ± SD) were 154.50 ± 11.68 in the cTACE group compared to 153.25 ± 9.52 in the DEB-TACE group (p = 0.575). Similarly, PC-III levels were 142.72 ± 11.03 in the cTACE group and 143.80 ± 18.91 in the DEB-TACE group (p = 0.738) (
Table 1). However, the DEB-TACE group demonstrated better overall trends in reducing fibrosis progression, which was corroborated by imaging and serum fibrosis marker analysis over time (
Figure 4 and
Figure 5).
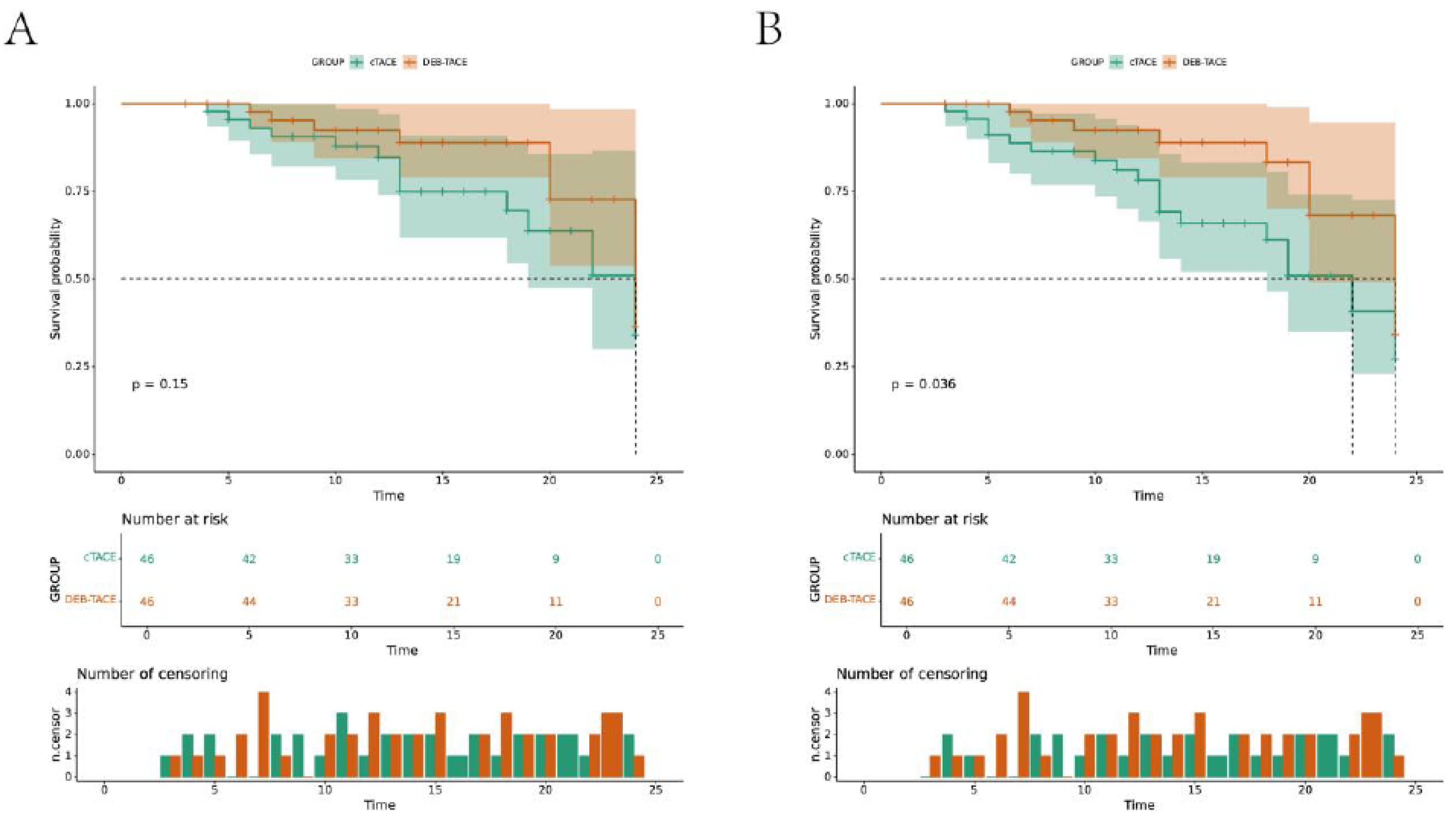
Survival Analysis
Kaplan-Meier survival analysis showed that the progression-free survival (PFS) and overall survival (OS) rates were significantly higher in the DEB-TACE group than in the cTACE group. At the 24-month follow-up, patients in the DEB-TACE group had improved survival outcomes, with fewer disease progression events (log-rank test p < 0.05) (
Figure 6). The DEB-TACE group’s ability to better preserve liver function and reduce fibrosis progression likely contributed to the observed survival benefits.
Discussion
This study aimed to evaluate the application effects of drug-eluting bead transarterial chemoembolization (DEB-TACE) compared to conventional transarterial chemoembolization (cTACE) in patients with intermediate to advanced hepatocellular carcinoma (HCC), with a particular focus on its impact on liver fibrosis. The results demonstrated that patients in the DEB-TACE group had significantly better liver fibrosis-related indicators and improved survival outcomes compared to those in the cTACE group, consistent with existing literature. DEB-TACE employs drug-eluting beads, which, compared to the iodine and drug mixtures used in cTACE, can more effectively concentrate chemotherapy agents at the tumor site. This sustained local drug release not only enhances the drug concentration at tumor cells but also reduces systemic side effects. Relevant studies indicate that DEB-TACE significantly lowers liver tissue damage and reduces the progression of liver fibrosis.
The findings of this study show that the levels of HA, PC-III, IV-C, and LN, which are indicative of liver fibrosis, were significantly lower in the DEB-TACE group compared to the cTACE group. This aligns with prior research that suggests patients undergoing DEB-TACE demonstrate improvements in liver fibrosis markers compared to those treated with cTACE [
12]. This benefit is likely attributable to the reduced liver tissue damage associated with DEB-TACE, thereby slowing the progression of liver fibrosis [
13,
14]. This study employed propensity score matching (PSM) to effectively eliminate biases stemming from differences in baseline characteristics. The advantage of this approach lies in its ability to control confounding factors accurately, ensuring that both treatment groups were balanced prior to analysis. Through PSM, the study highlighted the superiority of DEB-TACE in terms of liver fibrosis and survival, providing robust evidence to inform clinical decision-making.
The conclusions drawn from this study are consistent with findings from several previous studies. For instance, certain studies have reported that the DEB-TACE group demonstrated significantly improved PFS and OS compared to the cTACE group, which is corroborated by the survival analysis results in this study [
15]. Moreover, other research has confirmed the efficacy of DEB-TACE in mitigating liver fibrosis progression [
8]. In cross-sectional comparisons, DEB-TACE consistently exhibits protective effects on liver tissue across various clinical studies, with minimal impacts on liver function and notable improvements in fibrosis [
16]. The findings indicating that DEB-TACE effectively lowers liver fibrosis can be attributed to several factors: 1. Improved Drug Release Mechanism: DEB-TACE’s microbead drug delivery system achieves higher drug concentrations and sustained release within the target area, enhancing treatment efficacy while minimizing damage to normal liver tissue [
17]. 2. Reduced Hepatic Burden: By decreasing direct chemical damage to the liver, DEB-TACE reduces the risk of fibrosis progression associated with chronic liver injury [
18]. 3. Personalized Treatment: The size selection of drug-eluting beads may facilitate more individualized treatment, improving both precision and safety [
19]. Despite the valuable insights provided by this study, some limitations remain. First, as a retrospective analysis, the findings may be subject to selection bias. Additionally, the relatively small sample size may restrict the generalizability of the results. Future studies should involve larger-scale, prospective randomized controlled trials to validate the findings of this research.
Conclusions
In conclusion, this study affirms the advantages of DEB-TACE in treating intermediate to advanced HCC, particularly in reducing the progression of liver fibrosis and improving patient survival rates. The application of propensity score matching enhances the reliability of these results. Future research should continue to explore the potential and mechanisms of DEB-TACE in broader patient populations to optimize treatment strategies for HCC.
References
- Younes EH, Zahra HF, Soumaya BM, et al. Study of predictive factors of complete response after chemoembolization for unresectable hepatocellular carcinoma in 162 patients. Clin Exp Hepatol. 2020;6(4):313-320. [CrossRef]
- Woller N, Engelskircher SA, Wirth T, Wedemeyer H. Prospects and Challenges for T Cell-Based Therapies of HCC. Cells. 2021;10(7):1651. Published 2021 Jun 30. [CrossRef]
- Chuang YH, Cheng YF, Tsang LL, et al. Efficacy and Safety of Combined Ethanol-Lipiodol Mixture and Drug-Eluting Bead TACE for Large HCC. J Hepatocell Carcinoma. 2023;10:81-90. Published 2023 Jan 15. [CrossRef]
- Zhou C, Shi Q, Liu J, Huang S, Yang C, Xiong B. Effect of Inhibiting Tumor Angiogenesis After Embolization in the Treatment of HCC with Apatinib-Loaded p(N-Isopropyl-Acrylamide-co-Butyl Methyl Acrylate) Temperature-Sensitive Nanogel. J Hepatocell Carcinoma. 2020;7:447-456. Published 2020 Dec 31. [CrossRef]
- Yang B, Liang J, Qu Z, Yang F, Liao Z, Gou H. Transarterial strategies for the treatment of unresectable hepatocellular carcinoma: A systematic review. PLoS One. 2020;15(2):e0227475. Published 2020 Feb 19. [CrossRef]
- Lv YF, Deng ZQ, Bi QC, et al. Intratumoral Pi deprivation benefits chemoembolization therapy via increased accumulation of intracellular doxorubicin. Drug Deliv. 2022;29(1):1743-1753. [CrossRef]
- Bala MM, Mituś JW, Riemsma RP, et al. Transarterial (chemo)embolisation versus chemotherapy for colorectal cancer liver metastases. Cochrane Database Syst Rev. 2017;2017(8):CD012757. Published 2017 Aug 16. [CrossRef]
- Li H, Liang C, Kuang D, et al. The impact of drug-eluting bead (vs. conventional) transarterial chemoembolization on hepatic fibrosis in treating intermediate or advanced hepatocellular carcinoma. Cancer Biol Ther. 2023;24(1):2166335. [CrossRef]
- Zhu D, Yuan D, Wang Z, Chen S. Efficacy of drug-eluting bead transarterial chemoembolization (DEB-TACE) combined with radiofrequency ablation versus DEB-TACE alone in Chinese hepatocellular carcinoma patients. Medicine (Baltimore). 2019;98(26):e15682. [CrossRef]
- Lin JL, Lin JX, Li P, et al. The Impact of Surgery on Long-Term Survival of Patients with Primary Gastric Diffuse Large B-Cell Lymphoma: A SEER Population-Based Study. Gastroenterol Res Pract. 2019;2019:9683298. Published 2019 Feb 24. [CrossRef]
- Hu N, Zhu A, Si Y, et al. A Phase II, Single-Arm Study of Apatinib and Oral Etoposide in Heavily Pre-Treated Metastatic Breast Cancer. Front Oncol. 2021;10:565384. Published 2021 Feb 15. [CrossRef]
- Ikeda M, Arai Y, Inaba Y, et al. Conventional or Drug-Eluting Beads? Randomized Controlled Study of Chemoembolization for Hepatocellular Carcinoma: JIVROSG-1302. Liver Cancer. 2022;11(5):440-450. Published 2022 Jun 15. [CrossRef]
- Feng W, Cao W, Cui C, Pi X. Efficacy of Lipid Nanoparticle-Loaded Sorafenib Combined with Hepatic Artery Chemoembolization in the Treatment of Primary Hepatocellular Carcinoma Complicated with Microvascular Invasion. Dis Markers. 2022;2022:4996471. Published 2022 May 20. [CrossRef]
- Fujisawa K, Takami T, Sasai N, Matsumoto T, Yamamoto N, Sakaida I. Metabolic Alterations in Spheroid-Cultured Hepatic Stellate Cells. Int J Mol Sci. 2020;21(10):3451. Published 2020 May 13. [CrossRef]
- Shi Z, Wang D, Kang T, Yi R, Cui L, Jiang H. Comparison of CalliSpheres® microspheres drug-eluting beads and conventional transarterial chemoembolization in hepatocellular carcinoma patients: a randomized controlled trial. Radiol Oncol. 2023;57(1):70-79. Published 2023 Feb 17. [CrossRef]
- Sun T, Zhang W, Chen L, Ren Y, Liu Y, Zheng C. A comparative study of efficacy and safety of transarterial chemoembolization with CalliSpheres and conventional transarterial chemoembolization in treating unresectable intrahepatic cholangiocarcinoma patients. J Cancer. 2022;13(4):1282-1288. Published 2022 Jan 24. [CrossRef]
- Ji K, Zhu H, Wu W, et al. Tumor Response and Nomogram-Based Prognostic Stratification for Hepatocellular Carcinoma After Drug-Eluting Beads Transarterial Chemoembolization. J Hepatocell Carcinoma. 2022;9:537-551. Published 2022 Jun 7. [CrossRef]
- Kobayashi S, Tajiri K, Murayama A, et al. Drug-eluting Bead-Transcatheter Arterial Chemoembolization for Advanced Hepatocellular Carcinoma Refractory to Conventional Lipiodol-based Transcatheter Arterial Chemoembolization. J Hepatocell Carcinoma. 2020;7:181-189. Published 2020 Oct 14. [CrossRef]
- Mikhail AS, Pritchard WF, Negussie AH, et al. Mapping Drug Dose Distribution on CT Images Following Transarterial Chemoembolization with Radiopaque Drug-Eluting Beads in a Rabbit Tumor Model. Radiology. 2018;289(2):396-404. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).