1. Introduction
According to the European Society for Clinical Nutrition and Metabolism (ESPEN), malnutrition is defined as “a state resulting from lack of intake or uptake of nutrition that leads to altered body composition (decreased fat-free mass) and body cell mass, causing diminished physical and mental function and impaired clinical outcomes” [
1]. The goal of convalescent rehabilitation is to improve activities of daily living (ADL) and facilitate home discharge for patients with acute diseases, such as cerebrovascular and orthopedic diseases, including hip and vertebral fractures; malnutrition poses a significant barrier to this process. Over 40% of patients in convalescent rehabilitation settings experience malnutrition [
2], and appropriate nutritional management can improve rehabilitation outcomes [3-5].
After admission to a convalescent rehabilitation ward (CRW), patients with malnutrition must be identified as soon as possible to ensure effective convalescent rehabilitation and nutritional management. Therefore, the first nutritional assessment should be conducted within 1 or 2 days of admission [
6]. Nutritional assessment is routinely performed using nutritional screening methods that can be performed quickly and easily and whose adequacy and credibility have been certified [
6]. Several screening methods have been developed and are available for clinical use, each with advantages and disadvantages. ESPEN recommends Nutritional Risk Screening 2002 (NRS-2002), the Malnutrition Universal Screening Tool (MUST), and the Mini Nutritional Assessment Short Form (MNA-SF) for hospitalized patients, community-dwelling individuals, and community-dwelling older adults, respectively [
7]. The MNA-SF is considered an effective nutritional screening tool for older adults, as low MNA-SF scores can predict functional decline in patients [8-12]. However, MNA-SF requires a medical interview, making it unsuitable for patients with impaired consciousness, cerebrovascular disease, or dementia. Another widely used tool, the Geriatric Nutritional Risk Index (GNRI), assesses malnutrition risk based on body mass index (BMI) and serum albumin levels; however, it does not fully reflect the patient's nutritional status [
13], raising concerns about its suitability for convalescent rehabilitation patients. To our knowledge, no nutritional screening method has been specifically designed for patients in CRWs.
Therefore, we propose establishing a new nutritional screening method for CRW. Since a precise nutritional assessment needs to be evaluated using several strategies [
14,
15], we attempted to develop a new method comprising six items extracted from several known methods (body weight [BW] loss, food intake, BMI, serum albumin concentration, decubitus, and digestive symptoms). This method uses all information from our medical records without the need for patient interviews, making it more accessible than other methods. This study presents the results of applying this method to patients in our CRW and compares its effectiveness to MNA-SF. Additionally, this study discusses the potential advantages of our J-Method over the MNA-SF.
2. Materials and Methods
2.1. Patients
We collected data from patients with cerebrovascular diseases aged > 65 years admitted to our CRW between February 2020 and February 2022. The following were the exclusion criteria: 1) patients who refused to participate in this study and 2) patients transferred to an acute hospital due to a sudden deterioration of their health that was too severe to continue rehabilitation.
2.2. Establishment of a New Nutritional Screening Method (J-Method)
Based on several nutritional screening methods, we attempted to establish a new nutritional screening method (J-Method) suitable for our CRW. The J-Method comprised six items: 1) BW loss of >1% in a week; 2) < 50% of daily food intake for 3 days after admission; 3) BMI < 18.5 kg/m
2; 4) serum albumin concentration ≤ 3.0 g/dL; 5) the presence of decubitus anywhere in the body; and 6) miscellaneous factors, including digestive symptoms and total parenteral nutrition. A patient was diagnosed with malnutrition if they had decubitus or met more than two of the remaining criteria. The rate of BW loss was calculated using the following formula:
Each patient's average daily food intake was calculated based on the meal contents (principal food and accompanying dishes), and the intake amounts for 3 days after admission. BMI was calculated using the height (m) and the weight (kg) of each patient at the admission in our CRW. Serum albumin concentration was measured using blood collected from each patient during the examination on admission. Decubitus was detected through physical examination by an attending physician. Additionally, digestive symptoms such as nausea, vomiting, and/or diarrhea, as well as total parenteral nutrition, were collected from each patient's physician’s and nursing reports. Based on the J-Method’s results, the participants were classified into the “malnutrition” and “non-malnutrition” groups.
2.3. Nutritional Screening by MNA-SF
Within 1 week of admission to our CRW, MNA-SF was performed by KO (the first author). MNA-SF comprised of the following six items, each scored between 0 and 2 or 3: 1) food intake decline for the last 3 months [severe, 0; moderate, 1; no decrease, 3]; 2) BW loss over the last 3 months [> 3 kg, 0; unknown, 1; 1–3 kg, 2; no change or increase, 3]; 3) mobility [bedridden or using wheel chair, 0; walking indoors, 1; walking outdoors, 2], 4) physical and/or mental stress [under stress, 0; no stress, 2]; 5) neurological and/or psychological diseases [severe dementia or depression, 0; moderate dementia, 1; none, 2]; and 6) BMI (< 19 kg/m2, 0; 19–21 kg/m2, 1; 21–23 kg/m2, 2; > 23 kg/ m2, 3). Nutritional status was classified based on total score: 0–7 indicates malnutrition, 8–11 indicates risk of malnutrition, and ≥ 12 indicates good nutrition. Patients with an MNA-SF score < 7 were classified in the malnutrition group, while others were classified in the non-malnutrition group.
2.4. Coincidence Analysis between J-Method and MNA-SF
Based on the J-Method and MNA-SF results, patients were divided into the following four groups; 1) The “definite malnutrition group” (D), which included patients identified as being malnourished by both the J-Method and MNA-SF; 2) The “possible malnutrition group by J-Method” (P-J), included patients identified as being malnourished only by the J-Method; 3) The “possible malnutrition group by MNA-SF” (P-M), included patients identified as being malnourished only by the MNA-SF; and 4) The “no malnutrition group” (N), included patients determined as not being malnourished by both screening methods. Four items were compared across these groups: 1) food intake decline over the last 3 months, 2) intake rate of principal food and accompanying dish in the 3 three days post-admission, 3) BMI at admission to our CRW, and 4) serum albumin concentration at admission to our CRW. In addition, to verify the coincidence of the results between the J-Method and MNA-SF, the kappa coefficient (κ) was calculated. The coincidence of the results evaluated by κ were categorized as follows: slight, 0.0–0.20; fair, 0.21–0.40; moderate, 0.41–0.60; substantial, 0.61–0.80, substantial; and almost perfect, 0.81–1.00.
2.5. Statistical Analysis
All analyses were performed using IBM®SPSS Statistics 28 (IBM Corporation, Armonk, NY, USA). For normally distributed data, results were expressed as mean ± standard deviation (SD), and differences among the four groups were determined using a two-way analysis of variance (ANOVA), with the significance of individual differences evaluated using the Tukey–Kramer post hoc test. Fornon-normally distributed data, results were reported as medians with interquartile ranges, and differences among the four groups were analyzed using the Kruskal–Wallis test. The significance of individual differences was evaluated using the Bonferroni method as a post-hoc test. P-values < 0.05 were considered statistically significant.
2.5. Ethics
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committees of Doshisha Women’s College (2020-01) and Jyujyo Takeda Rehabilitation Hospital (20191118-1). All patients and/or their family members were informed of the study's protocols and had the right to withdraw from the study at any time without facing any negative consequences. Personal data were fully anonymized and securely managed throughout the study.
3. Results
Of the 1,161 patients hospitalized in our CRW between February 2020 and February 2022, 168 patients with cerebrovascular diseases aged > 65 years agreed to participate in this study. However, 20 patients (five transferred to an acute hospital due to the deterioration of physical condition; 15 lacked necessary data during hospitalization) were excluded. Finally, 148 patients were included in this study (males, 78; females, 70).
3.1. Nutritional Screening Results by J-Method and MNA-SF
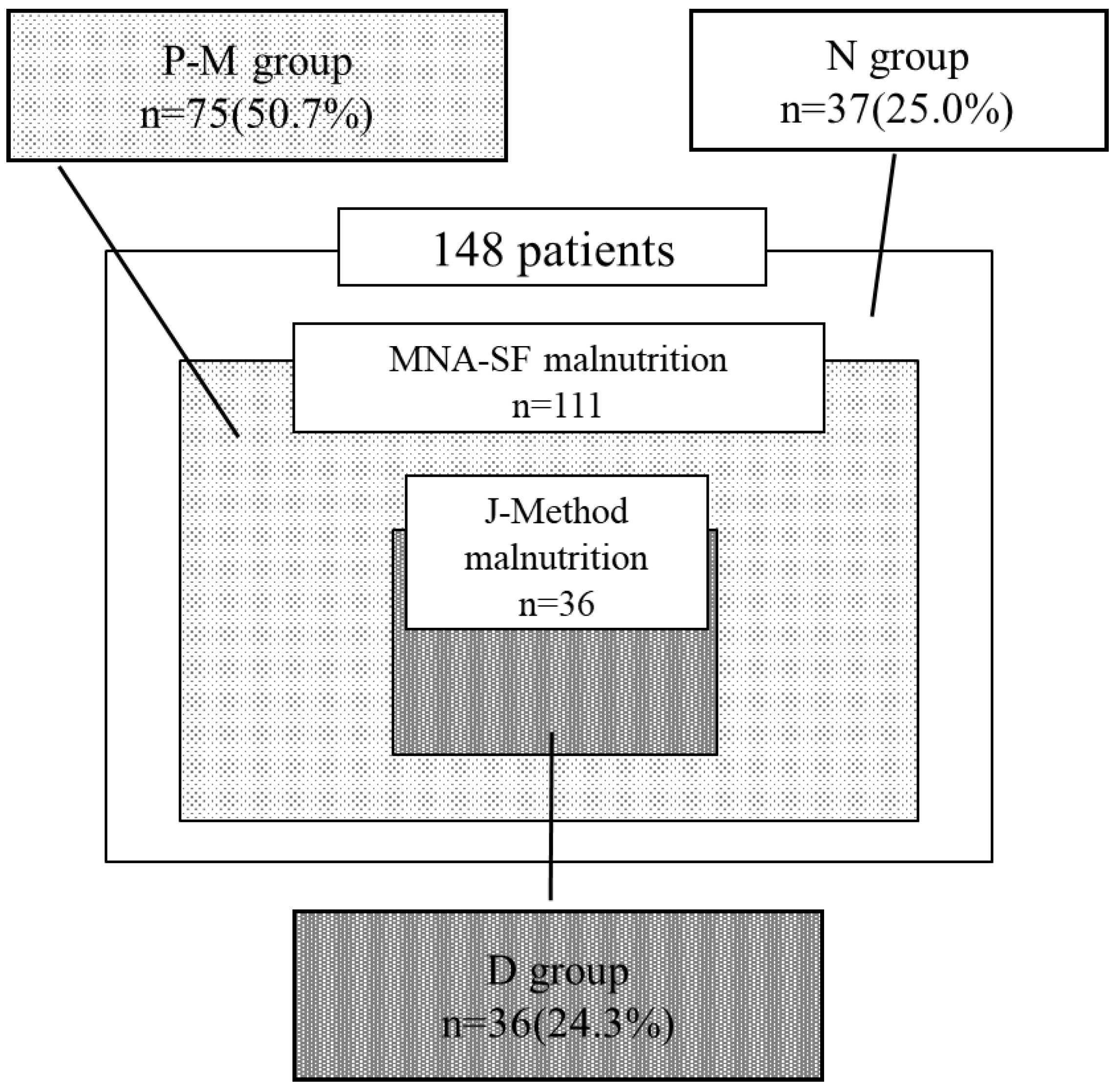
By nutritional screening using J-Method,36 and 112 patients were categorized into the malnutrition and non-malnutrition groups, respectively. Using the MNA-SF, 111 and 37 patients were categorized into the malnutrition and non-malnutrition groups, respectively. Of the patients, 37 (25.0%) and 36 (24.3%) were categorized into the non-malnutrition (N) and definite malnutrition (D) groups, respectively. Notably, 75 (50.4%) were classified as having malnutrition by the MNA-SF (P-M) but as non-malnutrition by the J-Method; however, no patients were in the opposite scenario (P-J) (
Figure 1). Therefore, patients were only classified into only three of the four groups (D, P-M, and N). The kappa coefficient (κ) for the coincidence between the two methods was 0.19 (
Table 1), indicating that the result of nutritional screening by J-Method slightly coincided with those by MNA-SF.
3.2. Decrease of Food Intake in the Three Groups, D, P-M, and N
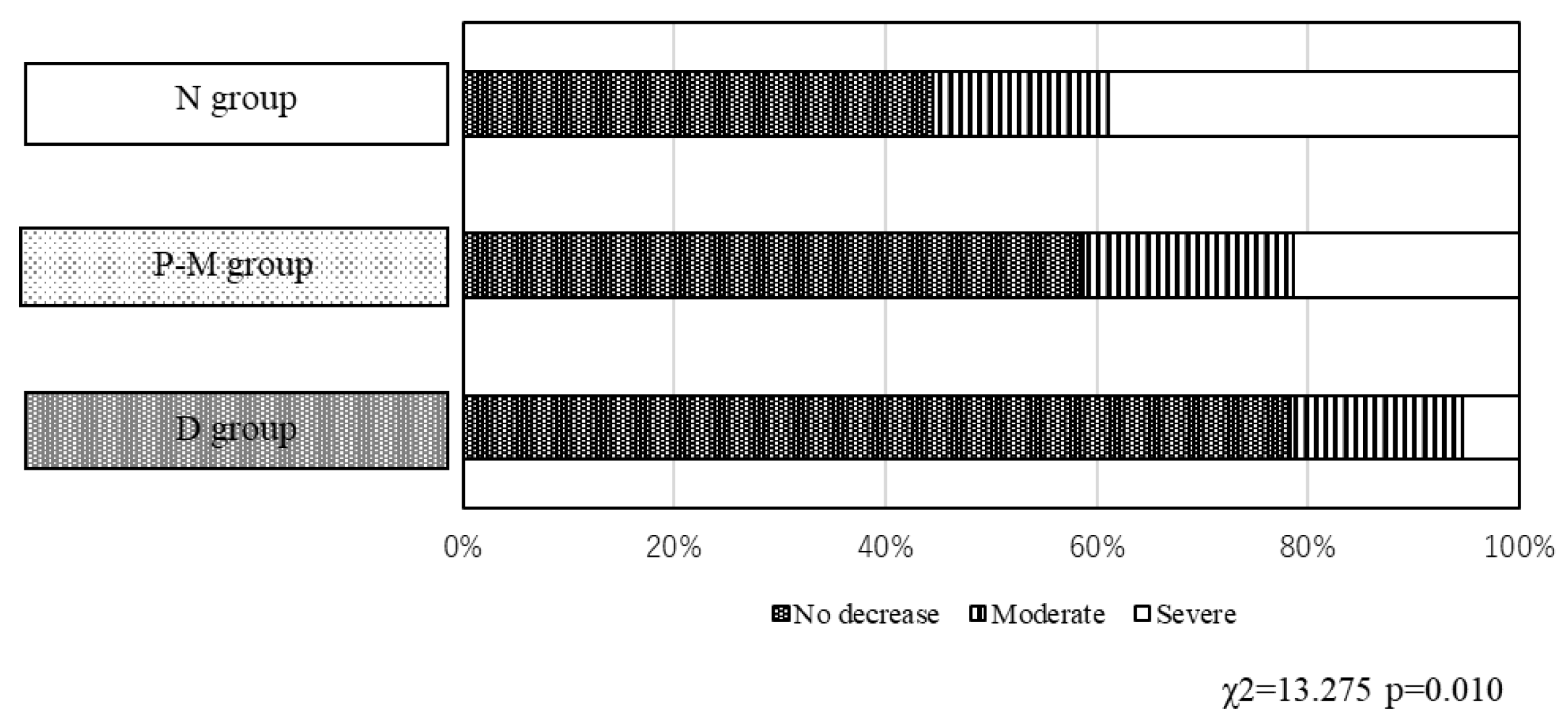
In the N group, 29 of 37 patients (78.4%) reported no changes in their food intake in the last 3 months before admission. While the rate of patients who reported no changes in their food intake decreased in the P-M (44/75, 58.7%) and D (16/36, 44.4%) groups. Conversely, severe decreases in food intake were observed only in the N (2/37, 5.4%) and D (14/36, 38.9%) groups (p = 0.010,
Figure 2), while the proportion of patients with moderate decreases in food intake was similar across the three groups.
3.3. Intake Rate of Principal Food and Accompanying Dish in the Three Groups
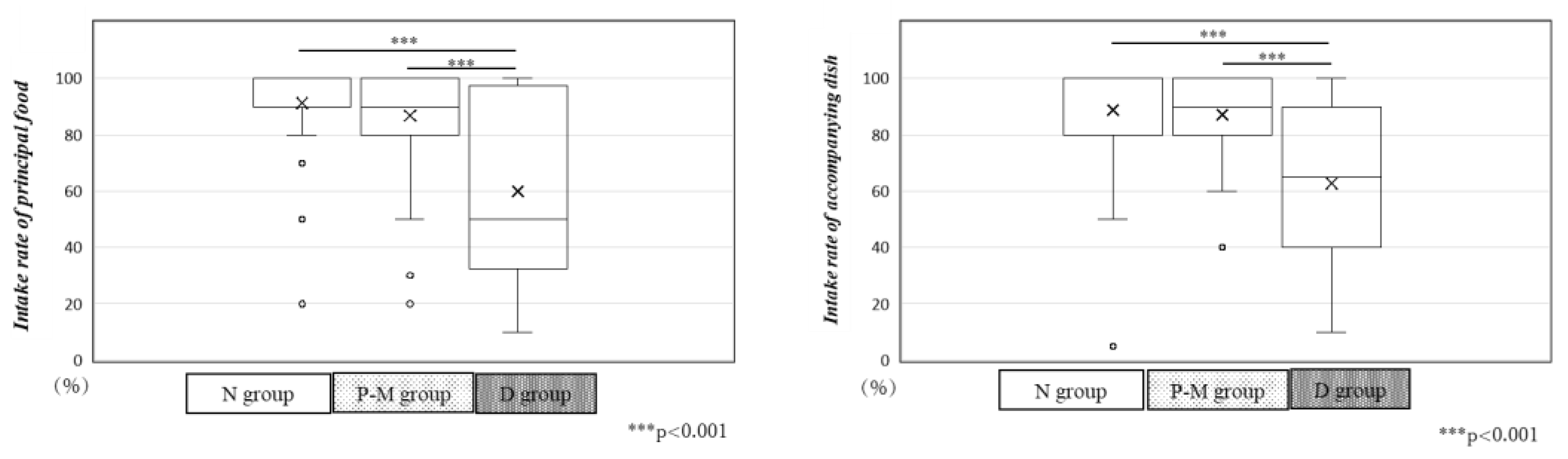
After admission to our CRW, patients in the N group consumed almost all of the principal food and accompanying dishes provided by our hospital. Patients in the P-M group consumed 90%, while those in the D group consumed only 50% of the principal food and 65% of the accompanying dishes, representing a significant decrease (p < 0.001,
Figure 3).
3.4. BMI at Admission to Our CRW in the Three Groups
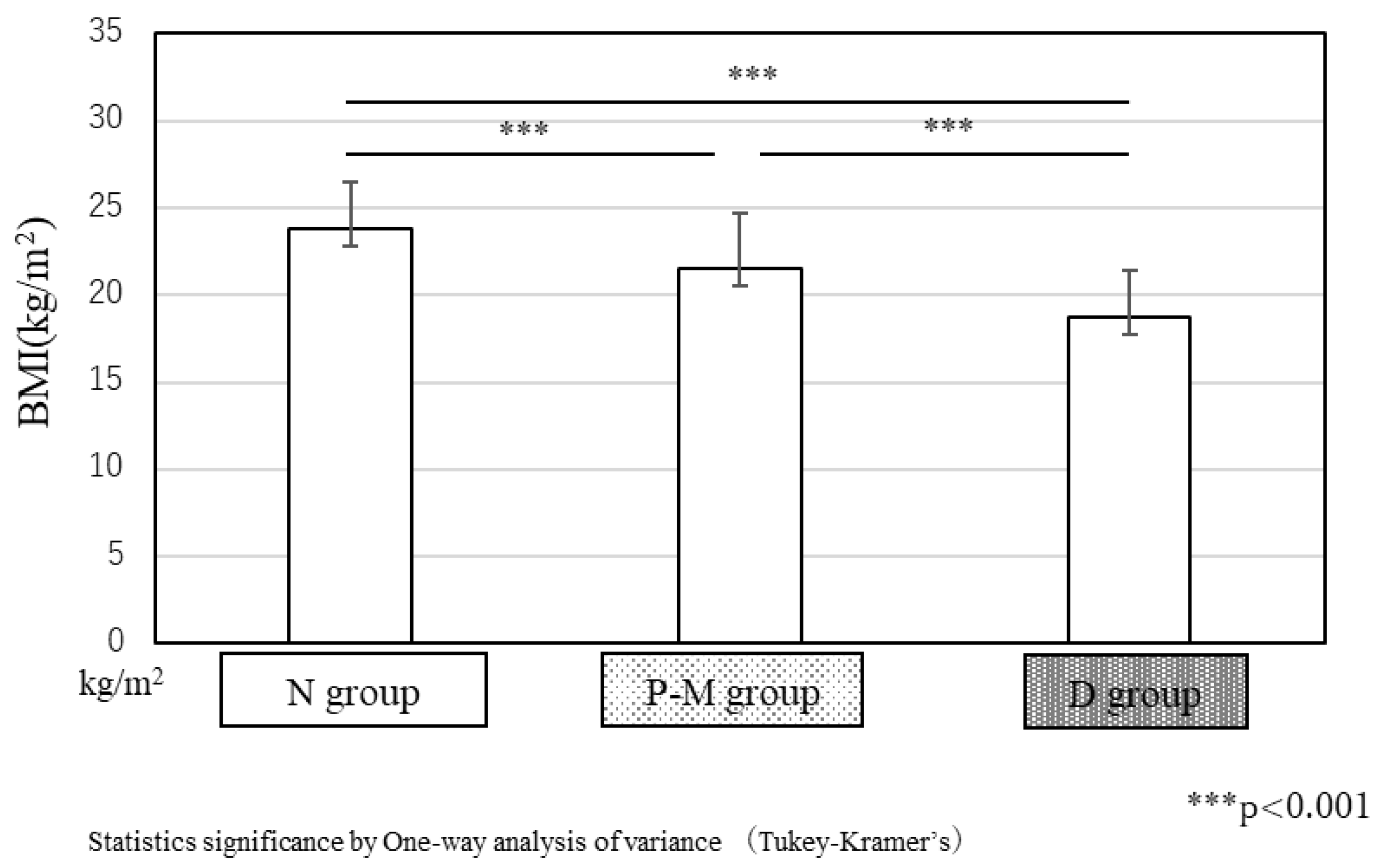
The average BMI of patients at admission was 23.8 ± 2.7 kg/m
2, in the N group, above ideal body weight. In the P-M group, the average BMI was 21.5 ± 3.2 kg/m
2, slightly below ideal BW but within the normal range. In the D group, the average BMI was significantly lower (18.7 ± 2.7 kg/m², p < 0.001,
Figure 4), indicating low body weight.
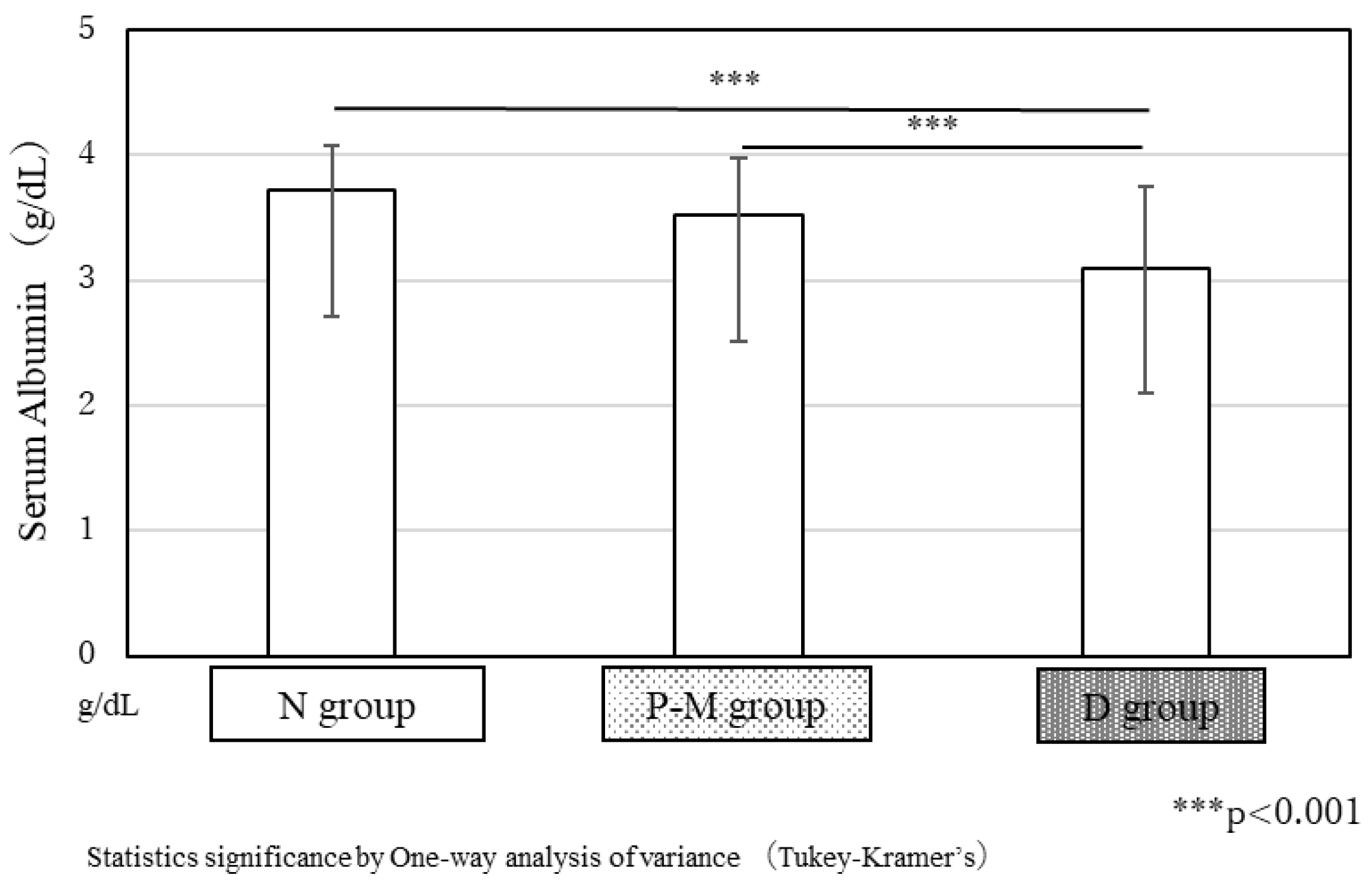
3.5. Concentration of Serum Albumin at the Admission in Our CRW in the 3 Groups
In the N and P-M groups, the average serum albumin concentration at the admission was 3.7 ± 0.4 g/dL and 3.5 ± 0.5 g/dL, respectively, showing no significant differences. However, in the D group, the average serum albumin concentration was 3.1 ± 0.7 g/dL, significantly lower than that in the other two groups (p < 0.001,
Figure 5).
4. Discussion
With the J-Method, we were able to conveniently obtain nutritional information without patient interviews, which was a key advantage. To establish the J-Method, we referred to several nutritional screening methods to obtain the best results. In contrast, the MNA-SF is a widely used screening method [
16] with proven validity for older adults [
17,
18,
19,
20]. The Global Leadership Initiative on Malnutrition (GLIM) [
21] also recommends the MNA-SF as a primary nutritional screening method. Moreover, patients with malnutrition diagnosed using the MNA-SF are more likely to develop sarcopenia [
22], with their malnutrition severity of malnutrition being inversely related to their Barthel Index scores [
23], which can show independence in ADL. Therefore, we compared the results of our J-Method with those of the MNA-SF in this study.
The kappa coefficient (κ = 0.19) indicated slight agreement between the two methods. Several factors may explain this. All patients classified as having malnutrition by the J-Method were also categorized as such by the MNA-SF (D group), but no patients were identified as having malnutrition by the J-Method but not by the MNA-SF. Moreover, > 50% of patients were classified as having malnutrition by the MNA-SF only. This may be due to MNA-SF’s inclusion of items like “walking independently” and “experience of mental stress and/or acute diseases during the last 3 months.” Most of the patients admitted to our CRW after acute diseases, such as cerebrovascular diseases and/or orthopedic disorders, including femoral neck fracture and vertebral compression fracture. In other words, most of the convalescent rehabilitation patients had experienced acute diseases and could not walk independently at the time of admission to our CRW. Thus, when the MNA-SF was used in these patients, it is likely that the MNA-SF scores would be low and that the patients were determined as having malnutrition. The MNA-SF has recently been reported to have high sensitivity but low specificity [
24], making it a less suitable nutritional screening method for convalescent rehabilitation patients. In contrast, 24.3% of patients were diagnosed as having malnutrition by the J-Method. As mentioned above, > 40% of patients in rehabilitation settings may suffer from malnutrition. Although our results were lower than expected, the J-Method shows potential as a specialized nutritional screening method for convalescent rehabilitation patients.
While food intake for the last 3 months before admission did not change in > 50% of the patients in the P-M group, it moderately or severely decreased in > 50% of the patients in the D group. These trends persisted after admission, with the D group consuming significantly less of the principal food and accompanying dishes provided by the hospital than the N and P-M groups. This result suggests that the patients’ food intake declined gradually before admission rather than immediately upon admission. Moreover, patients in the D-group had lower BMI and serum albumin concentration than those in the N and P-M groups. It has been reported that low BMI and/or serum albumin concentration diminishes the effect of convalescent rehabilitation. Therefore, it is crucial to identify patients who have malnutrition accurately and intervene early; however, improvements in food intake should also be prioritized in the acute hospital.
The question items in the J-Method were extracted from several nutritional screening methods recommended in nutritional assessment guidelines (
Table 2), with each item being individually significant in those screening methods. When performing nutritional screening, combining several methods is recommended [
13]. This indicates that, by combining various nutritional screening methods, we can develop a novel and effective tool like the J-Method. To verify the efficacy of the J-Method, it was initially tested only in our rehabilitation setting. We do not consider the J-Method the best method for the CRW, and its question items may be revised in the future. For broader application, the J-Method should be implemented in other institutions. Nonetheless, it has significant potential for improving the evaluation of nutritional status in convalescent rehabilitation patients.
5. Conclusions
In conclusion, we developed a novel nutritional screening method specifically for convalescent rehabilitation settings and compared its results with those of the MNA-SF. In these settings, the J-Method may prove to be more effective than the MNA-SF. Continuous efforts to refine nutritional screening methods will help improve nutrition management in convalescent rehabilitation patients.
Author Contributions
KO designed this study. YT, SI, and HI collected and evaluated clinical data. KO collected nutritional data. KO and MK interpreted the data, and then KO and HI prepared this draft manuscript. MK managed this study, and MK, YT, and SI revised this manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committees of Doshisha Women’s College (2020-01) and Jyujyo Takeda Rehabilitation Hospital (20191118-1).
Informed Consent Statement
All patients and/or their family members were informed of the study's protocols and had the right to withdraw from the study at any time without facing any negative consequences. Personal data were fully anonymized and securely managed throughout the study.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Editage (
www.editage.com) for English language editing.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; Jensen, G.L.; Malone, A.; Muscaritoli, M.; Nyulasi, I.; Pirlich, M.; Rothenberg, E.; Schindler, K.; Schneider, S.M.; De van der Schueren, M.A.E.; Sieber, C.; Valentini, L.; Yu, J.C.; Van Gossum, A.; Singer, P. ESPEN Guidelines on Definitions and Terminology of Clinical Nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Bauer, J.; Rämsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.; Anthony, P.; Charlton, K.; Maggio, M.; Tsai, A.; Vellas, B.; Sieber, C.; Mini Nutritional Assessment International Group. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J. Am. Geriatr. Soc. 2010, 58, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, S.; Wakabayashi, H.; Nishioka, E.; Yoshida, T.; Mori, N.; Watanabe, R. Nutritional Improvement correlates with Recovery of Activities of Daily Living among Malnourished Elderly Stroke Patients in the Convalescent Stage: A Cross-Sectional Study. J. Acad. Nutr. Diet. 2016, 16, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Morioka, K.; Okamoto, K.; Tsutsumi, H.; Ishino, S.; Kiritani, N.; Kubo, H.; Nara, M.; Tsuji, Y. Energy requirements for patients in convalescent rehabilitation using motor scores as in the Functional Independent Measure. Asia Pac. J. Clin. Nutr. 2019, 28, 31–34. [Google Scholar]
- Okamoto, K.; kogirima, M.; Ishino, S.; Tsuji, Y.; Inoue, H. The impact of energy and protein intake on rehabilitation efficiency in convalescent patients. Asia Pac. J. Clin. 2024, 33, 33–38. [Google Scholar]
- Schueren, M.; Guaitoli, P.; Jansma, E.; Vet, H. Nutrition screening tools: does one size fit all? A systematic review of screening tools for the hospital setting. Clin. Nutr. 2014, 33, 39–58. [Google Scholar] [CrossRef]
- Kondrup, J. , Allison, S., Elia, M.; B Vellas, B.; Plauth, M.; Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Phillips, M.B.; Foley, A.L.; Barnard, R.; Isenring, E.A.; Miller, M.D. Nutritional Screening in Community-Dwelling Older Adults: A Systematic Literature Review. Asia Pac. J. Clin. Nutr. 2010, 19, 440–449. [Google Scholar]
- Salvi, F.; Giorgi, R.; Grilli, A.; Morichi, V.; Espinosa, E.; Spazzafumo, L.; Marinozzi, M.L.; Dessì-Fulgheri, P. Mini Nutritional Assessment (Short Form) and Functional Decline in Older Patients Admitted to an Acute Medical Ward Aging Clinical and Experimental Research. Aging Clin. Exp. Res. 2008, 20, 322–328. [Google Scholar] [CrossRef]
- Dent, E.; Chapman, I.M.; Piantadosi, C.; Visvanathan, R. Performance of Nutritional Screening Tools in Predicting Poor Six-Month Outcome in Hospitalised Older Patients. Asia Pac. J. Clin. Nutr. 2014, 23, 394–399. [Google Scholar]
- Dent, E.; Chapman, I.; Piantadosi, C.; Visvanathan, R. Nutritional Screening Tools and Anthropometric Measures Associate with Hospital Discharge Outcomes in Older People. Australas. J. Ageing. 2015, 34, E1–E6. [Google Scholar] [CrossRef] [PubMed]
- Raslan, M.; Gonzalez, M.C.; Gonçalves Dias, M.C.; Nascimento, M.; Castro, M.; Marques, P.; Segatto, S.; Torrinhas, R.S.; Cecconello, I.; Waitzberg, D.L. Comparison of Nutritional Risk Screening Tools for Predicting Clinical Outcomes in Hospitalized Patients. Nutrition. 2010, 26, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Rasheedy, D.; El-Kawaly, W.H. The Accuracy of the Geriatric Nutritional Risk Index in Detecting Frailty and Sarcopenia in Hospitalized Older Adults. Aging Clin. Exp. Res. 2020, 32, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Skipper, A.; Ferguson, M.; Thompson, K.; Castellanos, V.; Porcari, J. Nutrition screening tools: an analysis of the evidence. JPEN. J. Parenter. Enter. Nutr. 2012, 36, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Skipper, A.; Coltman, A.; Tomesko, J.; Charney, P.; Porcari, J.; Piemonte, T.; Handu, D.; Cheng, F. Adult Malnutrition (Undernutrition) Screening: An Evidence Analysis Center Systematic Review. J. Acad. Nutr. Diet. 2020, 120, 669–708. [Google Scholar] [CrossRef]
- Rubenstein, LZ.; Harker, J.O.; Salva, A.; Guigoz, Y.; Vellas, B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. A-Biol. 2001, 56, 366–372. [Google Scholar] [CrossRef]
- Kaiser, M.; Bauer, J.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.; Anthony, P.; Charlton, E.; Maggio, M.; Tsai, A.; Grathwohl, D.; Vellas, B.; Sieber, C. MNA-International Group. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J. Nutr. Health Aging. 2009, 13, 782–8. [Google Scholar] [CrossRef]
- Guigoz, Y. The Mini Nutritional Assessment (MNA) review of the literature--What does it tell us? J Nutr Health Aging. 2006, 10, 466–485. [Google Scholar]
- Rubenstein, L.; Harker, J.; Salvà, A.; Y Guigoz, Y.; Vellas, B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. 2001, 56, M366–M372. [Google Scholar] [CrossRef]
- Vellas, B.; Villars, H.; Abellan, G.; Abellan; Soto, M.; Rolland, Y.; Guigoz, Y.; Morley, J.; Chumlea, W.; Salva, A.; Rubenstein, L.; Garry, P. Overview of the MNA--Its history and challenges. J. Nutr. Health Aging. 2006, 10, 456–463. [Google Scholar]
- Morley, J.; Muscaritoli, M.; Nyulasi, I.; Pirlich, M.; Pisprasert, V.; Schueren, M.; Siltharm, S.; Singer, P.; Tappenden, K.; Velasco, N.; Waitzberg, D.; Yamwong, P.; Gossum, J.; Compher, C.; GLIM Core Leadership Committee; GLIM Working Group. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 10, 207–217. [Google Scholar]
- Yürüyen, M.; Yavuzer, H.; Yavuzer, S.; Cengiz, M.; Demirdağ, F.; Kara, Z.; Avci, S.; Yurttaş, N.; İslamoğlu, M.; İmre, E.; Taşkin, A.; Döventaş, A.; Erdinçlerş, D. Comparison of nutritional risk screening tools for predicting sarcopenia in hospitalized patients. Turk. J. Med. Sci. 2017, 47, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Lelli, D.; Calle, A.; Pérez, L.; Onder, G.; Morandi, A.; Ortolani, E.; Colominas, M.; Pedone, C.; Inzitari, M. Nutritional Status and Functional Outcomes in Older Adults Admitted to Geriatric Rehabilitations: The SAFARI Study. J. Am. Coll Nutr. 2019, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, S.; Omagari, K.; Nishioka, E.; Mori, N.; Taketani, Y.; Kayashita, J. Concurrent and predictive validity of the Mini Nutritional Assessment Short-Form and the Geriatric Nutritional Risk Index in older stroke rehabilitation patients. J. Hum. Nutr. Diet. 2020, 33, 12–22. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Figure 1.
The results of nutritional screening using the J-Method and MNA-SF. The J-Method and the MNA-SF identified 36 and 111 patients as having malnutrition, respectively. While 37 patients (25.0%) were classified as “N,” 36 (24.3%) were classified as “D” when combining the J-Method and MNA-SF. Notably, 75 patients (50.4%) were classified as having malnutrition by the MNA-SF but as non-malnutrition by J-Method (P-M).
Figure 1.
The results of nutritional screening using the J-Method and MNA-SF. The J-Method and the MNA-SF identified 36 and 111 patients as having malnutrition, respectively. While 37 patients (25.0%) were classified as “N,” 36 (24.3%) were classified as “D” when combining the J-Method and MNA-SF. Notably, 75 patients (50.4%) were classified as having malnutrition by the MNA-SF but as non-malnutrition by J-Method (P-M).
Figure 2.
Food intake in the D, P-M, and N groups.The percentage of patients who reported no changes in food intake over the last three months before admission were as follows: 78.4% (29/37 patients), 58.7% (44/75 patients), and 44.4% (16/36 patients) in the N, P-M, and D groups, respectively. Significant differences were observed between the three groups (p = 0.010).
Figure 2.
Food intake in the D, P-M, and N groups.The percentage of patients who reported no changes in food intake over the last three months before admission were as follows: 78.4% (29/37 patients), 58.7% (44/75 patients), and 44.4% (16/36 patients) in the N, P-M, and D groups, respectively. Significant differences were observed between the three groups (p = 0.010).
Figure 3.
Principal food and accompanying dish intake rates in the three groups. In the N and P-M groups, the intake of principal food and accompanying dishes were 100% and 90%, respectively. However, in the D group, the intake of principal food and accompanying dishes was only 50% and 65%, respectively, significantly lower than that of the other two groups (p < 0.001).
Figure 3.
Principal food and accompanying dish intake rates in the three groups. In the N and P-M groups, the intake of principal food and accompanying dishes were 100% and 90%, respectively. However, in the D group, the intake of principal food and accompanying dishes was only 50% and 65%, respectively, significantly lower than that of the other two groups (p < 0.001).
Figure 4.
BMI at admission to our CRW in the three groups.The average BMI in the N and P-M groups were 23.8 ± 2.7 kg/m² and 21.5 ± 3.2 kg/m², respectively. However, in the D group, the average BMI was only 18.7 ± 2.7 kg/m², significantly lower than that in the other groups (p < 0.001).
Figure 4.
BMI at admission to our CRW in the three groups.The average BMI in the N and P-M groups were 23.8 ± 2.7 kg/m² and 21.5 ± 3.2 kg/m², respectively. However, in the D group, the average BMI was only 18.7 ± 2.7 kg/m², significantly lower than that in the other groups (p < 0.001).
Figure 5.
Serum albumin concentration at admission to our CRW in the three groups.In the N and P-M groups, average serum albumin concentrations at the admission were 3.7 ± 0.4 g/dL and 3.5 ± 0.5 g/dL, respectively, showing no significant difference. However, in the D group, the average serum albumin concentration was only 3.1 ± 0.7 g/dL, significantly lower than that in the other two groups (p < 0.001).
Figure 5.
Serum albumin concentration at admission to our CRW in the three groups.In the N and P-M groups, average serum albumin concentrations at the admission were 3.7 ± 0.4 g/dL and 3.5 ± 0.5 g/dL, respectively, showing no significant difference. However, in the D group, the average serum albumin concentration was only 3.1 ± 0.7 g/dL, significantly lower than that in the other two groups (p < 0.001).
Table 1.
The results of nutritional screening by J-Method and MNA-SF.
Table 1.
The results of nutritional screening by J-Method and MNA-SF.
| |
|
MNA-SF |
Total |
| |
|
malnutrition |
non-malnutrition |
| J-Method |
malnutrition |
36 |
0 |
36 |
| non-malnutrition |
75 |
37 |
112 |
| |
Total |
111 |
37 |
148 |
| |
|
|
|
κ=0.19 |
Table 2.
Combination of several nutritional screening methods recommended in nutrition assessment guidelines.
Table 2.
Combination of several nutritional screening methods recommended in nutrition assessment guidelines.
| Nutritional screening method |
Rate of body weight loss |
Decrease in food intake |
BMI |
Ideal weight ratio(BW/IBW) |
Acute disease |
Serum Alb |
TLC |
TC |
Digestive symptoms |
TPN |
Decubitus |
| GNRI |
|
|
|
〇 |
|
〇 |
|
|
|
|
|
| CONUT |
|
|
|
|
|
〇 |
〇 |
〇 |
|
|
|
| MST |
〇 |
〇 |
|
|
|
|
|
|
|
|
|
| MUST |
〇 |
〇 |
〇 |
|
|
|
|
|
|
|
|
| NRS2002 |
〇 |
〇 |
〇 |
|
〇 |
|
|
|
|
|
|
| J-Method |
〇 |
〇 |
〇 |
Substituted
by BMI
|
|
〇 |
|
|
〇 |
〇 |
〇 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).