Submitted:
21 October 2024
Posted:
21 October 2024
Read the latest preprint version here
Abstract
Keywords:
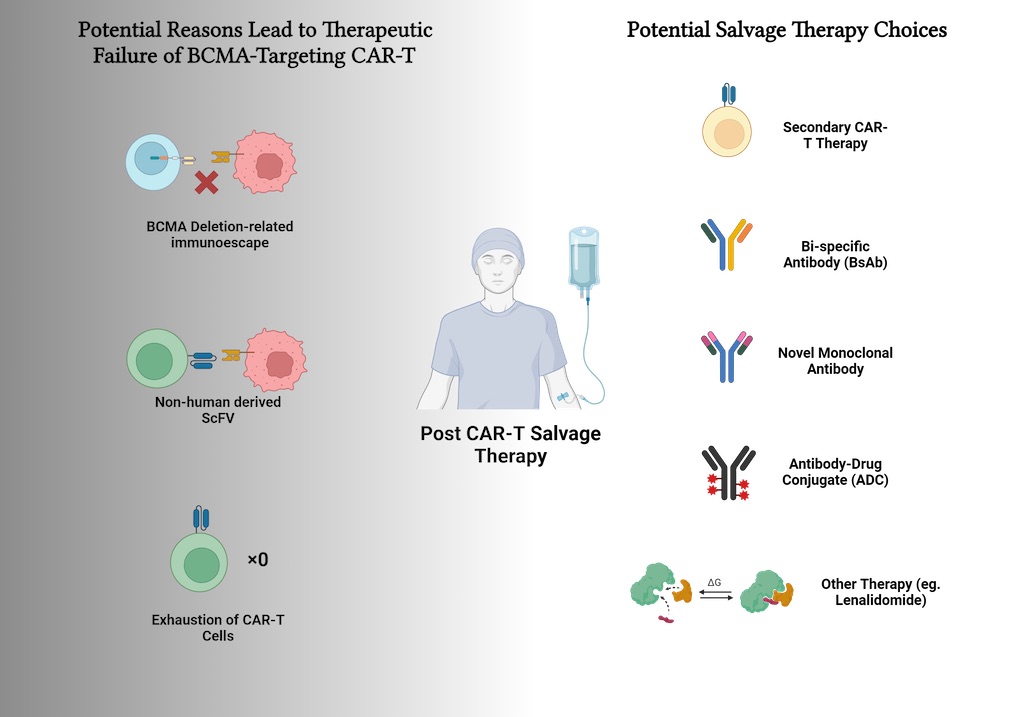
1. Introduction
2. Literature Review
2.1. Current Landscape of CAR-T Cell Therapy towards Multiple Myeloma
2.2. Potential Factors Contributing to CAR-T Therapy Failure
2.3. Salvage Therapies
2.3.1. Secondary CAR-T Cell Therapy
2.3.2. Bispecific Antibodies
2.3.3. Novel Monoclonal Antibodies (PD-1/PD-L1)
2.3.4. Antibody-Drug Conjugates (ADCs)
2.3.5. Other Treatments
2.4. Recommended Strategies
2.4.1. Moving CAR-T Therapy to Earlier lines Of Treatment
2.4.2. Exploring CAR-T Cells with Different Targets and Origins
2.4.3. Rapid-Manufactured CAR-T
2.4.4. Sequential Selection of T-Cell Redirecting Therapies
2.4.5. Combination Therapy
2.4.6. Maintenance Therapy
3. Conclusions
This study is funded by Technical Innovation Guidance Program of Jiangxi Province, China, (20212BDH80014) and the Science and Technology Innovation Action Plan of Shanghai, China, (23S11905500).
Acknowledgments
Conflicts of Interest
References
- Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N and Chen W (2022) Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 135:584-590. [CrossRef]
- Kumar SK, Callander NS, Adekola K, Anderson LD, Jr., Baljevic M, Baz R, Campagnaro E, Castillo JJ, Costello C, D'Angelo C, Devarakonda S, Elsedawy N, Garfall A, Godby K, Hillengass J, Holmberg L, Htut M, Huff CA, Hultcrantz M, Kang Y, Larson S, Lee HC, Liedtke M, Martin T, Omel J, Robinson T, Rosenberg A, Sborov D, Schroeder MA, Sherbenou D, Suvannasankha A, Valent J, Varshavsky-Yanovsky AN, Kumar R and Snedeker J (2023) Multiple Myeloma, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 21:1281-1301. [CrossRef]
- Hu D, Chen L, Yan D, Dong W, Chen M, Niu S, Wang S, Zhang J, Nie X and Fang Y (2023) Effectiveness and safety of anti-BCMA chimeric antigen receptor T-cell treatment in relapsed/refractory multiple myeloma: a comprehensive review and meta-analysis of prospective clinical trials. Front Pharmacol 14:1149138. [CrossRef]
- Wang Q, Wei R, Guo S, Min C, Zhong X, Huang H and Cheng Z (2024) An alternative fully human anti-BCMA CAR-T shows response for relapsed or refractory multiple myeloma with anti-BCMA CAR-T exposures previously. Cancer Gene Ther 31:420-426. [CrossRef]
- Keller AL, Parzych SE, Reiman LT, Walker ZJ, Forsberg PA and Sherbenou DW (2023) BCMAxCD3 Bispecific Antibody Elranatamab Is Effective in Patient Myeloma Relapsed after BCMA CAR-T. Blood 142:4684. [CrossRef]
- Reyes KR, Liu Y-C, Huang C-Y, Banerjee R, Martin T, Shah N, Wong SW, Wolf JL, Arora S and Chung A (2022) Clinical Outcomes and Salvage Therapies in Patients with Relapsed/Refractory Multiple Myeloma Following Progression on BCMA-Targeted CAR-T Therapy. Blood 140:617-619.
- Van Oekelen O, Nath K, Mouhieddine TH, Farzana T, Aleman A, Melnekoff DT, Ghodke-Puranik Y, Shah GL, Lesokhin A, Giralt S, Thibaud S, Rossi A, Rodriguez C, Sanchez L, Richter J, Richard S, Cho HJ, Chari A, Usmani SZ, Jagannath S, Shah UA, Mailankody S and Parekh S (2023) Interventions and outcomes of patients with multiple myeloma receiving salvage therapy after BCMA-directed CAR T therapy. Blood 141:756-765. [CrossRef]
- Liu R, Yang R, Xu X, Zhao W, Wang F, Zhang W, Lei B, Yang R, Wang Y, He A and Wang J (2024) Outcomes in patients with multiple myeloma receiving salvage treatment after BCMA-specific CAR-T therapy: A retrospective analysis of LEGEND-2. Br J Haematol. [CrossRef]
- Snyder J, Bellman P, Alsaddi Z, Alkharabsheh O, Paul B, Hashmi H, Lutfi F, Ahmed N, Mahmoudjafari Z, Abdallah A-O and Atrash S (2024) Patient Outcomes Following First and Second Exposure to BCMA-Directed Therapies Including CAR-T Cell Therapy in Relapsed/Refractory Multiple Myeloma. Transplantation and Cellular Therapy 30:S394-S395. [CrossRef]
- Bernabei L, Garfall AL, Melenhorst JJ, Lacey SF, Stadtmauer EA, Vogl DT, Gonzalez V, Plesa G, Young RM, Waxman A, Levine BL, June CH, Milone MC and Cohen AD (2018) PD-1 Inhibitor Combinations As Salvage Therapy for Relapsed/Refractory Multiple Myeloma (MM) Patients Progressing after Bcma-Directed CAR T Cells. Blood 132:1973-1973.
- Chung A, Huang C-Y, Martin T, Wolf JL, Wong SW and Shah N (2022) Phase II Study of Pembrolizumab in Multiple Myeloma Patients Relapsing after or Refractory to Anti-BCMA CAR-T Therapies. Blood 140:12651-12652.
- Jakubowiak AJ, Anguille S, Karlin L, Chari A, Schinke C, Rasche L, San-Miguel J, Campagna M, Hilder BW, Masterson TJ, Qin X, Renaud T, Tolbert J, Vishwamitra D, Skerget S and Moreau P (2023) Updated Results of Talquetamab, a GPRC5D×CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Multiple Myeloma with Prior Exposure to T-Cell Redirecting Therapies: Results of the Phase 1/2 MonumenTAL-1 Study. Blood 142:3377. [CrossRef]
- Grajales-Cruz AF, Castaneda O, Hansen DK, Vazquez-Martinez MA, Blue B, Khadka S, Liu H, Ochoa-Bayona JL, Freeman CLL, Locke FL, Nishihori T, Shain K, Baz R and Alsina M (2023) Teclistamab Induces Favorable Responses in Patients with Relapsed and Refractory Multiple Myeloma after Prior BCMA-Directed Therapy. Blood 142:3351. [CrossRef]
- Ferreri CJ, Hildebrandt MAT, Hashmi H, Shune LO, McGuirk JP, Sborov DW, Wagner CB, Kocoglu MH, Rapoport A, Atrash S, Voorhees PM, Khouri J, Dima D, Afrough A, Kaur G, Anderson LD, Jr., Simmons G, Davis JA, Kalariya N, Peres LC, Lin Y, Janakiram M, Nadeem O, Alsina M, Locke FL, Sidana S, Hansen DK, Patel KK and Castaneda Puglianini OA (2023) Real-world experience of patients with multiple myeloma receiving ide-cel after a prior BCMA-targeted therapy. Blood Cancer J 13:117. [CrossRef]
- Popat R, Zweegman S, Cavet J, Yong K, Lee L, Faulkner J, Kotsopoulou E, Al-Hajj M, Thomas S, Cordoba SP, Pule M, Cerec V, Peddareddigari VGR, Khokhar NZ and Menne TF (2019) Phase 1 First-in-Human Study of AUTO2, the First Chimeric Antigen Receptor (CAR) T Cell Targeting APRIL for Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 134:3112-3112.
- Reyes KR, Liu YC, Huang CY, Banerjee R, Martin T, Wong SW, Wolf JL, Arora S, Shah N, Chari A and Chung A (2024) Salvage therapies including retreatment with BCMA-directed approaches after BCMA CAR-T relapses for multiple myeloma. Blood Adv 8:2207-2216. [CrossRef]
- Smith EL, Harrington K, Staehr M, Masakayan R, Jones J, Long TJ, Ng KY, Ghoddusi M, Purdon TJ, Wang X, Do T, Pham MT, Brown JM, De Larrea CF, Olson E, Peguero E, Wang P, Liu H, Xu Y, Garrett-Thomson SC, Almo SC, Wendel HG, Riviere I, Liu C, Sather B and Brentjens RJ (2019) GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med 11. [CrossRef]
- Zhang X, Zhang H, Lan H, Wu J and Xiao Y (2023) CAR-T cell therapy in multiple myeloma: Current limitations and potential strategies. Front Immunol 14:1101495. [CrossRef]
- Xu J, Wang BY, Yu SH, Chen SJ, Yang SS, Liu R, Chen LJ, Hou J, Chen Z, Zhao WH, He AL, Mi JQ and Chen SJ (2024) Long-term remission and survival in patients with relapsed or refractory multiple myeloma after treatment with LCAR-B38M CAR T cells: 5-year follow-up of the LEGEND-2 trial. J Hematol Oncol 17:23. [CrossRef]
- Lin Y, Raje NS, Berdeja JG, Siegel DS, Jagannath S, Madduri D, Liedtke M, Rosenblatt J, Maus MV, Massaro M, Petrocca F, Yeri A, Finney O, Caia A, Yang Z, Martin N, Campbell TB, Rytlewski J, Fuller J, Hege K, Munshi NC and Kochenderfer JN (2023) Idecabtagene vicleucel for relapsed and refractory multiple myeloma: post hoc 18-month follow-up of a phase 1 trial. Nat Med 29:2286-2294. [CrossRef]
- Li C, Wang D, Song Y, Huang H, Li J, Chen B, Liu J, Dong Y, Hu K, Liu P, Zhang X, Mi J-Q, Li Z, Ding K, Xu A-n, Cai S-b, Guo J-j, Gui H-y, Wang W and Qiu L (2023) CT103A, a novel fully human BCMA-targeting CAR-T cells, in patients with relapsed/refractory multiple myeloma: Updated results of phase 1b/2 study (FUMANBA-1). Journal of Clinical Oncology 41:8025-8025. [CrossRef]
- Fu C, Chen W, Cai Z, Yan L, Wang H, Shang J, Wu Y, Yan S, Gao W, Shi X, Han X, Tang F, Zheng G, Wen Y, Meng X, Zheng W, Wang H and Li Z (2023) Three-Year Follow-up on Efficacy and Safety Results from Phase 1 Lummicar Study 1 of Zevorcabtagene Autoleucel in Chinese Patients with Relapsed or Refractory Multiple Myeloma. Blood 142:4845-4845. [CrossRef]
- Hashmi H, Hansen DK, Peres LC, Puglianini OC, Freeman C, De Avila G, Sidana S, Shune L, Sborov DW, Davis J, Wagner C, Kocoglu MH, Atrash S, Voorhees P, Simmons G, Ferreri C, Kalariya N, Anderson LD, Jr., Afrough A, Dima D, Khouri J, McGuirk J, Locke F, Baz R, Patel KK and Alsina M (2024) Factors associated with refractoriness or early progression after idecabtagene vicleucel in patients with relapsed/ refractory multiple myeloma: US Myeloma Immunotherapy Consortium real world experience. Haematologica 109:1514-1524. [CrossRef]
- Huang H, Hu Y, Zhang M, Wei G, Zhou L, Fu S, Feng J, Hong R, Cui J, Huang S, Zhou J, Tang Y, Ding X, Zhuo L, Zhang Y, Xu R and He X (2024) OriCAR-017, a novel GPRC5D-targeting CAR-T, in patients with relapsed/refractory multiple myeloma: Long term follow-up results of phase 1 study (POLARIS). 42:7511-7511. [CrossRef]
- Xia J, Li H, Yan Z, Zhou D, Wang Y, Qi Y, Cao J, Li D, Cheng H, Sang W, Zhu F, Sun H, Chen W, Qi K, Yan D, Qiu T, Qiao J, Yao R, Liu Y, Wang X, Zhang Y, Peng S, Huang CH, Zheng J, Li Z, Chang AH and Xu K (2023) Anti-G Protein-Coupled Receptor, Class C Group 5 Member D Chimeric Antigen Receptor T Cells in Patients With Relapsed or Refractory Multiple Myeloma: A Single-Arm, Phase Ⅱ Trial. J Clin Oncol 41:2583-2593. [CrossRef]
- Mei H, Li C, Jiang H, Zhao X, Huang Z, Jin D, Guo T, Kou H, Liu L, Tang L, Yin P, Wang Z, Ai L, Ke S, Xia Y, Deng J, Chen L, Cai L, Sun C, Xia L, Hua G and Hu Y (2021) A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma. J Hematol Oncol 14:161. [CrossRef]
- Li C, Xu J, Luo W, Liao D, Xie W, Wei Q, Zhang Y, Wang X, Wu Z, Kang Y, Zheng J, Xiong W, Deng J, Hu Y and Mei H (2024) Bispecific CS1-BCMA CAR-T cells are clinically active in relapsed or refractory multiple myeloma. Leukemia 38:149-159. [CrossRef]
- Du J, Fu W-J, Jiang H, Dong B, Gao L, Liu L, Ge J, He A, Li L and Lu JJJCO (2023) Updated results of a phase I, open-label study of BCMA/CD19 dual-targeting fast CAR-T GC012F for patients with relapsed/refractory multiple myeloma (RRMM). 41:8005.
- Da Via MC, Dietrich O, Truger M, Arampatzi P, Duell J, Heidemeier A, Zhou X, Danhof S, Kraus S, Chatterjee M, Meggendorfer M, Twardziok S, Goebeler ME, Topp MS, Hudecek M, Prommersberger S, Hege K, Kaiser S, Fuhr V, Weinhold N, Rosenwald A, Erhard F, Haferlach C, Einsele H, Kortum KM, Saliba AE and Rasche L (2021) Homozygous BCMA gene deletion in response to anti-BCMA CAR T cells in a patient with multiple myeloma. Nat Med 27:616-619. [CrossRef]
- Perica K, Nataraj S, Sjojstrand ML, Nagel K, Pavkovic E, Payson E, Cuenca A, Patel A, Chung D, Mailankody S, Shah GL, Usmani SZ, Giralt SA and Sadelain M (2023) Low Target Antigen Expression Mediates Resistance to BCMA CAR T Cell Therapy. Blood 142:2124. [CrossRef]
- Mailankody S, Devlin SM, Landa J, Nath K, Diamonte C, Carstens EJ, Russo D, Auclair R, Fitzgerald L, Cadzin B, Wang X, Sikder D, Senechal B, Bermudez VP, Purdon TJ, Hosszu K, McAvoy DP, Farzana T, Mead E, Wilcox JA, Santomasso BD, Shah GL, Shah UA, Korde N, Lesokhin A, Tan CR, Hultcrantz M, Hassoun H, Roshal M, Sen F, Dogan A, Landgren O, Giralt SA, Park JH, Usmani SZ, Riviere I, Brentjens RJ and Smith EL (2022) GPRC5D-Targeted CAR T Cells for Myeloma. N Engl J Med 387:1196-1206. [CrossRef]
- Zhang M, Wei G, Zhou L, Zhou J, Chen S, Zhang W, Wang D, Luo X, Cui J, Huang S, Fu S, Zhou X, Tang Y, Ding X, Kuang J, He XP, Hu Y and Huang H (2023) GPRC5D CAR T cells (OriCAR-017) in patients with relapsed or refractory multiple myeloma (POLARIS): a first-in-human, single-centre, single-arm, phase 1 trial. Lancet Haematol 10:e107-e116. [CrossRef]
- Caillot L, Sleiman E, Lafon I, Chretien M-L, Gueneau P, Payssot A, Pedri R, Lakomy D, Bailly F, Guy J, Quenot J-P, Avet-Loiseau H and Caillot D (2024) Early Chimeric Antigen Receptor T Cell Expansion Is Associated with Prolonged Progression-Free Survival for Patients with Relapsed/Refractory Multiple Myeloma Treated with Ide-Cel: A Retrospective Monocentric Study. Transplantation and Cellular Therapy. [CrossRef]
- Lam N, Trinklein ND, Buelow B, Patterson GH, Ojha N and Kochenderfer JN (2020) Anti-BCMA chimeric antigen receptors with fully human heavy-chain-only antigen recognition domains. Nat Commun 11:283. [CrossRef]
- Zelle-Rieser C, Thangavadivel S, Biedermann R, Brunner A, Stoitzner P, Willenbacher E, Greil R and Johrer K (2016) T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. J Hematol Oncol 9:116. [CrossRef]
- Rodriguez-Marquez P, Calleja-Cervantes ME, Serrano G, Oliver-Caldes A, Palacios-Berraquero ML, Martin-Mallo A, Calvino C, Espanol-Rego M, Ceballos C, Lozano T, San Martin-Uriz P, Vilas-Zornoza A, Rodriguez-Diaz S, Martinez-Turrillas R, Jauregui P, Alignani D, Viguria MC, Redondo M, Pascal M, Martin-Antonio B, Juan M, Urbano-Ispizua A, Rodriguez-Otero P, Alfonso-Pierola A, Paiva B, Lasarte JJ, Inoges S, Lopez-Diaz de Cerio A, San-Miguel J, Fernandez de Larrea C, Hernaez M, Rodriguez-Madoz JR and Prosper F (2022) CAR density influences antitumoral efficacy of BCMA CAR T cells and correlates with clinical outcome. Sci Adv 8:eabo0514. [CrossRef]
- Gandhi UH, Cornell RF, Lakshman A, Gahvari ZJ, McGehee E, Jagosky MH, Gupta R, Varnado W, Fiala MA, Chhabra S, Malek E, Mansour J, Paul B, Barnstead A, Kodali S, Neppalli A, Liedtke M, Narayana S, Godby KN, Kang Y, Kansagra A, Umyarova E, Scott EC, Hari P, Vij R, Usmani SZ, Callander NS, Kumar SK and Costa LJ (2019) Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 33:2266-2275. [CrossRef]
- Chinese Medical Doctor Association HB and Chinese Society of Hematology CMA (2022) [The Chinese consensus for the CAR-T cell therapy in multiple myeloma (2022 version)]. Zhonghua Xue Ye Xue Za Zhi 43:265-271. [CrossRef]
- Subramanian NG, Garcia Pleitez H, Pasvolsky O, Kalariya N, Patel KK and Ferreri CJ (2023) Clinical outcomes of patients with multiple myeloma following disease progression after BCMA-targeted CAR T-cell therapy. Journal of Clinical Oncology 41:e20002-e20002. [CrossRef]
- Brudno JN, Maric I, Hartman SD, Rose JJ, Wang M, Lam N, Stetler-Stevenson M, Salem D, Yuan C, Pavletic S, Kanakry JA, Ali SA, Mikkilineni L, Feldman SA, Stroncek DF, Hansen BG, Lawrence J, Patel R, Hakim F, Gress RE and Kochenderfer JN (2018) T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J Clin Oncol 36:2267-2280. [CrossRef]
- Guoxing Z, CHENG Z, Runhong W, Yi W, Lei F, Qiuling M and Xianhui L (2022) Efficacy and safety of anti-B cell maturation antigen chimeric antigen receptor T-cell for retreatment of relapsed/refractory multiple myeloma. Journal of Leukemia Lymphoma:229-234. [CrossRef]
- Bal S, Htut M, Nadeem O, Anderson LD, Koçoğlu H, Gregory T, Rossi AC, Martin T, Egan DN, Costa L, Hu H, Chen Y, Li S, Kelly LM, Sarkis N, Ziyad S, Kao W-M, Kaeding AJ, Burgess MR and Berdeja JG (2023) BMS-986393 (CC-95266), a G Protein-Coupled Receptor Class C Group 5 Member D (GPRC5D)-Targeted Chimeric Antigen Receptor (CAR) T-Cell Therapy for Relapsed/Refractory Multiple Myeloma (RRMM): Updated Results from a Phase 1 Study. Blood 142:219. [CrossRef]
- Shi M, Wang J, Huang H, Liu D, Cheng H, Wang X, Chen W, Yan Z, Sang W, Qi K, Li D, Zhu F, Li Z, Qiao J, Wu Q, Zeng L, Fei X, Gu W, Miao Y, Xu K, Zheng J and Cao J (2024) Bispecific CAR T cell therapy targeting BCMA and CD19 in relapsed/refractory multiple myeloma: a phase I/II trial. Nat Commun 15:3371. [CrossRef]
- Li C, Wang X, Xu J, Liu J and Mei H (2024) Treatment of multiple myeloma: What is the impact on T-cell function? Ther Adv Hematol 15:20406207241245194. [CrossRef]
- Metelo AM, Jozwik A, Luong LA, Dominey-Foy D, Graham C, Attwood C, Inam S, Dunlop A, Sanchez K, Cuthill K, Rice C, Streetly M, Bentley T, Boldajipour B, Sommer C, Sasu B and Benjamin R (2022) Allogeneic Anti-BCMA CAR T Cells Are Superior to Multiple Myeloma-derived CAR T Cells in Preclinical Studies and May Be Combined with Gamma Secretase Inhibitors. Cancer Res Commun 2:158-171. [CrossRef]
- Dholaria B, Shune L, Kin A, McArthur K, Eskew JD, Martin CE, Haag S, McCaigue J, Namini H, DePrimo S, Cranert S, Coronella J, Shedlock D and Belani R (2024) Abstract CT071: Clinical activity of P-BCMA-ALLO1, a B-cell maturation antigen (BCMA) targeted allogeneic chimeric antigen receptor T-cell (CAR-T) therapy, in relapsed refractory multiple myeloma (RRMM) patients (pts) following progression on prior BCMA targeting therapy. Cancer Research 84:CT071-CT071.
- Moreau P, Garfall AL, van de Donk N, Nahi H, San-Miguel JF, Oriol A, Nooka AK, Martin T, Rosinol L, Chari A, Karlin L, Benboubker L, Mateos MV, Bahlis N, Popat R, Besemer B, Martinez-Lopez J, Sidana S, Delforge M, Pei L, Trancucci D, Verona R, Girgis S, Lin SXW, Olyslager Y, Jaffe M, Uhlar C, Stephenson T, Van Rampelbergh R, Banerjee A, Goldberg JD, Kobos R, Krishnan A and Usmani SZ (2022) Teclistamab in Relapsed or Refractory Multiple Myeloma. N Engl J Med 387:495-505. [CrossRef]
- Chari A, Minnema MC, Berdeja JG, Oriol A, van de Donk N, Rodriguez-Otero P, Askari E, Mateos MV, Costa LJ, Caers J, Verona R, Girgis S, Yang S, Goldsmith RB, Yao X, Pillarisetti K, Hilder BW, Russell J, Goldberg JD and Krishnan A (2022) Talquetamab, a T-Cell-Redirecting GPRC5D Bispecific Antibody for Multiple Myeloma. N Engl J Med 387:2232-2244. [CrossRef]
- Lesokhin AM, Tomasson MH, Arnulf B, Bahlis NJ, Miles Prince H, Niesvizky R, Rodriotaguez-Otero P, Martinez-Lopez J, Koehne G, Touzeau C, Jethava Y, Quach H, Depaus J, Yokoyama H, Gabayan AE, Stevens DA, Nooka AK, Manier S, Raje N, Iida S, Raab MS, Searle E, Leip E, Sullivan ST, Conte U, Elmeliegy M, Czibere A, Viqueira A and Mohty M (2023) Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat Med 29:2259-2267. [CrossRef]
- Varshavsky-Yanovsky A, Jethava Y, Stevens DA, Nooka AK, Stiff PJ, Perez-Cruz I, Leip E and Lesokhin A (2023) Efficacy and Safety of Elranatamab in Black Patients with Relapsed/Refractory Multiple Myeloma (RRMM): A Subgroup Analysis of the Magnetismm Studies. Blood 142:3333. [CrossRef]
- Trudel S, Cohen AD, Krishnan AY, Fonseca R, Spencer A, Berdeja JG, Lesokhin A, Forsberg PA, Laubach JP, Costa LJ, Rodriguez-Otero P, Kaedbey R, Richter J, Mateos M-V, Thomas SK, Wong C, Li M, Choeurng V, Vaze A, Samineni D, Sumiyoshi T, Cooper J and Harrison SJ (2021) Cevostamab Monotherapy Continues to Show Clinically Meaningful Activity and Manageable Safety in Patients with Heavily Pre-Treated Relapsed/Refractory Multiple Myeloma (RRMM): Updated Results from an Ongoing Phase I Study. Blood 138:157-157.
- Schinke CD, Touzeau C, Minnema MC, van de Donk NW, Rodríguez-Otero P, Mateos M-V, Rasche L, Ye JC, Vishwamitra D and Ma X (2023) Pivotal phase 2 MonumenTAL-1 results of talquetamab (tal), a GPRC5DxCD3 bispecific antibody (BsAb), for relapsed/refractory multiple myeloma (RRMM).
- Mouhieddine TH, Van Oekelen O, Melnekoff DT, Li J, Ghodke-Puranik Y, Lancman G, Thibaud S, Pan D, Rajeeve S, Agte S, Aleman A, Sanchez L, Richard S, Rossi A, Richter J, Cho HJ, Rodriguez C, Lagana A, Moshier E, Chari A, Jagannath S and Parekh S (2023) Sequencing T-cell redirection therapies leads to deep and durable responses in patients with relapsed/refractory myeloma. Blood Adv 7:1056-1064. [CrossRef]
- Banerjee R, Lynch RC, Wu QV, Simon S, Ujjani CS, Till BG, Wuliji N, Gausman D, Dizon J, Kwok ML, Lee SS, Silbermann RW, Medvedova E, Maloney DG, Ramos J, Shadman M, Gauthier J, Turtle CJ, Gopal AK, Green DJ, Riddell SR and Cowan AJ (2024) Post-CAR-T Checkpoint Inhibition Can Result in Durable Responses in a Minority of Patients with Multiple Myeloma (MM) or Non-Hodgkin's Lymphoma (NHL): Results of a Phase 2 Study of Nivolumab after CAR-T Failure. Transplantation and Cellular Therapy 30:S399-S400. [CrossRef]
- Vaxman I, Abeykoon J, Dispenzieri A, Kumar SK, Buadi F, Lacy MQ, Dingli D, Hwa Y, Fonder A, Hobbs M, Reeder C, Sher T, Hayman S, Kourelis T, Warsame R, Muchtar E, Leung N, Go R, Gonsalves W, Siddiqui M, Kyle RA, Rajkumar SV, Kristen M, Kapoor P and Gertz MA (2021) "Real-life" data of the efficacy and safety of belantamab mafodotin in relapsed multiple myeloma-the Mayo Clinic experience. Blood Cancer J 11:196. [CrossRef]
- Gazeau N, Beauvais D, Yakoub-Agha I, Mitra S, Campbell TB, Facon T and Manier S (2021) Effective anti-BCMA retreatment in multiple myeloma. Blood Adv 5:3016-3020. [CrossRef]
- Agte S, Elnaggar M, Adamopolous C, Melnekoff D, Adleman A, Kappes K, Restrepo P, Oekelen OV, Leshchenko V, Poulikakos PI, Lagana A, Verina D, Jagannath S and Parekh S (2021) P-090: BRAF V600E multiple myeloma patient salvaged with triple MAPK inhibition after CAR T relapse. Clinical Lymphoma Myeloma and Leukemia 21:S88. [CrossRef]
- Qian Y, Qian Z, Zhao X, Pan W, Wei X, Meng H, Yang L and Xiao H (2021) Successful Treatment of Relapsed/Refractory Extramedullary Multiple Myeloma With Anti-BCMA CAR-T Cell Therapy Followed by Haploidentical Hematopoietic Stem Cell Transplantation: A Case Report and a Review of the Contemporary Literature. Front Med (Lausanne) 8:649824. [CrossRef]
- Garfall AL, Dancy EK, Cohen AD, Hwang WT, Fraietta JA, Davis MM, Levine BL, Siegel DL, Stadtmauer EA, Vogl DT, Waxman A, Rapoport AP, Milone MC, June CH and Melenhorst JJ (2019) T-cell phenotypes associated with effective CAR T-cell therapy in postinduction vs relapsed multiple myeloma. Blood Adv 3:2812-2815. [CrossRef]
- Dima D, Ullah F, Mazzoni S, Williams L, Faiman B, Kurkowski A, Chaulagain C, Raza S, Samaras C, Valent J, Khouri J and Anwer F (2023) Management of Relapsed-Refractory Multiple Myeloma in the Era of Advanced Therapies: Evidence-Based Recommendations for Routine Clinical Practice. Cancers (Basel) 15. [CrossRef]
- Abecassis A, Roders N, Fayon M, Choisy C, Nelson E, Harel S, Royer B, Villesuzanne C, Talbot A, Garrick D, Goodhardt M, Fermand JP, Burbridge M, Arnulf B and Bories JC (2022) CAR-T cells derived from multiple myeloma patients at diagnosis have improved cytotoxic functions compared to those produced at relapse or following daratumumab treatment. EJHaem 3:970-974. [CrossRef]
- Rodriguez-Otero P, Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, Manier S, Callander N, Costa LJ, Vij R, Bahlis NJ, Moreau P, Solomon SR, Delforge M, Berdeja J, Truppel-Hartmann A, Yang Z, Favre-Kontula L, Wu F, Piasecki J, Cook M and Giralt S (2023) Ide-cel or Standard Regimens in Relapsed and Refractory Multiple Myeloma. N Engl J Med 388:1002-1014. [CrossRef]
- Wu JF and Dhakal B (2023) BCMA-targeted CAR-T cell therapies in relapsed and/or refractory multiple myeloma: latest updates from 2023 ASCO Annual Meeting. J Hematol Oncol 16:86. [CrossRef]
- Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, Stewart AK, Hari P, Htut M, Lesokhin A, Deol A, Munshi NC, O'Donnell E, Avigan D, Singh I, Zudaire E, Yeh TM, Allred AJ, Olyslager Y, Banerjee A, Jackson CC, Goldberg JD, Schecter JM, Deraedt W, Zhuang SH, Infante J, Geng D, Wu X, Carrasco-Alfonso MJ, Akram M, Hossain F, Rizvi S, Fan F, Lin Y, Martin T and Jagannath S (2021) Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 398:314-324. [CrossRef]
- Lin Q, Zhao J, Song Y and Liu D (2019) Recent updates on CAR T clinical trials for multiple myeloma. Mol Cancer 18:154. [CrossRef]
- Ahmed N, Wesson W, Mushtaq MU, Bansal R, AbdelHakim H, Bromert S, Appenfeller A, Ghazal BA, Singh A, Abhyankar S, Ganguly S, McGuirk J, Abdallah AO and Shune L (2023) "Waitlist mortality" is high for myeloma patients with limited access to BCMA therapy. Front Oncol 13:1206715. [CrossRef]
- Afrough A, Hashmi H, Hansen DK, Sidana S, Ahn C, Dima D, Freeman CL, Castaneda Puglianini OA, Kocoglu MH, Atrash S, Voorhees PM, Shune L, Simmons G, Sborov DW, Ferreri CJ, Wagner CB, Patel KK, Khouri J, Anderson JLD and Lin Y (2023) Impact of bridging therapy (BT) on outcome of relapsed refractory multiple myeloma (RRMM) with Ide-cel CAR T-cell therapy: Real-world experience from the US myeloma CAR T consortium. Journal of Clinical Oncology 41:8013-8013. [CrossRef]
- Sperling AS, Derman BA, Nikiforow S, Im S-Y, Ikegawa S, Prabhala RH, Rodriguez DH, Li Y, Quinn DS, Pearson D, Bu D, Mataraza J, Liegel J, D'Souza A, Rispoli L, Credi M, Ritz J, Jakubowiak AJ, Vita SD and Munshi NC (2023) Updated phase I study results of PHE885, a T-Charge manufactured BCMA-directed CAR-T cell therapy, for patients (pts) with r/r multiple myeloma (RRMM). 41:8004-8004. [CrossRef]
- Costa LJ, Kumar SK, Atrash S, Liedtke M, Kaur G, Derman BA, Bergsagel PL, Mailankody S, McCarthy PL, Limones J, Chen Y, Das S, Thorpe J, Cacciatore J, Navarro G, Koegel AK, Burgess MR, Hege K and Richard S (2022) Results from the First Phase 1 Clinical Study of the B-Cell Maturation Antigen (BCMA) Nex T Chimeric Antigen Receptor (CAR) T Cell Therapy CC-98633/BMS-986354 in Patients (pts) with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 140:1360-1362. [CrossRef]
- Liu H, Wang T, Yang Y, Feng R, Li J, Zhang C, Bai J, Ding Y, Liu G, Wu F, Lu X, Li J and He T (2022) Phase I Study of a BCMA-Directed CAR-T Cell Therapy for Relapsed/Refractory Multiple Myeloma Manufactured in <3 Days Using the High-Performance Platform. Blood 140:10307-10308. [CrossRef]
- Choi T and Kang Y (2022) Chimeric antigen receptor (CAR) T-cell therapy for multiple myeloma. Pharmacol Ther 232:108007. [CrossRef]
- Kegyes D, Constantinescu C, Vrancken L, Rasche L, Gregoire C, Tigu B, Gulei D, Dima D, Tanase A, Einsele H, Ciurea S, Tomuleasa C and Caers J (2022) Patient selection for CAR T or BiTE therapy in multiple myeloma: Which treatment for each patient? J Hematol Oncol 15:78. [CrossRef]
- Dima D, Rashid A, Davis JA, Shune L, Abdallah AO, Li H, DeJarnette S, Khouri J, Williams L, Hashmi H, Raza S, McGuirk J, Anwer F and Ahmed N (2024) Efficacy and safety of idecabtagene vicleucel in patients with relapsed-refractory multiple myeloma not meeting the KarMMa-1 trial eligibility criteria: A real-world multicentre study. Br J Haematol 204:1293-1299. [CrossRef]
- Cohen AD, Mateos MV, Cohen YC, Rodriguez-Otero P, Paiva B, van de Donk N, Martin T, Suvannasankha A, De Braganca KC, Corsale C, Schecter JM, Varsos H, Deraedt W, Wang L, Vogel M, Roccia T, Xu X, Mistry P, Zudaire E, Akram M, Nesheiwat T, Pacaud L, Avivi I and San-Miguel J (2023) Efficacy and safety of cilta-cel in patients with progressive multiple myeloma after exposure to other BCMA-targeting agents. Blood 141:219-230. [CrossRef]
- Wang X, Walter M, Urak R, Weng L, Huynh C, Lim L, Wong CW, Chang WC, Thomas SH, Sanchez JF, Yang L, Brown CE, Pichiorri F, Htut M, Krishnan AY and Forman SJ (2018) Lenalidomide Enhances the Function of CS1 Chimeric Antigen Receptor-Redirected T Cells Against Multiple Myeloma. Clin Cancer Res 24:106-119. [CrossRef]
- Works M, Soni N, Hauskins C, Sierra C, Baturevych A, Jones JC, Curtis W, Carlson P, Johnstone TG, Kugler D, Hause RJ, Jiang Y, Wimberly L, Clouser CR, Jessup HK, Sather B, Salmon RA and Ports MO (2019) Anti-B-cell Maturation Antigen Chimeric Antigen Receptor T cell Function against Multiple Myeloma Is Enhanced in the Presence of Lenalidomide. Mol Cancer Ther 18:2246-2257. [CrossRef]
- Zhao G, Wei R, Feng L, Wu Y, He F, Xiao M and Cheng Z (2022) Lenalidomide enhances the efficacy of anti-BCMA CAR-T treatment in relapsed/refractory multiple myeloma: a case report and revies of the literature. Cancer Immunol Immunother 71:39-44. [CrossRef]
- Pont MJ, Hill T, Cole GO, Abbott JJ, Kelliher J, Salter AI, Hudecek M, Comstock ML, Rajan A, Patel BKR, Voutsinas JM, Wu Q, Liu L, Cowan AJ, Wood BL, Green DJ and Riddell SR (2019) gamma-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood 134:1585-1597. [CrossRef]
- Cowan AJ, Pont MJ, Sather BD, Turtle CJ, Till BG, Libby EN, 3rd, Coffey DG, Tuazon SA, Wood B, Gooley T, Wu VQ, Voutsinas J, Song X, Shadman M, Gauthier J, Chapuis AG, Milano F, Maloney DG, Riddell SR and Green DJ (2023) gamma-Secretase inhibitor in combination with BCMA chimeric antigen receptor T-cell immunotherapy for individuals with relapsed or refractory multiple myeloma: a phase 1, first-in-human trial. Lancet Oncol 24:811-822. [CrossRef]
- Aleman A, Kogan-Zajdman A, Upadhyaya B, Van Oekelen O, Chen L, Leshchenko V, Jagannath S and Parekh S (2023) P-175 Improving Anti-BCMA CAR-T functionality with novel immunomodulatory agent Iberdomide (CC220) in Multiple Myeloma. Clinical Lymphoma Myeloma and Leukemia 23:S131-S132. [CrossRef]
- Hassan H, Samur MK, Cohen AD, Moreau P, Rodríguez Otero P, Kumar SK, Mateos MV, Avigan D, Sperling AS, Nadeem O, Hudecek M, Fernández de Larrea C, Martínez-López J, Themeli M, Yong K, Berdeja JG, Chari A, Sonneveld P, Gay F, Krishnan A, Usmani SZ, Anderson KC, Jagannath S, Einsele H, Munshi NC and Midha S (2023) Heterogeneity in Access and Toxicity Management of Commercially Available BCMA-Directed CAR-T and Bispecific T-Cell Engager Therapy Among the International Myeloma Community. Blood 142:7248-7248.
- Hashmi H, Kumar A, Kharfan-Dabaja MA, Munshi PN, Inamoto Y, DeFilipp ZM, Dholaria B, Jain T, Perales MA, Carpenter PA, Hamadani M, Dhakal B and Usmani SZ (2024) ASTCT Committee on Practice Guidelines Survey on Evaluation & Management of Relapsed Refractory Multiple Myeloma after Failure of Chimeric Antigen Receptor T-Cell Therapy. Transplant Cell Ther. [CrossRef]
- Deng J, Lin Y, Zhao D, Tong C, Chang AH, Chen W and Gao W (2022) Case report: Plasma cell leukemia secondary to multiple myeloma successfully treated with anti-BCMA CAR-T cell therapy. Front Oncol 12:901266. [CrossRef]
- Arnulf B, Kerre T, Agha ME, Delforge M, Braunschweig I, Shah N, Richard S, Alsina M, Einsele H, Mistry P, Varsos H, Corsale C, Schecter JM, Braganca KCD, Jethava Y, Song Q, Koneru M, Akram M, Cohen YC and Roeloffzen W (2024) Efficacy and safety of ciltacabtagene autoleucel ± lenalidomide maintenance in newly diagnosed multiple myeloma with suboptimal response to frontline autologous stem cell transplant: CARTITUDE-2 cohort D. 42:7505-7505. [CrossRef]
| Trial Code/Number | CAR-T Product | CAR-T Target | Median Follow-up (months) | Efficacy | Safety | |||
|---|---|---|---|---|---|---|---|---|
| ORR | Median PFS (months) | Median OS (months) | CRS | ICANS | ||||
| LEGEND-2 (NCT03090659) |
LCAR-B38M [19] | BCMA | 65.4 | 87.8% | 18 | 55.8 | - | - |
| CRB401 (NCT02658929) |
bb2121 [20] | BCMA | 18.1 (1.5-44.5) | 75.8% | 8.8 (95% CI, 5.9-11.9) | 34.2 (95% CI, 19.2-NE) | 75.8% | - |
| FUMANBA-1 (NCT05066646) |
CT103A [21] | BCMA | 13.8 (0.4-27.2) | 96% | NR | - | - | - |
| LUMMICAR-1 (NCT03975907) |
CT053 [22] | BCMA | 37.7 (14.8-44.2) | 100% | 25 | NR | 92.9% | 0 |
| POLARIS (NCT05016778) |
OriCAR-017 [24] | GPRC5D | >24 (all patients) | 100% | 11.37 (95% CI, 5.93-18.00) | NR | 100% | 0 |
| ChiCTR2100048888 | anti-GPRC5D CAR-T [25] | GPRC5D | 5.2 (3.2-8.9) | 91% | NR | NR | 76% | 6% |
| ChiCTR1800018143 | BM38 CAR-T [26] | BCMA & CD38 | 9.0 (0.5-18.5) | 87% | 17.2 (95% CI, 7.5-26.8) | NR | 87% | - |
| NCT04662099 | CS1-BCMA CAR-T [27] | CS1 & BCMA | 246 days (55-547) | 81% | 9.0 (95% CI, 2.1-NR) | NR | 38% | 0 |
| NCT04236011 & NCT04182581 | GC012F [28] | BCMA & CD19 | 30.7 (14.6-43.6) | 93.1% | 38.0 (95% CI, 11.8-NE) | - | 86.2% | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





