1. Introduction
Malaria remains a major global health concern resulting in 249 million cases annually and 608,000 deaths in children under the age of 5 [
1]. RTS,S and R21 vaccines approved for preventing malaria infection are modestly effective against malaria [
2]. However, malaria remains endemic in many parts of the world due to drug resistant parasites and insecticide resistant mosquitoes. The parasite is introduced into humans by the bite of a female
Anopheles mosquito. The parasite invades liver cells and undergoes asexual development. Merozoite stages released from the liver invade red blood cells (RBC) and immediately remodel the host cell by establishing membranous transport and export pathways as well as new nutrient uptake channels [
3,
4,
5]. The newly formed parasitophorous vacuole membrane (PVM) and the Maurer’s clefts participate in export of proteins from the parasite into the host cell cytoplasm and export of virulence markers such as Knob-associated histidine rich protein 1 (KAHRP1),
Plasmodium falciparum Erythrocyte Membrane Protein 1 (PfEMP1) [
6] and trophozoite export protein 1 (Tex1) [
7] to the infected red cell surface to aid the parasite in immune evasion. Resident and transient MC proteins facilitating the transport of PfEMP1 and other proteins trafficked to the infected red cell surface were identified through proteomic analysis [
8]. RhopH3 protein and Pf130 were distributed in MC membranes in trophozoite and schizont stages colocalized with Bodipy-ceramide, indicating the presence of both proteins in a lipid environment [
9,
10]. However, the role of RhopH3 in MC organization and function is unknown. Additionally, the topology of RhopH3 associated with the MC membranes has not been investigated. The high molecular weight rhoptry proteins consisting of the proteins RhopH1/Clag, RhopH2 and RhopH3 (RhopH) are transferred to the red blood cell cytoplasm where individual RhopH proteins associate transiently with the Maurer’s clefts (MCs) [
10,
11]. RhopH2 was identified in domains of the erythrocyte cytoplasm in proximity to the red cell membrane and associated with the MC, with the N-terminus protected from protease treatment [
8]. RhopH3 is required for merozoite invasion and nutrient import and is exported to the MC like RhopH2 in the red cell cytoplasm [
10]. Both RhopH2 and RhopH3 participate in the formation and function of new permeability pathways (NPPs), particularly the formation of the plasmodial surface anion channel (PSAC) important for nutrient uptake in the host cell [
12]. Although interaction of the RhopH proteins with the
Plasmodium translocon of exported proteins (PTEX) on the PVM has been shown, the mechanism of RhopH protein transit through PTEX is unclear and the conformational organization of the RhopH protein complex in association with the MC during transit into the cytoplasm and to the erythrocyte membrane is unknown [
13]. Chaperone proteins and MC proteins are implicated in the insertion and translocation of the RhopH complex into the cytoplasm through the PVM [
10]. In our previous studies, we showed that the 20 kDa integral membrane MC protein PfMC2TM and RhopH3, were synthesized and trafficked through the classical protein traffic pathway following brefeldin A (BFA) treatment [
14]. Pf130 is a soluble MC protein identified using monoclonal antibodies and shown by IEM to be distributed in longitudinal compartments associated with the MC underneath knobs in the infected red cell membrane [
9]. Due to the dual role of the RhopH3 protein in merozoite invasion and nutrient traffic through formation of PSAC and the association with the red blood cell cytoskeleton and the MC, we investigated the topology of RhopH3 and Pf130 within the infected red cell using SLO permeabilization and protease accessibility approaches. These types of studies aim to provide important insights regarding the interaction of high molecular weight rhoptry proteins with MC compartments en route to the host cell membrane.
2. Materials and Methods
Plasmodium falciparum (3D7) was cultured in type A+ human erythrocytes (Interstate Blood Bank, Memphis, TN) at 5 % hematocrit in RPMI-1640-HEPES complete media supplemented with 10 % A+ human serum (Interstate Blood Bank) and 20 mM glucose as described previously [
15]. Ring- and schizont- infected erythrocytes were synchronized using 5% sorbitol lysis of mature parasites [
15] and 65% Percoll gradient [
16] respectively.
Eighty percent synchronized
Plasmodium falciparum (strains 3D7) segmented schizonts were mixed with uninfected erythrocytes to obtain a parasitemia of 3% schizonts for merozoite invasion and processed for collection of ring stages at 4, 8, 12 and 16 hpi, followed by collections of trophozoites and schizonts at 30, 42 and 51 hpi as described previously. Percoll synchronized segmented schizont - infected erythrocytes were processed for Brefeldin A treatment by incubating schizonts with 5 % sorbitol at 2 hours post invasion (hpi), to obtain young rings. Stage specific parasites were treated with 10 µg/ml BFA as described previously [
14]. Ring stage parasites were treated with BFA and cultured for 16 h, trophozoites for 12 h and schizonts for 9 h. One set of BFA treated cells was collected after the drug treatment, washed three times using RPMI-1640 and incubated with fresh media to recover protein expression and translocation. Following drug treatment, parasites were collected, centrifuged and pellets separated from supernatant for IFA and electrophoresis as described [
17].
Infected erythrocytes were treated with streptolysin O (SLO) activated using 10 mM DTT in 1X PBS as described [
14]. SLO treated cells were incubated at RT for 6 min as described [
18] , centrifuged to separate supernatant from pellets and the pellets washed in 1 X PBS. Infected erythrocytes were fixed with 0.5% formalin on ice for 30 min, followed by washing with 1X PBS. Four aliquots of cells containing ring infected or trophozoite infected erythrocytes were incubated with 0.09% saponin and 4 units SLO in 1X PBS. The first aliquot was treated with SLO alone. The second aliquot was treated with SLO followed by 0.09% saponin on ice for 15 min, the third aliquot was treated with SLO followed by 1mg/ml trypsin for 15 min at 37
oC and the fourth aliquot was treated with SLO, saponin, followed by 1mg/ml trypsin [
18]. Smears from the four treatments were prepared on glass slides for IFA as described previously [
14].
Immunofluorescence and confocal microscopy was performed as previously described [
17]. Smears of infected erythrocytes on glass slides fixed in cold methanol and acetone (1:1) for 5 min at -20°C were incubated with rabbit or mouse polyclonal antibodies or mouse monoclonal antibodies either individually or together in colocalization experiments. Anti-Pf130, anti-RhopH3 (686), anti-SERA1 antibodies (685) and anti-REX antibodies were used for IFA. Normal mouse and rabbit antibodies were used as negative controls. Following 1X PBS washes secondary antibodies conjugated to Alexa 488, Alexa 568, Alexa 594 and Alexa 633/647 (Molecular Probes) directed to both species, were applied to slides for detection of primary antibodies. Incubation with primary and secondary antibodies was carried out for 1 h at room temperature (RT) followed by three washes with 1XPBS, and one wash in distilled water supplemented with 8µg/ml bis-benzimide. Vectashield containing 4′, 6-diamidino-2-phenylindole (DAPI) (Vector, Burlingame, CA, USA) or DAPI Fluoromount-G (Southern Biotech) was used as a mounting agent. Images were collected using a Leica TCS-SP5II upright laser scanning confocal microscope (Leica Microsystems, GmbH, Wetzlar, Germany). In addition, an SP8 True Scanning Confocal (TCS) on a DMI8 inverted microscope was used to generate differential interference contrast (DIC) images. Microscopy was performed at the Cleveland Clinic Imaging Core, The Lerner Research Institute, Cleveland, OH, USA.
Results and Discussion
In this study, we investigated the topological orientation and organization of the Maurer’s clefts protein Pf130 and the RhopH3 rhoptry protein. The structure of the individual proteins of the RhopH protein complex was solved using cryoelectron microscopy and the organization of the complex was described [
5]. The function of RhopH3 in merozoite invasion and nutrient transport was also described, showing that the individual RhopH proteins (RhopH1/Clag, RhopH2, RhopH3) are synthesized as soluble proteins with roles in merozoite invasion and through conformational changes insert into the host cell membrane for PSAC formation [
4]. However, the contributions of the tubular membranes and the MCs in the erythrocyte cytoplasm to rhoptry protein export and nutrient transport is unknown. Questions remain regarding the topology of the MC markers and associated proteins such as RhopH3 during the transport of RhopH proteins in the erythrocyte cytoplasm. In previous studies, we reported the stage specific expression of the Pf130 and RhopH3 rhoptry proteins, showing that protein expression begins early in the ring stage and can be detected by 4 hours post invasion (hpi) [
9,
14]. RhopH3 can be found distributed initially in the cytoplasm of the parasite followed by detection in the erythrocyte cytoplasm as the parasite matures [
14,
19]. Using monoclonal antibodies against Pf130 and anti-REX1 antibodies, tight colocalization was observed between the resident MC protein REX1 and Pf130 [
17]. Association of RhopH3 with membranous structures and detection in membranous compartments including the MC has been described in the cytoplasm along with Pf130 colocalized with Bodipy-ceramide in trophozoite and early schizont-infected erythrocytes [
9].
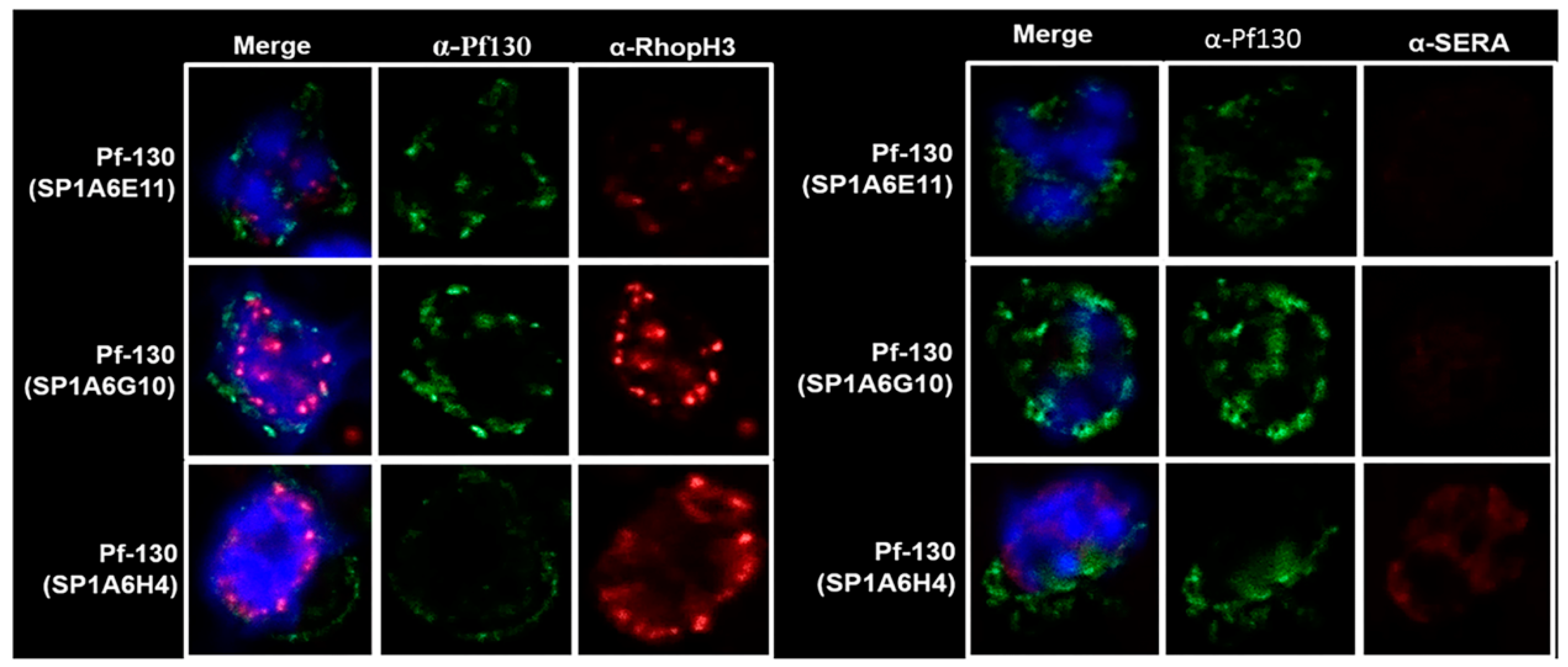
Figure 1.
Confocal microscopy of P. falciparum schizont-infected erythrocytes incubated with antibodies against RhopH3, SERA1 and Pf130.
Figure 1.
Confocal microscopy of P. falciparum schizont-infected erythrocytes incubated with antibodies against RhopH3, SERA1 and Pf130.
Three monoclonal antibodies specific for Pf130 were used in IFA with the anti-RhopH3 antibody 686 and the antiSERA1 antibody 685 (
Figure 1). Antibody reactivity targeting Pf130 in the schizont shows Pf130 localized in the periphery of the host cell membrane with few areas where RhopH3 and Pf130 are proximal (reactivity of Mab SP1A6G10). RhopH3 is expressed and trafficked in the classical secretory pathway [
14] following BFA treatment of infected erythrocytes. Pf130 was insensitive to BFA treatment as observed by IFA and by western blotting using pooled anti-Pf130 monoclonal antibodies (
Supplementary Figure 1, [
14]).
Due to the distribution of Pf130 detected in the periphery of the infected erythrocyte membrane, within the erythrocyte cytoplasm and distributions of Pf130 near RhopH3 (
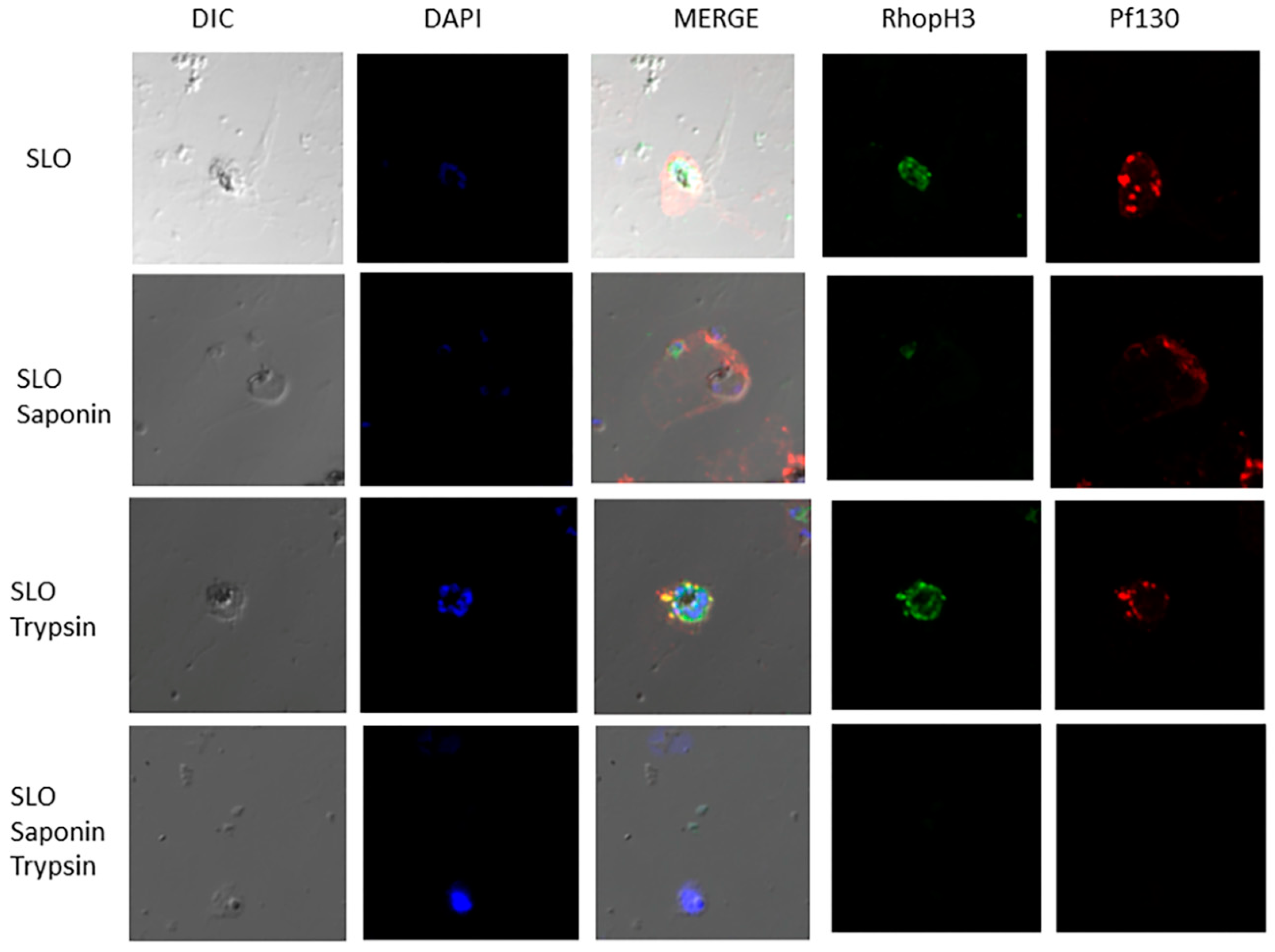
Figure 1), and to determine accessibility of the proteins on associated membranes, we investigated the topology of the proteins. In
Figure 2, late ring and trophozoite-infected erythrocytes were permeabilized with either SLO, SLO followed by saponin, SLO followed by trypsin treatment or SLO followed by saponin and trypsin treatment. SLO permeabilizes the erythrocyte membrane, while saponin permeabilizes the PVM and MC membranes. Following SLO permeabilization, RhopH3 and Pf130 reactivity was detected in the erythrocyte cytoplasm. Soluble proteins would have been lost from the erythrocyte cytoplasm following SLO treatment. RhopH3 reactivity was localized in the PVM, with Pf130 colocalization (
Figure 2). In addition, Pf130 was seen distributed in discrete punctate structures in the erythrocyte cytoplasm. Following SLO and saponin permeabilization, where the PVM and MC membranes would have been permeabilized, RhopH3 and Pf130 reactivity was still detected faintly in the erythrocyte cytoplasm. However, when SLO permeabilization was followed by trypsin treatment, Pf130 and RhopH3 were detected in the PVM and on membrane extensions into the erythrocyte cytoplasm (
Figure 2). RhopH3 and Pf130 were detected colocalized on the structures extending from the PVM into the erythrocyte cytoplasm. Anti-REX1 and anti-Pf130 reactivity showed tight colocalization of REX-1 and Pf130 following permeabilization with SLO, SLO followed by saponin and SLO followed by trypsin treatment demonstrating that the cytoplasmic structures are MCs (
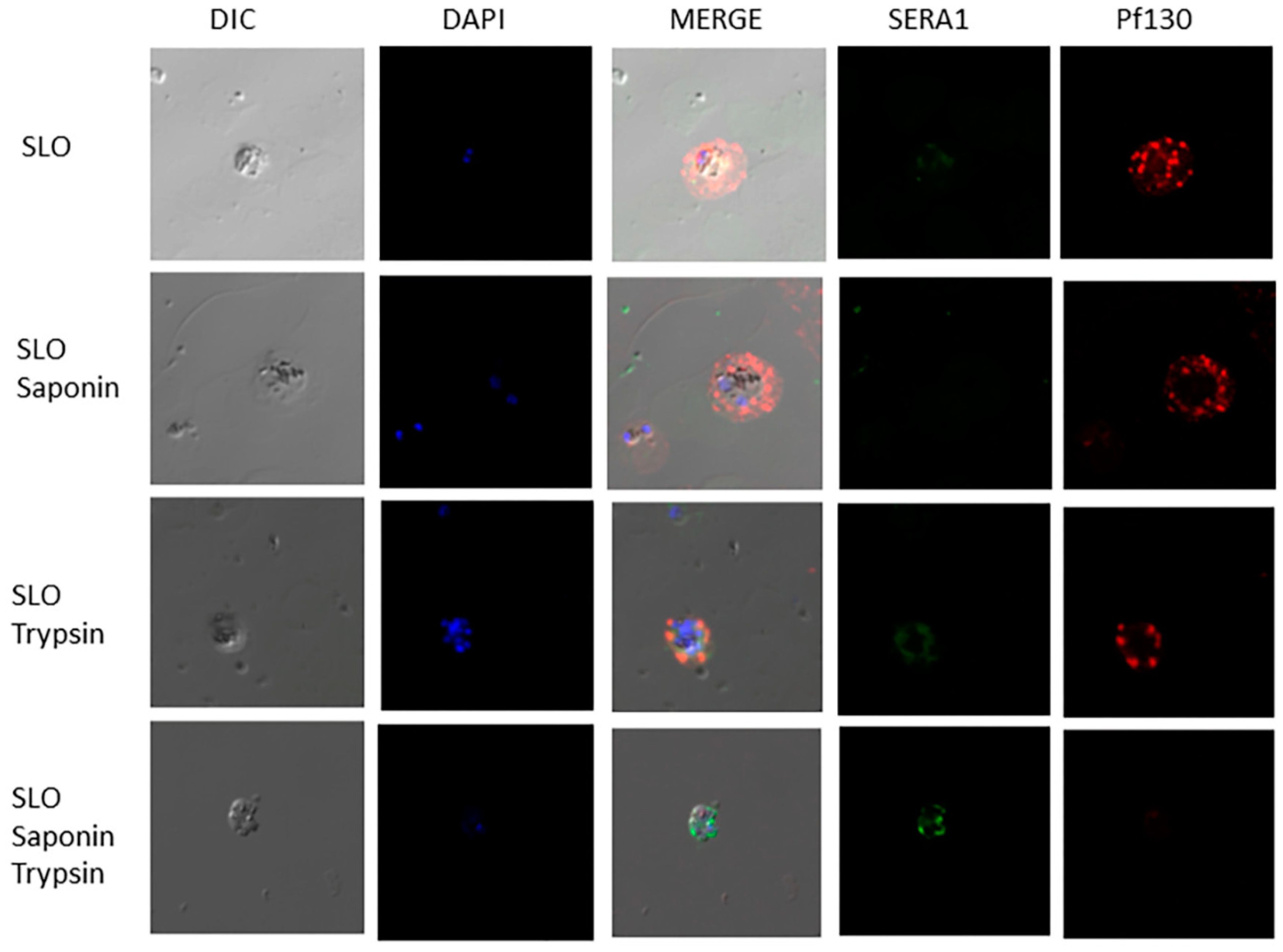
Figure S2). Antibody reactivity with anti-SERA1 antibody confirms the PVM is intact after SLO permeabilization of the erythrocyte membrane. SERA1 was detected in the PVM near Pf130 reactivity (
Figure 3). RhopH3, SERA1 and Pf130 are distributed in a lipid environment indicated by Bodipy-ceramide colocalization in trophozoite and schizont stages [
9]. Following SLO, saponin and trypsin treatment, diminished antibody reactivity with Pf130, RhopH3 and SERA1 was detected (
Figure 2,
Figure 3 and
Figure S3) indicating protease access to the proteins following PVM and MC membrane permeabilization.
Investigations of the native endogenous structure of the RhopH complex determined that the complex is trafficked to the erythrocyte membrane in a soluble form and that allosteric disulfide bonds present in RhopH2 and RhopH3 facilitate channel formation through the host cell membrane [
5]. However, the transport mechanisms utilized by the rhoptry proteins are unknown and interacting proteins associated with tubular membranous structures or MCs in the host cell cytoplasm have not been described. We were interested in determining the topology of RhopH3 and the MC protein Pf130 in the host cell cytoplasm following our previous data that PfMC2TM localizes in the same compartments with RhopH3 [
9]. Both RhopH3 and Pf130 are soluble in the host cell cytoplasm [
4,
9] but interact with the MC following their expression and translocation across the PVM into the host cell cytoplasm where they interact with MC and other membranous compartments such as J-dots [
18]. Modifications of RhopH3 and Pf130 may occur through phosphorylation by FIKK kinases associated with the MCs to facilitate transport and function of RhopH3 and associated Clag and RhopH2 proteins in the host cell membrane [
19]. The gene encoding Pf130 has not been identified. Efforts to use immunoproteome analysis to identify the gene were unsuccessful due to weak reactivity of the antibodies in immunoprecipitation assays [
9]. We performed LC-MS/MS on protease digested Pf130 followed by Mascot and Sequest analysis. The peptide mixture predominantly possessed the high molecular weight rhoptry protein 2 (RhopH2) and Falcilysin (
Supplementary Table 1). RhopH2 and RhopH3 are required for transport of RhopH1/Clag to the rhoptries [
3,
4,
5,
20] and to the host cell membrane [
20].
Plasmodium falciparum falcilysin is distributed in different parasite compartments including the apicoplast [
23]. It is unclear what relationship exists between Pf130 and the identified proteins as Pf130 localizes to the MCs and has not been identified in the rhoptries or apicoplast. Currently the identity of the gene encoding Pf130 is unknown and needs to be identified so that the structure of the gene and encoded protein can be studied.
Taken together, our data shows that RhopH3 and Pf130 are located on the MCs within the erythrocyte cytoplasm and are protected from protease digestion following SLO permeabilization and trypsin treatment. The change in conformation of RhopH3 from a soluble protein to a transmembrane (TM) protein able to insert into the MC may be due to the presence of predicted TM domains buried within the RhopH complex [
5]. However, the TM domains in the complex are available for interaction with the MC membrane during transit of RhopH3 along with RhopH2 and RhopH1/Clag to the erythrocyte membrane. While there are conflicting reports of surface exposure of the RhopH proteins on the surface of the infected host erythrocyte, the change from soluble to transmembrane conformation in the MC and in the erythrocyte membrane shares the properties of pore-forming proteins [
5,
10,
19]. Our novel results show that Pf130 and RhopH3 were tightly colocalized on the MC membranes and inaccessible to trypsin treatment. Both proteins in the membrane were still detected following SLO and saponin permeabilization, demonstrating that conformational changes required to transport the RhopH proteins may depend on transit through the MC to minimize extended presence of the proteins in the aqueous erythrocyte cytoplasm [
5]. The mechanism by which RhopH translocates across the PTEX in the PVM to enter the host cell cytoplasm involves disassembling of the complex to traffic through PTEX [
5]. The mechanism of RhopH complex disassembly to cross the PTEX and to assemble again once the RhopH components are in the erythrocyte cytoplasm are unknown. There is a need for further investigations of the parasite proteins associated with the RhopH proteins during transport across the erythrocyte cytoplasm and the role of the MCs in the transport of proteins involved in PSAC formation on the host cell membrane. Knowledge of the export and transport of RhopH proteins would provide important insights for a clearer understanding of the cell biology of
P. falciparum and the development of new anti-malarial therapeutic agents aimed at the transport pathways. MC and RhopH proteins are unique to
Plasmodium falciparum and
Plasmodium species, respectively, thereby constituting important targets for therapeutic attack.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, T.S-Y. and R.Y.; methodology, R.Y., J.W.P. and T.S-Y.; software, J.W.P and R.Y..; validation, J.W.P and R.Y.; formal analysis, T.SY, R.Y. and J. W. P.; investigation, R. Y. and T.S-Y.; resources, T.SY. and J. W. P.; data curation, R. Y. and J.W.P.; writing—original draft preparation, T.S-Y.; writing—review and editing, T.S-Y. and R.Y..; visualization, R.Y.; supervision, T.S-Y. and J. W. P.; project administration, T. S-Y..; funding acquisition, T.S-Y. and R. Y. All authors have read and agreed to the published version of the manuscript.