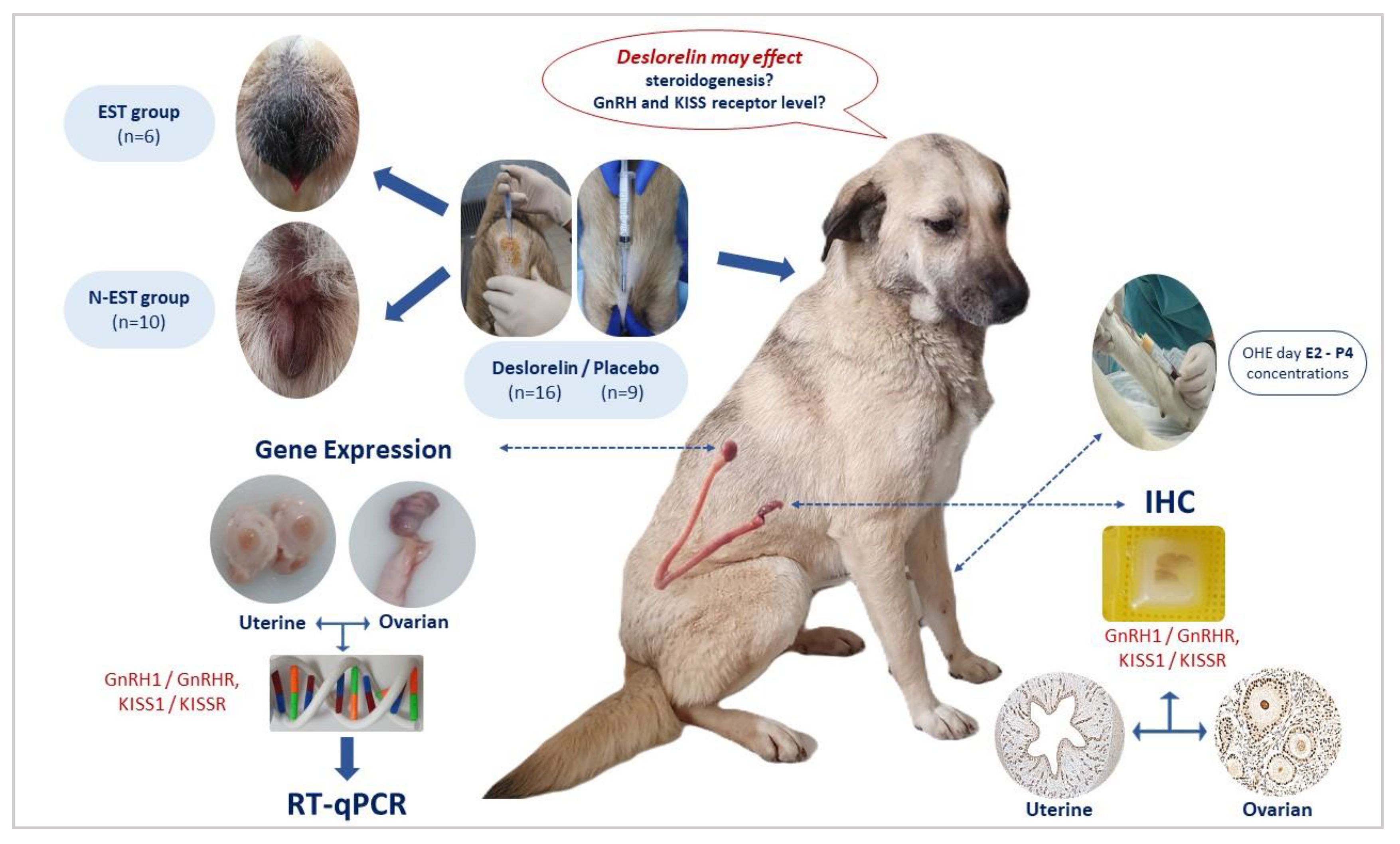
1. Introduction
The physiological initiation of puberty and its regulation is still poorly understood. Few studies address the endocrine changes during puberty of female dogs [
1,
2,
3,
4] and the morphological development of the genital tract [
5,
6,
7,
8]; and only few of these address estrogen, progesterone [
7] and follicle-stimulating hormone (FSH) [Mc Bride et al. [
5] receptor expression. Many details are still derived from other mammals. However, in prepubertal beagle dogs, at the age of 4 months, chronic infusion of a GnRH agonist prevented the increase in gonadotropins, which stayed at the level of anestrus bitches [
3]. This pioneer finding initiated the development of long-term gonadotropin-releasing hormone (GnRH) agonists for delay of estrus and puberty of dogs [
9,
10,
11,
12,
13,
14,
15].
GnRH is a neuroendocrine decapeptide crucial for the onset of puberty and maintenance of reproductive function in mammals [
16,
17]. During pubescence, the strong central inhibition on gene activation decreases and increasing amounts of GnRH traverse the portal system to reach the anterior pituitary gland, where it selectively binds to the GnRH receptor (GnRHR) located on gonadotropic cells. Activation of the GnRHR initiates intracellular signaling cascades, subsequently promoting the biosynthesis and secretion of gonadotropins, namely LH and FSH [
18,
19,
20]. Kisspeptins, a family of RF-amide peptides varying in length from 10 to 54 amino acids, play a crucial role in this essential step of sexual ripening [
21]. Encoded by the KISS1 gene, kisspeptins exert potent stimulatory effects on gonadotropin release across diverse mammalian species, including humans [
22], monkeys [
23], sheep [
24], rats [
25,
26], mice [
27], and dogs [
28].
Kisspeptin is responsible for the regulation of pulsatile GnRH secretion and the onset of puberty [
29]. The responsiveness of GnRH neurons to kisspeptin undergoes developmental regulation, with the proportion of responsive GnRH neurons increasing from 25% in prepubertal animals to exceeding 90% in adults. This augmentation in GnRH responsiveness during the pubertal phase is attributable to an upsurge in KISS1 gene expression [
30].
Shortly before puberty, the slow increase in kisspeptin and GnRH pulses secreted from the hypothalamus is accompanied by increased sensitivity of their receptors and desensibilization of steroid hormone receptors in the hypothalamus. During this period up-regulation of estrogen receptors in the central nervous system occurs, as well as receptor-level up-regulation of other organs that become increasingly sensitive to estrogen [
3]. In beagles, at an average age of 4 months, increasing gonadotropin secretion finally stimulates the ovary to generate gametes, peptides, and steroid hormones in the pubescent animal [
3]. The intricate interplay of positive and negative feedback mechanisms in the hypothalamus and pituitary gland then orchestrates the post-pubertal regulation of the reproductive hormone axis [
31]. Kisspeptin cells play a key role in the steroid negative-feedback control of GnRH neurons and there is a high degree of co-receptor localization for gonadal sex steroids in these cells. Most kisspeptin cells co-localize the estrogen receptor (ER-alpha-responsible for the negative-feedback actions of estradiol on GnRH secretion), the progesterone receptor and the androgen receptor [
31,
32,
33].
KISS1 has furthermore been proven to act on peripheral reproductive tissues, and the expression of KISS1 and KISS1R genes in canine follicles (granulosa, techia, oocyte), uterus and trophoblast cells has been determined [
34,
35]; similarly in lifestock [
36]. KISS1 and KISS1R proteins were found in the anteroventral periventricular nucleus and the arcuate nucleus. KISS1 appears to have autocrine and paracrine effects in follicle and oocyte maturation, trophoblast development as well as implantation and placentation in mammals [
36].
GnRH analogs, which are synthetically produced GnRH, are produced to interact with the GnRH receptor and act on the release of the pituitary gonadotropins FSH and LH. The use of long-acting GnRH agonists for the delay of puberty, duration of action and flare-up in female dogs is a current research topic; however there are few scientific studies investigating their clinical and histopathologic/molecular effects on ovarian/uterine function of pubescent dogs [
10,
12,
13,
14,
15]. Previous studies on expression of GnRH and KISS1 and their receptors in the uterus and ovaries after Deslorelin implant insertion were conducted with very young prepubertal animals, at the age of 4 months [
13,
14]. The assumption was that in these animals, no flare-up should occur. But even at such an early age, results varied considerably pointing towards different degrees of ripening of the HPG axis and peripheral receptor expression and/or additional factors.
Deslorelin, employed for the long-term suppression of puberty, does not have adverse effects on subsequent reproductive activity and ovarian functionality in dogs [
13]; however, there is still insufficient research on the stimulation of puberty and local receptor expression in reproductive organs. Therefore, this study aims to address this gap by investigating the expression and localization of GnRHR and KISS1R in genital tissues following the application of Deslorelin in still prepubertal dogs at the age of 7 months.
2. Materials and Methods
2.1. Animals
In the present study, ovarian and uterine tissues of a total of 25 clinically healthy female dogs were utilized. The tissue samples were derived from our previous study and also utilized in this work [
15]. Briefly, dogs of mixed breeds with an average age of 8 months (7.8±0.2), and an average body weight of 21.8±0.7 kg, were brought to Kafkas University Veterinary Faculty Education, Practice and Research Hospital by their owners for ovariohysterectomy (OHE). All dogs were still in the prepubertal period as was confirmed by anamnesis and clinical/laboratory findings (vaginal inspection, cytology, serum-P4 and -E2 measurements, x-rays of epiphyseal plates). Animal owners were informed about the study before the operation and their consent was obtained.
Both the clinical part of the study and the tissue examination was approved by Kafkas University Animal Experiments Local Ethics Committee with the number KAÜ-HAYDEK-2018/033 and 2022/166.
2.2. Experimental Design and Tissue Collection
Following the clinical examination, a subcutaneous implant containing Deslorelin acetate (Suprelorin
®, 4.7 mg, Virbac, France) was administered into the interscapular region of sixteen late prepubertal dogs [
15]. In order to determine estrus, daily clinical observations were done (vulvar appearance/swelling and bloody vaginal discharge, behavioral changes) and sampling (vaginal cytology and serum estradiol 17β (E2) and progesterone (P4) concentrations) was conducted at 2-day intervals. Dogs exhibiting clinical proestrus/estrus comprised the estrus group (EST, n=6), while dogs without clinical proestrus/estrus constituted the non-estrus group (N-EST, n=10). The cycle stages were defined as described by Karadağ et al. [
15]. The control group consisted of prepubertal dogs that received placebo implants (CONT, n=9), with 1 ml of sterile NaCl injected subcutaneously into the interscapular region using an implant applicator. They were otherwise treated like the experimental dogs. Between day 30 and 45 after implant application, ovariohysterectomy (OHE) was performed in all dogs, and tissues were subsequently collected as described below (
Figure 1). All operations were performed under general anesthesia. Atropine sulfate (Atropine
®, 2 mg/ml concentration; Vetas, Türkiye) was administered at a dose of 0.05 mg/kg, SC before anesthesia to support induction of anesthesia. Premedication was administered with xylazine HCl (Rompun
® 23.32 mg/ml; Bayer, Türkiye) at a dose of 2.35 mg/kg, IM. Fifteen minutes after sedation was achieved, ketamine HCl (Ketasol
®, 100 mg/ml; Interhas, Türkiye) was administered at a dose of 10 mg/kg IM. Anesthesia was then maintained with IV ketamine HCl injection. All animals received analgesic (meloxicam; 0.2 mg/kg, SC, Maxicam
® 5 mg/ml; Sanovel, Türkiye) treatment for 3 days after OHE.
2.3. Blood Sampling and Hormone Measurements
Blood samples were collected from all dogs before OHE via the Vena cephalica antebrachii, either into serum tubes without anticoagulant or plasma tubes (BD Vacutainer®, New Jersey, USA) containing EDTA anticoagulant. The samples were then centrifuged (Nüve NF400, Türkiye) at 3500 rpm for 10 minutes, and serum samples were separated.
The samples were stored at -20℃ until hormone measurements were performed. Estradiol (E2, pg/ml) and Progesterone (P4, ng/ml) concentrations were measured by direct chemiluminescence (CLIA) method using Siemens (Siemens
®, Tarrytown, New York, USA) fully automatic hormone analyzer and ADVIA Centaur test analysis kits. The ADVIA Centaur test is a measurement technique with a sensitivity of 100% and a specificity of 95.5% [
15].
2.4. Tissue Processing
Following sectioning based on their anatomical structure, tissues designated for Real-Time qPCR analysis were promptly frozen in liquid nitrogen and stored at -80 °C. The remaining portions of the ovarian and uterine tissues underwent a fixation protocol for immunohistochemical analysis. These tissues were initially placed in a 4% buffered formalin solution at +4 °C for 24 hours and subsequently washed with phosphate-buffered saline (PBS) for 7 days. Post-washing, dehydrated in a graded ethanol series, and embedded in paraffin as described previously [
37].
2.5. qRT-PCR Analysis
Total RNA extraction was performed using Trizol reagent (TRIzol®, Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. The concentrations of RNAs were determined using a NanoDrop™ 2000c spectrophotometer (Thermo Fisher Scientific AG, Reinach, CH). In the next step, RNA concentrations of uterine and ovarian tissues were adjusted to 1000 ng. Reverse transcriptase was performed with Oligo (dT) and Random Hexamer primers. Reverse transcriptase enzyme (Sensifat cDNA Kit) (Bioline; Meridian Biosince, USA) was used for cDNA synthesis. Primer designs of these kits were made as exon-exon junctions. In case of any DNA contamination, primers do not bind to DNA. Therefore, DNAse treatment was not necessary to prevent contamination. cDNA Component Volumes; cDNA Buffer 5X 2 µl, RT Enzyme (Sensifat cDNA Kit) 0.5 µl and RNA + ddH2O=7.5 µl, the total volume was adjusted to 10 µl. The ABI Veriti 96 (Applied Biosystems. Veriti™ 96-Well Fast Thermal Cycler) facilitated the reverse transcription process with the following temperature steps: 25 °C for 10 minutes for primer binding, 42 °C for 15 minutes for reverse transcription, and 85 °C for 5 minutes for enzyme inactivation. Following this, the enzyme was further inactivated at 85 °C for 5 minutes, and the cDNA products were stored at -20 °C for subsequent use in PCR analysis.
In RT-qPCR analyses, the expression levels of 4 target genes (GnRH1, GnRHR, KISS1 and KISS1R) were analyzed in uterine and ovarian tissues. Three different reference genes (EIF4H, KDM4A, PTK2) were used as an internal control (
Table 1). Primer design for each gene was performed using Primer Express Software version 3.0 (Applied Biosystems by Thermo Fischer). After primer design, synthesis of target and reference genes was performed at Biomers company (Ulm, Germany).
The mRNA transcript levels of each gene of interest were determined using the SensiFAST™ SYBR® RT-qPCR Kit (SensiFAST SYBR No-ROX Kit, (Meridian Bioscience)). The mRNA expression levels of each gene of interest were determined following the Bio-RAD protocol using the CFX®96 Real-Time system. The 10 μl reaction mixture consisted of 5 μl Master mix (SensiFAST SYBR® No-ROX Mix (Meridian Bioscience)), 2.5 μl mRNA MixB (Primer F&R) and 2.5 μl cDNA sample corresponding to 1000 ng total RNA. As negative controls (NTC), 2.5 μl sterile distilled water (PCR Water, DNase/RNase free, Bioline; Meridian) was used instead of cDNA to screen for DNA contamination. All samples were performed in 96-well plates and duplicates. Amplification conditions were as follows: Initial denaturation at 95°C for 1 min, followed by 40 cycles of GnRH1 at 61°C, GnRHR at 57°C, KISS1 at 65°C, KISS1R at 59°C for 30 s and 1 cycle of Melting Curve analysis at 95°C for 10 s, 70°C for 5 s, 95°C for 5 s. Upon completion of the procedure, the assessment of target genes (GnRH1, GnRHR, KISS1, KISS1R) was carried out using the comparative CT method (ΔΔCT method or Livak method). This method involves the calculation of the ΔΔCt value using an arithmetic formula, comparing the CT value of the target gene with the result of the endogenous reference gene, ultimately expressed as 2^-(ΔΔCt).
2.6. Immunohistochemistry
In order to determine the immunolocalization of GnRH1, GnRHR, KISS1 and KISS1R in ovarian and uterine tissues, 2 µm thick sections were taken with a microtome device Leica Rm 2125Rt (Leica Biosystems, US) from the paraffin blocks. The sections were then mounted on HistoBond
® slides (Marienfeld, Germany) and kept in the oven at 37 °C for 24 hours. Streptavidin-biotin immunperoxidase complex technique was used for the detection of GnRH1, GnRHR, KISS1 and KISS1R. Sections were deparaffinized in xylene and slides were rehydrated in a graded sequence of ethanol. Epitope/antigen recovery of the deparaffinized slides was performed by heating in 10 mM citrate buffer (pH 6.0, Citrate, Sodium citrate (tri-sodium citrate dihydrate)) at 600 W in a microwave oven for 15 min. Then the sections were allowed to cool at room temperature for 20 min and washed with PBS. Endogenous peroxidase activity (to prevent false positive detection and high background staining) was blocked with 3% hydrogen peroxide in absolute methanol for 20 min at room temperature and then slides were washed with PBS. To prevent non-specific binding of antibodies, 1 drop of blocking solution (Ultra V Block, Thermo Fisher Scientific, LabVision Corporation, Fremont, CA, USA) was added to the sections and they were incubated for 5 minutes at RT. Tissue sections were then incubated with the primary antibodies (
Table 2) overnight at 4 °C. All antibodies were diluted in an antibody diluent solution (Antibody Diluent OP Quanta, Thermo Fisher Scientific, LabVision Corporation, Fremont, CA, USA). For negative controls, samples incubated with nonimmunized IgGs (isotype controls) of the same species and at the same protein concentration as the primary antibody, as well as without the primary antibodies (negative control), were used. The next day, after rinsing the samples with PBS, the slides were incubated with secondary antibodies, i.e. Biotinylated Goat Anti Rabbit Secondary (Thermo Fisher Scientific, LabVision Corporation, Fremont, CA, USA) for 20 min. After rinsing the slides with PBS, signal intensity was increased by incubating the tissue samples with streptavidin peroxidase (Thermo Fisher Scientific, TS-125-HR) for 20 min at room temperature. Peroxidase activity was obtained by waiting for 10 min using the DAB plus substrate system (Dako, Agilent Technologies, Santa Clara, USA). Slides were counterstained with Gills haematoxylin for 5 min and rinsed under tap water. Finally, slides were mounted in Entellan (Entellan
®, Merck, 1079610500, Darmstadt, Germany).
Immunostaining in the slides was examined using a light microscope (Olympus BX51), and the results were systematically tabulated based on the localization of stained cells within the tissues. Both descriptive and quantitative assessments of brown precipitation and immunostaining were conducted by the same investigator, considering the expression intensity. The evaluation criteria were as follows: no immunoreactivity observed in cells at high magnification (bar: 20μm-X40) was classified as a negative (-) reaction. Immunoreactivity observed solely in cells at high magnification (bar: 20μm-X40) was categorized as a weak reaction (+/-). Immunoreactivity in cells at low magnification (bar: 50/100μm-X10/X20) was considered a strong reaction (+). Clear observation of immunoreactivity in cells at low magnification (bar: 50/100μm-X10/X20) was designated as a very strong reaction (++).
2.7. Statistical Analysis
Statistical analyses were performed using the IBM SPSS 22 package program (SPSS®, IL, USA). Results are presented as mean ± standard error of the mean (SEM). The normal distribution of steroid hormone values was assessed using Shapiro-Wilk analysis. Since serum E2 concentrations of the comparison of groups showed normal distribution, one-way analysis of variance (One-Way ANOVA), and P4 concentrations did not show normal distribution, Kruskal-Wallis analysis was used. In the determination of gene expression levels, One-Way Analysis of Variance (One-Way ANOVA) was used for comparisons since the group analyzes provided normal distribution. Post-Hoc multiple comparison test Tukey was used to determine the differences between groups. Differences were considered statistically significant if P < 0.05.
3. Results
In this study, 6 (37.5%) of 16 dogs showed signs of proestrus/estrus within 8.6 ± 0.6 days after implant application, while in 10 dogs, no clinical signs of proestrus/estrus were observed within this period (62.5%). For time to proestrus/estrus and diestrus, and duration of proestrus/estrus in individual dogs of EST group after implant insertion, see Karadag et al. 2023 [
15],
Table 2. None of the CONT dogs showed clinical or cytological signs of beginning proestrus/estrus.
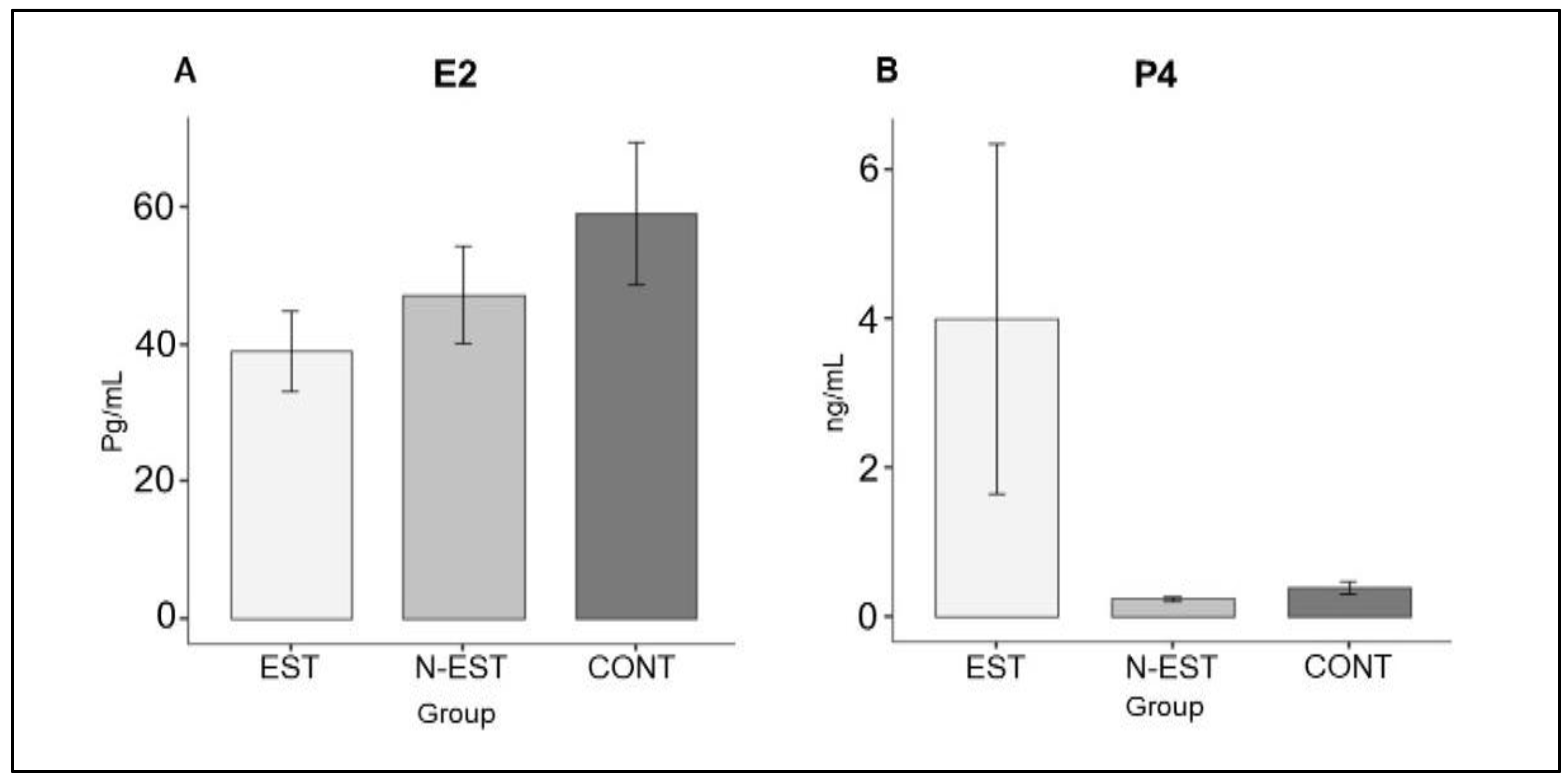
3.1. Serum Estradiol and Progesterone Concentration
The course of the average serum concentrations of E2 and P4 have been published previously [Karadağ et al. [
15]. Within the frame of this study, only the values at the day of OHE were of interest; at this time point, only 4/6 dogs in the EST group and none of the other groups had P4 values >2 ng/ml, indicating ovulations. However, the average serum steroid hormone values (E2 and P4) did not differ significantly between groups on the day of ovariohysterectomy (p>0.05,
Figure 2).
3.2. Gene Expression of GnRH1, GnRHR, KISS1 and KISS1R in the Uterus and Ovary
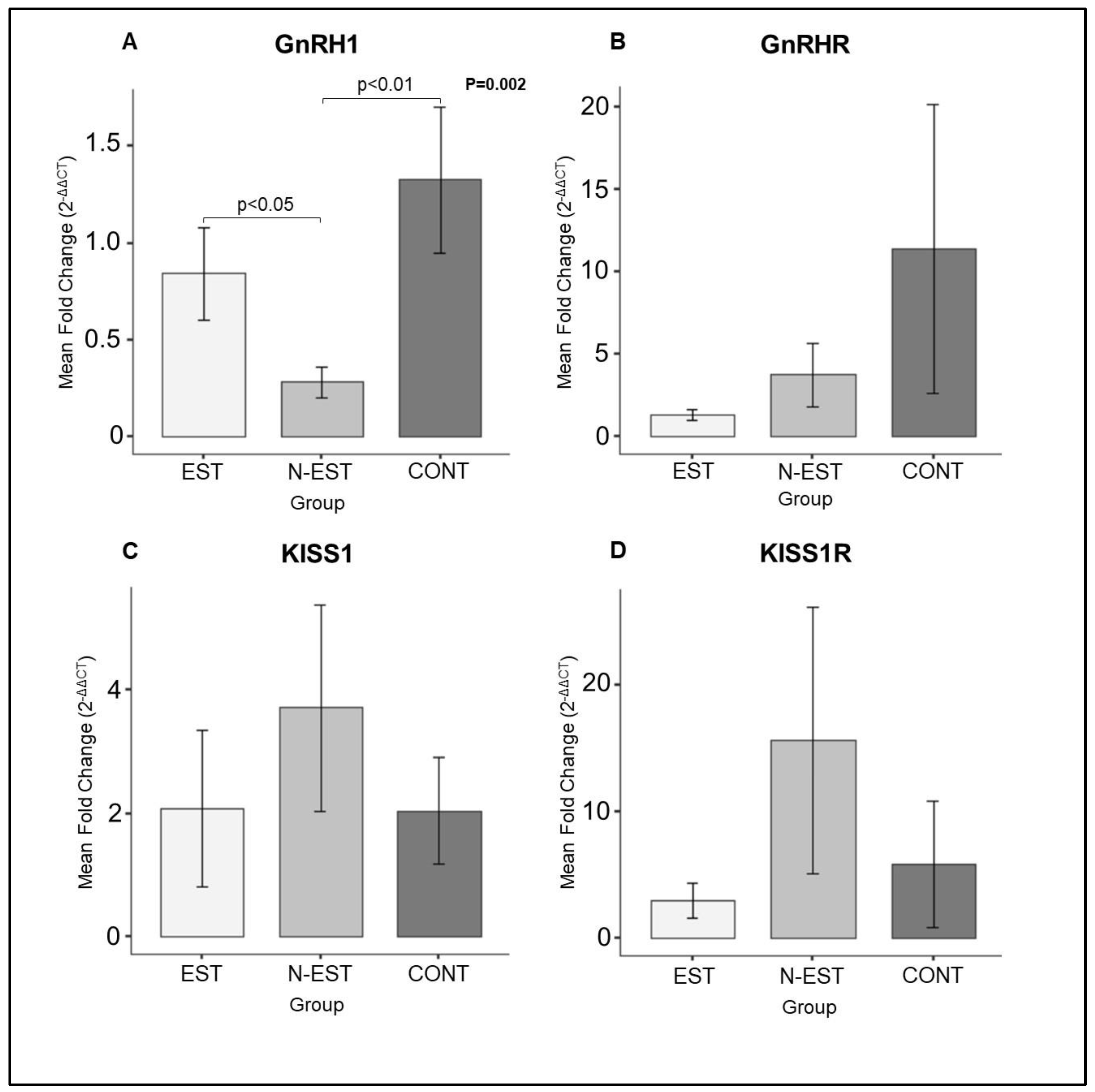
Expression of GnRH1, KISS1 and their receptors GnRHR and KISS1R was detectable in all uterine and ovarian samples in each group investigated in the present study. As for the uterine samples, expression of GnRH1 mRNA was affected by the deslorelin treatment. Thus, whereas it was unaffected in EST, it decreased significantly in N-EST compared with the CONT animals. However, the abundance of KISS1, GnRHR and KISS1R transcripts did not differ significantly in each group investigated (
Figure 3).
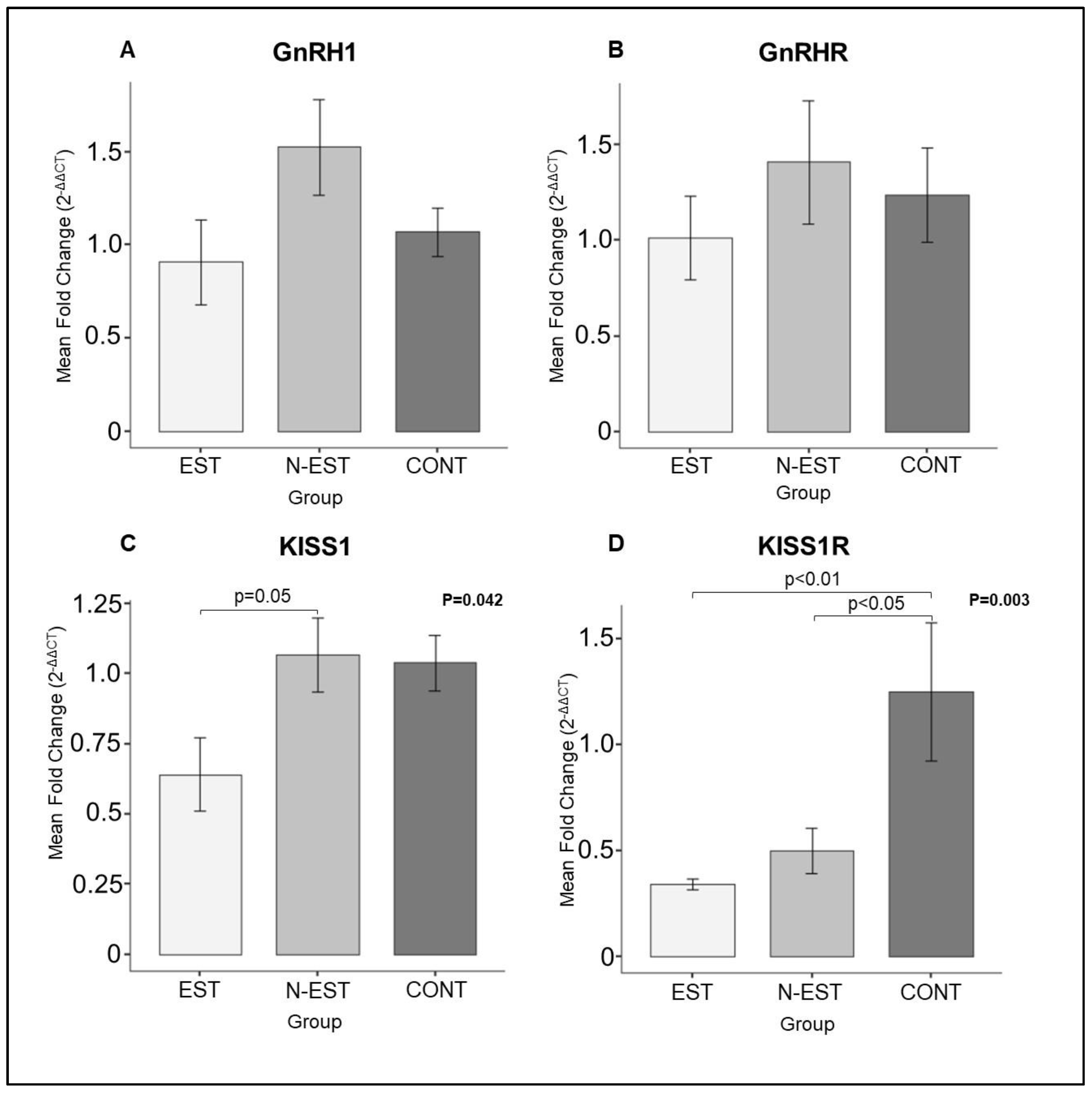
As for the ovarian samples, whereas the expression of GnRH and GnRHR mRNA did not change significantly in all groups investigated, the expression of KISS1 and KISS1R was significantly affacted by deslorelin treatment. Accordingly, the expression of KISS1 mRNA was downregulated in EST group compared with the N-EST animals. Similiarly, in EST animals KISS1R mRNA decreased significantly compared with the control group (
Figure 4).
3.3. Localization of GnRH1, GnRHR, KISS1 and KISS1R in the Uterus and Ovary
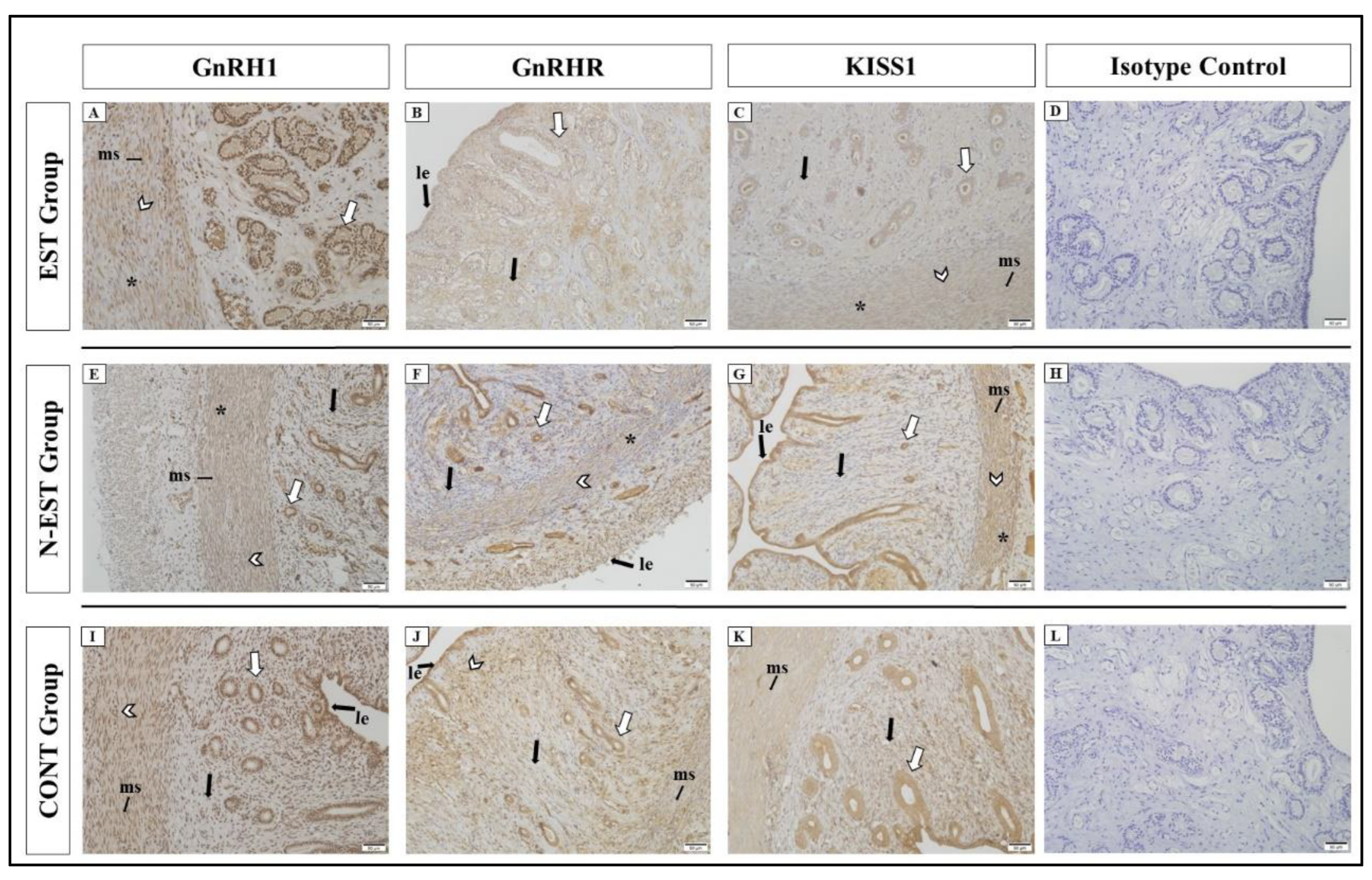
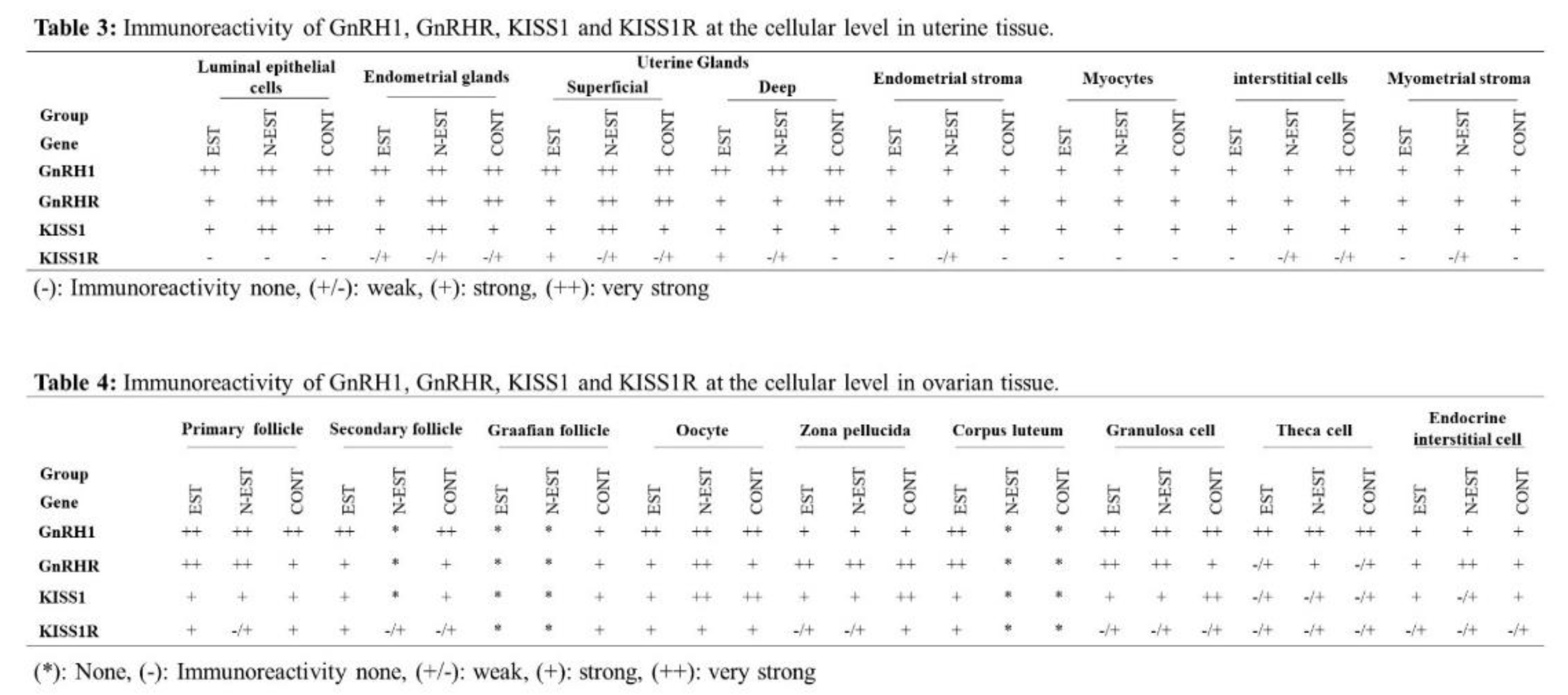
In all groups, strong GnRH1 immunoreactivity was detected in luminal epithelial cells, endometrial glands, superficial and deep uterine glands of the uterus except the endometrial stroma. Weaker reaction was seen in the myometrium (myocytes, interstitial cells and myometrial stroma). Similar to GnRH1, GnRHR also showed positive immunoreactivity in immunohistochemical sections in all groups (Fig. 5).
All groups showed different degrees of positive immunoreactivity for KISS1 in luminal epithelial cells, endometrial glands, superficial and deep uterine glands, myocytes, interstitial cells and endometrial stroma. While similar positive immunoreactivity was observed in cellular localizations of the uterus between groups, strong immunoreactivity was seen in luminal epithelial cells and superficial uterine glands in the N-EST group. For KISS1R, overall negative and weak positive immunoreactivity was observed in cellular localizations of the uterus. Very weak and weak immunoreactivity was observed in endometrial glands, superficial and deep uterine glands, endometrial stroma and interstitial cells, while KISS1R showed no immunoreactivity in luminal epithelial cells, myocytes and endometrial stroma in some groups (
Figure 5, Table 3).
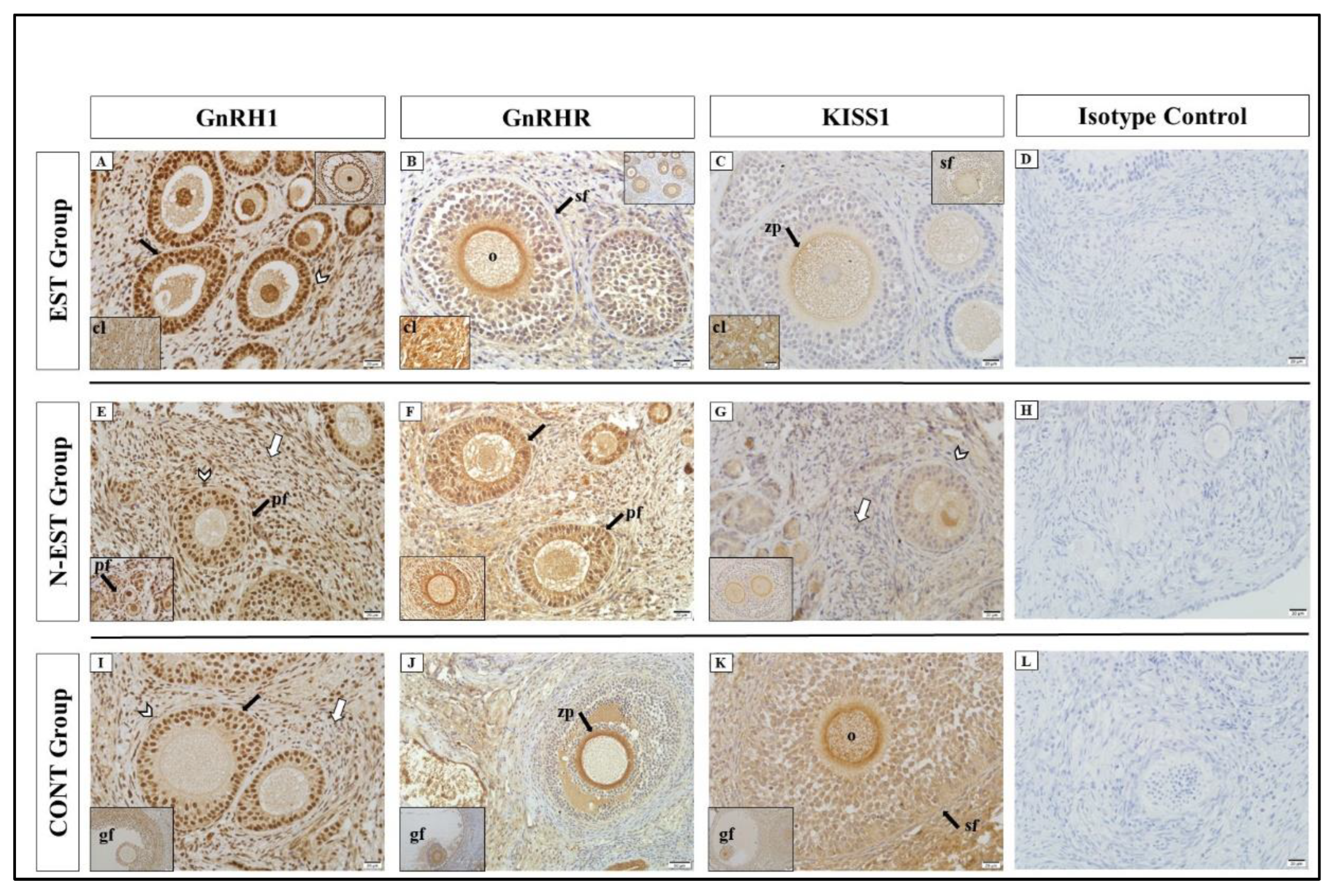
GnRH1 showed positive immunoreactivity in cellular localizations of the ovary in all groups. Similar to GnRH1, positive immunoreactivity for GnRHR was determined at different degrees (strong, very strong) in the groups. In all groups, very strong GnRH1 immunoreactivity was detected in primary, secondary and graafian follicles, oocyte, granulosa and theca cell and strong immunoreactivity zona pellucida and endocrine interstitial cells. For KISS1, the weakest immunoreactivity was seen in theca cells in all groups. In general, KISS1R showed negative and weak positive immunoreactivity in all cellular localizations of the ovary in all groups. The weakest immunoreactivity was seen in theca cells and endocrine interstitial cells (
Figure 6, Table 4).
4. Discussion
4.1. Steroid Hormone Concentrations
In various studies involving adult dogs, both stimulation and suppression of estrus have been successfully achieved through the administration of GnRH agonists, mainly dependant on the cycle stage at the time of application but in addition on sometimes unknown, individual factors. In prepubertal dogs, the mechanism of how the initation of the hypothalamus-pituitary-gonadal axis (HPG) is prevented/delayed by deslorelin is not yet fully understood. Most probably the individual grade of immaturity of GnRH neurons, the kisspeptin/GnRH pulse-producing neuronal circuit, and further unknown control mechanisms regulating the secretory activity of neurons [
38] result in distinct clinical findings. Clinical studies have unveiled variable suppression durations and inconsistent flare-up findings even when application took place as early as 3-4 months of age [
9,
10,
12,
13,
39] but also when implants were inserted at the age of 6-8 months [
9,
15,
40]. These results may suggest that the effects of prepubertal administration of the GnRH agonist Deslorelin on the HPG axis are not limited to central GnRH release alone and that other factors such as peripheral expression of GnRH, Kisspeptin (KISS) and their receptors, which are essential for reaching puberty, should also be investigated. Therefore, in this study, the presence and localization of GnRH1 and KISS1 and their receptors in ovarian/uterine tissues after insertion of Deslorelin in late prepubertal dogs were investigated. The most critical point of the studies on the prepubertal period is to determine with clinical and hormonal analyzes that the animals are actually prepubertal [
41]. The prepubertal period was confirmed by clinical reproductive examinations, vaginal cytology and determination of E2/P4 levels in all female dogs used in this study.
In studies on application of Deslorelin in prepubertal dogs at the age of 3-4 months, showed a variable response in serum E2 concentrations [
12,
13,
14]. Some animals exhibited an increase followed by a rapid decrease below 18 pmol/L, maintaining a low level throughout the treatment period without showing clinical signs of proestrus. Only 4/9 animals entered proestrus and only 2 out of these ovulated as indicated by the P4 values. Mean E2 and P4 concentrations at the time of tissue removal in control and Deslorelin-treated bitches 30-45 days after they had entered proestrus, did not differ significantly between Deslorelin-treated bitches and controls [
12,
13,
14]. Authors explained the possible reasons for this with individual differences in drug absorption and/or metabolism between dogs. Differences in individual deslorelin concentrations during the first four weeks in dogs administered different doses of deslorelin acetate support this view [
12]. In this study, which solely included late prepubertal dogs at the age of 7 months, the serum E2 values in the EST group were lowest at 30-45 days after inplant insertion, indicating post flare-up decrease, whereas in the CONT group, the higher average value may indicate approaching proestrus. Average progesterone values were relatively high in the EST group since 4/6 dogs had ovulated, others not; the latter may be related to inadequate release of neuropeptides, hormones and mediators effective in the stimulation of puberty rather than the effect of deslorelin, since anovulatory cycles rather frequently occur in young bitches during their first cycle; however, this occurred both after Deslorelin administration at the age of 4 and 7 months. Finally, these results so far indicate that the effect of Deslorelin on prepubertal dogs cannot be completely foreseen; clinical parameters, E2 and P4 values do not sufficiently indicate, how the individual HPG axis will react, even not in 3-4 months old animals; on the other hand, insertion of Deslorelin in older dogs not necessarily results in a flare-up. This rectifies the search for other indicators of approaching puberty.
4.2. Expression of GnRH1, GnRHR, KISS1 and KISS1R Genes and Proteins in the Ovary
4.2.1. KISS1 and KISS1R
The findings from our study show the presence of KISS1 and KISS1R proteins and mRNA in the ovarian tissue of dogs before and after puberty. In the ovary, the presence of KISS1 and its receptor is thought to fulfill multiple functions during the female cycle. Its role in follicular development is recognized by the fact that in rats, the mRNA of KISS1 in the ovary gradually increases from birth to puberty [
42]. A high expression of KISS1 and KISS1R in the ovaries of different mammals such as rodents, hamsters and humans during the pubertal period has been previously reported [
43,
44,
45,
46,
47]. A recent study showed that kisspeptin controls ovulation and follicular dynamics in mice and its deficiency is one of the main factors responsible for ovarian aging in women [
48,
49]. Gaytan et al. [
48] reported in their study in mice that Kisspeptin receptor deficiency causes early ovulatory failure; KISS1R hypomorph mice showed an early decline in ovulation rate, followed by progressive loss of antral follicles, oocyte loss and a decrease in all categories of preantral follicles. They also reported that the failure of follicular development and ovulation due to KISS1R deficiency cannot be completely corrected by (prolonged) gonadotropin replacement. These findings point to a direct active role of kisspeptin signaling in the ovary.
The similarity between the groups in the distribution of KISS1 and KISS1R in the ovary at 30-45 days after implant insertion and irrespective of a flare-up and whether dogs had ovulated or not, indicates the physiological site of kisspeptin signaling, especially in the oocyte, in the control of ovulation and follicle survival, and independent of the agonistic effect of Deslorelin; the latter might mainly change the degree of expression in case of a flare-up by promoting physiological downregulation towards the luteal phase. The latter was supposed, since the ovarian KISS1 and KISS1R expression in the EST group was significantly lower than in the other groups, probably suggesting that KISS1 had exerted its local effect earlier, whereas at the time of tissue collection, Deslorelin or another mechanism had already promoted local downregulation of these genes. However, this hypothesis has to be proven.
Ruohonen et al. 2022 [
49] proposed that the expression of KISS1 and KISS1R, along with the presence of KISS1 and KISS1R proteins in follicles (granulosa, theca, oocyte), and corpus lutea in mice, exerts significant autocrine and paracrine effects on local KISS1, influencing ovarian function. In a study by Cielesh et al. 2017 [
34] on dogs, the immunohistochemical localization of KISS1 and its receptor in ovaries was investigated. The study involved pre-pubertal (n = 5), anoestrus (n = 5), and cyclic animals (proestrus, estrus, and dioestrus; n= 14), ranging from 10 weeks to 10 years of age. Unfortunately, the average age of the prepubertal group is not given; puberty was excluded post operation by examination of the uterus and the ovaries. Expression of KISS1 was found in primordial follicles of one prepubertal bitch, and expression of KISS1R in primordial follicles, oocytes and granulosa cells, of all bitches. However, no positive reaction was detected in theca cells. In postpubertal dogs with corpora lutea, staining of KISS1 and KISS1R was inconsistently detectable in granulosa cells, oocytes and corpora lutea [
34]. In our study, KISS1 showed weak immunoreactivity in theca cells in all groups, also in those that had not entered proestrus at the time of OHE, due to either a delay of puberty or naturally late occurrence of puberty (N-EST and CONT); KISS1R showed negative or weak immunoreactivity in all cellular localizations of the ovary in all groups with weakest immunoreactivity in theca cells and endocrine interstitial cells. The discrepancy in theca cell expression of KISS1 and KISS1R may be due to different stages of ovarian development in dogs of both studies. The role of kisspeptins in oocyte development and survival may increase as puberty approaches. Concerning the corpus luteum in dogs that received Deslorelin during the early prepubertal phase, KISS1R expression in mid-luteal phase did not differ between treated dogs and non-treated controls; KISS1 protein expression was low in both groups [
13]. Similarly, in this study on late prepubertal dogs, protein expression of both KISS1 and KISS1R was weak but detectable in the corpus luteum of the EST group during the first mid-luteal phase. Thus in dogs, kisspeptin, produced in and/or acting on granulosa cells of the ovary, may be important for progesterone production/secretion. In a recent study, a bolus injection of Kiss-10 in adult, anestrus beagle dogs, did not induce estrus but seemed to prevent preovulatory increase in progesterone [
50]. It remains to be proven, whether this was caused by local KISS1R downregulation.
4.2.2. GnRH and GnRHR
In the present study using late prepubertal dogs at 30-45 days after implant insertion, the ovarian expression of both GnRH and GnRHR was proven and comparable between groups of mid-luteal post-pubertal (EST) and prepubertal dogs (N-EST, CONT), irrespective of Deslorelin treatment. Protein expression of GnRH1 and -R was variable; in case of GnRH1 strong/very strong, in all structures of the ovary, except the Graafian follicles of the treated groups. While the significant lower levels of KISS1 and KISS1R expression in the EST group may suggest that deslorelin treatment accelerates local downregulation of these genes, this does not seem to apply to ovarian GnRH and its receptor; mRNA expression at day 30-45 after implant insertion was comparable between groups. In parallel with the findings of this study in late prepubertal dogs, Kaya et al. 2017 [
13] reported that the relative expression of GnRHR and KISS1 was found to be low in ovarian tissue in both deslorelin-treated and control early prepubertal animals. The GnRH/GnRHR system is supposed to contribute to follicle development and atresia and to luteinization as well as luteolysis, and, in a more autocrine/paracrine way also in ovarian epithelial cell proliferation of different mammals [
51,
52,
53,
54]. These processes are supposed to be still inactive or not fully active, especially in very young prepubescent dogs, even though receptors and hormones are already there. However, after puberty, a role of this system in follicular and corpus luteum steroidogenesis and apoptosis [
55,
56,
57,
58,
59,
60] has been demonstrated in several mammalian species; in the canine species, there is no proof. The present results implicate activities of the GnRH/GnRHR system during canine follicular development before puberty and during the first estrus thereafter, as has been described in humans [
61]. Results of this study and earlier findings [
13] furthermore point towards a direct endocrine regulatory involvement of GnRH within the canine corpus luteum, as in corpora lutea of dogs during their first estrus, GnRH and –R protein and mRNA expression was detected (EST). Time-dependent relative expression of GnRHR in the corpus luteum of adult dogs throughout diestrus and decreasing relative expression with luteal regression has been reported [
13]. The effect of Deslorlin on canine ovarian GnRH/GnRHR expression may be doubted since no differences between groups were observed.
However, more studies are necessary to investigate the local effect of GnRH/GnRH1R and GnRH agonists in the canine ovary, especially as in other species, differences at the molecular level between the central and the peripheral system were supposed; different intracellular signaling and post-translational processing are possible [
62,
63].
4.3. Expression of GnRH1, GnRHR, KISS1 and KISS1R Genes and Proteins in the Uterus
KISS1 and KISS1R
In the uterus, KISS1 protein expression was found in all uterine cells, whereas KISS1R protein was inconsistently expressed and either weak or absent. KISS1 and KISS1R mRNA expression was comparable between groups but a tendency for higher values were found in treated dogs that did not enter proestrus (N-EST). During a previous study on Deslorelin application in early prepubertal dogs, similar results were found as protein expression of KISS1R and ERα,β was especially high in bitches ovariohysterectomy was performed prepubertally [
14], which corresponds to the N-EST group of this study; for KISS1, immunoreactivity scores were highest in the post-pubertal controls. Expression of KISS and KISS1R mRNA in the human endometrium has been reported; however, in contrary to the present study, not in the myometrium [
45]. In mice, kisspeptin binding to intrauterine KISS1R was shown to affect on uterine growth and especially on gland function [
64], and participates in endometrial homeostasis. A lack of of KISS1 and KISS1R in the endometrium can cause abnormal expression of matrix metalloproteinases (MMPs), vascular endothelial growth factor (VEGF), and other molecules, leading to disturbed invasion, migration, and angiogenesis of the endometrium; furthermore abnormal endometrial hyperplasia, endometriosis and uterine endometrial carcinoma were related to this abnormality. Kisspeptin thus seems to be an important factor for maintainance of the normal structure and function of the endometrium, also during pregnancy [
65]. It was previously stated that in humans, the expression of KISS1 and KISS1R increases significantly during the late secretory phase, coinciding with ongoing decidualization in the pregnant uterus [
66]; KISS1 and its receptor were also detected in the canine pregnant uterus and trophoblast cells [
35]. In our study, still prepubertal (N-EST, CONT) and meanwhile postpubertal bitches (EST) were obviously ovariohysterectomy before this increase. However, uterine expression of KISS1 and KISS1R seems to be higher in prepubertal than postpubertal dogs, highly variable and not to be effected by stage of prebubescence at the time of Deslorelin application.
In contrast to KISS1 and KISS1R, GnRH1 expression in the uterus was significantly lower in the N-EST group than in the other groups, whereas GnRH1R expression did not differ between groups; however, both GnRH1 and GnRH1R expression was highest in the still prepubertal CONT group. This may suggest that exposure of GnRH neurons to an agonist for approximately 2-3 weeks was sufficient to initiate down-regulation of both GnRH and its receptor in the treatment groups. This is supposed since in humans, an increase in the endometrial expression of GnRH and –R during the luteal phase was observed, especially in glandular and stromal cells [
67]; however, this is not proven in dogs and the here observed effect might also be related to physiological changes during the mid-luteal phase. The protein expression differed between cells but was on average more intense for GnRH1 than for its receptor. GnRH1 showed strong positive immunoreactivity in all layers of the uterus except the endometrial stroma in all groups. However, GnRHR immunoreactivity was especially weak in the EST group and in all endometrial layers, which might be due to the flare-up effect of Deslorelin or a local effect of Deslorelin. Deslorelin in our study might have overcome the progesterone effect by down-regulation of local GnRH- and R; however, this remains to be proven.
5. Conclusions
Finally, in this study, we found signs that GnRH, KISS and supposedly also the GnRH agonist deslorelin may have a direct effect at both the ovarian and uterine level in dogs. All may directly affect steroidogenesis through ovarian GnRH- and KISS- receptors, which could be part of a local autoregulation system, as in some other mammalian species. The significantly decreased KISS1 and KISS1R expression in ovarian tissues of dogs in EST group following deslorelin administration and similar numerical findings in GnRH1/GnRHR expression, may indicate that the mutual regulatory mechanism between GnRH and KISS is affected by agonist applications in the late prepubertal period. Our data furthermore revealed that the molecular responses of deslorelin in female dogs in the late prepubertal period showed individual differences, similar to the clinical responses and similar to the effects in early prepubertal dogs. This may be related to the fact that puberty is a complex and multifactorial biological process. More animals and better indicators of real prepubescence, before the “brake” in the central nervous system allows KISS- and GnRH-pulsatility to increase, would allow to better study the effect of deslorelin in prepubertal dogs. Furthermore, the role of ovarian and uterine GnRH and KISS1 and receptors in dogs deserves more research in the future.
Author Contributions
D.K, S.S.S designed and planned the project, analyzed the data, and D.K, S.A, S.S.S, A.G. and M.A.K drafted and write the initial manuscript. M.A.K performed the experiments and ovariohysterectomy operations, D.K provided data control. A.G. and M.A.K. performed PCR and IHC analyses, and A.G. and D.K. provided analysis control. All authors edited and proofread the manuscript.
Funding
This study was partially supported by Kafkas University Scientific Research Projects Coordination Office (KAU-BAP, Project No: 2021-TS-36).
Acknowledgments
The authors would like to thank Prof.Dr. Bülent POLAT for valuable comments.
Declaration of competing interest
The authors declare that there are no conflicts of interest. All coauthors have seen and agree with the contents of the manuscript.
References
- Chakraborty, P.K.; Stewart, A.P.; Seager, S.W. Relationship of growth and serum growth hormone concentration in the prepubertal labrador bitch. coLab Anim Sci. 1983, 33(1), 51-5.
- Lacoste, L.; Dubé D, Trudel C.; Bélanger A.; Labrie F. Normal gonadal functions and fertility after 23 months of treatment of prepubertal male and female dogs with the GnRh agonist [D-Trp6.; des-Gly-NH2(10)] GnRH ethylamide. J Androl. 1989, 10(6), 456-65. [CrossRef]
- Concannon, P.W. Biology of gonadotrophin secretion in adult and prepubertal female dogs. J Reprod Fertil Suppl. 1993, 47, 3–27.
- Conzemius, M.G.; Brown, D.C.; Brabec, M.; Smith, G.K.; Washabau, R.; LaFond, E.; Chakraborty P.K. Correlation between longitudinal bone growth.; growth hormone.; and insulin-like growth factor-I in prepubertal dogs. Am J Vet Res. 1998, 59(12), 1608-12. [CrossRef]
- Mc Bride, M.W.; Aughey, E.; O'Shaughnessy, P.J.; Jeffcoate, I.A. Ovarian function and FSH receptor characteristics during canine anoestrus. J Reprod Fertil Suppl. 2001, 57, 3-10.
- Haenisch-Woehl, A.; Kölle, S.; Neumüller, C.; Sinowatz, F.; Braun, J. Morphology of canine cumulus-oocyte complexes in pre-pubertal bitches. Anat Histol Embryol. 2003, 32(6), 373-7. [CrossRef]
- Cooke, P.S.; Borsdorf, D.C.; Ekman, G.C.; Doty, K.F.; Clark, S.G.; Dziuk, P.J.; Bartol F.F. Uterine gland development begins postnatally and is accompanied by estrogen and progesterone receptor expression in the dog. Theriogenology. 2012, 78(8), 1787-95. [CrossRef]
- Lunardon, N.T.; Silva-Santos, K.C.; Justino, R.C.; Dessunti, G.T.; Seneda, M.M.; Martins M.I. Population estimate of the preantral follicles and frequency of multioocyte follicles in prepubertal and adult bitches. Theriogenology. 2015, 83(6), 1015-20. [CrossRef]
- Trigg, T.E.; Doyle, A.G.; Walsh, J.D.; Swangchan-uthai, T. A review of advances in the use of the GnRH agonist deslorelin in control of reproduction. Theriogenology. 2006, 66(6), 1507-1512. [CrossRef]
- Kaya, D.; Aslan, S.; Kaya, S.; Kuru, M.; Kaçar, C. Clinical and endocrine short-term effects of GnRH analogue deslorelin in prepubertal bitches: Does a “flare-up” occur? Kafkas Univ Vet Fak Derg. 2013, 19, 299. [CrossRef]
- Marino, G.; Rizzo, S.; Quartuccio, M.; Macrì, F.; Pagano, G.; Taormina A.; Cristarella S.; Zanghì A. Deslorelin implants in pre-pubertal female dogs: short- and long-term effects on the genital tract. Reprod Domest Anim. 2014, 49(2), 297-301. [CrossRef]
- Kaya, D.; Schäfer-Somi, S.; Kurt, B.; Kuru, M.; Kaya, S.; Kaçar, C.; Aksoy, Ö.; Aslan, S. Clinical use of deslorelin implants for the long-term contraception in prepubertal bitches: Effects on epiphyseal closure.; body development.; and time to puberty. Theriogenology. 2015, 83, 1147–1153. [CrossRef]
- Kaya, D.; Gram, A.; Kowalewski, M.P.; Schäfer-Somi, S.; Kuru, M.; Boos, A.; Aslan, S. Expression of GnRH receptor in the canine corpus luteum.; and luteal function following deslorelin acetate-induced puberty delay. Reprod Domest Anim. 2017, 52, 1104–1112. [CrossRef]
- Schäfer-Somi, S.; Kaya, D.; Sözmen, M.; Kaya, S.; Aslan, S. Pre-pubertal treatment with a GnRH agonist in bitches-Effect on the uterus and hormone receptor expression. Reprod Domest Anim. 2018, 53(3), 103–109. [CrossRef]
- Karadağ, M.A.; Schäfer-Somi, S.; Demir, M.C.; Kuru, M.; Aslan, S.; Kaya, D. Short-term clinical and hormonal effects of a deslorelin implant on late-prepubertal bitches–Based on flare-up signs. Theriogenology. 2023, 209, 162-169. [CrossRef]
- Millar, R.P. GnRHs and GnRH receptors. Anim Reprod Sci. 2005, 88, 5-28. [CrossRef]
- Whitlock, K.E.; Postlethwait, j.; and Ewer, j. Neuroendocrinology of reproduction: Is gonadotropin-releasing hormone (GnRH) dispensable? Front Neurol. 2019, 53, 100738. [CrossRef]
- Hanoune, J.; Messager, S.; Chatzidaki, E.E.; Ma, D. Kisspeptin directly stimulates gonadotropinreleasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci. 2005, 102, 1761–1766. [CrossRef]
- Keen, K.L.; Wegner, F.H.; Bloom, S.R.; Ghatei, M.A.; Terasawa, E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008, 149, 4151–4157. [CrossRef]
- Tsutsumi, R.; Webster, N.J. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J. 2009, 56, 729 –737. [CrossRef]
- Popa, S.M.; Clifton, D.K.; Steiner, R.A. The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu Rev Physiol. 2008, 70, 213–238. [CrossRef]
- Dhillo, W.S.; Chaudhri, O.B.; Thompson, E.L.; Murphy, K.G.; Patterson, M.; Ramachandran, R.; Bloom, S.R. Kisspeptin-54 stimulates gonadotrophin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab. 2007, 92, 3958–3966. [CrossRef]
- Plant, T.M.; Ramaswamy, S.; DiPietro, M.J. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006, 147, 1007–1013. [CrossRef]
- Caraty, A.; Smith, J.T.; Lomet, D.; Ben Said, S.; Morrissey, A.; Cognie, J.; Clarke, I.J. Kisspeptin synchronizes preovulatory surges in cyclic ewes and causes ovulation in seasonally acyclic ewes. Endocrinology. 2007, 148, 5258–5267. [CrossRef]
- Navarro, V.M.; Castellano, J.M.; Fernandez-Fernandez, R.; Tovar, S.; Roa, J.; Mayen, A.; Tena-Sempere, M. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology. 2005, 146, 1689–1697. [CrossRef]
- Thompson, E.L.; Patterson, M.; Murphy, K.G.; Smith, K.L.; Dhillo, W.S.; Todd, J.F.; Bloom, S.R. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic–pituitary–gonadal axis. J Neuroendocrinol. 2004, 16, 850–858. [CrossRef]
- Gottsch, M.L.; Cunningham, M.J.; Smith, J.T.; Popa, S.M.; Acohido, B.V.; Crowley, W.F.; Steiner, R.A. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004, 145, 4073–4077. [CrossRef]
- Albers-Wolthers, K.H.; De Gier, J.; Kooistra, H.S.; Rutten, V.P.; Van Kooten, P.J.; De Graaf, J.J.; Schaefers-Okkens, A.C. Identification of a novel kisspeptin with high gonadotrophin stimulatory activity in the dog. Neuroendocrinology. 2014, 99(3-4), 178-189. [CrossRef]
- Roa, J.; Navarro, V.M.; Tena-Sempere, M. Kisspeptins in reproductive biology: consensus knowledge and recent developments1. Biol Reprod. 2011, 85(4), 650-660. [CrossRef]
- Han, S.K.; Gottsch, M.L.; Lee, K.J.; Popa, S.M.; Smith, J.T.; Jakawich, S.K.; Herbison, A.E. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005, 25, 11349–11356. [CrossRef]
- Pielecka-Fortuna, J.; Chu, Z.; Moenter, S.M. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008, 149(4), 1979-1986. [CrossRef]
- Wintermantel, T.M., Campbell, R.E., Porteous, R., Bock, D., Gröne, H.-J., Todman, M.G., Korach, K.S., Greiner, E., Pérez, C.A., Schütz, G., Herbison, A.E. Definition of Estrogen Receptor Pathway Critical for Estrogen Positive Feedback to Gonadotropin-Releasing Hormone Neurons and Fertility. Neuron. 2006, 52, 271–280.. [CrossRef]
- Lehman, M.N.; Merkley, C.M.; Coolen, L.M.; Goodman, R.L: Anatomy of the kisspeptin neural network in mammals. Brain Res J. 2010, 1364, 90-102. [CrossRef]
- Cielesh, M.; McGrath, B.; Scott, C.; Norman, S.; Stephen, C. The localization of kisspeptin and kisspeptin receptor in the canine ovary during different stages of the reproductive cycle. Reprod Domest Anim. 2017, 52, 24-28. [CrossRef]
- Schäfer-Somi, S.; Ay, S.S.; Kaya, D.; Sözmen, M.; Beceriklisoy, H.B.; Ağaoğlu, A.R.; Aslan, S. Kisspeptin-10 and the G protein-coupled receptor 54 are differentially expressed in the canine pregnant uterus and trophoblast cells. Reprod Domest Anim. 2017, 52, 123-129. [CrossRef]
- D’Occhio, M.J.; Campanile, G.; Baruselli, P.S. Peripheral action of kisspeptin at reproductive tissues-role in ovarian function and embryo implantation and relevance to assisted reproductive technology in livestock: a review. Biol Reprod. 2020, 103(6), 1157-1170. [CrossRef]
- Kowalewski, M.P.; Beceriklisoy, H.B.; Pfarrer, C.; Aslan, S.; Kindahl, H.; Kücükaslan, I.; Hoffmann, B. Canine placenta: a source of prepartal prostaglandins during normal and antiprogestin-induced parturition. Reproduction. 2010, 139, 655–664. [CrossRef]
- Herbison, A.E.; Plant, T.M. The GnRH neuron and its control. Pittsburg USA; John Wiley & Sons; 2018, p: 1-150.
- Schäfer-Somi, S.; Kaya, D.; Aslan, S. Prepubertal Use of Long-Term GnRH Agonists in Dogs: Current Knowledge and Recommendations. Animals. 2022, 12, 2267. [CrossRef]
- Palm, J.; Reichler, I.M. The use of deslorelin acetate (Suprelorin®) in companion animal medicine. Schweiz Arch Tierheilkd. 2012, 154(1), 7-12. [CrossRef]
- Gobello, C. Prepubertal and Pubertal Canine Reproductive Studies: Conflicting Aspects. Reprod Domest Anim. 2014, 49(6), e70-e73. [CrossRef]
- Ricu, M.A.; Ramirez, V.D.; Paredes, A.H.; Lara, H.E. Evidence for a celiac ganglion-ovarian kisspeptin neural network in the rat: intraovarian anti-kisspeptin delays vaginal opening and alters estrous cyclicity. Endocrinology. 2012, 153(10), 4966-4977. [CrossRef]
- Terao, Y.; Kumano, S.; Takatsu, Y.; Hattori, M.; Nishimura, A.; Ohtaki, T.; Shintani, Y. Expression of KiSS-1, a metastasis suppressor gene, in trophoblast giant cells of the rat placenta. Biochim Biophys Acta. 2004, (2-3)1678, 102-110. [CrossRef]
- Gaytán, F.; Gaytán, M.; Castellano, J.M.; Romero, M.; Roa, J.; Aparicio, B.; Garrido, N.; Sánchez-Criado, J.E.; Millar, R.P.; Pellicer, A.; Fraser, H.M.; Tena-Sempere, M. KiSS-1 in the mammalian ovary: distribution of kisspeptin in human and marmoset and alterations in KiSS-1 mRNA levels in a rat model of ovulatory dysfunction. Am J Physiol Endocrinol Metab. 2009 296(3), e520-531. [CrossRef]
- Cejudo Roman, A.; Pinto, F.M.; Dorta, I.; Almeida, T.A.; Hernández, M.; Illanes, M.; Tena-Sempere, M.; Candenas, L. Analysis of the expression of neurokinin B, kisspeptin, and their cognate receptors NK3R and KISS1R in the human female genital tract. Fertil Steril. 2012, 97(5), 1213-9. [CrossRef]
- Peng Jing, M.D.; Min Tang, M.D.; Bao-Ping Zhang, M.D.; Peng Zhang, B.S.; Ting Zhong, M.D.; Teng Zong, B.S.; Bei Yang, M.D.; Hai-Bin Kuang, M.D: Kisspeptin stimulates progesterone secretion via the Erk1/2 mitogen-activated protein kinase signaling pathway in rat luteal cells. Fertil Steril. 2013, 99(5), 1436-1443. [CrossRef]
- Nakamura, S.; Watanabe, Y.; Goto, T.; Ikegami, K.; Inoue, N.; Uenoyama, Y.; Tsukamura, H. Kisspeptin neurons as a key player bridging the endocrine system and sexual behavior in mammals. Front Neurol. 2022, 64, 100952. [CrossRef]
- Gaytan, F.; Garcia-Galiano, D.; Dorfman, M.D.; Manfredi-Lozano, M.; Castellano, J.M.; Dissen, G.A.; Tena-Sempere, M. Kisspeptin receptor haplo-insufficiency causes premature ovarian failure despite preserved gonadotropin secretion. Endocrinology 2014, 155(8), 3088-3097. [CrossRef]
- Ruohonen, S.T.; Gaytan, F.; Usseglio Gaudi, A.; Velasco, I.; Kukoricza, K.; Perdices-Lopez, C.; Franssen D.; Guler I.; Mehmood, A.; Elo LL.; Ohlsson, C.; Poutanen, M.; Tena-Sempere M. Selective loss of kisspeptin signaling in oocytes causes progressive premature ovulatory failure. H Repro. 2022, 37(4), 806-821. [CrossRef]
- Bozkurt, G.; Ağaoğlu, A.R.; Wehrend, A.; Çortu, A.; Aslan, S.; Schäfer Somi, S. Effect of cabergoline with or without kisspeptin on the estrus cycle and reproductive hormones of bitches. 1st ESAR congress Nantes, 2023.
- Kang, S.K.; Choi, K.C.; Yang, H.S.; Leung, P.C. Potential role of gonadotrophin-releasing hormone (GnRH)-I and GnRH-II in the ovary and ovarian cancer. Endocr Relat Cancer. 2003, 10, 169–177. [CrossRef]
- Limonta, P.; Moretti, R.M.; Marelli, M.M.; Motta, M. The biology of gonadotropin hormone-releasing hormone: role in the control of tumor growth and progression in humans. Front Neuroendocrinol. 2003, 24, 279–295. [CrossRef]
- Cheung, L.W.; Wong, A.S. Gonadotropin-releasing hormone: GnRH receptor signaling in extrapituitary tissues. FEBS J. 2008, 275, 5479 –5495. [CrossRef]
- Limonta P.; Montagnani Marelli M.; Moretti R.; Marzagalli M.; Fontana F.; Maggi R. GnRH in the Human Female Reproductive Axis. Vitam Horm 2018;107:27-66. [CrossRef]
- Srivastava RK.; Krishna A.; Sridaran R.Follicular development.; steroidogenesis and gonadotrophin secretion in response to long-term treatment with a gonadotrophin-releasing hormone agonist in the rat. J Endocrinol 1995, 146(2), 349-57. https://joe.bioscientifica.com/view/journals/joe/joe-overview.xml.
- Krishna A.; Srivastava, R.K.; Sridaran, R. Effects of short-term treatment of a gonadotropin-releasing hormone agonist on the follicular development and gonadotropin secretion in the rat. Endocr Res. 1996, 22(3), 299-310. [CrossRef]
- Papadopoulos, V.; Dharmarajan, A.M.; Li, H.; Culty, M.; Lemay, M.; Sridaran, R Mitochondrial peripheral-type benzodiazepine receptor expression. Correlation with gonadotropin-releasing hormone (GnRH) agonist-induced apoptosis in the corpus luteum. Biochem Pharmacol. 1999, 58(9), 1389-93. [CrossRef]
- Sridaran, R.; Hisheh, S.; Dharmarajan, A.M. Induction of apoptosis by a gonadotropin-releasing hormone agonist during early pregnancy in the rat. Apoptosis. 1998, 3(1), 51-7. [CrossRef]
- Sridaran, R.; Lee, M.A.; Haynes, L.; Srivastava, R.K.; Ghose, M.; Sridaran, G.; Smith, C.J. GnRH action on luteal steroidogenesis during pregnancy. Steroids. 1999a, 64(9), 618-23. [CrossRef]
- Sridaran, R.; Philip, G.H.; Li, H.; Culty, M.; Liu, Z.; Stocco, D.M.; Papadopoulos, V. GnRH agonist treatment decreases progesterone synthesis.; luteal peripheral benzodiazepine receptor mRNA.; ligand binding and steroidogenic acute regulatory protein expression during pregnancy. J Mol Endocrinol. 1999b, 22(1), 45-54. [CrossRef]
- Maggi, R.; Cariboni, A.M.; Marelli, M.M.; Moretti, R.M.; Andre, V.; Marzagalli M.; Limonta, P. GnRH and GnRH receptors in the pathophysiology of the human female reproductive system. Hum Reprod Update. 2016, 22(3), 358-381. [CrossRef]
- Rama, S.; Rao, A.J. Embryo implantation and GnRH antagonists: the search for the human placental GnRH receptor. Hum Reprod. 2006, 16(2), 201-205. [CrossRef]
- Millar, R.P.; Pawson, A.J.; Morgan, K.; Rissman, E.F.; Lu, Z.L. Diversity of actions of GnRHs mediated by ligand-induced selective signaling. Front Neuroendocrinol. 2008, 29(1), 17-35. [CrossRef]
- León, S.; Fernandois, D.; Sull, A.; Sull, J.; Calder, M.; Hayashi, K.; Bhattacharya, M.; Power, S.; Vilos, G.A.; Gilos, A.G.; Tena-Sempere, M.; Babwah, A.V. Beyond the brain-Peripheral kisspeptin signaling is essential for promoting endometrial gland development and function. Sci Rep. 2016, 1(6), e29073. [CrossRef]
- Zhang, J.; Jin, L.; Kong, L.; Nie, L.; Yuan, D. Physiological and pathological roles of locally expressed kisspeptin and KISS1R in the endometrium. Hum Reprod. 2023, 38(7), 1253-1260. [CrossRef]
- Baba, T.; Kang, H.S.; Yuko, Hosoe, Y.; Kharma, B.; Abiko, K.; Matsumura, N.; Hamanishi, J.; Yamaguchi, K.; Yoshioka, Y.; Koshiyama, M.; Mandai, M.; Murphy, S.K.; Konishi, I. Menstrual cyclic change of metastin/GPR54 in endometrium. Med Mol Morpho. 2015, 48(2), 76-84. http://dx.doi.org/10.1007/s00795-014-0081-0.
- Raga, F.; Casañ, E.M.; Kruessel, J.S.; Wen, Y.; Huang, H.Y.; Nezhat, C.; Polan ML Quantitative gonadotropin-releasing hormone gene expression and immunohistochemical localization in human endometrium throughout the menstrual cycle. Biol Reprod. 1998, 59(3), 661-9. [CrossRef]
Figure 2.
Mean serum E2 (A) and P4 (B)concentrations of the groups at the day of ovariohysterectomy. (EST: Estrus, N-EST: Non- Estrus, CONT: Control, the sensitivity of the measurements is 100% and the specificity is 95.5%.).
Figure 2.
Mean serum E2 (A) and P4 (B)concentrations of the groups at the day of ovariohysterectomy. (EST: Estrus, N-EST: Non- Estrus, CONT: Control, the sensitivity of the measurements is 100% and the specificity is 95.5%.).
Figure 3.
Expression of GnRH1 (A), GnRHR (B), KISS1 (C) and KISS1R (D) in uterine tissue. (EST: Estrus, N-EST: Non- Estrus, CONT: Control). All numerical data are presented as mean ± SEM. p values < 0.05 were considered significant and indicated. Comparison between groups Parametric one-way ANOVA was followed by Tukey multiple comparison post hoc-test.
Figure 3.
Expression of GnRH1 (A), GnRHR (B), KISS1 (C) and KISS1R (D) in uterine tissue. (EST: Estrus, N-EST: Non- Estrus, CONT: Control). All numerical data are presented as mean ± SEM. p values < 0.05 were considered significant and indicated. Comparison between groups Parametric one-way ANOVA was followed by Tukey multiple comparison post hoc-test.
Figure 4.
Expression of GnRH1 (A), GnRHR (B), KISS1 (C) and KISS1R (D) in ovarian tissue. (EST: Estrus, N-EST: Non- Estrus, CONT: Control). All numerical data are presented as mean ± SEM. p values < 0.05 were considered significant and indicated. Comparison between groups Parametric one-way ANOVA was followed by Tukey multiple comparison post hoc-test.
Figure 4.
Expression of GnRH1 (A), GnRHR (B), KISS1 (C) and KISS1R (D) in ovarian tissue. (EST: Estrus, N-EST: Non- Estrus, CONT: Control). All numerical data are presented as mean ± SEM. p values < 0.05 were considered significant and indicated. Comparison between groups Parametric one-way ANOVA was followed by Tukey multiple comparison post hoc-test.
Figure 5.
Immunolocalization of GnRH1, GnRHR and KISS1 in uterine tissue. Immunoreactivity of GnRH1 in different areas of the uterus by groups; EST (A), N-EST (E), CONT (I), (bar: 50μm-X20). Immunoreactivity of GnRHR in different areas of the uterus by groups: EST (B), N-EST (F), CONT (J), (bar: 50μm-X20). Immunoreactivity of KISS1 in different areas of the uterus by groups: EST (C), N-EST (G), CONT (K), (bar: 50μm-X20). No reaction was observed in the isotype controls (D, H, L, bar: 50μm-20x) (ms: myometrial stroma, le: Luminal epithelial cells, black thick arrow: endometrial stroma, arrowhead: myocytes, white arrow: endometrial glands, *: interstitial cells).
Figure 5.
Immunolocalization of GnRH1, GnRHR and KISS1 in uterine tissue. Immunoreactivity of GnRH1 in different areas of the uterus by groups; EST (A), N-EST (E), CONT (I), (bar: 50μm-X20). Immunoreactivity of GnRHR in different areas of the uterus by groups: EST (B), N-EST (F), CONT (J), (bar: 50μm-X20). Immunoreactivity of KISS1 in different areas of the uterus by groups: EST (C), N-EST (G), CONT (K), (bar: 50μm-X20). No reaction was observed in the isotype controls (D, H, L, bar: 50μm-20x) (ms: myometrial stroma, le: Luminal epithelial cells, black thick arrow: endometrial stroma, arrowhead: myocytes, white arrow: endometrial glands, *: interstitial cells).
Figure 6.
Immunolocalization of GnRH1, GnRHR and KISS1 in ovarian tissue. Immunoreactivity of GnRH1 in different areas of the ovarian by groups; EST (A), N-EST (E), CONT (I), (bar: 20μm-X40). Immunoreactivity of GnRHR in different areas of the ovarian by groups: EST (B), N-EST (F), CONT (J), (bar: 20/50μm-X20/40). Immunoreactivity of KISS1 in different areas of the ovarian by groups: EST (C), N-EST (G), CONT (K), (bar: 20μm-X40). No reaction was observed in the isotype controls (D, H, L, bar: bar: 20/50μm-X20/40). (cl: corpus luteum, gf: Graafian follicle, sf: secondary follicle, pf: primary follicle, zp: zona pellucida, o: oocyt, black arrow: Granulosa cell, arrowhead: theca cell, white arrow: endocrine interstitial cell).
Figure 6.
Immunolocalization of GnRH1, GnRHR and KISS1 in ovarian tissue. Immunoreactivity of GnRH1 in different areas of the ovarian by groups; EST (A), N-EST (E), CONT (I), (bar: 20μm-X40). Immunoreactivity of GnRHR in different areas of the ovarian by groups: EST (B), N-EST (F), CONT (J), (bar: 20/50μm-X20/40). Immunoreactivity of KISS1 in different areas of the ovarian by groups: EST (C), N-EST (G), CONT (K), (bar: 20μm-X40). No reaction was observed in the isotype controls (D, H, L, bar: bar: 20/50μm-X20/40). (cl: corpus luteum, gf: Graafian follicle, sf: secondary follicle, pf: primary follicle, zp: zona pellucida, o: oocyt, black arrow: Granulosa cell, arrowhead: theca cell, white arrow: endocrine interstitial cell).
Table 1.
List of primer sequence and accession numbers used for real time (SYBR No-ROX) qPCR. F; forward primer, R; reverse primer.
Table 1.
List of primer sequence and accession numbers used for real time (SYBR No-ROX) qPCR. F; forward primer, R; reverse primer.
| Primer Sequence |
|---|
| Gene |
Primer Sequences |
Product length (bp) |
Accesion Numbers
(canis lupus familaris) |
| GnRH1 |
F: 5'-AGTCTGATTGAAGAGGAA-3' |
157 |
XM_038434631 |
| R: 5'-AATATGTGAACTTAGCGTAA-3' |
| GnRHR |
F: 5'-ACACGAGTCCTTCATCAG-3' |
129 |
AF206513 |
| R: 5'-AGTCCAGCACACAGTAAA-3' |
| KISS1 |
F: 5'-CCTGGTTTCTTGGCAGCTAATG-3' |
81 |
KJ512885 [13] |
| R: 5'-GTCTCCATGGGTGCCACCTT-3' |
| KISS1R |
F: 5'-GGAACTCTCTGGTCCTTT-3' |
73 |
NM_001314087 |
| R: 5'-TTGGCTATGTAAAAGTTGGT-3' |
| EIF4H |
F: 5'-TAAGGTCTCAGCAATTAC-3' |
101 |
XM_014114129 |
| R: 5'-AAGTTAAGTATTGGTGTCA-3' |
| KDM4A |
F: 5'-TCACAGAGAAGGAAGTTAAG-3' |
82 |
XM_005629107 |
| R: 5'-GCAGGCTCAATGTAATCT-3' |
| PTK2 |
F: 5'-ACCTGGCTGACTTCAATC-3' |
85 |
XM_005627993 |
| R: 5'-ATCTTCAACTGTAGCATTCCT-3' |
Table 2.
List of antibodies and dilutions used in immunohistochemical staining.
Table 2.
List of antibodies and dilutions used in immunohistochemical staining.
| Target Protein |
Product reference and manufacturer |
Species/Type |
Dilution |
| GnRH1 |
ORB585773 (Biorbyt, Cambridge, UK) |
Rabbit polyclonal |
1:100 |
| GnRHR |
AGR030 (Alomone Labs, Jerusalem, Israel) |
Rabbit polyclonal |
1:100 |
| KISS1 |
Obtained from Prof.Dr. Alain Caraty (INRA, France) |
Rabbit polyclonal |
1:100 |
| KISS1R |
NLS1927 (Novus, Centennial, USA) |
Rabbit polyclonal |
1:100 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).