Submitted:
21 October 2024
Posted:
22 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
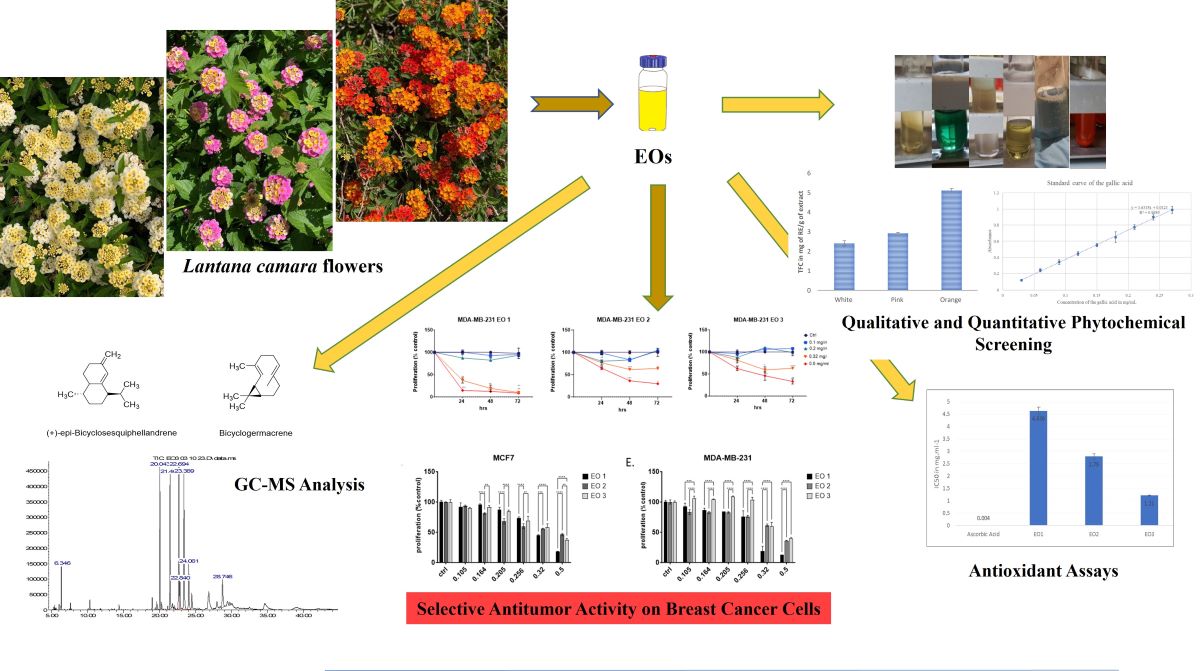
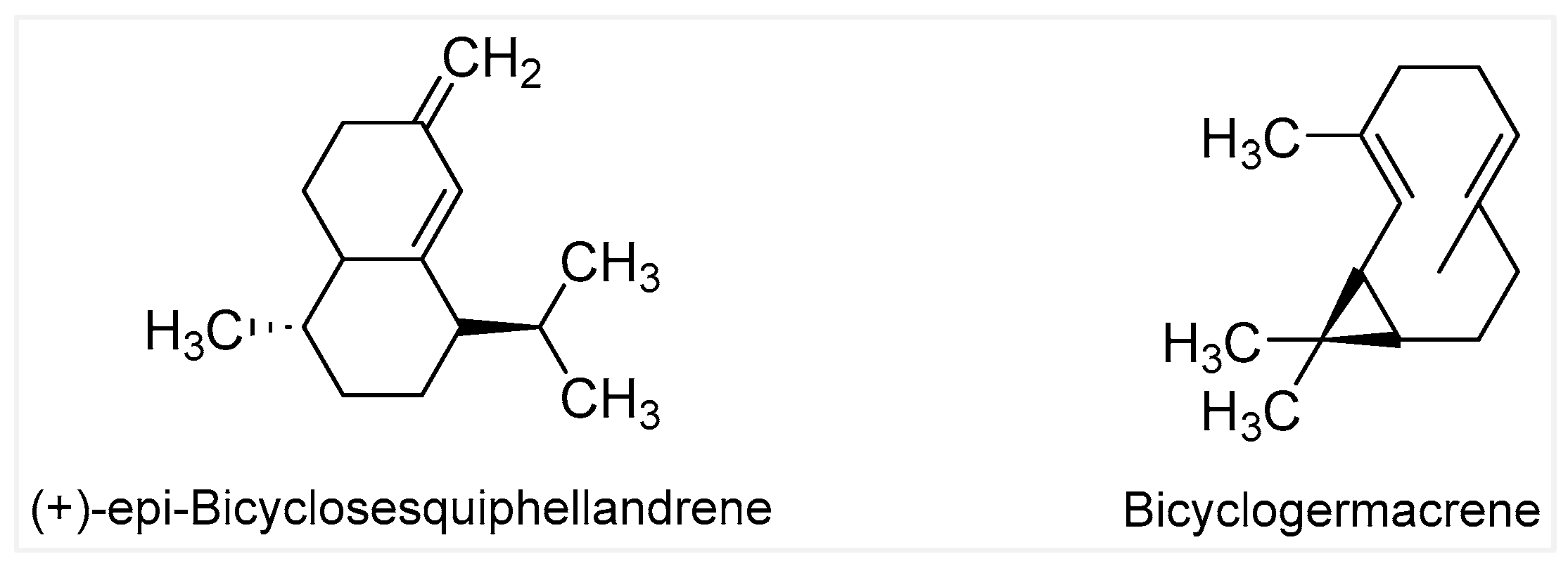
2.1. GC-MS Analysis Results and Yields of Extractions
2.2. Total Flavonoid Content (TFC) and Total Phenol Content (TPC)
2.3. Antioxidant Activities
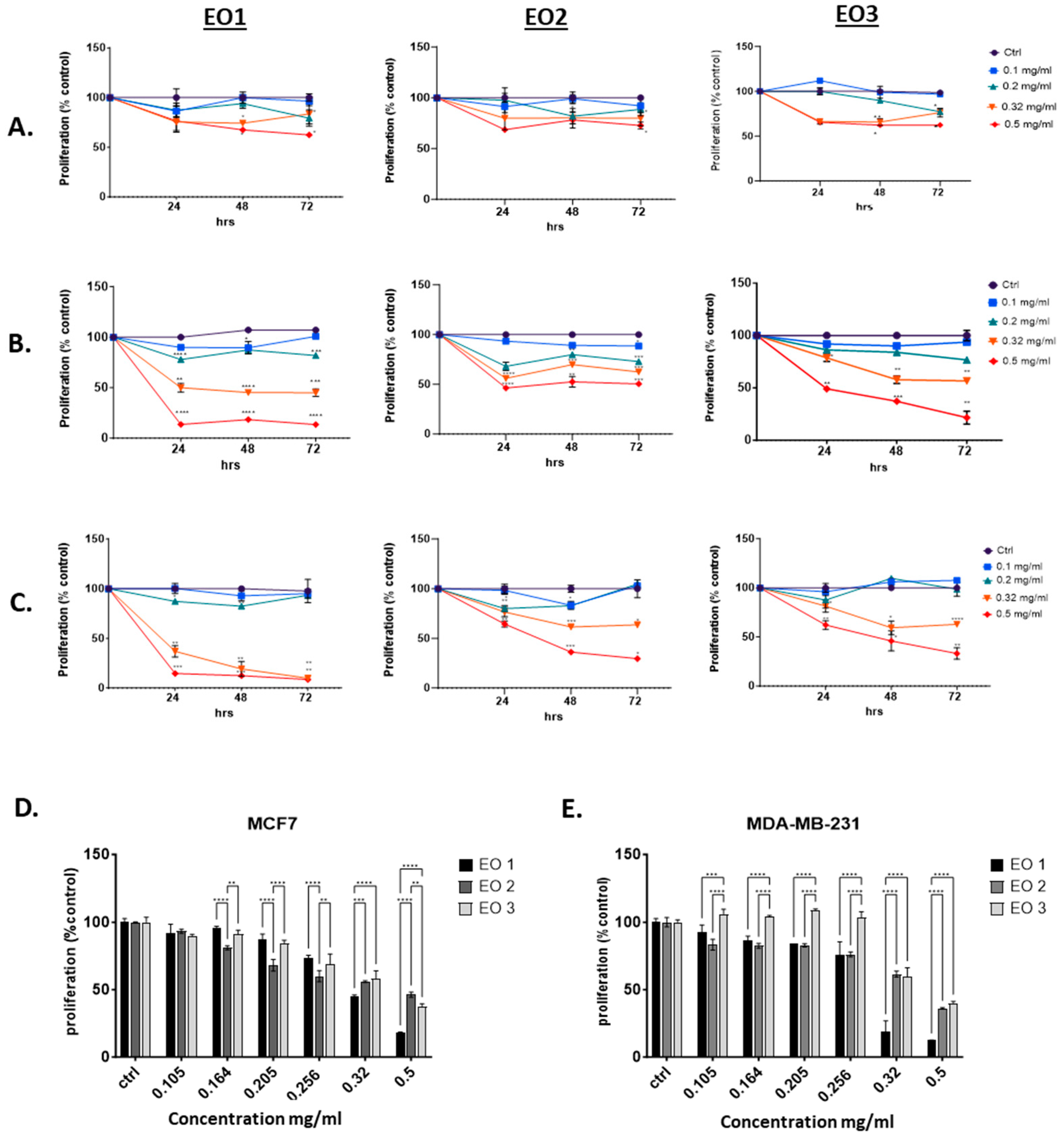
2.4. Reduction of MCF-7 and MDA-MB-231 Cell Proliferation in a Dose-dependent Manner
2.5. Pearson’s Correlation Coefficient Analysis
3. Materials and Methods
3.1. Collection of the Petals of L. camara
3.2. Hydrodistillation with Clevenger
3.3. GC-MS analysis
3.4. TFC analysis
3.5. TPC analysis
3.6. DPPH Assay
3.7. FRAP Assay
3.8. Cell Culture
3.9. Cell Proliferation Assay
3.10. Statistical Analyses
4. Conclusion and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Dedication
Conflicts of Interest
List of Abbreviations
References
- H. A. Parimoo, R. Sharma, R. D. Patil, V. Patial, Comp. Clin. Pathol., 2015, 24, 1541–1552. [CrossRef]
- S. Kalita, G. Kumar, L. Karthik, K.V.B. Rao, Research J. Pharm. and Tech. 2012, 5, 6, 711-715.
- B.S. Nayak, S.S. Raju, M. Eversley, A. Ramsubhag, Phytother. Res. 2009, 23, 241–245. [CrossRef]
- T. Ganesh, S. Saikat, E. Thilagam, G. Thamotharan, T. Loganathan, R. Chakraborty, International Journal of Research in Pharmaceutical Sciences, 2010, 1, 3, 247–252.
- O.P. Sharma, H.P. Makkar, R.K. Dawra, Toxicon, 1988, 26, 11, 975-987. [CrossRef]
- M. Shah, H. F. Alharby, K. R. Hakeem, Letters in Applied NanoBioScience, 2020, 9, 3, 1199–1207. [CrossRef]
- A. Mansoori, N. Singh, S. K. Dubey, T. K. Thakur, N. Alkan, S. N. Das, A. Kumar, Front. Agron., 2020, 2, 582268. [CrossRef]
- K. Aisha, N. U. Visakh, B. Pathrose, N. Mori, R. S. Baeshen, R. Shawer, Molecules 2024, 29, 344. [CrossRef]
- F. Nea, D. Kambiré, M. Genva, E. Tanoh, E. Wognin, H. Martin, Y. Brostaux, F. Tomi, G. C. Lognay, Z. Tonzibo, M.-L. Fauconnier, Molecules 2020, 25, 2400;. [CrossRef]
- S. El Kantar, A. Yassin, B. Nehmeh, L. Labaki, S. Mitri, F. Naser Aldine, A. Hirko, S. Caballero, E. Monck, A. Garcia-Maruniak, E. Akoury. Sci. Rep. 2022, 12, 15489. [CrossRef]
- M. Sharma, P.D. Sharma, M.P. Bansal, J. Singh, Chem. and Biodivers., 2007, 4, 5, 932–939. [CrossRef]
- M. Sharma, P.D. Sharma, M.P. Bansal, J. Nat. Prod., 2008, 71, 7, 1222–1227. [CrossRef]
- V.B. Babar, P.R. Khapale, S. Nagarale, Journal of Pharmacognosy and Phytochemistry, 2019, 8, 5, 2524-2527.
- E. B. Han, B. Y. Chang, Y. S. Jung, S. Y. Kim, Pathol. Oncol. Res., 2015, 21, 325–331. [CrossRef]
- H. Wang, T.O. Khor, L. Shu, Z.-Y. Su, F. Fuentes, J.-H. Lee, A.-N. T. Kong, Anticancer Agents Med Chem. 2012, 12, 10, 1281–1305. [CrossRef]
- D. Ochwang’i, C. Kimwele, J. Oduma, P. Gathumbi, J. Mbaria, S. Kiama, J. Ethnopharmacol. 2014, 151, 3, 1040-1055. [CrossRef]
- M. Greenwell, P.K.S.M. Rahman, Int. J. Pharm. Sci. Res. 2015, 6, 10, 4103–4112. [CrossRef]
- P. Aiello, M. Sharghi, S.M. Mansourkhani, A. P. Ardekan, L. Jouybari, N. Daraei, K. Peiro, S. Mohamadian, M. Rezaei, M. Heidari, I. Peluso, F. Ghorat, A. Bishayee, W. Kooti, Oxid. Med. Cell. Longev. 2019, 2019, 2075614. [CrossRef]
- M. S. O'Reilly, T. Boehm, Y. Shing, N. Fukai, G. Vasios, W. S. Lane, E. Flynn, J.R. Birkhead, B.R. Olsen, J. Folkman, Cell, 1997, 88, 2, 277-85. [CrossRef]
- The American Cancer Society medical and editorial content team: Chemotherapy Side Effects. Retrieved from https://www.cancer.org/cancer/managing-cancer/treatment-types/chemotherapy/chemotherapy-side-effects.html Accessed on 07.10.2024.
- G.M. Cragg, D.J. Newman, J. Ethnopharmacol. 2005, 100, 1-2, 72-9. [CrossRef]
- R. Sivaraj, P. Rahman, P. Rajiv, S. Narendhran, R. Venckatesh, Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 129, 255-258. [CrossRef]
- B. Hassan, Plants and Cancer Treatment, Medicinal Plants - Use in Prevention and Treatment of Diseases. IntechOpen, 2020. [CrossRef]
- Nea, F.; Kambiré, D.A.; Genva, M.; Tanoh, E.A.; Wognin, E.L.; Martin, H.; Brostaux, Y.; Tomi, F.; Lognay, G.C.; Tonzibo, Z.F.; et al. Molecules 2020, 25, 2400. [CrossRef]
- S. Zoubiri, B. Aoumeur, Journal of Essential Oil Research 2012, 24.4, 377-383.
- J. U. Chowdhury, N. C. Nandi, I. Nazrul, M. N.I. Bhuiyan, Bangladesh Journal of Botany, 2007, 36.2, 193-194.
- K. Aisha, N.U. Visakh, B. Pathrose, N. Mori, R.S. Baeshen, R. Shawer, Molecules 2024, 29, 2, 344. [CrossRef]
- L. Gushiken, F. Beserra, M. Hussni, M. Gonzaga, V. Ribeiro, P. Fernanda de Souza, J. Campos, T. Massaro, C. Hussni, R. Takahira, P. Marcato, J. Bastos, C. Pellizzon, Oxidative Medicine and Cellular Longevity, 2022, 2022, 9004014. [CrossRef]
- J.-H. Wang, F. Luan, X.-D. He, Y. Wang, M.-X. Li, Journal of Traditional and Complementary Medicine, 2018, 8, 1, 24-38. [CrossRef]
- A.R. Do, S.A. Emami, M. Akaberi, Phytochem. Rev. 2024. [CrossRef]
- Sundufu, A.J., Shoushan, H., Flavour and Fragrance Journal, 2004, 19, 3, 229-232. [CrossRef]
- E.O. Sousa, A.V. Colares, F.F.G. Rodrigues, A.R. Campos, S.G. Lima, J.G.M. Costa, Records of Natural Products, 2010, 4, 1, 31-37.
- R. Singh, B. Tiwari, U. Sharma, S. Singh, Asian Journal of Chemistry, 2012, 24, 12, 12.
- R. Liambila, J. Wesonga, C. Ngamau, W. Wallyambillah, Afr. J. Agric. Res. 2021, 17, 9, 1198-1208. [CrossRef]
- A.N. Mamadaliev, Kh.Kh. Kushiev, Z.R. Abdullaeva, Austrian Journal of Technical and Natural Sciences, 2022, 5-6, 3. [CrossRef]
- C.A. Semeniuc, M.-I. Socaciu, S.A. Socaci, V. Mureșan, M. Fogarasi, A.M. Rotar, Molecules, 2018, 23, 9, 2261. [CrossRef]
- A.M. Shraim, T.A. Ahmed, M. Rahman, Y.M. Hijji, LWT, 2021, 150, 111932. [CrossRef]
- K. Zhang, Y. Zuo, J. Agric. Food Chem. 2004, 52, 2, 222–227. [CrossRef]
- A.E. Al Snafi, Asian Journal of Pharmaceutical and Clinical Research, 2019, 12, 12, 10-20. [CrossRef]
- J. Kapali, K.R. Sharma, J. Med. Plants 2021, 20, 80, 102-116. [CrossRef]
- M. Li, P.W. Paré, J. Zhang, T. Kang, Z. Zhang, D. Yang, K. Wang, H. Xing, Rec. Nat. Prod. 2018, 12, 239–250. [CrossRef]
- G.S. El Baroty, H.M. Goda, E.A. Khalifa, H. H. Abd El Baky, Der Pharma Chemica, 2014, 6, 6, 246-255.
- A. Sajid, Q. Manzoor, M. Imran, F. Aslam, T.A. Gondal, R.S. Ahmad, et al. Sains Malaysiana, 2021, 50, 10, 2923-2936. [CrossRef]
- A. Mahdi-Pour, S.L. Jothy, L.Y. Latha, Y. Chen, S. Sasidharan, Asian Pacific Journal of Tropical Biomedicine, 2012, 2, 12, 960-965. [CrossRef]
- H. Annaz, K. El Fakhouri, W. Ben Bakrim, I. Mahdi, M. El Bouhssini, M. Sobeh, Critical Reviews in Food Science and Nutrition, 2024, 64, 21, 7343-7362. [CrossRef]
- M. Radice, A. Durofil, R. Buzzi, E. Baldini, A.P. Martínez, L. Scalvenzi, S. Manfredini, Life 2022, 12, 1602. [CrossRef]
- B. Ivanescu, A. Miron, A. Corciova, J. Anal. Methods Chem., 2015, 2015, 247685. [CrossRef]
- Prithviraj Karak, International Journal of Pharmaceutical Sciences and Research, 2019, 10, 4, 1567-1574. [CrossRef]
- F. Guesmi, A.K. Tyagi, S. Prasad, A. Landoulsi, Oncotarget. 2018, 9, 32305-32320. [CrossRef]
- M.M. Montalvão, F.B. Felix, E.W. Propheta dos Santos, et al., BMC Complement. Med. Ther. 2023, 23, 139.
- R.P. Pereira, R. Fachinetto, A. de Souza Prestes, et al., Neurochem. Res. 2009, 34, 973–983. [CrossRef]
- R. San Miguel-Chávez, Phenolic Compounds-Biological Activity, 2017, 8, 54-79.
- A. Jaber, R. Soukariyeh, A. Khalil, F. Abdel-Sater, E. Cheble, Int. J. Pharm. Sci. Rev. Res., 2020, 61, 1, 70-77.
- D. Barreca, G. Laganà, U. Leuzzi, A. Smeriglio, D. Trombetta, E. Bellocco, Food Chemistry, 2016, 196, 493-502. [CrossRef]
- V.L. Singleton, R. Orthofer, R.M. Lamuela-Raventós, Methods in Enzymology, 1999, 299, 152-178. [CrossRef]
- N. Awada, A. Ayoub, A. Jaber, F. Ibrahim, N. El Ghotmi, E. Cheble, Pharm Sci. 2023, 29, 3, 384–394. [CrossRef]
- M. Oyaizu, Jpn. J. Nutr. Diet., 1986, 44, 6, 307-315. [CrossRef]
- Balouiri M, Sadiki M, Ibnsouda SK, Journal of Pharmaceutical Analysis, 2016, 6, 2, 71-79. [CrossRef]
- J. Semaan, S. El-Hakim, J.-N. Ibrahim, R. Safi, A. Elnar, C. El Boustany, Breast Cancer 2020, 27, 696–705. [CrossRef]
- J.-N. Ibrahim, S. El-Hakim, J. Semaan, S. Ghosn, H. El Ayoubi, A.A. Elnar, N. Tohme, C. El Boustany, Biomedicines 2024, 12, 1779. [CrossRef]


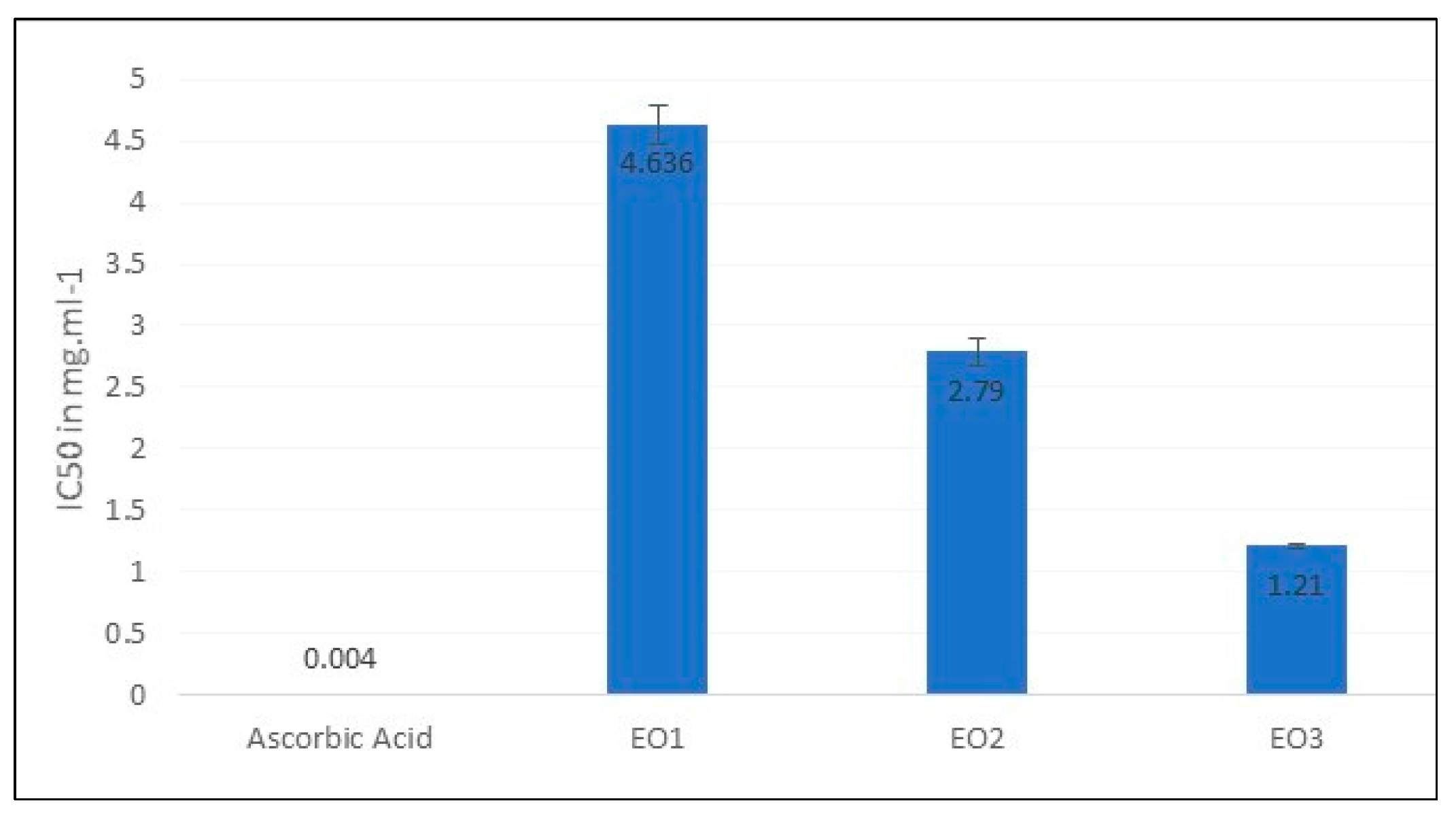
| Sample | TFC (mg of RE/g of extract) |
TPC (mg of GAE/g of extract) |
IC50 (mg/ml) 1 |
|---|---|---|---|
| EO1 | 2.41 | 16.80 | 4.64 |
| EO2 | 2.93 | 24.63 | 2.79 |
| EO3 | 5.12 | 26.71 | 1.21 |
| Cell line | Time (hours) |
IC50 (g.ml-1) | ||
|---|---|---|---|---|
| EO1 | EO2 | EO3 | ||
| MCF-7 (BC) |
24 | 0.3061±0.091 | 0.3630±0.057 | 0.5927±0.03 |
| 48 | 0.3021±0.043 | 0.4101±0.109 | 0.3937±0.09 | |
| 72 | 0.3179±0.002 | 0.4144±0.09 | 0.3397±0.027 | |
| MDA-MB-231 (BC) |
24 | 0.3099±0.001 | 0.7398±0.012 | 0.6473±0.005 |
| 48 | 0.2820±0.022 | 0.4015±0.01 | 0.4150±0.06 | |
| 72 | 0.2800±0.023 | 0.3931±0.058 | 0.3951±0.08 | |
| MCF-10A (normal) |
24 | > 1 | > 1 | > 1 |
| 48 | > 1 | > 1 | > 1 | |
| 72 | > 1 | > 1 | > 1 | |
| TFC | TPC | DPPH | MCF-7 | MDA-MB-231* | |
|---|---|---|---|---|---|
| TFC | 1 | ||||
| TPC | 0.7869 | 1 | |||
| DPPH | -0.9260 | -0.9617 | 1 | ||
| MCF-7 | 0.99997 | 0.7913 | -0.9286 | 1 | |
| MDA-MB-231 | 0.4790 | 0.9186 | -0.7750 | 0.4853 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





