Submitted:
21 October 2024
Posted:
22 October 2024
You are already at the latest version
Abstract
Background Small intestinal neuroendocrine tumors (SI-NETs) are characterized by carcinoid syndrome and carcinoid heart disease (CHD). The aim of study was to identify early risk markers for carcinoid heart disease and survival in a prospective median-term follow-up setting. Methods We measured 5-HIAA, cumulative 5-HIAA exposure (Cum-5-HIAA) based on repeat measurements, proBNP, vascular function, hepatic tumor load and transthoracic echocardiography (TTE) were assessed at baseline and during median 5-year follow-up. Of 65 patients with SI-NETs; 54 patients underwent prospective follow-up. In addition, survival was evaluated during median follow-up of 6 years. Results At baseline three patients had CHD. During median follow-up of 5 years, two patients (4%) developed CHD. Cum-5-HIAA and proBNP correlated with CHD (Westberg Score, Spearman’s ρ = 0.32 and 0.31, respectively). Cum-5-HIAA had a superior diagnostic capability, predicting CHD in receiver operator characteristics analysis with AUC of 0.98 (95% CI: 0.94–1.00) and outperformed proBNP, chromogranin A (CgA), as well as individual serum 5-HIAA measurements (AUC = 0.75, 0.85, and 0.91, respectively). Minor changes in valve regurgitation were frequently detected but did not correlate with vascular function. In 29% of tricuspid and 30% of pulmonic valves regurgitation increased or decreased. CHD, hepatic tumor load, serum 5-HIAA, and elevated aortic pulse wave velocity (PWV) associated with increased mortality in SI-NET patients. Conclusions Cum-5-HIAA is a promising biomarker for CHD risk and outperformed other biomarkers. CHD and hepatic tumor load are strongest predictors of mortality. PWV is a novel predictor of survival. The incidence of CHD was small among the SI-NET patients, probably reflecting successful treatment regimens.
Keywords:
1. Introduction
2. Patients and Methods
2.1. Patients and Data
2.2. Echocardiography
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Echocardiographic Features Observed During the Follow-Up
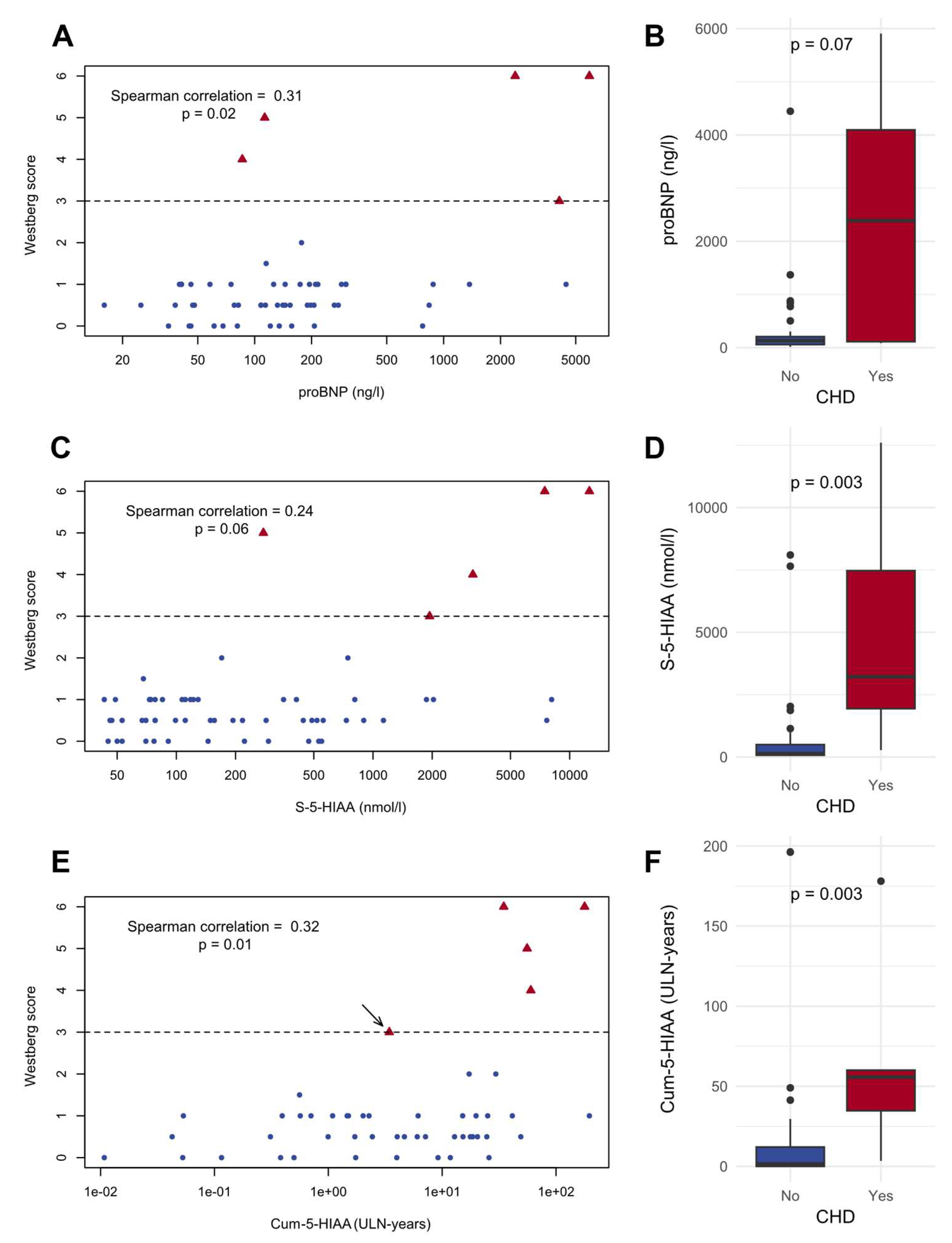
3.3. Correlations between Biomarkers and Westberg Score
3.4. Assessment of Patients Who Did Not Have Follow-Up TTE
3.5. Biomarker Performance of the Assessed Variables for the Detection of CHD
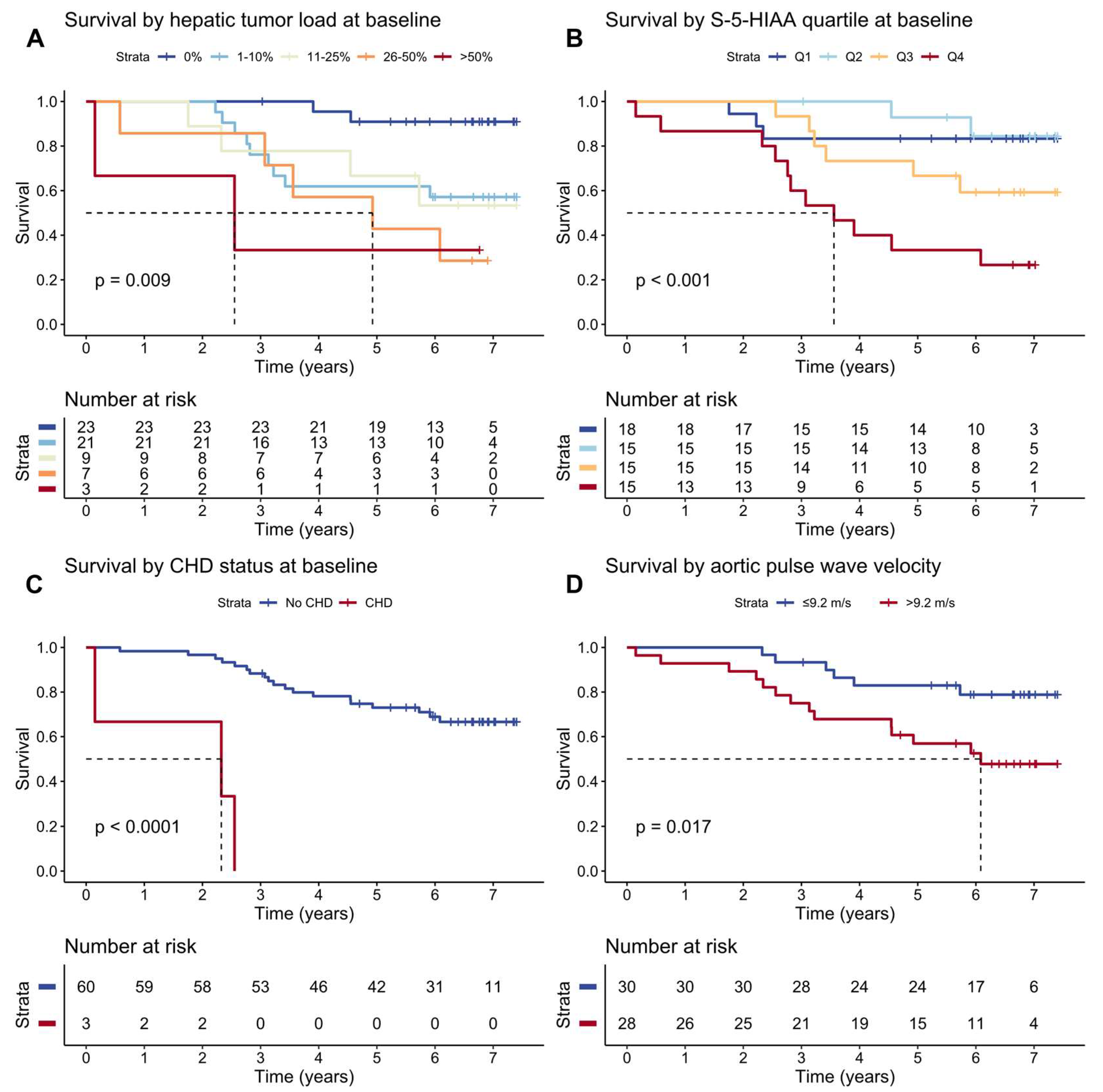
3.6. Survival and Predictors of Mortality in SI-NET Patients
4. Discussion
Author Contributions
Funding
Data Availability Statement
Statement of Ethics
Conflicts of Interest Statement
References
- Niederle, B.; Pape, U.-F.; Costa, F.; Gross, D.; Kelestimur, F.; Knigge, U.; Öberg, K.; Pavel, M.; Perren, A.; Toumpanakis, C.; et al. ENETS Consensus Guidelines Update for Neuroendocrine Neoplasms of the Jejunum and Ileum. Neuroendocrinology 2016, 103, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Grozinsky-Glasberg, S.; Davar, J.; Hofland, J.; Dobson, R.; Prasad, V.; Pascher, A.; Denecke, T.; Tesselaar, M.E.T.; Panzuto, F.; Albåge, A.; et al. European Neuroendocrine Tumor Society (ENETS) 2022 Guidance Paper for Carcinoid Syndrome and Carcinoid Heart Disease. J Neuroendocrinol 2022, 34, e13146. [Google Scholar] [CrossRef] [PubMed]
- Mulders, M.C.F.; de Herder, W.W.; Hofland, J. What Is the Carcinoid Syndrome? A Critical Appraisal of Its Proposed Mediators. Endocr Rev 2023, bnad035. [Google Scholar] [CrossRef]
- Buchanan-Hughes, A.; Pashley, A.; Feuilly, M.; Marteau, F.; Pritchard, D.M.; Singh, S. Carcinoid Heart Disease: Prognostic Value of 5-Hydroxyindoleacetic Acid Levels and Impact on Survival: A Systematic Literature Review. Neuroendocrinology 2021, 111, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fijalkowski, R.; Reher, D.; Rinke, A.; Gress, T.M.; Schrader, J.; Baum, R.P.; Kaemmerer, D.; Hörsch, D. Clinical Features and Prognosis of Patients with Carcinoid Syndrome and Carcinoid Heart Disease: A Retrospective Multicentric Study of 276 Patients. Neuroendocrinology 2022, 112, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.; Kilgallen, A.B.; Korse, C.M.; Oerlemans, M.I.F.J.; Sluijter, J.P.G.; van Laake, L.W.; Valk, G.D.; Tesselaar, M.E.T. Elevated Serotonin and NT-proBNP Levels Predict and Detect Carcinoid Heart Disease in a Large Validation Study. Cancers (Basel) 2022, 14, 2361. [Google Scholar] [CrossRef]
- Blažević, A.; Hofland, J.; Hofland, L.J.; Feelders, R.A.; de Herder, W.W. Small Intestinal Neuroendocrine Tumours and Fibrosis: An Entangled Conundrum. Endocr Relat Cancer 2018, 25, R115–R130. [Google Scholar] [CrossRef]
- Gustafsson, B.I.; Tømmerås, K.; Nordrum, I.; Loennechen, J.P.; Brunsvik, A.; Solligård, E.; Fossmark, R.; Bakke, I.; Syversen, U.; Waldum, H. Long-Term Serotonin Administration Induces Heart Valve Disease in Rats. Circulation 2005, 111, 1517–1522. [Google Scholar] [CrossRef]
- Mekontso-Dessap, A.; Brouri, F.; Pascal, O.; Lechat, P.; Hanoun, N.; Lanfumey, L.; Seif, I.; Benhaiem-Sigaux, N.; Kirsch, M.; Hamon, M.; et al. Deficiency of the 5-Hydroxytryptamine Transporter Gene Leads to Cardiac Fibrosis and Valvulopathy in Mice. Circulation 2006, 113, 81–89. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Schapira, A.H.; Mikhailidis, D.P.; Davar, J. Drug-Induced Fibrotic Valvular Heart Disease. Lancet 2009, 374, 577–585. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Jagroop, A.; Gujral, D.M.; Hayward, C.; Toumpanakis, C.; Caplin, M.; Mikhailidis, D.P.; Davar, J. Circulating Plasma and Platelet 5-Hydroxytryptamine in Carcinoid Heart Disease: A Pilot Study. J Heart Valve Dis 2013, 22, 400–407. [Google Scholar] [PubMed]
- Dobson, R.; Burgess, M.I.; Banks, M.; Pritchard, D.M.; Vora, J.; Valle, J.W.; Wong, C.; Chadwick, C.; George, K.; Keevil, B.; et al. The Association of a Panel of Biomarkers with the Presence and Severity of Carcinoid Heart Disease: A Cross-Sectional Study. PLoS One 2013, 8, e73679. [Google Scholar] [CrossRef] [PubMed]
- Zuetenhorst, J.M.; Bonfrer, J.M.G.M.; Korse, C.M.; Bakker, R.; van Tinteren, H.; Taal, B.G. Carcinoid Heart Disease: The Role of Urinary 5-Hydroxyindoleacetic Acid Excretion and Plasma Levels of Atrial Natriuretic Peptide, Transforming Growth Factor-Beta and Fibroblast Growth Factor. Cancer 2003, 97, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Møller, J.E.; Connolly, H.M.; Rubin, J.; Seward, J.B.; Modesto, K.; Pellikka, P.A. Factors Associated with Progression of Carcinoid Heart Disease. N Engl J Med 2003, 348, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Cuthbertson, D.J.; Jones, J.; Valle, J.W.; Keevil, B.; Chadwick, C.; Poston, G.P.; Burgess, M.I. Determination of the Optimal Echocardiographic Scoring System to Quantify Carcinoid Heart Disease. Neuroendocrinology 2014, 99, 85–93. [Google Scholar] [CrossRef]
- Hofland, J.; Lamarca, A.; Steeds, R.; Toumpanakis, C.; Srirajaskanthan, R.; Riechelmann, R.; Panzuto, F.; Frilling, A.; Denecke, T.; Christ, E.; et al. Synoptic Reporting of Echocardiography in Carcinoid Heart Disease (ENETS Carcinoid Heart Disease Task Force). J Neuroendocrinol 2022, 34, e13060. [Google Scholar] [CrossRef]
- Johnson, K.K.N.; Stemann Lau, T.; Mark Dahl Baunwall, S.; Elisabeth Villadsen, G.; Guldbrand Rasmussen, V.; Grønbaek, H.; Oksjoki, R.K.; Dam, G. The Role of N-Terminal pro-Brain Natriuretic Peptide, Chromogranin A, and 5-Hydroxyindoleacetic Acid in Screening for Carcinoid Heart Disease. J Neuroendocrinol 2023, 35, e13327. [Google Scholar] [CrossRef]
- Kostiainen, I.; Karppinen, N.; Simonen, P.; Rosengård-Bärlund, M.; Lindén, R.; Tarkkanen, M.; Gordin, D.; Rapola, J.; Schalin-Jäntti, C.; Matikainen, N. Arterial Function, Biomarkers, Carcinoid Syndrome and Carcinoid Heart Disease in Patients with Small Intestinal Neuroendocrine Tumours. Endocrine 2022, 77, 177–187. [Google Scholar] [CrossRef]
- Becker, A.; Schalin-Jäntti, C.; Itkonen, O. Comparison of Serum and Urinary 5-Hydroxyindoleacetic Acid as Biomarker for Neuroendocrine Neoplasms. J Endocr Soc 2021, 5, bvab106. [Google Scholar] [CrossRef]
- Westberg, G.; Wängberg, B.; Ahlman, H.; Bergh, C.H.; Beckman-Suurküla, M.; Caidahl, K. Prediction of Prognosis by Echocardiography in Patients with Midgut Carcinoid Syndrome. Br J Surg 2001, 88, 865–872. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J Thorac Oncol 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Toumpanakis, C.; Chilkunda, D.; Caplin, M.E.; Davar, J. Risk Factors for the Development and Progression of Carcinoid Heart Disease. Am J Cardiol 2011, 107, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Uema, D.; Alves, C.; Mesquita, M.; Nuñez, J.E.; Siepmann, T.; Angel, M.; Rego, J.F.M.; Weschenfelder, R.; Rocha Filho, D.R.; Costa, F.P.; et al. Carcinoid Heart Disease and Decreased Overall Survival among Patients with Neuroendocrine Tumors: A Retrospective Multicenter Latin American Cohort Study. J Clin Med 2019, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Mattig, I.; Franke, M.R.; Pschowski, R.; Brand, A.; Stangl, K.; Knebel, F.; Dreger, H. Prevalence, One-Year-Incidence and Predictors of Carcinoid Heart Disease. Cardiovasc Ultrasound 2023, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Snorradottir, S.; Asgeirsdottir, A.; Rögnvaldsson, S.; Jonasson, J.G.; Björnsson, E.S. Incidence and Prognosis of Patients with Small Intestinal Neuroendocrine Tumors in a Population Based Nationwide Study. Cancer Epidemiol 2022, 79, 102197. [Google Scholar] [CrossRef]
- Stensbøl, A.B.; Krogh, J.; Holmager, P.; Klose, M.; Oturai, P.; Kjaer, A.; Hansen, C.P.; Federspiel, B.; Langer, S.W.; Knigge, U.; et al. Incidence, Clinical Presentation and Trends in Indication for Diagnostic Work-Up of Small Intestinal and Pancreatic Neuroendocrine Tumors. Diagnostics (Basel) 2021, 11, 2030. [Google Scholar] [CrossRef]
- Pellikka, P.A.; Tajik, A.J.; Khandheria, B.K.; Seward, J.B.; Callahan, J.A.; Pitot, H.C.; Kvols, L.K. Carcinoid Heart Disease. Clinical and Echocardiographic Spectrum in 74 Patients. Circulation 1993, 87, 1188–1196. [Google Scholar] [CrossRef]
- de Mestier, L.; Savagner, F.; Brixi, H.; Do Cao, C.; Dominguez-Tinajero, S.; Roquin, G.; Goichot, B.; Hentic, O.; Dubreuil, O.; Hautefeuille, V.; et al. Plasmatic and Urinary 5-Hydroxyindolacetic Acid Measurements in Patients With Midgut Neuroendocrine Tumors: A GTE Study. J Clin Endocrinol Metab 2021, 106, e1673–e1682. [Google Scholar] [CrossRef]
- Krenning, E.P.; Kooij, P.P.; Bakker, W.H.; Breeman, W.A.; Postema, P.T.; Kwekkeboom, D.J.; Oei, H.Y.; de Jong, M.; Visser, T.J.; Reijs, A.E. Radiotherapy with a Radiolabeled Somatostatin Analogue, [111In-DTPA-D-Phe1]-Octreotide. A Case History. Ann N Y Acad Sci 1994, 733, 496–506. [Google Scholar] [CrossRef]
- Hofland, J.; Brabander, T.; Verburg, F.A.; Feelders, R.A.; de Herder, W.W. Peptide Receptor Radionuclide Therapy. J Clin Endocrinol Metab 2022, 107, 3199–3208. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Zandee, W.T.; Brabander, T.; Blažević, A.; Minczeles, N.S.; Feelders, R.A.; de Herder, W.W.; Hofland, J. Peptide Receptor Radionuclide Therapy With 177Lu-DOTATATE for Symptomatic Control of Refractory Carcinoid Syndrome. J Clin Endocrinol Metab 2021, 106, e3665–e3672. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xing, A.; Hidru, T.H.; Li, J.; Yang, X.; Chen, S.; Xia, Y.-L.; Wu, S. The Association between Arterial Stiffness and Cancer Occurrence: Data from Kailuan Cohort Study. Front Cardiovasc Med 2023, 10, 1112047. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, S.S.; Kim, I.J.; Kim, J.H.; Kim, B.H.; Kim, M.K.; Lee, S.H.; Lee, C.W.; Kim, M.C.; Ahn, J.H.; et al. Arterial Stiffness Is an Independent Predictor for Risk of Mortality in Patients with Type 2 Diabetes Mellitus: The REBOUND Study. Cardiovasc Diabetol 2020, 19, 143. [Google Scholar] [CrossRef] [PubMed]


| Variable | All patients (n = 65), at baseline | Patients who had follow-up TTE (n = 54), at baseline | Patients who had follow-up TTE (n = 54), at follow-up TTE | Patients who did not have follow-up TTE (n = 11), at baseline | CHD patients (n = 5), at CHD diagnosis |
|---|---|---|---|---|---|
| Age (years) | 66 (59–72) | 64 (58–70) | 70 (61–74) | 69 (66–75) | 62 (57–67) |
| Sex, female:male (n) | 33:32 (51%:49%) | 27:27 (50%:50%) | 27:27 (50%/50%) | 6:5 (55%:45%) | 1:4 (20%:80%) |
| Time from the initial SI-NET diagnosis at assessment (months) | 72 (32–108) | 70 (31–107) | 130 (79–169) | 87 (38–132) | 32 (32–78) |
| Primary tumor Ki-67 (%) | 2 (1–5) | 2 (1–5) | 2 (1–5) | 2 (2–7) | 2 (1–3) |
| Hepatic tumor burden (n) | |||||
| 0% | 23 (35%) | 20 (37%) | 13 (24%) | 3 (27%) | 0 (0%) |
| 1–10% | 23 (35%) | 18 (33%) | 24 (44%) | 5 (45%) | 0 (0%) |
| 10–25% | 9 (14%) | 8 (15%) | 9 (17%) | 1 (9%) | 2 (40%) |
| 26–50% | 7 (11%) | 6 (11%) | 6 (11%) | 1 (9%) | 1 (20%) |
| >50% | 3 (5%) | 2 (4%) | 2 (4%) | 1 (9%) | 2 40%) |
| Serum 5-HIAA (nmol/L) | 138 (78–424) | 135 (78–372) | 147 (74–533) | 286 (78–525) | 3220 (1940–7470) |
| Cum-5-HIAA (ULN-years) | 0.8 (0.0–4.8) | 0.7 (0.0–4.3) | 1.9 (0.0–15) | 1.0 (0.1–11) | 57 (35–60) |
| Plasma proBNP (ng/L) | 81 (35–194) | 73 (35–176) | 135 (65–237) | 128 (56–214) | 1283 (113–2391) |
| CgA (proportion of ULN) | 1.7 (0.9–5.5) | 1.6 (0.9–5.1) | 1.1 (0.6–8.9) | 2.3 (1.0–9.0) | 83 (32–133) |
| Treatment (n) | |||||
| Resection of the primary tumor | 57 (87%) | 47 (87%) | 49 (91%) | 10 (91%) | 2 (40%) |
| Resection of recurrence | 3 (5%) | 2 (4%) | 2 (4%) | 1 (9%) | 0 (0%) |
| Non-systemic treatment for metastases1 | 23 (35%) | 20 (37%) | 25 (46%) | 3 (27%) | 2 (40%) |
| Somatostatin analog | 56 (86%) | 47 (87%) | 49 (91%) | 9 (82%) | 5 (100%) |
| PRRT2 | 18 (28%) | 15 (28%) | 30 (56%) | 3 (27%) | 3 (60%) |
| PRRT, retreatment2 | 3 (5%) | 2 (4%) | 14 (26%) | 1 (9%) | 0 (0%) |
| PRRT, second retreatment2 | 0 (0%) | 0 (0%) | 6 (11%) | 0 (0%) | 0 (0%) |
| Telotristat ethyl | 0 (0%) | 0 (0%) | 3 (6%) | 0 (0%) | 0 (0%) |
| Interferon alfa-2b | 12 (18%) | 9 (17%) | 10 (19%) | 3 (27%) | 0 (0%) |
| Chemotherapy3 | 3 (5%) | 2 (4%) | 11 (20%) | 1 (9%) | 0 (0%) |
| Variable | Baseline TTE, all patients (n = 63) | Baseline TTE for patients with follow-up TTE (n = 54) | Follow-up TTE (n = 54) | |||
|---|---|---|---|---|---|---|
| Result | Data available | Result | Data available | Result | Data available | |
| Tricuspid valve thickening (n) | 62/63 (98%) | 51/54 (94%) | 48/54 (89%) | |||
| None | 58 (94%) | 49 (96%) | 46 (96%) | |||
| Mild | 1 (2%) | 0 (0%) | 0 (0%) | |||
| Moderate | 3 (5%) | 2 (4%) | 1 (2%) | |||
| Severe | 0 (0%) | 0 (0%) | 1 (2%) | |||
| Tricuspid valve mobility (n) | 62/63 (98%) | 51/54 (94%) | 48/54 (89%) | |||
| Increased | 0 (0%) | 0 (0%) | 1 (2%) | |||
| Normal | 58 (94%) | 48 (94%) | 45 (94%) | |||
| Mildly reduced | 2 (3%) | 2 (4%) | 0 (0%) | |||
| Moderately reduced | 1 (2%) | 1 (2%) | 1 (2%) | |||
| Severely reduced | 1 (2%) | 0 (0%) | 1 (2%) | |||
| Tricuspid valve regurgitation (n) | 61/63 (97%) | 50/54 (93%) | 50/54 (93%) | |||
| None | 9 (15%) | 7 (14%) | 10 (20%) | |||
| Trace | 23 (38%) | 16 (32%) | 17 (34%) | |||
| Mild | 24 (39%) | 23 (46%) | 17 (34%) | |||
| Moderate | 3 (5%) | 3 (6%) | 2 (4%) | |||
| Severe | 2 (3%) | 1 (2%) | 4 (8%) | |||
| Pulmonic valve thickening (n) | 60/63 (95%) | 51/54 (94%) | 47/54 (87%) | |||
| None | 55 (92%) | 47 (92%) | 45 (96%) | |||
| Mild | 3 (5%) | 3 (6%) | 2 (4%) | |||
| Moderate | 2 (3%) | 1 (2%) | 0 (0%) | |||
| Severe | 0 (0%) | 0 (0%) | 0 (0%) | |||
| Pulmonic valve mobility (n) | 59/63 (94%) | 50/54 (93%) | 47/54 (87%) | |||
| Increased | 0 (0%) | 0 (0%) | 0 (0%) | |||
| Normal | 56 (95%) | 48 (96%) | 46 (98%) | |||
| Mildly reduced | 2 (3%) | 1 (2%) | 0 (0%) | |||
| Moderately reduced | 0 (0%) | 0 (0%) | 1 (2%) | |||
| Severely reduced | 1 (2%) | 1 (2%) | 0 (0%) | |||
| Pulmonic valve stenosis (n) | 59/63 (94%) | 51/54 (94%) | 47/54 (87%) | |||
| None | 57 (97%) | 49 (96%) | 46 (98%) | |||
| Mild | 1 (2%) | 1 (2%) | 1 (2%) | |||
| Moderate | 1 (2%) | 1 (2%) | 0 (0%) | |||
| Severe | 0 (0%) | 0 (0%) | 0 (0%) | |||
| Pulmonic valve regurgitation (n) | 61/63 (97%) | 51/54 (94%) | 48/54 (89%) | |||
| None | 39 (64%) | 33 (65%) | 27 (56%) | |||
| Trace | 6 (10%) | 5 (10%) | 9 (19%) | |||
| Mild | 13 (21%) | 11 (22%) | 11 (23%) | |||
| Moderate | 1 (2%) | 1 (2%) | 0 (0%) | |||
| Severe | 2 (3%) | 1 (2%) | 1 (2%) | |||
| Right ventricle area, systolic (cm2)1 | 12 (8–16) | 56/63 (89%) | 12 (9–16) | 47/54 (87%) | 11 (8–17) | 47/54 (87%) |
| Right ventricle basal dimension, diastolic (mm)1 | 34 (31–40) | 59/63 (94%) | 34 (31–40) | 49/54 (91%) | 35 (30–39) | 44/54 (81%) |
| Right ventricle mid-cavity dimension, diastolic (mm)1 | 31 (27–36) | 58/63 (92%) | 31 (28–35) | 49/54 (91%) | 32 (29–36) | 47/54 (87%) |
| Right ventricle longitudinal dimension, diastolic (mm)1 | 63 (59–67) | 58/63 (92%) | 63 (60–67) | 49/54 (91%) | 65 (60–69) | 49/54 (91%) |
| Right atrium area, systolic (cm2)1 | 13 (15–19) | 58/63 (92%) | 15 (13–18) | 48/54 (89%) | 15 (14–19) | 44/54 (81%) |
| Tricuspid annular plane systolic excursion, TAPSE (mm) | 20 (20–25) | 57/63 (90%) | 22 (21–25) | 47/54 (87%) | 22 (20–24) | 50/54 (93%) |
| Westberg score | 1 (0.5–1) [0-6] | 61/63 (97%) | 1 (0.5–1) [0-4] | 50/54 (93%) | 0.5 (0.5–1) [0-6] | 47/54 (87%) |
| Variable | Alive (n = 42) | Deceased (n = 22) | P-value |
|---|---|---|---|
| Age (years) | 65 (60–70) | 66 (59–74) | 0.31 |
| Sex, female/male (n) | 24/18 (57%/43%) | 8/14 (36%/64%) | 0.19 |
| Time from the initial SI-NET diagnosis at assessment (months) | 75 (47–109) | 77 (32–108) | 0.85 |
| Primary tumor Ki-67 (%) | 2 (1–5) | 2 (2–5) | 0.24 |
| Hepatic tumor burden (n) | 0.006 | ||
| 0% | 20 (47%) | 2 (9%) | |
| 1–10% | 14 (33%) | 9 (41%) | |
| 10–25% | 5 (12%) | 4 (18%) | |
| 26–50% | 2 (5%) | 5 (23%) | |
| >50% | 1 (2%) | 2 (9%) | |
| Serum 5-HIAA (nmol/L) | 95 (70–183) | 433 (174–746) | <0.001 |
| Cum-5-HIAA (ULN-years) | 0.3 (0.0–1.2) | 6.3 (0.7–14) | <0.001 |
| Plasma proBNP (ng/L) | 55 (35–176) | 109 (57–214) | 0.10 |
| CgA (proportion of ULN) | 1 (1–3) | 6 (2–14) | <0.001 |
| Treatment (n) | |||
| Resection of the primary tumor | 39 (93%) | 17 (77%) | 0.11 |
| Resection of recurrence | 2 (5%) | 1 (5%) | 1.0 |
| Non-systemic treatment for metastases1 | 17 (40%) | 6 (27%) | 0.41 |
| Somatostatin analog | 35 (83%) | 21 (95%) | 0.25 |
| PRRT2 | 10 (24%) | 8 (36%) | 0.38 |
| PRRT, retreatment2 | 1 (2%) | 2 (9%) | 0.27 |
| PRRT, second retreatment2 | 0 (0%) | 0 (0%) | n/a |
| Telotristat ethyl | 0 (0%) | 0 (0%) | n/a |
| Interferon alfa-2b | 4 (10%) | 8 (36%) | 0.04 |
| Chemotherapy3 | 1 (2%) | 2 (9%) | 0.27 |
| Variable | Univariate Hazard Ratio (95% CI) | P-value | Multivariate Hazard ratio (95% CI) | P-value |
|---|---|---|---|---|
| Sex | ||||
| Female | 1 | 1 | ||
| Male | 1.88 (0.79-4.47) | 0.16 | 1.29 (0.46-3.61) | 0.62 |
| Age at TTE | 1.03 (0.97-1.09) | 0.20 | 1.05 (0.98-1.13) | 0.20 |
| Serum 5-HIAA (nmol/L) | 1.00 (1.00-1.00) | 0.01 | 1.00 (1.00-1.00) | 0.60 |
| Aortic pulse wave velocity (m/s) | 1.23 (1.09-1.40) | 0.001 | 1.22 (1.04-1.43) | 0.01 |
| Metastases at baseline | ||||
| No | 1 | 1 | ||
| Yes | 7.02 (1.68-30.8) | 0.008 | 5.28 (1.09-25.6) | 0.04 |
| CHD at baseline | ||||
| No | 1 | 1 | ||
| Yes | 24.8 (5.43-113.4) | <0.001 | 36.1 (5.36-243) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





