1. Introduction
Over the past decades, starting with the first trials in 1951 in France by Charles Eyries and Andre Djourno and the single-channel implants of Dr. William House in 1965 in the United States [
1,
2], the indication of cochlear implantation - being the most successful active prosthesis stimulating a cranial nerve - has been changed over and over again. In the early days, only completely deaf patients were considered for a cochlear implant (= CI) on one ear [
3]. Today, more and more patients with better hearing were considered for CI due to the great success of CI [
4]. The hearing loss indicating CI moved from complete deafness in both ears to severe hearing loss (based on a national consensus) in the ear that could possibly be implanted [
5]. Nowadays patient groups that have high residual hearing in the low frequency spectrum are possible CI candidates. This is enabled by soft intracochlear electrode arrays, different types of these electrode arrays (especially straight vs pre-curved and forms for malformed cochleae), different lengths of these electrode arrays to cover the wide range of cochlear sizes across patients’ individual anatomy, new surgical and robotic techniques and also live monitoring of the residual hearing of the lower frequencies during the insertion of the electrode array in the cochlear duct [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19]. This special group with residual hearing in the lower frequencies can receive so-called electro-acoustic-stimulation processors stimulating the lower frequencies with an integrated hearing aid and the mid to high frequencies electrically using the electrode array [
20]. Also, patients with very little word understanding can be considered for CI though their hearing threshold alone would indicate a hearing aid instead.
But there is not only a trend of implanting patients with a CI who have much better hearing than CI users had some decades ago. In contrast to this, also patients with insecure CI candidacy get implanted with CI more often [
21,
22]. In these patients, where the success of hearing and speech understanding after cochlear implantation is questionable, additional measurements can be performed to check the chances of these candidates and avoid a frustrating future for patients and clinicians [
23,
24].
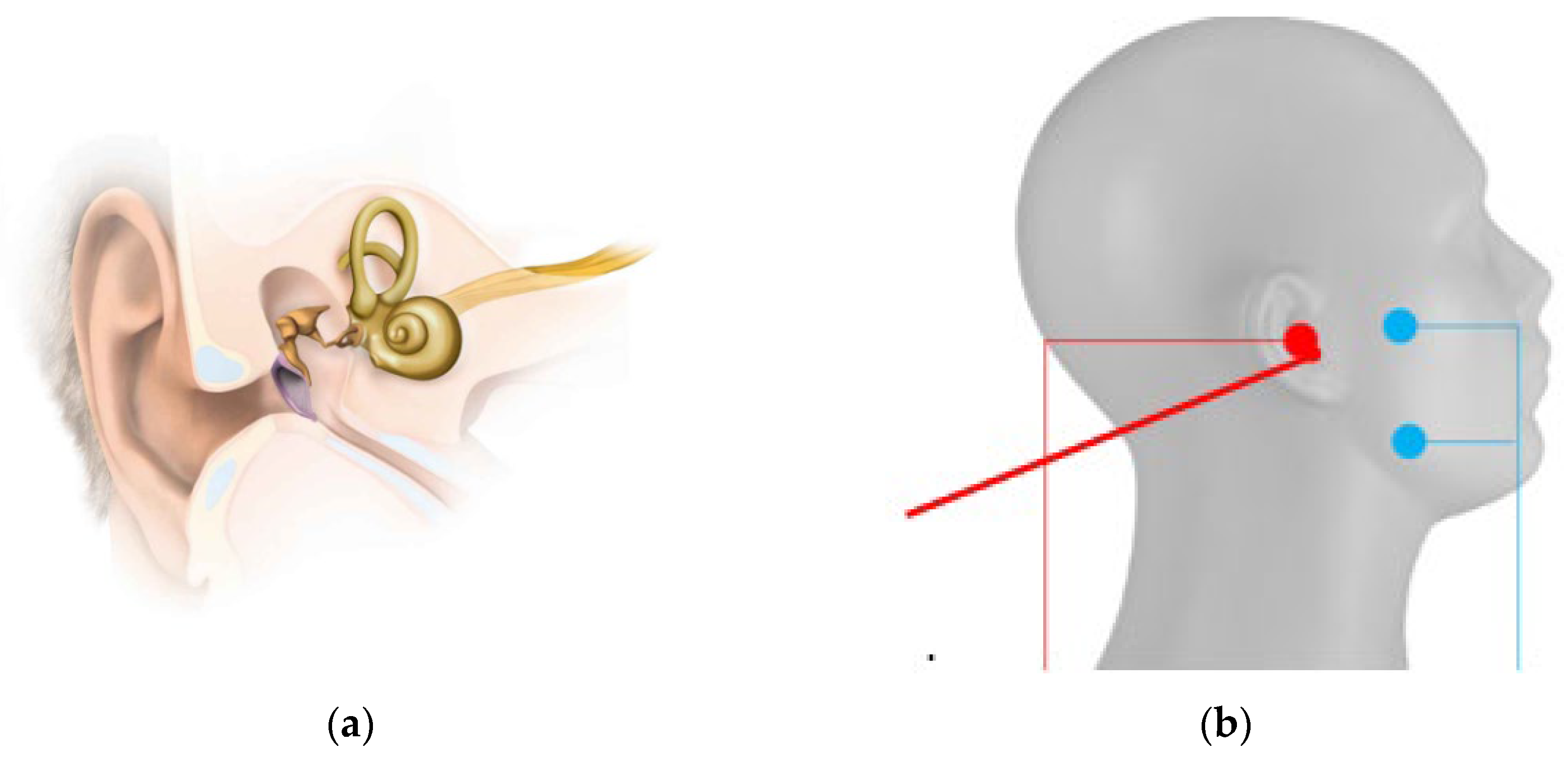
During the pre-investigations for cochlear implantation, numerous audiological tests are conducted to determine the suitability of the respective patient for a CI. Among these tests, the auditory brainstem response to acoustic stimuli is used, mainly through auditory brainstem response (= ABR) recording. In some patients, even with maximum acoustic stimulation, no response can be detected. An alternative is the promontory test, where the patient is electrically stimulated near the auditory nerve and has to associate it with a sensation. Ideally, the electrode is placed in the middle ear for electrical stimulation after the tympanic membrane is opened. In this trans-tympanic setup, electrically evoked ABR (=EABR), electrically evoked auditory mid-late responses (= EAMLR), and electrically evoked auditory late response (= EALR) can be recorded when using local anesthesia similar to results from intra-cochlear test electrodes like the auditory nerve test electrode “ANTS” or the gold standard cochlear implant stimulation [
25,
26,
27,
28,
29,
30,
31,
32]. Using general anesthesia offers EABR [
33] and EAMLR [
34,
35,
36] while EALR can only be recorded in awake patients. Trans-tympanic EABR, EALMR, and EALR (TT-EABR resp. TT-EAMLR resp. TT-EALR) are useful tools to examine the existence of the auditory nerve and thus the excitability of the auditory pathway [
25,
33,
34,
35,
37,
38,
39,
40,
41,
42,
43]. Compared to a trans-tympanic promontory test, only a small additional effort is required when performing a tympanic promontory test. However, both options allow the activation of the auditory cortex as shown in fMRI research [
44]. Previous results have shown that deriving brainstem potentials and cortical potentials is possible with trans-tympanic electrical stimulation. It allows for the objective integrity assessment of the auditory nerve. Insecure sensations of the patients can potentially be objectified in this way. Now, an alternative measurement is to be carried out with the help of a tympanic PromCERA, the so-called “TympEALR” also known as the “PromCERA light” [
20]. Choosing EALR, the auditory pathway can be analyzed up to the cortex objectively [compare 46] with less interfering stimulation artifacts, myogenic artifacts, or facial nerve responses compared to EABR recording [
47].
2. Materials and Methods
For TympEALR, we used established systems for stimulation and measurement of evoked potentials. For the stimulation, we used the electro-stimulator “inomed Neurostimulator ISIS”. We recorded response signals by the EP system “Nihon Kohden Neuropack S1 MEB-9400”. A BNC-Trigger cable (TTL 5V signal) is connected between the electro-stimulator and the EP system to enable the synchronization of stimulation impulses and timing of response recording. The stimulation counter electrodes (“ambu neuroline 720-00s”) were positioned on the mandibular and zygomatic arch short-circuited with each other as known from trans-tympanic EABR, EAMLR, and EALR in local anesthesia [
25,
37,
48]. One out of twelve medical doctors put the active stimulation electrode (“Sanibel TM electrode” or” inomed disposable Tympanon electrode 530 453”) on the tympanic membrane. To ease the insertion of the tympanic electrode, it was surrounded by electrode gel manually (Parker signa gel). The stimulation setup for TympEALR is shown in
Figure 1.
Initially, as a pre-test, an electrostimulation without a recording of evoked potentials was performed as known from a conventional tympanic promontory test. As standard stimulation parameters for the subjective pre-test, we selected 50Hz stimulation frequency using a biphasic pulse of 10.5ms pulse width per phase (i.e., 21ms full pulse width). Before the stimulation, a stimulation impedance test integrated into the electro-stimulator was performed to ensure proper contact with the tissue. By this, we collect data about the subjective sensation(s) given by the electrical stimulation (no sensation, hearing, pain, uncomfortable feeling, other, or unknown sensation) and the area of sensation (ear, face, throat/neck, or other). In addition, individual stimulation levels for the threshold (SL), the maximum comfortable level (MCL), and the uncomfortable level (UCL) were acquired in the health records.
For TympEALR, we expected a similar EALR as known from trans-tympanic or cochlear implant stimulation [
46,
48,
49]. Therefore, the stimulation frequency was reduced to 0.9Hz to enable the recording of the desired time window after stimulation in the EEG. To be able to record responses we chose alternating polarity. Alternating polarity is known to reduce artifacts, especially the stimulation artifact in EPs when using electrical stimulators [
50]. Because of software restrictions, the electro-stimulator “inomed Neurostimulator ISIS” was not able to use alternating polarity. Therefore, we recorded alternating polarity by stimulation with a positive initial phase first and with a negative initial phase second. The mean of these two curves gave us one manually created alternating polarity waveform. To record during tympanic electrical stimulation, we used the clinical recording setting for EALR in our experimental attempt (see
Table 1).
To differentiate between clear response (= positive TympEALR), unclear response, and absent response (= negative TympEALR) the mean of 2 to 3 manually averaged waveforms was visually analyzed for ipsilateral and contralateral recording. As there is not yet enough data about response amplitude in preoperative EALR, we chose postoperative EALR as a reference. Postoperative EALR in adult cochlear implant patients as well as basal ALR in normal hearing adults show N1-P2 response amplitudes at 3µV or more [
51,
52]. Therefore, a lower limit of 3µV was set for response amplitudes in TympEALR. The response latencies in preoperative EALR are N1, P2, P3 (not always clearly separate from P2), and N2 is located at 100ms for N1 and 200-300ms for N2 with P2 and sometimes P3 around 150-200ms [
48]. We chose to use these values as a reference for the detection of TympEALR with an acceptance window of ±50ms for each latency marker.
If ipsilateral and contralateral mean waveforms were matching in morphology similar to other EALR and the amplitude of N1-P2/P3 was ≥3µV we defined the result as a positive TympEALR. If the morphology was matching but the amplitude was <3µV we defined the result as an insecure TympEALR. All other cases were defined as negative TympEALR.
3. Results
From 2018 to 2022, we performed TympEALR recording parallel to standard tympanic promontory nerve test in 16 cochlear implant candidates. In one of these patients, we performed TympEALR on both sides during one session. The other patients were tested solely unilaterally. Seven right ears and ten left ears were tested. Seven patients were male and nine patients were female. When we performed TympEALR the mean age was 46.94±17.48 years with a range from 16 years to 77 years of age.
In eight patients, the etiology of their deafness was unknown. The bilaterally tested patient was deafened by toxic antibiotic treatment. Two patients were deafened by a vestibular schwannoma and one by vestibular schwannoma resection. Two patients were deafened by meningitis. One patient was deafened after an infection with herpes zoster. One patient was deafened by a traumatic brain trauma.
The mean duration of hearing loss was 13.54 ± 17.66 years with a minimum of 0.25 years and a maximum of 63 years. The mean duration of deafness was 9.84 ± 12.66 years with a minimum of 0.25 years and a maximum of 47 years.
All patients reported some kind of subjective sensation during the pre-test or the recording of TympEALR. An overview of the patients’ sensations is shown in
Table 2. Mostly an unknown sensation in the ear was reported (N = 12). If a patient reported “other sensation”, we additionally noted his or her individual description. In these seven cases, we found four times some kind of pressure feeling, once a vibration and once a temperature change. All these “other sensations” were located in the ear. The one patient tested bilaterally reported an unknown sensation in both ears.
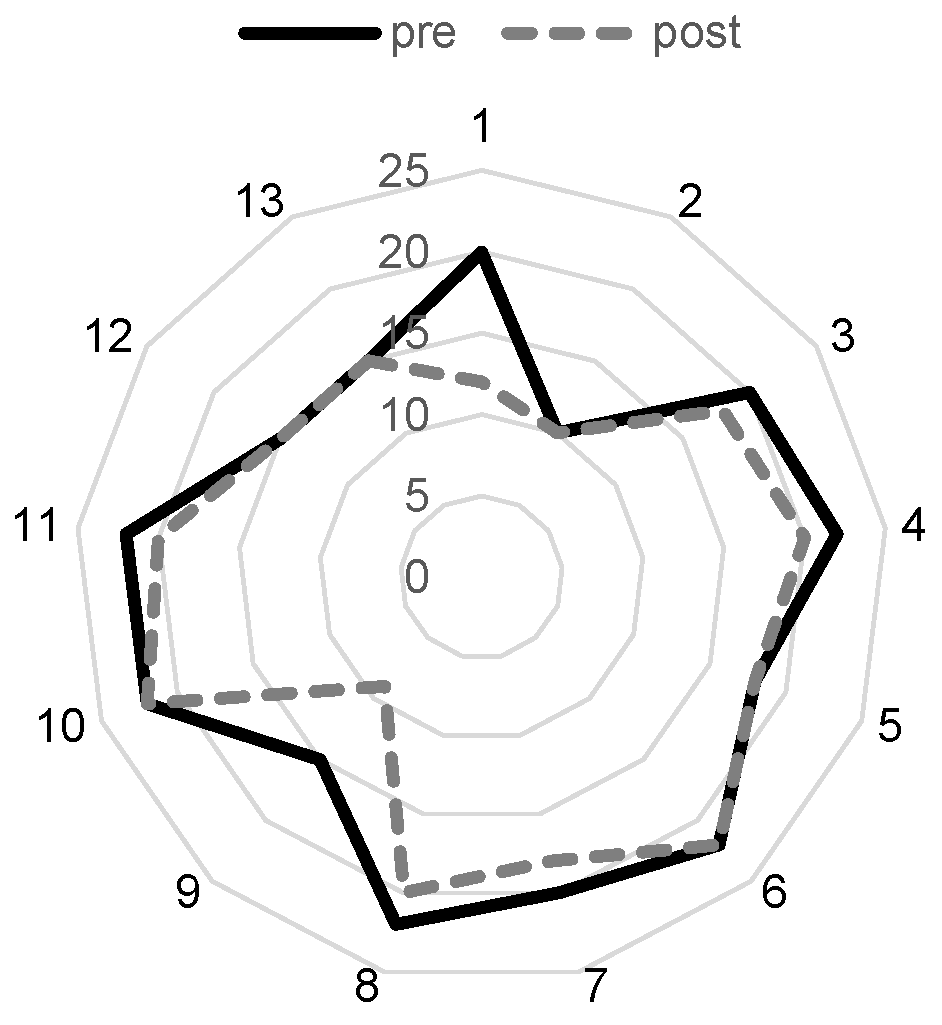
We analyzed data about the subjective stimulation levels and stimulation impedances. The mean UCL was 187.69 ± 78.66 µA with a minimum of 60 µA and a maximum of 300 µA (N = 14). The mean MCL was 170.00 ± 68.87 µA with a minimum of 50 µA and 280 µA (N = 15). The SL was 107.33 ± 59.27 with a minimum of 20 µA and a maximum of 200 µA (N = 16). The mean stimulation impedance before the first stimulation at pre-testing was 19.00 ± 3.67 kΩ with a minimum of 10 kΩ and a maximum of 22 kΩ and 16.00 ± 4.02 kΩ with a minimum of 9 kΩ and a maximum of 20 kΩ after the last TympEALR recording (N = 13; see
Figure 2a).
3.1. Positive, insecure, or negative TympEALR
In seven cases we found a positive response in TympEALR recording. In nine ears including both sides of the bilaterally tested patient, we found an insecure response. In the one remaining case, we found no clear response maybe caused by unusually high EEG variation and a very prominent stimulus artifact in the EP recording.
For the positive TympEALR cases, we found a response amplitude N1-P2/P3 of 4.64 ± 1.94 µA with a minimum of 3 µV and a maximum of 8.9 µV. The latency response for N1 was 90.04 ± 23.00 ms with a minimum of 55 ms and a maximum of 120 ms. The latency response for P2 was 146.52 ± 42.11 ms with a minimum of 108 ms and a maximum of 229.5 ms. In two cases, we found P3 with a latency of 185.37 ms in one case and 180.5 in the other case. The latency response for N2 was 351.5 ± 59.15 with a minimum of 166 ms and a maximum of 351.5 ms.
For insecure TympEALR cases, we found a response amplitude N1-P2/P3 of 1.79 ± 0.84 µA with a minimum of 0.41 µV and a maximum of 2.9 µV. The latency response for N1 was 89.17 ± 23.70 ms with a minimum of 59.72 ms and a maximum of 131.5 ms. The latency response for P2 was 137.43 ± 30.26 ms with a minimum of 86.84 ms and a maximum of 200.5 ms. In five cases, we found P3 with a latency of 182.36 ± 28.45 ms with a minimum of 156.86 ms and a maximum of 236 ms. The latency response for N2 was 226.18 ± 52.13 ms with a minimum of 172.55 ms and a maximum of 349.5 ms.
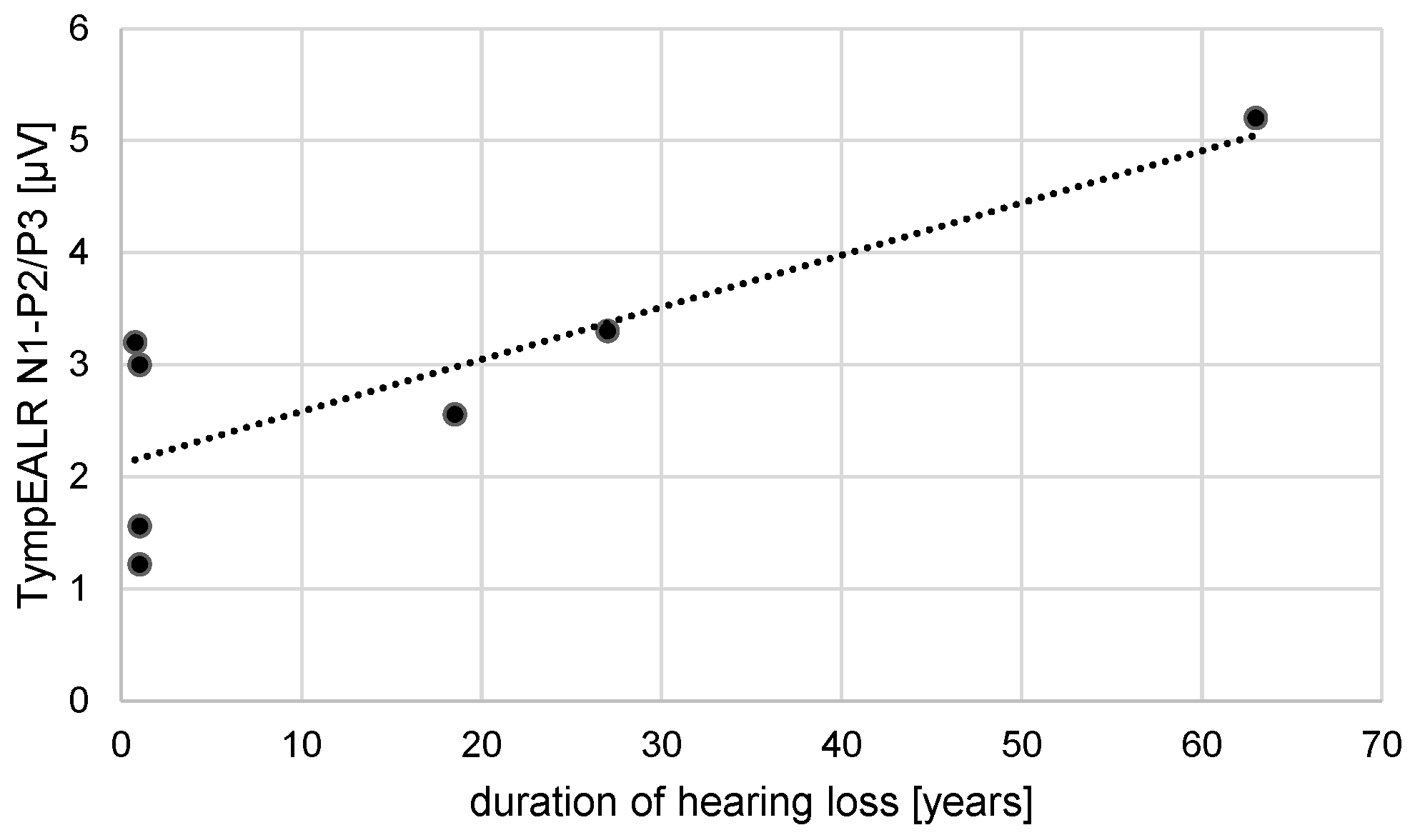
A correlation test was performed for the duration of hearing loss vs. N1-P2/P3 response amplitude and for the duration of deafness vs. N1-P2/P3 response amplitude. A Pearson correlation coefficient assesses the linear relationship between the duration of hearing loss and N1-P2/P3 amplitude. There was a positive correlation between the two variables, r(5) = 0.83, p = 0.02 (see
Figure 3). A Pearson correlation coefficient was computed to assess the linear relationship between the duration of deafness and N1-P2/P3 amplitude. There is no significant correlation between the two variables, r(4) = 0.45, p = 0.38.
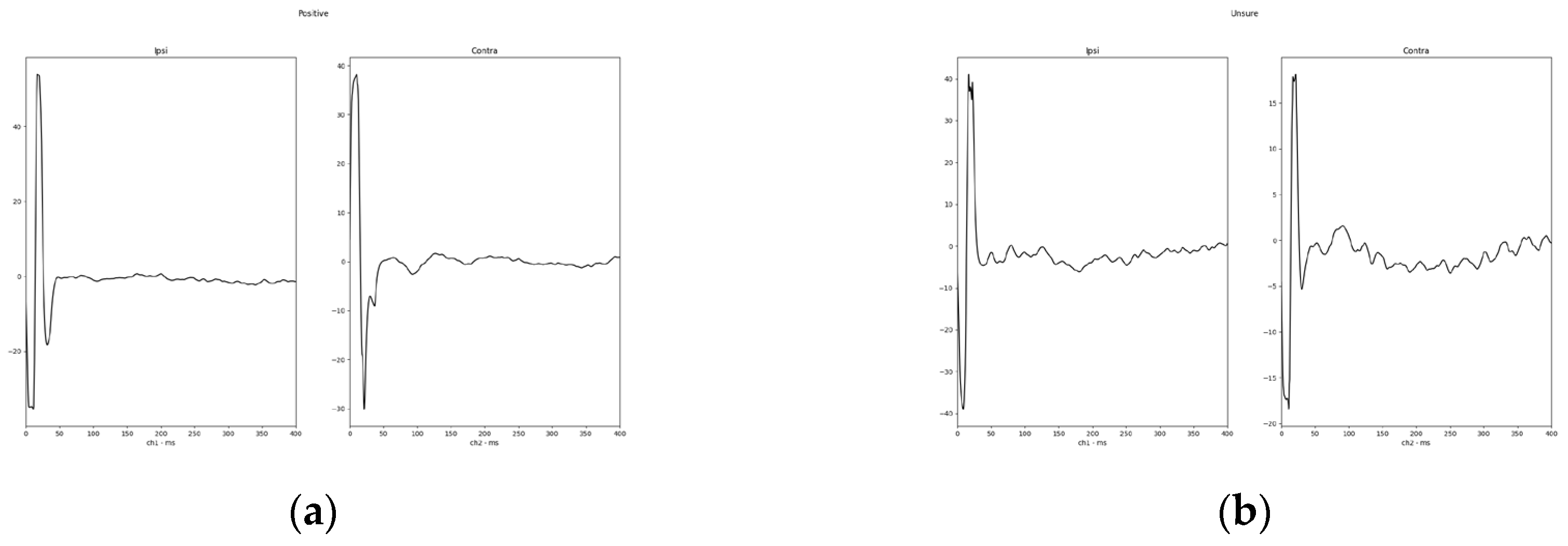
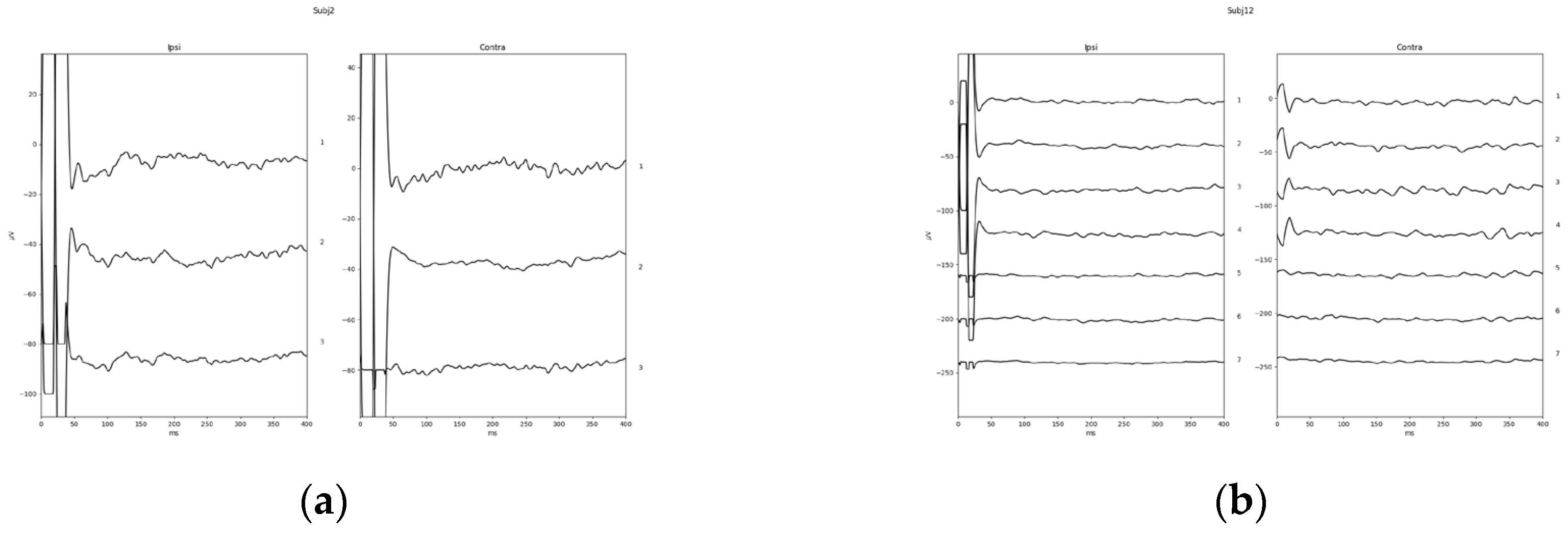
Finally, to show a typical response waveform, a total mean was plotted from a direct export of the response waveform selected as described before. The total mean of the response waveforms grouped in positive and insecure are shown in
Figure 4. One example of a positive response (subject 02) and the only negative response in the investigated study group (subject 12) are shown in
Figure 5.
3.2. Clinical procedure after TympEALR testing
Eight of these seventeen ears (four positive, three insecure, and one negative TympEALR) including both sides of the bilaterally tested patient received a cochlear implant. All of these eight patients are having hearing sensations with their cochlear implant systems. Seven also have a speech understanding. Six months after the initial cochlear implant activation, the Freiburg monosyllable word recognition score at 65dB SPL in quiet was 41.25 ± 29.02 % with a minimum of 0 % and a maximum of 70 %. There were two patients with no word recognition six months after cochlear implant activation. The one patient (positive TympEALR) with 0 %-word recognition score six months after initial CI activation (later data is missing) could understand items in the Freiburg multi-syllable number test. Another patient (positive TympEALR) with 0 %-word recognition score one month after initial CI activation (later speech understanding was not documented in our database) also couldn’t understand items in the Freiburg multisyllable number test but was hearing tones using the CI.
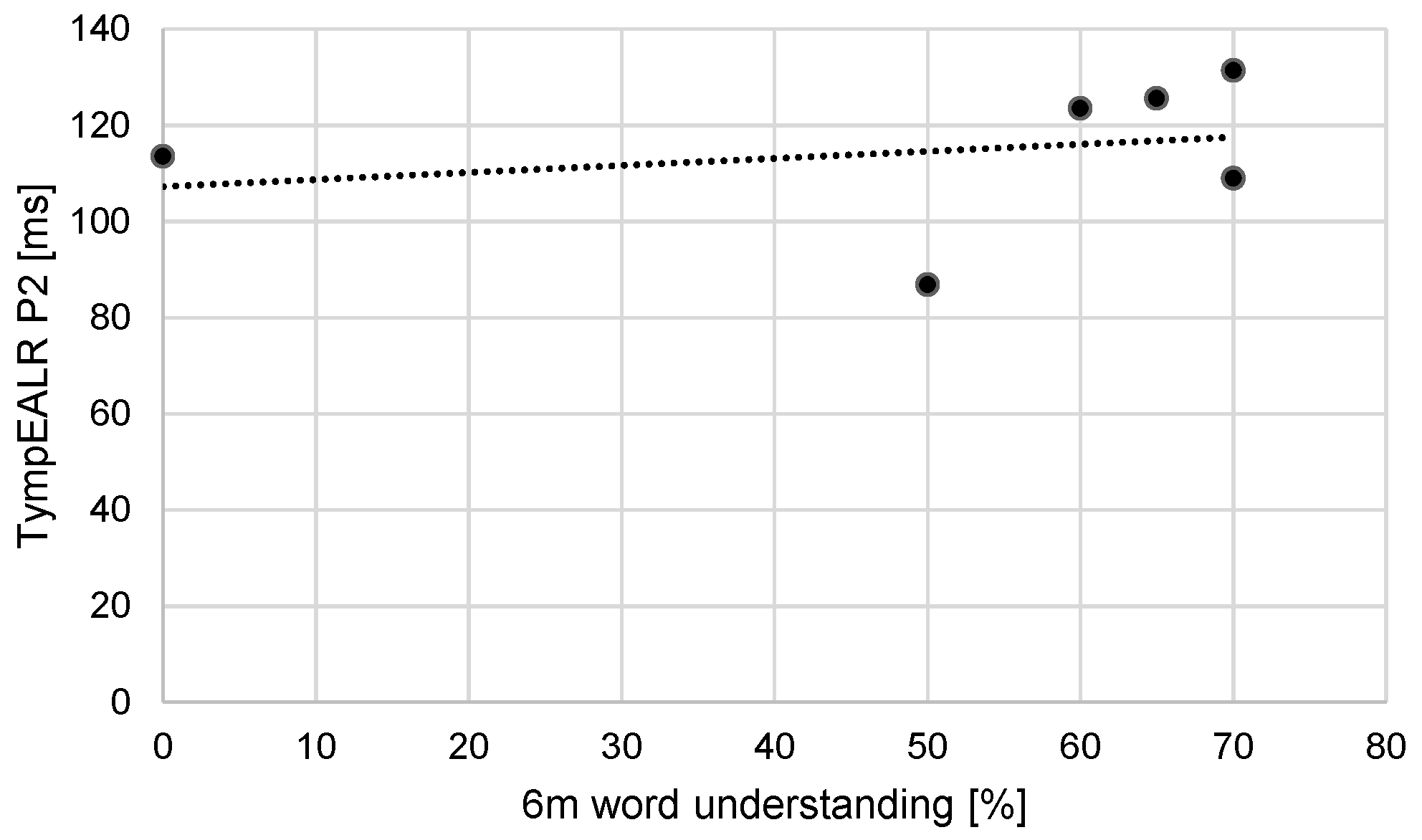
A Pearson correlation coefficient was computed to assess the linear relationship between word understanding six months after the first fitting of the cochlear implant system and P2 latency. There is no significant correlation between the two variables, r(4) = - 0.25, p = 0.64. The data is shown in
Figure 6.
4. Discussion
In the past, the subjective promontory stimulation test was used to evaluate the indication of CI in insecure candidates (especially in retro-cochlear etiologies). Due to the low specificity of the subjective promontory test, it is used rarely today. With trans-tympanic EABR an objective promontory test was found as a good alternative to the subjective promontory test. Especially the testing in local anesthesia offers an interesting setup excluding general anesthesia and includes the possibility to record EAMLR and EALR in addition and being able to assess the patient’s subjective sensation during stimulation. Based on the data presented in this study, the TympEALR looks like another step forward as no anesthesia is needed at all.
To our best knowledge, this study is the first about TympEALR or similar measurement. Therefore, the small number of patients gives us a limited possibility to compare the reliability of TympEALR with the trans-tympanic equivalent in local anesthesia (LA-TT-EALR) or measurements of brainstem resp. midbrain responses in local or general anesthesia (TT-EABR/EAMLR). A larger dataset is needed to finally prove the concept of TympEALR and compare it, especially to LA-TT-EALR as a pre-test checking for a clear response in TympEALR and in other cases going for LA-TT-EALR. In case of insecure or negative LA-TT-EALR, LA-TT-EABR and LA-TT-EAMLR can be assessed in the same session within a short time.
Besides the mentioned benefits of TympEALR to objective promontory tests using the classical trans-tympanic approach, we need to address also the critical points. First, the stimulation is delivered from the tympanic membrane instead of the area around the round window niche. By this, there is a need for a higher stimulation charge to stimulate the auditory path which enlarges the stimulation artifact in the recorded waveforms and additionally the possibility and strength of co-stimulations (e.g., facial nerve). The enlarged stimulation artifact brought us to solely being able to record TympEALR from the EEG and impeded the recording of TympEABR. In future studies, TympEAMLR could be evaluated. The enlarged co-stimulation in TympEALR is limiting the maximum tolerance level of stimulation charge. This potentially leads to a non-positive TympEALR though the auditory pathway is electrically excitable. In addition, this effect is in line with the several unsecure subjective sensations at TympEALR reported within our study. In the cases with non-positive TympEALR, LA-TT-EALR would be the next logical step.
In the case of TympEALR, the electrical stimulation is delivered from the tympanic membrane to the auditory nerve. In the case of LA-TT-EABR/-EAMLR/-EALR, the stimulation is delivered from the round window niche to the auditory nerve. As LA-TT-EABR stimulating in the round windows niche is known to show waveforms like “very basal” EABR using CI stimulation, we hypothesize that TympEALR stimulating “even more basal” therefore shows waveforms like an “even more basal” CI stimulation. This leads us to the limiting factor known from CI stimulation evoked potentials, i.e., EABR, EAMLR, and EALR: The more apical the stimulation the lower the response latency and the larger the amplitudes of the response waveforms [
53]. Luckily, EALRs are known to give relatively large response waveforms also slightly above the threshold which for sure is a big factor why TympEALR works. As no cognitive impairment was known in our tested patients within this study no reduction of response amplitude by this can be assumed [
54].
Looking at stimulation impedances, we found stability or a slight reduction over the time of TympEALR (before vs. after testing). This finding is similar to the results known from trans-tympanic testing and also with experiences from subjective tympanic promontory testing. All over, the stimulation in TympEALR seems to be stable and in regular cases, no continuous checking of stimulation impedance should be needed.
In our study dataset, we checked for correlations of hearing loss vs. N1-P2/P3 response amplitude and for duration of deafness vs. N1-P2/P3 response amplitude. For the duration of hearing loss, a positive linear relationship was found. In contrast, for the duration of deafness, no significant correlation was found. First, we have to keep in mind the relatively small dataset of our study and the variability within patients’ demography. Secondly, the correlation of hearing loss relies on several complex etiologies like vestibular schwannoma (resection). Therefore, this significant correlation needs to be re-evaluated in future studies.
Looking at the postoperative word recognition tests after cochlear implantation, we focused on the one patient with no speech understanding (no words and no Freiburg multisyllables). Though later data is missing (only one month after the initial cochlear implant fitting session was available) this patient had the highest latency for P2 (i.e., 229,50ms) and also the highest latency for N2 (i.e., 351,50ms) which could indicate that the response may not be auditory. The patient had a sudden hearing loss by traffic accident followed by a dens axis fracture and received a SYNCHRONY 2 S-VECTOR (Mi1250) with a STANDARD electrode array (compare estimated preoperative CT-based cochlear duct length of 33.7mm [
19,
55]). During surgery impedance measurement has shown contact of the stimulation electrodes of the CI and the cochlear tissue were in normal range. No electrically evoked compound action potentials could be recorded nor could electrically evoked stapedius reflexes could be recognized. Due to this, we also performed an intra-operative eABR using the test electrode system “ANTS” giving a reproducible wave V and therefore confirming the connection of the CI to the patient’s auditory brainstem [
27,
56]. Besides the missing word and number understanding the patient though achieved the hearing and the discrimination of tones. On the non-implanted ear, the patient had a CPT-AMA of 13.1%. Therefore, single-sided deafness may also be part of the problem as chances for achieving speech understanding can vary a lot when comparing different hearing histories/etiologies [
57]. Most likely, we suppose that the trauma causing the single-sided deafness also caused an auditory neuropathy. In those cases, instead of traumatic damage of the synaptic connection at the modiolus, poor performance with cochlear implants is reported [
58].
5. Conclusions
In the end, TympEALR did result in clear positive results in seven of sixteen cases. Assuming that positive TympEALR does avoid invasive procedures like trans-tympanic promontory testing, we have avoided such a procedure in almost half the patients of our study group. Though not reaching the reliability of trans-tympanic electrically evoked auditory potential measurements due to the distance between the stimulation electrode and cochlear nerve and resulting problems of co-stimulation and also in recording responses, TympEALR could avoid the need for its trans-tympanic alternatives in many with a positive response. If a clear negative TympEALR may speak against cochlear implantation should be investigated in further studies.
Author Contributions
Conceptualization, M.N. and D.P.; methodology, M.N., and D.P.; validation, M.N., and D.P.; formal analysis, M.N., and D.P.; investigation, M.N., and D.P.; resources, D.P.; data curation, D.P.; writing—original draft preparation, D.P.; writing—review and editing, M.N., D.P., and F.S..; visualization, D.P.; supervision, D.P.; project administration, D.P. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by MDPI & LMU Munich.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The study was performed anonymously and retrospectively, according to the current statement of the Ethics Committee (EC) of LMU Munich and is approved by the Ethics Committee of LMU Munich (protocol code 17-227 approved at 04th Jul 2017 using extending protocol code 24-0923-KB).
Informed Consent Statement
As the study was performed anonymously and retrospectively, according to the current statement of the Ethics Committee (EC) of LMU Munich, researchers do not require informed consent from subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
We thank Giacomo Mandruzzato (MED-EL, Innsbruck, Austria) for support in creating the waveform plots.
Conflicts of Interest
D.P. received travel support from Cochlear Ltd. (Australia), INNOFORCE Est. (Liechtenstein) and MED-EL GmbH (Austria), free hardware loan from HZ Haase & Zeisberg (Germany), inomed Medizintechnik GmbH (Germany), Mack Medizintechnik GmbH (Germany) and PATH MEDICAL GmbH, research support from MED-EL GmbH (Austria) and publication discount from Georg Thieme Verlag KG and MDPI AG.
References
- Eshraghi AA, Nazarian R, Telischi FF, Rajguru SM, Truy E, Gupta C. The Cochlear Implant: Historical Aspects and Future Prospects. The Anatomical Record. 2012;295: 1967–1980. [CrossRef]
- Edgerton BJ, Brimacombe JA, House WF. Auditory Capabilities of Single-Channel Cochlear Implant Patients: Etiologic Considerations. Archives of Otolaryngology. 1985;111: 255–258. [CrossRef]
- National Institutes of Health. Cochlear Implants. Consensus Development Conference Statement. 1988. [CrossRef]
- Müller J, Molenda C, Polterauer D. Aktuelle Trends und Entwicklungen bei der Cochlea-Implantat-Versorgung (Current Trends and Developments in Cochlear Implantation). Sprache · Stimme · Gehör. 2024;48: 22–31. [CrossRef]
- Van de Heyning P, Távora-Vieira D, Mertens G, Van Rompaey V, Rajan GP, Müller J, et al. Towards a unified testing framework for single-sided deafness studies: a consensus paper. Audiology and Neurotology. 2016;21: 391–398. [CrossRef]
- Friedland DR, Venick HS, Niparko JK. Choice of ear for cochlear implantation: the effect of history and residual hearing on predicted postoperative performance. Otol Neurotol. 2003;24: 582–589.
- Gfeller KE, Olszewski C, Turner C, Gantz B, Oleson J. Music Perception with Cochlear Implants and Residual Hearing. Audiol Neurotol. 2006;11(suppl 1): 12–15.
- Mick P, Amoodi H, Shipp D, Friesen L, Symons S, Lin V, et al. Hearing Preservation With Full Insertion of the FLEXsoft Electrode. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2013;35. [CrossRef]
- Usami S-I, Moteki H, Tsukada K, Miyagawa M, Nishio S, Takumi Y, et al. Hearing preservation and clinical outcome of 32 consecutive electric acoustic stimulation (EAS) surgeries. Acta oto-laryngologica. 2014;134: 1–11. [CrossRef]
- Schraivogel S, Aebischer P, Weder S, Caversaccio M, Wimmer W. Cochlear implant electrode impedance subcomponents as biomarker for residual hearing. Frontiers in neurology. 2023;14: 1183116. [CrossRef]
- Gibson W. Acoustic Neural Response Telemetry: The Equipment and Methodology Needed to Measure Residual Hearing. CI2016. 2016.
- Yin L, Barnes J, Saoji A, Carlson M. Clinical Utility of Intraoperative Electrocochleography (ECochG) During Cochlear Implantation: A Systematic Review and Quantitative Analysis. Otology & Neurotology. 2020;Publish Ahead of Print. [CrossRef]
- Haumann S, Mynarek (née Bradler) M, Maier H, Helmstaedter V, Büchner A, Lenarz T, et al. Does Intraoperative Extracochlear Electrocochleography Correlate With Postoperative Audiometric Hearing Thresholds in Cochlear Implant Surgery? A Retrospective Analysis of Cochlear Monitoring. Trends in Hearing. 2024;28: 23312165241252240. [CrossRef]
- Sijgers L, Sorensen T, Soulby A, Boyle P, Dalbert A, Röösli C, et al. Classification of Acoustic Hearing Preservation After Cochlear Implantation Using Electrocochleography. Trends in Hearing. 2023;27. [CrossRef]
- O’Leary S, Mylanus E, Venail F, Lenarz T, Birman C, Di Lella F, et al. Monitoring Cochlear Health With Intracochlear Electrocochleography During Cochlear Implantation: Findings From an International Clinical Investigation. Ear & Hearing. 2022;Publish Ahead of Print. [CrossRef]
- Harris MS, Riggs WJ, Koka K, Litvak LM, Malhotra P, Moberly AC, et al. Real-Time Intracochlear Electrocochleography Obtained Directly Through a Cochlear Implant. Otology & Neurotology. 2017;38: e107. [CrossRef]
- Dhanasingh A, Hochmair I. Thirty Years of Translational Research Behind MED-EL. Acta Oto-Laryngologica. 2021;141: (i)-(cxcvi). [CrossRef]
- Schreier A, Molenda C, Draut S, Hempel J-M, Volgger V, Polterauer D, et al. Individualized cochlear implantation – first experience with a new 34 mm electrode for patients with very long cochleae. Laryngo-Rhino-Otologie. Georg Thieme Verlag KG; 2024. [CrossRef]
- Spiegel JL, Polterauer D, Hempel J-M, Canis M, Spiro JE, Müller J. Variation of the cochlear anatomy and cochlea duct length: analysis with a new tablet-based software. Eur Arch Otorhinolaryngol. 2022;279: 1851–1861. [CrossRef]
- Spitzer E, Waltzman S, Landsberger D, Friedmann DR. Acceptance and Benefits of Electro-Acoustic Stimulation for Conventional-Length Electrode Arrays. Audiology and Neurotology. 2020;26: 1–10. [CrossRef]
- Gadenstaetter A, Auinger A, Gerlitz M, Riss D, Yildiz E, Roessler K, et al. Long-Term Follow-Up After Translabyrinthine IAC Tumor Removal With Simultaneous Cochlear Implantation. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2024. [CrossRef]
- Dahm V, Gadenstaetter AJ, Arnoldner C. “To implant or not to implant”: electrically evoked auditory brainstem response audiometry for decision-making in vestibular schwannoma resection with CI. HNO. 2024 [cited 15 Jul 2024]. [CrossRef]
- Carlson ML, Neff BA, Sladen DP, Link MJ, Driscoll CL. Cochlear Implantation in Patients With Intracochlear and Intralabyrinthine Schwannomas. Otology & Neurotology. 2016;37: 647. [CrossRef]
- Carlson ML, Breen JT, Driscoll CL, Link MJ, Neff BA, Gifford RH, et al. Cochlear Implantation in Patients With Neurofibromatosis Type 2: Variables Affecting Auditory Performance. Otology & Neurotology. 2012;33: 853. [CrossRef]
- Polterauer D, Neuling M. Transtympanic electrically evoked auditory midbrain response in local anesthesia (= LA-TT-EAMLR) as a pre-operative tool for checking cochlear implant (= CI) candidacy in an intrasubject comparison to established equivalent measurements of the auditory brainstem (= LA-TT-EABR) and auditory cortex (= LA-TT-EALR). 2023 Sep 17; Köln.
- Causon A, O’Driscoll M, Stapleton E, Lloyd S, Freeman S, Munro KJ. Extracochlear Stimulation of Electrically Evoked Auditory Brainstem Responses (eABRs) Remains the Preferred Pre-implant Auditory Nerve Function Test in an Assessor-blinded Comparison. Otology & Neurotology. 2019;40: 47–55. [CrossRef]
- Lassaletta L, Polak M, Huesers J, Díaz-Gómez M, Calvino M, Varela-Nieto I, et al. Usefulness of electrical auditory brainstem responses to assess the functionality of the cochlear nerve using an intracochlear test electrode. Otology & Neurotology. 2017;38: e413–e420.
- Hosoya M, Nagaoka Y, Wakabayashi T, Shimanuki MN, Nishiyama T, Ueno M, et al. A novel intraoperative continuous monitoring method combining dorsal cochlear nucleus action potentials monitoring with auditory nerve test system. J of Otolaryngol - Head & Neck Surg. 2023;52: 67. [CrossRef]
- Cinar BC, Yarali M, Atay G, Bajin MD, Sennaroglu G, Sennaroglu L. The role of eABR with intracochlear test electrode in decision making between cochlear and brainstem implants: preliminary results. European Archives of Oto-Rhino-Laryngology. 2017;274: 3315–3326. [CrossRef]
- Kasbekar A, Tam Y, Carlyon R, Deeks J, Donnelly N, Tysome J, et al. Intraoperative Monitoring of the Cochlear Nerve during Neurofibromatosis Type-2 Vestibular Schwannoma Surgery and Description of a “Test Intracochlear Electrode.” Journal of Neurological Surgery Reports. 2019;80: e1–e9. [CrossRef]
- Medina MM, Polo R, Amilibia E, Roca-Ribas F, Díaz M, Pérez M, et al. Diagnostic Accuracy of Intracochlear Test Electrode for Acoustic Nerve Monitoring in Vestibular Schwannoma Surgery. Ear & Hearing. 2020;Publish Ahead of Print. [CrossRef]
- Polterauer D. Intraoperative and postoperative measurement of brainstem responses through electrical stimulation of the auditory nerve via implantable neurostimulators. 2020 Jul. [CrossRef]
- Kileny PR, Zwolan TA. Pre-perioperative, transtympanic electrically evoked auditory brainstem response in children. Int J Audiol. 2004;43 Suppl 1: 16–21.
- Kileny PR, Kemink JL. Electrically Evoked Middle-Latency Auditory Potentials in Cochlear Implant Candidates. Archives of Otolaryngology - Head and Neck Surgery. 1987;113: 1072–1077. [CrossRef]
- Kileny PR, Kemink JL, Miller JM. An intrasubject comparison of electric and acoustic middle latency responses. The American journal of otology. 1989;10: 23–27.
- Huen M, Lee J, Westerberg B. Use of auditory evoked potentials with electrical stimulation at the round window niche pre-operatively on a brain-injured patient: A case study. Cochlear implants international. 2020;22: 1–7. [CrossRef]
- Polterauer D, Mandruzzato G, Neuling M, Polak M, Müller J, Hempel J. Evaluation of auditory pathway excitability using a pre-operative trans-tympanic electrically evoked auditory brainstem response under local anesthesia in cochlear implant candidates. International Journal of Audiology. 2022; 1–11. [CrossRef]
- Dutt S, Kumar A, Mittal A, Vadlamani S, Gaur S. Cochlear implantation in auditory neuropathy spectrum disorders: role of transtympanic electrically evoked auditory brainstem responses and serial neural response telemetry. The Journal of Laryngology & Otology. 2021; 1–8. [CrossRef]
- Kileny PR, Kim AH, Wiet RM, Telian SA, Arts HA, El-Kashlan H, et al. The predictive value of transtympanic promontory EABR in congenital temporal bone malformations. Cochlear implants international. 2010;11: 181–186. [CrossRef]
- Polterauer D, Mandruzzato G, Neuling M, Polak M, Müller J, Hempel J-M. PromCERA: praeoperative ECERA – Erweiterter objektiver PromTest zur Integritätsprüfung des Hörnervs bei CI-Kandidaten. 50 Jahrestagung der Deutschen Gesellschaft für Medizinische Physik. DGMP; 2019.
- Polterauer D, Neuling M, Müller J, Hempel J-M, Mandruzzato G, Polak M. PromBERA: A preoperative eABR: An update. Current Directions in Biomedical Engineering. 2018;4: 563–565. [CrossRef]
- Pau H, Gibson WP, Sanli H. Trans-tympanic electric auditory brainstem response (TT-EABR): the importance of the positioning of the stimulating electrode. Cochlear implants international. 2006;7: 183–187. [CrossRef]
- Dutt SN, Kumar A. The Methodology and Electro-physiological Classification of Pre-operative Trans-tympanic Electrically-Evoked Auditory Brainstem Response (TT-EABR). Indian Journal of Otolaryngology and Head & Neck Surgery. 2019. [CrossRef]
- Neumann K, Raab P, Preibisch C, Lanfermann H, Reimold I, Kiefer J. Aktivierung des auditiven Cortex durch Gehörgangs-Elektrostimulation bei gehörlosen Erwachsenen–eine fMRI-Studie. 5 DGA Jahrestagung. DGA; 2002. Available: http://www.uzh.ch/orl/dga2002/programm/Neumann_a.pdf.
- Neuling M, Polterauer D, Mandruzzato G, Müller J, Hempel J. PromCERA light (tympanale praeop. ECERA) vs. PromCERA (transtympanale praeop. ECERA in Lokalanästhesie). Köln; 2020. [CrossRef]
- Kosaner J, Van Dun B, Yigit O, Gultekin M, Bayguzina S. Clinically recorded cortical auditory evoked potentials from paediatric cochlear implant users fitted with electrically elicited stapedius reflex thresholds. International Journal of Pediatric Otorhinolaryngology. 2018;108: 100–112. [CrossRef]
- Polterauer D, Neuling M, Stoecklein S, Mueller J. Cochlear Implantation in a Patient with Implanted Trigeminus Stimulator—Clinical Considerations for Using Two Different Electrical Stimulators in the Same Patient and Our Results. Journal of Otorhinolaryngology, Hearing and Balance Medicine. 2024;5: 2. [CrossRef]
- Polterauer D, Mandruzzato G, Neuling M, Polak M, Müller J, Hempel J. LA-TT-EALR / PromCERA: Comparison of preoperatively performed electrically evoked auditory potentials at the brainstem and cortical level during local anesthesia. Current Directions in Biomedical Engineering. 2022;8: 233–236. [CrossRef]
- Tavora-Vieira D, Ffoulkes E. Direct Elicitation of Cortical Auditory Evoked Potentials by Electrical Stimulation and Their Use to Verify the Most Comfortable Level of Stimulation in Cochlear Implant Users. Audiology and Neurotology. 2023; 1–14. [CrossRef]
- Hu H, Kollmeier B, Dietz M. Reduction of stimulation coherent artifacts in electrically evoked auditory brainstem responses. Biomedical Signal Processing and Control. 2015;21: 74–81. [CrossRef]
- Hughes ML. Objective Measures in Cochlear Implants. Plural Publishing; 2012.
- Lightfoot G. Background information on Cortical ERA. Oct 2014. Available: http://corticalera.com/basics.html.
- Firszt JB, Chambers RD, Kraus N, Reeder RM. Neurophysiology of Cochlear Implant Users I: Effects of Stimulus Current Level and Electrode Site on the Electrical ABR, MLR, and N1-P2 Response. Ear and Hearing. 2002;23: 502–515. [CrossRef]
- Eggermont JJ. The Auditory Brain and Age-Related Hearing Impairment. Academic Press; 2019. Available: https://shop.elsevier.com/books/the-auditory-brain-and-age-related-hearing-impairment/eggermont/978-0-12-815304-8.
- Müller-Graff F-T, Spahn B, Herrmann D, Kurz A, Voelker J, Hagen R, et al. Comprehensive literature review on the application of the otological-surgical planning software OTOPLAN® for cochlear implantation. German version. HNO. 2024. [CrossRef]
- Polterauer D, Mandruzzato G, Neuling M, Polak M, Müller J, Hempel J. Intra-operative test electrode and electrical auditory brainstem response after preoperative assessment in cochlear implant candidacy. Current Directions in Biomedical Engineering. 2023;9: 725–728. [CrossRef]
- Jakob TF, Aschendorff A, Arndt S. Single-Sided Deafness – Mit dem „Zweiten“ hört man besser. Sprache · Stimme · Gehör. 2024;48: 32–37. [CrossRef]
- Shearer AE, Hansen MR. Auditory synaptopathy, auditory neuropathy, and cochlear implantation. Laryngoscope Investigative Otolaryngology. 2019;4: 429–440. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).