1. Introduction
The concept of soil quality was first defined as “the capacity of soil to function within an ecosystem and under various land uses in such a way that it sustains biological productivity, maintains water and air quality, and promotes the health of animals and plants” [
1]. Larson and Pierce [
2] further argued that the combination of a soil’s physical, chemical, and biological properties enables it to fulfill three key functions: (1) provide a medium for plant growth, (2) control and regulate water flow in the environment, and (3) serve as an environmental filter.
Although soil quality appears to be a straightforward concept, defining and quantifying it presents significant challenges [
3]. Some researchers argue that the notion of “quality” cannot be easily applied to such a complex, dynamic, and diverse system as soil [
4,
5,
6]. However, a growing number of studies highlight the importance of soil quality for environmental sustainability and human well-being e.g [
7,
8,
9,
10,
11,
12,
13]. Soil quality offers a holistic approach to understanding the relationships between soil’s biological, chemical, and physical properties, which is crucial for sustainable land use and the management of non-renewable soil resources [
1,
14].
Assessing soil quality requires consideration of both inherent and dynamic soil properties and processes. For any given region, soil quality assessment is influenced by a combination of factors, including management practices like crop rotation and manure application, as well as climate and soil type [
13]. The first step in soil quality assessment is selecting appropriate soil quality indicators (SQIs) to form a minimum data set (MDS) for evaluation [
15]. It is essential to choose indicators that represent a broad spectrum of physical, chemical, and biological properties to accurately assess soil quality, while ensuring that the selected parameters effectively capture the information provided by all relevant indicators.
Many soil attributes that contribute to soil quality, however, are highly correlated, working in concert with other soil properties [
2,
16]. Given this correlation, a stronger assessment of soil quality can be achieved through statistical methods that account for relationships among these attributes. Multivariate statistical analyses, for instance, allow for the simultaneous evaluation of correlated variables, offering insights that might be overlooked if each variable were analyzed individually [
17]. Numerous researchers have focused on using multivariate statistical methods to identify a minimal number of soil quality indices capable of describing changes in soil quality [
18,
19,
20,
21]. Zhou et al., [
22] used ANOVA and factor analysis to identify a subset of four key soil indicators from an initial group of 26 to build an MDS for evaluating soil quality in wheat-producing regions of China. Similarly, Brejda et al. [
23] employed principal component and discriminant analyses to identify sensitive soil indicators at a regional scale.
Research on soil quality assessments based on MDS has been effectively applied to various land use types, including coastal areas [
24], agricultural zones [
23], and grasslands [
20]. However, in the Mediterranean region, only a few studies have developed specific sets of soil quality indicators [
25,
26,
27,
28,
29], and even fewer have incorporated biological parameters [
29].
Nevertheless, soil function is significantly influenced by seasonal variations in temperature and moisture, as well as by management practices in agricultural systems. The majority of studies aimed at identifying MDS do not account for the seasonal variability of soil quality indicators, as soil samplings is typically conducted during one specific season. This is particularly important in Mediterranean regions, which are characterized by a pronounced seasonal contrast in rainfall between winter and summer. Soil quality indicators in such regions often exhibit significant seasonal variability that is frequently overlooked in efforts to establish MDS at a regional scale [
30,
31]. Furthermore, Mediterranean agroecosystems are notable for their highly variable soil cover, spatial diversity, and long history of continuous human settlement and intensive cultivation [
32], which further influence soil quality.
To address these challenges, the present study introduces a statistics-based methodology for identifying an MDS for soil quality assessment, incorporating seasonal samplings across five different land use types over two consecutive years. The objectives of the study were: (i) to identify regional-scale soil quality “factors” from a set of 23 physicals, chemicals, and biologicals soil quality indicators, (ii) to determine which soil quality factors vary significantly with land use, and (iii) to identify sensitive soil attributes that can serve as reliable indicators for monitoring soil quality at a regional scale, accounting for the seasonal variation of soil functions in Mediterranean agroecosystems.
2. Materials and Methods
2.1. Study Area
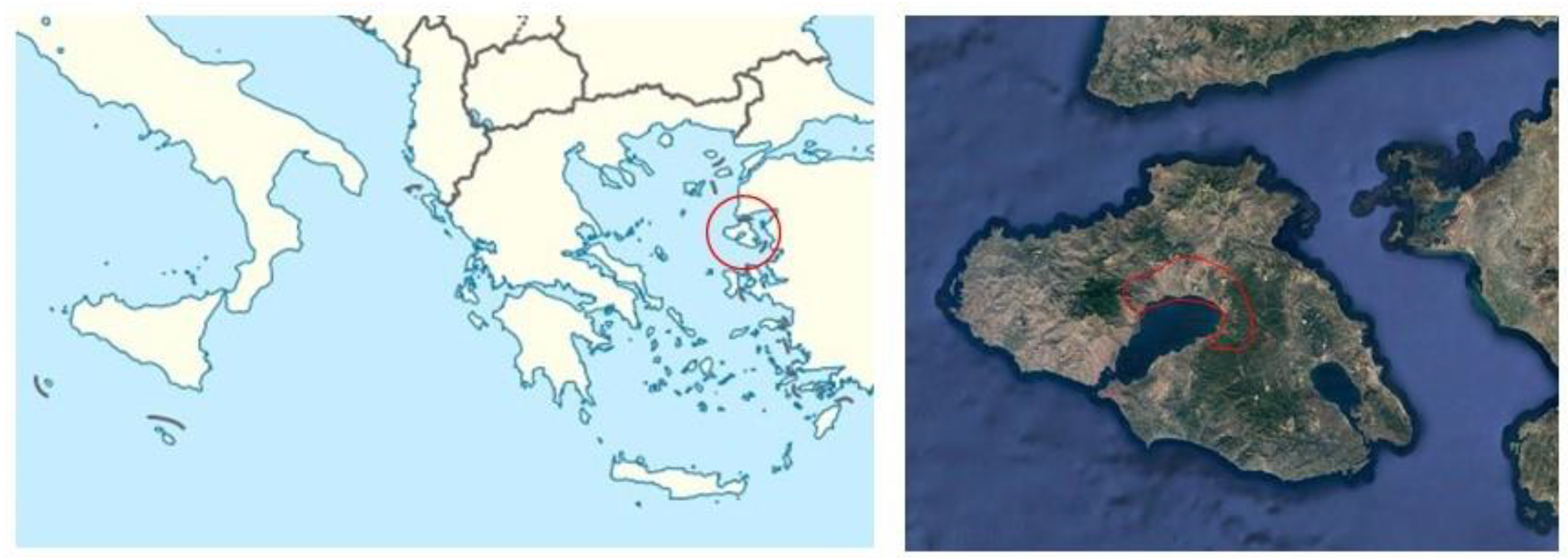
The study was conducted in the Kaloni Gulf watershed, located on Lesvos Island (Longitude: 26° 06ʹ 51 E; Latitude: 39° 12ʹ 40 N), in the northern Aegean Sea (
Figure 1).
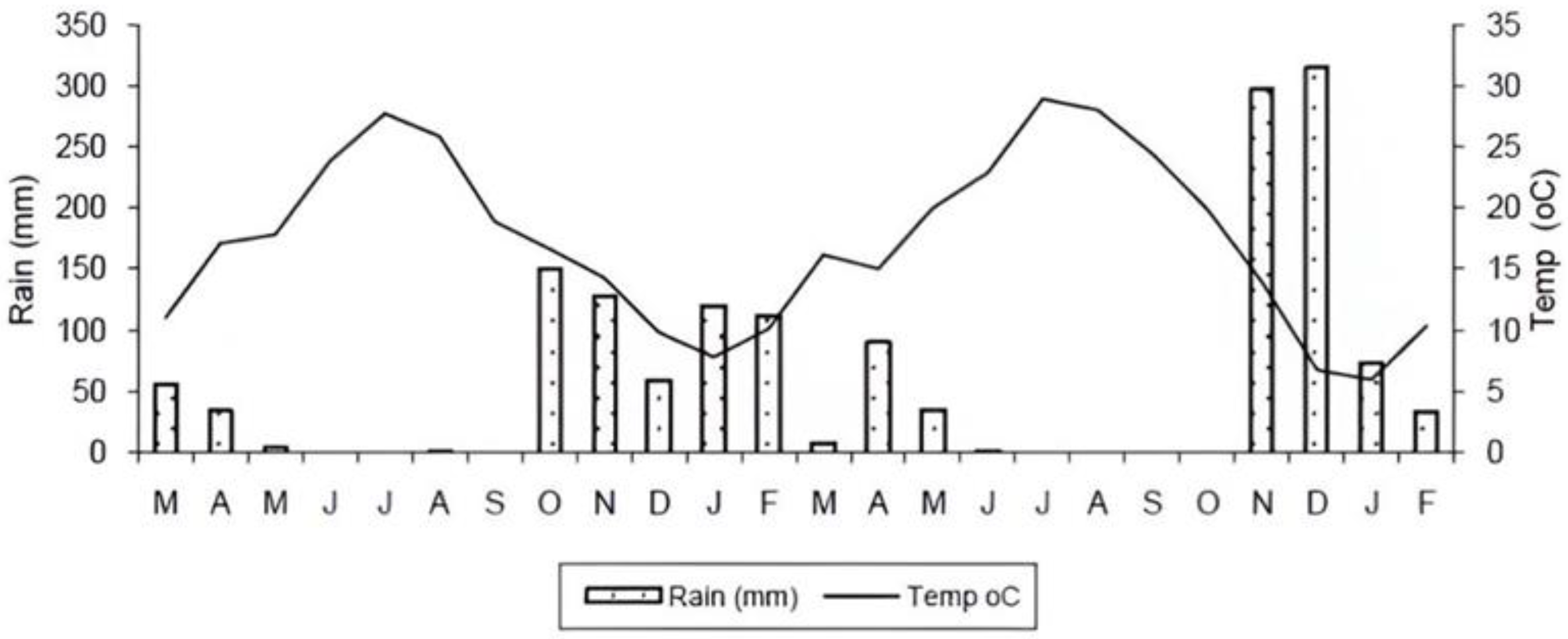
Lesvos, the third-largest Greek island and seventh-largest in the Mediterranean, has a dry to sub-humid Mediterranean climate. The area experiences moderate water surplus in winter, with an annual rainfall of 650 mm and a mean annual temperature of 17.7°C. (
Figure 2).
The total watershed area is 49,260 hectares, consisting of 33.5% cropland, 39.4% pasture, 21.6% forest, and 5.5% for other uses. Olive cultivation accounts for 70% of croplands, with the remaining areas dedicated to arable crops, mainly wheat. The region’s soils derive from Mio-Pliocene volcanic pyroclastics, with common soil-forming factors across agricultural lands surrounding the Kalloni Gulf.
Five land covers representing forest, cropland, and pasture were selected to reflect the island’s ecosystem diversity: pine forests (Pinus brutia), olive groves, wheat fields, crop rotations of corn-wheat (referred to as double cultivation), and shrubland pastures. Preliminary soil samples were collected from 75 representative fields (20 pastures, 15 forests, 40 arable) covering all land use/cover types, with slopes of less than 3% to minimize erosion effects. Three fields (3 hectares each) for each land cover were selected after cluster analysis to ensure similar soil texture and pH (data not shown). All selected soils were classified as Entisols, with Typic Xerofluvents for forest, olive trees, wheat, and wheat/maize double cultivation soils, and Lithic Xerorthents for pastures. All soil textures were sandy-clay-loam.
Soil samples were collected from conventionally farmed fields according to local practices. Forest sites received minimal management, except for resin collection that ceased thirty years ago. Olive grove sites were tilled to a depth of 15 cm in April to incorporate annual vegetation and minimize water competition. Composite 15–15–15 fertilizer was applied every 3 years to each tree (45 kg N ha−1), with soil samples taken before fertilizer application.
For wheat cultivation, seedbeds were prepared using a moldboard plow to invert the soil to a depth of 20–30 cm at the end of October, incorporating plant residues from previous cultivation. No preplant fertilization was applied, with in-season ammonium nitrate (NH4NO3) applied at a rate of 90–100 kg N ha−1 in February. Wheat is harvested in June, and fields remain bare until the next seeding in autumn.
Crop rotation was used for forage production in a restricted area, with cereals sown in October after deep tillage with a moldboard plow (40–50 cm). Pre-plant fertilization with 11–15–15 fertilizer was applied (110 kg N ha−1), and no in-season fertilization was performed, as harvest occurred in late April. Second deep tillage was performed between May 10th and 20th before maize seedling, with pre-plant fertilization of 110 kg ha−1 applied using composite 11–15–15 fertilizer. Maize cultivation was drip irrigated every 8–10 days from the end of June until the end of August, receiving a total volume of 550–600 mm water. In-season N fertilization was performed through irrigation water (fertigation) 3–4 times, totaling 165 kg N ha−1 as NH4NO3. Maize was harvested in late September.
Pasture sites, dominated by “Sarcopoterium spinosum”, had shallow soils in a region with severe degradation through erosion. These soils did not receive any particular management except for grazing by sheep and goats.
2.2. Soil Sampling and Analysis
Over two consecutive years, soil sampling was conducted eight times, seasonally, on the same day and under consistent precipitation conditions for all land uses. Soil samplings performed in early May, August, October, and February. At each sampling site, three composite surface soil samples (0–15 cm depth) were collected randomly from the central area of the site, with each composite sample comprising 8 soil cores (2.5 cm diameter). Prior to sampling, all vegetation and plant residues were cleared from soil surface. The collected soil sub-samples were thoroughly mixed to ensure homogeneity, roots and visible plant residues were removed, and the composite samples were stored at 4°C for 3 days before soil microbial biomass determinations. Additionally, subsamples of the soils were air-dried, ground, sieved through a 2 mm sieve, and stored separately for chemical analysis.
Bulk density (BD) /porosity (Vp) estimated after sampling undisturbed soil samples. Soil moisture (gravimetric water content) was determined by drying at 105°C of triplicate 10 g samples. Water Holding Capacity (WHC) estimated by the Gardner, 1986 method [
33]. Soil texture was determined by physical fractionation (particle- size analysis, PSA) using the Bouyoucos method, after the destruction of organic matter with hydrogen peroxide and dispersion with sodium hexametaphosphate [
34]. Organic C was determined by the Walkley – Black procedure [
35] and total N by the semimicro-Kjeldahl method [
36]. Nitrate and ammonium nitrogen estimated chromatographically by the “cadmium reduction” and indophenol blue method respectively [
37]. Soil-available P was extracted by employing the method suggested by Olsen and Sommers [
38] and determined using spectrophotometry. Soil pH and electrical conductivity (EC) was measured in 1:1 suspension with water. Microbial biomass carbon (Cmic) and nitrogen (Nmic) were determined using the fumigation-extraction method developed by Vance, Brookes, and Jenkinson [
39] for Cmic, and Brookes, Landman, and Jenkinson [
40] for Nmic. Active carbon (Cact) estimated by the permanganate-oxidizable carbon method [
41]. All data are expressed on an oven-dry (at 105°C) soil weight basis.
2.3. Statistical Analysis
The statistical analysis was conducted using the SPSS statistical software. In order to identify differences among physical, chemical and biological soil quality indicators in different land uses, analysis of variance (ANOVA) was chosen. The identification of samples showing significant statistical differences was performed using the LSD post hoc test for multiple comparisons. The Pearson correlation test was used to examine the relationships among SQIs in all land uses. Prior to these analyses, the data were tested for ANOVA assumptions, and were log-transformed when necessary. Principal component analysis (PCA) was used to extract a small number of factors from the set of 23 soil quality indicators studied and identify the most significant properties explaining the majority of the variance in the original data. PCA was used to extract the factors, as it does not require prior assessment of the variance of each soil property explained by the factors [
23]. PCA was performed on standardized variables using the correlation matrix to neutralize the effects of different measurement units on determining the weight of each factor loading [
42,
43]. Factors with eigenvalues > 1 were distinguished from the analysis using “varimax” rotation. Discriminant analysis (DA) was performed on the full set of physical, chemical, and biological soil quality indicators, which includes the effects of land use and season as recorded by seasonal measurements throughout the entire measurement period (8 samples per land use). DA was used to identify the distinction between land uses in relation to their physical, chemical, and biological soil quality indicators, analyze their spatial relationships, and recognize the properties that predominantly affect this distinction.
4. Discussion
In this study, the dataset used in the multivariate analyses incorporates the effects of both land use and season on the examined SQI. Land use within the same climate and soil type influences soil function, not only through climatic conditions but also through management practices such as tillage, irrigation, fertilization, and biomass removal. Additionally, species composition and vegetation cover play a role, as they determine the quantity and quality of plant residues. While land use appears to affect individual SQIs, it significantly influences the combined set of indices (physical, chemical, and biological), categorizing soil functions of each land use into distinct groups. For example, crop soil functions, though varying among crops, stand out more significantly compared to those of forests and pastures.
PCA identified NO
3-N and P as key indicators that differentiate soil functions among land uses. Differences observed, may be attributed to soil management practices: systematic fertilization, which accumulates inorganic phosphorus through continuous use of chemical fertilizers (e.g., 11-15-15 type), animal excretions in pastures that increase nitrate nitrogen, and closed nutrient cycling in forests. Two other indicators, Cmic and Cact, representing labile organic carbon in soils [
44,
45,
46,
47], also distinguish soil functions among land uses. These indicators are influenced by practices such as tillage and biomass removal in crops, animal excretions in pastures, and nutrient cycling in forests, which affect labile carbon pools in the soil. Crop residues serve as a crucial source of energy and nutrients for microbial proliferation, contributing to the formation of soil organic carbon. Labile organic carbon pools represent a small part of soil organic carbon but serve as sensitive indicators of soil biogeochemical processes under agricultural management [
48].
In the current study, six key soil quality factors were identified: “organic matter”, “microbial biomass”, “nutrients”, “compaction”, “C/N ratio”, and “Cact/Corg ratio”. Each of these factors plays a role in supporting one or more essential soil functions. The “organic matter” factor, in particular, reflects both long-term and short-term changes associated with land use transitions [
49]. Soil organic matter (SOM) underpins crucial ecosystem services, such as food production, climate regulation, water filtration, erosion control, nutrient cycling, and providing energy for soil organisms [
50,
51]. It is widely regarded as a vital indicator of soil quality for the Mediterranean agroecosystems [
52,
53,
54,
55].
The “microbial biomass” factor governs ecological processes that drive carbon and nutrient cycles, making it a sensitive measure of soil management impacts. It has been extensively recognized as a critical soil quality indicator [
56,
57,
58] and has been reported among the most important ecological indicators of soil quality in the Mediterranean ecosystem [
30]. The “nutrients” factor affects nutrient availability, while the “compaction” factor influences water retention, aeration, and soil physical, chemical, and biological properties.
The C/N and Cact/Corg ratios, while unidimensional in factor analysis, represent complex and dynamics soil functions. The C/N ratio is a key indicator of the quality of organic substrates available for decomposition [
59], while the Cact/Corg ratio reflects the mineralization dynamics of organic matter [
41]. Together, these factors provide a comprehensive assessment of soil quality in Mediterranean agroecosystems.
The differentiation of these factors based on land use reflects dynamic soil qualities [
16] and assesses the impact of land use and management practices on soil quality. The “organic matter” and “compaction” factors have been previously recognized by other researchers [
23,
60]. In this study, four additional factors are identified: “microbial biomass”, “nutrients”, “C/N”, and “Cact/Corg”. Evaluating these soil quality factors identifies five of the six as significant for assessing changes in soil quality due to land use changes. The Cact/Corg factor appears less important, as it does not effectively express soil quality dynamics.
In general, soil quality factors exhibit similar behavior across different land uses, consistent with the dominant indicators that comprise them. The “organic matter” factor is significantly affected by cultivation practices within the same agro-climatic zone. The “microbial biomass” factor is influenced by carbon and nitrogen incorporation into microbial biomass, while the “nutrients” factor is impacted by nitrogen availability (via fertilization or mineralization). Soil management practices affect the “compaction” factor, and the “C/N” factor is influenced by the quantity and composition of plant residues. Among these, the “organic matter”and “C/N” factors, which reflect the quantity and quality of soil organic matter, appear to be the most crucial determinants of soil quality in Mediterranean agroecosystems. Changes in soil quality, resulting from land use and management practices, are reflected in all components of each factor [
61]. In this study, Ntot highlighed as critical for determining shifts in soil quality within the soil organic matter factor. Its significance as a fundamental property for soil quality is noted in numerous studies [
23,
62,
63] due to its incorporation of a large portion of the information related to interacting soil parameters. In this study forest soils show the highest Ntot stocks, followed by grasslands and croplands similar to other researchers findings [
64,
65]. Ntot was significantly correlated with soil moisture, clay, Corg, Cmic, and Cact, indicating its influence on both labile and stable forms of soil organic matter. Ntot is a SQI that incorporates soil organic matter dynamics, and furthermore, is a significant and direct contributor to plant nitrogen nutrition, even in agricultural contexts [
66]. Ntot as a sensitive SQI among diferent land uses has been reported by Zhao et al., [
67] for other types of climatic zones.
In this study, the soil C/N ratio emerged as a second crucial factor for assessing changes in soil quality. Its significance lies in its ability to reflect the dynamics of organic matter decomposition, which plays a pivotal role in overall soil quality. The soil C/N ratio has long been recognized as a key indicator of organic matter quality and nitrogen mineralization-immobilization processes [
68]. Shifts in soil C/N stoichiometry are known to significantly influence carbon dynamics in agroecosystems [
59]. Microorganisms use labile carbon as an energy source to produce extracellular enzymes, facilitating nitrogen extraction from soil organic matter (SOM) and leading to SOM mineralization [
69,
70]. Thus, the C/N ratio, though often underestimated, plays a fundamental role in regulating soil organic matter decomposition, indirectly impacting soil quality. The C/N ratio also serves as a common proxy for organic matter stability [
71], offering insights into soil quality changes. While interpreting shifts in C/N ratios in bulk soils is complex, especially in response to land use or climate change, it is essential for understanding potential soil organic carbon (SOC) sequestration or losses, as well as nutrient cycling and availability in agroecosystems. This makes the C/N ratio a valuable indicator for tracking soil quality changes in agricultural systems.
From a set of 23 physical, chemical, and biological SQIs, this study identifies soil properties of total nitrogen and C/N ratio as sensitive key indicators, capturing most of the variability across the all 23 SQIs among land uses. These two indicators provide valuable insights into soil quality changes resulting from land use changes or the application of specific management practices. The proposed indicators are sufficient for assessing long-term soil quality changes.
5. Conclusions
The concept of soil quality, which encompasses the holistic relationships and functions of physical, chemical, and biological soil properties, aligns with the sustainable management of non-renewable soil resources. In this study, the influence of land use on soil function and overall SQIs is emphasized, particularly under the unique conditions imposed by different land uses incorporating any seasonal variation of the SQIs in the Mediterranean agroecosystems. Agricultural use, in particular, is shown to significantly affect soil parameters, differentiating them from the natural ecosystem of the forest. Moreover, considerable differences are observed even within agricultural systems. Cultivation practices appear to have a consistent impact on soil parameters, with olive trees, wheat, and double-cropping systems grouped in a distinct manner, indicating that land cultivation affects soil function and differentiates it from other agricultural uses such as pasture.
The study identifies five key factors that depict soil function: “organic matter”, “microbial biomass”, “nutrients”, the “C/N ratio”, and “compaction”. These factors influence one or more soil functions and can be used to comparatively reflect changes in soil quality due to land use change. Soil properties of total soil nitrogen (Ntot) and C/N ratio, which determine the quantity and quality of organic matter in the soil, emerge as particularly sensitive indicators of soil quality changes in Mediterranean agroecosystems. By assessing Ntot and the C/N ratio, valuable insights into soil quality can be obtained, incorporating valuable evidence that can be derived by a biger set of physical, chemical, and biological indicators. The comparison of soils based on these two indicators reveals the impact of land use changes and management practices on soil quality.
The proposed indicators, which condense complex information about changes in soil quality, can serve as practical tools for assessing the sustainability of soil resources. Their use enables producers and land management entities to swiftly and accurately determine shifts in soil quality following land use changes or the adoption of new cultivation practices, providing an opportunity to reassess and optimize management strategies.
Figure 1.
The Study area in the watershed of Kaloni Gulf (Lesvos Island,Greece).
Figure 1.
The Study area in the watershed of Kaloni Gulf (Lesvos Island,Greece).
Figure 2.
Mean monthly air temperature (line) and rainfall (bars), starting from March (M) 1st year to February (F) 2nd year.
Figure 2.
Mean monthly air temperature (line) and rainfall (bars), starting from March (M) 1st year to February (F) 2nd year.
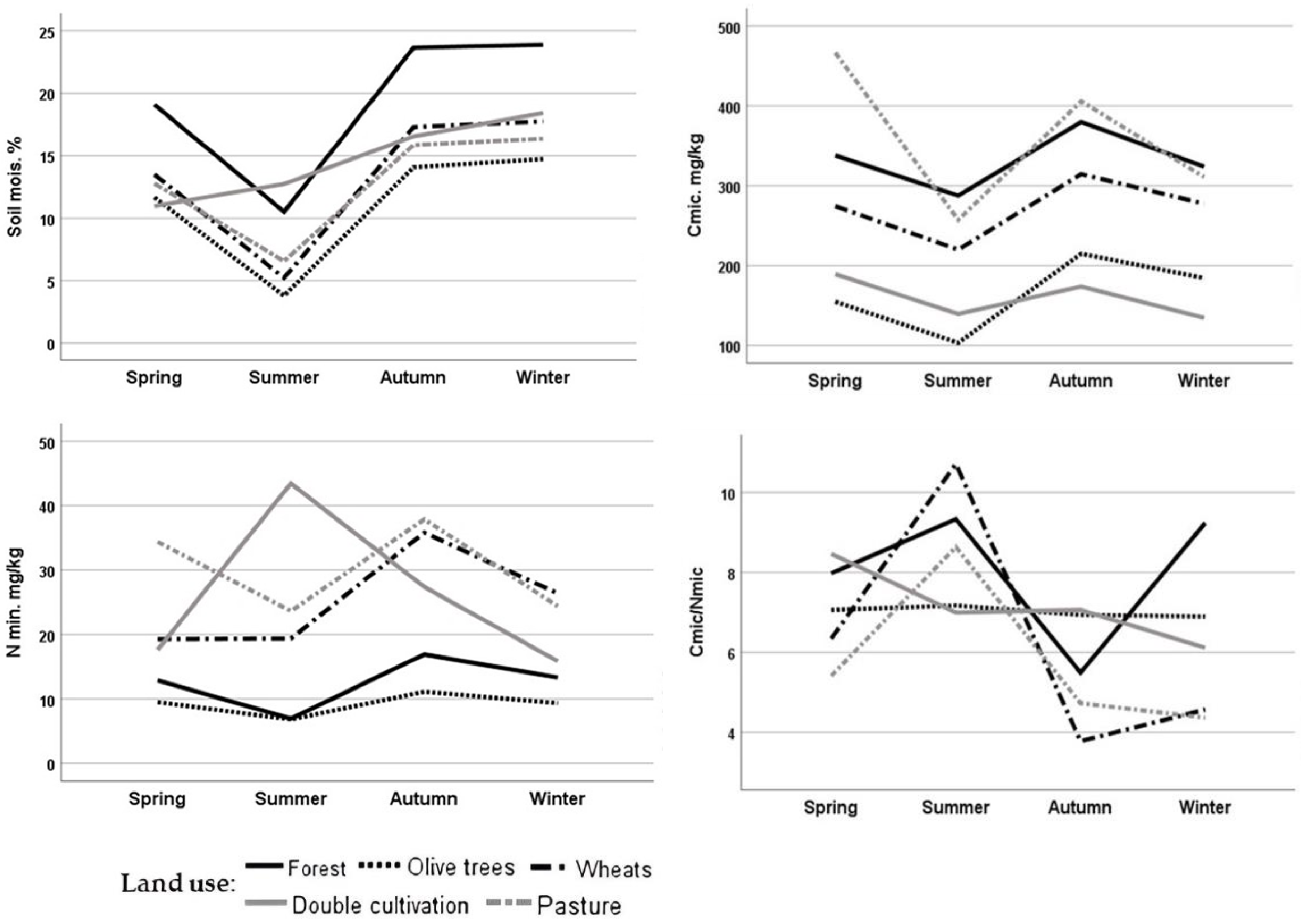
Figure 3.
Seasonal variation of soil moisture Cmic, Nmic and the Cmic/Nim ratio. The values represent the means of two samplings conducted during the same season across two consecutive years of the study.
Figure 3.
Seasonal variation of soil moisture Cmic, Nmic and the Cmic/Nim ratio. The values represent the means of two samplings conducted during the same season across two consecutive years of the study.
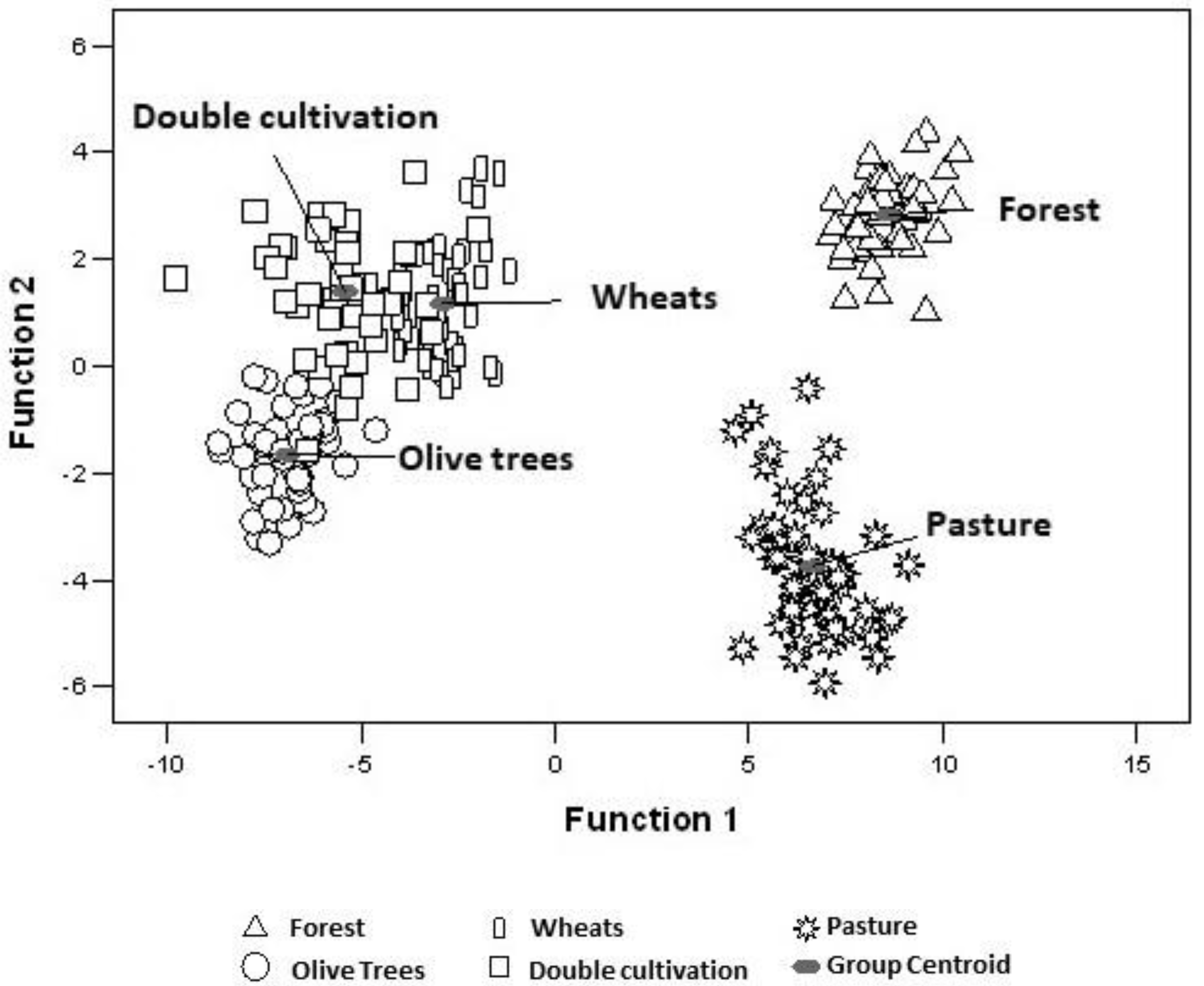
Figure 4.
Scatter plot of land uses regarding the discriminant scores of the first two functions.
Figure 4.
Scatter plot of land uses regarding the discriminant scores of the first two functions.
Table 1.
Soil quality indicators for each land use. Average values of the 8 soil samplings.
Table 1.
Soil quality indicators for each land use. Average values of the 8 soil samplings.
| |
Soil quality indicator |
Forest |
Olive Trees |
Wheats |
Double Cultivation |
Pasture |
| Physical SQIs |
Bulk density, g/cm3
|
1,25 c |
1,39 b |
1,21 c |
1,49 a |
1,40 b |
| Porosity, % |
52,65 a |
47,67 b |
54,38 a |
43,64 c |
47,00 b |
| moisture, % |
19,28 a |
11,07 c |
13,44 bc |
14,67 b |
12,89 bc |
| WHC, % |
79,09 a |
49,13 c |
60,29 b |
56,99 b |
51,93 c |
| Clay, % |
30,85 a |
22,96 b |
26,86 a |
24,42 a |
29,87 a |
| Silt, % |
23,88 ab |
24,48 ab |
24,92 |
27,47 a |
21,43 b |
| Sand, % |
45,27 b |
52,56 a |
48,22 a |
48,11 a |
48,71 a |
| Chemical SQIs |
Corg, g kg-1
|
17,49 a |
9,68 d |
10,55 c |
8,77 e |
13,44 b |
| Ntot, g kg-1
|
1,14 a |
0,76 c |
0,97 b |
0,78 c |
0,95 b |
| CN |
15,64 a |
13,08 bc |
11,81 c |
11,60 c |
14,31 ab |
| NO3_N, mg kg-1
|
3,75 b |
6,53 b |
19,52 a |
20,75 a |
20,59 a |
| NH4_N, mg kg-1
|
8,75 a |
2,66 c |
5,68 b |
5,32 b |
9,49 a |
| Nmin, mg kg-1 |
12,50 c |
9,19 c |
25,21 b |
26,07 ab |
30,07 a |
| P, mg kg-1
|
2,93 d |
20,76 b |
24,17 a |
24,74 a |
6,45 c |
| EC, dS m-1
|
0,40 a |
0,19 b |
0,39 a |
0,45 a |
0,38 a |
| Ph |
7,00 a |
6,17 b |
6,13 b |
6,27 b |
6,03 b |
| Biological SQIs |
Cmic, mg kg-1
|
332,26 a |
164,31 c |
271,57 b |
159,26 c |
360,13 a |
| Cmic/Corg % |
1,90 b |
1,70 b |
2,64 a |
1,85 b |
2,70 a |
| Nmic, mg kg−1
|
49,95 b |
24,48 c |
53,28 b |
23,31 c |
69,62 a |
| Nmic/Ntot, % |
4,44 c |
3,34 cd |
5,68 b |
3,13 d |
7,29 a |
| Cmic/Nmic |
8,01 a |
7,02 ab |
6,35 ab |
7,16 ab |
5,78 b |
| Cact, mg kg−1
|
411,27 a |
291,64 bc |
331,57 b |
265,55 c |
374,23 a |
| Cact/Corg % |
2,36 b |
3,03 a |
3,15 a |
3,07 a |
2,81 a |
Table 2.
Correlation coefficients for soil quality indicators (n=120).
Table 2.
Correlation coefficients for soil quality indicators (n=120).
| |
BD |
Vp |
moist |
WHC |
clay |
silt |
sand |
Corg |
Ntot |
C/N |
NO3-N |
NH4-N |
Nmin |
P |
EC |
pH |
Cmic |
Cmc/
Corg |
Nmic |
Nmic/Ntot |
Cmic/Nmic |
Cact |
| Vp |
-1,00** |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| moist |
-0,13*
|
0,13*
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| WHC |
-0,43**
|
0,43**
|
0,39**
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| clay |
-0,35**
|
0,35**
|
0,28**
|
0,48**
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| silt |
ns |
ns |
ns |
ns |
-0,52** |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| sand |
0,38**
|
-0,38**
|
-0,32**
|
-0,62** |
-0,65** |
-0,31**
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Corg |
-0,41**
|
0,41**
|
0,33**
|
0,69** |
0,77** |
-0,37**
|
-0,53** |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Ntot |
-0,32**
|
0,32**
|
0,40**
|
0,60** |
0,54** |
-0,29**
|
-0,34**
|
0,65** |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| C/N |
-0,15*
|
0,15*
|
ns |
0,13*
|
0,29**
|
ns |
-0,23**
|
0,38**
|
-0,30**
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NO3-N |
ns |
ns |
ns |
-0,13*
|
ns |
ns |
ns |
-0,25**
|
ns |
-0,29**
|
|
|
|
|
|
|
|
|
|
|
|
|
| NH4-N |
ns |
ns |
0,27**
|
0,27**
|
0,61** |
-0,30**
|
-0,41**
|
0,47**
|
0,33**
|
0,16*
|
ns |
|
|
|
|
|
|
|
|
|
|
|
| Nmin |
ns |
ns |
ns |
ns |
0,15*
|
ns |
-0,20**
|
ns |
0,19**
|
-0,23**
|
0,95**
|
0,33**
|
|
|
|
|
|
|
|
|
|
|
| P |
0,14*
|
-0,14*
|
-0,19**
|
-0,36**
|
-0,77** |
0,45**
|
0,45**
|
-0,78** |
-0,36**
|
-0,42**
|
0,41**
|
-0,47**
|
0,24**
|
|
|
|
|
|
|
|
|
|
| EC |
-0,15*
|
0,15*
|
0,27**
|
0,26**
|
0,20**
|
ns |
-0,29**
|
ns |
0,24**
|
ns |
0,39**
|
0,24**
|
0,45**
|
ns |
|
|
|
|
|
|
|
|
| pH |
-0,27**
|
0,27**
|
0,25**
|
0,52** |
0,29**
|
ns |
-0,38**
|
0,44**
|
0,33**
|
ns |
-0,28**
|
ns |
-0,23**
|
-0,34**
|
0,23**
|
|
|
|
|
|
|
|
| Cmic |
-0,27**
|
0,27**
|
0,38**
|
0,36**
|
0,68** |
-0,47**
|
-0,33**
|
0,60** |
0,59** |
ns |
0,16*
|
0,57** |
0,33**
|
-0,52** |
0,19**
|
ns |
|
|
|
|
|
|
| Cmic/Corg |
ns |
ns |
0,20**
|
ns |
0,25**
|
-0,28**
|
ns |
ns |
0,24**
|
-0,16*
|
0,36**
|
0,31**
|
0,43**
|
ns |
ns |
-0,19**
|
0,75** |
|
|
|
|
|
| Nmic |
-0,22**
|
0,22**
|
0,43**
|
ns |
0,55** |
-0,42**
|
-0,23**
|
0,39**
|
0,45**
|
ns |
0,28**
|
0,51**
|
0,43**
|
-0,35**
|
0,16*
|
ns |
0,72** |
0,56** |
|
|
|
|
| Nmic/Ntot |
ns |
ns |
0,27**
|
-0,15*
|
0,39**
|
-0,34**
|
-0,13*
|
0,16*
|
ns |
0,35**
|
0,22**
|
0,42**
|
0,34**
|
-0,25**
|
ns |
-0,19**
|
0,53** |
0,52** |
0,85** |
|
|
|
| Cmic/Nmic |
ns |
ns |
-0,19**
|
0,24**
|
ns |
ns |
ns |
ns |
ns |
ns |
-0,15*
|
-0,14*
|
-0,18**
|
ns |
ns |
0,13*
|
ns |
ns |
-0,52** |
-0,53** |
|
|
| Cact |
-0,28**
|
0,28**
|
0,21**
|
0,60** |
0,52** |
-0,29**
|
-0,32**
|
0,64** |
0,62** |
ns |
ns |
0,35**
|
ns |
-0,47**
|
ns |
0,18**
|
0,56** |
0,20**
|
0,30**
|
ns |
0,19**
|
|
| Cact/Corg |
0,18**
|
-0,18**
|
-0,13*
|
ns |
-0,28**
|
ns |
0,22**
|
-0,40**
|
ns |
-0,37**
|
0,25**
|
-0,13*
|
0,20**
|
0,33**
|
ns |
-0,27**
|
ns |
0,28**
|
-0,16*
|
-0,19**
|
0,20**
|
0,41**
|
Table 3.
Standardized coefficients and properties of the discriminant analysis for physical, chemical, and biological SQI.
Table 3.
Standardized coefficients and properties of the discriminant analysis for physical, chemical, and biological SQI.
| Soil quality indicator |
Function 1 |
Function 2 |
Function 3 |
Function 4 |
| BD |
0,511 |
0,307 |
-0,353 |
0,734 |
| moist |
-0,146 |
0,244 |
-0,486 |
0,186 |
| WHC |
0,356 |
0,754 |
-0,073 |
0,020 |
| clay |
0,690 |
0,595 |
0,495 |
0,306 |
| silt |
-0,318 |
0,857 |
0,229 |
0,417 |
| Corg |
0,511 |
0,406 |
-0,638 |
-0,266 |
| Ntot |
0,281 |
0,371 |
0,410 |
-0,358 |
| C/N |
-0,284 |
0,483 |
-0,130 |
0,123 |
| NO3-N |
0,397 |
-0,952 |
-0,161 |
0,687 |
| NH4-N |
0,402 |
-0,180 |
0,081 |
0,215 |
| P |
-1,096 |
0,767 |
0,510 |
-0,118 |
| EC |
0,438 |
0,094 |
0,074 |
0,234 |
| pH |
-0,161 |
0,105 |
-0,224 |
-0,063 |
| Cmic |
-1,053 |
-0,515 |
-1,229 |
0,546 |
| Cmic/Corg |
0,849 |
0,523 |
1,306 |
-0,572 |
| Nmic |
-0,217 |
0,491 |
0,067 |
0,182 |
| Nmic/Ntot |
0,599 |
-0,584 |
0,575 |
-0,656 |
| Cmic/Nmic |
0,235 |
-0,030 |
0,182 |
-0,093 |
| Cact |
0,474 |
-1,087 |
1,581 |
-0,456 |
| Cact/Corg |
-0,786 |
0,682 |
-1,442 |
0,245 |
| Cmic/Nmic |
8,01 a |
7,02 ab |
-0,353 |
0,734 |
| Cact |
411,27 a |
291,64 bc |
-0,486 |
0,186 |
| |
|
|
|
|
| Eigenvalues |
41,596 |
5,727 |
2,362 |
1,744 |
Commulative
variation % |
80,9 |
92,0 |
96,6 |
100,0 |
| Sig. |
< 0,001 |
< 0,001 |
< 0,001 |
< 0,001 |
Table 4.
Pairwise comparison of land uses for existence significant difference of their centroids.
Table 4.
Pairwise comparison of land uses for existence significant difference of their centroids.
| Land Use |
|
Forest |
Olive Trees |
Wheats |
Double Cultivation |
| Forest |
F |
357,378 |
|
|
|
| Sig. |
,000 |
|
|
|
| Olive trees |
F |
207,950 |
57,095 |
|
|
| Sig. |
,000 |
,000 |
|
|
| Wheats |
F |
281,162 |
36,172 |
34,197 |
|
| Sig. |
,000 |
,000 |
,000 |
|
| Double cultivation |
F |
70,362 |
260,748 |
165,884 |
230,242 |
| Sig. |
,000 |
,000 |
,000 |
,000 |
Table 5.
The first 10 factors of principal component analysis.
Table 5.
The first 10 factors of principal component analysis.
| Component |
Initial Eigenvalues |
| |
Total |
% of Variance |
Cumulative % |
| 1 |
5,57 |
29,34 |
29,34 |
| 2 |
3,46 |
18,19 |
47,53 |
| 3 |
2,38 |
12,51 |
60,04 |
| 4 |
1,83 |
9,64 |
69,68 |
| 5 |
1,36 |
7,17 |
76,85 |
| 6 |
1,15 |
6,04 |
82,89 |
| 7 |
0,87 |
4,58 |
87,47 |
| 8 |
0,73 |
3,86 |
91,33 |
| 9 |
0,55 |
2,90 |
94,24 |
| 10 |
0,42 |
2,18 |
96,42 |
Table 6.
“Weights” and percentage of explained variance of the SQI of the 6 factors.
Table 6.
“Weights” and percentage of explained variance of the SQI of the 6 factors.
| Soil Properties |
Factors |
Communalities |
| 1 |
2 |
3 |
4 |
5 |
6 |
| Ntot |
0,82 |
0,16 |
0,10 |
0,19 |
-0,26 |
-0,09 |
0,82 |
| Corg |
0,82 |
0,08 |
-0,10 |
0,22 |
0,44 |
-0,05 |
0,93 |
| WHC |
0,80 |
-0,18 |
0,08 |
0,30 |
0,08 |
0,13 |
0,79 |
| Cact |
0,73 |
0,30 |
0,00 |
0,15 |
-0,07 |
0,36 |
0,78 |
| P |
-0,61 |
-0,24 |
0,34 |
0,05 |
-0,52 |
0,02 |
0,81 |
| Soil moist |
0,54 |
0,11 |
0,05 |
0,01 |
-0,16 |
-0,50 |
0,58 |
| NH4-N |
0,49 |
0,41 |
0,22 |
-0,22 |
0,35 |
-0,14 |
0,65 |
| Cmic/Corg |
0,07 |
0,85 |
0,16 |
-0,02 |
-0,21 |
0,13 |
0,82 |
| Nmic/Ntot |
-0,07 |
0,78 |
0,10 |
0,04 |
0,32 |
-0,45 |
0,93 |
| Nmic |
0,29 |
0,75 |
0,15 |
0,12 |
0,06 |
-0,52 |
0,95 |
| Cmic |
0,56 |
0,74 |
0,11 |
0,12 |
0,13 |
0,04 |
0,90 |
| Nmin |
-0,01 |
0,39 |
0,87 |
-0,05 |
-0,12 |
-0,03 |
0,93 |
| NO3-N |
-0,18 |
0,28 |
0,85 |
0,01 |
-0,24 |
0,02 |
0,89 |
| EC |
0,29 |
-0,15 |
0,76 |
0,07 |
0,05 |
-0,14 |
0,70 |
| VP |
0,21 |
0,05 |
0,01 |
0,96 |
0,08 |
-0,04 |
0,98 |
| BD |
-0,21 |
-0,05 |
-0,01 |
-0,96 |
-0,08 |
0,04 |
0,98 |
| C/N |
-0,05 |
0,08 |
-0,15 |
0,12 |
0,88 |
0,12 |
0,84 |
| Cact/Corg |
-0,06 |
0,22 |
0,07 |
-0,13 |
-0,61 |
0,52 |
0,73 |
Table 7.
Effect of land use on the factors score derived from principal component analysis.
Table 7.
Effect of land use on the factors score derived from principal component analysis.
| |
Factor score |
ANOVA |
| Factors |
Forest |
Olive trees |
Wheats |
Double Cultiv. |
Pasture |
F |
Sig. |
| Organic Matter |
1,53 a |
-0,80 d |
-0,38 c |
-0,46 c |
0,10 b |
121,24 |
0,00 |
| Microbial Biomass |
-0,36 c |
-0,41 cd |
0,43 b |
-0,79 d |
1,12 a |
52,76 |
0,00 |
| Nutrients |
-0,47 c |
-0,96 d |
0,31 b |
0,78 a |
0,33 b |
37,99 |
0,00 |
| Compaction |
0,23 b |
0,04 b |
0,99 a |
-0,74 c |
-0,51 c |
34,41 |
0,00 |
| C/N |
0,63 a |
-0,33 b |
-0,59 b |
-0,31 b |
0,60 a |
21,04 |
0,00 |
| Active Carbon |
-0,11a |
0,13 a |
-0,01 a |
0,02 a |
-0,03 a |
0,35 |
0,84 |
Table 8.
Eigenvalues and percentages of the explained variance of the four functions when discriminating land uses by soil quality factors.
Table 8.
Eigenvalues and percentages of the explained variance of the four functions when discriminating land uses by soil quality factors.
| Functions |
Eigenvalue |
% of Variance |
Cumulative % |
| 1 |
7,903 |
75,9 |
75,9 |
| 2 |
1,237 |
11,9 |
87,8 |
| 3 |
,924 |
8,9 |
96,7 |
| 4 |
,347 |
3,3 |
100,0 |
Table 9.
Unstandardized coefficients of discrete functions of soil quality factors.
Table 9.
Unstandardized coefficients of discrete functions of soil quality factors.
Factos |
Functions |
| 1 |
2 |
3 |
4 |
| 1 Organic matter |
2,504 |
-0,150 |
-0,117 |
0,385 |
| 2 Microbial biomass |
0,416 |
1,069 |
0,756 |
-0,346 |
| 3 Nutrients |
-0,460 |
0,903 |
-0,406 |
0,799 |
| 4 Compaction |
0,104 |
-0,383 |
1,045 |
0,527 |
| 5 C/N |
1,431 |
0,264 |
-0,221 |
-0,366 |
| 6 Active carbon |
-0,203 |
-0,046 |
0,003 |
-0,082 |
| (Constant) |
,000 |
,000 |
,000 |
,000 |
Table 10.
Eigenvalues and percentages of the explained variance of the four functions when discriminating land uses by organic matter factor.
Table 10.
Eigenvalues and percentages of the explained variance of the four functions when discriminating land uses by organic matter factor.
| Function |
Eigenvalue |
% of Variance |
Cumulative % |
| 1 |
13,646 |
84,2 |
84,2 |
| 2 |
2,078 |
12,8 |
97,0 |
| 3 |
0,313 |
1,9 |
99,0 |
| 4 |
0,169 |
1,0 |
100,0 |
Table 11.
Unstandardized coefficients of discrete functions of the organic matter factor.
Table 11.
Unstandardized coefficients of discrete functions of the organic matter factor.
| SQI |
Functions |
| |
1 |
2 |
3 |
4 |
| Corg |
0,324 |
-0,098 |
-0,237 |
0,271 |
| Ntot |
2,000 |
-0,099 |
3,187 |
3,318 |
| WHC |
0,019 |
0,169 |
0,008 |
-0,039 |
| moist |
0,004 |
0,035 |
-0,036 |
-0,064 |
| NH4-N |
0,136 |
-0,021 |
0,312 |
-0,116 |
| P |
-0,234 |
0,043 |
0,031 |
0,089 |
| Cact |
-0,003 |
-0,010 |
0,000 |
0,004 |
| (Constant) |
-3,201 |
-6,596 |
-2,564 |
-5,163 |