Submitted:
22 October 2024
Posted:
22 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
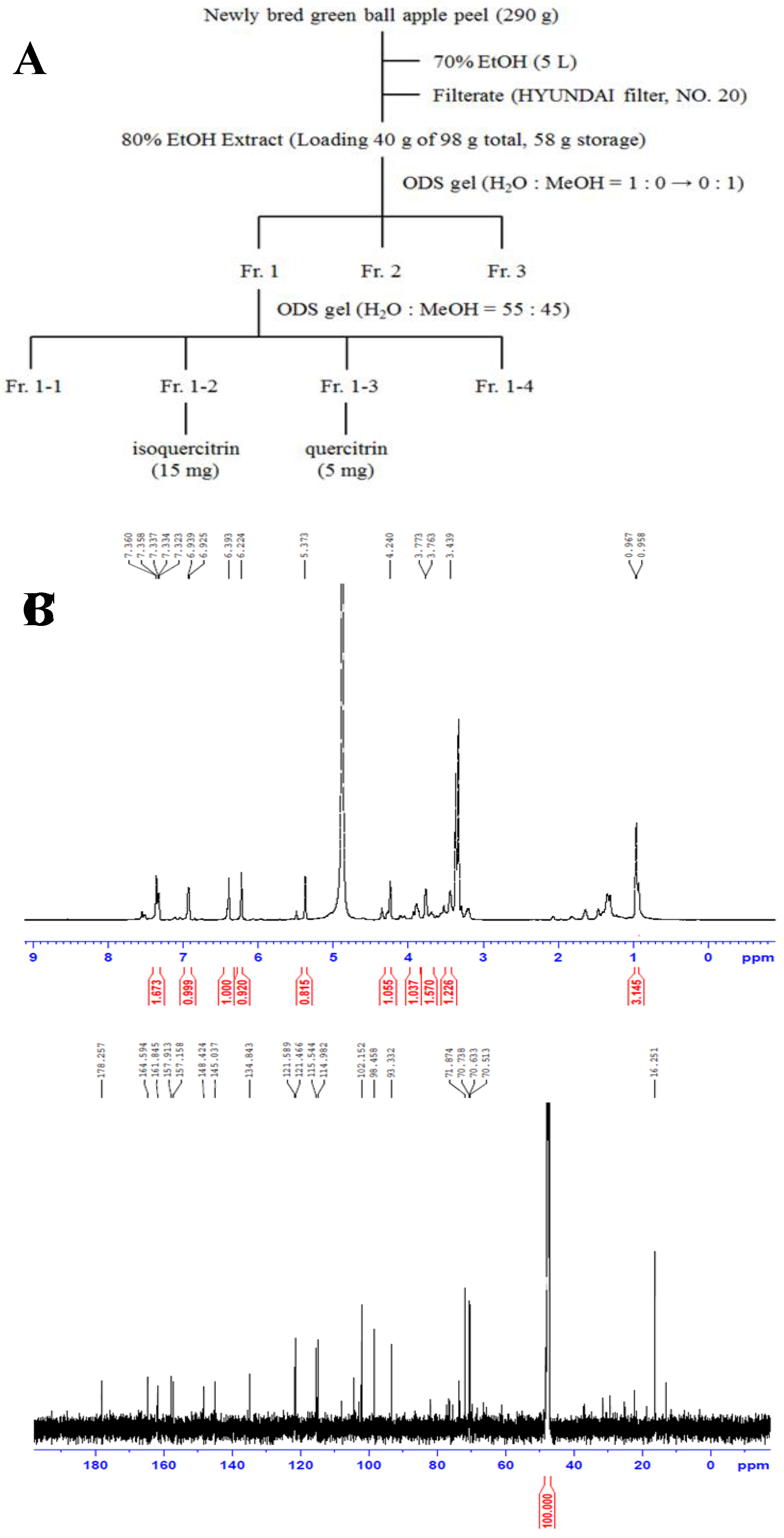
2.1. Plant Material
2.2. Green Ball ApplePpeelEextraction
2.3. Isolation and Identification of Bioactive Substances
2.4. Cell Culture and Maintenance
2.5. Stimulation and Quercitrin Treatment
2.6. Cytotoxicity Measurement by MTT Assay
2.7. Western Blot Assay
2.8. Cytoplasmic and Nuclear Fractionation
2.9. Measurement of Nitric Oxide (NO) Production
2.10. Measurement of Suppression of mRNA Expression by Real Time-PCR
| Gene | Accession | primer | Sequence (5' -3') | Amplicon (bp) |
| COL1A2 | NM_000089.3 | Forward | AGAAACACGTCTGGCTAGGAG | 105 |
| Reverse | GCATGAAGGCAAGTTGGGTAG | |||
| MMP-1 | NM_002421.4 | Forward | TGGGAGGCAAGTTGAAAAGC | 135 |
| Reverse | CATCTGGGCTGCTTCATCAC | |||
| MMP-9 | NM_004994.3 | Forward | CCTGGGCAGATTCCAAACCT | 172 |
| Reverse | GTACACGCGAGTGAAGGTGA | |||
| HAS2 | NM_005328.3 | Forward | GAGGACGACTTTATGACCAAGAGC | 121 |
| Reverse | TAAGCAGCTGTGATTCCAAGGAGG | |||
| TGFB1 | NM_000660.6 | Forward | CAATTCCTGGCGATACCTCAG | 86 |
| Reverse | GCACAACTCCGGTGACATCAA | |||
| TIMP-1 | NM_003254.3 | Forward | CTTCTGCAATTCCGACCTCGT | 79 |
| Reverse | ACGCTGGTATAAGGTGGTCTG | |||
| TNF-α | NM_001278601.1 | Forward | TCTACTGAACTTCGGGGTGA | 87 |
| Reverse | AGGGTCTGGGCCATAGAACT | |||
| IL-1β | NM_008361.4 | Forward | CAACCAACAAGTGATATTCTCCATG | 152 |
| Reverse | GATCCACACTCTCCAGCTGCA | |||
| IL-6 | NM_031168.2 | Forward | TAGTCCTTCCTACCCCAATTTCC | 76 |
| Reverse | TTGGTCCTTAGCCACTCCTTC | |||
| MCP-1 | NM_011333.3 | Forward | TTCCTCCACCACCATGCAG | 64 |
| Reverse | CCAGCCGGCAACTGTGA | |||
| PTGES2 | NM_133783.2 | Forward | CCGTGAGAAGGACTGAGATC | 162 |
| Reverse | AAGTGATGACCTCTTCCAGG | |||
| GAPDH | NM_008084.3 | Forward | TGCACCACCAACTGCTTAGC | 87 |
| Reverse | GGCATGGACTGTGGTCATGAG | |||
| β-actin | NM_007393.4 | Forward | CGTGCGTGACATCAAAGAGAA | 137 |
| Reverse | GCTCGTTGCCAATAGTGATGA |
2.11. Statistical Analysis
3. Results
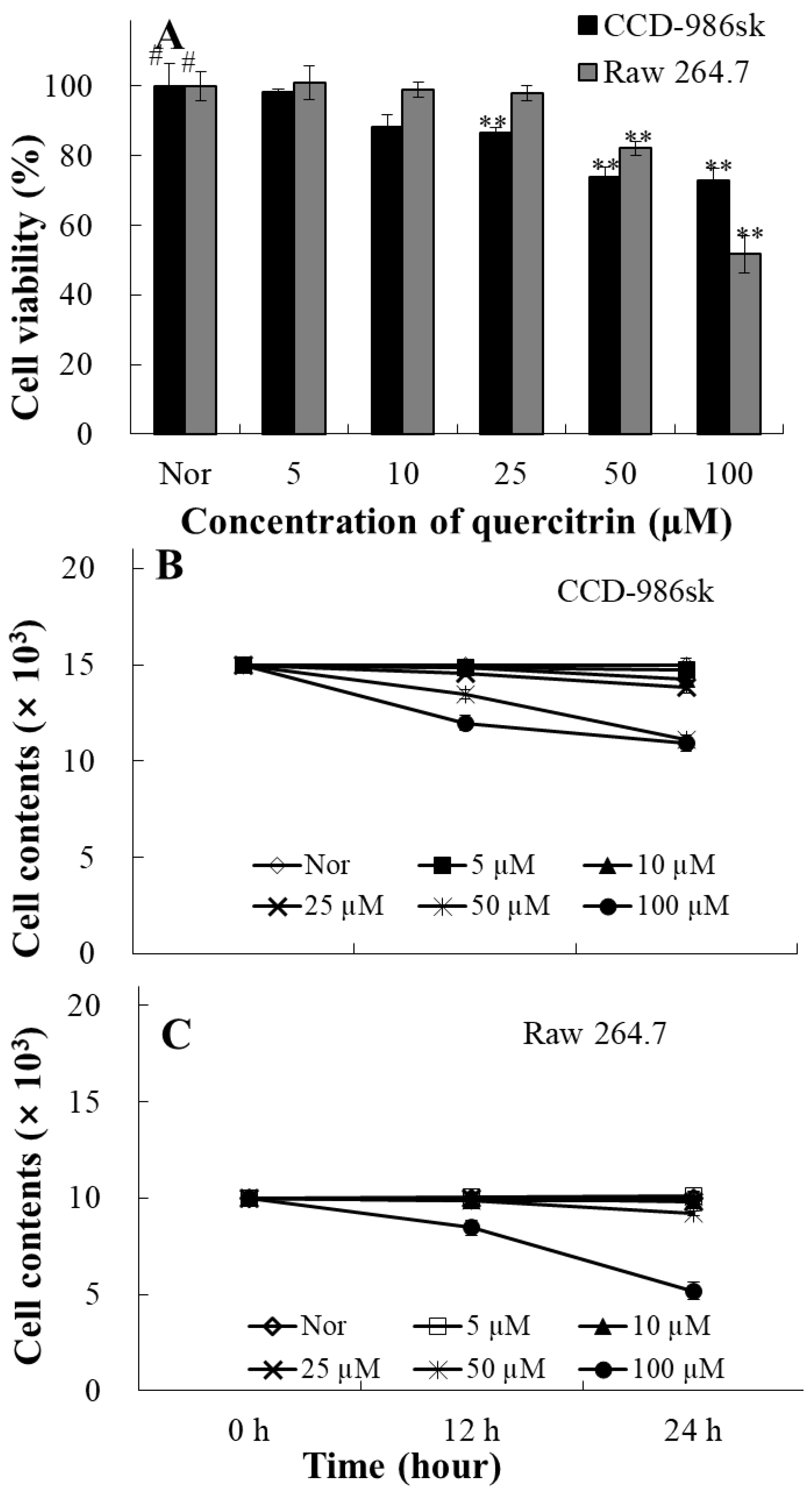
3.1. MTT Assay of Quercitrin on CCD-986sk Cells and Raw 264.7 Cells
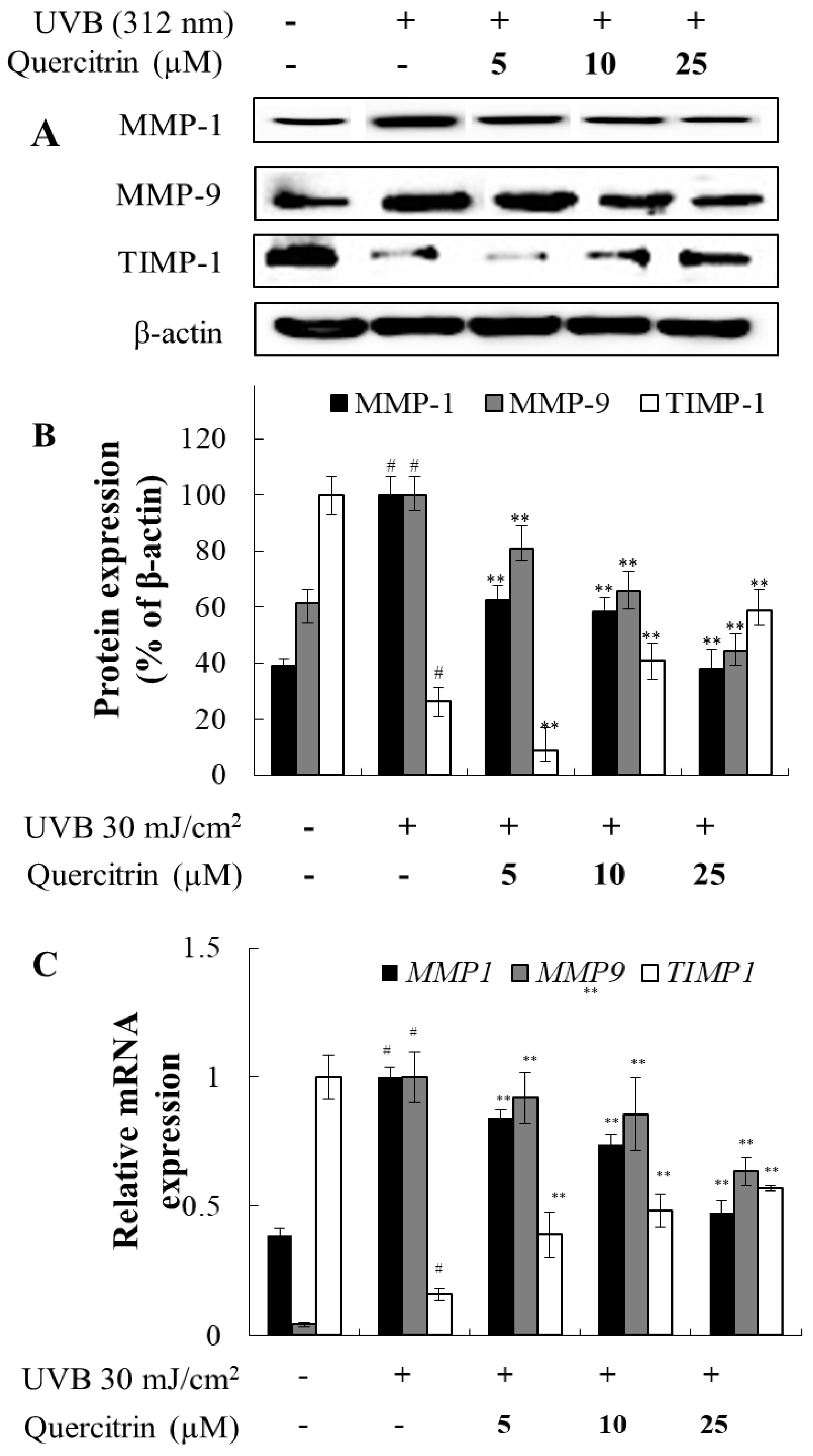
3.2. Effects of Quercitrin on MMPs and TIMP-1 Expression in UVB-Irradiated CCD-986sk Cells
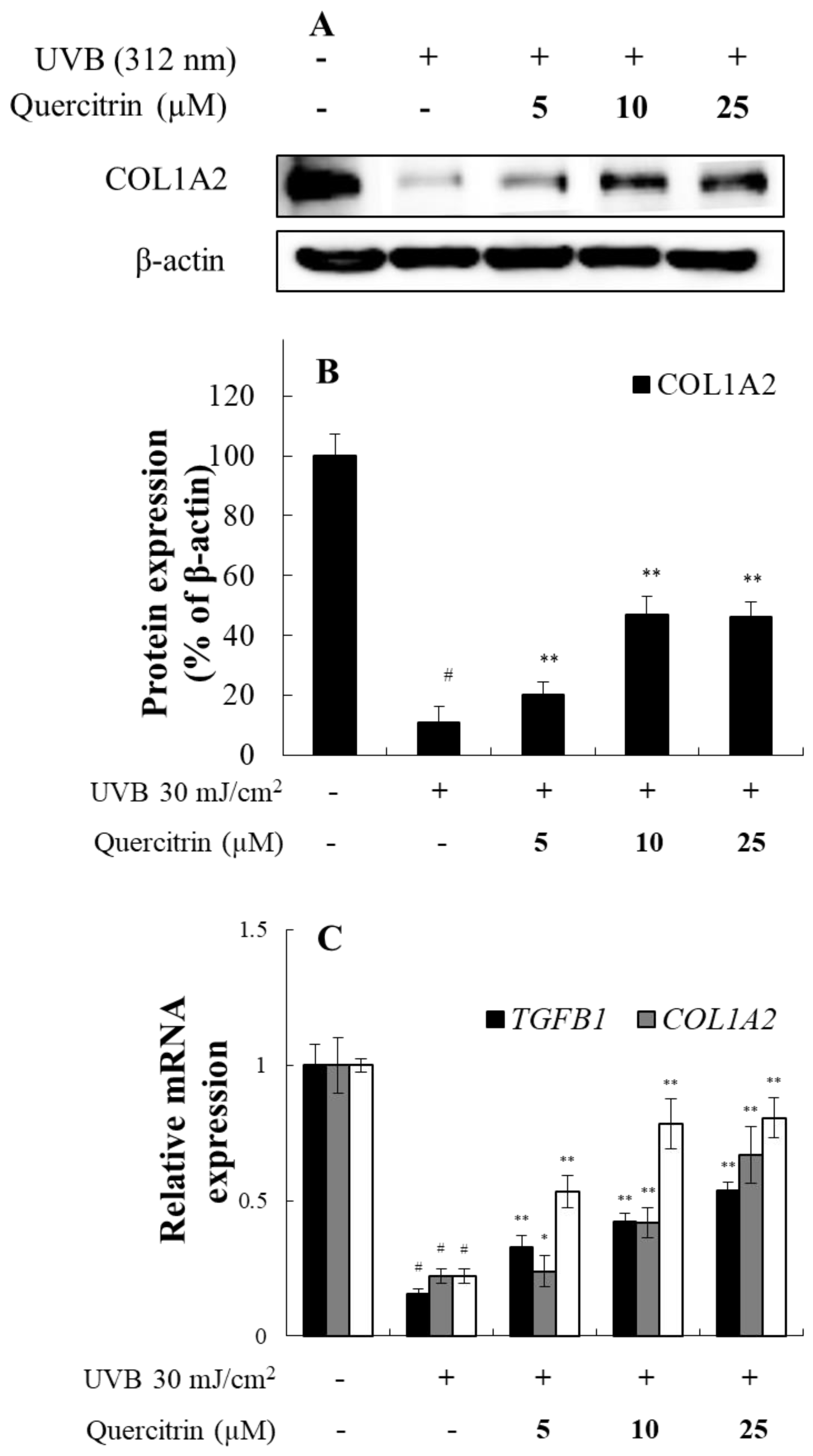
3.3. Effects of Quercitrin on COL1A2, HAS2, and TGFB1 Expression in UVB-Irradiated CCD-986sk Cells
3.4. Effects of Quercitrin on NF-κB Signaling Pathway in LPS-Stimulated Raw Cells
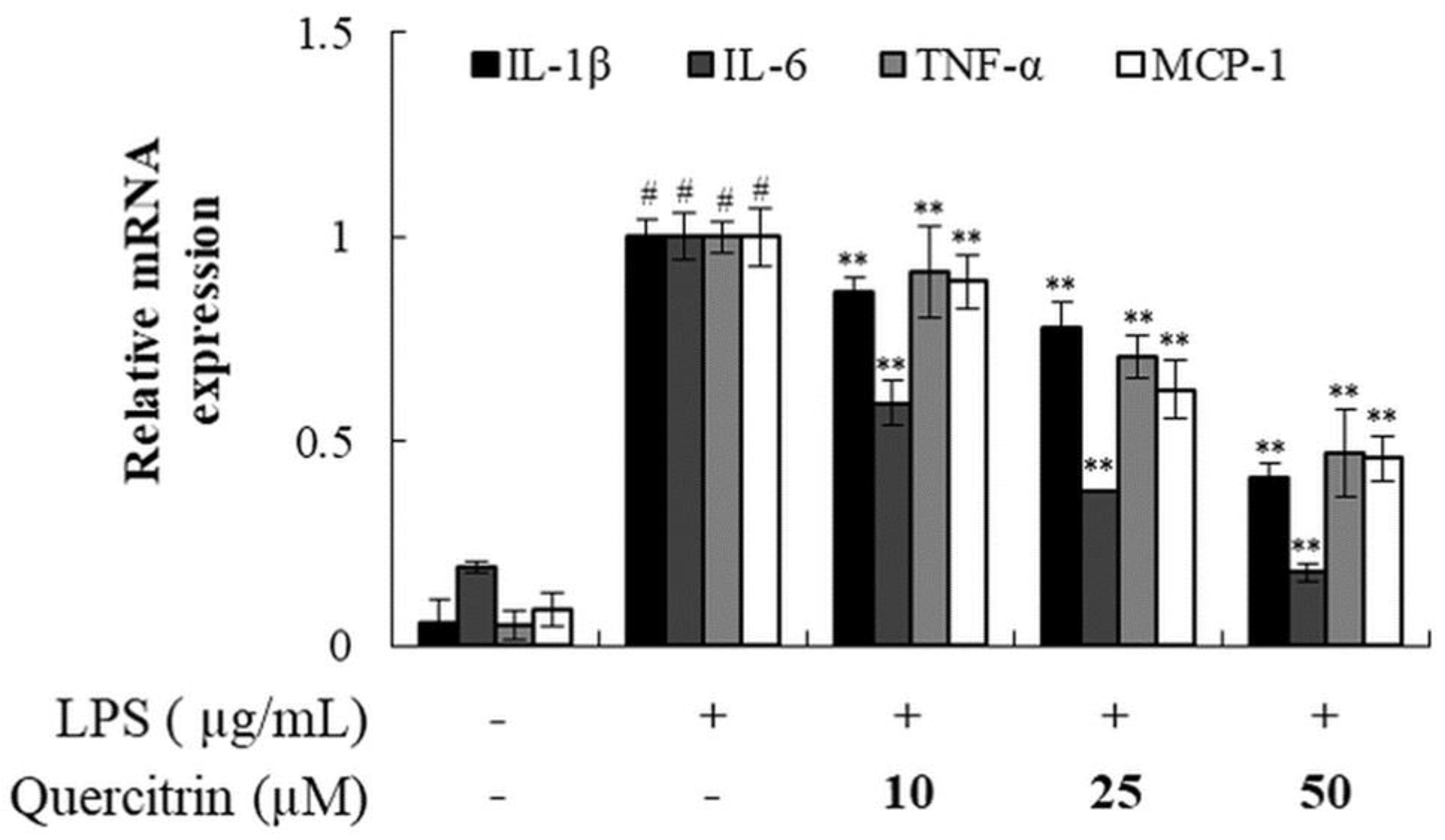
3.5. Effects of Quercitrin on Cytokines and Chemokine Expression in LPS-Stimulated Raw Cells
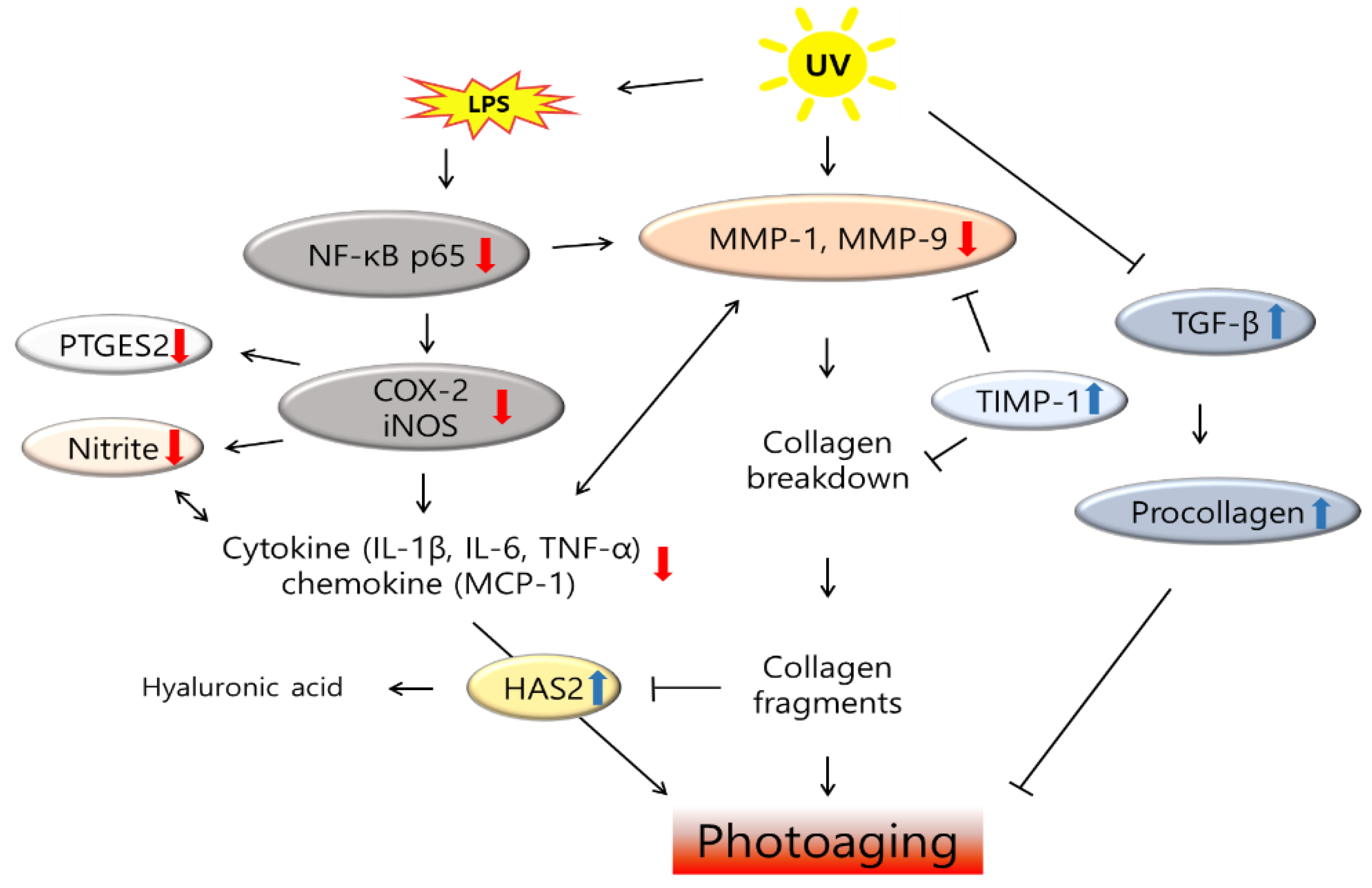
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aguirre, L.; Portillo, M.P.; Hijona, E.; Bujanda, L. Effects of resveratrol and other polyphenols in hepatic steatosis. World J. Gastroenterol. 2014, 20, 7366–7380. [Google Scholar] [CrossRef] [PubMed]
- Andoh, T.; Nagasawa, T.; Satoh, M.; Kuraishi, Y. Substance P induction of itch associated response mediated by cutaneous NK1 tachykinin receptors in mice. J. Pharmacol. Exp. Ther. 1998, 286, 1140–1145. [Google Scholar] [PubMed]
- Bosch, R.; Philips, N.; Suarez-Perez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; Gonzalez, S. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Forslind, B. A domain mosaic model of the skin barrier. Acta Derm. Venereol. 1994, 74, 1–6. [Google Scholar] [CrossRef]
- Hosoi, J.; Abe, E.; Suda, T.; Kuroki, T. Regulation of melanin synthesis of B16 mouse melanoma cell by 1a,25-dihydroxyvitamin D3 and retinoic acid. Cancer Res. 1985, 45, 1474–1478. [Google Scholar]
- Ikoma, A.; Steinhoff, M.; Stander, S.; Yosipovitch, G.; Schmelz, M. The neurobiology of itch. Nat. Rev. Neurosci. 2006, 7, 535–547. [Google Scholar] [CrossRef]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, anti-inflammatory, and anti-aging properties of mycosporine-like amino acids: Molecular and Cellular Mechanisms in the Protection of Skin-aging. Mar. Drugs. 2019, 17, 222. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.B.; Yun, J.G.; Hwang, J.K. Protective effects of standardized Siegesbeckia glabrescens extract and its active compound kirenol against UVB-induced photoaging through inhibition of MAPK/NF-κB pathways. J. Microbiol. Biotechnol. 2017, 27, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, Y.M.; Ahn, K.M. Effect of the indoor environment on atopic dermatitis in children. Allergy Asthma Respir. Dis. 2020, 8, 175–183. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Cho, E.B.; Kim, B.O.; Jung, H.Y.; Lee, S.Y.; Yoo, J.G.; Kang, I.K.; Cho, Y.J. Functional properties of newly-bred ‘Summer King’ apples. Hortic. Sci. Technol. 2020, 38, 405–417. [Google Scholar] [CrossRef]
- Lee, E.H.; Cho, J.H.; Kim, D.H.; Hong, S.H.; Kim, N.H.; Park, M.J.; Hong, E.J.; Cho, Y.J. Anti-inflammatory activity of manassantin A from ultra-fine ground Saururus chinensis in lipopolysaccharide-stimulated RAW 264.7 cells. Appl. Biol. Chem. 2017, 60, 63–71. [Google Scholar] [CrossRef]
- Lee, J.C.; Lee, E.; Oh, H.; Yoon, H.S.; Ha, T.K.; Hong, E.H.; Lee, Y.C. Effects of Fructus foeniculi extract on recovering liver function. Kor. J. Herbol. 2007, 22, 213–218. [Google Scholar] [CrossRef]
- Lee, Y.R.; Noh, E.M.; Han, J.H.; Kim, J.M.; Hwang, J.K.; Hwang, B.M.; Chung, E.Y.; Kim, B.S.; Lee, S.H.; Lee, S.J. Brazilin inhibits UVB-induced MMP-1/3 expressions and secretions by suppressing the NF-κB pathway in human dermal fibroblasts. Eur. J. Pharmacol. 2012, 674, 80–86. [Google Scholar] [CrossRef]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef]
- Puglia, C.; Offerta, A.; Saija, A.; Trombetta, D.; Venera, C. Protective effect of red orange extract supplementation against UV-induced skin damages: Photoaging and solar lentigines. J. Cosmet. Dermatol. 2014, 13, 151–157. [Google Scholar] [CrossRef]
- Quan, T.; Shao, Y.; He, T.; Voorhees, J.J.; Fisher, G.J. Reduced expression of connective tissue growth factor (CTGF/CCN2) mediates collagen loss in chronologically aged human skin. J. Investig. Dermatol. 2010, 130, 415–424. [Google Scholar] [CrossRef]
- Rittie, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Shiga, A.; Yoshida, Y.; Furuhashi, T.; Fugita, Y.; Niki, E. Effects of novel gaseous antioxidative system containing a rosemary extract on the oxidation induced by nitrogen dioxide and ultraviolet radiation. Biosci. Biotechnol. Biochem. 2004, 68, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.; Garcia, C.C. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.Y.; Simonyi, A.; Sun, G.Y. The “French Paradox” and beyond: neuroprotective effects of polyphenols. Free Radic. Biol. Med. 2002, 32, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Tsareva, S.A.; Moriggl, R.; Corvinus, F.M.; Wiederanders, B.; Schutz, A.; Kovacic, B.; Friedrich, K. Signal transducer and activator of transcription 3 activation promotes invasive growth of colon carcinomas through matrix metalloproteinase induction. Neoplasia 2007, 9, 279–291. [Google Scholar] [CrossRef]
- Willenbrock, F.; Murphy, G. Structure-function relationships in the tissue inhibitors of metalloproteinases. Am. J. Respir. Crit. Care Med. 1994, 150, S165–S170. [Google Scholar] [CrossRef]
- Yun, H.J.; Lim, S.Y.; Hur, J.M.; Jeong, J.W.; Yang, S.H.; Kim, D.H. Changes of functional compounds in, and texture characteristics of, apples, during post-irradiation storage at different temperatures. Korean J. Food Preserv. 2007, 14, 239–246. [Google Scholar]
- Zhu, J.X.; Wang, Y.; Kong, L.D.; Yang, C.; Zhang, X. Effects of Biota orientalis extract and its flavonoid constitutions, quercetin and rutin on serum uric acid levels on oxonate-induced mice and xanthine dehydrogenase and xanthine oxidase activities in mouse liver. J. Ethnopharmacol. 2004, 93, 133–140. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





