1. Introduction
The circular economy is a relatively new production-consumption model that focuses on recycling, remanufacturing and repairing materials, thereby extending the life cycle of products and reducing waste generation. Closing the material and energy cycle can help protect the environment by minimizing the use of natural resources and reducing greenhouse gas emissions. Product and processing modifications can contribute to innovation and competitiveness in various industrial sectors, economic growth, create new workplaces, which pays off for both entrepreneurs and consumers [
1]. Due to the aforementioned significant environmental and economic benefits, the European Union is encouraging economic transformation. This is further prompted by the fact that most raw materials are imported into the EU, and recycling waste could alleviate issues related to availability and price [
2].
1.1. Closing the Loop in Tanning Waste
For years, the tanning industry has been struggling with the problem of waste, due to its huge amount, type as well as diversity of composition. Changing from a linear to a circular model would reduce costs associated with waste disposal and emissions control, and improve the public image of the tanning business as more environmentally friendly [
1]. Solid tannery wastes can be an energetic secondary feedstock because of their high heating value (17-20 MJ/kg), but high-temperature treatment can lead to the release of nitrogen oxides [
3] or the formation of toxic Cr(VI) [
4]. Tannery shavings and sludge have potential to be reused to produce biogas, which could meet growing energy needs and simultaneously reduce fossil fuel consumption [
5]. Tannery waste is a source of fats, which can be utilized in biofuel production using supercritical ethanol [
6]. It is possible to obtain proteases from tannery waste that find applications in medicine, biotechnology or the food industry. For this purpose, the waste is subjected to biological degradation using Synergistes sp., Pseudomonas aeruginosa, Selenomonas ruminantium [
7]. Treating the remains of goat skins with organic acids (such as acetic or propionic acid) can provide type I collagen, which is a valuable substance in pharmacy and cosmetics [
8]. Collagen from leather waste can also be a component of hybrid polymer films for medical applications [
9], an ingredient in high-strength composites with Al2O3 [
10], and even building materials that retard the bonding of gypsum [
11]. The agricultural sector can also be a recipient of processed tannery waste. Tanning waste provide a renewable source of organic nitrogen (protein), which can be converted to short peptides and free amino acids through hydrolysis [
12]. Amino acids such as proline, glycine, arginine, lysine, valine, aspartic acid and glutamic acid can be present in hydrolyzed leather trimmings, which promote healthy plant development [
13].
Different treatment methods (thermal, biological, chemical) recover valuable components from tannery residues, and generate new and useful products for various industries. However, the ongoing fertilizer crisis (related to the availability of raw materials, the high price of natural gas and, consequently, the high price of commercial products) makes the exploration of alternative raw materials for the production of agrochemicals seem particularly important [
14].
1.2. Hydrolysis and/ or Carbonization?
Hydrolysates based on leather waste are an intermediate for the preparation of fertilizers. They are obtained by the results of temperature, enzymes or chemicals. The last type of hydrolysis is most frequently practiced because it is inexpensive, easy and quick [
15]. Various mineral acids (H
2SO
4, H
3PO
4, HCl), organic acids (acetic, citric), acid mixtures or alkali (mainly NaOH or CaOH) are employed as hydrolyzing agents [
16]. Considering the fertilizer direction of waste valorization, it is most beneficial to apply phosphoric acid(V) or potassium hydroxide for treatment. The achieved hydrolysate will be a more valuable product, rich not only in nitrogen (in the form of amino acids and short peptides), but also in the essential macronutrients P and K [
12]. Fertilizers based on hydrolysate from wet blue shavings mixed with poultry bone meal and hyacinth ash [
17], or by mixing hydrolysate with ash (from biomass combustion or wastewater treatment plants) have been successfully produced [
12]. Fertilizer was also made as compost from tannery sludge mixed with manure and straw [
18]. Carbonization of tanned leather waste fraction via pyrolysis was already studied, but the application of wet-greens as a novel feedstock material, amendment with biochars followed by the microbial treatment, constitutes a novel approach.
The valorization of tanning wastes for fertilizer purposes is not yet widely explored. Residues from tanning leather with chromium(III) salts are mainly reused for the production of agrochemicals [
19]. After fertilizer application, chromium can be released into the soil and accumulate there or in the plant, causing a threat to the environment and future food consumers. In vivo usefulness studies (pot tests, greenhouse tests, field tests) are particularly important in this case. They will provide information about the assimilation of nutrients from the fertilizer into the plant, and also verify the possibility of transferring hazardous contaminants into the environment. Additionally, the tanning industry is opening up to green technologies and using plant extracts for leather processing. There is a lack of information on their potential use in the agricultural sector.
1.3. The Objective of the Study
The objective of this study was to investigate the effects of various tannery waste-based fertilizers on ryegrass, specifically focusing on their impact on nitrogen use efficiency, plant growth, and soil properties. This was carried out by conducting a five-month greenhouse study evaluating the effects of various tannery waste-based fertilizers on ryegrass. In this study, 7 fertilizers were applied, including acid hydrolysates of shavings (containing or not containing chromium), as well as wet-green shavings (alone, with biochar from "wet white" shavings, or with biochar and a consortium of microorganisms), which is the novelty. Yield and nitrogen uptake were checked at monthly intervals to assess total nitrogen consumption and growth dynamics of ryegrass over the harvest. In this study, an innovative approach of utilizing tannery waste shavings by preparing different types of hydrolysates and treating wet-greens was applied. The new organic nitrogen-based fertilizers were evaluated for nitrogen use efficiency. This study intends to show the significant potential for the use of tannery waste-based fertilizers in improving nitrogen use efficiency in ryegrass cultivation. This work lays the groundwork for further investigations into sustainable and cost-effective agricultural practices, contributing to the broader efforts in moving towards a circular economy. Taking into account the quantitative aspects of global tannery industry production, where only ca. 20% of the feedstock material (bovine shavings) is transferred to the final product, any processing of the waste stream accounting for the remaining 80% is valuable.
2. Materials and Methods
A series of in vitro tests (such as extraction tests simulating soil solution, for example, sodium citrate and water solubility) and in vivo plant tests are used in a standardized approach to evaluating the functional properties of fertilizers. The in vivo sequence includes germination tests (which serve as initial dose-effect studies of functional properties), pot trials, and field trials where the crop yield-generating effect is tested under real conditions. This standardized approach allows the comparison of the obtained results with those of other authors. It also complies with the law on fertilizer registration.
The effect of tanned waste fractions on the growth of ryegrass was studied in pot experiments performed in a glasshouse in Gdynia Wiczlino, Northern Poland (54.5048 N, 18.4038 E). The study lasted from April 2022 to September 2022.
2.1. Fertilisers
Several types of bovine leather waste were used in the research. The mentioned waste is generated during the processing of tanned leather for upholstery purposes in BADER Polska sp. z o.o. located in Bolesławiec, south-west Poland. The global BADER group covers 20% of the automotive upholstery market worldwide. The process of preparing the leather waste for use as fertilizer is critical to our study. We started by collecting bovine leather waste from BADER Polska LtD. This waste is generated during the processing of tanned leather for upholstery purposes. To improve the fertilizing properties of the leather waste, we implemented a pretreatment process. A detailed description of the fertilisers used in tests is presented in
Table 1. Seven fertiliser materials were investigated in a glasshouse experiment and compared with the commercially available mineral NP fertiliser.
Table 1 below shows the specification of materials and pre-treatments used. Finally,
Table 2 shows the descriptions of the pre-treatments. The pretreatment involved both hydrolysing the shavings with a mixture of acids (below) and amending the wet-greens with biochar derived from wet-whites (shavings free of Cr) and additionally with a commercial microbial consortia (BactoFos).
HC and HFOC: Shavings were hydrolysed with a mixture of the following acids: phosphoric (V) (12%), fumaric (2%), oxalic (1.7%), and citric (1.7%). 200 g of the solution and microelement sulfate salts (Cu, Zn, Mn) in the amount of 0.25% were added to 200 g of shavings and left for 1 h at a temperature of 100 ° C. The pH of the prepared hydrolysate was corrected with potassium hydroxide to pH 2.5. Subsequently, the hydrolysate was granulated with biomass combustion in the ratio of 350 g of suspension solution to 450 g of ash. According to EU legislation, the chromium (VI) content is limited in organic-mineral fertilizers. The maximum chromium (VI) content is 2 mg/kg. Speciation analysis of leather waste fertilizers shows that in most cases the chromium (VI) content in formulations reaches values of <1 mg/kg. Fertilizers based on hydrolysates using sulfuric (VI) acid and phosphoric (V) acid granulated with biomass combustion ashes show acceptable levels of chromium(VI) content [
12,
20].
HYDC and HYDFOC. These hydrolysates were obtained while extracting collagen from shavings using strong HCl to maximise the collagen recovery. Thus, they only constitute just a byproduct after the collagen extraction. This approach was chosen to rather test this process waste as fertiliser without any further pretreatment and compare them with dedicated hydrolysates, described in previous paragraph.
As reference fertiliser an NP mineral fertiliser FLOROVIT (MF) was used: 19.0% (total N); 5.4% (nitrate N), 13.6% (ammonium N); 6.0% (P2O5 in neutral ammonium citrate and water), 3.9% (P2O5 in water); 2.5% (MgO total) 4.0% (Fe total). Total N contents as referred to dry matter of the tanned waste fractions were as follows: HC 11; HFOC 7,5; HYDC 20,37; HYDFOC 18,14; OLIV 63,04; OLIVb 63,04; and OLIVB_bio 63,04 gN/ kg. The FLOROVIT standard (fast effect) was chosen as the most typical mineral fertilizer purchased from widely available garden stores. We know that the phosphorus content in the tannery waste fractions is limited (<0.5% in all fractions tested, except HC, where it was over 8%, because the material was further supplemented).
It was presumed that the plant responds to rich-N organic waste, fertiliser dosages were applied at rates ranging from 20 to 370 kg N/ha to reach the plateau on the N response curve. The levels of N added are shown in
Table 2. It shows the experimental plan expressed by the fertiliser dosage assumption and the amounts of the corresponding calculated nitrogen and fertiliser per pot. It was decided to start with the normal dose (20 kg N/ ha) that is recommended for ryegrass according to the mineral fertiliser requirements. The dose was increased by 50 kg N/ ha, till it reached 170 kg N/ ha, which is the maximum allowed N yearly for natural fertilisers on Polish agricultural land (
Regulation of the Council of 12 Ministers of July 12, 2018 on the adoption of the "Action Program to reduce water pollution with nitrates from agricultural sources and prevent further pollution"). Then the dose was increased further; the last dose was increased by 100 kg N/ ha as referred to the previous one and equalled 370 kg N/ ha in order to reach the plateau of over-fertilisation.
2.2. Soil and Plants
The plants were grown in the <2 mm sieved fraction of a sandy soil mixed with peat in w/w ratio sand:peat = 5:1, which corresponds to v/v ratio 1:1.5. Soil properties were as follows: dry matter (d.m.), 88.77%, organic matter (o.m.), 6.08% , Total Kjeldahl Nitrogen (TKN) 1.32 gN/ kg d.m., Total Phosphorus (TP) 185.58 mgP/ kg d.m., P-Olsen 19.04 mgP/ kg d.m., Total Potassium (TK) 610 mgK/ kg d.m., K-Olsen 64.94 mgK/ kg d.m., pH 8.287, Redox potential 63.8 mV, Electrical conductivity EC 159.7 mS/ cm. Approximately, 1.75 kg of prepared soil was placed in a 14.5 cm internal diameter pot (surface area: 0.0165 m2); supplemental nutrient solutions (except N) were added to each pot according to the recipe: K2SO4 (42 g/L) 12 ml/ pot and 6 ml/ pot in solution: CaCl2·2H2O (90 g/L), MgSO4·7H2O (24 g/L), MnSO4·H2O (6 g/L), ZnSO4·7H2O (5.4 g/L), CuSO4·5H2O (1.2 g/L), H3BO3 (0.42 g/L), CoSO4·7H2O (0.16 g/L), Na2Mo4·2H2O (0.12 g/L). The soil and nutrients were thoroughly mixed in the top 5-cm soil layer.
Then 80 annual ryegrass seeds, that is, 0.5 g (a mixture of lolium perenne 40%, lolium multiflorum-estanzuela 284 20%, festuca rubra 25%, lolium hybridum 15%) were placed in the pots on the surface of the soil and covered with an additional 80 g of soil. Experiments were done in duplicate and the pots were rerandomized each 7 days to eliminate differences in insolation and kept at a constant weight with (DIW), at field capacity (20% g H2O/g soil d.m., that is, approximately 26.4% (cm3 H2O/cm3 soil). Harvesting was carried out every month over a 4-months period by cutting the tops at about 1 cm above the soil surface. The harvested plants were then placed in paper bags and dried at 105 °C until the constant weight.
2.3. Soil and Plant Analysis
Soil samples were analysed for pH, EC and Redox potential (1:5 H
2O) before planting and after the last harvest [
21]. The phosphorus concentration in liquid samples was determined on a portable spectrophotometer (Hach DR3900, Hach Company) using the Hach Method 8048, with mineralization step. Before analysis, water soil samples were filtered on a paper filter, followed with a 0.45 µm syringe-filter. The ryegrass tops after each of the 4 harvests were dried and ground. The samples were analysed for total N. Total Kjeldahl Nitrogen (TKN) was determined by the Kjeldahl method. The samples were digested (SpeedDigester K-436, Büchi) in concentrated H
2SO
4 acid in the presence of a titanium-based catalyst, the next step was the steam distillation step (K-355 distillation unit, Büchi) into boric acid solution with Tashiro indicator, then titrated with HCl acid to measure the released ammonia.
2.4. Theory/ Calculation
Agronomic effectiveness
Absolute agronomic effectiveness (AAE) and relative agronomic effectiveness (RAE) of the materials were calculated for each of the 4 harvests and cumulatively after the end of the experiment, using the total N uptake data (1-4 harvests and the cumulatively) and dry matter yield data (only cumulatively). AAE is expressed by a slope of the best-fit line of the relation between plant N uptake and N application rate and RAE is expressed by the ratio of AAE of the material to AAE of the reference fertiliser. This is a standard method to evaluate the performance of various fertilisers [
22].
Nitrogen use efficiency
Nutrient/ nitrogen use efficiency (NUE) refers to the ability of crops to take up and utilise nutrients for optimal yields, therefore, the concept involves three major processes in plants: uptake, assimilation, and utilisation of nutrients. It is calculated by the difference between nitrogen use in kg N/ ha for a given dose and the nitrogen use in kg N/ ha for the control scenario (without fertiliser) as referred to the input N with the fertiliser applied (kg N/ ha). This parameter is widely used in the similar studies [
23,
24].
2.5. Limitations
The research was performed with the following limitations: (1) carried out in the glasshouse, (2) using small-diameter pots, (3) under semi-controlled meteorological circumstances, (4) for the limited amount of harvests, (5) for one crop and (6) one soil type.
3. Results
3.1. Ryegrass Biomass Yield and Nitrogen Uptake Responses to Applied Waste Based Fertilisers
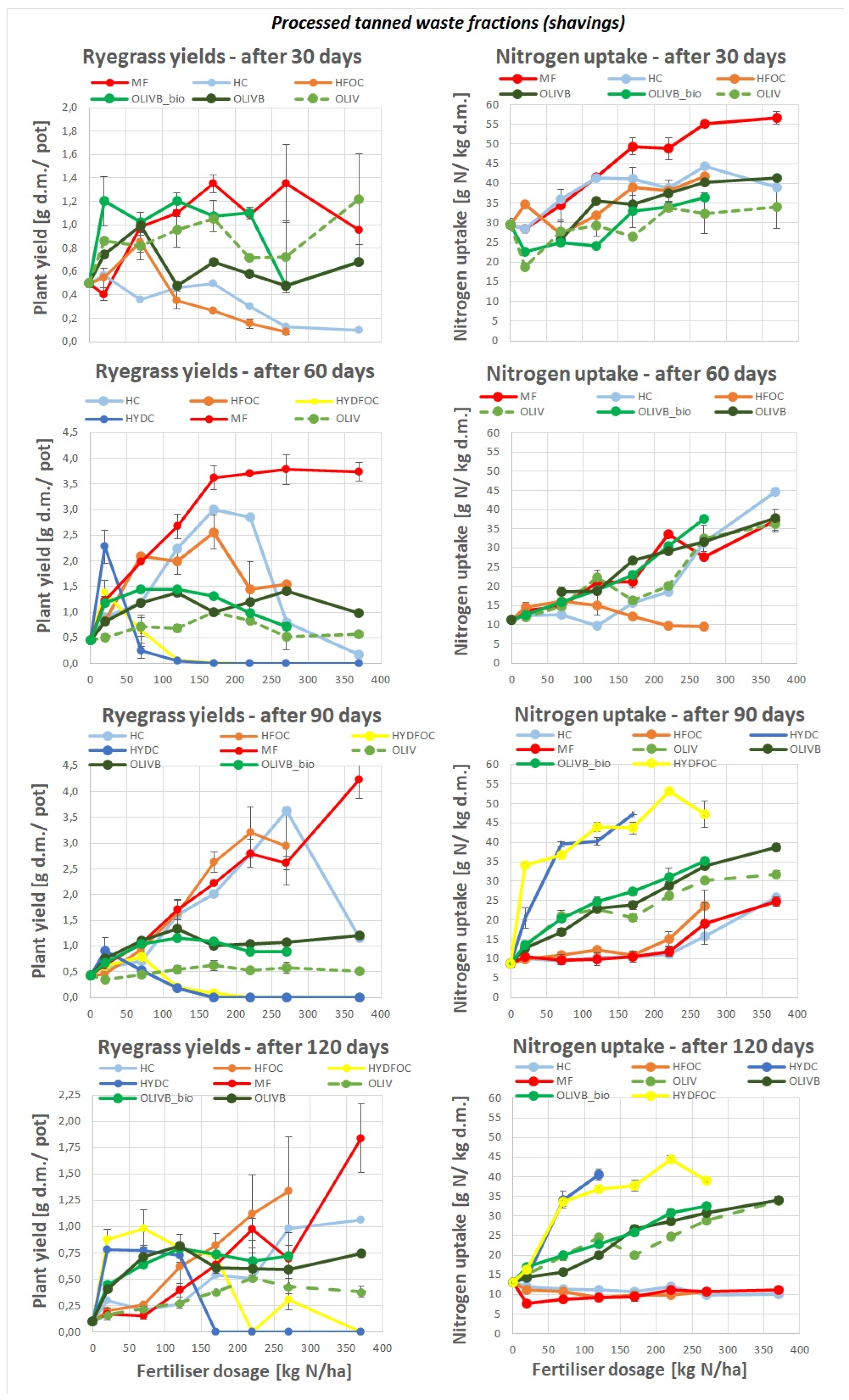
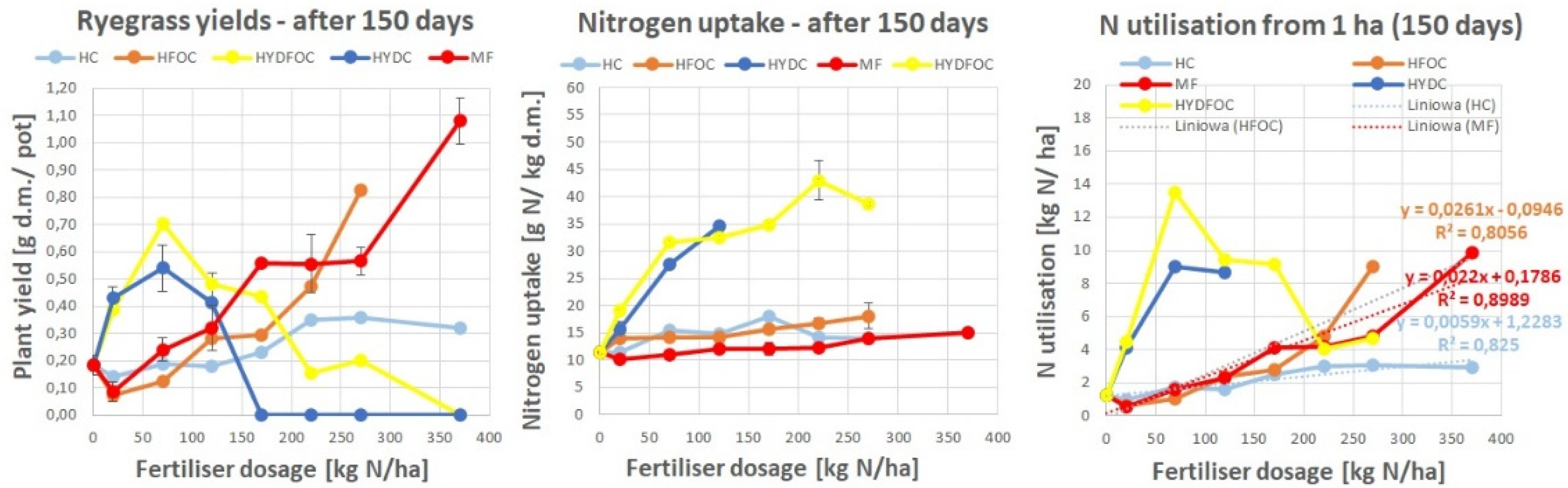
Figure 1.
Ryegrass biomass yields response (left) and nitrogen uptake (right) to tanned leather waste based fertilisers after 4 subsequent harvests, as compared to the mineral fertiliser (MF) for harvests after 30, 60, 90 and 120 days.
Figure 1.
Ryegrass biomass yields response (left) and nitrogen uptake (right) to tanned leather waste based fertilisers after 4 subsequent harvests, as compared to the mineral fertiliser (MF) for harvests after 30, 60, 90 and 120 days.
3.1.1. Dry Matter Ryegrass Yield
Generally, dry grass yields ranged from 1.7 to 4.5 g of dry matter per pot. The maximum yields were reached after 90 days. Only "light" hydrolysates reached the biomass yields comparable to mineral fertilizer after 90 and 120 days whereas wet-green based fertilizers remained at a stable plant yield level in most of the time, not exceeding 1.5 g d.m./ pot. Biochar amended wet-green fertilizers provided up to 3 times better yields than untreated wet-greens expressing the maximum of growth around dosage 120 kg N/ ha. Heavy hydrolysates allowed the growth of biomass later, only after 60 days and further showing maximums for small doses around 70 kg N/ ha with slight advantage of hydrolysates without chromium. These materials provided similar yields to the wet-green ones for smaller doses and exceeded the light hydrolysates-provided yields after 120 days to be even 3 times better than all other materials after 150 days. After a prolonged period, heavy hydrolysates without chromium continued to provide yields after 170 kg N/ha. In contrast, those with chromium completely inhibited growth after this dose, irrespective of the growth time.
These findings indicate that hydrochloric acid, used for heavy hydrolysates, likely dissolved many other pollutants into the soil. The soil likely neutralized part of the elements, providing some availability of nitrogen to the plants, but only for the first half range of doses. For the remaining higher doses, the growth was inhibited.
In general the findings are in line with the hypothesis where hydrolysates behave similarly to mineral fertilizer after certain time and organic materials such wet-green provide smaller yields but in a more stable manner over longer time and the plant response is more stable and less driven by the application dose.
3.1.2. Nitrogen Uptake
Nitrogen concentration in plants dry mass varied from 10 to 55 g N/ha. Mineral fertilizer provided the highest N concentrations among all materials only in the beginning of growth. Then, after 60 days, concentrations for MF and wet-greens fertilised plants were similar and after 90 and 120 days showed the highest amounts for heavy hydrolysates (up to 55 g N/ha), medium range amounts (ca. 30-50% less) for wet-green ones. The lowest (ca. 60-90% less) and the least dosage-dependent amounts were observed for light hydrolysates and mineral fertilizer. The effect of chromium on nitrogen uptake is unclear. Nonetheless, heavy hydrolysates without chromium provided improved nitrogen concentrations, particularly after 120 kg N/ha, and only in the later growth stages after 90 days.
This is in line with dry matter yields which started only after 90 days due to nitrogen uptake. Generally observed in the literature phenomena on fast exploitation of nitrogen source from highly available mineral fertiliser and light hydrolysates is supported. Wet-green based materials with no clear difference between untreated ones and biochar/ biochar and microbes amended ones show moderate increase of nitrogen concentration with dosage application, however still way higher than mineral one and light hydrolysates after 90 days.
3.2. Total Ryegrass Growth Dynamics Across Harvests
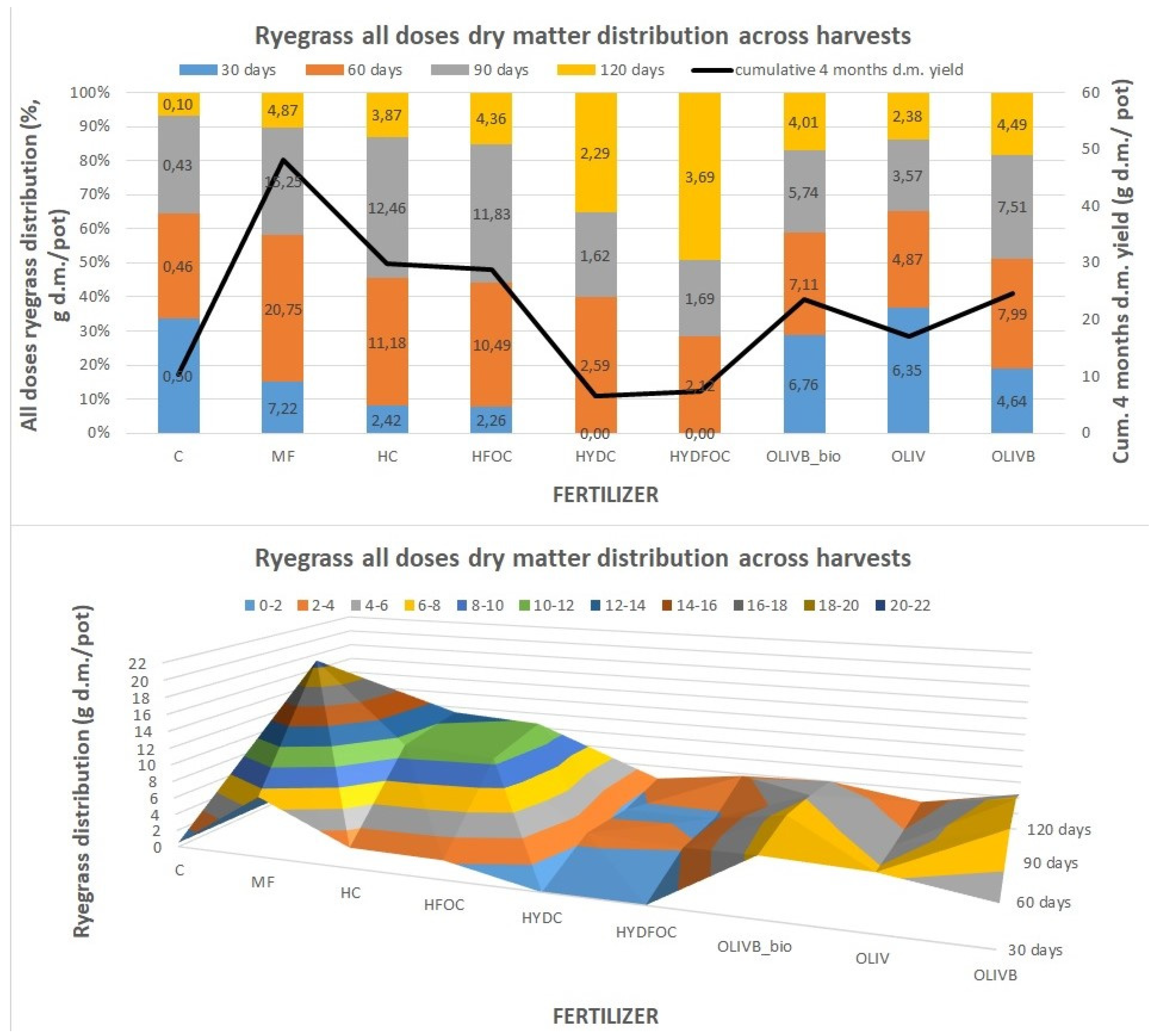
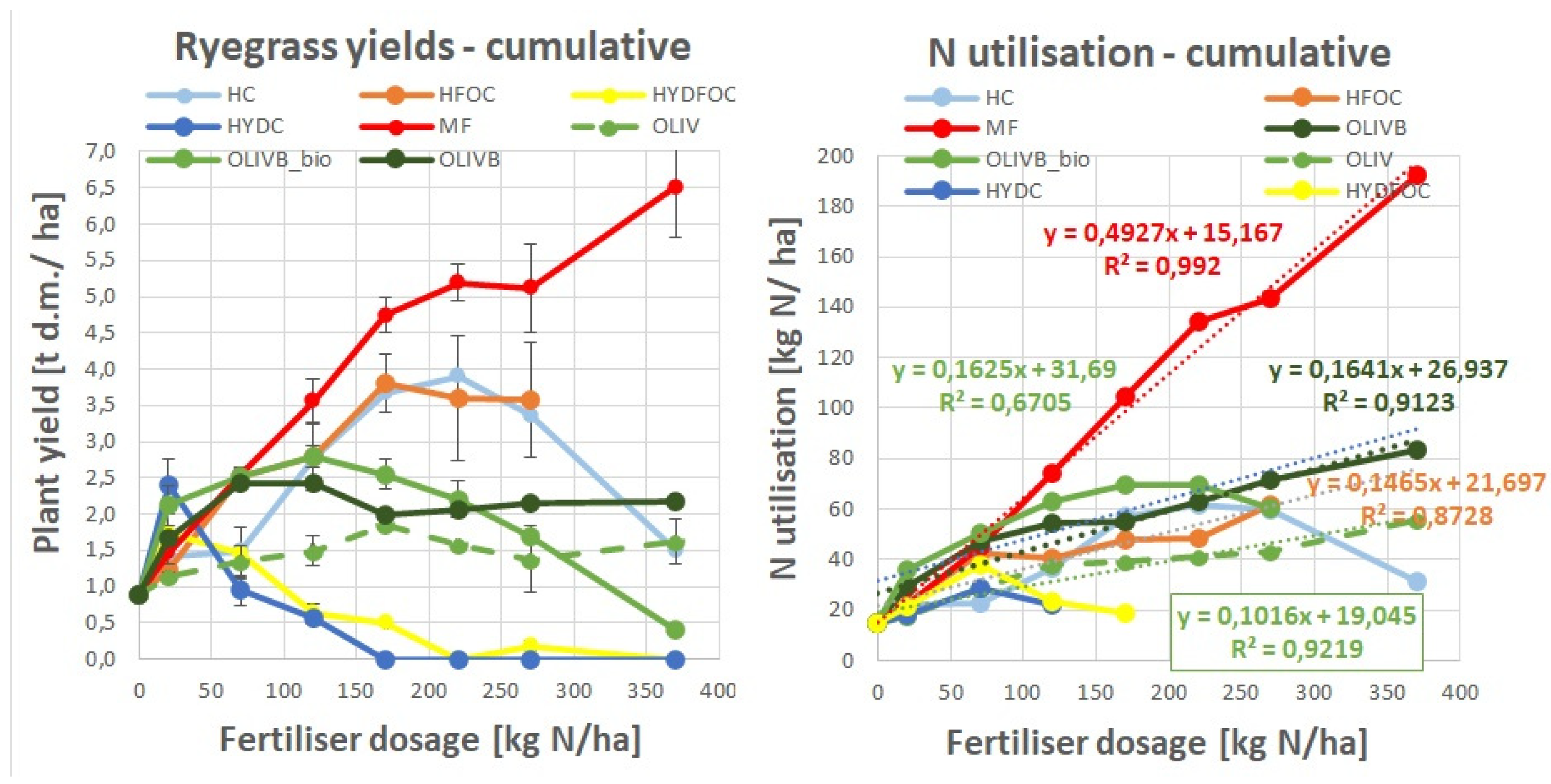
This figure was created to visualise the distribution of ryegrass total dry matter yield across harvests (as a sum for all doses) to show which materials provided the most of the yields in a given month of growth. For mineral fertilizer and light hydrolysates, most of the biomass is generated at month 2 and 3 (70-75%), whereas the rest is formed after month 1 and 4 (25-30%). In total, mineral fertiliser provided 48 g d.m./pot and light hydrolysates provided 30 g d.m./ pot. In contrary wet-greens provide more gradual growth of biomass over three first months providing ca. 20-35% of total biomass each month. Wet greens still provide up to 20% of total biomass in the last month that is relatively even twice more than mineral fertilizer in this month. It seems that the presence of biochar amendment in this treatment shifts the biomass growth to later months as the maximum biomass growth for the month 1 was only observed for untreated wet-greens (38% of total biomass). Biochar amendments and biochar amendments with microbial treatment also provided 60-110% higher yields than untreated wet-greens especially after month 2, 3 and 4. In total, during the first 3 months, wet-greens provided 3,6-8 g d.m./ pot vs. mineral fertiliser and light hydrolysates, providing up to 3 times more during month 2 and 3 (10,5-20,8 g d.m./ pot). No biomass increase was found after month 1 for the heavy hydrolysates, where the most of the growth (up to 50% of the total biomass) was found only at the last month 4, especially for the chromium free hydrolysate. In absolute terms this was from 22 to 23 times higher than control treatment but still lower than most of other materials in this time of growth. The lower part of
Figure 2 visualises the differences in dry matter distribution dynamics between harvests in absolute terms. The total dry matter yield in g d.m./ pot presented from the highest to the lowest values is as follows: MF 48,08; HC 29,93; HFOC 28,93; OLIVB 24,62, OLIVB_bio 23,60; OLIV 17,17; CONTROL 10,4; HYDFOC 7,50; HYDC 6,50.
3.3. Nitrogen Utilisation by Ryegrass
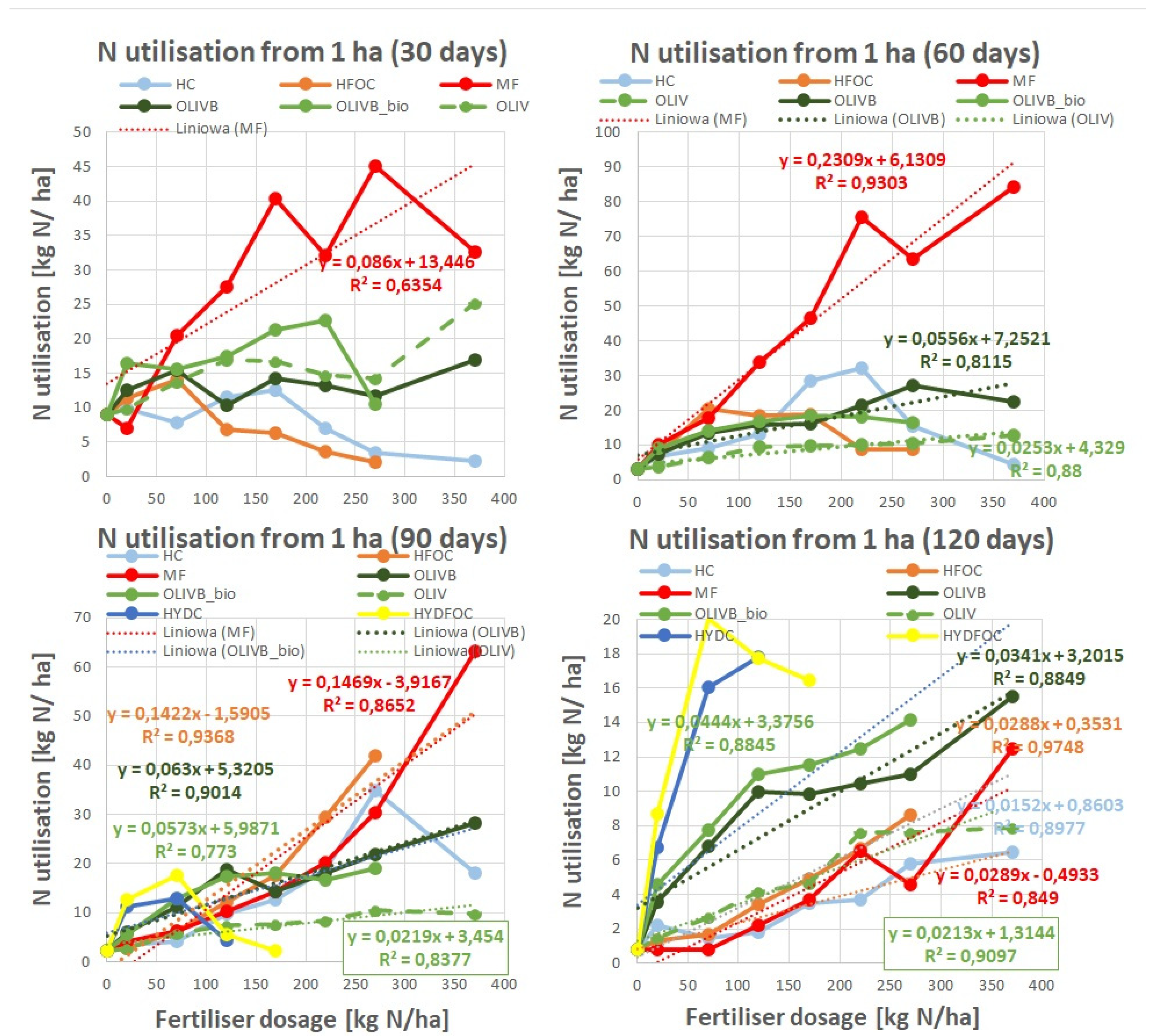
Figure 3 shows nitrogen utilization expressed as total nitrogen extracted by ryegrass per hectare. In general highest amounts of nitrogen (up to 80 kg N/ha) are utilised by plants fertilized with mineral fertilizer after 60 days of growth and they decrease dramatically after 120 days to mostly values less than 10 kg N/ ha. This is in opposition to studied materials that express N utilization in a more gradual manner up to 30 kg N/ ha across first three months to decrease under 20 kg N/ ha after the last month.
The dynamics of N utilization across growth time present as follows. First, wet-greens express up to 2-5 times better N utilization than light hydrolysates after 30 and 120 days. This is especially true for the amendment with microbially treated biochar. After 60 and 90 days, the N utilization of all studied materials, apart from heavy hydrolysates and untreated wet-greens, is more or less the same. Second, chromium-containing light hydrolysates show a maximum of N utilization at 60 days at 220 kg N/ha and for 90 days at 270 kg N/ha. Third, after 90 days, heavy hydrolysates start showing N utilization up to 20 kg N/ha. Compared to others, they still express very high N utilization for small doses (less than 170 kg N/ha) after 120 days. This is up to 2 times higher than biochar amended wet-greens and up to 10 times higher than light hydrolysates, mineral fertilizer and untreated wet-greens, (4) microbiologically treated biochar amendments of wet-greens express highest N utilization among all wet-greens especially after 120 kg N/ha dose after 120 days.
Where the linear regression model was possible to apply, the trend lines were showed with correlation factors and equations on the plots. Absolute agronomic effectiveness (AAE) is expressed by the slope of the best-fit linear regression model for the response of plant growth to fertiliser dosage. This could be expressed as the relationship between (1) N use or (2) dry matter yield and fertiliser dosage. In other words, N-based AAE describes how much nitrogen (in %) is taken up in relation to nitrogen input (introduced and supplied with the fertiliser) across the increasing dosages. Calculating AAE is a common way to assess fertiliser performance throughout the literature and has been found in other studies [
25,
26].
3.4. Total Nitrogen Utilisation Dynamics Across Harvests
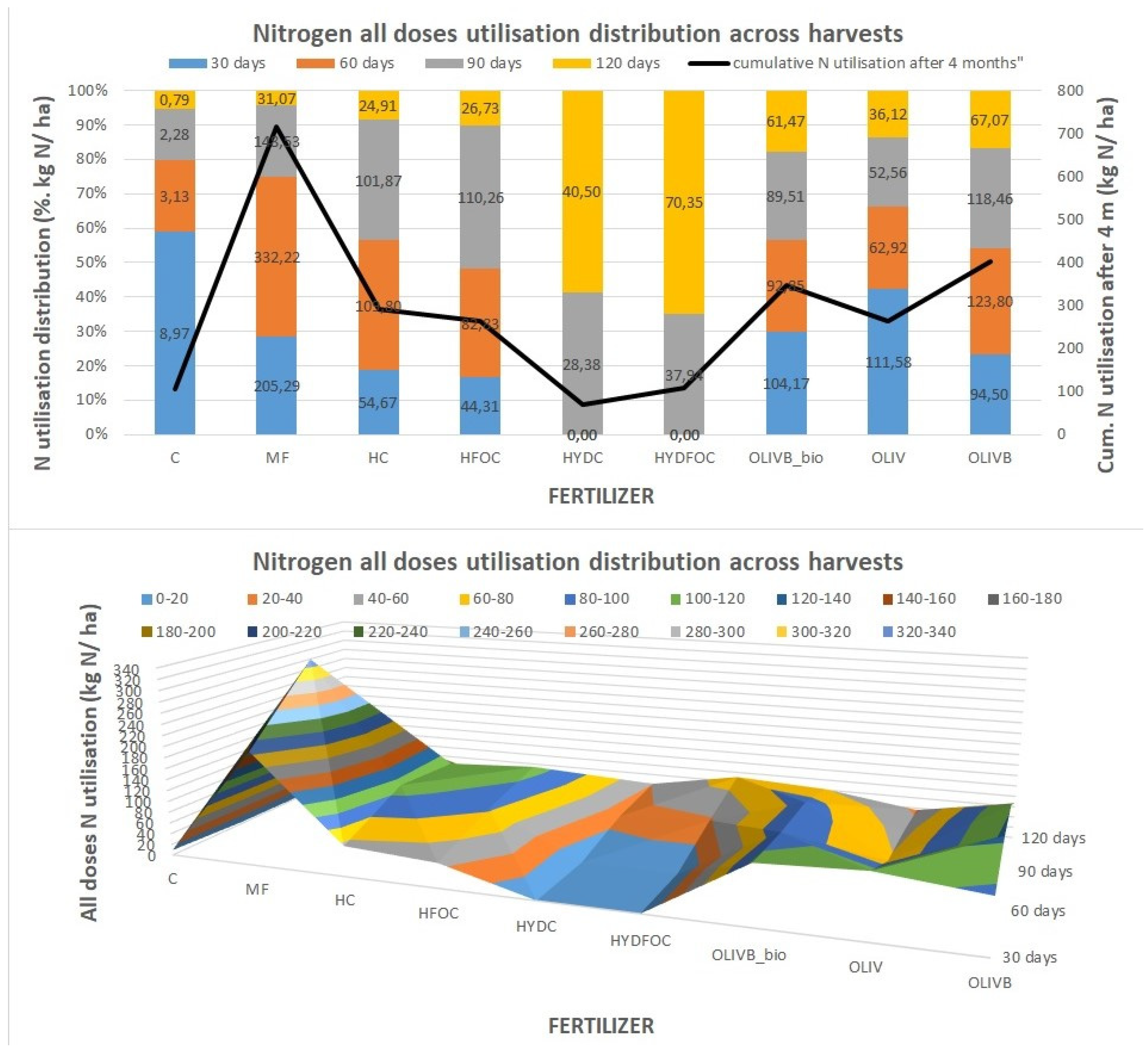
Figure 4 was created to best visualize nitrogen utilization dynamics across months of growth (presented as the sum of all doses N utilisations in each month). It shows that control treatment provides up to 60% of nitrogen utilization very early, already after 30 days, whereas mineral fertilizer represents 30% in the same time and 45% of nitrogen utilized later from 30 to 60 days. Nitrogen utilization from light hydrolysates is more evenly distributed over time with maximums (35-40%) pointing from 60 to 90 days. The difference in nitrogen utilization between chromium containing light hydrolysates and those ones without chromium is insignificant, slightly shifting the N utilization more to month 3 for the non-chromium ones. In general light hydrolysates behave similarly to mineral fertilizer in terms of fast exploitation of the majority of nitrogen during first 3 months, however in the last month these materials still provide up to 10% of N utilisation vs. 3% for the mineral fertilizer. Biochar amendments of wet-greens shifts the nitrogen utilization further to the last three months (70% vs. 58% for pure wet-greens) without significant differences between solely biochar amendment and this amendment after microbial treatment. Nitrogen utilization from soils amended with heavy hydrolysates is strongly retarded due to potential toxicity resulted from strong hydrolysis of many pollutants with HCl. It only shows some measurable numbers after 90 days (35-40%) with the greatest values after 120 days (60-65%) and even after 150 days (only shown on
Figure 5). In absolute terms after month 4 they provided up to 3 times more nitrogen than mineral fertilizer, 2 times more than light hydrolysates and up to 10% more than biochar amended wet-greens. Heavy hydrolysates without chromium provided around 10% more nitrogen utilization after month 3 and up to 80% more nitrogen after month 4 as compared to chromium containing heavy hydrolysates.
Lower part of the
Figure 4 visualises the dynamics of cumulative value of nitrogen utilisation per each harvest. The total N utilisation in kg N/ ha presented from the highest to the lowest values is as follows: MF 717,10; OLIVB 403,84, OLIVB_bio 348,00, HC 291,26; HFOC 264,14; OLIV 263,18; HYDFOC 108,29; CONTROL 106,2; HYDC 68,88. This order is almost in line with the order of total dry matter yields discussed under the
Figure 2 (with the advantage of biochar amended wet greens being before light hydrolysates in N utilisation), evidencing the nitrogen being the main growth stimulating factor.
3.5. Ryegrass Yield, Nitrogen Uptake and Nitrogen Utilisation After Additional Harvest
Because heavy hydrolysates started showing both dry matter yields and nitrogen utilization after 90 and 120 days rapidly, it was decided to keep the growth for these materials for one more month and compare it with the growth on light hydrolysates.
Figure 5 shows three different parameters starting from plant dry matter yield, then nitrogen concentration in plants and finally nitrogen utilization after 150 days of growth. Heavy hydrolysates express maximums for all parameters visible for small doses range below 120 kg N/ ha. The only exception is chromium-free heavy hydrolysate that shows the maximum of nitrogen concentration for 220 kg N/ ha. Plant dry matter yields are up to 7 times higher, nitrogen concentrations up to 3 times higher, nitrogen utilization up to 7 times higher, than scenarios with light hydrolysates. The trend of having better parameters for non-chromium heavy hydrolysates is maintained. It seems like all three parameters follow similar character for mineral fertilizer and light hydrolysates and they are less fertilizer dosage dependent - only for nitrogen uptake and utilization. Chromium containing heavy hydrolysates provide growth parameters only until 120 kg N/ ha dose. After that does, the growth is strongly inhibited by some unidentified pollutants. Another comprehensive study is currently underway to verify the exact compounds responsible for that. The overall conclusion is that small doses of heavy hydrolysates shift the growth parameters to month 3rd and 4th and later to 5th with an increasing tendency proving about high soil self-purification efficiency and mineralization capability of initially plants unavailable compounds and potentially toxic compounds that inhibit the growth after a month 1 and 2.
3.6. Cumulative Ryegrass Yield and Nitrogen Utilisation
Figure 6 summarizes all the data from
Figure 1 (left) referred to plant dry matter yield and from
Figure 3 referred to nitrogen utilization. The cumulative values of plant yield show maximums (4-4,5 g d.m./ pot) of dry matter yield for light hydrolysates around 170 to 270 kg N/ ha being still ca. 20% lower than mineral fertilizer- provided plant yields. Then the response of the plant to wet-greens is up to 3 g d.m./ pot being more stable across doses showing maximums for smaller doses (< 170 kg N/ ha) and making the microbially treated biochar amendments up to 20% better than amendments with only biochar and pure wet-greens being in the last place (< 2 g d.m./ pot). Heavy hydrolysates generate minimum amounts of dry matter only for small doses (< 120 kg N/ ha) what was mentioned earlier. Cumulative values of nitrogen utilization for waste-based materials is rather similar apart from heavy hydrolysates and untreated wet-greens which show clearly lower nitrogen utilization. Where it was possible, linear regression model was applied to show minor differences in the response of N utilisation in a mathematical way. Please note that correlation coefficients higher than 0.87 were mostly found with the only one exception of 0.67 for OLIVB_bio. The regression for chromium containing light hydrolysate was not displayed due to non-linear trend. These values will be used to further calculate relative agronomic effectiveness.
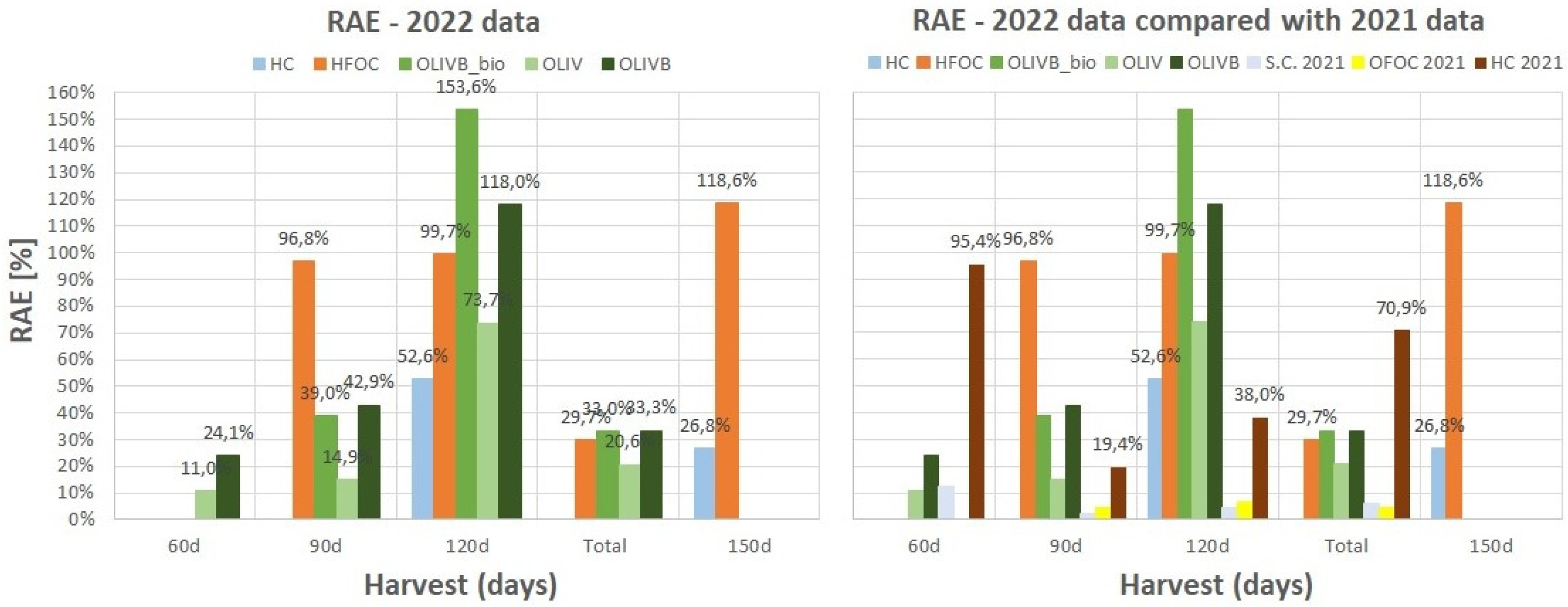
3.7. Relative Agronomic Effectiveness and Nutrients Use Efficiency
Figure 7 summarizes all previously presented data on
Figure 3,
Figure 5 and
Figure 6 by showing the calculated Relative Agronomic Effectiveness (RAE) for the fertiliser materials, where the calculation of AAE was possible. RAE is essentially the AAE compared to the AAE of the reference mineral fertiliser (MF). The RAE based on the utilization of N (RAE(N)) was calculated and presented for each subsequent harvest and also as a total value after adding all harvest data. However, the d.m. yield-based RAE (Y) was calculated only as a total value. The N-utilisation-based RAE(N) better characterizes the fertiliser material (according to authors’ opinion) as it also contains the dry matter yield when calculating the total N-utilisation per area. The RAE values for processed waste-based materials can be determined only for the regressions (
Figure 3,
Figure 5 and
Figure 6) with relatively good correlation coefficient, eg. R
2 > 0,85.
Figure 7 shows relative agronomic effectiveness data based on the linear regressions of nitrogen utilization data from figures 3, 5 (right) and 6 (right). The left plot refers to data from 2022, while the right plot incorporates some data from 2021 for comparison. The highest values were noticed after 120 days reaching 100% and even more for chromium-free light hydrolysates and biochar amended wet-greens, respectively. Microbially-treated biochar amendments of wet-greens exceeded 150% after 120 days followed by chromium-free light hydrolysates and biochar amendments of wet-greens after 120 days reaching almost 120% after 150 days. Chromium containing light hydrolysates were not that efficient and did not exceed 53%. Chromium free light hydrolysates were more consistent in keeping RAE close to 100% over time of growth than biochar amendments of wet-greens, which apart from 120 days growth, did not exceed 43%. Microbial treatment with BactoFos product of biochar amended wet-greens provided up to 30% better RAE than solely biochar amendments. Excluding the RAE values in particular months of growth, their cumulative values for 4 months for the studied materials did not exceed 34% with biochar amended wet-greens having the highest values, followed by chromium-free light hydrolysates.
Comparing the data from 2022 to data for some of the materials studied in 2021 (chromium containing light hydrolysates, ground bovine shavings and ground splits and offcuts) it seems like chromium containing light hydrolysates in 2021 provided much better RAE reaching 95% for 30 days compared to twice poorer data for 2022 reaching as low as 27% for 150 days. These inconsistent results may be due to the heterogeneous structure of the different feedstock materials used by the upholstery industry between 2021 and 2022, or possibly related to the sampling process. As demonstrated in previous study [
27], only mechanical and effective microbial treatment of the leather waste fractions have been found to increase RAE by no more than 10%.
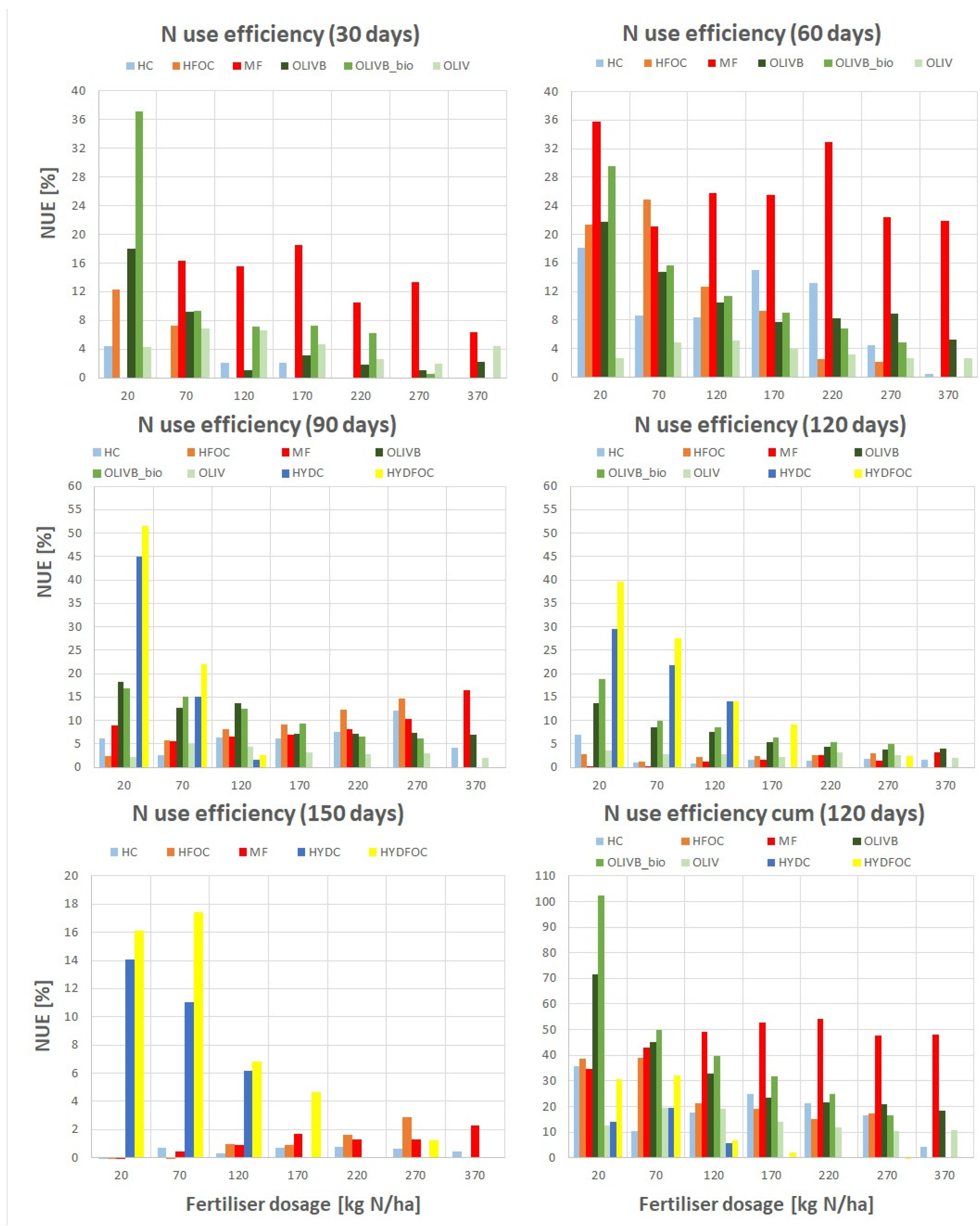
It is important to highlight that relative agronomic effectiveness tries to capture the fertilizer performance taking into account the response of nitrogen utilization to fertilizer dose across all applied doses and calculates a single output value information for the whole trend. However nutrient use efficiency (NUE) represents simpler approach that only shows how much nitrogen is extracted from the agricultural system for the given fertiliser material and given dose as compared to control treatment. This is why NUE are calculated for the single dose separately unlike RAE that tries to capture the whole trend. These differences in approach enable a comprehensive understanding of the fertilizer performance of alternative waste-based materials.
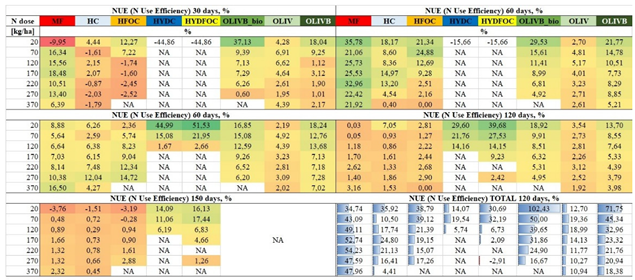
Table 3 shows NUE values for all the studied materials and a reference material for the singular dose in each month of growth and finally the total NUE after the whole experiment (4 months). The colours from dark red to dark green help to visualize the levels of NUE. The highest values were observed for chromium-free heavy hydrolysates, reaching 51,5% after 60 days. However, considering previous observations, these materials perform only at small doses and during later months, which renders their use impractical and potentially toxic. This is also backed up by looking at total NUE where the values did not exceed 32% for chromium-free and 20% for chromium containing ones. Excluding heavy hydrolysates, the best fertilizer material identified was microbially treated biochar amendment of wet-greens reaching 37% after 30 days and in total even over 100% for the smallest dose (20 kg N/ ha). These microbial product provided up to 30-50% more total NUE than biochar amendment of wet-greens solely but only for the small dose (20 kg N/ ha). For higher doses this microbial treatment effect was less visible reaching few % in particular months of growth and up to 20% in total. Pure untreated wet-greens showed the smallest NUE values not exceeding 7% in each harvest and not even 20% in total. Light hydrolysates showed up to 25% of NUE for the advantage of chromium free ones and only after 60 days but in total up to 40% and the total N use efficiency was slowly decreasing over fertilizer dose. The NUE for the mineral fertilizer exceeded 35% for the single month (60 days) and reached 54% in total for the 220 kg N/ ha. In general total NUE values were decreasing with increasing fertilizer dose for the alternative materials whereas for the reference one, the maximum was around 200 kg N/ ha. These also gives a practical information on optimal use of waste materials as fertilisers providing the highest N use efficiencies with the smallest possible amounts of waste being applied on land.
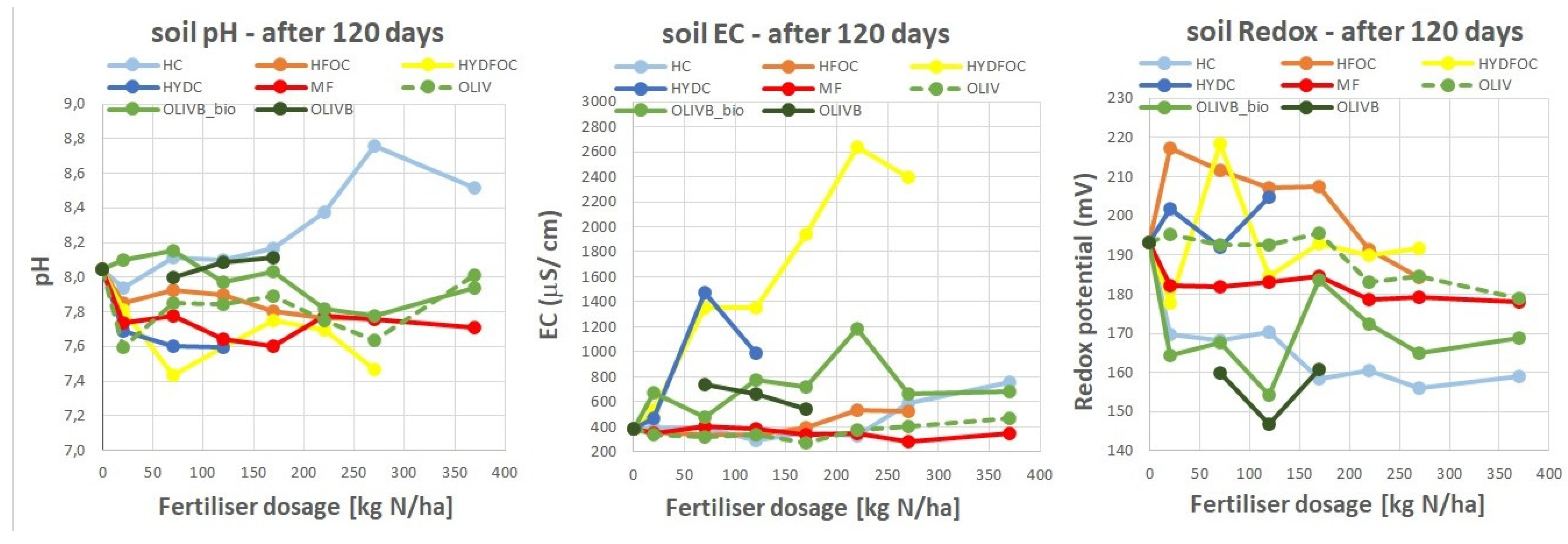
3.8. Residual Soil Properties After 120 Days of Ryegrass Growth
Figure 9 shows basic soil physical properties after termination of the 120 days glasshouse experiment. For most materials pH slightly decreased from ca. 8,0 to 7,4 for Cr-free heavy hydrolysates. Biochar amended wet-greens kept pH rather in a stable level although biochars are rather alkaline, however their dose was not that significant to alter the pH. The highest pH reaching 8,8 was observed for Cr-containing light hydrolysate. Soil EC altered 2-3 times after application of biochar amended wet-greens and quite considerably after application of Cr-containing heavy hydrolysates (almost 4 times more) and significantly after application of Cr-free heavy hydrolysates (6,5 times more). This is due to usage of hydrochloric acid as very strong reagent hydrolysing many ions to the soil solution. The redox potential of the soil solution, also known as the Eh (oxidation-reduction potential), refers to the tendency of the soil to either gain or lose electrons in its chemical reactions. It is a measure of the soil's ability to undergo oxidation (loss of electrons) or reduction (gain of electrons) processes. Application of the tested waste materials mostly decreased the Redox potential of the soil solution with the increased application rate from max 220 to 145 (mV), meaning soil being in a reduced state and turning into more anaerobic conditions. In a reduced soil environment, the availability of oxygen is limited, as it must have been consumed by soil microflora when adding more and more fertilizer to the soil. The low redox potential in the soil can have significant implications for various biogeochemical processes. For example (1) Plant Growth: some plants may not thrive or may even die in anaerobic conditions due to the lack of oxygen necessary for their root respiration, (2) Nutrient Availability: the availability of certain nutrients may be influenced by redox conditions. For instance, in reduced soils, iron and manganese may become more soluble and available to plants, while under oxidized conditions, these nutrients may precipitate and become less accessible. (3) Decomposition: the breakdown of organic matter can be slowed down in anaerobic conditions, leading to the accumulation of organic residues in the soil, (4) Greenhouse Gas Emissions: anaerobic conditions in soils can promote the production of greenhouse gases like methane (CH
4) and nitrous oxide (N
2O), (5) Contaminant Mobility: redox conditions can affect the mobility and bioavailability of various contaminants, such as heavy metals and organic pollutants.
The regulations on the content of Cr in fertilizers in the European Union only regulate the content of Cr (VI), while Cr (III) is generally acceptable without limitation, based on literature that considers it not to be dangerous. It is expected that in Europe the maximum allowable Cr (VI) content will remain at 2 mg/kg, while Cr (III) will remain unlimited (Fertilizers, Regulation (EC) 2003/2003 of the European Parliament and of the Council Referred to Fertilizers). The scientific literature gives a safe maximum Cr (III) content of 5000 mg/kg.
4. Discussion
Leather waste is a very valuable source of nitrogen, a key element for proper plant growth [
28]. It is worth noting that the use of nitrogen mineral fertilizers causes rapid dissolution of granules and fast release of element. Niedziński et al. (2021) in their work noted that within 35 days 70% of nitrogen is released from ammonium granules, while urea granules release as much as 98% [
29]. In the case of organic fertilizers, this value ranges from 15-28%, significantly reducing potential emissions in the form of ammonia and NOx [
29]. However, direct application of waste from tanning industry as organic preparation does not allow efficient use of nitrogen, due to the presence of nutrients in forms not available to plants [
30]. The use of untreated wastes makes it possible to increase yields, but the process of natural decomposition and increased nutrient availability takes a long time. This paper proposes two main treatment methods, hydrolysis and carbonization, as safe technologies for processing waste for fertilizer purposes. The developed processes do not generate by-products, thus fitting closely with the assumptions of a closed-loop economy and green production. Acid hydrolysis leads to the production of short peptides and free amino acids, thus increasing the market value of the proposed products [
31]. The use of sulfuric(VI) and phosphoric(V) acids additionally leads to the valorization of fertilizer materials in phosphorus and sulfur, which play a key role in the proper plant growth [
32,
33].
Carbonization of waste tends to produce materials rich in nitrogen and carbon [
34]. The process is a very good alternative to combustion, allowing safe processing, eliminating nitrogen emissions in the form of NOx into the air [
35]. Biochar has an extremely beneficial effect on crop production, increasing the sorption capacity of the soil and regulating its pH, and reducing the leaching of nutrients into ground and surface water [
36]. In the case of chromium-containing waste, its use is limited, but it has been proven that the chromium present in biochar from chromium materials is in the highly stable form of chromium carbide, which is not available to plants, and is therefore safe for the environment, [
37].
Particularly noteworthy is the designed combination with an introduced mixture of live soil bacteria (BactoFos), which naturally decompose phosphates contained in the substrate. The increase in the concentration of available phosphorus has a beneficial effect on the proper development of plants, supporting their proper rooting [
38]. The proposed composition naturally increases crop yield with preservation of plant quality and reduction of carbon footprint by reducing the amount of applied chemicals - organic farming [
39].
The key problem that arises with the use of waste in fertilizer production is the presence of chromium from the tanning medium. The element, entering the soil and water, is easily incorporated into the digestive pathway and causes toxic effects on plants, and causes many diseases in animals and humans [
40]. According to the EU Regulation, only the Cr(VI) content is limited (<2 mg/kg s.m.). Given this, there are no legal barriers to using fertilizers containing higher Cr(III) contents, but due to the possibility of oxidation of Cr(III) to Cr(VI), care must be taken [
41]. The safe solution is to use this type of fertilizer in closed (greenhouse) crops, where it is possible to control the transfer of the element into the environment (avoiding potential accumulation in the soil or leaching into groundwater). Controlled plantations using Cr-containing formulations would enable the production of specialty foods (functional hypoglycemic foods) [
42,
43].
The positive aspect in the case of leather production is the slow abandonment of treating materials with Cr-containing substances and replacing them with natural tannins in the form of oils, [
44]. This allows the waste to be safely processed into fertilizers that meet EU requirements with high efficiency and a safe environmental aspect. Recycling this type of waste can replace inorganic fertilizers reducing greenhouse gas emissions and carbon footprint.
Quantitatively speaking, our industrial partner globally covers 19% of world car upholstery production (23% in Europe). The above solution could help managing the substantial part of the waste stream amounting for 90 t/ day globally resulting from 116 000 m2 of input bovine leather material a day. Assuming 40% of this amount as bovine shavings from the feedstock material (both Cr containing and Cr-free) and the amount of nitrogen reaching 10% (w/w) and average NUE (for both light hydrolysates and microbially altered and biochar amended wet-greens) being ca. 70%, that gives 919,8 t of recycled nitrogen per year, which comes back to the environment in plant biomass.
On top of sustainability aspects, any transformation of the tanned leather waste fractions into the secondary products to be implemented in agriculture is worth investigating as nowadays the utilisation costs of leather waste are within 500 - 1000 EUR/ tonne.
5. Conclusions
Seven differently treated tanned leather waste fractions were tested for rye grass growth as alternative fertilizers. Although plant dry matter yields were generally better for light hydrolysates, the final information containing the nitrogen use efficiency shows that microbially incubated, biochar amended wet-greens provided the highest nitrogen use efficiencies exceeding 100% after 4 months growth (for 20 kg N/ha) and varying from 17% to 37% in particular months. This is backed up by another parameter (relative agronomic effectiveness) that for these materials exceeded 150% for single month and in total around 33%. Biochar amendments significantly increased agronomic parameters for wet-greens and their microbial treatment enhanced them even further. Light hydrolysates expressed also quite good performance as the relative economic effectiveness reached 118% for a single month and nutrient use efficiency reached in total 39%. In spite of higher nitrogen concentrations in harvested plant tops and consequently higher NUE values for heavy hydrolysates (up to 51,5%), they totally inhibited the growth after 170 kg N/ ha dose, especially the ones with Cr. In general residual soil physical properties were not affected considerably, apart from treatments with heavy hydrolysates that increased the soil electrical conductivity substantially.
Summing up, although good availability of nutrients in the first stage of growth from hydrolysates is a good point when farmers count on fast effect, this research shows that minimally treated wet-greens with no Cr provide even better results, in the same time avoiding additional chemicals (acids) used for the treatment. This helps keep the carbon footprint of the whole process as low as possible while contributing to the real principle of a circular economy where no additional chemicals are introduced to the system. It is further recommended to verify the very long term effect of such waste usage on the ryegrass growth, N uptake and soil properties, eg. over the full year vegetation period including winter. The soil once fertilised in the first season would probably hold part of the nutrients in waste for subsequent utilisation in second season, without the need for re-fertilisation. The next dosages could be then applied in third season and every second season thereafter. This approach would have given the full picture of tanned waste fractions usage as N-fertilisers [
22]
Processed leather waste fractions show promise as organic nitrogen fertilizers, offering an opportunity to repurpose a problematic waste stream while providing essential nutrients for plant growth. This was observed especially for the microbially treated biochar amendment of wet-greens reaching 37% after 30 days and in total even over 100% for the smallest dose (20 kg N/ ha). However, further research is needed to optimize their use, address environmental concerns, and develop guidelines for their safe and effective application. Integrating processed leather waste fractions into agricultural practices could contribute to sustainable waste management and enhance soil fertility, fostering a more circular and environmentally friendly approach to agriculture.
Author Contributions
Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing - original draft K.K.; Investigation, Methodology, Data curation, Validation, Writing - review & editing D.S.; Investigation, Methodology, Validation, Writing - review & editing K.C.; Investigation, Methodology, Validation, Data curation, Writing - review & editing K.M.; Data curation, Resources, Validation, Writing - review & editing P.B.; Methodology, Validation R.T.; Methodology, Validation S.M.; Methodology, Validation A.W.; Project administration, Supervision, Validation, Writing - review & editing A.C. All authors have read and agreed to the published version of the manuscript.”
Funding
The research was carried out under the project nr POIR.04.01.04-00-0071/ 20–00 co-financed by the European Regional Development Fund, entitled: “Development of technologies for rational management of bovine shavings from leather processing (MIZDRA 2.0)" co-financed by the National Centre for Research and Development from the Smart Development Operational Program, Action 4.1.4 “Application Projects”. Beneficiaries: The Institute of Fluid-Flow Machinery Polish Academy of Sciences, Wroclaw University of Technology, Institute of Technology and Life Sciences/ Poznań, BADER Polska Ltd.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The Authors would like to express their gratitude to the technological partner and tanned leather waste fractions provider – BADER Polska LtD. from Bolesławiec, Poland, a supplier of the BactoFos microbial product – BactoTech from Toruń, Poland and the lab technician Sabina Szymańska for her contribution to harvests assistance, sample preparation and analyses (dry matter and N contents).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”.
References
- Korhonen, J.; Honkasalo, A.; Seppälä, J. Circular Economy: The Concept and Its Limitations. Ecol. Econ. 2018, 143, 37–46. [Google Scholar] [CrossRef]
- Circular Economy: Definition, Importance and Benefits | News | European Parliament, (n.D.). Available online: https://www.europarl.europa.eu/news/en/headlines/economy/20151201STO05603/circular-economy-definition-importance-and-benefits?&at_campaign=20234-Economy&at_medium=Google_Ads&at_platform=Search&at_creation=RSA&at_goal=TR_G&at_audience=benefits of a circula.
- Forero-Núñez, C.A.; Méndez-Velásquez, J.A.; Sierra-Vargas, F.E. Energetic Improvement of Tanned Leather Solid Wastes by Thermal Treatment. Ing. y Desarro. 2015, 33, 1–17. [Google Scholar] [CrossRef]
- Kluska, J.; Turzyński, T.; Kardaś, D. Experimental Tests of Co-Combustion of Pelletized Leather Tannery Wastes and Hardwood Pellets. Waste Manag. 2018, 79, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Agustini, C.; da Costa, M.; Gutterres, M. Biogas Production from Tannery Solid Wastes–Scale-up and Cost Saving Analysis. J. Clean. Prod. 2018, 187, 158–164. [Google Scholar] [CrossRef]
- Yuliana, M.; Santoso, S.P.; Soetaredjo, F.E.; Ismadji, S.; Ayucitra, A.; Angkawijaya, A.E.; Ju, Y.-H.; Tran-Nguyen, P.L. A One-Pot Synthesis of Biodiesel from Leather Tanning Waste Using Supercritical Ethanol: Process Optimization. Biomass and Bioenergy 2020, 142, 105761. [Google Scholar] [CrossRef]
- Kumar, A.G.; Nagesh, N.; Prabhakar, T.G.; Sekaran, G. Purification of Extracellular Acid Protease and Analysis of Fermentation Metabolites by Synergistes Sp. Utilizing Proteinaceous Solid Waste from Tanneries. Bioresour. Technol. 2008, 99, 2364–2372. [Google Scholar] [CrossRef]
- Masilamani, D.; Madhan, B.; Shanmugam, G.; Palanivel, S.; Narayan, B. Extraction of Collagen from Raw Trimming Wastes of Tannery: A Waste to Wealth Approach. J. Clean. Prod. 2016, 113, 338–344. [Google Scholar] [CrossRef]
- Murali, R.; Anumary, A.; Ashokkumar, M.; Thanikaivelan, P.; Chandrasekaran, B. Hybrid Biodegradable Films from Collagenous Wastes and Natural Polymers for Biomedical Applications. Waste and Biomass Valorization 2011, 2, 323–335. [Google Scholar] [CrossRef]
- Dwivedi, S.P.; Saxena, A. Extraction of Collagen Powder from Chrome Containing Leather Waste and Its Composites with Alumina Employing Different Casting Techniques. Mater. Chem. Phys. 2020, 253, 123274. [Google Scholar] [CrossRef]
- Ding, X.; Shan, Z.; Long, Z.; Chen, Z. Utilization of Collagen Protein Extracted from Chrome Leather Scraps as a Set Retarders in Gypsum. Constr. Build. Mater. 2020, 237, 117584. [Google Scholar] [CrossRef]
- Mikula, K.; Konieczka, M.; Taf, R.; Skrzypczak, D.; Izydorczyk, G.; Moustakas, K.; Kułażyński, M.; Chojnacka, K.; Witek-Krowiak, A. Tannery Waste as a Renewable Source of Nitrogen for Production of Multicomponent Fertilizers with Biostimulating Properties. Environ. Sci. Pollut. Res. 2023, 30, 8759–8777. [Google Scholar] [CrossRef] [PubMed]
- Pahlawan, I.F.; Sutyasmi, S.; Griyanitasari, G. Hydrolysis of Leather Shavings Waste for Protein Binder. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing, 2019; Vol. 230, p. 12083. [Google Scholar]
- Chojnacka, K.; Skrzypczak, D.; Szopa, D.; Izydorczyk, G.; Moustakas, K.; Witek-Krowiak, A. Management of Biological Sewage Sludge: Fertilizer Nitrogen Recovery as the Solution to Fertilizer Crisis. J. Environ. Manage. 2023, 326, 116602. [Google Scholar] [CrossRef] [PubMed]
- Yorgancioglu, A.; Başaran, B.; Sancakli, A. Value Addition to Leather Industry Wastes and By-Products: Hydrolyzed Collagen and Collagen Peptides. In Waste in textile and leather sectors; IntechOpen London, 2020; pp. 1–26. ISBN 1789852447. [Google Scholar]
- ELSAYED, H.M.; ATTIA, R.Z.; MOHAMED, O.A.; EL-SAYED, N.H.; IBRAHIM, S.A. High Bloom Gelatin Strength from White Leather Shavings. Rev. Piel. Incaltaminte 2018, 18, 259. [Google Scholar] [CrossRef]
- Majee, S.; Halder, G.; Mandal, T. Formulating Nitrogen-Phosphorous-Potassium Enriched Organic Manure from Solid Waste: A Novel Approach of Waste Valorization. Process Saf. Environ. Prot. 2019, 132, 160–168. [Google Scholar] [CrossRef]
- Silva, J.D.C.; Leal, T.T.B.; Araújo, A.S.F.; Araujo, R.M.; Gomes, R.L.F.; Melo, W.J.; Singh, R.P. Effect of Different Tannery Sludge Compost Amendment Rates on Growth, Biomass Accumulation and Yield Responses of Capsicum Plants. Waste Manag. 2010, 30, 1976–1980. [Google Scholar] [CrossRef]
- China, C.R.; Maguta, M.M.; Nyandoro, S.S.; Hilonga, A.; Kanth, S. V; Njau, K.N. Alternative Tanning Technologies and Their Suitability in Curbing Environmental Pollution from the Leather Industry: A Comprehensive Review. Chemosphere 2020, 254, 126804. [Google Scholar] [CrossRef]
- Skrzypczak, D.; Szopa, D.; Mikula, K.; Izydorczyk, G.; Baśladyńska, S.; Hoppe, V.; Pstrowska, K.; Wzorek, Z.; Kominko, H.; Kułażyński, M. Tannery Waste-Derived Biochar as a Carrier of Micronutrients Essential to Plants. Chemosphere 2022, 294, 133720. [Google Scholar] [CrossRef]
- Rayment, G.E.; Higginson, F.R. Australian Laboratory Handbook of Soil and Water Chemical Methods; Inkata Press Pty Ltd, 1992; ISBN 0909605688. [Google Scholar]
- Bolland, M.; Gilkes, R.J. The Poor Performance of Rock Phosphate Fertilizers in Western Australia: Part I. The Crop and Pasture Responses. Agric. Sci. 1990, 3, 43–48. [Google Scholar]
- Adhikari, S.; Anuragi, H.; Chandra, K.; Tarte, S.H.; Dhaka, S.R.; Jatav, H.S.; Hingonia, K. Molecular Basis of Plant Nutrient Use Efficiency - Concepts and Challenges for Its Improvement. In Sustainable Plant Nutrition; Elsevier, 2023; pp. 107–151. [Google Scholar]
- Prieto, K.R.; Echaide-Aquino, F.; Huerta-Robles, A.; Valério, H.P.; Macedo-Raygoza, G.; Prado, F.M.; Medeiros, M.H.G.; Brito, H.F.; da Silva, I.G.N.; Cunha Felinto, M.C.F.; et al. Endophytic Bacteria and Rare Earth Elements; Promising Candidates for Nutrient Use Efficiency in Plants. In Plant Macronutrient Use Efficiency; Elsevier, 2017; pp. 285–306. [Google Scholar]
- Kuligowski, K.; Gilkes, R.J.; Poulsen, T.G.; Yusiharni, B.E. Ash from the Thermal Gasification of Pig Manure—Effects on Ryegrass Yield, Element Uptake, and Soil Properties. Soil Res. 2012, 50, 406–415. [Google Scholar] [CrossRef]
- Yusiharni, B.E.; Ziadi, H.; Gilkes, R.J. A Laboratory and Glasshouse Evaluation of Chicken Litter Ash, Wood Ash, and Iron Smelting Slag as Liming Agents and P Fertilisers. Soil Res. 2007, 45, 374–389. [Google Scholar] [CrossRef]
- Kuligowski, K.; Cenian, A.; Konkol, I.; Świerczek, L.; Chojnacka, K.; Izydorczyk, G.; Skrzypczak, D.; Bandrów, P. Application of Leather Waste Fractions and Their Biochars as Organic Fertilisers for Ryegrass Growth: Agri-Environmental Aspects and Plants Response Modelling. Energies 2023, 16. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Lemaire, G.; Gastal, F. Nitrogen, Plant Growth and Crop Yield. Plant nitrogen 2001, 343–367. [Google Scholar]
- Niedziński, T.; Sierra, M.J.; Łabętowicz, J.; Noras, K.; Cabrales, C.; Millán, R. Release of Nitrogen from Granulate Mineral and Organic Fertilizers and Its Effect on Selected Chemical Parameters of Soil. Agronomy 2021, 11, 1981. [Google Scholar] [CrossRef]
- Nogueira, F.G.E.; Castro, I.A.; Bastos, A.R.R.; Souza, G.A.; de Carvalho, J.G.; Oliveira, L.C.A. Recycling of Solid Waste Rich in Organic Nitrogen from Leather Industry: Mineral Nutrition of Rice Plants. J. Hazard. Mater. 2011, 186, 1064–1069. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Mikula, K.; Witek-Krowiak, A.; Izydorczyk, G.; Kuligowski, K.; Bandrów, P.; Kułażyński, M. Progress in Sustainable Technologies of Leather Wastes Valorization as Solutions for the Circular Economy. J. Clean. Prod. 2021, 313. [Google Scholar] [CrossRef]
- Bhattacharya, A. Changing Climate and Resource Use Efficiency in Plants; Academic Press, 2018; ISBN 0128168374. [Google Scholar]
- Muscolo, A.; Marra, F.; Canino, F.; Maffia, A.; Mallamaci, C.; Russo, M. Growth, Nutritional Quality and Antioxidant Capacity of Lettuce Grown on Two Different Soils with Sulphur-Based Fertilizer, Organic and Chemical Fertilizers. Sci. Hortic. (Amsterdam). 2022, 305, 111421. [Google Scholar] [CrossRef]
- Stejskal, J.; Ngwabebhoh, F.A.; Sáha, P.; Prokeš, J. Carbonized Leather Waste: A Review and Conductivity Outlook. Polymers (Basel). 2023, 15, 1028. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Zhang, G.; Kang, G.; Liu, Z.; Yu, J.; Gao, S. Research on the Influence of Combustion Methods on NO x Emissions from Co-Combustion of Various Tannery Wastes. ACS omega 2022, 7, 4110–4120. [Google Scholar] [CrossRef]
- Rashid, M.; Hussain, Q.; Khan, K.S.; Alwabel, M.I.; Hayat, R.; Akmal, M.; Ijaz, S.S.; Alvi, S. Carbon-Based Slow-Release Fertilizers for Efficient Nutrient Management: Synthesis, Applications, and Future Research Needs. J. Soil Sci. Plant Nutr. 2021, 21, 1144–1169. [Google Scholar] [CrossRef]
- Wells, H.C.; Sizeland, K.H.; Edmonds, R.L.; Aitkenhead, W.; Kappen, P.; Glover, C.; Johannessen, B.; Haverkamp, R.G. Stabilizing Chromium from Leather Waste in Biochar. ACS Sustain. Chem. Eng. 2014, 2, 1864–1870. [Google Scholar] [CrossRef]
- Loudari, A.; Mayane, A.; Naciri, R.; Zeroual, Y.; Colinet, G.; Oukarroum, A. Root Morphological and Anatomical Responses to Increasing Phosphorus Concentration of Wheat Plants Grown under Salinity. Plant Stress 2022, 6, 100121. [Google Scholar] [CrossRef]
- Holka, M.; Kowalska, J.; Jakubowska, M. Reducing Carbon Footprint of Agriculture—Can Organic Farming Help to Mitigate Climate Change? Agriculture 2022, 12, 1383. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N. Toxic and Genotoxic Effects of Hexavalent Chromium in Environment and Its Bioremediation Strategies. J. Environ. Sci. Heal. Part C 2016, 34, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Apte, A.D.; Tare, V.; Bose, P. Extent of Oxidation of Cr (III) to Cr (VI) under Various Conditions Pertaining to Natural Environment. J. Hazard. Mater. 2006, 128, 164–174. [Google Scholar] [CrossRef]
- Li, P.; Cao, Y.; Song, G.; Zhao, B.; Ma, Q.; Li, Z.; He, C. Anti-Diabetic Properties of Genistein-Chromium (III) Complex in Db/Db Diabetic Mice and Its Sub-Acute Toxicity Evaluation in Normal Mice. J. Trace Elem. Med. Biol. 2020, 62, 126606. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Z.; Pan, Y.; Gao, X.; Chen, H. Anti-Diabetic Effects of Inonotus Obliquus Polysaccharides-Chromium (III) Complex in Type 2 Diabetic Mice and Its Sub-Acute Toxicity Evaluation in Normal Mice. Food Chem. Toxicol. 2017, 108, 498–509. [Google Scholar] [CrossRef]
- Sahu, B.; Jayakumar, G.C.; Alla, J.P. Recent Trends in Oil Tanning and Its Applications-A Way Forward towards Cleaner Approach in Chamois Leather Making. J. Clean. Prod. 2022, 356, 131755. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).