Submitted:
22 October 2024
Posted:
24 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- ⮚
- To assess the current and future alternative antiviral drugs used to treat IAV
- ⮚
- To describe the various mechanisms by which IAV develops resistance to commonly used anti-influenza drugs
2. Literature Review
2.1. Genomic Organization of the Influenza a Virus RNA
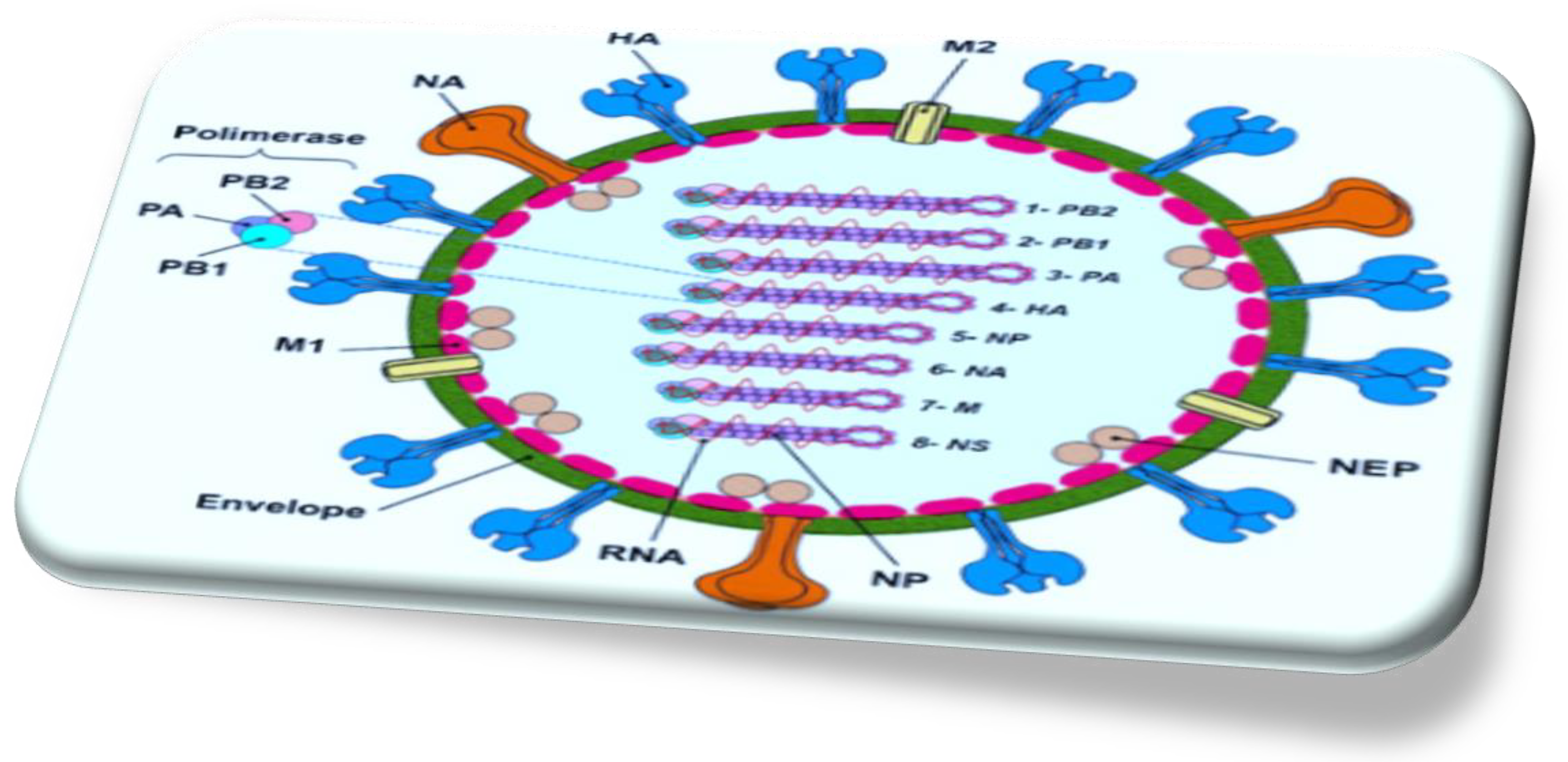
2.2. Mechanism of Action of IAV Replication
2.3. Natural History and Epidemiology of IAV
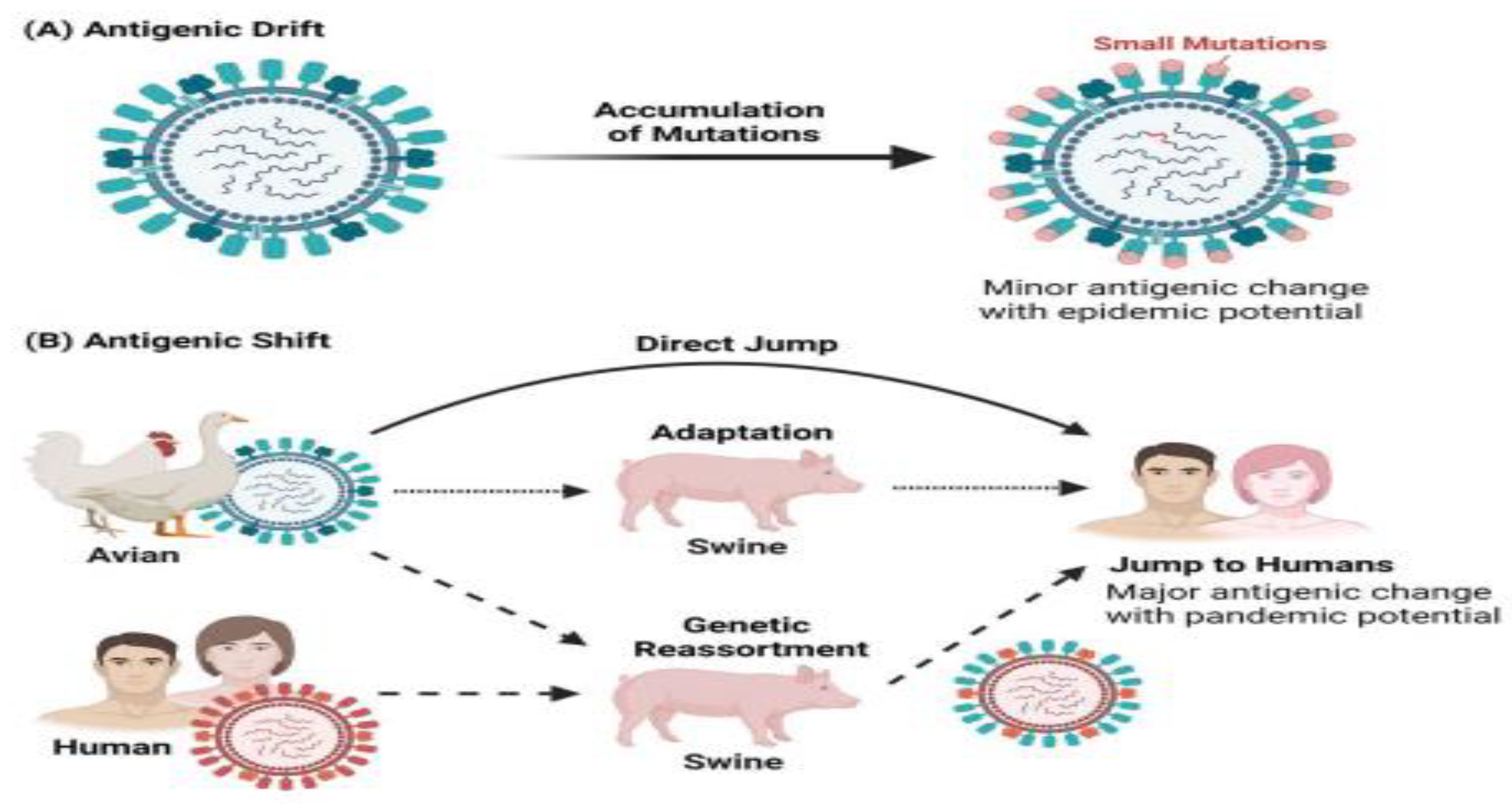
2.4. Spread and Epidemiology of Drug-Resistant Human Influenza Viruses
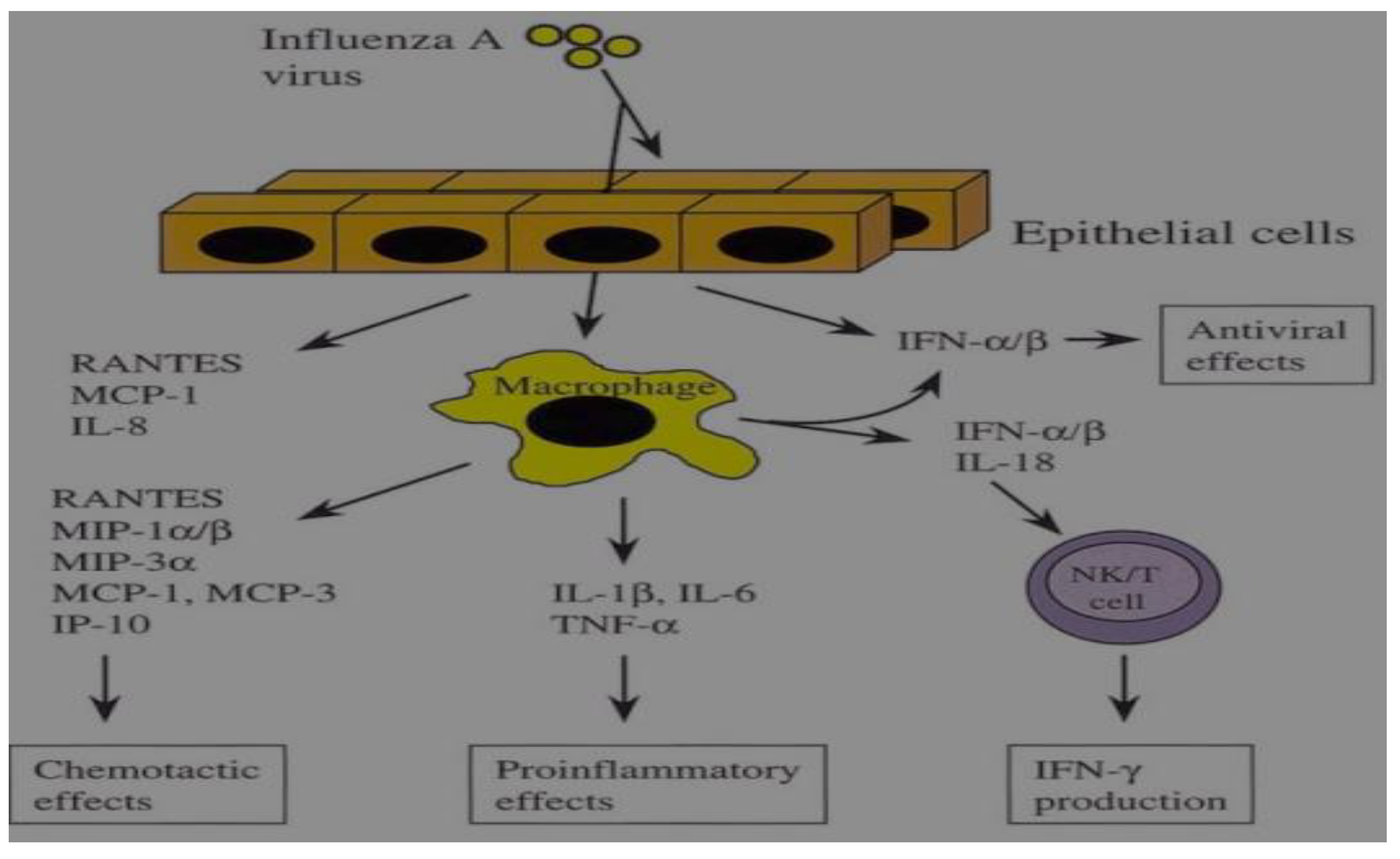
2.5. Pathogenesis of IAV
Production of Cytokines in IAV
2.6. Assortment of Coding Strategies in Influenza a Viruses
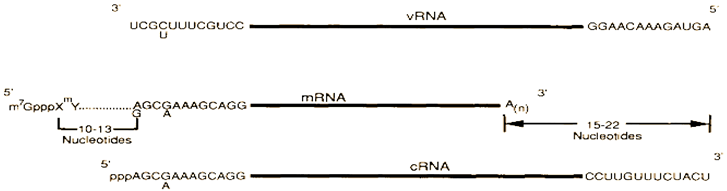
mRNAs Splicing and Reading Frames Overlapping
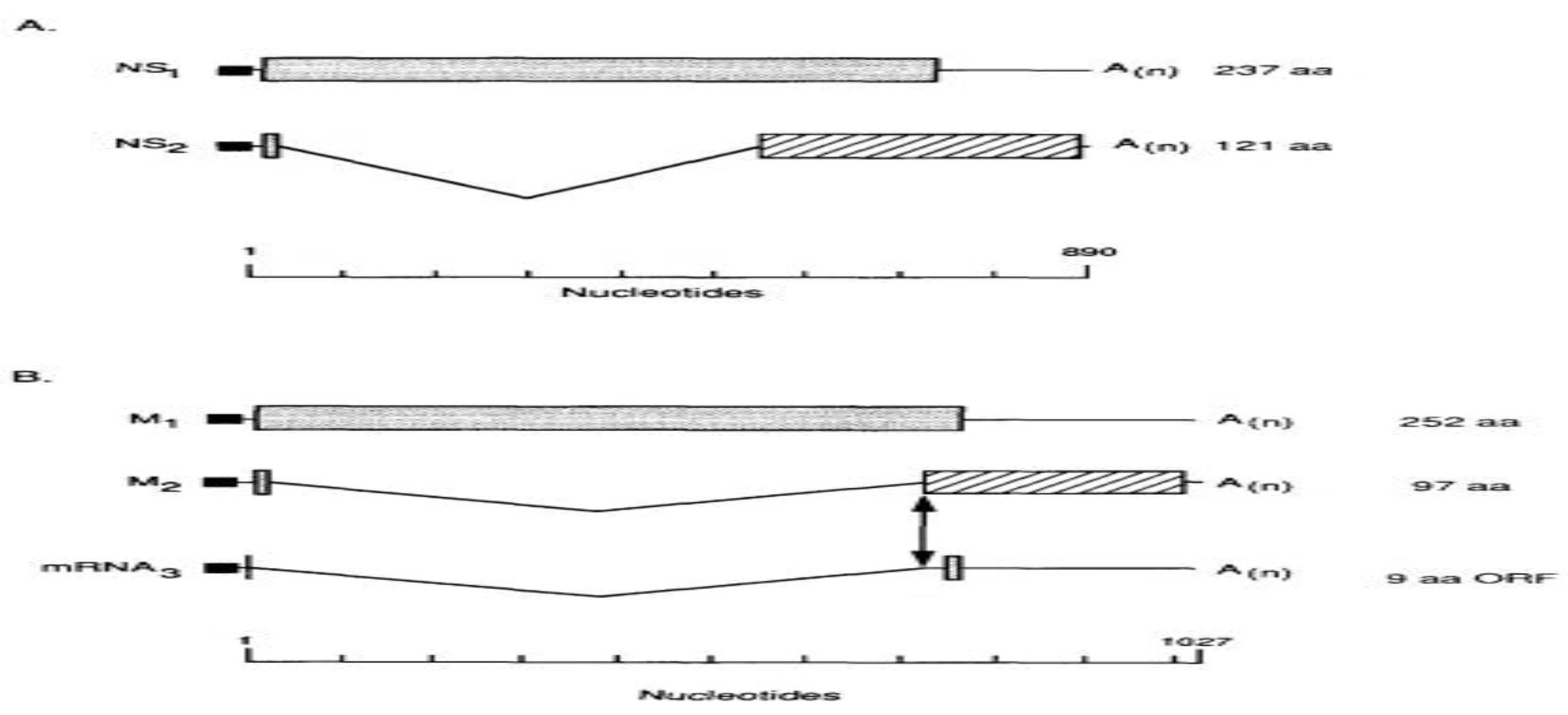
2.7. Clinical Presentation of IAV Infection
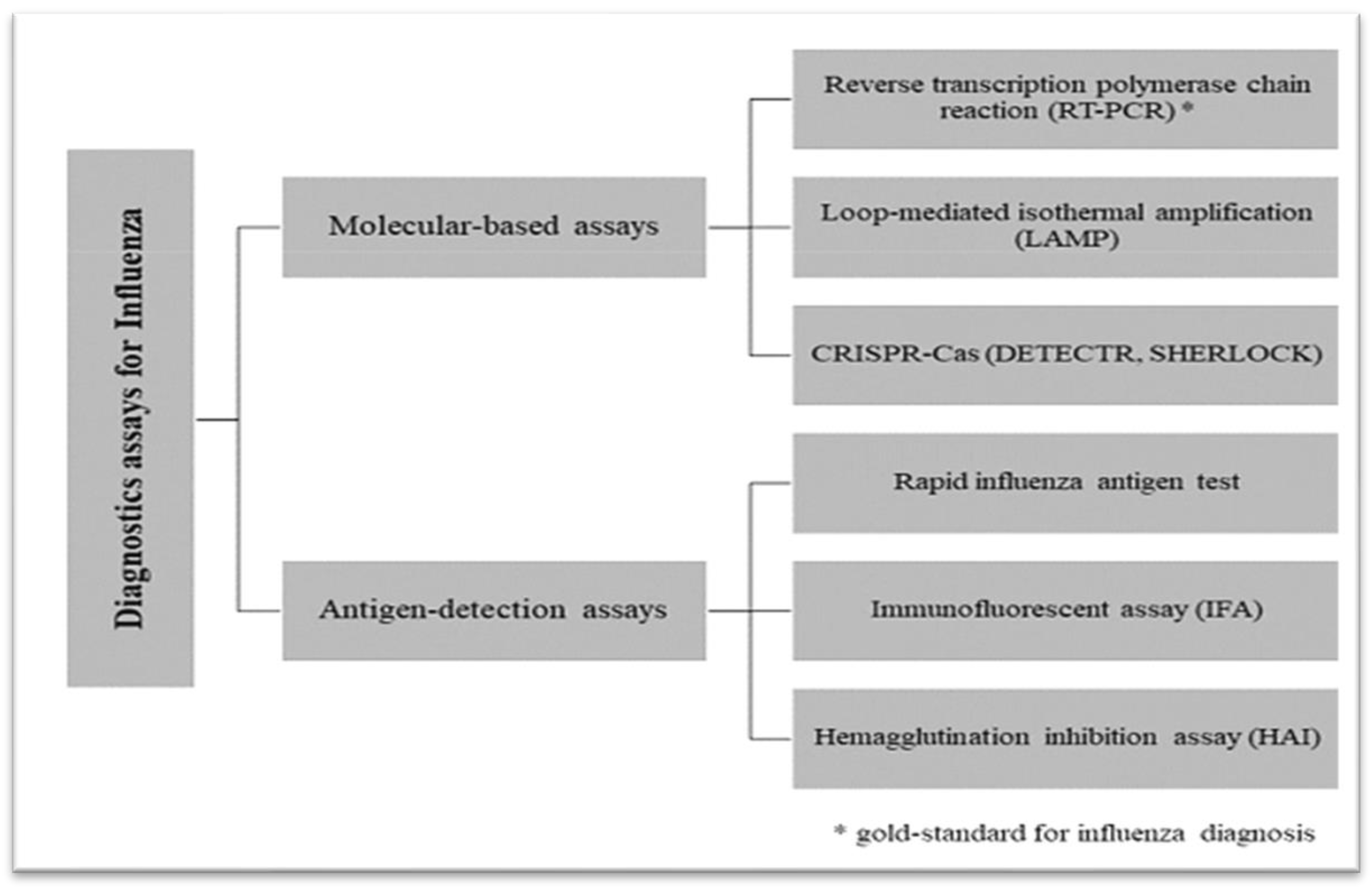
2.8. Diagnosis of IAV
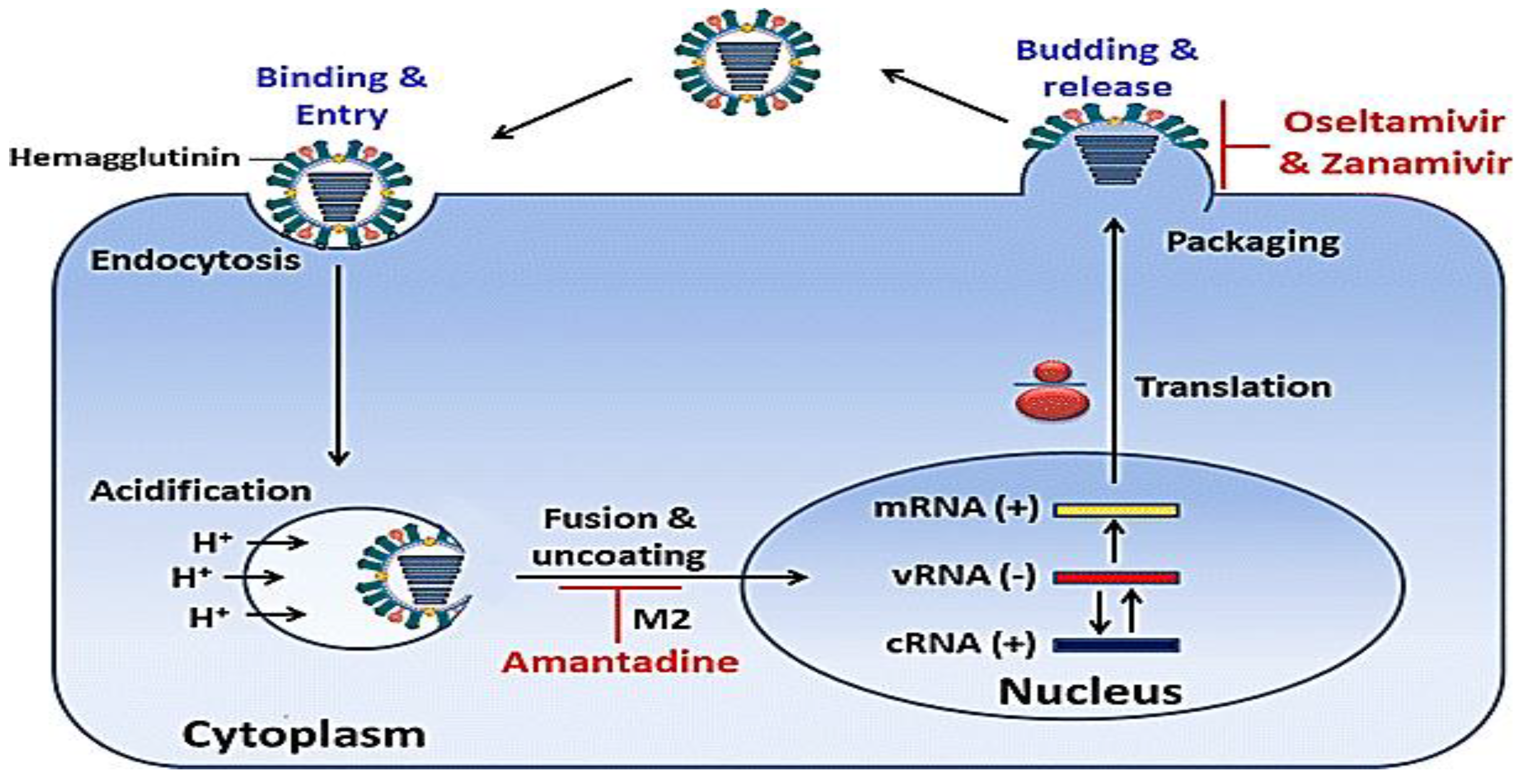
2.9. Antiviral Drugs for IAV
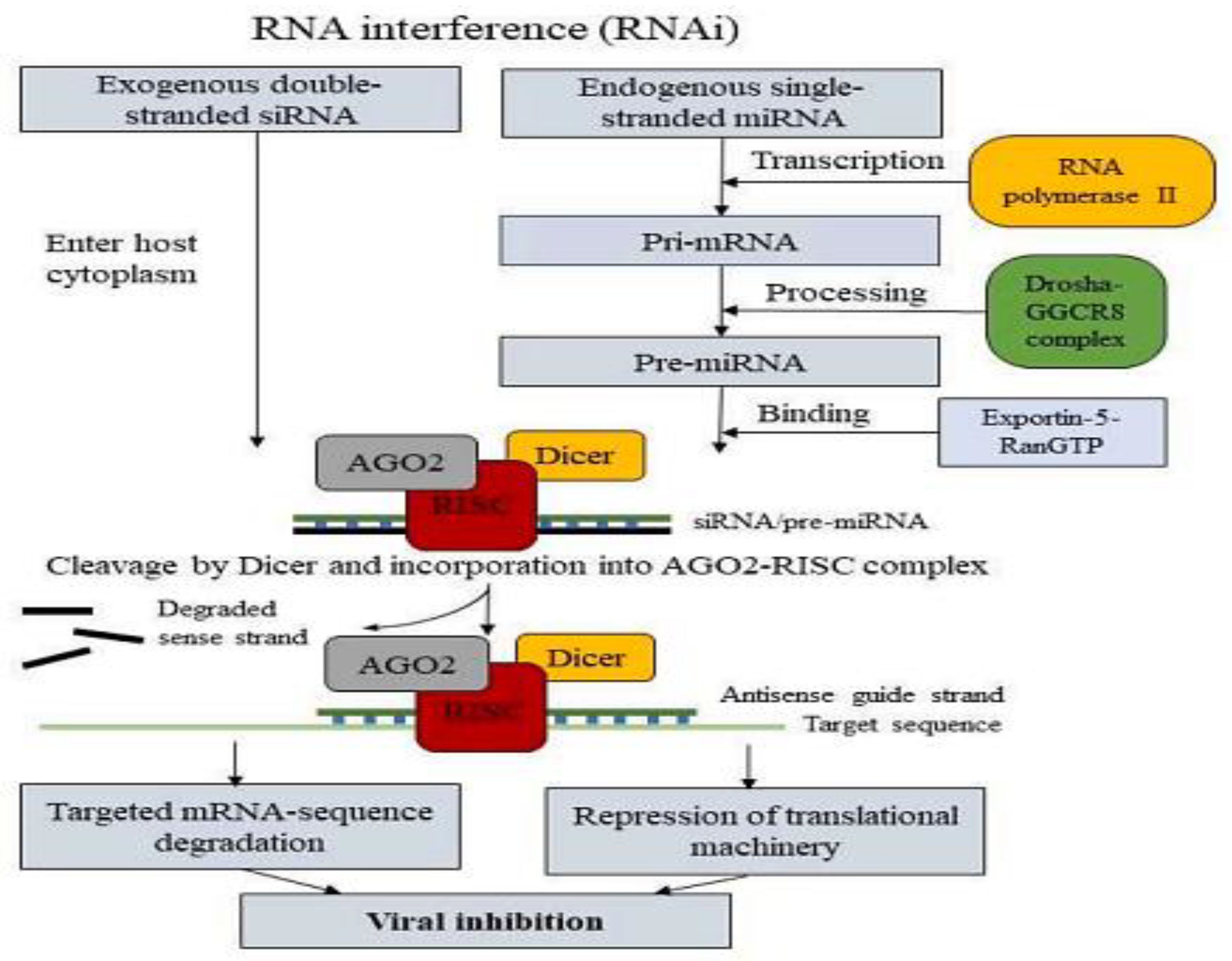
2.10. Alternative Antiviral Approach That Targets the Genome
2.10.1. Interference RNA (RNAi)
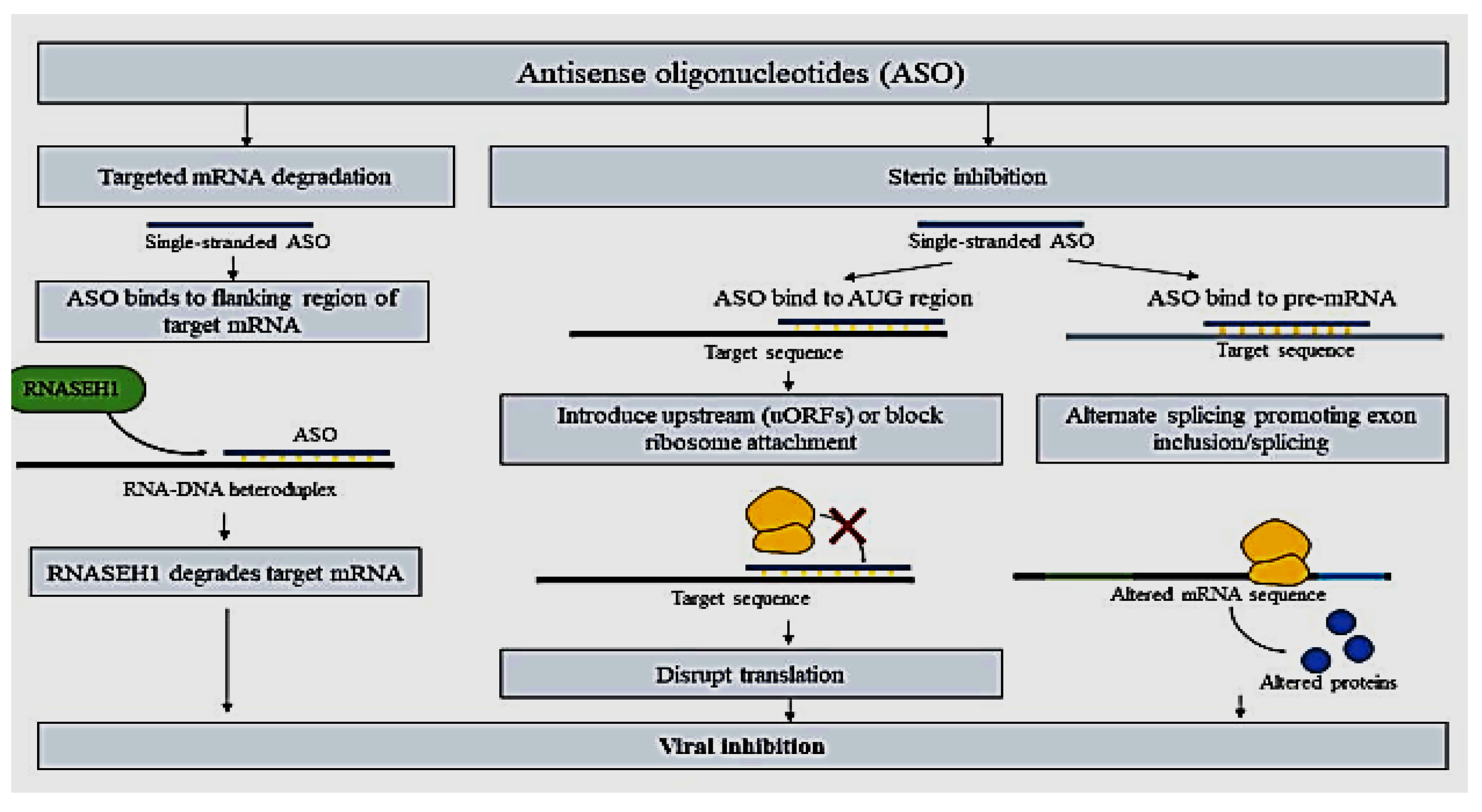
2.10.2. ASO
2.11. Evolution of Influenza a Virus
2.12. Significance of Anti-IAV Resistance
2.13. Factors Driving Antiviral Resistance
| Factors driven by virus | Factors driven by a host | ||
| Issue | Potential Explication | Problem | Solution |
| Error-prone polymerase, quasispecies, antigenic drift | Target host pathways | Chemoprophylaxis, use of sub-therapeutic doses | Confined and checked the use of chemoprophylaxis with full dosage |
| Antigenic shift | Watchful surveillance | Persistent shedding owing to virulent strain or contagion of a high-risk group. | Hospitalized isolation, treatment, and maintenance to prevent nosocomial communication |
2.14. Resistance to Anti-Influenza Therapy
| Subtypes of Influenza | M2 Ion Channel Inhibitors (Adamantanes) | Neuraminidase Inhibitors | ||||||
| Oseltamivir | Zanamivir | |||||||
| %Resistant | Major M2 Mutation | Refs | %Resistant to NAIs | Major NA Mutations | Compensatory Mutations | Major NA Mutation* | Refs. | |
| H3N2 | >96% | S31N | [119] | 0.2% | R292K E119V N294S | Unknown | Q136K | [120] |
|
Pre-2009 seasonal H1N1 |
16% | S31N | [121] | >99% | H274Y | R354G K329E V234M, R222Q, D344N, |
Q136K | [122] |
| 2009 Pandemic H1N1 | 100% (USA) | S31N | [119] | 0.5% | H274Y I223R | V240I, N368K | I223R | [123] |
| H5N1 | Differs by clade | S31N V27A | [124] | Rare | H274Y N294S | Unknown | Unknown | [125] |
2.15. Mode of Resistance to NAIs and M2
2.16. Alternate Options Contrary to the Increasing Resistance of Influenza a Antivirals
2.16.1. CRISPR
2.16.2. Natural Occurring Compounds
2.16.3. Directing Towards Host Molecules
3. Conclusion and Recommendation
References
- Long, J.S., et al., Host and viral determinants of influenza A virus species specificity. 2019. 17(2): p. 67-81. [CrossRef]
- Taubenberger, J.K., J.C.J.C.h. Kash, and microbe, Influenza virus evolution, host adaptation, and pandemic formation. 2010. 7(6): p. 440-451. [CrossRef]
- Jang, Y.H. and B.L.J.Y.m.j. Seong, Cross-protective immune responses elicited by live attenuated influenza vaccines. 2013. 54(2): p. 271-282.
- Tong, S., et al., New world bats harbor diverse influenza A viruses. 2013. 9(10): p. e1003657. [CrossRef]
- Pizzorno, A., Y. Abed, and G. Boivin. Influenza drug resistance. In Seminars in respiratory and critical care medicine. 2011. © Thieme Medical Publishers.
- Ferguson, N.M., et al., Strategies for containing an emerging influenza pandemic in Southeast Asia. 2005. 437(7056): p. 209-214. [CrossRef]
- Abed, Y., et al., Generation and characterization of recombinant influenza A (H1N1) viruses harboring amantadine resistance mutations. 2005. 49(2): p. 556-559. [CrossRef]
- Cheung, C.-L., et al., Distribution of amantadine-resistant H5N1 avian influenza variants in Asia. 2006. 193(12): p. 1626-1629. [CrossRef]
- Bright, R.A., et al., Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. 2005. 366(9492): p. 1175-1181. [CrossRef]
- Bright, R., et al., High levels of adamantane resistance among influenza A (H3N2) viruses and interim guidelines for use of antiviral agents--United States, 2005-06 influenza season. 2006. 55(2).
- Hutchinson, E.C., et al., Conserved and host-specific features of influenza virion architecture. 2014. 5(1): p. 1-11. [CrossRef]
- Rossman, J.S. and R.A.J.V. Lamb, Influenza virus assembly and budding. 2011. 411(2): p. 229-236. [CrossRef]
- Van Reeth, K., I.H. Brown, and C.W.J.D.o.s. Olsen, Influenza virus. 2012. 10: p. 557-571.
- Ison, M.G. and F.G.J.C.o.i.i.d. Hayden, Viral infections in immunocompromised patients: what's new with respiratory viruses? 2002. 15(4): p. 355-367.
- Webster, R.G. and E.A.J.A.o.t.N.Y.A.o.S. Govorkova, Continuing challenges in influenza. 2014. 1323(1): p. 115-139.
- Eisfeld, A.J., G. Neumann, and Y.J.N.R.M. Kawaoka, At the center: influenza A virus ribonucleoproteins. 2015. 13(1): p. 28-41.
- Moeller, A., et al., Organization of the influenza virus replication machinery. 2012. 338(6114): p. 1631-1634. [CrossRef]
- Wandzik, J.M., T. Kouba, and S.J.C.S.H.p.i.m. Cusack, Structure and function of influenza polymerase. 2021. 11(9): p. a038372. [CrossRef]
- Ferhadian, D., et al., Structural and functional motifs in influenza virus RNAs. 2018. 9: p. 354580. [CrossRef]
- Michalak, P., et al., Secondary structure of the segment 5 genomic RNA of influenza A virus and its application for designing antisense oligonucleotides. 2019. 9(1): p. 3801. [CrossRef]
- Wang, J., et al., Dual roles of the hemagglutinin segment-specific noncoding nucleotides in the extended duplex region of the influenza A virus RNA promoter. 2017. 91(1): p. 10.1128/jvi. 01931-16. [CrossRef]
- Pflug, A., et al., Structural insights into RNA synthesis by the influenza virus transcription-replication machine. 2017. 234: p. 103-117. [CrossRef]
- Anchisi, S., et al., Mismatches in the influenza A virus RNA panhandle prevent retinoic acid-inducible gene I (RIG-I) sensing by impairing RNA/RIG-I complex formation. 2016. 90(1): p. 586-590. [CrossRef]
- Soszynska-Jozwiak, M., et al., Influenza virus segment 5 (+) RNA-secondary structure and new targets for antiviral strategies. 2017. 7(1): p. 15041. [CrossRef]
- Chauhan, R.P. and M.L.J.V.G. Gordon, An overview of influenza A virus genes, protein functions, and replication cycle highlighting important updates. 2022. 58(4): p. 255-269. [CrossRef]
- Ellis, D., et al., Structure-based design of stabilized recombinant influenza neuraminidase tetramers. 2022. 13(1): p. 1825. [CrossRef]
- Sun, Y., et al., Hemagglutinin Glycosylation Pattern-Specific Effects: Implications for The Fitness of H9. 4.2. 5-branched H9N2 Avian Influenza Viruses: Effect of HA glycosylation on the fitness of H9N2 AIV. 2024(just-accepted): p. 2364736.
- Gaymard, A., et al., Functional balance between neuraminidase and haemagglutinin in influenza viruses. 2016. 22(12): p. 975-983. [CrossRef]
- Richard, M., et al., Rescue of a H3N2 influenza virus containing a deficient neuraminidase protein by a hemagglutinin with a low receptor-binding affinity. 2012. 7(5): p. e33880. [CrossRef]
- Lo, C.-Y., et al., Structure and function of influenza virus ribonucleoprotein. 2018: p. 95-128.
- Domínguez, L.M., et al., Antiviral resistance in influenza viruses. 2023. 69(13): p. 16-23. [CrossRef]
- Noda, T. and Y.J.R.i.m.v. Kawaoka, Structure of influenza virus ribonucleoprotein complexes and their packaging into virions. 2010. 20(6): p. 380-391.
- Dawson, A.R., et al., Post-translation regulation of influenza virus replication. 2020. 7(1): p. 167-187. [CrossRef]
- McNicholl, I.R. and J.J.J.A.o.P. McNicholl, Neuraminidase inhibitors: zanamivir and oseltamivir. 2001. 35(1): p. 57-70.
- Geraci, J., et al., Mass mortality of harbor seals: pneumonia associated with influenza A virus. 1982. 215(4536): p. 1129-1131. [CrossRef]
- Hinshaw, V., et al., Characterization of two influenza A viruses from a pilot whale. 1986. 58(2): p. 655-656. [CrossRef]
- Yuanji Guo, Y.G., et al., Characterization of a new avian-like influenza A virus from horses in China. 1992. [CrossRef]
- Guan, Y., et al., Emergence of avian H1N1 influenza viruses in pigs in China. 1996. 70(11): p. 8041-8046. [CrossRef]
- Beare, A., and R.J.A.o.v. Webster, Replication of avian influenza viruses in humans. 1991. 119: p. 37-42. [CrossRef]
- Cox, N. and Y. Kawaoka, Thomyxo Viruses: Influenza in Topely and Will Sons Microbiology and Microbial Infections. 1998.
- Ito, T., et al., Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. 1998. 72(9): p. 7367-7373. [CrossRef]
- Nayak, D.P., et al., Influenza virus morphogenesis and budding. 2009. 143(2): p. 147-161. [CrossRef]
- Shtykova, E.V., et al., Structural analysis of influenza A virus matrix protein M1 and its self-assemblies at low pH. 2013. 8(12): p. e82431. [CrossRef]
- Webster, R.G., et al., Evolution and ecology of influenza A viruses. 1992. 56(1): p. 152-179. [CrossRef]
- Tong, S., et al., A distinct lineage of influenza A virus from bats. 2012. 109(11): p. 4269-4274. [CrossRef]
- Wu, Y., et al., Bat-derived influenza-like viruses H17N10 and H18N11. 2014. 22(4): p. 183-191.
- Hope-Simpson, R., D.J.E. Golubev, and Infection, A new concept of the epidemic process of influenza A virus. 1987. 99(1): p. 5-54. [CrossRef]
- Rambaut, A., et al., The genomic and epidemiological dynamics of human influenza A virus. 2008. 453(7195): p. 615-619. [CrossRef]
- Neuzil, K.M., et al., Illness among schoolchildren during influenza season: effect on school absenteeism, parental absenteeism from work, and secondary illness in families. 2002. 156(10): p. 986-991.
- Worobey, M., G.-Z. Han, and A.J.P.o.t.N.A.o.S. Rambaut, Genesis and pathogenesis of the 1918 pandemic H1N1 influenza A virus. 2014. 111(22): p. 8107-8112. [CrossRef]
- Husain, M.J.I., Genetics and Evolution, Avian influenza A (H7N9) virus infection in humans: epidemiology, evolution, and pathogenesis. 2014. 28: p. 304-312. [CrossRef]
- Bouvier, N.M. and P.J.V. Palese, The biology of influenza viruses. 2008. 26: p. D49-D53. [CrossRef]
- Tamerius, J., et al., Global influenza seasonality: reconciling patterns across temperate and tropical regions. 2011. 119(4): p. 439-445. [CrossRef]
- Paules, C.I. and A.S.J.T.J.o.i.d. Fauci, Influenza vaccines: good, but we can do better. 2019. 219(Supplement_1): p. S1-S4.
- Clayville, L.R.J.P. and Therapeutics, Influenza update: a review of currently available vaccines. 2011. 36(10): p. 659.
- Shao, W., et al., Evolution of influenza a virus by mutation and re-assortment. 2017. 18(8): p. 1650. [CrossRef]
- Hurt, A.C.J.C.o.i.v., The epidemiology and spread of drug-resistant human influenza viruses. 2014. 8: p. 22-29.
- Short, K.R., et al., Influenza-induced inflammation drives pneumococcal otitis media. 2013. 81(3): p. 645-652. [CrossRef]
- Kaiser, L., et al., Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. 2003. 163(14): p. 1667-1672. [CrossRef]
- Lê, V.B., et al., Platelet activation and aggregation promote lung inflammation and influenza virus pathogenesis. 2015. 191(7): p. 804-819. [CrossRef]
- Koutsakos, M., K. Kedzierska, and K.J.t.J.o.I. Subbarao, Immune responses to avian influenza viruses. 2019. 202(2): p. 382-391. [CrossRef]
- Zlotnik, A. and O.J.I. Yoshie, Chemokines: a new classification system and their role in immunity. 2000. 12(2): p. 121-127.
- Pirhonen, J., et al., Virus infection activates IL-1β and IL-18 production in human macrophages by a caspase-1-dependent pathway. 1999. 162(12): p. 7322-7329. [CrossRef]
- Gong, J.-H., et al., Influenza A virus infection of macrophages. Enhanced tumor necrosis factor-alpha (TNF-alpha) gene expression and lipopolysaccharide-triggered TNF-alpha release. 1991. 147(10): p. 3507-3513. [CrossRef]
- Le Goffic, R., et al., Cutting Edge: Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. 2007. 178(6): p. 3368-3372. [CrossRef]
- Jewell, N.A., et al., Lambda interferon is the predominant interferon induced by influenza A virus infection in vivo. 2010. 84(21): p. 11515-11522. [CrossRef]
- Kuiken, T., et al., Pathogenesis of influenza virus infections: the good, the bad and the ugly. 2012. 2(3): p. 276-286. [CrossRef]
- Marion, T., et al., Respiratory mucosal proteome quantification in human influenza infections. 2016. 11(4): p. e0153674. [CrossRef]
- Guo, X.-z.J. and P.G. Thomas. New fronts emerge in the influenza cytokine storm. In Seminars in immunopathology. 2017. Springer. [CrossRef]
- Lamb, R.A. and C.M.J.T.i.G. Horvath, Diversity of coding strategies in influenza viruses. 1991. 7(8): p. 261-266.
- Hale, B.G., et al., The multifunctional NS1 protein of influenza A viruses. 2008. 89(10): p. 2359-2376. [CrossRef]
- Lamb, R.A. and C.-J.J.V. Lai, Expression of unspliced NS1 mRNA, spliced NS2 mRNA, and a spliced chimera mRNA from cloned influenza virus NS DNA in an SV40 vector. 1984. 135(1): p. 139-147. [CrossRef]
- Gibson, S., et al., Three cases of acute myositis in adults following influenza-like illness during the H1N1 pandemic. 2013. 4(1): p. 51. [CrossRef]
- Taubenberger, J.K. and D.M.J.A.R.P.M.D. Morens, The pathology of influenza virus infections. 2008. 3: p. 499-522.
- Chughtai, A., et al., The presence of fever in adults with influenza and other viral respiratory infections. 2017. 145(1): p. 148-155. [CrossRef]
- Popescu, C.P., et al., Neurologic complications of influenza B virus infection in adults, Romania. 2017. 23(4): p. 574. [CrossRef]
- Milne, M., et al., Influenza, hay fever, and sinusitis: Know the differences. 2018. 85(6): p. 19-26.
- Kong, W.J.T.L.I.D., Influenza virus associated with ocular complications. 2018. 18(6): p. 602-603. [CrossRef]
- Wilhelm, M.J.T.A.j.o.m.c., Influenza in older patients: a call to action and recent updates for vaccinations. 2018. 24(2 Suppl): p. S15-S24.
- Rello, J. and A.J.C.C. Pop-Vicas, Clinical review: primary influenza viral pneumonia. 2009. 13: p. 1-6.
- Odio, C.D., et al., Severe influenza A (H1N1) virus infection complicated by myositis, refractory rhabdomyolysis, and compartment syndrome. 2019. 2019(1): p. 1540761.
- Pandey, Y., et al., Acute influenza infection presenting with cardiac tamponade: a case report and review of the literature. 2019. 23. [CrossRef]
- Henrickson, K.J.J.C.m.r., Parainfluenza viruses. 2003. 16(2): p. 242-264.
- Noor, A. and E.J.O.J. Gradidge, A case of Reye syndrome caused by influenza A virus. 2018. 18(4): p. 425-427. [CrossRef]
- Schrör, K.J.P.D., Aspirin and Reye syndrome: a review of the evidence. 2007. 9: p. 195-204.
- Binnicker, M.J., et al., Direct detection of influenza A and B viruses in less than 20 minutes using a commercially available rapid PCR assay. 2015. 53(7): p. 2353-2354. [CrossRef]
- Anderson, K.B., et al., Clinical and laboratory predictors of influenza infection among individuals with influenza-like illness presenting to an urban Thai hospital over five years. 2018. 13(3): p. e0193050. [CrossRef]
- Ghebrehewet, S., P. MacPherson, and A.J.T.B. Ho, Clinical Updates: Influenza. 2016. 355.
- Mahony, J., et al., Multiplex loop-mediated isothermal amplification (M-LAMP) assay for the detection of influenza A/H1, A/H3, and influenza B can provide a specimen-to-result diagnosis in 40 min with single genome copy sensitivity. 2013. 58(1): p. 127-131.
- Zhang, H., et al., Development of a multiplex real-time RT-PCR assay for simultaneous detection and differentiation of influenza A, B, C, and D viruses. 2019. 95(1): p. 59-66.
- Trombetta, V.K., et al., Are rapid influenza antigen tests still clinically useful in today's molecular diagnostics world? 2018. 77(9): p. 226.
- Low, Z.Y., et al., The convergent evolution of influenza A virus: Implications, therapeutic strategies and what we need to know. 2023: p. 100202. [CrossRef]
- Eyers, S., et al., The effect on mortality of antipyretics in the treatment of influenza infection: a systematic review and meta-analysis. 2010. 103(10): p. 403-411.
- Ng, K.E.J.P. and Therapeutics, Xofluza (Baloxavir marboxil) for the treatment of acute uncomplicated influenza. 2019. 44(1): p. 9.
- Kosik, I. and J.W.J.V. Yewdell, Influenza hemagglutinin, and neuraminidase: Yin–Yang proteins coevolving to thwart immunity. 2019. 11(4): p. 346. [CrossRef]
- McLean, H.Q., et al. Impact of late oseltamivir treatment on influenza symptoms in the outpatient setting: results of a randomized trial. In Open forum infectious diseases. 2015. Oxford University Press. [CrossRef]
- Eiland, L.S., E.H.J.T. Eiland, and C.R. Management, Zanamivir for the prevention of influenza in adults and children age 5 years and older. 2007. 3(3): p. 461-465.
- Pielak, R.M., K. Oxenoid, and J.J.J.S. Chou, Structural investigation of rimantadine inhibition of the AM2-BM2 chimera channel of influenza viruses. 2011. 19(11): p. 1655-1663. [CrossRef]
- Baker, D.E.J.H.P., Baloxavir marboxil. 2019. 54(3): p. 165-169.
- Han, J., et al., Influenza virus: small molecule therapeutics and mechanisms of antiviral resistance. 2018. 25(38): p. 5115-5127. [CrossRef]
- Kumar, B., et al., Potent intracellular knock-down of influenza A virus M2 gene transcript by DNAzymes considerably reduces viral replication in host cells. 2015. 57: p. 836-845. [CrossRef]
- Tarn, W.-Y., et al., Antisense oligonucleotide-based therapy of viral infections. 2021. 13(12): p. 2015. [CrossRef]
- Svoboda, P.J.F.i.p.s., Key mechanistic principles and considerations concerning RNA interference. 2020. 11: p. 1237.
- Takahashi, T., S.M. Heaton, and N.F.J.P.p. Parrish, Mammalian antiviral systems directed by small RNA. 2021. 17(12): p. e1010091. [CrossRef]
- McMillen, C.M., et al., Inhibition of influenza A virus matrix and nonstructural gene expression using RNA interference. 2016. 497: p. 171-184. [CrossRef]
- Henahan, S.J.I.W., Fomivirsen focuses on the future of CMV retinitis. 1998. 1138(1): p. 11-12. [CrossRef]
- Roberts, T.C., R. Langer, and M.J.J.N.r. D.d. Wood, Advances in oligonucleotide drug delivery. 2020. 19(10): p. 673-694. [CrossRef]
- Chen, Y.-Q., et al., Influenza infection in humans induces broadly cross-reactive and protective neuraminidase-reactive antibodies. 2018. 173(2): p. 417-429. e10.
- Palese, P.J.V., Orthomyxoviridae. 2007: p. 1647-1740.
- Szewczyk, B., K. Bieńkowska-Szewczyk, and E.J.A.B.P. Król, Introduction to molecular biology of influenza A viruses. 2014. 61(3): p. 397-401.
- Dugan, V.G., et al., The evolutionary genetics and emergence of avian influenza viruses in wild birds. 2008. 4(5): p. e1000076.
- Garten, R.J., et al., Antigenic and genetic characteristics of swine-origin 2009 A (H1N1) influenza viruses circulating in humans. 2009. 325(5937): p. 197-201.
- Hussain, M., et al., Drug resistance in influenza A virus: the epidemiology and management. 2017: p. 121-134.
- Kessler, S., et al., Influenza A viruses and zoonotic events—are we creating our reservoirs? 2021. 13(11): p. 2250.
- Van der Vries, E., Influenza Resistance to Antiviral Drugs: Virus characterization, mechanism and clinical impact. 2014.
- Whittle, J.R., et al., Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. 2011. 108(34): p. 14216-14221.
- LeGoff, J., et al., I223R mutation in influenza A (H1N1) pdm09 neuraminidase confers reduced susceptibility to oseltamivir and zanamivir and enhanced resistance with H275Y. 2012.
- Wang, D., et al., Neuraminidase inhibitor susceptibility testing of influenza type B viruses in China during 2010 and 2011 identifies viruses with reduced susceptibility to oseltamivir and zanamivir. 2013. 97(3): p. 240-244.
- Deyde, V.M., et al., Surveillance of Resistance to Adamantanes among Influenza A(H3N2) and A(H1N1) Viruses Isolated Worldwide. The Journal of Infectious Diseases, 2007. 196(2): p. 249-257.
- Hurt, A.C., et al., Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors, 2014–2015. 2016. 132: p. 178-185.
- Deyde, V.M., et al., Surveillance of resistance to adamantanes among influenza A (H3N2) and A (H1N1) viruses isolated worldwide. 2007. 196(2): p. 249-257.
- Dharan, N.J., et al., Infections with oseltamivir-resistant influenza A (H1N1) virus in the United States. 2009. 301(10): p. 1034-1041.
- Butler, J., et al., Estimating the fitness advantage conferred by permissive neuraminidase mutations in recent oseltamivir-resistant A (H1N1) pdm09 influenza viruses. 2014. 10(4): p. e1004065.
- Baranovich, T., R.G. Webster, and E.A.J.C.O.i.V. Govorkova, Fitness of neuraminidase inhibitor-resistant influenza A viruses. 2011. 1(6): p. 574-581.
- Schünemann, H.J., et al., WHO Rapid Advice Guidelines for pharmacological management of sporadic human infection with avian influenza A (H5N1) virus. 2007. 7(1): p. 21-31.
- Duan, S., et al., Novel genotyping and quantitative analysis of neuraminidase inhibitor resistance-associated mutations in influenza viruses by single-nucleotide polymorphism analysis. 2011. 55(10): p. 4718-4727.
- Russell, R.J., et al., The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. 2006. 443(7107): p. 45-49.
- van der Vries, E., M. Schutten, and C.A.J.C.o.i.i.d. Boucher, The potential for multidrug-resistant influenza. 2011. 24(6): p. 599-604.
- Dong, G., et al., Adamantane-resistant influenza viruses in the world (1902–2013): frequency and distribution of M2 gene mutations. 2015. 10(3): p. e0119115.
- Omoto, S., et al., Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. 2018. 8(1): p. 9633.
- Donatelli, I., et al., Human-animal interface: the case for influenza interspecies transmission. 2017: p. 17-33.
- Lyall, J., et al., Suppression of avian influenza transmission in genetically modified chickens. 2011. 331(6014): p. 223-226.
- Lee, H.J., et al., Germline modification and engineering in avian species. 2015. 38(9): p. 743-749.
- Furuse, Y., et al., Large-scale sequence analysis of M gene of influenza A viruses from different species: mechanisms for the emergence and spread of amantadine resistance. 2009. 53(10): p. 4457-4463.
- Yi, C., et al., Genome-wide CRISPR-Cas9 screening identifies the CYTH2 host gene as a potential therapeutic target of influenza viral infection. 2022. 38(13).
- Abbott, T.R., et al., Development of CRISPR as an antiviral strategy to combat SARS-CoV-2 and influenza. 2020. 181(4): p. 865-876. e12.
- Owen, L., K. Laird, and M.J.L.i.A.M. Shivkumar, Antiviral plant-derived natural products to combat RNA viruses: Targets throughout the viral life cycle. 2022. 75(3): p. 476-499.
- Perovic, V., et al., Exploring the Antiviral Potential of Natural Compounds against Influenza: A Combined Computational and Experimental Approach. 2024. 25(9): p. 4911.
- Pizzorno, A., et al., Repurposing of drugs as novel influenza inhibitors from clinical gene expression infection signatures. 2019. 10: p. 60.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




