Introduction
Cryopreservation and chilling are the predominant strategies for sperm storage in various species, each with inherent advantages and limitations. Although, cryopreservation offers extended storage durations, but it severely affecting sperm motility, viability, acrosomal integrity, and fertilizing capacity (Küçük et al. 2021). Accordingly, the success rates of rabbit sperm cryopreservation vary with the use of different proposed extenders (DMSO (Küçük et al. 2021), dextran (Martínez-Pastor et al. 2021), luteolin (Akarsu et al. 2023), and curcumin (Abdelnour et al. 2020).
Lower conception rates of frozen semen compared to fresh semen present a significant challenge in achieving optimal pregnancy rates in rabbits, necessitating strategies to protect sperm against cryo-injuries (Y. Chen et al. 1989). Daniel and Renard (2010) also favored reliance fresh semen AI in rabbits as suboptimal fertility rates of frozen semen present a significant bottleneck at industrial level. Conversely, chilling semen at temperatures ranging from 4 to 15°C presents a less deleterious alternative for short-term storage, though it is not without challenges. Storing semen at higher temperatures (5 to 25°C) has its potential benefits, including ease of dilution, storage, transport, and efficient revival of sperm function in field conditions. Mocé et al. (2010) documented higher pregnancy rates with rabbit semen chilled to 4 to 22°C, which is more practical in field conditions. The primary concern in chilling rabbit semen is the production of reactive oxygen species (ROS) and bacterial proliferation (Martínez-Pastor et al. 2021). The use of antibiotics in chilled semen also raises the concern of antibiotic resistance, which urges the exploration of alternative extenders for sperm storage (Martínez-Pastor et al. 2021; Viudes-de-Castro et al. 2021). Previous studies have investigated the use of l-carnitine (Sarıözkan et al. 2014), honey (Gardela et al. 2023), and melatonin (Fadl et al. 2021) to protect rabbit sperm in different methods of freezing. During chilling of rabbit sperm, various alternative agents have been included in extender to protect sperm under storage conditions by supplying nutrients for energy, pH modulation, cold shock resistant, antioxidants and bacterial growth inhibitors (Kubovicova et al. 2022). Addition to these, chilling temperatures also affect bacterial growth and sperm metabolism (Hozbor et al. 2016) which, in turn lead to oxidative stress and the premature depletion of energy resources in the semen. To mitigate the adverse effects on sperm during low temperature storage, sericin, a silk-derived protein, has been utilized as a cryoprotective, antioxidant, and antimicrobial agent (Zhang 2002). Sericin possesses adhesive properties, rich in amino acids, and has water solubility along with potential cryoprotective properties for sperm and embryos (Sasaki et al. 2005). This study aims to investigate the effects of sericin and different storage temperatures on sperm through in vivo and in vitro experiments, offering new insights into alternative preservation strategies for rabbit sperm storage.
Materials and Methods
Extender Preparation
A basic tris-citric acid-glucose (TCG) extender was prepared for semen dilution, comprising tris (250.04 mM), citric acid (79.76 mM), glucose (69.38 mM), streptomycin (75.00 IU), and penicillin-G (166.20 IU). The pH and osmolarity of the extender were meticulously adjusted to 7.14 and 299 mOsm/kg, respectively. The extenders were immediately stored at −20°C until use.
Animal Selection and Husbandry Practices
The present study was sanctioned by Aydin Adnan Menderes University Animal Ethics Committee for the welfare and use of experimental animals (ADÜ-HADYEK No. 64583101/2020/007). For this purpose, twelve (n=12) male New Zealand white rabbits with average body weight of 2.9±0.1 kg, and aged 10–12 months were selected. The bucks were separately confined in galvanized wire cages (70×50×35 cm) in a naturally ventilated environment with standard daylight (12–14 h) at a room temperature of 16–28°C. They were offered commercially prepared feed with free access to clean fresh water. The rabbit bucks underwent proper training for semen collection using teaser does, and only high-quality semen donor bucks were selected for further experiments.
Semen Collection and Evaluation
The semen was collected from rabbit bucks by a single operator using an artificial vagina mounted over a teaser doe. Following collection, the gel plug was immediately discarded, and the semen was placed in a water bath at 37°C for further evaluation. All individual ejaculates were examined for motility, concentration and morphology through standard procedures. Ejaculates with ≥ 70% motile, 200×106 mL-1, and 80 % morphologically normal sperm were selected for further experimentation.
Sperm Treatment and Processing
Briefly, the best qualifying ejaculates (six semen samples) after the initial evaluation were pooled (one ejaculate/male; six males/pool) and diluted with the TCG extender to achieve a final sperm concentration of 50×106 mL-1. Afterward, the diluted semen was split into three aliquots and categorized into control, 0.1 and 0.5% sericin groups (Sericin Bombyx mori-silkworm, S5201, Sigma-Aldrich, USA). Each treatment group was stored at either 4°C or 15°C for a period of 72 hours. All the samples were observed for sperm motility, sperm kinematics, acrosome integrity, sperm viability, and plasma membrane integrity at 0, 24, 48, and 72 hours of the storage period at 4°C or 15°C. The experiment was replicated at least five times for observation recording.
Sperm Motility and Kinematics
The motility assessment of each sample was performed using the CASA system (SCA®-Sperm Class Analyzer, Microptic S.L. Viladomat, Barcelona, Spain) connected to a phase-contrast microscope (Olympus, CX41, Japan) with an adjustable heated stage and camera. The semen samples were subjected to motility assessment using a 3 µL drop by placed over a pre-warmed glass slide covered with a coverslip (18 × 18 mm2). The CASA system specifications were adjusted for rabbit sperm motility properties, and standardized accordingly. To detect only sperm head and exclude other particles or gel droplets, a particle size > 10 µm was used. During the analysis, at least five fields were randomly selected on the slide (100 sperms/field at ×10).
Evaluation of Sperm Viability
A small drop of semen and 1% nigrosin–eosin stain were combined over a prewarmed glass slide to prepare thin and uniform smears. The air-dried smears were then observed using phase contrast microscopy at ×1000 (oil emersion lens) for unstained heads (live sperm) and stained or partially stained heads (dead sperm).
Assessment of Sperm Membrane Integrity
A small volume of semen sample (25 µL) was exposed to a hypo-osmotic solution (475 µL, 100 mOsm/kg) and incubated for 15 min at 35°C. After the osmotic challenge, the percentage of membrane-intact sperm were calculated by preparing a wet mounts. Sperm sub-populations were counted based on the curliness of sperm tail portion to assess the integrity or damage to the plasma membrane. Approximately 200 sperm populations were counted using a bright-field microscope (40×).
Evaluation of Acrosome Integrity
A Coomassie Blue G-250 staining method was adopted from a previous study to assess the percentage of acrosome-intact sperm (Larson and Miller 1999). Firstly, 50 µl of sperm were fixed for 10 min in a 4% paraformaldehyde solution (110 mM Na2HPO4, 2.5 mM NaH2PO4, 4% paraformaldehyde, pH 7.4) at room temperature. The samples were then centrifuged, and the pellet was washed twice using ammonium acetate (100 mM; pH 9.0). Subsequently, a 25–50 µl sperm suspension was transferred onto a glass slide, smeared, and air-dried. Coomassie stain (0.22% Coomassie Blue G-250, 50% methanol, 10% glacial acetic acid, 40% water) was poured over the smeared area on the slide for 2 min. The excess stain was washed off with distilled water, and the cells were counted by using a bright-field microscope at 100× magnification. A total of 200 sperm were counted for each group per replicate.
Assessment of Bacterial Growth
The semen samples stored at 15°C for 72 hours were processed to assess the bacterial load. The samples were homogenized with physiological saline at a 1:9 (v/v) ratio, and 100 µl of appropriate dilutions were seeded in parallel using plate count agar (Oxoid CM 325) medium by streaking in duplicates. Petri dishes were incubated for 48 hours at 36 °C, and the developing colonies were evaluated according to the OIE (1998) protocol. Plates containing between 15 and 300 colony forming units (CFU) were counted, while those with >300 CFU were graded as “+300 UFC”. The CFU mL-1 for each sample was calculated by multiplying the average number of colonies counted in duplicates by the inverse of the highest dilution, considering a 100 µL inoculum per plate. All colonies grown on petri dishes containing plate count agar medium were counted following the procedure described by Gączarzewicz et al. (2016), and typical total coliform colonies were considered for culture using violet red bile agar (1-2 mm in diameter, red with a pinkish precipitation halo).
Evaluation of In Vivo Fertility
To observe the effect of sericin-treated sperm stored for 72 hours at 4 or 15 °C on fertility, ninety-six New Zealand white rabbit does were inseminated. Receptive multiparous does (3 to 4.5 kg) with reddened to purple vaginal mucosa were selected and inseminated transvaginally using 12.5×106 sperm. The does were held in either dorsal or ventral recumbency, and the semen was transferred at the uterine bifurcation. To induce ovulation, the does were injected with GnRH injection (Buserin, 0.2ml = 0.004mg/ml, i.m, Turkey) at the time of insemination. The pregnancy status of the does was assessed using transabdominal ultrasonography with a micro-convex probe (Mylab 30-Esaote®, Genova, Italy) on day 9 post-insemination. During the ultrasound scanning, the presence of fetal heartbeat and gestational sacs were considered positive signs of pregnancy.
Statistical Analyses
All the data in this study were analyzed using the R-Studio. In experiment 1, the variables, extender type (control, 0.1 or 0.5% sericin), storage temperature (4 or 15°C), storage time (0, 24, 48, 72 h) were incorporated and subjected to Generalized Linear Mixed Model GLMM) for main effects, and interactions of variables. The sperm quality parameters were then compared using Tukey HSD test. In Experiment 2, data from both storage temperature conditions (4 or 15°C) were analyzed through the logistic regression to compare the bacterial load and pregnancy rate. In both experiments, a P value of <0.05 was deemed statistically significant. Results were expressed as the model-derived mean ± standard error of the mean (SEM).
Results
Experiment 1
Sperm motility characteristics (Motility and kinematics)
The results given in
Table 1 indicate that progressive and total motility of rabbit sperm were significantly influenced by sericin treatment, storage temperature, and storage duration. Interactions were observed between storage duration and storage temperature for both progressive and total motilities, as well as between sericin treatment and storage temperature for total motility. As shown in
Table 1, storage temperature significantly affected all sperm kinematic variables (VCL, VSL, VAP, LIN, STR, WOB, ALH, and BCF). The sperm VCL, VSL, VAP, ALH, and BCF were significantly influenced by sericin treatment, while VCL, VAP, STR, WOB, and BCF were significantly affected by storage duration. Interaction between storage duration and storage temperature was observed for VCL, VAP, and BCF. The rest of the sperm kinematic variables did not show interactions between sericin treatment and storage temperature or between sericin treatment and storage duration. However, WOB showed an interaction among storage temperature, sericin treatment, and storage duration.
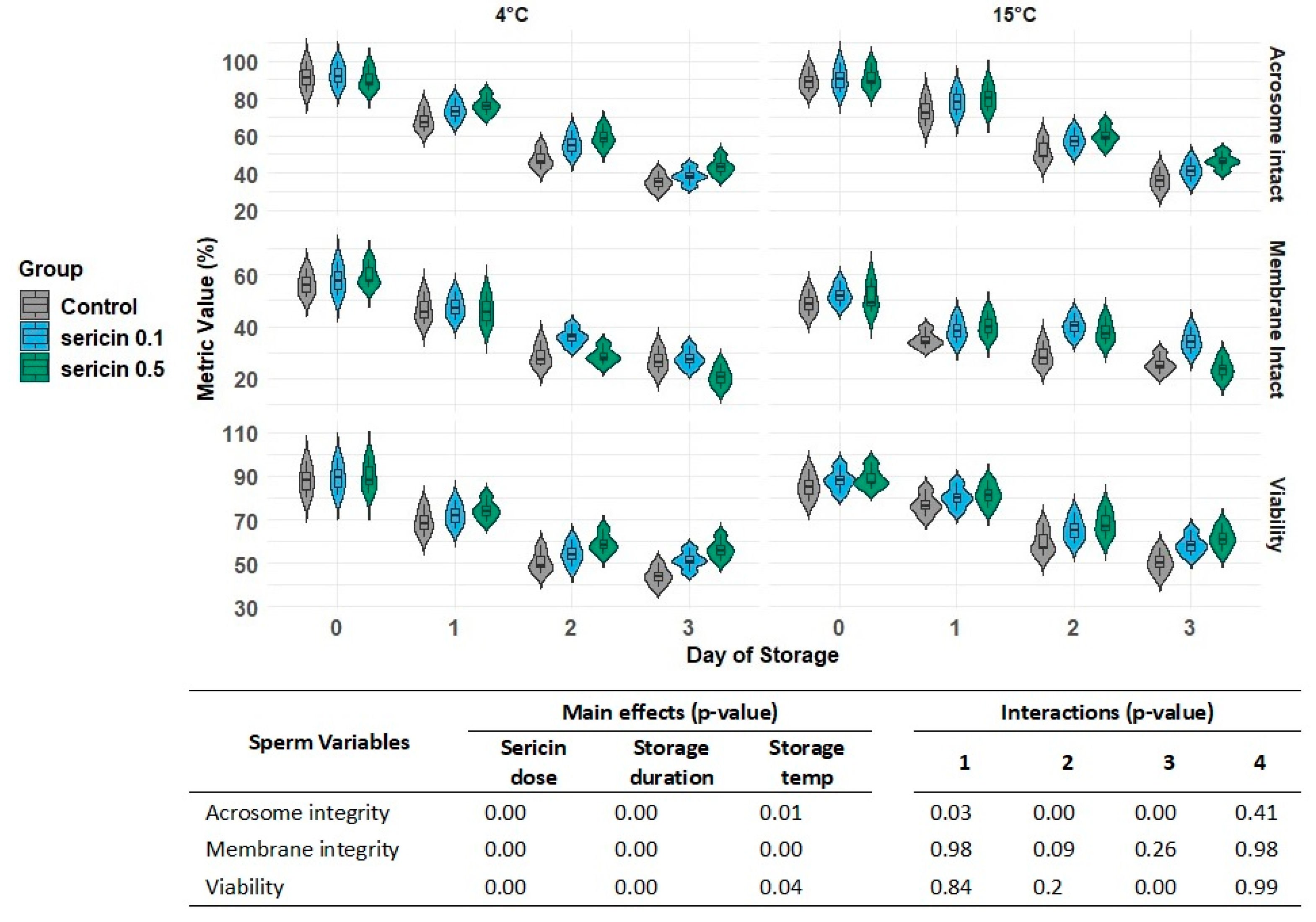
Sperm viability, plasma membrane integrity and acrosome integrity
Based on the results for acrosome integrity, membrane integrity, and sperm viability staining, significant effects of sericin treatment, storage temperature, and storage duration were observed. Interactions were found between sericin treatment and storage temperature, sericin treatment and storage duration, and storage temperature and storage duration concerning membrane integrity. However, interactions were non-significant for acrosome integrity, although an interaction between storage temperature and storage duration was found for sperm viability rate (
Figure 1).
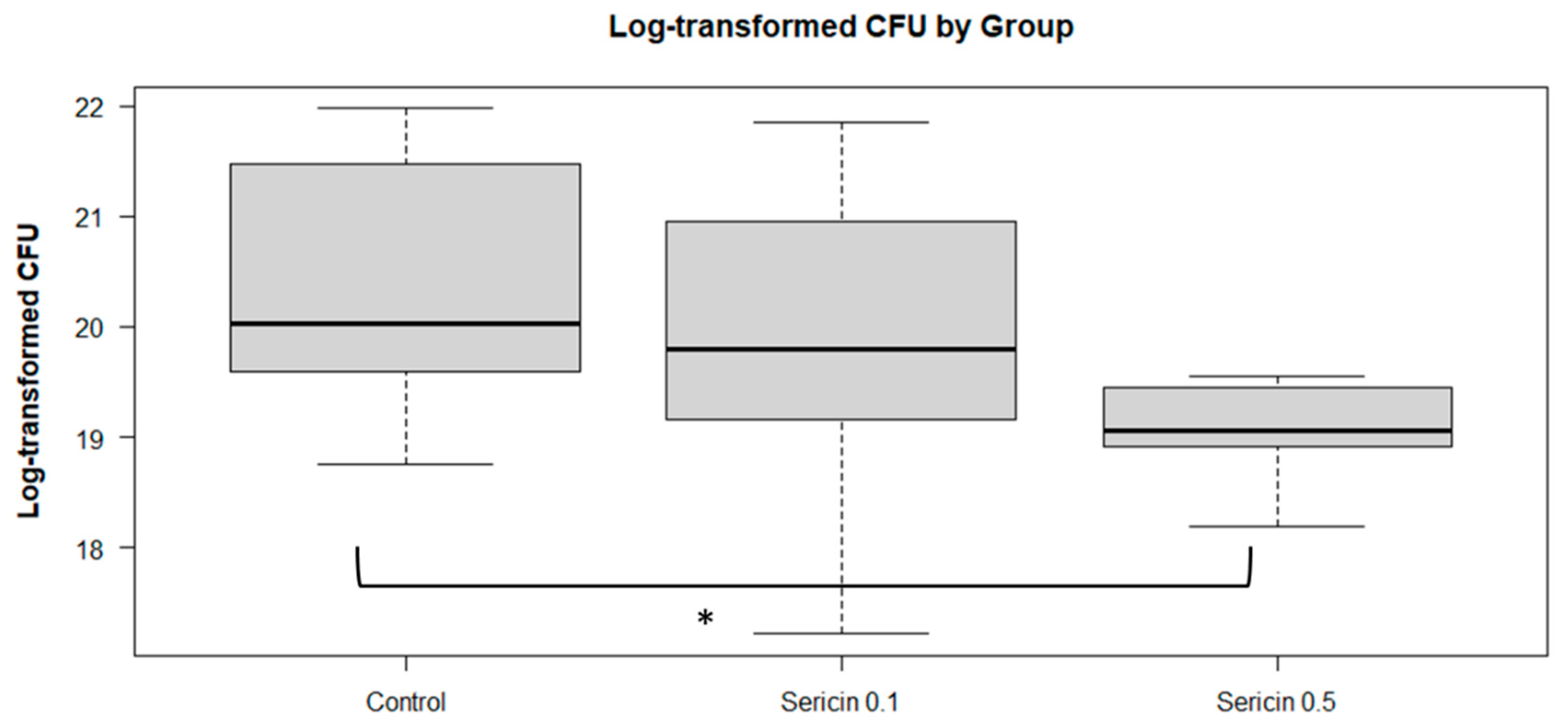
Bacterial load
The estimated marginal means of the log CFU count, presented in
Figure 2, indicate that the log CFU count for the control group was significantly different from that of the sericin 0.5 group (p = 0.0463). However, comparisons between the control group and the sericin 0.1 group (p = 0.6538), or between sericin treatment groups (sericin 0.1 vs. sericin 0.5, p = 0.2603), did not show any statistical difference.
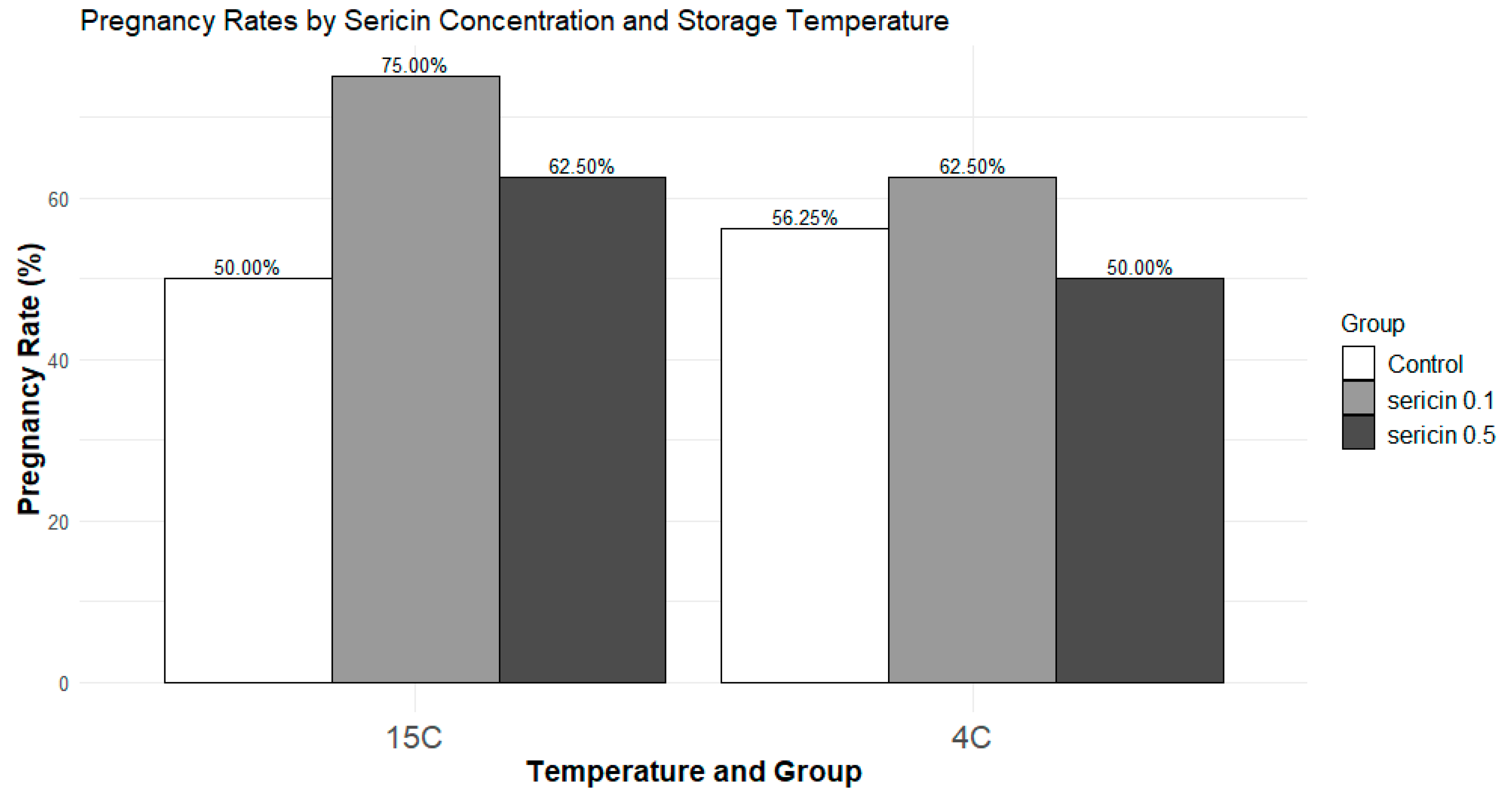
In vivo fertility
A numerically higher percentage of rabbit does become pregnant when inseminated by semen treated with sericin 0.1% (75%) compared to the control or sericin 0.5% groups (50 and 62.5%, respectively), particularly at 15°C. Logistic regression analysis showed no significant effect of sericin concentrations (0.1 or 0.5%) or storage temperatures (4 or 15֯ C) and their interaction on the likelihood of achieving pregnancy (
Figure 3).
Discussion
The present study results show that sericin supplementation appears to maintain progressive and total motility of sperm stored at 4°C and 15°C over a period of three days. However, a significant decrease in sperm quality parameters was observed at day 2 and 3 of storage at these temperatures. This sharp decline is consistent with previous research attributing the vulnerability of rabbit sperm to oxidative damage, primarily due to the high content of polyunsaturated fatty acids and limited tolerance to chilling (Roca et al. 2000; Rosato and Iaffaldano 2011). Several additives, including caffeine, gelatin, quercetin, methionine, and various antioxidants, have been investigated to prolong the storage time and fertility of rabbit semen at 5 or 15°C. These substances have shown promising results in maintaining sperm motility and other quality parameters, extending sperm lifespan up to 3–4 days (Johinke et al. 2014; 2015; López and Alvariño 2000; Nagy et al. 2002).
The sustained pattern motility (both total and progressive) of rabbit sperm stored at 15°C was observed with sericin treatment (0.1%) over an extended period (72 hours). This effect is attributed to the bioactive properties of sericin, which enhance sperm resilience to cold stress. Numerous reports describe the beneficial effects of sericin with same dose level during cryopreservation, while maintaining the motility during liquid storage has been documented in rooster sperm using a smaller dose of sericin (Ratchamak et al. 2023). Additionally, the consistent pattern of sperm motility in sericin-treated samples indicates that sericin may be involved in metabolic activity of sperm, although further investigations are needed.
The relationship between sperm kinematics and fertility outcomes has long been recognized in rabbits. This study posited that supplementing sericin could enhance sperm kinematics, thereby potentially improving fertility outcomes (Johinke et al. 2014). A similar trend in rabbit sperm kinematics variables, as observed in motility, was observed by sericin treatment in response to storage temperature and duration. These results suggest that sericin exerts a protective effect on sperm kinematics during storage at cooler temperatures by providing antioxidant and antibiotic properties, thereby helping rabbit sperm sustain their viability for a longer duration.
One major challenge encountered in rabbit liquid semen storage is the "dilution effect," whereby extending semen beyond a certain threshold leads to alterations in sperm plasma membrane structure and function (M. Aksoy et al. 2010). While some reports suggest that increased dilution rates provide substantial nutrients for extended storage, the dilution effect can reduce the availability of plasma proteins for sperm, resulting in lower fertility (Di Iorio et al. 2023). Sericin, a biomolecule with its unique molecular properties, could be utilized as a first aid to repair damages to the plasma membrane (D. Aksoy et al. 2024), especially when addressing dilution factors. Interestingly, sericin-treated sperm exhibited slightly higher levels of membrane and acrosomal integrity from days 1 to 3 at both storage temperatures. The exact mechanism by which sericin protects rabbit sperm during refrigeration is not yet known. Assessing the antioxidants and biochemical profile of sericin-treated rabbit sperm pre- and post-exposure at cooler temperatures for different time points could reveal the underlying mechanism. Similar improvements in sperm membrane and acrosomal integrity have been reported in dairy bulls and boars semen supplemented with sericin during the cryopreservation process (Ratchamak et al. 2020; Yangnam et al. 2021).
In the present study, the antimicrobial activity of sercicn was evaluated by selecting a storage temperature of 15°C, which is more conducive to bacterial growth compared to refrigeration. The mean log CFU count was significantly reduced in the sericin 0.5 supplemented rabbit semen after 72 hours of storage, suggesting a potential inhibitory effect of sericin treatment on microbial growth. Due to its antimicrobial effect, sericin is used in sericin-based hydrogels in wound healing (Xue et al. 2016) and cryogels for wound dressing materials (C. Chen et al. 2023). Sericin could be a promising supplement in semen extenders and culture media for the maturation and fertilization of oocytes (Isobe et al. 2015; Yasmin et al. 2015).
Interestingly, the does inseminated with sperm treated with a 0.1% sericin concentration exhibited a numerically higher pregnancy rate after 72 hours of storage at 15°C. However, logistic regression analysis did not reveal a significant effect of sericin concentration, storage temperature, or their interaction on the likelihood of achieving pregnancy. Our findings shed light on the intricate relationship between sericin concentration, temperature, and potential increase in in-vivo fertility. Including a larger sample of rabbit does in insemination trials could provide a better understanding of how rabbit sperm responds to different storage conditions and durations.
In conclusion, treating rabbit sperm with sericin and storing it at 4 and 15°C for 48 to 72 hours holds promising effects on sperm quality. Furthermore, the antibacterial role of sericin during liquid semen storage at lower temperatures have been elucidated. Treatment with sericin could be a potential way to enhance pregnancy outcomes when rabbit sperm is refrigerated for extended durations.
Funding
This study was has been support by the Directorate of Scientific Research Projects of Aydın Adnan Menderes University through VTF-20031.
Authors contributions
Sanan Raza: Investigation, experiment, writing and analysis; Ugur Ucan and Melih Aksoy: Supervision, writing, revision and analysis; Gunes Erdogan and Komal Khan: Conceptualization and writing; Zahid Naseer: Writing, review and submission .
Conflicts of Interest
We confirm that there are no conflicts of interest to disclose or related to the content discussed in this manuscript with any financial organization.
References
- Abdelnour SA, Hassan MA, Mohammed AK et al (2020) The effect of adding different levels of curcumin and its nanoparticles to extender on post-thaw quality of cryopreserved rabbit sperm. Animals 9:1508. [CrossRef]
- Akarsu S, Acısu T, Güngör İ et al (2023) The effect of luteolin on spermatological parameters, apoptosis, oxidative stress rate in freezing rabbit semen. Pol J Vet Sci:91-98-91-98. [CrossRef]
- Aksoy D, Turan DN, Bayraktar ZB (2024) Cholesterol and Sericin as First Aid for Damaged Cells. J Biosci Med 04:79-88. [CrossRef]
- Aksoy M, Cankat Lehimcioglu N, Akman O (2010) Effect of seminal plasma on functional integrity of rabbit sperm membranes during storage at 4ºC or freezing. World Rabbit Sci 1. [CrossRef]
- Chen C, Chen L, Mao C et al (2023) Natural Extracts for Antibacterial Applications. Nano Micro Small:2306553. [CrossRef]
- Chen Y, Li J, Simkin M, Yang X, Foote R (1989) Fertility of Fresh and Frozen Rabbit Semen Inseminated at Different Times is Indicative of Male Differences in Capacitatlon Time. Biol Reprod 5:848-853. [CrossRef]
- Daniel N, Renard J-P (2010) Artificial insemination in rabbits. Cold Spring Harb Protoc 1:pdb. prot5358. [CrossRef]
- Di Iorio M, Lauriola F, Rusco G, Antenucci E, Schiavitto M, Iaffaldano N (2023) Cryopreserving Rabbit Semen: Impact of Varying Sperm Concentrations on Quality and the Standardization of Protocol. Vet Sci 1:9. [CrossRef]
- Fadl AM, Ghallab ARM, Abou-Ahmed MM, Moawad AR (2021) Melatonin can improve viability and functional integrity of cooled and frozen/thawed rabbit spermatozoa. Reprod Domest Anim 1:103-111. [CrossRef]
- Gączarzewicz D, Udała J, Piasecka M, Błaszczyk B, Stankiewicz T (2016) Bacterial Contamination of Boar Semen and its Relationship to Sperm Quality Preserved in Commercial Extender Containing Gentamicin Sulfate. Pol J Vet Sci 3:451-459. [CrossRef]
- Gardela J, Ruiz-Conca M, Palomares A et al (2023) Effect of Honey, Coenzyme Q10, and β-Carotene/α-Tocopherol as Novel Additives in Rabbit-Sperm Cryopreservation Extender. Animals 14:2392. [CrossRef]
- Hozbor F, Ledesma A, Manes J et al (2016) Improve intra-uterine insemination in rabbits using ultra-high temperature skim milk as extender to keep semen at room temperature. Andrologia 2:231-234. [CrossRef]
- Isobe T, Ikebata Y, Do LTK, Tanihara F, Taniguchi M, Otoi T (2015) In vitro development of OPU-derived bovine embryos cultured either individually or in groups with the silk protein sericin and the viability of frozen-thawed embryos after transfer. Anim Sci J 7:661-665. [CrossRef]
- Johinke D, De Graaf S, Bathgate R (2014) Quercetin reduces the in vitro production of H2O2 during chilled storage of rabbit spermatozoa. Anim Reprod Sci 3-4:208-219. [CrossRef]
- Johinke D, de Graaf S, Bathgate R (2015) The Effect of Sperm Concentration and Storage Vessel on Quercetin-Supplemented Rabbit Semen During Chilled Storage. Reprod Domest Anim 4:567-573. [CrossRef]
- Kubovicova E, Makarevich AV, Balazi A, Vasicek J, Chrenek P (2022) Factors affecting rabbit sperm cryopreservation: a mini-review. Zygote 1:1-8. [CrossRef]
- Küçük N, Raza S, Matsumura K et al (2021) Effect of different carboxylated poly l-lysine and dimethyl sulfoxide combinations on post thaw rabbit sperm functionality and fertility. Cryobiology:127-132. [CrossRef]
- Larson JL, Miller DJ (1999) Simple histochemical stain for acrosomes on sperm from several species. Mol Reprod Develop 4:445-449. [CrossRef]
- López F, Alvariño J (2000) Effects of added caffeine on results following artificial insemination with fresh and refrigerated rabbit semen. Anim Reprod Sci 1-2:147-154. [CrossRef]
- Martínez-Pastor F, Lacalle E, Martínez-Martínez S et al (2021) Low density Porcicoll separates spermatozoa from bacteria and retains sperm quality. Theriogenology:28-36. [CrossRef]
- Mocé E, Lavara R, Vicente JS (2010) Effect of cooling rate to 5 C, straw size and farm on fertilizing ability of cryopreserved rabbit sperm. Reprod Domest Anim 5:e1-e7. [CrossRef]
- Nagy S, Sinkovics G, Kovács A (2002) Viability and acrosome integrity of rabbit spermatozoa processed in a gelatin-supplemented extender. Anim Reprod Sci 3-4:283-286. [CrossRef]
- Ratchamak R, Authaida S, Boonkum W, Chankitisakul V (2023) Improvement of rooster semen freezability and fertility rate after sericin supplementation in freezing semen extender. Anim Biosci 10:1530. [CrossRef]
- Ratchamak R, Ratsiri T, Kheawkanha T, Vongpralub T, Boonkum W, Chankitisakul V (2020) Evaluation of cryopreserved boar semen after supplementation sericin form silkworm (Bombyx mori) in semen extender. Anim Sci J 1:e13428. [CrossRef]
- Roca J, Martınez S, Vázquez J, Lucas X, Parrilla I, Martınez E (2000) Viability and fertility of rabbit spermatozoa diluted in Tris-buffer extenders and stored at 15 C. Anim Reprod Sci 1-2:103-112. [CrossRef]
- Rosato M, Iaffaldano N (2011) Effect of chilling temperature on the long-term survival of rabbit spermatozoa held either in a tris-based or a jellified extender. Reprod Domest Anim 2:301-308. [CrossRef]
- Sarıözkan S, Özdamar S, Türk G, Cantürk F, Yay A (2014) In vitro effects of l-carnitine and glutamine on motility, acrosomal abnormality, and plasma membrane integrity of rabbit sperm during liquid-storage. Cryobiology 3:349-353. [CrossRef]
- Sasaki M, Kato Y, Yamada H, Terada S (2005) Development of a novel serum-free freezing medium for mammalian cells using the silk protein sericin. Biotechnol appl biochem 2:183-188. [CrossRef]
- Viudes-de-Castro MP, Marco-Jimenez F, Vicente JS, Marin C (2021) Antibacterial activity of some molecules added to rabbit semen extender as alternative to antibiotics. Animals 4:1178. [CrossRef]
- Xue R, Liu Y, Zhang Q et al (2016) Shape changes and interaction mechanism of Escherichia coli cells treated with sericin and use of a sericin-based hydrogel for wound healing. Appl Environ Microbiol 15:4663-4672. [CrossRef]
- Yangnam Y, Chapanya S, Vongpralub T, Boonkum W, Chankitisakul V (2021) Effect of semen extender supplementation with sericin on post-thaw dairy bull sperm quality and lipid peroxidation. Czech J Anim Sci 1:13-20. [CrossRef]
- Yasmin C, Otoi T, Setiadi M, Karja N (2015) Maturation and fertilisation of sheep oocytes cultured in serum-free medium containing silk protein sericin. Acta Vet Hung 1:110-117. [CrossRef]
- Zhang Y-Q (2002) Applications of natural silk protein sericin in biomaterials. Biotechnol Adv 2:91-100. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).