1. Introduction
The stored product ecosystem is considered as a man-made environment, which is not affected much by abiotic conditions that are determinative in the case of other ecosystems, such as those in the agroforestry environments (Odum 1989, Dunkel 1992). For instance, in bulked grains, heat conduction is considered to occur much more gradually than that of the surrounding air, a phenomenon that has been characterized as “hysteresis” (Hagstrum 1987, 1989, Athanassiou et al. 2003). As a result, the grain cools down slowly during winter months, allowing insect acclimation to the extreme temperature conditions, constituting stored product insects tolerant to cold (Andreadis and Athanassiou 2017 and references therein). Indicatively, in an earlier study, Athanassiou and Arthur (2020) found that several stored product insects remained unaffected by the exposure at 0 oC for 7 days.
In a similar way with bulked grains, food processing facilities retain temperatures that are higher than those in the surrounding environment during the cold period of the year, given that these facilities usually operate throughout the year and have to be heated when this is considered necessary (Dowdy and McGaughey 1994, Campbell et al. 2010a, b, Semeao and Campbell 2013, Morrison et al. 2023). Hence, insect activity is continuous in these areas during the entire year (Trematerra and Sciarretta 2004, Trematerra et al. 2007, Semeao and Campbell 2013). For instance, Morrison et al. (2023) found that the population fluctuation of stored product insects in different processing facilities in Greece indicated larger insect numbers in summer and early fall, but there were considerable insect numbers during winter months as well.
Based on the above, and in contrast with pests that occur in different types of crops and orchards, stored product insects have a dynamic presence all year round, with often overlapping generations and, as such, their presence requires continuous trapping and sampling (Athanassiou and Buchelos 2001, 2020, Morrison et al. 2023). In fact, often detection during the cold period of the year is even more important than that in the warm period, in order to estimate hidden infestations and initial colonization foci (Athanassiou et al. 2005, Trematerra et al. 2007, Campbell et al. 2010a). For instance, Athanasiou et al. (2005) found that insect colonization in silos starts from the upper bulk part. Nevertheless, large insect populations may likely occur in the bottom layers in silos with false floors (Hagstrum 1989, Weston and Barney 1998, Athanassiou et al. 2005).
Floor traps have been proved important tools for the detection of insects in processing facilities, and have been tested with success in different application scenarios (Campbell et al. 2010a, b, Morrison et al. 2023). Not surprisingly, these traps can be even used unbaited, as take advantage of the stereotropism of crawling insects (Morrison et al. 2023). Several studies have shown that even the addition of a pheromone may not affect captures. At the same time, floor traps may capture and retain insects without the need of a killing agent (Trematerra and Sciarretta 2004, Trematerra et al. 2007, Athanassiou et al. 2016, 2017a, Morrison et al. 2023). In an extensive surveillance based on floor traps that was carried out a few years ago, Morrison et al. (2023) indicated that these traps could depict the spatio-temporal distribution of stored product insects in processing facilities, as well as the dominance and frequency of the main species, and eventually provide guidance on specific control measures. As a continuance of that work, we used floor traps in order to illustrate the population dynamics of major stored product insects in a feed mill, as an effort to locate increased insect presence, and time localized control measures.
2. Materials and Methods
2.1. Facility
The trapping was conducted in a feed mill in Northern Greece, which stored and processed soft and hard wheat, but also barley and maize in smaller quantities. The traps were deployed on the facility on 24 February 2022.
2.2. Experimental Design
The trap type that was used in our experiments was Dome Trap (Trécé Inc., USA), and the attractant was StorgardTM Oil kairomone food (Oil, Trécé Inc., USA). In total, 36 traps were used and were checked every 15 days, except for periods when access to trapping areas was limited due to periodical spraying with insecticides or fumigations. The attractant oil was refilled when it was considered necessary. All captured insects were transferred to the Laboratory of Entomology and Agricultural Zoology (LEAZ), Department of Agriculture, Crop Production and Rural Environment, at the University of Thessaly, where counting and identification were carried out. The insects found were identified using different taxonomic keys, such as Bousquets (1990), Gorham (1991), and Peacock (1993).
2.3. Data Analysis
The insect numbers captured were analyzed according to the criteria of “Dominance” and “Frequency” as used by Curry (1973) and Buchelos and Athanassiou (1993). The term Dominance signifies the percentage of individuals belonging to a particular species compared to the individuals of all species identified in total. Thus, a given species can be classified as Dominant, Influent, and Recedent, corresponding to >5, 2–5 and <2% of the total number of individuals found. A species “Frequency” is measured by the percentage of samples in which the particular species was reported. Thus, a given species can be classified as Constant, Accessory, and Accidental, when individuals of this species have been found to >50, 25-50 or less than 25% of the total number of samples (Buchelos and Athanassiou 1993). Additionally, the spatiotemporal distribution was visualized using Python, specifically employing the Matplotlib library for visualization and the SciPy library's interpolation module for handling data points in multiple dimensions.
3. Results
A total of 2.113 individuals were collected, corresponding the several taxa. The individuals found corresponded to 5 Orders, 15 Families, and at least 16 species. The most dominant species were the confused flour beetle,
Tribolium confusum Jacquelin DuVal (Coleoptera: Curculionidae), the red flour beetle,
Tribolium castaneum (Herbst) (Coleopetra: Tenebrionidae), the rice weevil,
Sitophilus oryzae (L.) (Coleoptera: Curculionidae), the granary weevil,
Sitophilus granarius (L.) (Coleoptera: Curculionidae), and members of the Orders of Lepidoptera and Diptera (
Table 1). The most frequently found species were
T. confusum (406 adults),
T. castaneum (319 adults),
O. surinamensis (79 adults), and
S. granarius (410 adults) (
Table 1).
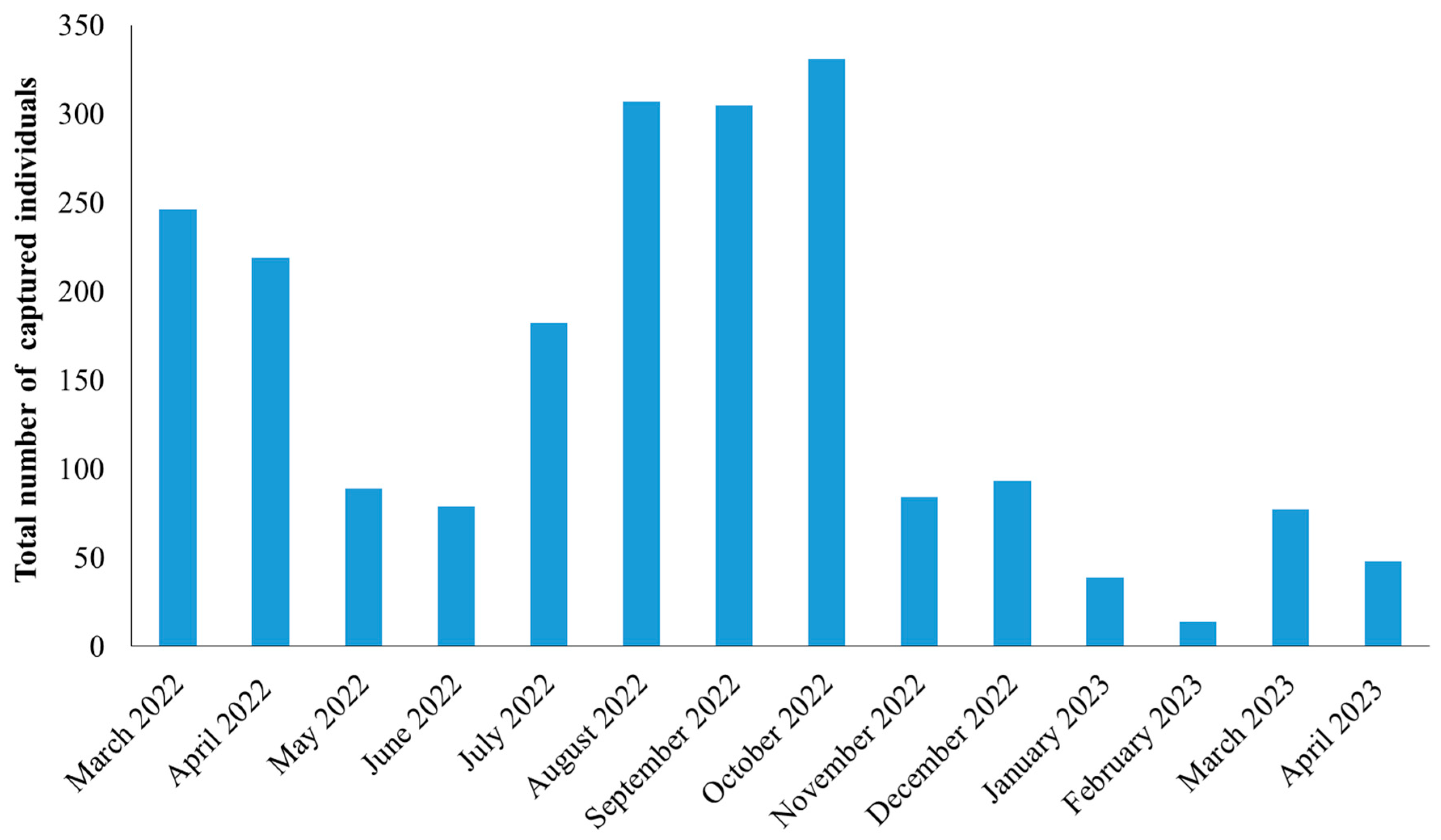
The highest number of stored product insect individuals was captured in August, September, and October 2022, with 307, 305, and 331 individuals to be recorded, respectively (
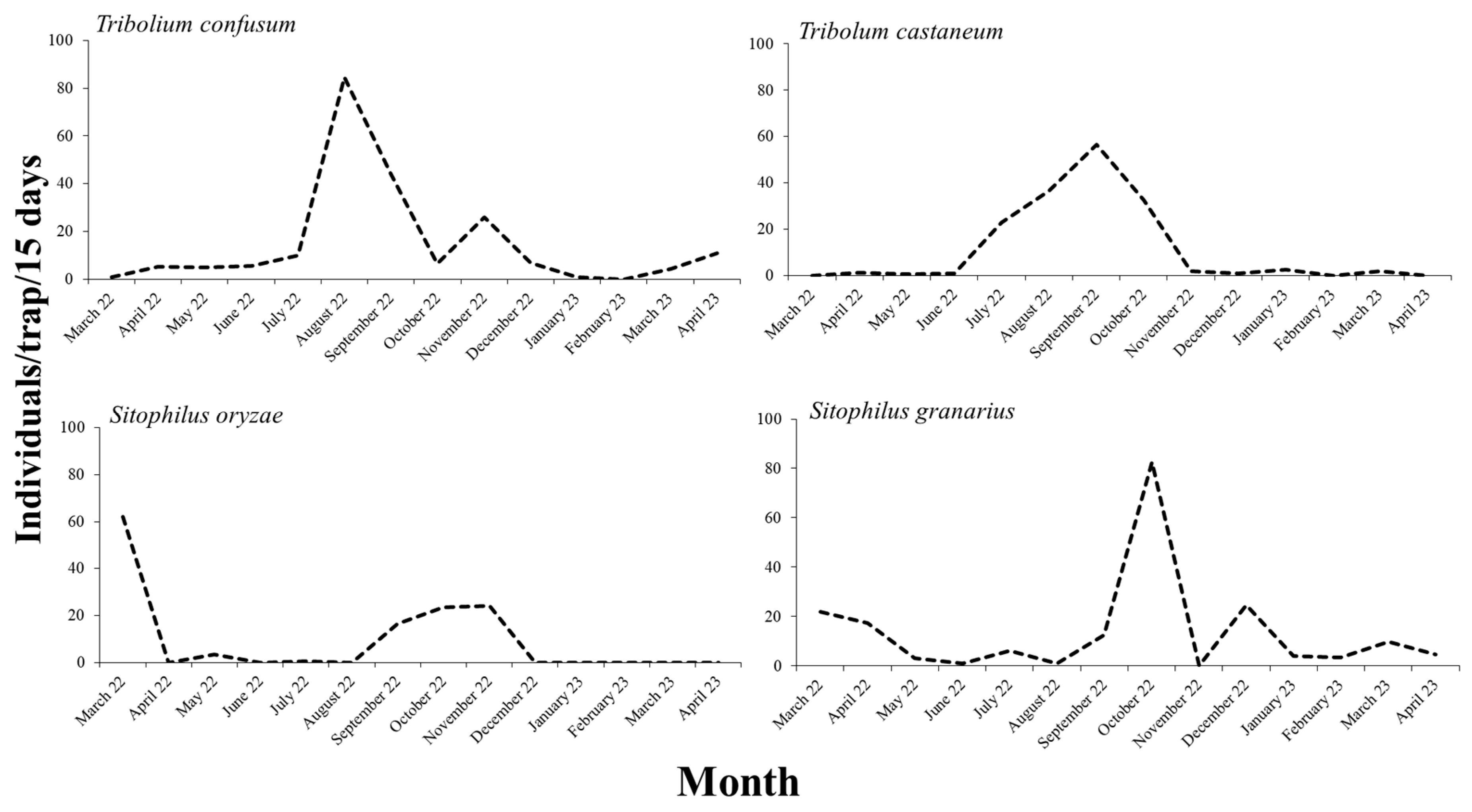
Figure 1). Regarding the dominant species, in the case of
T. confusum and
T. castaneum, the majority of the individuals were recorded in August and September, respectively (
Figure 2). On the other hand, the highest number of individuals for
S. oryzae was found in March, at the beginning of the trapping period, but high numbers were also recorded in autumn. In a similar pattern, the peak of
S. granarius individuals in the traps was noted in October (
Figure 2).
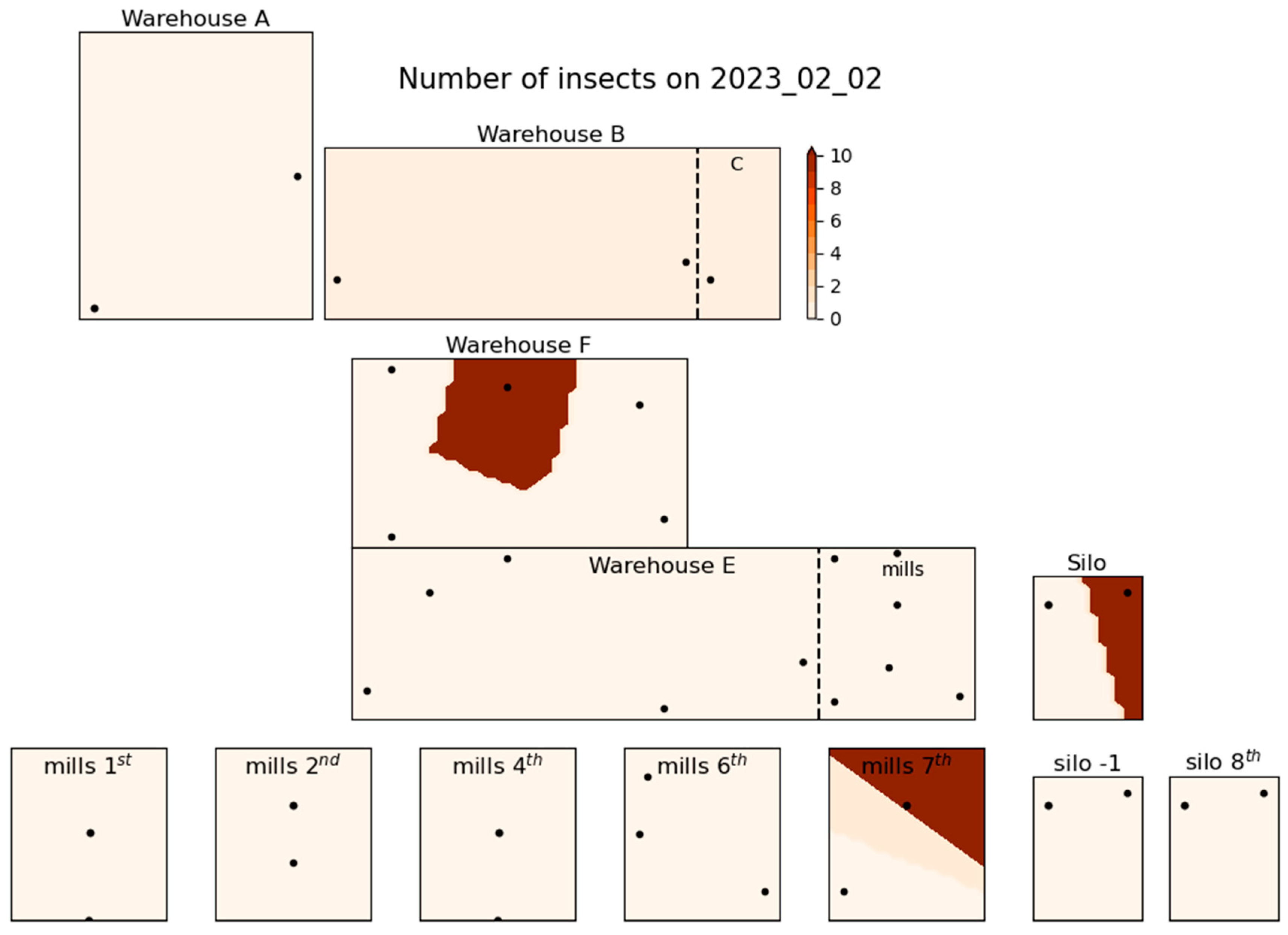
Figure 3,
Figure 4 and
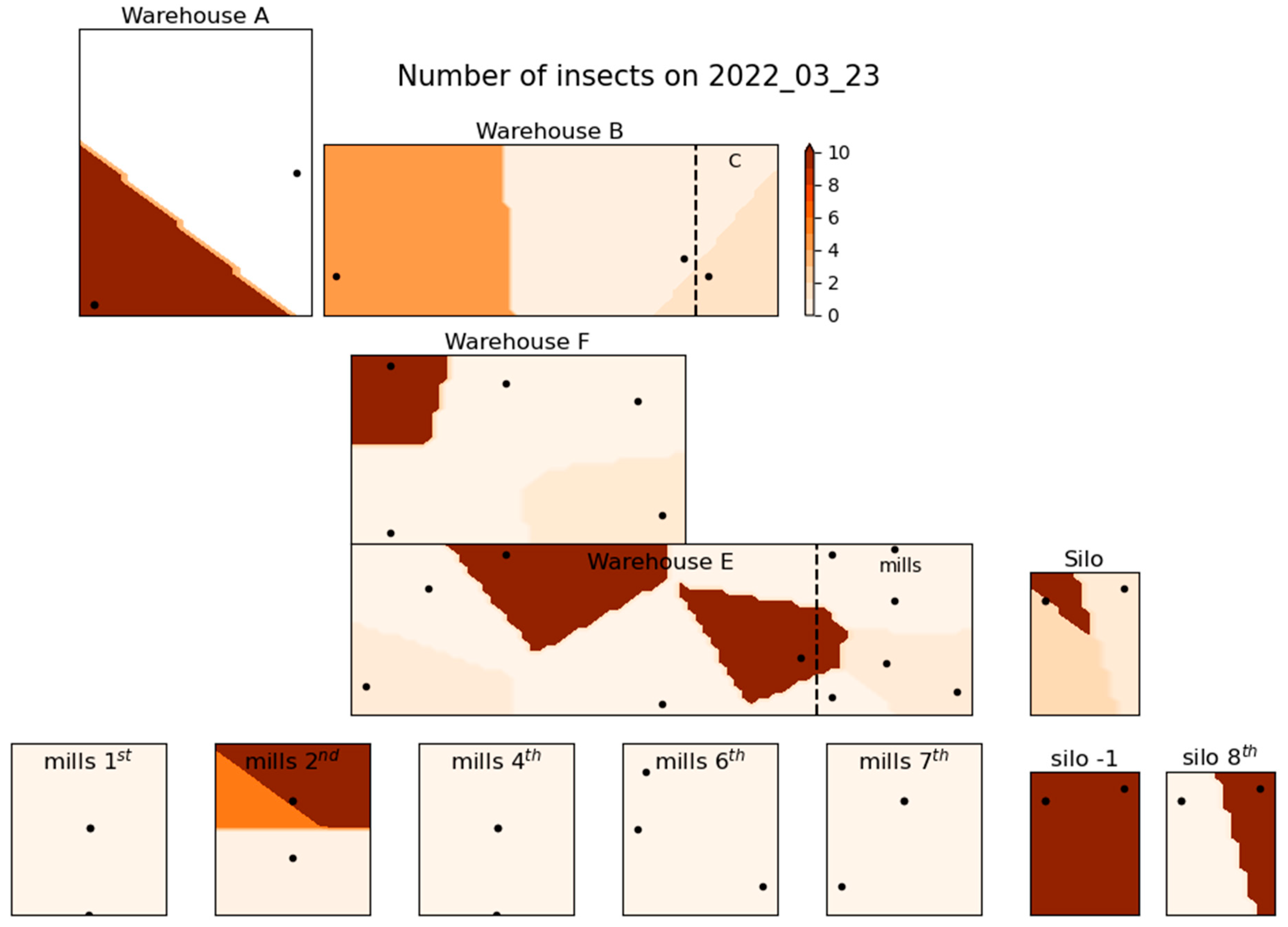
Figure 5 illustrate the spatial distribution of stored product insects in the feed mill at three different times during the trapping period: March 23, 2022 (
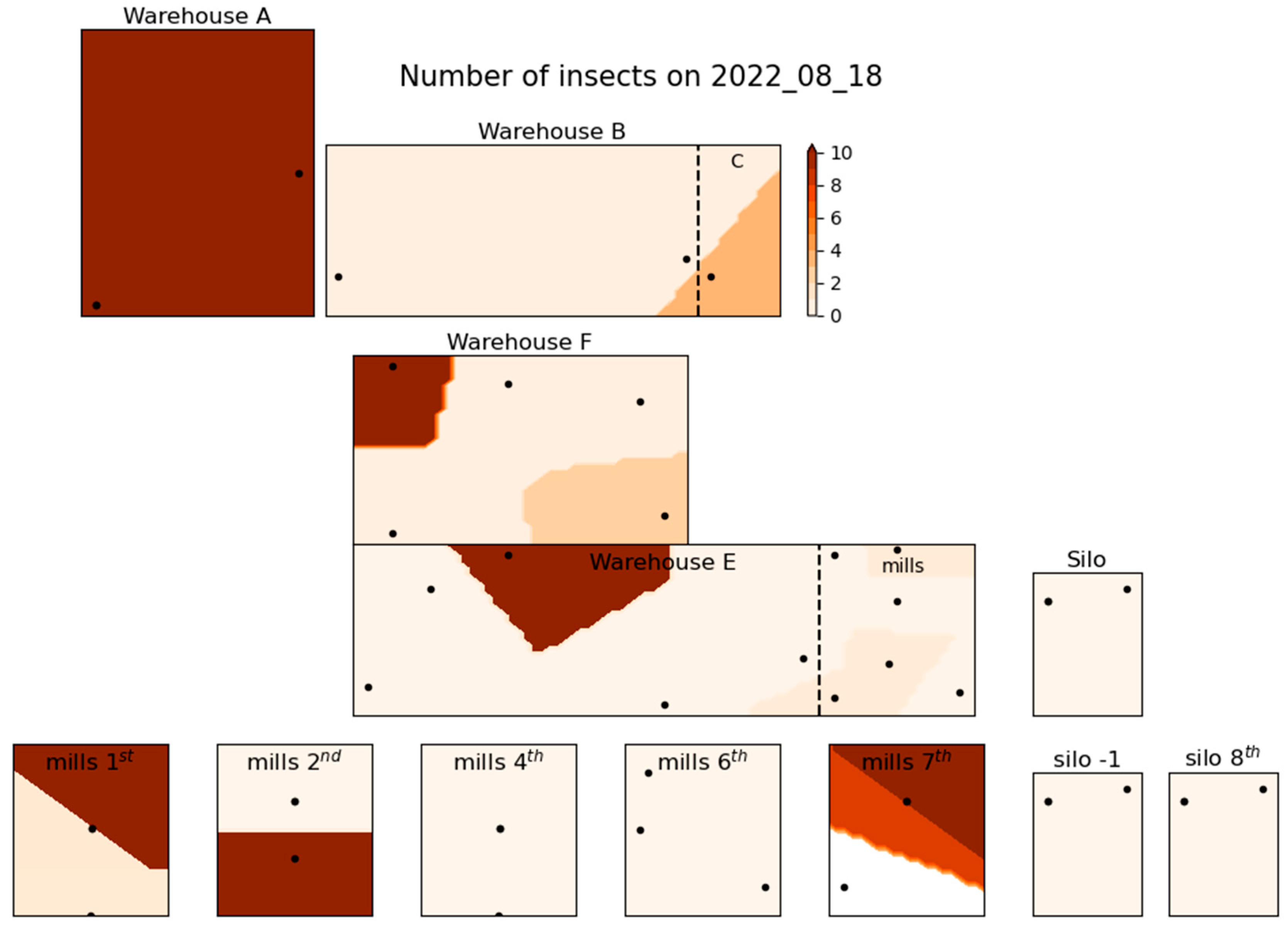
Figure 3), August 18, 2022 (
Figure 4), and February 2, 2023 (
Figure 5). These figures show how the presence of insects in the facility changed over time; the three time intervals that are presented here are selected as indicative of the spatial distribution of the species found throughout the trapping period. Similar figures for other dates were also generated during the study but could not all be presented in this paper for brevity. In
Figure 3, representing March 2022, insect distribution appears relatively uniform, and moderate insect activity throughout the facility. Higher insect counts are concentrated in the central and storage areas associated with product storage and processing. By August 2022, as shown in
Figure 4, insect numbers have increased significantly, with a notable concentration of insects in the central and northern parts of the facility. The spatial concentration in these areas suggests increased insect activity, particularly in the vicinity of product handling areas. This period marks the highest insect population density recorded during the entire trapping period, with a widespread presence throughout the facility. In contrast,
Figure 5 (February 2023) shows a decrease in insect numbers compared to August. However, certain zones, particularly near storage areas, still show moderate insect activity. The distribution in this figure highlights the persistence of insect presence in localized areas despite the overall reduction in numbers.
4. Discussion
Interestingly, the most abundant species that were found during our entire trapping period were two relative primary colonizers, S. orzyae and S. granarius, and two secondary colonizers, T. castaneum and T. confusum. This could be attributed to the fact that the same facility had quantities of both product categories, i.e. sound grain kernels, which are prone to infestation by primary colonizers, and bran and related processed commodities, which are prone to infestation by secondary colonizers. These two groups of species, although they are often found in a complementary way in the same locations, they are also competing for the same food source (Crombie 1945, Birch 1945a, b, c, Giga and Canhao 1993, Trematerra et al. 2000, Athanassiou et al. 2017b). Trematerra et al. (2000) found that secondary colonizers are more prone to infest kernels that have been previously infested by primary colonizers, rather than undamaged kernels. In this regard, this ecological succession can retain its dynamics in a given facility for a long period, with minor spatial segregations (Athanassiou et al. 2003, 2005, Trematerra et al. 2007, Semeao and Campbell 2013).
Surprisingly, the relative species that have been found here are direct competitors, and often only one is the superior colonizer. For instance, Athanassiou et al. (2017b) found that in vials with wheat and rice, the coexistence of S. oryzae, S. granarius and the maize weevil, Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) resulted in the gradual displacement of the latter species in both commodities. This could be attributed to the fact that the population growth and reproductive rates of S. oryzae were superior to those of S. granarius, which is expressed vigorously when both species are in a confined area with limited food availability (Athanassiou et al. 2017b). In contrast, T. castaneum and T. confusum may coexist for a long time at the same conditions without displacement, probably due to the fact that these species can easily switch infestation foci and food preferences within the same facility (Trematerra and Sciarretta 2004, Trematerra et al. 2007, Semeao and Campbell 2013). The co-occurrence of Sitophilus spp. may further contribute to this coexistence indirectly, due to the production of damaged kernels as a result of the infestation that are concomitantly suitable for the colonization of secondary colonizers. Periodically, insecticidal applications may favor the population outbursts of one of these species, that may be more tolerant/resistant than the other species found in certain active ingredients, but the population rebound for all four species has similar trends. At the same time, control measures may periodically change the spatio-temporal distribution of stored product insects (Scheff et al., 2022).
Although it is theoretically expected that, based on what has been mentioned above, the peaks of the primary colonizers are usually preceded than those of the secondary ones, we found that all peaks of the most abundant species found were pretty much the same period, and, in fact, often the peaks of the secondary colonizers preceded those of their primary counterparts. Apparently, this is partially due to the presence of processed products throughout the entire period, in most of the areas of the facility, which means that there was no need for the primary colonizers to produce cracked kernels. Nevertheless, there are records where species that are considered secondary colonizers are able to infest sound grain kernels (Aitken 1975). For instance, T. castaneum has been found to infest the embryos of wheat kernels (Tremattera and Throne, 2012). Furthermore, there are cases where primary colonizers can develop in cracked kernels or processed amylaceous commodities (Tremattera and Throne, 2012). For instance, Kavallieratos et al. (2012) found that adults of the lesser grain borer, Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae) could oviposit better in broken rice kernels as compared to whole rice kernels. Based on previous studies and the data reported here, it becomes evident that spatial segregation is temporary, and that secondary colonizers can establish high population outbursts in areas that had previously infested by primary colonizers, such as warehouses with grains, that lay much further than areas with processed commodities, such as flour or bran.
5. Conclusions
What we saw in this study is the dynamic succession of stored product insect populations in a confined area, and the potential “synchronization” of their population fluctuations, suggesting that abiotic conditions, such as temperature, may be more important than biotic ones, such as food resource. From a practical point of view, the results of the present work underline the importance of continuous trapping in a given area, which may reveal the need for localized control measures at the initial infestation locations and at certain periods, rather than treating blindly the entire facility.
Acknowledgments
This research was carried out as part of the project «Integrated management of insect infestations in stored animal feed: Feed without pesticides» (Project code: ΚΜΡ6-0088130) under the framework of the Action «Investment Plans of Innovation» of the Operational Program «Central Macedonia 2021-2027», that is co-funded by the European Regional Department Fund and Greece.
Data availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andreadis S. and Athanassiou C.G. (2016). A review of insect cold hardiness and its potential in stored product insect control. Crop Protection 91: 93-99. [CrossRef]
- Athanassiou C.G., Kavallieratos N.G. and Campbell J.F. (2016). Capture of Tribolium castaneum and Tribolium confusum (Coleoptera: Tenebrionidae) in floor traps: the effect of previous captures. Journal of Economic Entomology 109: 461-466.
- Athanassiou C.G., Kavallieratos N.G. and Campbell J.F. (2017a). Effect of the presence of live or dead insects on subsequent captures of six stored product beetle species: the relative species matters. Journal of Economic Entomology 110: 770-775. [CrossRef]
- Athanassiou C.G., Kavallieratos N.G. and Campbell J.F. (2017b). Competition of three species of Sitophilus on rice and maize. PLoS One 12(3): e0173377. [CrossRef]
- Athanassiou C.G., Kavallieratos N.G., Palyvos N.E., Sciarretta A., Trematerra P. Spatiotemporal distribution of insects and mites in horizontally stored wheat. J Econ Entomol. 2005; 98: 1058–1069. [CrossRef]
- Athanassiou C.G. and Buchelos C.Th. (2001b). Detection of stored-wheat beetle species and estimation of population density using unbaited probe traps and grain trier samples. Entomologia Experimentalis et Applicata 98: 67-78.
- Athanassiou C.G. and Buchelos C.T. (2020). Grain properties and insect distribution trends in silos of wheat. Journal of Stored Products Research 88: 101632. [CrossRef]
- Athanassiou C.G., Kavallieratos N.G., Palyvos P.E. and Buchelos C. T. (2003). Three-dimensional distribution and sampling indices of insects and mites in horizontally-stored wheat. Applied Entomology and Zoology 38-413-426. [CrossRef]
- Athanassiou C.G. and Arthur F.H. (2020). Cool down- Warm up: Gradual temperature changes do not affect the efficacy of cold treatment against stored product insects. Insects 11: 158.
- Birch L.C. The influence of temperature on the development of the different stages of Calandra oryzae L. and Rhyzopertha dominica Fab. (Coleoptera). Aust J Exp Biol Med Sci. 1945a; 23: 29–35.
- Birch LC. The mortality of the immature stages of Calandra oryzae (L.) (small strain) and Rhyzopertha dominica Fab. in wheat of different moisture contents. Aust J Exp Biol Med Sci. 1945b; 23: 141–145.
- Birch LC. A contribution to the ecology of Calandra oryzae (L.) and Rhyzopertha dominica Fab. (Coleoptera) in stored wheat. Trans R Soc S Aust. 1945c; 69: 140–149.
- Campbell J.F., M.D. Toews, F.H. Arthur, R.T. Arbogast Long-term monitoring of Tribolium castaneum populations in two flour mills: seasonal patterns and impact of fumigation J. Econ. Entomol., 103 (2010), pp. 991-1001.
- Campbell J.F., M.D. Toews, F.H. Arthur, R.T. Arbogast Long-term monitoring of Tribolium castaneum populations in two flour mills: rebound after fumigation J. Econ. Entomol., 103 (2010), pp. 1002-1011.
- Crombie AC. On competition between different species of graminivorous insects. Proc R Soc Lond B Biol Sci. 1945; 132: 362–395.
- Dunkel F.V., The stored grain ecosystem: A global perspective, Journal of Stored Products Research, 28, Issue 2: 1992, Pages 73-87. [CrossRef]
- Doud C. W. and Phillips T. W. Activity of Plodia interpunctella (Lepidoptera: Pyralidae) in and around flour mills J. Econ. Entomol., 93 (2000), pp. 1842-1847.
- Dowdy A.K., McGaughey W.H.Seasonal activity of stored-product insects in and around farm-stored wheat J. Econ. Entomol., 93 (1994), pp. 1842-1847.
- Hagstrum, D.W. (1987) Seasonal variation of stored wheat environment and insect populations. Environ. Entomol.16: 77–83. [CrossRef]
- Hagstrum, D.W. (1989) Infestation by Cryptolestes ferrugineus of newly harvested wheat stored on three Kansas farms. J. Econ. Entomol. 82: 655–659 Giga DP, Canhao SJ. Competition between Prostephanus truncatus (Horn) and Sitophilus zeamais (Motsch.) in maize at two temperatures. J Stored Prod Res. 1993; 29: 63–70.
- Kavallieratos N.G., Athanassiou C.G., Arthur F.H. and Throne J.E. (2012). Lesser grain borers, Rhyzopertha dominica, select rough rice kernels with cracked hulls for reproduction. Journal of Insect Science 12: 38. [CrossRef]
- Odum E.P., Ecology and Our Endangered Life-Support Systems, Sinauer, Sunderland, Mass (1989), p. 283. [CrossRef]
- Scheff D.S., Campbell J.F. and Arthur F.H. (2022). Seasonal, landscape, and attractant effects on lesser grain borer, Rhyzopertha dominica (F.), captures in northeast Kansas. Agronomy 12: 99.
- Semeao A.r A., Campbell J.F., Hutchinson J.M.S., Whitworth R.J., Sloderbeck P.E., Spatio-temporal distribution of stored-product insects around food processing and storage facilities, Agriculture, Ecosystems & Environment, 65, 2013, 51-162,. [CrossRef]
- Trematerra P., A. Sciarretta Spatial distribution of some beetles infesting a feed mill with spatio-temporal dynamics of Oryzaephilus surinamensis, Tribolium castaneum and Tribolium confusum J. Stored Prod. Res., 40 (2004), pp. 363-377. [CrossRef]
- Trematerra P., Throne, J. Insect and mites pests of durum wheat. Editors: Mike Sissons, Joël Abecassis, Brian Marchylo, Marina Carcea. In American Associate of Cereal Chemists International, Durum Wheat (Second Edition), AACC International Press, (2012), p.73-83, . [CrossRef]
- Trematerra, P. Gentile, A. Brunetti, L. Collins, J. Chambers Spatio-temporal analysis of trap catches of Tribolium confusum J. du Val in a semolina mill, with a comparison of female and male distribution J. Stored Prod. Res., 43 (2007), pp. 315-322. [CrossRef]
- Trematerra P., Sciarretta A. and Tamasi E. (2000). Behavioural responses of Oryzaephilus surinamensis, Tribolium castaneum and Tribolium confusum to naturally and artificially damaged durum wheat kernels. Entomoogia Experimentalis et Applicacata 94: 195-200.
- Weston P.A. and Barney R.J. (1998). Comparison of three trap types for monitoring of insect populations in stored grain. J. Econ. Entomol. 91: 1449-1457. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).