1. Introduction
Cerebral aneurysms and arteriovenous malformations (AVMs) are complex vascular pathologies of the brain that present significant risks of hemorrhage, stroke, and neurological deficits. Their intricate vascular anatomy, coupled with the critical nature of the brain's blood supply, makes accurate diagnosis and effective surgical intervention crucial to patient outcomes.
Traditionally, intraoperative navigation and visualization of these vascular anomalies rely on standard white-light endoscopy and advanced imaging modalities such as digital subtraction angiography (DSA) and magnetic resonance imaging (MRI). However, these techniques are limited by their inability to provide real-time, high-resolution visualization of the cerebral vasculature during surgery.
Near-infrared fluorescence (NIRF) imaging, an emerging technology, has demonstrated promise in enhancing intraoperative visualization of vascular structures. By utilizing fluorescent dyes such as indocyanine green (ICG) that fluoresce under near-infrared light, NIRF imaging allows real-time mapping of blood flow and vascular anatomy, providing superior contrast and specificity compared to traditional imaging techniques.
The SPY mode of the Stryker endoscope utilizes near-infrared fluorescence (NIRF) imaging to provide real-time visualization of cerebral vasculature during surgery for cerebral aneurysms and arteriovenous malformations (AVMs). This technology works by detecting fluorescence from indocyanine green (ICG) dye injected into the bloodstream, allowing surgeons to visualize blood flow and identify vascular structures with high precision [
1,
2]. ICG, inert and water-soluble nonradioactive contrast agent circulates only in the intravascular compartment, allows real-time assessment and direct imaging of tissue perfusion and vascularization when enhanced by the laser in NIRF modality; fatal allergies and reactions are rare [
3].
SPY mode offers an alternative, in selected cases and with a limited resolution, to traditional digital subtraction angiography (DSA) or ICG videoangiography, as it provides immediate, intraoperative feedback without the need for complex or expensive radiological setups. By enhancing vessel visualization, it aids in confirming aneurysm clipping or AVM resection, potentially reducing the dependence on angiography and improving surgical outcomes in context where DSA or intraoperative ICG videoangiography is not available.
In this paper, we report our preliminary experience on the use of the SPY mode of the Stryker endoscope in cerebral aneurysms and AVMs surgery. We aim to assess the benefits of this technique as a valid alternative to partially vicariate the afore mentioned devices in improving intraoperative identification of vascular structures, guiding surgical resection, and minimizing the risk of complications such as incomplete obliteration of aneurysms or missing residual nidus in AVMs management. By evaluating the current literature and presenting case studies, we seek to demonstrate the potential of NIRF to enhance the safety and efficacy of neurovascular surgeries, offering a valuable tool in the neurosurgeon's armamentarium.
2. Materials and Methods
ICG-VA can be integrated with a surgical microscope: in our series we used a Leica microscope (Leica Microsystems- Wetzlar, Germany) without ICG-VA filter and separately a Stryker endoscope (Kalamazoo, Michigan, USA) exploiting its SPY modality, able to illuminate the surgical field with a wavelength covering the ICG absorption band, to observe in real time angiographic images on the endoscope video screen after ICG intravenous injection (ICG is not reabsorbed into intestine or hepatic circulation).
Study Cohort and Surgical Equipment
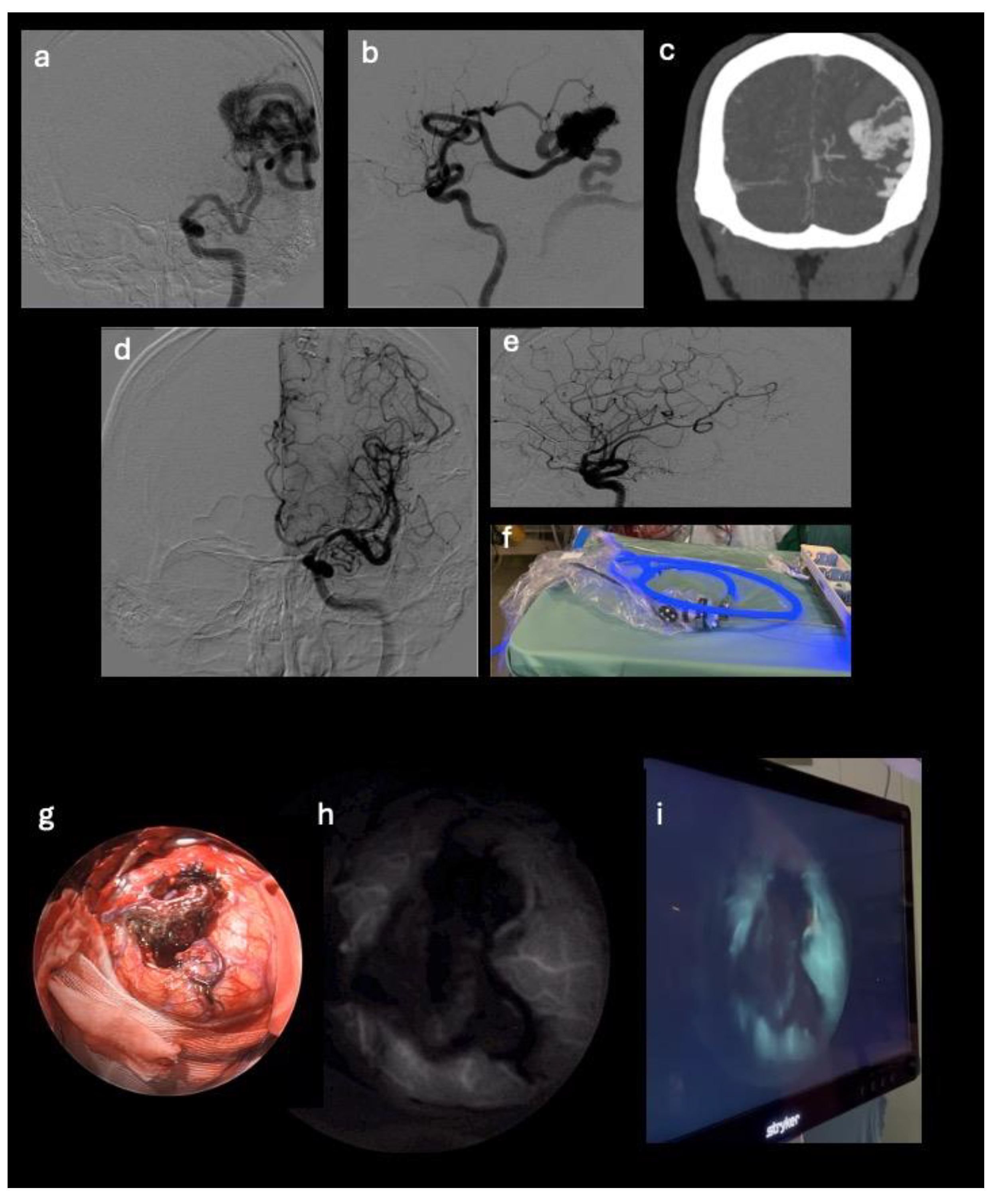
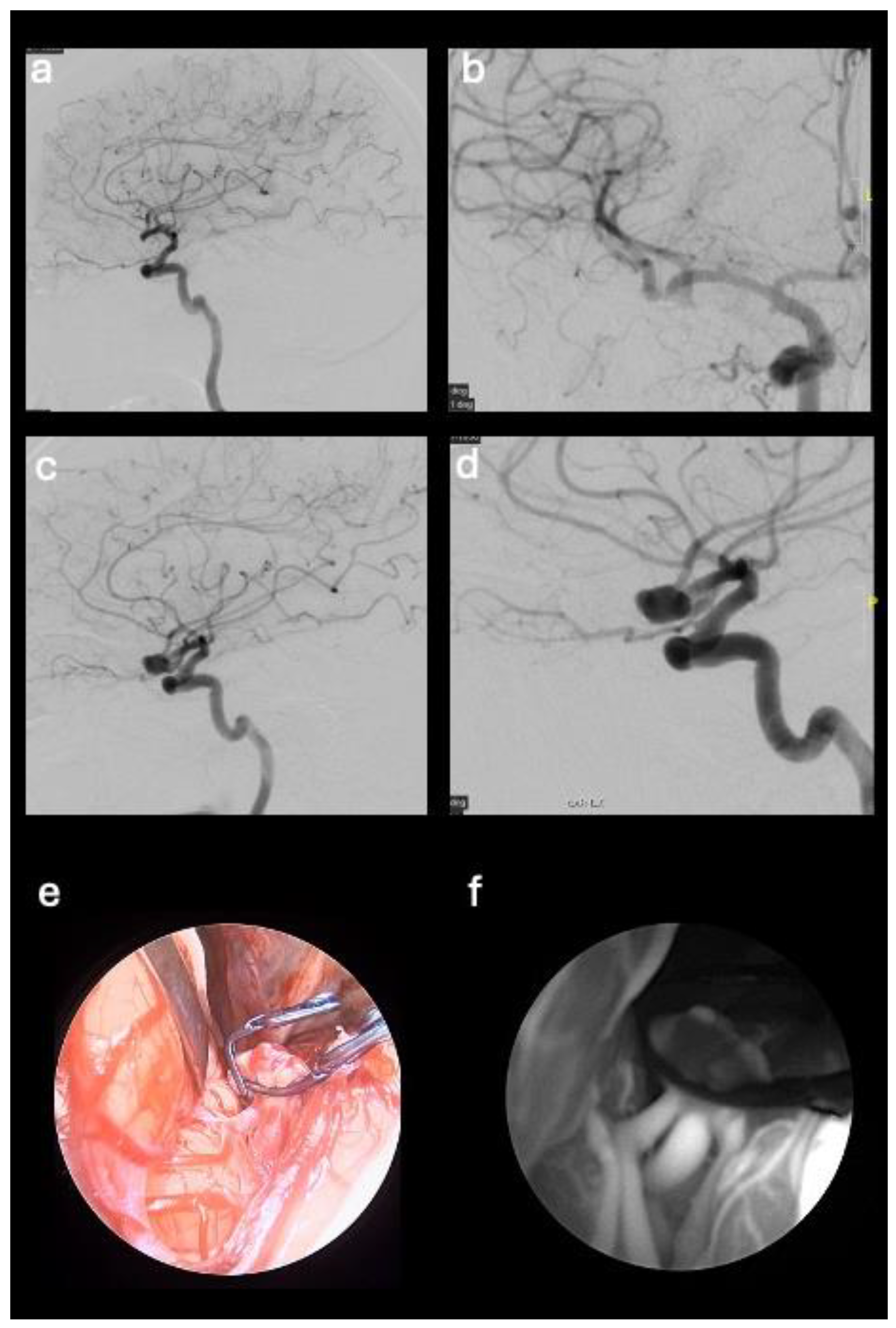
This retrospective observational study involved 5 patients suffering from cerebral aneurysm or AVM who underwent surgery with the aid of the microscope supported by the Stryker endoscope in SPY mode for the visualization of fluorescence emitted by indocyanine green at our institution during 2023. Patients’ data collection included clinical data and pre-/post-operative angiography and CTA images, together with intra-operative fluorescence images recorded using the endoscope. In each case the surgical treatment of the vascular neurosurgical pathology was performed with the aid of the microscope supported by the Stryker (Kalamazoo, Michigan, USA) endoscope in SPY mode for the visualization of fluorescence emitted by indocyanine green. Patients in whom fluorescence imaging equipment was used had regular aneurysms (3 cases) and AVM located superficially in the cerebral parenchyma (2 cases).
Preparation Protocol
A protocol has been established in our institution that provides for collaboration between departments: ICG, especially in small institutions, is often not available in Neurosurgery departments but is common equipment in Ophthalmology or Hepato-Biliary Surgery Departments. ICG is not routinely available in our operating room so in anticipation of the elective operation in which it will be used it is made available specifically; the intravenous injection is made mixing ICG with sterile water (its half-life is 3–5 min and elimination occurs within 15–20 min by the liver; maximum toxic dose is 5 mg/kg/day). Inject is made a few seconds (5-10 seconds) before aiming the endoscope at the affected vessel in SPY mode. The SPY mode of the stryker endoscope is activated through the laparoscopy mode and once in SPY mode, select the contrast modality (
Figure 1). The SPY mode is off by default and therefore the endoscope is initially in grayscale; to enhance the green, the operator from the sterile field will need to press the right botton of the sterile camera (there are 4 buttons on the camera head, press the right to turn on/off the SPY mode).
Use of the Endoscope with Fluorescence Visualization and Intra-Operative Evaluation
After clipping of the aneurysm or microsurgical exclusion of the AVM nidus, a single 25 mg bolus of ICG was injected by intravenous (iv) route. The endoscope in SPY mode for the visualization of fluorescence was positioned in the surgical field to visualize the vascular structures stained by the intravascular passage of the ICG. The angiographic images can be observed on the video screen in real time and recorded. In patients with cerebral aneurysm, the endoscope and fluorescence made possible to evaluate the patency of the vessel from which the aneurysm originates and of the surrounding ones, together with the absence of residual neck near the clip. In cases of AVM removal, endoscopic visualization was used to analyze the absence of lesional residues by exploring the cavity walls. No adverse reactions were observed following ICG infusion.
3. Results
Patients Overview
This retrospective observational study involved 5 patients suffering from cerebral aneurysm or AVM who underwent surgery at our institution during 2023. Patients’ data collection included clinical data and pre-/post-operative angiography and CTA images, together with intra-operative fluorescence images recorded using the endoscope. In each case the surgical treatment of the vascular neurosurgical pathology was performed with the aid of the Leica surgical microscope (Leica Microsystems- Wetzlar, Germany) supported by the Stryker endoscope (Kalamazoo, Michigan, USA) in SPY mode for the visualization of fluorescence emitted by indocyanine green. Patients in whom fluorescence imaging equipment was used had regular aneurysms (3 cases) and AVM located superficially in the cerebral parenchyma (2 cases).
The five patients who underwent surgery had the following clinical and neuroradiological characteristics:
- 1)
57-year-old male patient with middle cerebral artery aneurysm (M2 segment) with a diameter of 1.2 cm, neurologically intact. The patient underwent clipping of the aneurysm neck;
- 2)
73-year-old female patient with temporal AVM originating from the left middle cerebral artery and discharge into the ipsilateral transverse sinus, with a 3 cm nidus. The AVM manifested itself with an episode of fluent aphasia. The patient underwent pre-operative embolization and microsurgical removal of the nidus;
- 3)
46-year-old female patient with aneurysm of the anterior communicating artery with a diameter of 1 cm, neurologically intact. The patient underwent clipping of the aneurysm neck;
- 4)
44-year-old female patient with middle cerebral artery aneurysm (M1-M2 union) with a diameter of 1 cm, history of headache. The patient underwent clipping of the aneurysm neck;
- 5)
31-year-old male patient with frontal AVM originating from the right anterior cerebral artery and discharge into the sagittal sinus, with a 2,5 cm nidus. The patient presented with weakness of the left upper limb and underwent microsurgical removal of the nidus.
The intraoperative use of indocyanine green was a safe, rapid and effective technique, no adverse reactions occurred; the five patients who underwent surgery had the following clinical and neuroradiological characteristics summarized in
Table 1 and in the Illustrative case section (see Illustrative Cases section).
4. Discussion
Indocyanine Green (ICG) is a water-soluble dye; the first application in the medical field dates to 1956 for the study of cardiovascular and liver function, while in 1970 its fluorescence properties were exploited in the ophthalmological field; the advantage of ICG over fluorescein is that the light emission is more intense and easier to detect, and the adverse reactions are also very low. ICG was approved by US Food and Drug Administration in 1956 and 1975 for cardio circulatory measurements, liver function tests, and ophthalmic angiography. Only in 2003 it was introduced into the neurosurgical practice to perform angiographic studies, and it is frequently used in cerebrovascular surgery; the applications of ICG have expanded rapidly across different specialties since its initial development. Teng et al. described the application of ICG in neuro-oncology from gliomas, and not only, to pituitary adenomas; Mansour et al. reported its application in clipping a ruptured anterior spinal artery (ASA) aneurysm. [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11]
Endoscopy in our series resulted extremely useful because of its simple and reliable method for acquiring high spatial resolution images in real time. In addition, in a context where intraoperative angiography is not available, because of its large diffusion endoscopy can potentially reduce costs and be much more available than intraoperative angiography; it can be improved combining Doppler and can potentially overcome the dead angles of the microscope as reported by Hashimoto et al. [
11,
12,
13,
14,
15,
16,
17,
18]. Catapano et al. described a 40 patient endoscope-integrated indocyanine green fluorescence cases series with no perioperative complications, reporting endoscopy as a potentially critical tool in endonasal, intraventricular, aneurysm and brain tumor surgeries [
8]. Wong et al. demonstrated the possible application of endoscopic indocyanine green video angiography in challenging posterior circulation aneurysm cases while in anterior circulation ones Chen et al. reported a potentially reduced cerebral vasospasm combining neuroendoscopy with indocyanine green because of the decreased risk of misclipping perforating branches [
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30].
Eventhough the abovementioned promising features, especially in contexts where intraoperative video-angiography is not available, endoscopy has its own limitations and intraoperative DSA remains the gold standard technique in cerebrovascular surgery (especially with deep seated AVMs or when managing thick walled atherosclerotic vessels).
The main purpose of aneurysm surgery is to completely exclude them from the cerebral vascular circulation in the absence of residues in the collar and occlusion of adjacent vessels; similarly, in AVM surgery the goal is the early identification of the nidus and to avoid residues within it given the high risk of new rupture.
Indocyanine green videoangiography is often preferred, for cost or availability, to Doppler or intraoperative angiography DSA and with the same assumption it was possible, in our preliminary experience, to partially vicariate the aforementioned devices using the SPY mode of the Stryker endoscope allowing the visualization of fluorescence in high definition.
According to literature, the intraoperative use of indocyanine green was a safe, rapid and effective technique within a preliminary case study of "regular - not giant" aneurysms and AVM superficially located in the cerebral parenchyma. The endoscopic technique in SPY mode has allowed to partially vicariate, in selected cases and in our preliminary experience, the use of Doppler, intraoperative angiography and integrated microscope video angiography.
5. Conclusions
In contexts where intraoperative angiography is not available, in order to verify aneurysms’ exclusion and to early identify AVM residual nidus, we propose, in selected cases and keeping in mind the described limitations of the technique, the support of the endoscope in SPY mode during the microsurgical procedure in order to enhance and visualize the green fluorescence of indocyanine.
Author Contributions
Conceptualization, D.A., A.I., M.I., M.G.; methodology, A.R., M.D., G.P., R.G.; validation, M.I., M.G. and ; formal analysis, D.A., A.I., A.R., A.M.; data curation, D.A., A.I., A.R., A.M.; writing—original draft preparation, D.A., A.I., A.R., A.M..; writing—review and editing, D.A., A.I., A.R., A.M.,.; visualization, G.P., R.G., M.D., M.I.; supervision, M.I. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
ethical approval was waived because of the retrospective nature of the study and being all procedures part of the routine care. The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study and being all procedures part of the routine care.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Della Puppa A, Rossetto M, Volpin F, Rustemi O, Grego A, Gerardi A, Ortolan R, Causin F, Munari M, Scienza R. Microsurgical Clipping of Intracranial Aneurysms Assisted by Neurophysiological Monitoring, Microvascular Flow Probe, and ICG-VA: Outcomes and Intraoperative Data on a Multimodal Strategy. World Neurosurg. 2018 May;113:e336-e344. [CrossRef] [PubMed]
- Mansour A, Endo T, Inoue T, Sato K, Endo H, Fujimura M, Tominaga T. Clipping of an anterior spinal artery aneurysm using an endoscopic fluorescence imaging system for craniocervical junction epidural arteriovenous fistula: technical note. J Neurosurg Spine. 2019 Apr 26;31(2):279-284. [CrossRef] [PubMed]
- Devgan Y, Mayilvaganan S, Mishra A, Chand G, Agarwal G, Agarwal A. Comparison of indocyanine green angiography vs intraoperative parathyroid hormone in early prediction of risk of post-thyroidectomy hypocalcemia: a prospective cohort study. Ann Med Surg (Lond). 2024 Jan 3;86(2):678-688. [CrossRef] [PubMed] [PubMed Central]
- Teng CW, Huang V, Arguelles GR, Zhou C, Cho SS, Harmsen S, Lee JYK. Applications of indocyanine green in brain tumor surgery: review of clinical evidence and emerging technologies. Neurosurg Focus. 2021 Jan;50(1):E4. [CrossRef] [PubMed]
- Ferroli P, Acerbi F, Albanese E, Tringali G, Broggi M, Franzini A, Broggi G. Application of intraoperative indocyanine green angiography for CNS tumors: results on the first 100 cases. Acta Neurochir Suppl. 2011;109:251-7. [CrossRef] [PubMed]
- Cho SS, Salinas R, Lee JYK. Indocyanine-Green for Fluorescence-Guided Surgery of Brain Tumors: Evidence, Techniques, and Practical Experience. Front Surg. 2019 Mar 12;6:11. [CrossRef] [PubMed] [PubMed Central]
- Yannuzzi, LA. Indocyanine green angiography: a perspective on use in the clinical setting. Am J Ophthalmol. 2011 May;151(5):745-751.e1. [CrossRef] [PubMed]
- Lau CT, Au DM, Wong KKY. Application of indocyanine green in pediatric surgery. Pediatr Surg Int. 2019 Oct;35(10):1035-1041. [CrossRef] [PubMed]
- Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V. Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery. 2003 Jan;52(1):132-9; discussion 139. [CrossRef] [PubMed]
- Jeon JW, Cho SS, Nag S, Buch L, Pierce J, Su YS, Adappa ND, Palmer JN, Newman JG, Singhal S, Lee JYK. Near-Infrared Optical Contrast of Skull Base Tumors During Endoscopic Endonasal Surgery. Oper Neurosurg (Hagerstown). 2019 Jul 1;17(1):32-42. [CrossRef] [PubMed] [PubMed Central]
- Della Puppa A, Rustemi O, Scienza R. The "ICG Entrapment Sign" in Cerebral Aneurysm Surgery Assisted by Indocyanine Green Videoangiography. World Neurosurg. 2017 Jan;97:287-291. [CrossRef] [PubMed]
- Balamurugan S, Agrawal A, Kato Y, Sano H. Intra operative indocyanine green video-angiography in cerebrovascular surgery: An overview with review of literature. Asian J Neurosurg. 2011 Jul;6(2):88-93. [CrossRef] [PubMed] [PubMed Central]
- Chibbaro S, Tacconi L. Extracranial-intracranial bypass for the treatment of cavernous sinus aneurysms. J Clin Neurosci. 2006 Dec;13(10):1001-5. [CrossRef] [PubMed]
- Takagi Y, Samamura K, Hashimoto N. Intra operative Near-infrared Indocyanine green video angiography performed with surgical microscope – Applications in Cerebrovascular surgery. Eur Neurol Rev. 2008;3:66–8.
- Mery FJ, Amin-Hanjani S, Charbel FT. Is an angiographically obliterated aneurysm always secure? Neurosurgery. 2008 Apr;62(4):979-82; discussion 982. [CrossRef] [PubMed]
- Della Puppa A, Rustemi O, Rossetto M, Gioffrè G, Munari M, Charbel FT, Scienza R. The "squeezing maneuver" in microsurgical clipping of intracranial aneurysms assisted by indocyanine green videoangiography. Neurosurgery. 2014 Jun;10 Suppl 2:208-12; discussion 212-3. [CrossRef] [PubMed]
- Zitek H, Hejcl A, Sadeh M, Charbel FT, Sames M. Occipital artery to vertebral artery bypass for treatment of bilateral vertebral artery occlusion with QMRA as an adjunct to diagnostic assessment. Acta Neurochir (Wien). 2024 May 7;166(1):203. [CrossRef] [PubMed] [PubMed Central]
- Acerbi F, Prada F, Vetrano IG, Falco J, Faragò G, Ferroli P, DiMeco F. Indocyanine Green and Contrast-Enhanced Ultrasound Videoangiography: A Synergistic Approach for Real-Time Verification of Distal Revascularization and Aneurysm Occlusion in a Complex Distal Middle Cerebral Artery Aneurysm. World Neurosurg. 2019 May;125:277-284. [CrossRef] [PubMed]
- Hashimoto K, Kinouchi H, Yoshioka H, Kanemaru K, Ogiwara M, Yagi T, Wakai T, Fukumoto Y. Efficacy of Endoscopic Fluorescein Video Angiography in Aneurysm Surgery-Novel and Innovative Assessment of Vascular Blood Flow in the Dead Angles of the Microscope. Oper Neurosurg (Hagerstown). 2017 Aug 1;13(4):471-481. [CrossRef] [PubMed]
- Catapano G, Sgulò F, Laleva L, Columbano L, Dallan I, de Notaris M. Multimodal use of indocyanine green endoscopy in neurosurgery: a single-center experience and review of the literature. Neurosurg Rev. 2018 Oct;41(4):985-998. [CrossRef] [PubMed] [PubMed Central]
- Wong AK, Wong RH. Keyhole clipping of a low-lying basilar apex aneurysm without posterior clinoidectomy utilizing endoscopic indocyanine green video angiography. Surg Neurol Int. 2020 Feb 28;11:31. [CrossRef] [PubMed] [PubMed Central]
- Bruneau M, Appelboom G, Rynkowski M, Van Cutsem N, Mine B, De Witte O. Endoscope-integrated ICG technology: First application during intracranial aneurysm surgery. Neurosurg Rev. 2013;36:77–84. [CrossRef]
- De Oliveira JG, Beck J, Seifert V, Teixeira MJ, Raabe A. Assessment of flow in perforating arteries during intracranial aneurysm surgery using intraoperative near-infrared indocyanine green videoangiography. Neurosurgery. 2008;62(Suppl 3):1300–10. [CrossRef]
- Fischer G, Rediker J, Oertel J. Endoscope-versus microscope- integrated near-infrared indocyanine green videoangiography in aneurysm surgery. J Neurosurg. 2018. pp. 1–10. [CrossRef]
- Kalavakonda C, Sekhar LN, Ramachandran P, Hechl P. Endoscope-assisted microsurgery for intracranial aneurysms. Neurosurgery. 2002;51:1119–26. [CrossRef]
- Mielke D, Malinova V, Rohde V. Comparison of intraoperative microscopic and endoscopic ICG angiography in aneurysm surgery. Neurosurgery. 2014;10(Suppl 3):418–25. [CrossRef]
- Nishiyama Y, Kinouchi H, Senbokuya N, Kato T, Kanemaru K, Yoshioka H, et al. Endoscopic indocyanine green video angiography in aneurysm surgery: An innovative method for intraoperative assessment of blood flow in vasculature hidden from microscopic view. J Neurosurg. 2012;117:302–8. [CrossRef]
- Washington CW, Zipfel GJ, Chicoine MR, Derdeyn CP, Rich KM, Moran CJ, et al. Comparing indocyanine green videoangiography to the gold standard of intraoperative digital subtraction angiography used in aneurysm surgery. J Neurosurg. 2013;118:420–7. [CrossRef]
- Oda J, Kato Y, Chen SF, Sodhiya P, Watabe T, Imizu S, et al: Intraoperative near-infrared indocyanine green-videoangiog-raphy (ICG-VA) and graphic analysis of fluorescence intensity in cerebral aneurysm surgery. J Clin Neurosci 18:1097–1100,201. [CrossRef]
- Chen DY, Xu CS, Fu K, Ma YH, Zhang TB, Zou YC, Chen JC. [Application of neuroendoscopy combined with fluorescence angiography in anterior circulation aneurysm clipping]. Zhonghua Yi Xue Za Zhi. 2021 Jan 26;101(4):254-258. Chinese. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).