1. Introduction
Mycoplasma galisepticum (MG) is a bacterium belonging to the class of Mollicutes and the family Mycoplasmataceae. It is the causative agent of chronic respiratory disease (CRD) in chickens and infectious sinusitis in turkeys, chickens, game birds, pigeons, and passerine birds of all ages (Hennigana et al, 2011). Likewise, the disease is listed by the World Animal Health Organization (OIE) as one of the most pathogenic avian mycoplasmosis and the main cause of chronic respiratory disease in poultry (OIE, 2008). The infection caused by M. galisepticum becomes aggressive when accompanied by other poultry diseases, including Newcastle disease virus (NDV), Escherichia coli (E. coli), and infectious bronchitis virus (IBV) (Bwala, D.G. 2017, Nakamura et al., 1994, Valks, M. and Burch, D, 2002). The transmission of M. galisepticum can occur through direct and indirect, horizontal contact, aerosol transmission via the introduction of contaminated materials, or by people (Bradbury, J.M. et al., 2001a). The disease causes a loss of egg production, a reduction in the hatchability of eggs, and a decline in the quality of meat at slaughter (Stipkovits and Kempf, 1996; Kleven, 1997; Levisohn and Kleven, 2000). In developing countries, the eggs and meat of chickens are the major food components in the daily human diet and sources of income (Gari, 2004).
Despite the significant growth of the poultry industry in Ethiopia, major diseases affecting both commercial and local chickens remain understudied (Jibril et al, 2018). M. galisepticum is among the poultry diseases causing considerable economic losses in Ethiopia because of a lack of reliable diagnostic methods, treatment options, and effective control measures. Commercially available diagnostic kits are often expensive and time-consuming and fail to detect experimentally produced antibodies against M. galisepticum (unpublished). Hereafter, in this study, a homemade indirect ELISA kit that can detect antibodies produced against both natural and experimental infections was developed and evaluated.
2. Materials and Methods
2.1. Reference Serum
Positive serum samples were obtained from naturally infected chickens in the field and screened via a commercial kit, and strongly positive serum was selected and used as a positive control. Serum samples from non-vaccinated control chickens were also screened for antibodies against M. galisepticum and used as a negative control.
2.2. Chicken Anti-M. guisepticum Vaccine Serum
Sixty (n=60) local chickens were vaccinated with the M. galisepticum vaccine formulated from two different adjuvants: aluminum hydroxide and oil. The chickens received booster doses of the same vaccine, and blood samples were collected at weeks 4, 5, 6, 7 8, and 9 before the initial vaccination and during each subsequent vaccination. The separated serum was compared to a reference serum obtained from chickens with natural M. gallisepticum infections.
2.3. Mycoplasma Gallisepticum Isolation, Identification, and Preparation of the Coated Antigen
A local field strain of M. galisepticum was cultured on Frey’s medium. Mycoplasma colonies were observed under low magnification on agar plates via a light microscope with reduced light intensity. The isolated field strain revealed fried egg morphology with tiny, smooth colonies and dense elevated centers. The colonies were further allowed to grow on Hyflic medium supplemented with Mycoplasma selective supplement G (OXOID, code SR059C) to prepare a seed bank. Three vials of the seed bank were added to 200 mL of Hyflic medium and incubated at 37 °C with slow agitation. The growth was confirmed by turbidity and pH detection. Once the pH reached 6.5, the sterility and purity were verified. A 3-liter sterile medium was then prepared, inoculated with 20 % inoculum containing 20 % horse serum and incubated at 37 °C. After confirming growth, the culture was inactivated with 0.3% saponin.
2.3.1. Preparation of Bacterial Cell Lysate
The bacterial cells were centrifuged at 10,000 rpm for 20 minutes, and the supernatant was discarded without disturbing the pellet. To lyse bacterial cells, a freeze‒thaw cycle was used. The cell suspension was rapidly frozen on dry ice and then thawed at room temperature. The pellets were washed three times with phosphate-buffered saline (PBS) reconstituted with 5 ml of PBS and placed at -21 °C until protein quantification was conducted.
2.3.2. Protein Quantification
The protein concentration was determined via the bicinchoninic acid (BCA) method. A standard curve was created using bovine albumin, Cuso4, distilled water, and bicinconic acid.
2.3.3. DNA Extraction and PCR Detection
DNA was extracted from cultured organisms via a gSYNC™ Geneaid extraction kit (Korea). The sample was centrifuged at 16,000 × g for 2 minutes, the floating material was collected in a novel 1.5 ml tube, and 200 µl of GSB was added to the floating material. The mixture was then spun again for 10 seconds and mixed with 200 µl of absolute ethanol by vortexing. The blend was moved to the GS column and centrifuged at 16,000 × g for 1 min, after which 400 and 600 µl of W1 and W2 buffers were added, respectively, to the GS column with centrifugation, and 100 µl of warmed elution buffer was added to the tubes after thorough dehydration to elute the purified DNA. The resulting product was stored at −20 °C until use. For the amplification of MG DNA via PCR, two pairs of PCR primers were used on the basis of the National Veterinary Institute (NVI) molecular biology laboratory protocol. The primers used were MG 14-forw-5 pm/µl 5´GAGCTAA TCTGTAAAGTTGGTC-3´ and MG13-REV-5pm/µl 5´-GCTTCCTTGCGGTTAGCAAC. The primers were synthesized by Bioneer Co., Korea. The PCRs were performed with a total volume of 25 µl, as in the kit, and consisted of 5 µl of extracted DNA (template), 2 µl of each primer, 2 µl of MgCl2, 3 µl of RNA-free water, and 5 µl of prepared Mastermix solution. The positive control in both PCR runs was supplied with a kit. The thermocycler programs are explained in
Table 1. Electrophoresis was performed via the use of 10 µl of the amplified DNA in a 2% agarose gel. The bands were distinguished at 245–312 nm through a UV transilluminator (Biometra, Germany).
2.4. Optimization of Antigen Coating and Serum Dilution
Following protein estimation, the antigen was diluted to concentrations of 0.5 µg/µl, 0.75 µg/µl, 1 µg/ml, 1.5 µg/µl, and 2 µg/µl and coated onto a 96-well microplate with bicarbonate buffer (pH 9.6) (Sigma–Aldrich). After overnight incubation at +4 °C, the plate was washed with phosphate-buffered saline containing 0.05 % tween 20 (PBST) (Sigma–Aldrich). Nonspecific binding sites were blocked after 5 % skim milk solution was added to each well, and the samples were incubated at 37 °C for 2 hours. Following three washes with PBST, the antigen-coated plate was stored at 4 °C until use. Hyperimmune sera from vaccinated and control chickens were diluted 1:10, 1:50, 1:100, 1:200, and 1:400 in blocking buffer and added to individual wells. After a 30-minute incubation at 37 °C, excess antibodies were removed by washing with PBST. A 100 µL conjugate solution (1:2000) was then added to each well and incubated for 30 minutes at 37 °C, followed by another PBST wash. A TMB substrate solution was added to develop color in positive reactions. The reaction was stopped with 1 N H2SO4, and the optical density (OD) was measured at 450 nm.
2.5. Evaluation of Test Sensitivity and DIAGNOSTIC specificity
The serum samples of naturally infected chickens infected with M. galisepticum were obtained from the Addis Ababa University Faculty of Veterinary Medicine. Additionally, positive and negative sera were generated experimentally at the National Veterinary Institute. To evaluate the newly developed in-house indirect ELISA, 100 µL of each serum sample was added to individual wells of a precoated microplate. After a 30-minute incubation at 37 °C, excess antibodies were removed by washing with PBST. One hundred microliters of conjugate solution was added to all the wells, which were subsequently incubated for 30 minutes at 37 °C. Following a PBST wash, 100 µL of substrate solution was added, and the mixture was incubated for 30 min at room temperature. Positive reactions resulted in the development of an orange color. The reaction was stopped with 1 N H2SO4, and the optical density (OD) was measured at 450 nm.
2.6. Statistical Analysis
The method described by Samad et al. (1994) was used for the determination of test sensitivity and diagnostic specificity by comparison with the gold standard. The sensitivity and specificity of the newly developed in-house indirect ELISA and ID-vet indirect ELISA are described below.
2.6. Data Analysis
The data analysis was performed via GraphPad Prism (version 5.01) (GraphPad Software, San Diego, CA, USA), MedCalc (version 10.0.2.0) (MedCalc Software, Mariakerke, Belgium) and Microsoft Excel window 10.
3. Results
3.1. DNA Extraction and PCR Detection
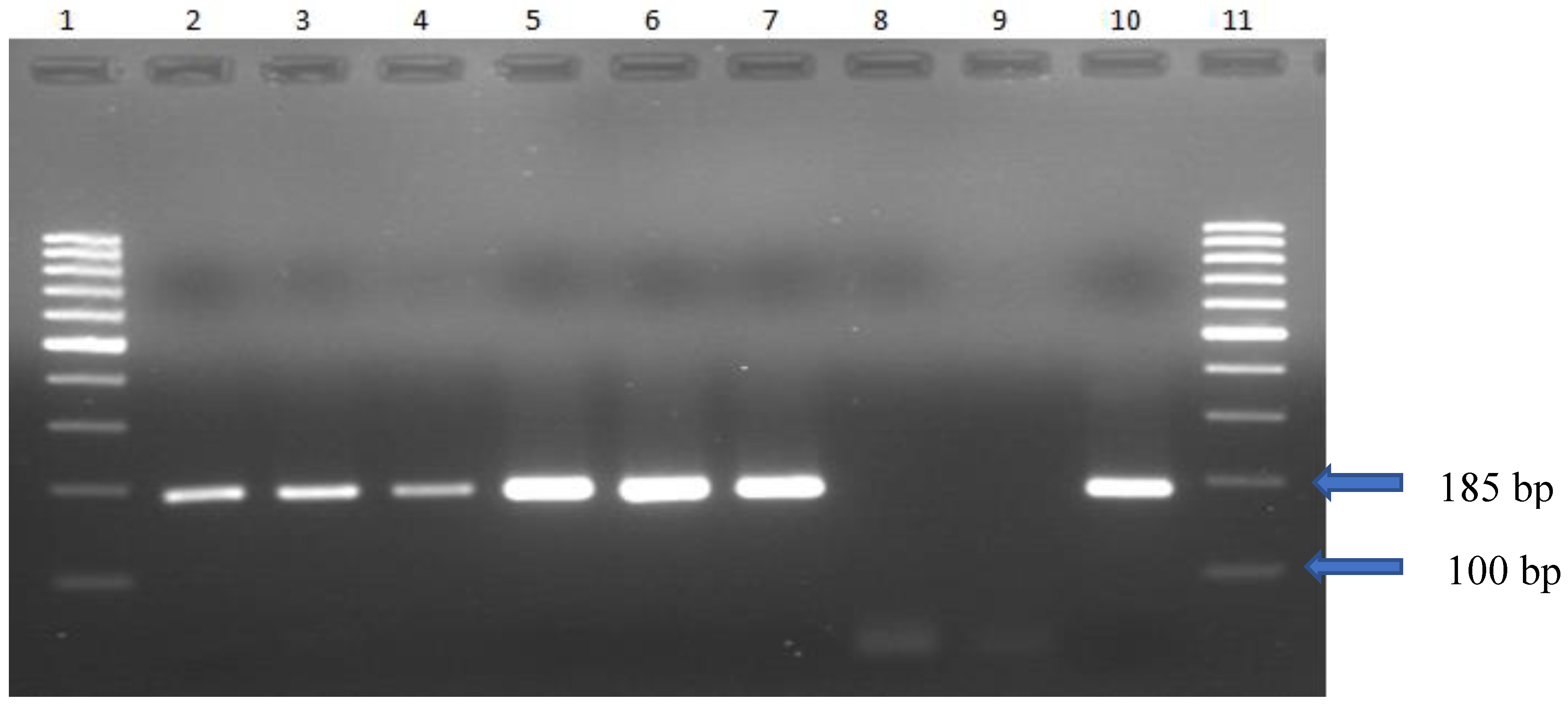
DNA was extracted from cultured organisms via the gSYNC™ Geneaid extraction kit. The PCR time, temperature, and cycle number are described in
Table 1. The electrophoretic profile of Mg DNA obtained from culture via PCR revealed 185 bp (
Figure 1).
3.2. Optimization of Antigen Coating and Serum Dilution
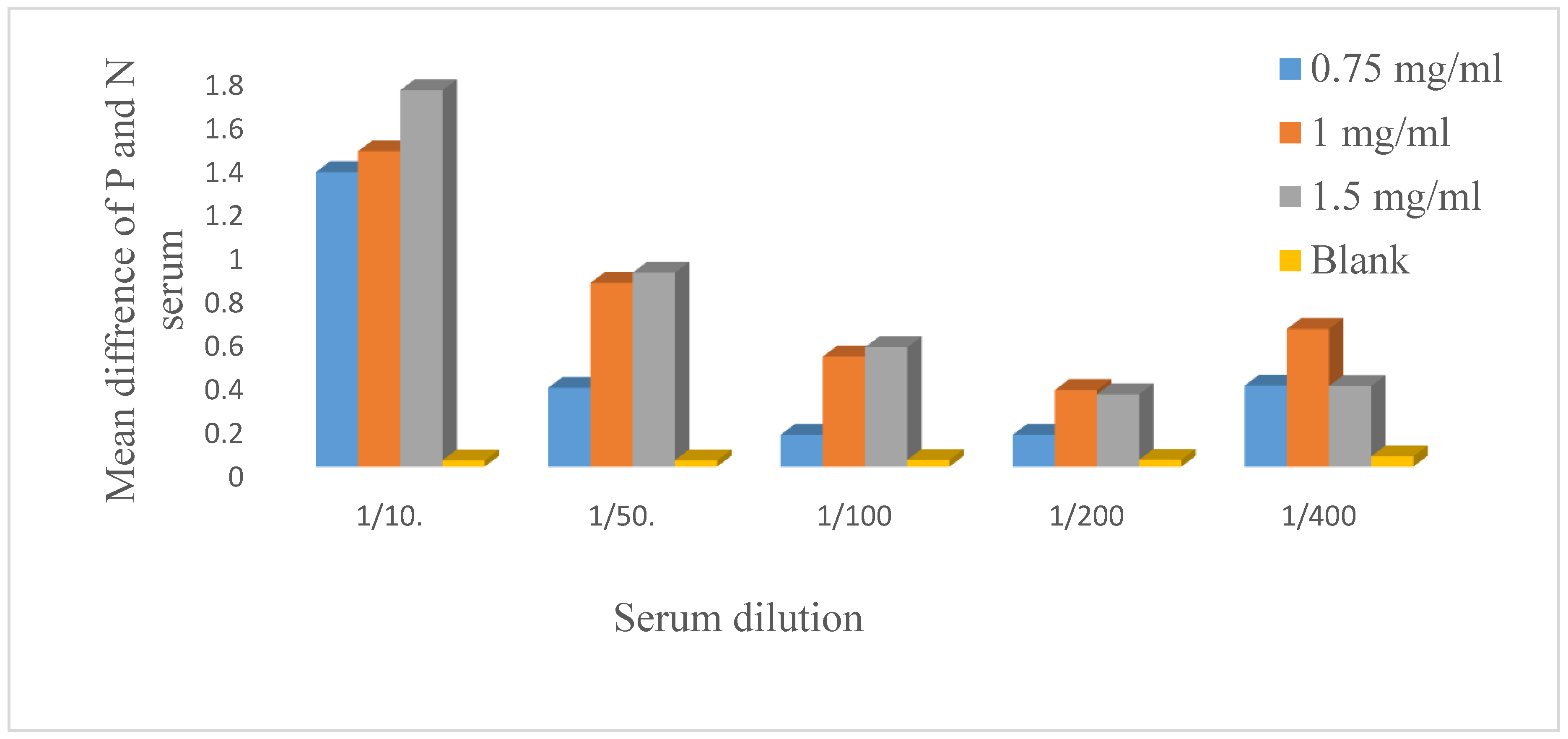
The antigen was diluted to concentrations of 0.5 µg/µl, 0.75 µg/µl, 1 µg/ml, 1.5 µg/µl, and 2 µg/µl and coated. The serum was diluted 1:10, 1:50, 1:100, 1:200, and 1:400 and incubated with a secondary antibody at a dilution of 1:2000, which was optimized earlier. After an indirect ELISA technique was applied, 1.5 µg/µl and 1:10 were selected as the best antigen and serum dilutions respectively.
Figure 2.
Optimization of the coated antigen and primary antibody dilution. The antigen was diluted to 0.5 µg/µl, 0.75 µg/ml, 1 µg/µl, 1.5 µg/µl and 2 µg/µl, and 100 µl of diluted antigen was dispensed horizontally on the microplate wells. The negative and positive sera were diluted 1:10.1:50, 1:100, 1:200, and 1:400 and added vertically. After the indirect ELISA method was applied, 1.5 µg/µl antigen and a 1:10 serum dilution were selected for the development of the in-house indirect ELISA. P=positive serum, N= negative serum, µg=microgram, µl=microliter.
Figure 2.
Optimization of the coated antigen and primary antibody dilution. The antigen was diluted to 0.5 µg/µl, 0.75 µg/ml, 1 µg/µl, 1.5 µg/µl and 2 µg/µl, and 100 µl of diluted antigen was dispensed horizontally on the microplate wells. The negative and positive sera were diluted 1:10.1:50, 1:100, 1:200, and 1:400 and added vertically. After the indirect ELISA method was applied, 1.5 µg/µl antigen and a 1:10 serum dilution were selected for the development of the in-house indirect ELISA. P=positive serum, N= negative serum, µg=microgram, µl=microliter.
3.3. Optimization of Incubation Time and Temperature
Figure 3.
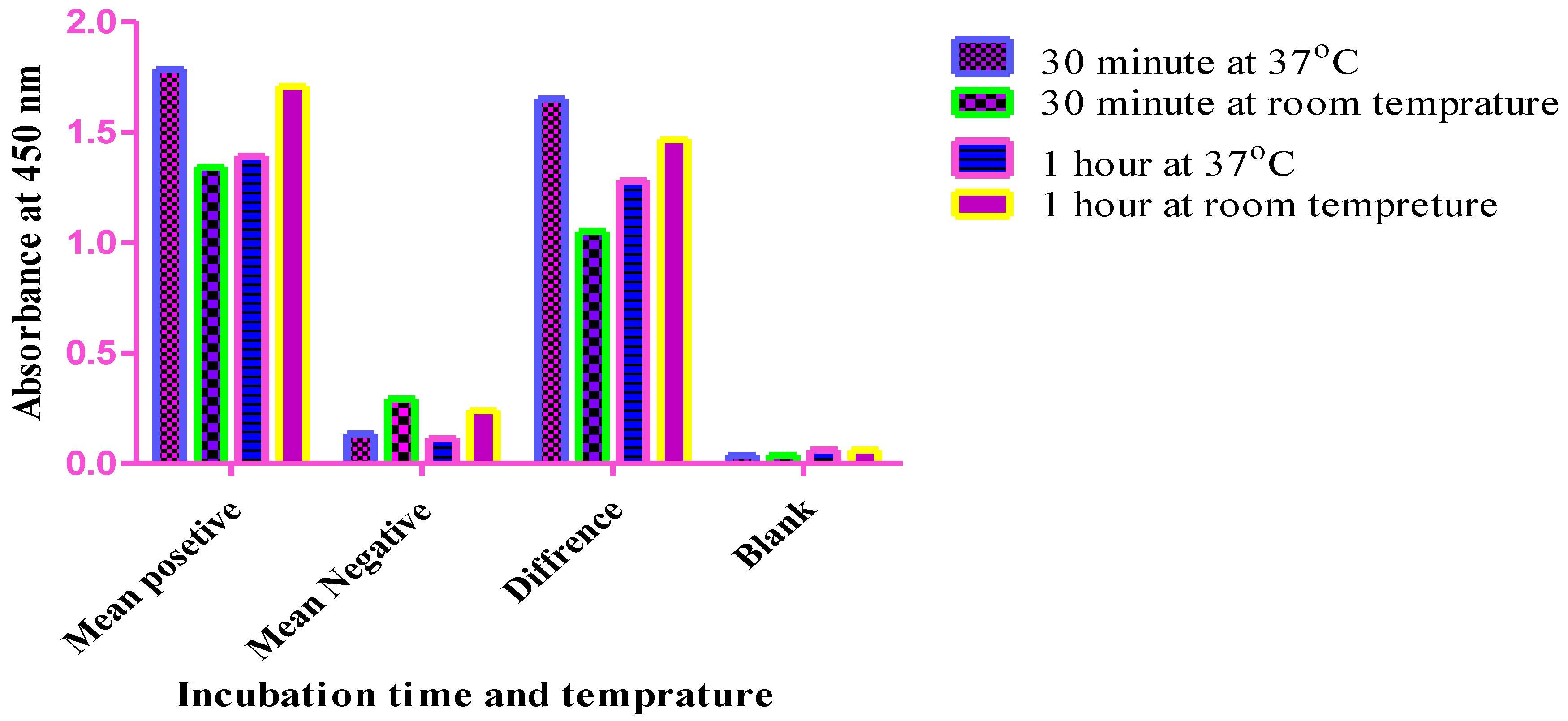
Optimization of the primary antibody incubation time. An in-house indirect ELISA method was used, and the primary antibody incubation times were compared after the samples were incubated for 30 minutes and 1 hr at 37 °C, 30 minutes, and 1 hr at room temperature. As shown in the graph, the best difference OD values of positive and negative sera were observed after an incubation time of 30 minutes at 37 °C, and these values were selected for the development of the current ELISA method.
Figure 3.
Optimization of the primary antibody incubation time. An in-house indirect ELISA method was used, and the primary antibody incubation times were compared after the samples were incubated for 30 minutes and 1 hr at 37 °C, 30 minutes, and 1 hr at room temperature. As shown in the graph, the best difference OD values of positive and negative sera were observed after an incubation time of 30 minutes at 37 °C, and these values were selected for the development of the current ELISA method.
3.3. Determination of the Cutoff Value
A cutoff value of 0.59 was established for the In-House Indirect ELISA Kit, which was calculated the method described by Kumar and Rao (1991): mean absorbance of negative controls + 3 standard deviations. Serum samples with antibody titers greater than 0.59 were considered positive for Mycoplasma gallisepticum, whereas those with values less than 0.59 were deemed negative.
Table 3. Sixteen (N=16) known negative controls were screened, and the means and standard deviations were determined. The cutoff value was calculated as 0.59. Calculation factor: Cutoff value = (mean ± 3 × standard deviation) of the negative control serum.
Table 3.
Determination of the cutoff value.
Table 3.
Determination of the cutoff value.
OD values of negative
serum |
Mean |
STDEV |
Cutoff value |
0.37
0.24
0.33
0.38
0.39
0.37
0.31
0.37
0.35
0.23
0.14
0.35
0.32
0.16
0.39
0.21 |
0.31 |
0.09 |
0.59 |
3.4. Determination of Test Sensitivity and Diagnostic Specificity
After optimization of the working antigen and serum, 16 known M. galisepticum-positive serum samples that were screened from naturally infected chickens and negative control serum samples identified from noninfected chickens were tested via the newly developed ELISA method and a commercial kit (ID vet indirect ELISA kit). The sensitivity and specificity of the current ELISA technique were 93.7 and 87.5, respectively.
Table 4. The test results of serum collected from naturally or experimentally infected animals (positive serum) and serum collected from noninfected animals (negative serum) are shown. The results revealed that out of the 16 positive and negative samples, the in-house indirect ELISA kit accurately detected both positive and negative sera. However, in the case of ID, vet Indirect ELISA kit out of 16 positive and Negative sera 1 sample was tested incorrectly.
3.5. Evaluation of the Kit Using Experimentally Produced Serum
The locally isolated M. gallisepticum was subsequently grown in culture media. The vaccine was prepared using oil and aluminum hydroxide adjuvants and was used to immunize chickens. Sera were collected 4 weeks after immunization, and the antibody response was evaluated with a newly developed ELISA kit.
Discussions
The demand for ELISA as a diagnostic method for serologically screening antibodies against
M. galisepticum in chickens is high. In developing countries, the commercial ELISA kits used for the detection of antibodies against
M. galisepticum are not adequate and are time-consuming for timely delivery. Furthermore, the sensitivity and specificity of the available kits are low. Accordingly, developing a more appropriate, highly sensitive and specific antibody detection method is compulsory. In the present study, a seemingly easily operational, less costly, more sensitive and specific in-house ELISA technique was developed and evaluated. For this developmental method, the incubation time was optimized to 37 °C for 30 minutes (
Figure 1).
Butler J et al. (1978) reported that ELISA is a fundamentally sensitive test that possibly overcomes at least some shortages of the remaining tests. In the present study, the
M. galisepticum antigen was purified from the culture after centrifugation and washed on the basis of the evidence that lysing the mycoplasmas with a high pH buffer may allow for a wider range of antibody recognition in the ELISA (Glenn F et al, 2002). Another report by Nicolet, J et al. (1980) reported that the selective digestion of nucleic acids also resulted in a better-quality product; indeed, it has been hypothesized that the presence of some of these internal cell components may be a cause of nonspecific serological binding. Consequently, after the purification process, the protein content was determined, followed by optimization of the working antigen and serum dilution (
Table 1).
The current ELISA method was further evaluated with serum collected from chickens immunized with the
M galisepticum vaccine using different adjuvants (aluminum hydroxide and oil). Since the antibody response against mycoplasma starts late, blood collection commenced on week 4, continued until week 9, and then was tested. On the basis of these data, the current kit detected negative and positive serum appropriately in both vaccine groups, with a slightly better antibody response against those chickens immunized with the oil adjuvant (
Figure 4).
In addition to the ELISA technique, the serum plate agglutination (SPA) test and hemagglutination inhibition (HI) test are the most commonly used assays for the serological detection of avian mycoplasmas (F. D. Talkington et al., 1985). In the report by Valentina A et al. (2022), the SPA test is a rapid and sensitive assay that detects immunoglobulin M (IgM) antibodies. Nevertheless, numerous factors related to the serum or antigen have an abundant effect on the specificity of this test method. Furthermore, the presence of a rheumatoid-like factor in avian serum also results in nonspecific agglutination and thus a lack of specificity (F. D. Talkington et al., 1985). Moreover, some commercial kits fail to detect experimentally produced antibodies. Therefore, the currently developed ELISA method is promising for overcoming the lack of antibody detection methods for Mycoplasma gallisepticum in chickens. The performance of this ELISA method was assessed by comparison with that of a commercial kit (ID-vet Indirect ELISA), which revealed a preeminent sensitivity and specificity. The method was well performed by testing positive and negative sera, which were experimentally produced and obtained from naturally infected animals, and this ELISA method will be used for the epidemiological study of M. galisepticum in chickens.
Conclusions
From this experimental work, we conclude that an economical and easily operational ELISA method was developed at the National Veterinary Institute and can be used for the serological detection of Mycoplasma gallisepticum in naturally and experimentally infected chickens.
Ethics statement
Not applicable
Statistical analysis
GraphPad Prism 5 and Microsoft Excell window 10 were used for the statistical analysis.
Consent to publish
Not applicable.
Availability of data and materials
Yes, available from the corresponding author upon request
Authors’ contributions
TT designed the study, performed the experiment and wrote the manuscript. TA, BB, TS and TT revised the manuscript.AL, KS, GA and AG performed experimental works. WW, WC and DD were supplied materials.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
We would like to thank the National Veterinary Institute for their overall support in this work.
Abbreviations
MG= Mycoplasma gallpticum, CRD=chronic respiratory disease, OIE= World Animal Health Organization, NDV=Newcastle disease virus, E. coli= Escherichia coli, IBV= infectious bronchitis virus, PBS=phosphate-buffered saline, BCA= Bicinconic acid, Cuso4=Cupper sulfate, PBST= phosphate-buffered saline Tween 20, TMB=3, 3’, 5, 5’-tetramethylbenzidine, H2SO4= sulfuric acid, OD=optical density, ELISA=enzyme-linked immunosorbent assay, STDEV=standard deviation, SPA= serum plate agglutination, HI= hemagglutination inhibition, IgM= immunoglobulin M,
References
- Hennigana, Suzanne L., Jeremy D, Driskellb., Naola, Ferguson-Noelc., Richard A, Dluhyd., Yiping, Zhaoe., Ralph A, Trippb., Duncan C, Krausea. 2011. "Detection and Differentiation of Avian Mycoplasmas by Surface-Enhanced Raman Spectroscopy Based on a Silver Nanorod Array". Applied and Environmental Microbiology. 78 (6): 1930–1935. [CrossRef] [PubMed]
- OIE. 2008. Avian mycoplasmosis (Mycoplasma gallisepticum, M. synoviae) is described in the manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees) in chapter 2. 3. 5. 6th ed. Paris: Office International Des Epizooties.
- Valks, M., and Burch, D. 2002. The Treatment and Control of Mycoplasma Infections in Turkey. Octagon Services Ltd., Old Windsor, Berks, United Kingdom.
- Bwala, D.G. 2017. Mycoplasma gallisepticum infection dynamics and vaccine protection in South African poultry (PhD dissertation) (pp. 40–69). Pretoria University of Pretoria. Google Scholar.
- Nakamura, K., Ueda, H., Tanimura, T. & Noguchi, K. 1994. Effect of mixed live vaccine (Newcastle disease and infectious bronchitis) and Mycoplasma galisepticum on the chicken respiratory tract and on Escherichia coli infection. Journal of Comparative Pathology, 111, 33–42. [CrossRef]
- Bradbury, J.M., Yavari, C.A. & Dare, C.M. 2001a. Mycoplasmas and respiratory disease in pheasants and partridges. Avian Pathology, 30, 391–396.
- Levisohn, S. and Kleven, S.H. 2000. Avian mycoplasmosis (Mycoplasma gallisepticum). Revue Scientifique et Technique Office International des Epizooties, 19, 425-442.
- Stipkovits, L. and Kempf, I.1996. Mycoplasmoses in poultry. Revue Scientifique et Technique Office International des Epizooties, 15, 1495-1525.
- Kleven, S.H.1997. Changing expectations in the control of Mycoplasma gallisepticum. Acta Veterinaria Hungarica, 45, 299-305.
- Gari, G., 2004. Studies on poultry Coccidiosis in TiyoWoredaArsi Zone, Oromia Re gional State. Addis Ababa University, Faculty of Veterinary Medicine, Debrezeit, Ethiopia, MSc Thesis.
- Bradbury, J.M., Yavari, C.A. & Dare, C.M. 2001a. Mycoplasmas and respiratory disease in pheasants and partridges. Avian Pathology, 30, 391–396.
- Yasmin, Jibril., Yilkal, Asfaw1., Berhe, Gebregziabher and Ahmed, Issa. 2018. Seroprevalence of Mycoplasma gallisepticum in do mestic chickens, East Shewa, Ethiopia. Ethiopian Veterinary Journal. [CrossRef]
- Tanimura, N., Tsukamoto, K., Nakamura, K., Narita, M. & Maeda M.1995. Association between pathogenicity of Infectious Bursal Disease Virus and viral antigen distribution detected by immu- nochemistry. Avian Diseases, 39, 9–20.
- Butler, J. E., T. L. Feldbush, P. L. McGivern, and N. Stewart. 1978. The enzymelinked immunosorbent assay (ELISA): A measure of antibody concentration or affinity? Immunochemistry 15:131-136.
- Glenn F. Browning., Peter J. Cowling., Denise O'Rourke, Kevin G. Whithear and Philip F. Markham 2002. Detection of Antibodies to Mycoplasma gallisepticum Vaccine ts-11 by an Autologous pMGA Enzyme-Linked Immunosorbent Assay. Avian Dis, 46 (2): 405–411.
- Nicolet, J., P. Paroz, and S. Bruggmann.1980. Tween 20 soluble proteins of Mycoplasma hyopneumoniae as antigen for an enzyme-linked immunosorbent assay. Res. Vet. Sci. 29:305-309.
- Samad, A., Awaz, KB and Sarkate, LB.1994. Diagnosis of bovine traumatic reticulo peritonitis I: strength of clinical signs in predicting correct diagnosis. Journal of Applied Animal Research 6: 13-18.
- Kumar, A and Rao, AT.1991. Double-antibody sandwich ELISA for detection of infectious bursal disease Virus. British Veterinary Journal 147: 251-255.
- F. D. Talkington., S. H. Kleven and J. Brown. 1985. An Enzyme-Linked Immunosorbent Assay for the Detection of Antibodies to Mycoplasma galisepticum in Experimentally Infected Chickens. American Association of Avian Pathologists, Avian Diseases, Vol. 29, pp. 53-70.
- Valentina, A., Schmidt, Victoria Rose Stevens., Javan, Esfandiari, and Konstantin P,. Lyashchenk.2022. Rapid Point-of-Care Tests Using Staphylococcal Protein A Can Detect Early IgM Responses in HIV-1 and Treponema pallidum Infections. Microbial Spectr.10 (6), e03309-22.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).